Real-Time Corrosion Monitoring of AISI 1010 Carbon Steel with Metal Surface Mapping in Sulfolane
Abstract
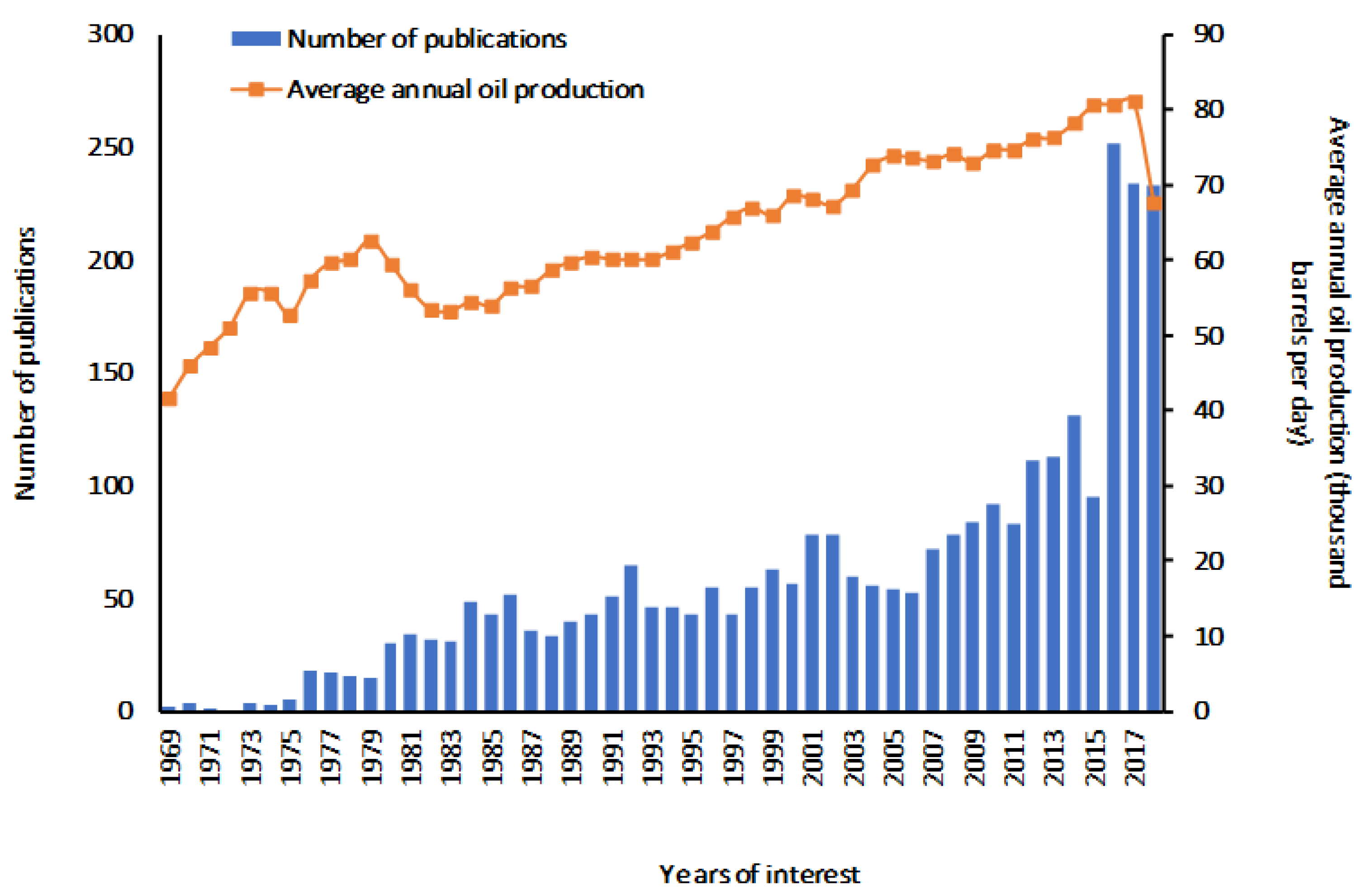
1. Introduction
Beware of Sulfolane
