Taguchi Method and Response Surface Methodology in the Treatment of Highly Contaminated Tannery Wastewater Using Commercial Potassium Ferrate
Abstract
1. Introduction
2. Materials and Methods
2.1. Apparatus and Experiment Conditions
2.2. Chemicals
2.3. Origin and Physicochemical Parameters of the Raw Tannery Wastewater
2.4. Analytical Procedures
2.5. Procedures for Experiment Optimization Using the Taguchi Method
2.6. Response Surface Methodology
3. Results and Discussion
3.1. Physicochemical Parameters of the Raw Tannery Wastewater and K2FeO4 (Envifer®)
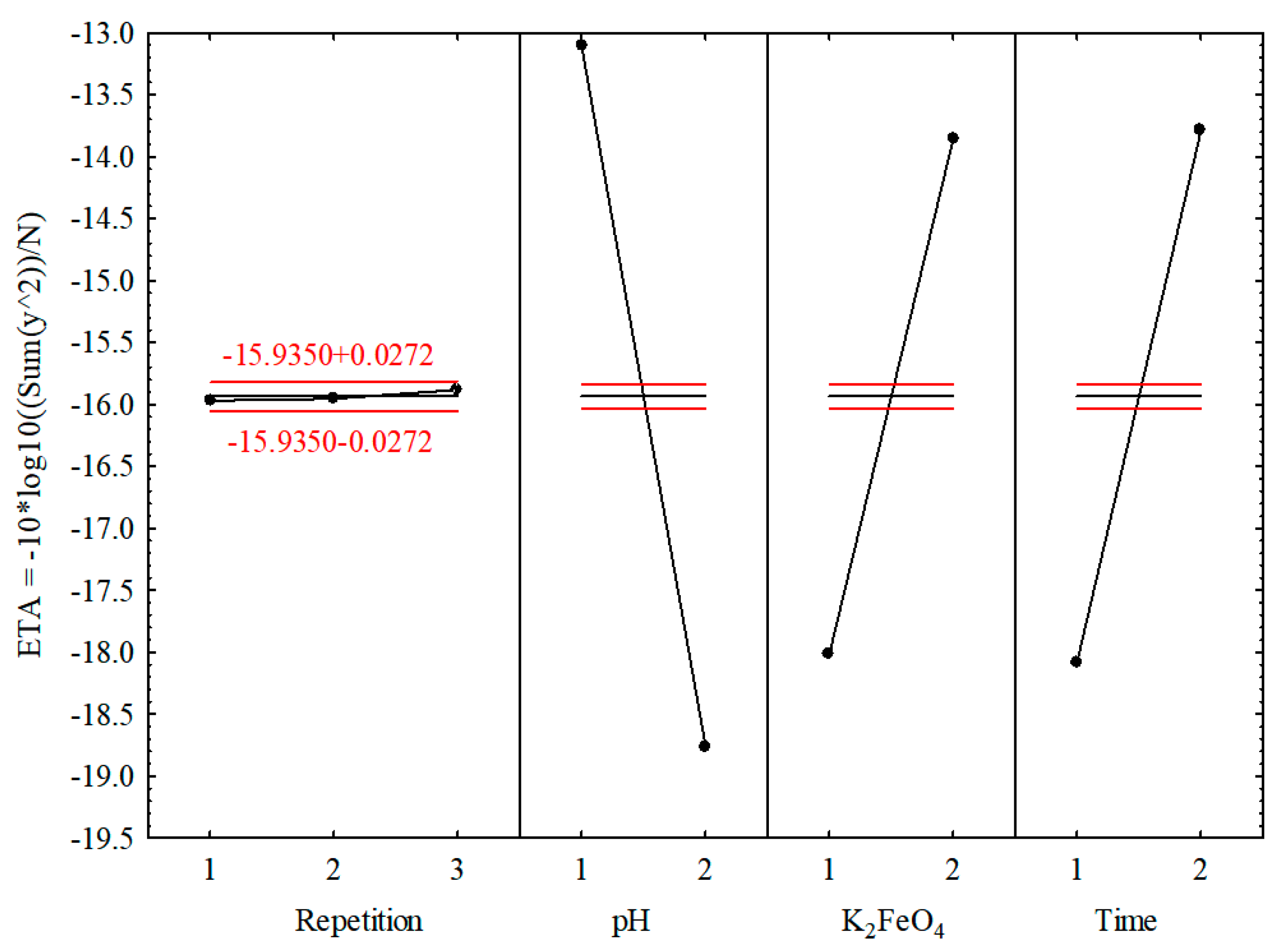
3.2. Taguchi Method
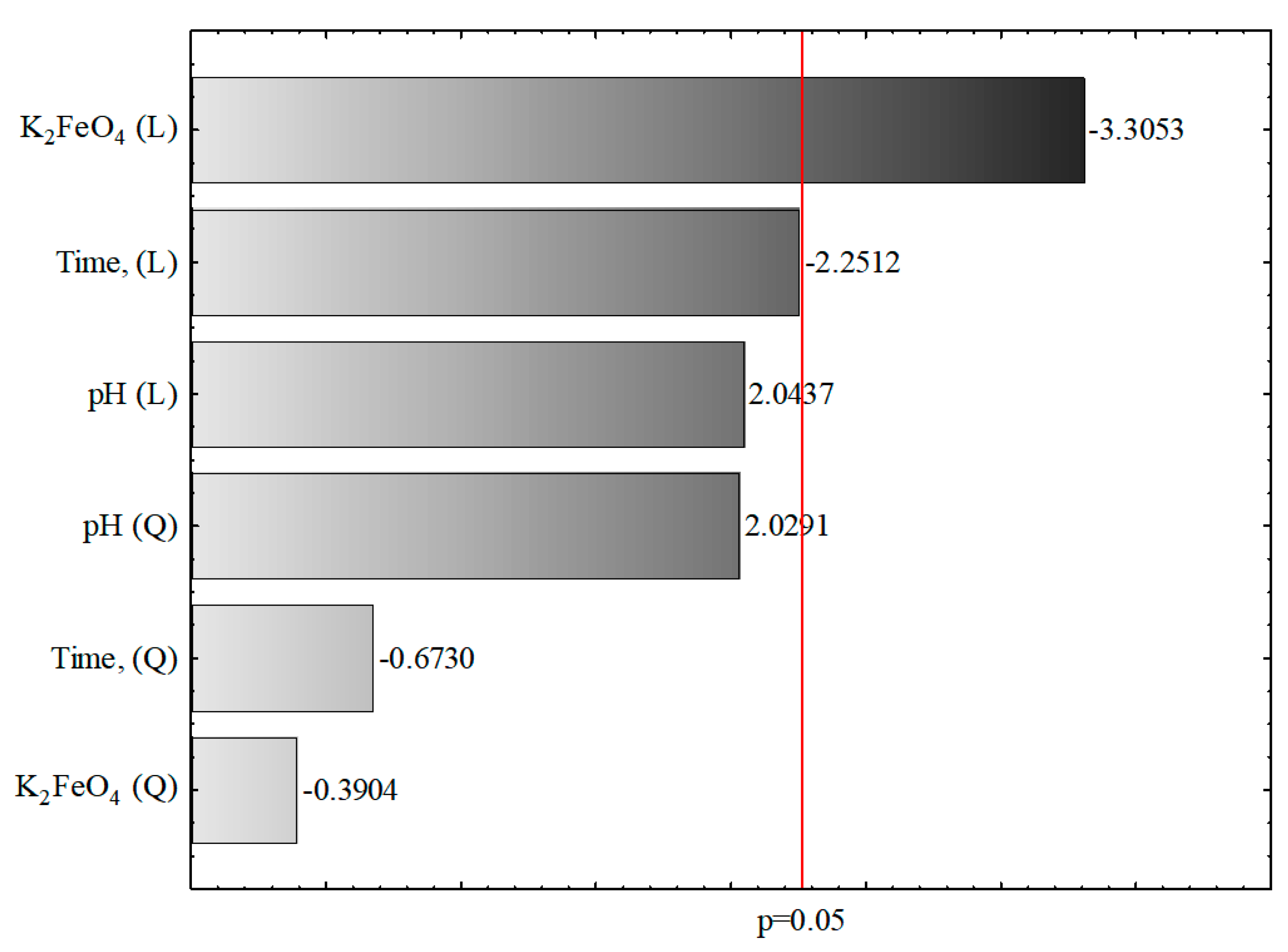
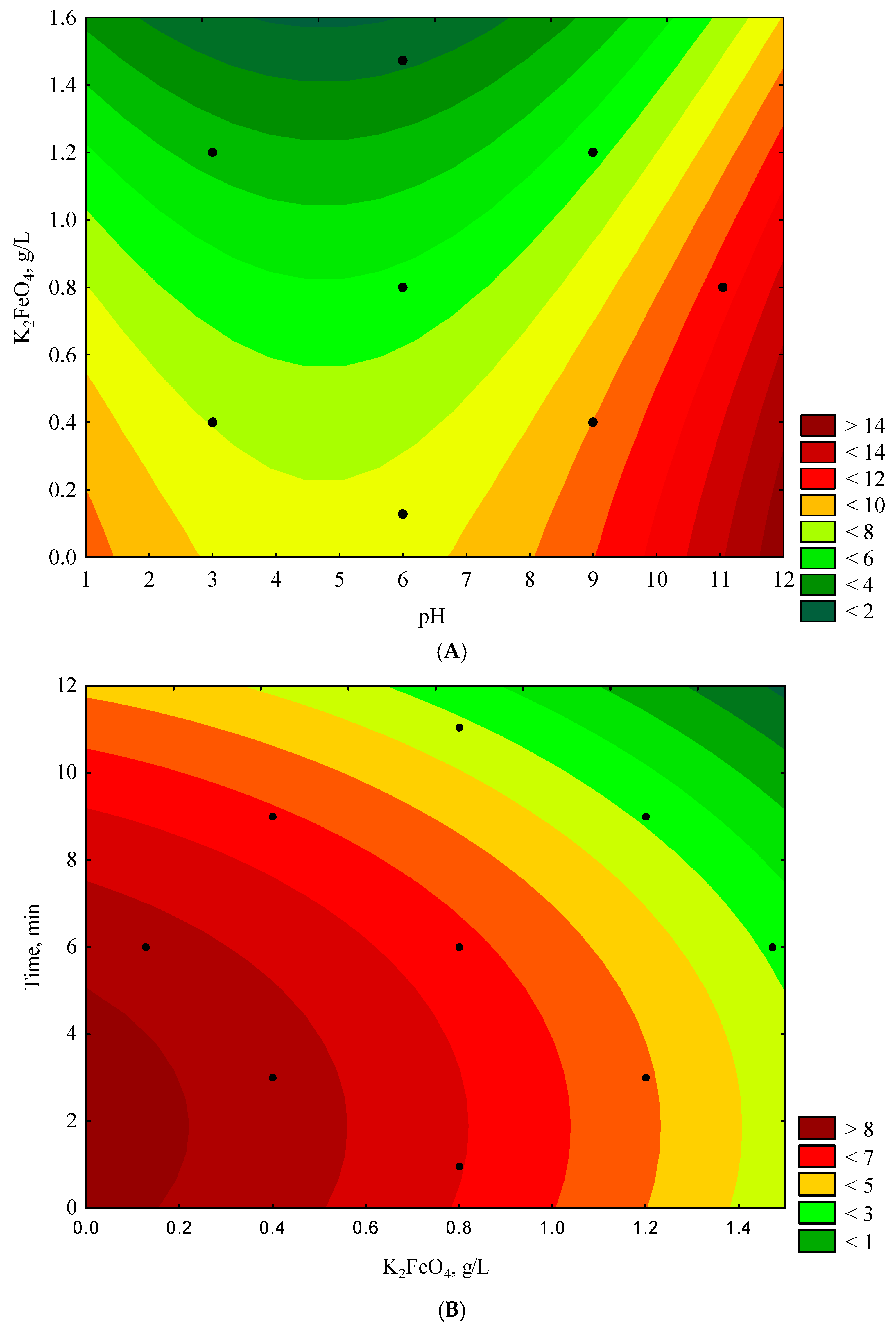
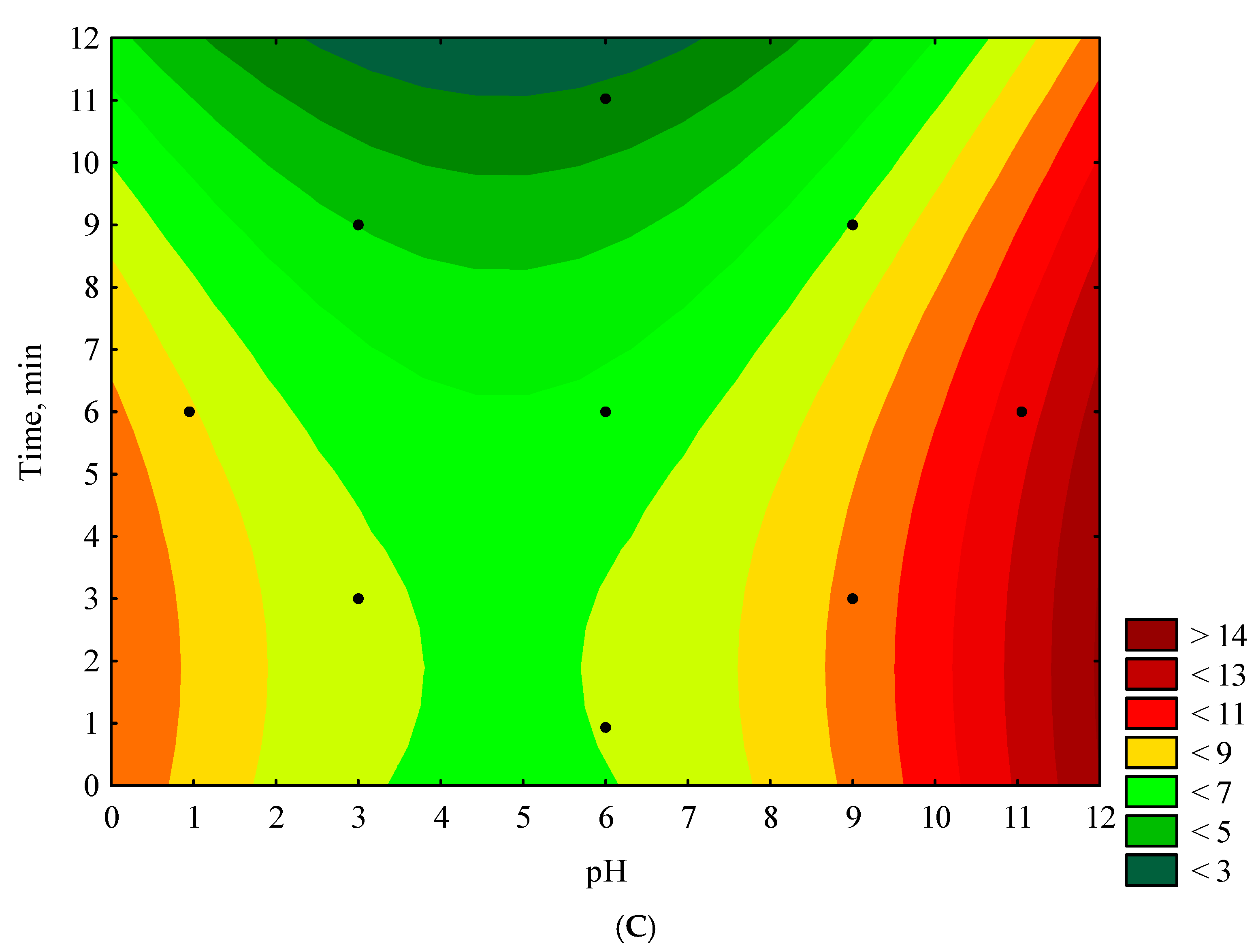
3.3. Response Surface Findings
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Leta, S.; Assefa, F.; Gumaelius, L.; Dalhammar, G. Biological nitrogen and organic matter removal from tannery wastewater in pilot plant operations in Ethiopia. Appl. Microbiol. Biotechnol. 2004, 66, 333–339. [Google Scholar] [CrossRef]
- Lefebvre, N.; Vasudevan, N.; Torrijosa, M.; Thanasekaran, K.; Moletta, R. Anaerobic digestion of tannery soak liquor with an aerobic post-treatment. Water Res. 2006, 40, 1492–1500. [Google Scholar] [CrossRef]
- Haydar, S.; Aziz, A. Characterization and treatability studies of tannery wastewater using chemically enhanced primary treatment (CEPT)-a case study of Saddiq Leather Works. J. Hazard. Mater. 2009, 163, 1076–1083. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, W.; Gong, X.; Li, X.; Liu, W.; He, C.; Wang, Z.; Minh, Q.N.; Chen, C.L.; Wang, J.Y. Biological pretreatment of tannery wastewater using a full-scale hydrolysis acidification system. Int. Biodeterior. Biodegrad. 2014, 95, 41–45. [Google Scholar] [CrossRef]
- Lofrano, G.; Meric, S.; Zengin, G.E.; Orhon, D. Chemical and biological treatment technologies for leather tannery chemicals and wastewaters: A review. Sci. Total Environ. 2013, 462, 265–281. [Google Scholar] [CrossRef] [PubMed]
- Lofrano, G.; Aydin, E.; Russo, F.; Guida, M.; Belgiorno, V.; Meric, S. Characterization, fluxes and toxicity of leather tanning bath chemicals in a large tanning district area (IT). Water Air Soil Pollut. Focus 2008, 8, 529–542. [Google Scholar] [CrossRef]
- Mannucci, A.; Munz, G.; Mori, G.; Lubello, C. Anaerobic treatment of vegetable tannery wastewaters: A review. Desalination 2010, 264, 1–8. [Google Scholar] [CrossRef]