2. Materials and Methods
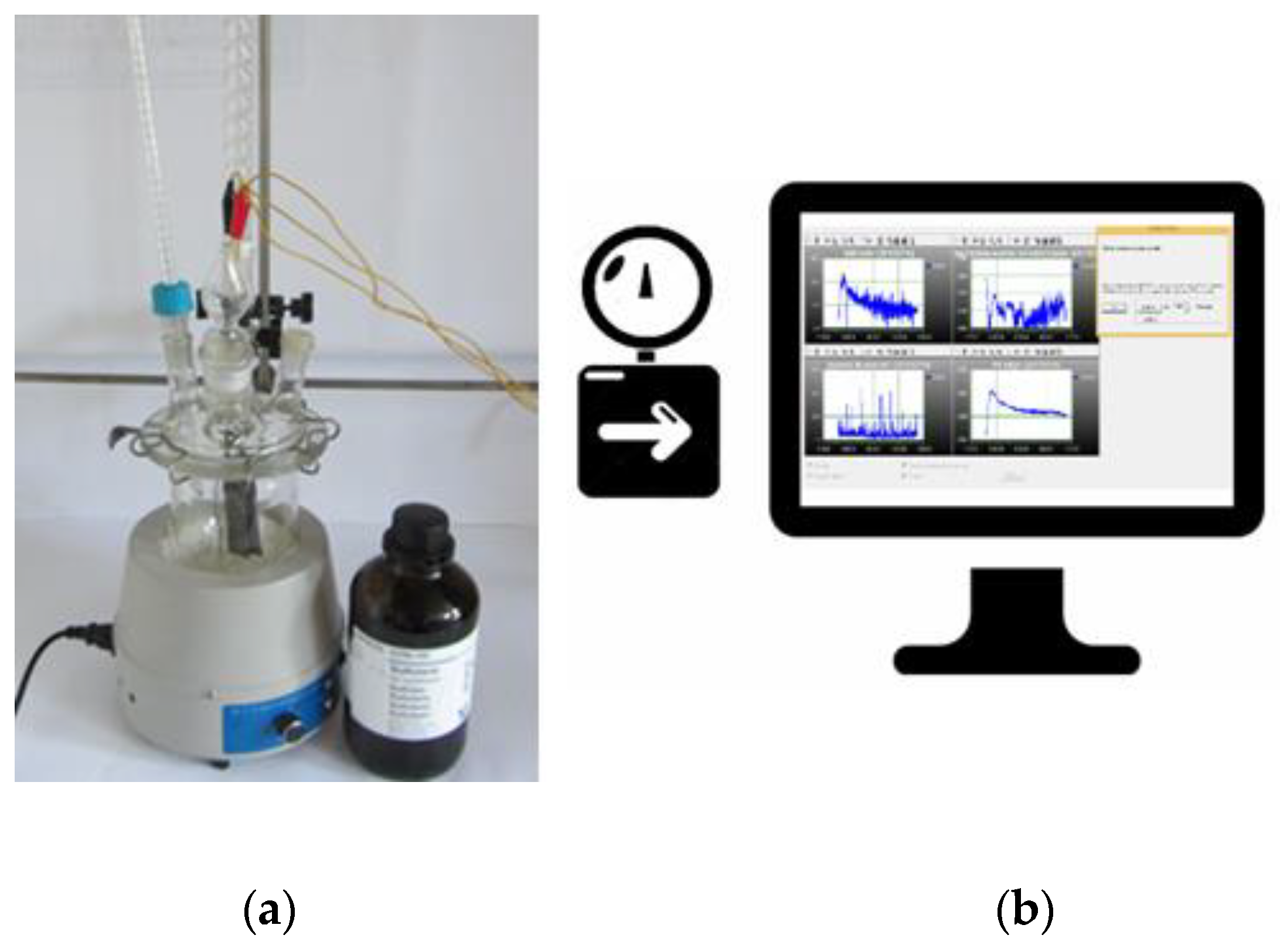
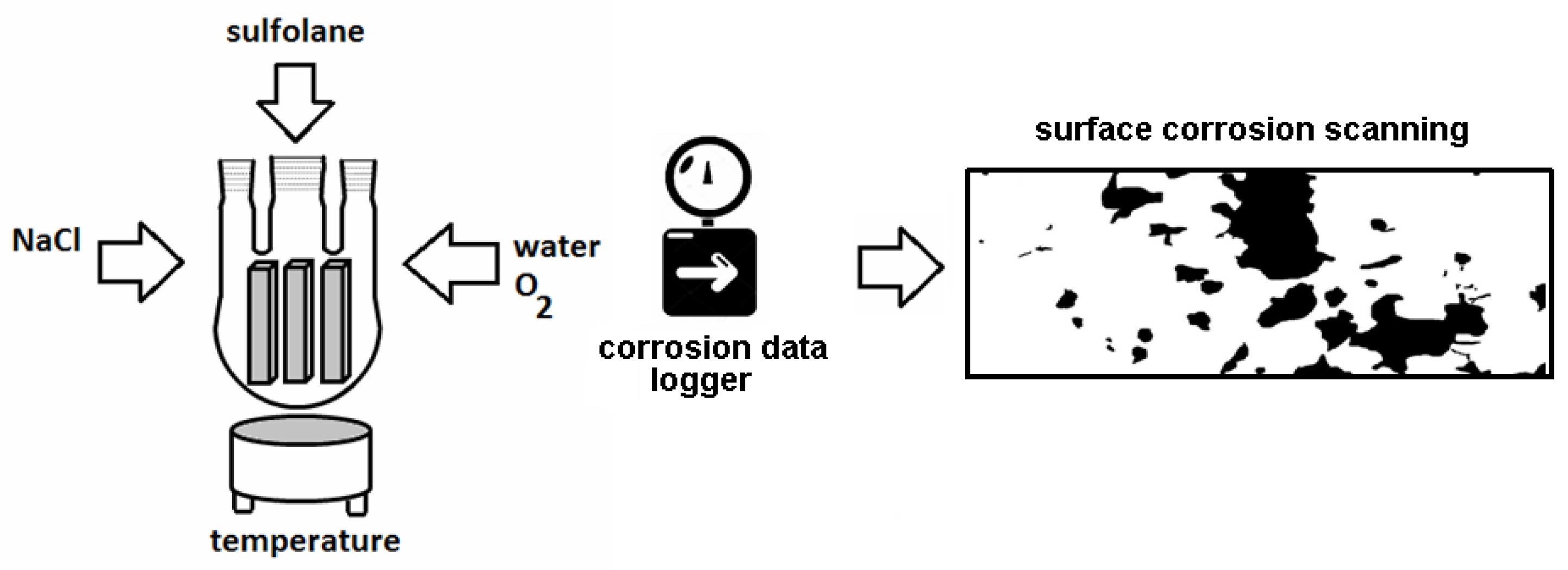