- De Nicola, E.; Meriç, S.; Gallo, M.; Iaccarino, M.; Della Rocca, C.; Lofrano, G. Vegetable and synthetic tannins induce hormesis/toxicity in sea urchin early development and in algal growth. Environ. Pollut. 2007, 146, 46–54. [Google Scholar] [CrossRef]
- Munz, G.; De Angelis, D.; Gori, R.; Mori, G.; Casarci, M.; Lubello, C. The role of tannins in conventional angogated membrane treatment of tannery wastewater. J. Hazard. Mater. 2009, 164, 733–739. [Google Scholar] [CrossRef]
- Tunay, O.; Kabdasli, I.; Orhon, D.; Ates, E. Characterization and pollution profile of leather tanning industry in Turkey. Water Sci. Technol. 1995, 32, 1–9. [Google Scholar] [CrossRef]
- Boujelben, R.; Ellouze, M.; Sayadi, S. Detoxification assays of Tunisian tannery wastewater under nonsterile conditions using the filamentous fungus Aspergillus niger. BioMed Res. Int. 2019, 2019, 9020178. [Google Scholar] [CrossRef] [PubMed]
- Durai, G.; Rajasimmam, M. Biological treatment of tannery wastewater: A review. J. Environ. Sci. Technol. 2011, 4, 1–17. [Google Scholar] [CrossRef]
- Kongjao, S.; Damronglerd, S.; Hunsom, M. Simultaneous removal of organic and inorganic pollutants in tannery wastewater using electrocoagulation technique. Korean J. Chem. Eng. 2008, 25, 703–709. [Google Scholar] [CrossRef]
- Kumar, V.; Majumdar, C.; Roy, P. Effects of endocrine disrupting chemicals from leather industry effluents on male reproductive system. J. Steroid Biochem. Mol. Biol. 2008, 111, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Abbas, M.; Nisar, J.; Nazir, A.; Qamar, A.Z. Bioassays based on higher plants as excellent dosimeters for ecotoxicity monitoring: A review. Chem. Int. 2019, 5, 1–80. [Google Scholar]
- Abbas, M.; Adil, M.; Ethisham-ul-Haque, S.; Munir, B.; Yameen, M.; Ghaffar, A.; Shar, G.A.; Tahir, M.A.; Iqbal, M. Vibrio fischeri bioluminescence inhibition assay for ecotoxicity assessment: A review. Sci. Total Environ. 2018, 626, 1295–1309. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M. Vicia faba bioassay for environmental toxicity monitoring: A review. Chemosphere 2016, 144, 785–802. [Google Scholar] [CrossRef]
- Montalvao, M.F.; de Souza, J.M.; Guimaraes, A.T.B.; Menezes, I.P.P.; Castro, A.L.S.; Rodriugues, A.S.L.; Malafaia, G. The genotoxicity and cytotoxicity of tannery wastewater effluent in bullfrog (Lithobates catesbeianus). Chemosphere 2017, 183, 491–502. [Google Scholar] [CrossRef]
- Yadav, A.; Raj, A.; Purchase, D.; Ferreira, L.F.R.; Saratale, G.D.; Bharagava, R.N. Phytotoxicity, cytotoxicity and genotoxicity evaluation of organic and inorganic pollutants rich tannery wastewater from a Common Effluent Treatment Plant (CETP) in Unnao district, India using Vigna radiata and Allium cepa. Chemosphere 2019, 224, 324–332. [Google Scholar] [CrossRef]
- Carpenter, J.; Sharma, S.; Sharma, A.K.; Verma, S. Adsorption of dye by using the solid waste from leather industry as an adsorbent. Int. J. Eng. Sci. Invent. 2013, 2, 64–69. [Google Scholar]
- Dixit, S.; Yadav, A.; Dwivedi, P.D.; Das, M. Toxic hazards of leather industry and technologies to combat threat: A review. J. Clean. Prod. 2015, 87, 39–49. [Google Scholar] [CrossRef]
- Bhatnagar, M.K.; Singh, R.; Gupta, S.; Bhatnagar, P. Study of tannery effluents and its effects on sediments of river Ganga in special reference to heavy metals at Jajmau, Kanpur, India. J. Environ. Res. Dev. 2013, 8, 56–59. [Google Scholar]
- Money, C.A. Salinity Reduction in Tannery Effluents in India and Australia; Final Report on Project AS1/2001/005; ACIAR: Canberra, ACT, Australia, 2008. [Google Scholar]
- Schilling, K.; Ulrike, B.; Helmut, K.; Zessner, M. Adapting the Austrian Edict on wastewater emissions for tanneries as consequence of foam formation on surface waters. Environ. Sci. Pollut. 2012, 23, 68–73. [Google Scholar] [CrossRef]
- Song, Z.; Williams, C.J.; Edyvean, R.G.J. Treatment of tannery wastewater by chemical coagulation. Desalination 2004, 164, 249–259. [Google Scholar] [CrossRef]
- Lofrano, G.; Meric, S.; Inglese, M.; Nikolaou, A.D.; Belgiorno, V. Fenton oxidation treatment of tannery wastewater and tanning agents: Synthetic tannin and nonylphenol ethoxylate based degreasing agents. Desalin. Water Treat. 2010, 23, 173–180. [Google Scholar] [CrossRef]
- Iqbal, M.; Muneer, M.; Hussain, S.; Parveen, B.; Javed, M.; Rehman, H.; Waquas, M.; Abid, M.A. Using combined UV and H2O2 treatments to reduce tannery wastewater pollution load. Pol. J. Environ. Stud. 2019, 28, 3207–3213. [Google Scholar] [CrossRef]
- Shanmugam, B.K.; Easwaran, S.N.; Mohanakrishnan, A.S.; Kalyanaraman, C.; Mahadevan, S. Biodegradation of tannery dye effluent using Fenton’s reagent and bacterial consortium: A biocalorimetric investigation. J. Environ. Manag. 2019, 15, 106–113. [Google Scholar] [CrossRef]
- Rodriquez-Rodriguez, J.; Ochando-Pulido, J.M.; Martinez-Ferez, A. The effect of pH in tannery wastewater by Fenton vs. heterogeneous Fenton process. Chem. Eng. Trans. 2019, 73, 205–210. [Google Scholar]
- Sekaran, G.; Chitra, K.; Mariappan, M.; Raghavan, K.V. Removal of sulphide in anaerobically treated tannery wastewater by wet air oxidation. J. Environ. Sci. Health A 1996, 31, 579–598. [Google Scholar] [CrossRef]