2.1. Design of Laboratory-Scale System
2.2. Sulfolane Corrosivity Parameters
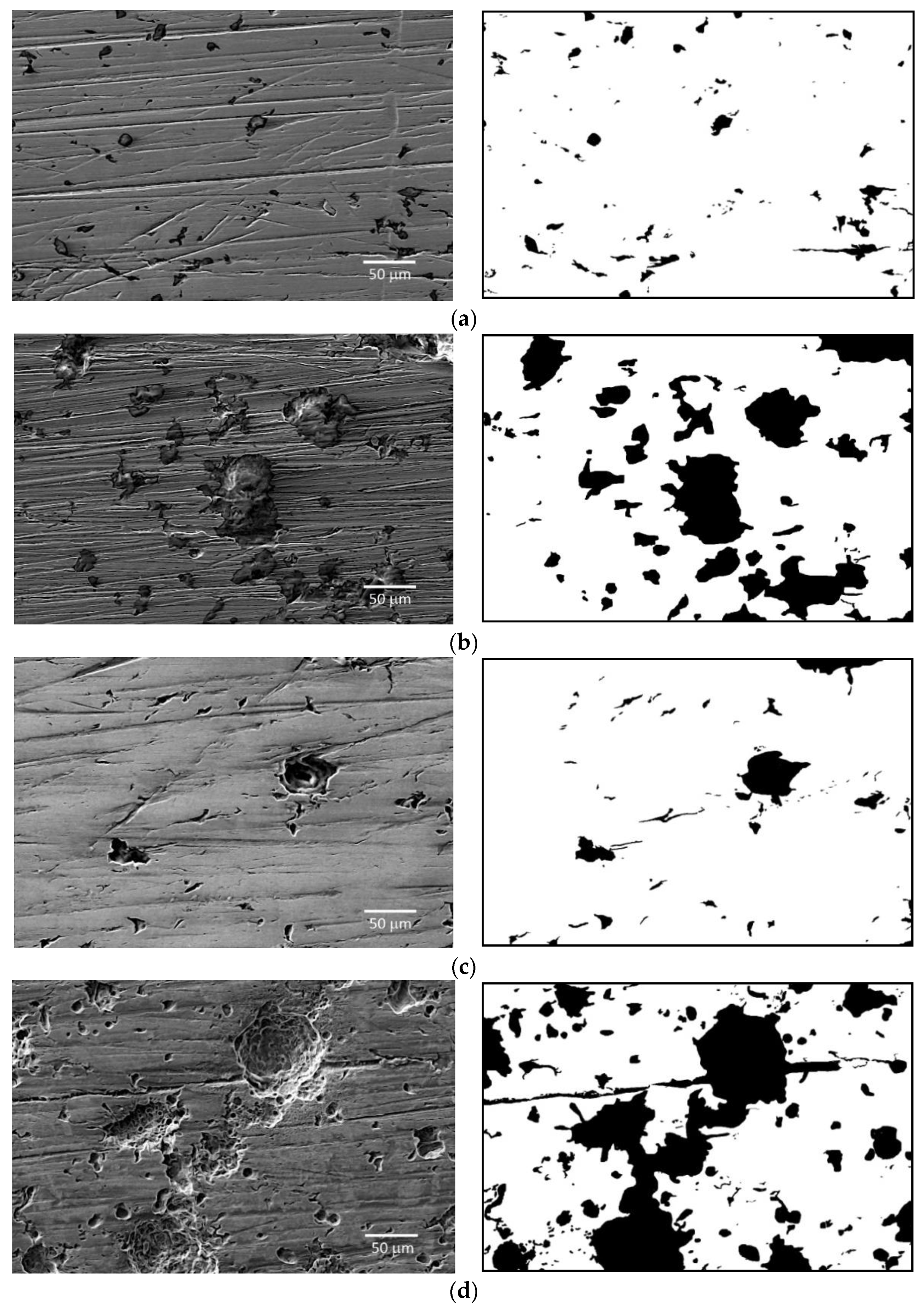
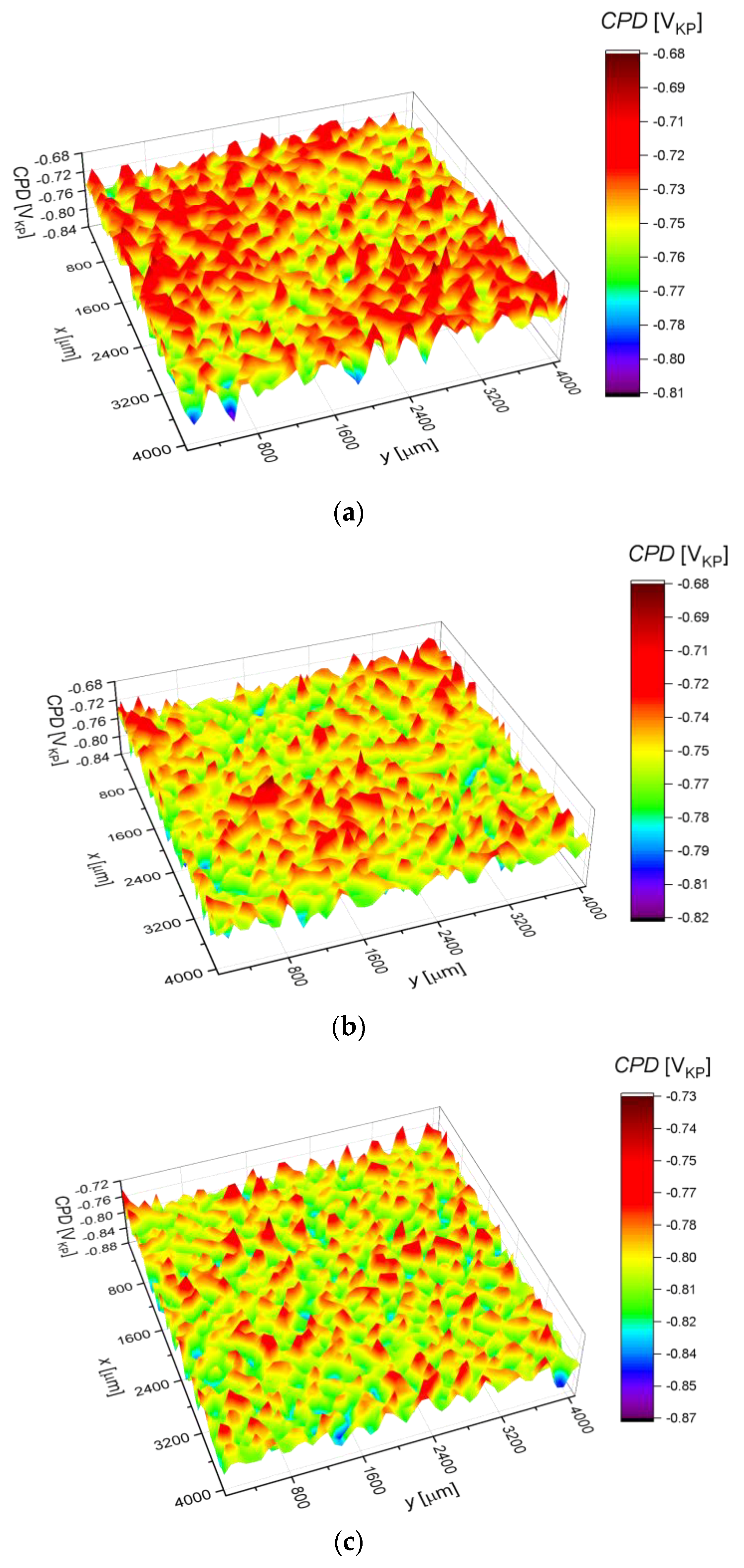