- Krishnomoorhi, S.; Sivakkumar, V.; Saravanan, K.; Prabhu, S. Treatment and reuse of tannery waste water by embedded system. Mod. Appl. Sci. 2009, 3, 129–134. [Google Scholar]
- Mohammed, K.; Sahu, O. Recovery of chromium from tannery industry waste water by membrane separation technology: Health and engineering aspects. Sci. Afr. 2019, 4, 1–9. [Google Scholar] [CrossRef]
- Roopa, D.; Divya, R.; Nathiya, S. Management of RO reject water from the tannery industry by solar tunnel dryer. Int. J. Adv. Res. 2019, 4, 15–20. [Google Scholar]
- Tare, V.; Gupta, S.; Bose, P. Case studies on biological treatment of tannery effluents in India. J. Air Waste Manag. Assoc. 2003, 53, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Audette, R.J.; Quail, J.W.; Smith, P.J. Ferrate (VI) ion, a novel oxidizing agent. Tetrahedron Lett. 1971, 3, 279–282. [Google Scholar] [CrossRef]
- Moradnia, M.; Panahifard, M.; Dindarlo, K.; Jamali, H.A. Optimizing potassium ferrate for textile wastewater treatment by RSM. Environ. Health Eng. Manag. J. 2016, 3, 137–142. [Google Scholar] [CrossRef]
- Thomas, M.; Barbusinski, K.; Klis, S.; Szpyrka, E.; Chyc, M. Synthetic textile wastewater treatment using potassium ferrate(VI)—Application of Taguchi method for optimisation of Experiment. Fibres Text. East. Eur. 2018, 3, 104–109. [Google Scholar] [CrossRef]
- Jiang, J.Q.; Panagoulopoulos, A.; Bauer, M.; Pearce, P. The application of potassium ferrate for sewage treatment. J. Environ. Manag. 2006, 79, 215–220. [Google Scholar] [CrossRef]
- Bartzat, R.; Nagel, D. Removal of nitrosamines from wastewater by potassium ferrate. Arch. Environ. Health Int. J. 1991, 46, 313–315. [Google Scholar] [CrossRef]
- Wu, K.; Wang, H.; Zhou, C.h.; Amina, Y.; Si, Y. Efficient oxidative removal of sulfonamide antibiotics from the wastewater by potassium ferrate. J. Adv. Oxid. Technol. 2018, 21, 97–108. [Google Scholar] [CrossRef]
- Rai, P.K.; Lee, J.; Kailasa, S.K.; Kwon, E.E.; Tsang, Y.F.; Ok, Y.S.; Kim, K.H. A critical review of ferrate(VI)-based remediation of soil and groundwater. Environ. Res. 2018, 160, 420–448. [Google Scholar] [CrossRef]
- Gokkus, O.; Yildiz, Y.S.; Yavuz, B. Optimization of chemical coagulation of real textile wastewater using Taguchi experimental design method. Desalin. Water Treat. 2012, 49, 263–271. [Google Scholar] [CrossRef]
- Madaeni, S.S.; Koocheki, S. Application of Taguchi method in the optimization of wastewater treatment using spiral-wound reverse osmosis element. Chem. Eng. J. 2006, 119, 37–44. [Google Scholar] [CrossRef]