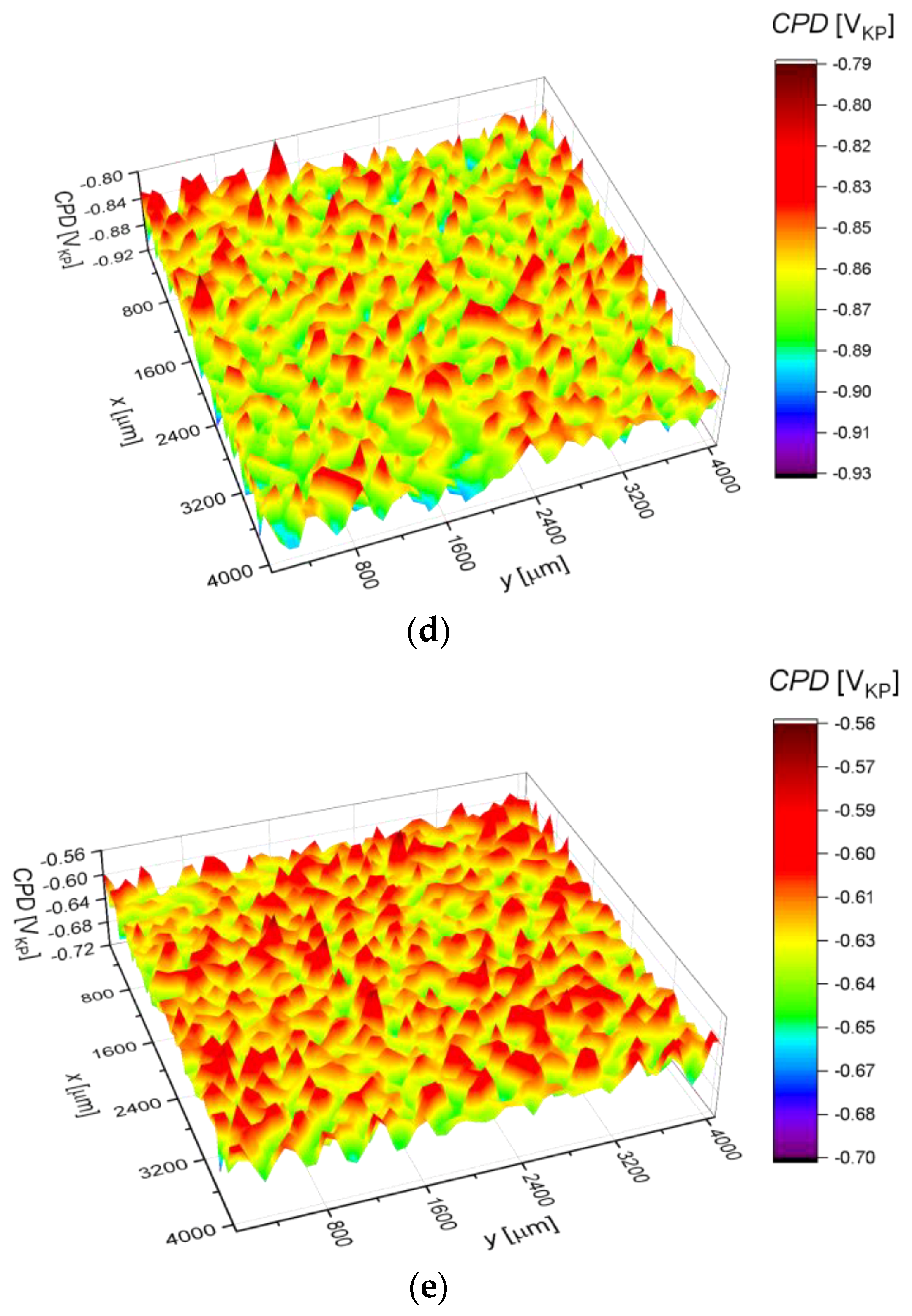
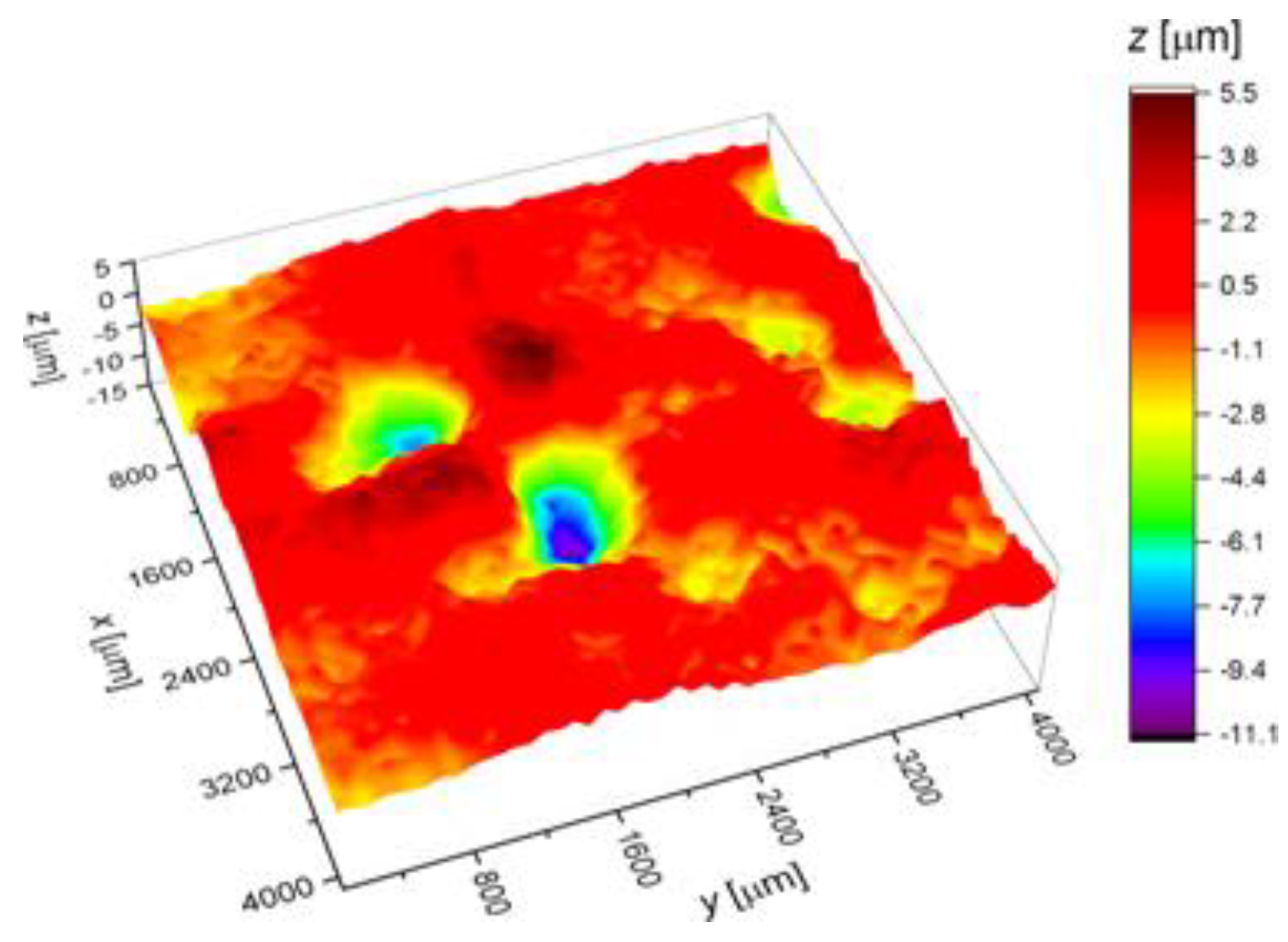