- Yousefi, Z.; Zafarzadeh, A.; Ghezel, A. Applicaion of Taguchi’s experimental design method for optimization of Acid Red 18 removal by electrochemical oxidation process. Environ. Health Eng. Manag. 2019, 5, 241–248. [Google Scholar] [CrossRef]
- Mosaddegh, M.R.; Shariati, F.P.; Yazdi, S.A.V.; Bidhendi, G.N. Application of response surface methodology (RSM) for optimizing coagulation process of paper recycling wastewater using Ocimum basilicum. Environ. Technol. 2018, 3, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Białecka, B.; Zdebik, D. Removal of organic compounds from wastewater originating from the production of printed circuit boards by UV-Fenton method. Arch. Environ. Prot. 2017, 43, 39–49. [Google Scholar] [CrossRef][Green Version]
- Bui, H.M. Optimization of electrocagulation of instant coffee production wastewater by using the response surface methodology. Pol. J. Chem. 2017, 19, 67–71. [Google Scholar] [CrossRef]
- He, Y.; Huang, G.; An, C.; Huang, J.; Zhang, P.; Chen, X.; Xin, X. Reduction of Escherichia Coli using ceramic disk filter decorated by nano-TiO2: A low-cost solution for household water purification. Sci. Total Environ. 2018, 616, 1628–1637. [Google Scholar] [CrossRef]
- Kakoi, B.; Kaluli, J.W.; Ndiba, P.; Thiong’o, G. Optimization of Maerua Decumbent bio-coagulant in paint industry wastewater treatment with response surface methodology. J. Clean. Prod. 2017, 164, 1124–1134. [Google Scholar] [CrossRef]
- Schreyer, J.M.; Thompson, G.W.; Ockerman, L.T. Oxidation of chromium(III) with potassium ferrate(VI). Anal. Chem. 1950, 22, 1426–1427. [Google Scholar] [CrossRef]
- Wei, Y.L.; Wang, Y.S.; Liu, C.H. Preparation of potassium ferrate from spent steel pickling liquid. Metals 2015, 5, 1770–1787. [Google Scholar] [CrossRef]
- PN-C-04541:1978. Water and Wastewater. Determination of Dry Residue, Roasting Residue, Loss after Roasting, Dissolved Substances, Mineral Dissolved Substances and Volatile Dissolved Substances; The Polish Committee for Standardization: Warsaw, Poland, 1978.
- PN-ISO 10523:2012. Water Quality. Determination of pH; The Polish Committee for Standardization: Warsaw, Poland, 2012.
- PN-ISO 7887:2012. Water Quality. Examination and Determination of Colour.; The Polish Committee for Standardization: Warsaw, Poland, 2011.
- PN-ISO 15705:2005. Water Quality. Determination of the Chemical Oxygen Demand Index (ST-COD). Small-Scale Sealed-Tube Method; The Polish Committee for Standardization: Warsaw, Poland, 2005.
- Nanocolor. Available online: http://ftp.mn-net.com/english/Instruction_leaflets/NANOCOLOR/985094en (accessed on 1 October 2018).
- Sreeja, P.H.; Sosamony, K.J. A comparative study of homogeneous and heterogeneous photo-fenton process for textile wastewater. Proc. Technol. 2016, 24, 217–223. [Google Scholar] [CrossRef]
- Andrew Liou, Y.H.; Lin, P.P.; Lindeke, R.R.; Chiang, H.D. Tolerance specification of robot kinematic parameters using an experimental design technique the Taguchi method. Robot. Comput. Integr. Manuf. 1993, 10, 199–207. [Google Scholar] [CrossRef]
- Thirugnanasambandham, K.; Sivakumar, V.; Shine, K. Optimization of reverse osmosis treatment process to reuse the distillery wastewater using Taguchi design. Desalin. Water Treat. 2016, 57, 24222–24230. [Google Scholar] [CrossRef]
- Thirugnanasambandham, K.; Sivakumar, V. Enzymatic catalysis treatment method of meat industry wastewater using lacasse. J. Environ. Health Sci. Eng. 2015, 13, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Thirugnanasambandham, K.; Sivakumar, V.; Prakash Maran, J. Performance evaluation and optimization of electrocoagulation process to treat grey wastewater. Desalin. Water Treat. 2015, 55, 1703–1711. [Google Scholar] [CrossRef]
- Thirugnanasambandham, K.; Sivakumar, V. An eco-friendly approach for copper (II) ion adsorption onto cotton seed cake and its characterization: Simulation and validation. J. Taiwan Inst. Chem. Eng. 2015, 50, 198–204. [Google Scholar] [CrossRef]
- Thirugnanasambandham, K.; Sivakumar, V. Optimization of treatment of grey wastewater using electro-Fenton technique—Modeling and validation. Process Saf. Environ. 2015, 95, 60–68. [Google Scholar] [CrossRef]
- Srinu Naik, S.; Pydi Setty, Y. Optimization for denitrification of wastewater using fluidized bed bioreactor by Taguchi method. Int. J. Biotechnol. Appl. 2011, 3, 97–101. [Google Scholar]
- Sahinkaya, S. Decolorization of reactive orange 16 via ferrate(VI) oxidation assisted by sonication. Turk. J. Chem. 2017, 41, 577–586. [Google Scholar] [CrossRef]
- Majid, D.; Kim, I.K. Degradation of toluene by liquid Ferrate(VI) and solid Ferrate(VI) in aqueous phase. J. Environ. Eng. 2018, 144, 04018093. [Google Scholar] [CrossRef]
- Yang, B.; Ying, G.G. Oxidation of benzophenone-3 during water treatment with ferrate(VI). Water Res. 2013, 47, 2458–2466. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Barbusiński, K.; Kalemba, K.; Piskorz, P.J.; Kozik, V.; Bak, A. Optimization of the Fenton oxidation of synthetic textile wastewater using response surface methodology. Fibres Text. East. Eur. 2017, 6, 108–113. [Google Scholar] [CrossRef]
- Saravanathamizhan, R.; Mohan, N.; Balasubramanian, N.; Ramamurthi, V.; Ahmed Basha, C. Evaluation of electro-oxidation of textile effluent using response surface methods. CLEAN Soil Air Water 2007, 35, 355–361. [Google Scholar] [CrossRef]
- Lei, B.; Zhou, C.; Cheng, T.; Du, J. Synthesis of Potassium Ferrate by chemical dry oxidation and its properties in degradation of methyl orange. Asian J. Chem. 2013, 25, 27–31. [Google Scholar] [CrossRef]
- Dong, X.L.; Wang, L.; Zhang, X.X.; Bai, L.; Zhang, X.F.; Ma, H.C.; Ma, C.; Xue, M. Oxidative degradation of azo dye reactive red 2BF by Potassium Ferrate. Adv. Mater. Res. 2012, 518, 2617–2620. [Google Scholar] [CrossRef]
- Hu, M.Z.; Shi, Z.H.; Zhao, H.Y. Study on treatment of m-cresol wastewater using potassium ferrate(VI). Adv. Mater. Res. 2013, 610, 2367–2371. [Google Scholar] [CrossRef]
- Mia, Z.; Wang, F.; Deng, D.; Wang, L.; Yang, J. Removal effect of Potassium Ferrate to COD in different wastewater. Adv. Mater. Res. 2012, 550, 2288–2290. [Google Scholar] [CrossRef]
- Jiang, J.Q.; Stanford, C.; Petri, M. Practical application off Ferrate(VI) for water and wastewater treatment—Site study’s approach. Water Energy Nexus 2018, 1, 42–46. [Google Scholar] [CrossRef]
- Pinto, B.M.; Samanamud, G.R.L.; Baston, E.P.; Franca, A.B.; Naves, L.L.R.; Lourdes, C.C.A.; Naves, F.L. Multivariate and multiobjective optimization of tannery industry effluent treatment using Musa sp flower extract in the coagulation and flocculation process. J. Clean. Prod. 2019, 219, 655–666. [Google Scholar] [CrossRef]
- Priya, K.; Abbasi, T.; Murugaiyn, V. Modeling the performance of a tannery common effluent treatment plant using artificial neural networks. Desalin. Water Treat. 2018, 122, 57–65. [Google Scholar] [CrossRef]