2.3. Surface Corrosion Evaluation
3. Results and Discussion
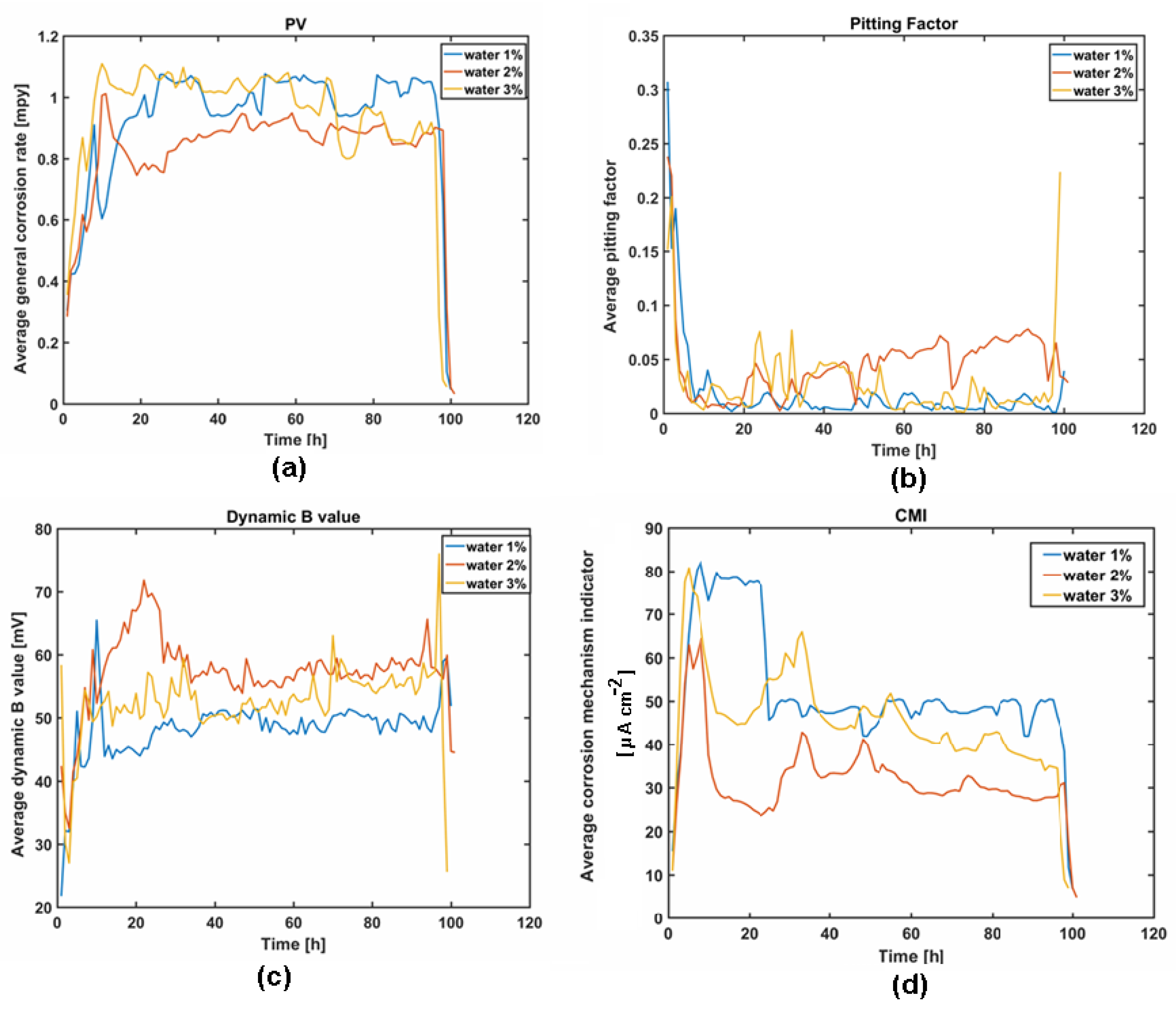