- Elabed, A.; El Khalfaouy, R.; Ibnsouda, S.; Basseguy, R.; Elabed, S.; Erable, B. Low-cost electrode modification to upgrade the bioelectrocatalytic oxidation of tannery wastewater using acclimated activated sludge. Appl. Sci. 2019, 9, 2259. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Peleka, E.N.; Ntolia, A. Treatment of tannery wastewater with vibratory shear-enhanced processing membrane filtration. Separations 2019, 6, 20. [Google Scholar] [CrossRef]
- Stoller, M.; Sacco, O.; Sannino, D.; Chianese, A. Successful integration of membrane technologies in a conventional purification process of tannery wastewater streams. Membranes 2013, 3, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Korpe, S.; Bethi, B.; Sonawane, S.H.; Jayakumar, K.V. Tannery wastewater treatment by cavitation combined with advanced oxidation process (AOP). Ultrason. Sonochem. 2019, 59, 104723. [Google Scholar] [CrossRef] [PubMed]
- Mella, B.; de Carvalho Barcellos, B.S.; da Silva Costa, D.E.; Gutterres, M. Treatment of leather dyeing wastewater with associated process of coagulation-flocculation/adsorption/ozonation. Ozone Sci. Eng. 2018, 2, 133–140. [Google Scholar] [CrossRef]
- Sathishkumar, K.; Narenkumar, J.; Madhavan, J.; Murugan, K.; Rajasekar, A. Electrochemical decolorization and biodegradation of tannery effluent for reduction of chemical oxygen demand and hexavalent chromium. J. Water Process Eng. 2017, 20, 22–28. [Google Scholar]
| Parameter | Unit | Result * |
|---|---|---|
| pH | - | 8.6 ± 0.1 |
| Total Dissolved Solids | mg/L | 18,250 ± 1825 |
| Total Solids | mg/L | 19,700 ± 1970 |
| Suspended Solids | mg/L | 1450 ± 145 |
| Color | mg Pt/L | 10,950 ± 2190 |
| Chemical Oxygen Demand | mg O2/L | 12,560 ± 1880 |
| Total Organic Carbon | mg/L | 4860 ± 729 |
| Run | Experimental Conditions | Experimental Results * | ||||||
|---|---|---|---|---|---|---|---|---|
| Repetition | pH | K2FeO4 | Time | pH | K2FeO4 (g/L) | Time (min) | COD (g O2/L) | |
| 1 | 1 | 1 | 1 | 1 | 3.0 | 0.400 | 3.00 | 7.340 ± 1.100 |
| 2 | 1 | 1 | 2 | 2 | 3.0 | 1.200 | 9.00 | 2.850 ± 0.430 |
| 3 | 1 | 2 | 1 | 2 | 9.0 | 0.400 | 9.00 | 8.570 ± 1.290 |
| 4 | 1 | 2 | 2 | 1 | 9.0 | 1.200 | 3.00 | 8.720 ± 1.310 |
| 5 | 2 | 1 | 1 | 1 | 3.0 | 0.400 | 3.00 | 7.390 ± 1.110 |
| 6 | 2 | 1 | 2 | 2 | 3.0 | 1.200 | 9.00 | 2.790 ± 0.420 |
| 7 | 2 | 2 | 1 | 2 | 9.0 | 0.400 | 9.00 | 8.600 ± 1.290 |
| 8 | 2 | 2 | 2 | 1 | 9.0 | 1.200 | 3.00 | 8.750 ± 1.310 |
| 9 | 3 | 1 | 1 | 1 | 3.0 | 0.400 | 3.00 | 7.340 ± 1.100 |
| 10 | 3 | 1 | 2 | 2 | 3.0 | 1.200 | 9.00 | 2.710 ± 0.410 |
| 11 | 3 | 2 | 1 | 2 | 9.0 | 0.400 | 9.00 | 8.640 ± 1.300 |
| 12 | 3 | 2 | 2 | 1 | 9.0 | 1.200 | 3.00 | 8.730 ± 1.310 |
| Run | Experimental Conditions | Experimental Results * | ||
|---|---|---|---|---|
| pH | K2FeO4 (g/L) | Time (min) | COD (g O2/L) | |
| 1 | 3.00 | 0.400 | 3.00 | 7.340 ± 1.100 |
| 2 | 3.00 | 0.400 | 9.00 | 5.570 ± 0.840 |
| 3 | 3.00 | 1.200 | 3.00 | 3.350 ± 0.500 |
| 4 | 3.00 | 1.200 | 9.00 | 1.850 ± 0.280 |
| 5 | 9.00 | 0.400 | 3.00 | 11.800 ± 1.770 |
| 6 | 9.00 | 0.400 | 9.00 | 8.570 ± 1.290 |
| 7 | 9.00 | 1.200 | 3.00 | 8.720 ± 1.310 |
| 8 | 9.00 | 1.200 | 9.00 | 6.980 ± 1.050 |
| 9 | 0.95 | 0.800 | 6.00 | 11.150 ± 1.670 |
| 10 | 11.05 | 0.800 | 6.00 | 8.850 ± 1.330 |
| 11 | 6.00 | 0.127 | 6.00 | 8.900 ± 1.340 |
| 12 | 6.00 | 1.473 | 6.00 | 2.710 ± 0.410 |
| 13 | 6.00 | 0.800 | 0.96 | 7.480 ± 1.120 |
| 14 | 6.00 | 0.800 | 11.05 | 3.150 ± 0.470 |
| 15 (C) | 6.00 | 0.800 | 6.00 | 6.280 ± 0.940 |
| 16 (C) | 6.00 | 0.800 | 6.00 | 6.270 ± 0.940 |
| Effect/Factor | Analysis of Variance, Mean = −15.9350, Sigma = 4.2932 | ||||
|---|---|---|---|---|---|
| SS | df | MS | F | p | |
| Repetition | 0.0180 | 2 | 0.0090 | 0.646 | 0.5569 |
| pH | 95.6632 | 1 | 95.6632 | 6855.424 | <0.05 |
| K2FeO4 | 51.8397 | 1 | 51.8396 | 3714.937 | <0.05 |
| Time | 55.1406 | 1 | 55.1405 | 3951.489 | <0.05 |
| Error | 0.0837 | 6 | 0.0136 | – | – |
| Effect/Factor | Predicted S/N Ratios under the Optimal Conditions Mean = −15.9350, Sigma = 4.2932 | ||
|---|---|---|---|
| Parameter Value | Significance of Effect | Standard Error | |
| Repetition | 3 | 0.0540 | 0.0591 |
| pH | 1 (pH = 3) | 2.8235 | 0.0482 |
| K2FeO4 | 2 (1.200 g/L) | 2.0785 | 0.0482 |
| Time | 2 (9 min) | 2.1436 | 0.0482 |
| Expected S/N ratio | - | −8.8354 | - |
| Parameter | Unit | Results * | Effect (%) ** |
|---|---|---|---|
| pH | - | 8.5 ± 0.1 | Non-significant |
| Total Dissolved Solids | mg/L | 19,430 ± 1940 | ↑6.5 |
| Total Solids | mg/L | 19,920 ± 1990 | ↑1.1 |
| Suspended Solids | mg/L | 45.0 ± 4.5 | ↓96.9 |
| Color | mg Pt/L | 175 ± 35 | ↓98.4 |
| Chemical Oxygen Demand | mg O2/L | 2860 ± 430 | ↓77.2 |
| Total Organic Content | mg/L | 1180 ± 180 | ↓75.7 |
| Parameter | Evaluation of Effects, COD, g O2/L, R2 = 0.7565, R2adj = 0.5941, 3 Parameter, 1 Block, 16 Experiments, MS = 3.4812 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Effect | Standard Error | p-Value * | −95% Confidence Interval | +95% Confidence Interval | Factor | Standard Error of Factor | Lower Confidence Interval | Upper Confidence Interval | |
| Constant value | 6.3052 | 1.3155 | 0.0010 | 3.3295 | 9.2811 | 6.3053 | 1.3155 | 3.3295 | 9.2811 |
| pH (L) | 2.0637 | 1.0098 | 0.0713 | −0.2205 | 4.3480 | 1.0319 | 0.5049 | −0.1103 | 2.1740 |
| pH (Q) | 2.4877 | 1.2260 | 0.0730 | −0.2857 | 5.2612 | 1.2439 | 0.6130 | −0.1429 | 2.6306 |
| K2FeO4 (L) | −3.3375 | 1.0098 | 0.0092 | −5.6218 | −1.0533 | −1.6688 | 0.5049 | −2.8109 | −0.5267 |
| K2FeO4 (Q) | −0.4786 | 1.2260 | 0.7054 | −3.2520 | 2.2949 | −0.2393 | 0.6130 | −1.6260 | 1.1474 |
| Time (L) | −2.2732 | 1.0098 | 0.0509 | −4.5574 | 0.0111 | −1.1366 | 0.5049 | −2.2787 | 0.0055 |
| Time (Q) | −0.8251 | 1.2260 | 0.5179 | −3.5985 | 1.9484 | −0.4125 | 0.6130 | −1.7993 | 0.9742 |
| Parameter | Evaluation of Effects, COD, g O2/L, R2 = 0.7565, R2adj = 0.5941, 3 Parameter, 1 Block, 16 Experiments, MS = 3.4812 | |||
|---|---|---|---|---|
| SS | MS | F | P * | |
| pH (L) | 14.5408 | 14.5408 | 4.1769 | 0.0713 |
| pH (Q) | 14.3335 | 14.3335 | 4.1173 | 0.0730 |
| K2FeO4 (L) | 38.0320 | 38.0320 | 10.9249 | 0.0092 |
| K2FeO4 (Q) | 0.5305 | 0.5305 | 0.1524 | 0.7054 |
| Time (L) | 17.6422 | 17.6422 | 5.0678 | 0.0509 |
| Time (Q) | 1.5766 | 1.5766 | 0.4529 | 0.5179 |
| Error | 31.3312 | 3.4812 | - | - |
| Parameter | Unit | Result * | Effect (%) ** |
|---|---|---|---|
| pH | - | 8.5 ± 0.1 | Non-significant |
| Total Dissolved Solids | mg/L | 19,590 ± 1960 | ↑7.3 |
| Total Solids | mg/L | 20,560 ± 2060 | ↑4.4 |
| Suspended Solids | mg/L | 30.0 ± 3.0 | ↓97.9 |
| Color | mg Pt/L | 80 ± 16 | ↓99.3 |
| Chemical Oxygen Demand | mg O2/L | 1740 ± 260 | ↓86.1 |
| Total Organic Carbon | mg/L | 950 ± 140 | ↓80.5 |
| Parameter | Results (This Study) | Results (Other Studies) |
|---|---|---|
| COD | ↓86.1% (oxidation using K2FeO4) | ↓78.7 ± 1.3% (carbon felt); ↓93.8 ± 1.7% (LTA zolite-modified anode); ↓96.3 ± 2.1% (bentonite-modified anode); bioelectrocatalytic oxidation [77] |
| ↓80–87% (UF membranes); ↓65% (MF membranes); ↓96% (RO; reverse osmosis) [78] | ||
| ↓96.33% (primary treatment process); ↓99.81% (NF; nanofiltration); ↓99.84% (RO; reverse osmosis) [79] | ||
| TOC | ↓80.5% (oxidation using K2FeO4) | ↓52%; simulated tannery wastewater (MF membranes) [78]; ↓87%; (hydrodynamic cavitation with addition of H2O2) [80]; ↓50.0%; (coagulation-flocculation/adsorption; CF-ADS) [81]; ↓46.5%; (coagulation-flocculation/ozonation; CF-OZ) [81] |
| Color | ↓99.3% (oxidation using K2FeO4) | ↓61.13%; (coagulation-flocculation/adsorption; CF-ADS) [81] ↓85.34%; (coagulation-flocculation/ozonation; CF-OZ) [81] ↓87%; (electrochemical oxidation; EO) [82] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozik, V.; Barbusinski, K.; Thomas, M.; Sroda, A.; Jampilek, J.; Sochanik, A.; Smolinski, A.; Bak, A. Taguchi Method and Response Surface Methodology in the Treatment of Highly Contaminated Tannery Wastewater Using Commercial Potassium Ferrate. Materials 2019, 12, 3784. https://doi.org/10.3390/ma12223784
Kozik V, Barbusinski K, Thomas M, Sroda A, Jampilek J, Sochanik A, Smolinski A, Bak A. Taguchi Method and Response Surface Methodology in the Treatment of Highly Contaminated Tannery Wastewater Using Commercial Potassium Ferrate. Materials. 2019; 12(22):3784. https://doi.org/10.3390/ma12223784
Chicago/Turabian StyleKozik, Violetta, Krzysztof Barbusinski, Maciej Thomas, Agnieszka Sroda, Josef Jampilek, Aleksander Sochanik, Adam Smolinski, and Andrzej Bak. 2019. "Taguchi Method and Response Surface Methodology in the Treatment of Highly Contaminated Tannery Wastewater Using Commercial Potassium Ferrate" Materials 12, no. 22: 3784. https://doi.org/10.3390/ma12223784
APA StyleKozik, V., Barbusinski, K., Thomas, M., Sroda, A., Jampilek, J., Sochanik, A., Smolinski, A., & Bak, A. (2019). Taguchi Method and Response Surface Methodology in the Treatment of Highly Contaminated Tannery Wastewater Using Commercial Potassium Ferrate. Materials, 12(22), 3784. https://doi.org/10.3390/ma12223784