3.1. Pilot Study of Sulfolane-Induced Corrosion
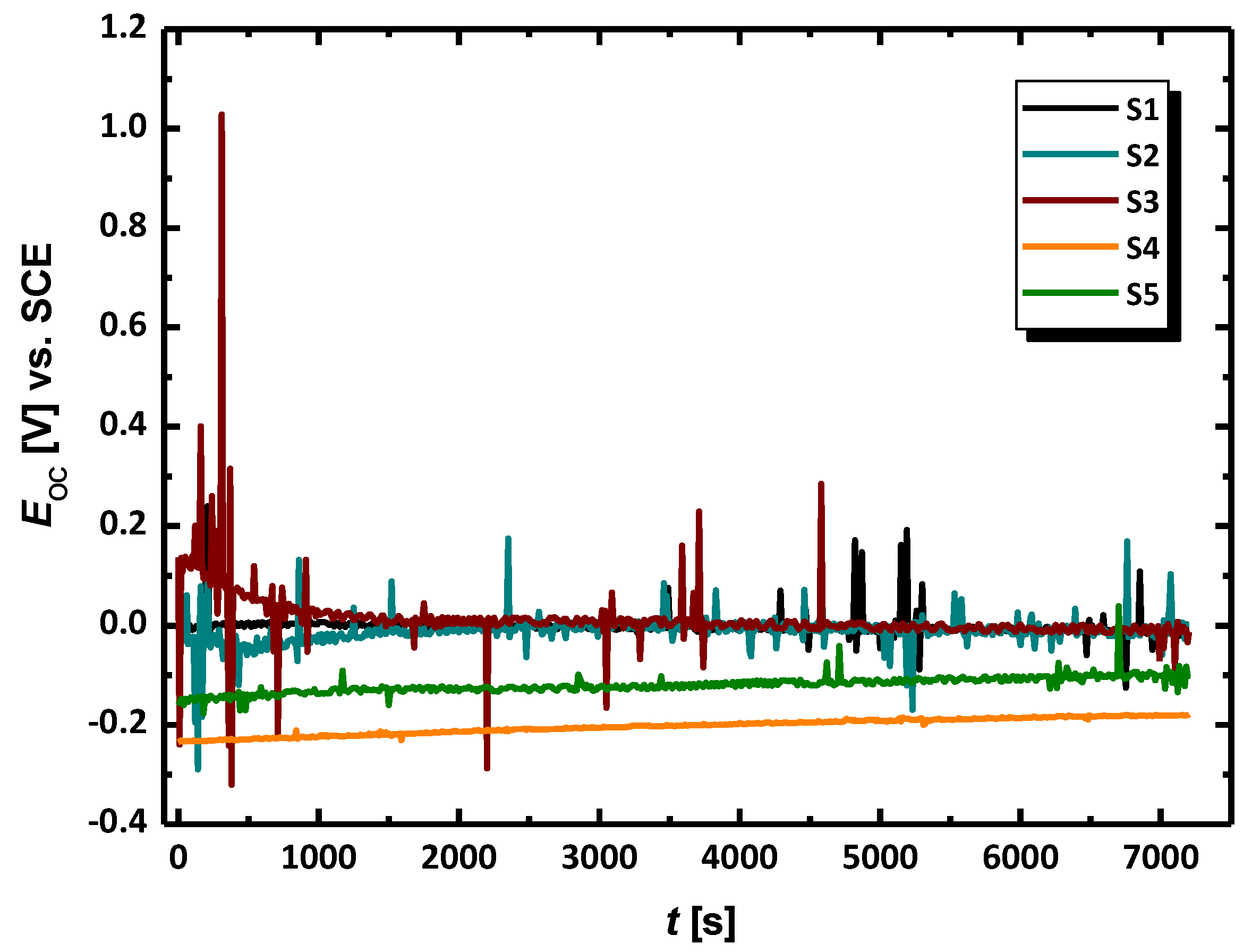
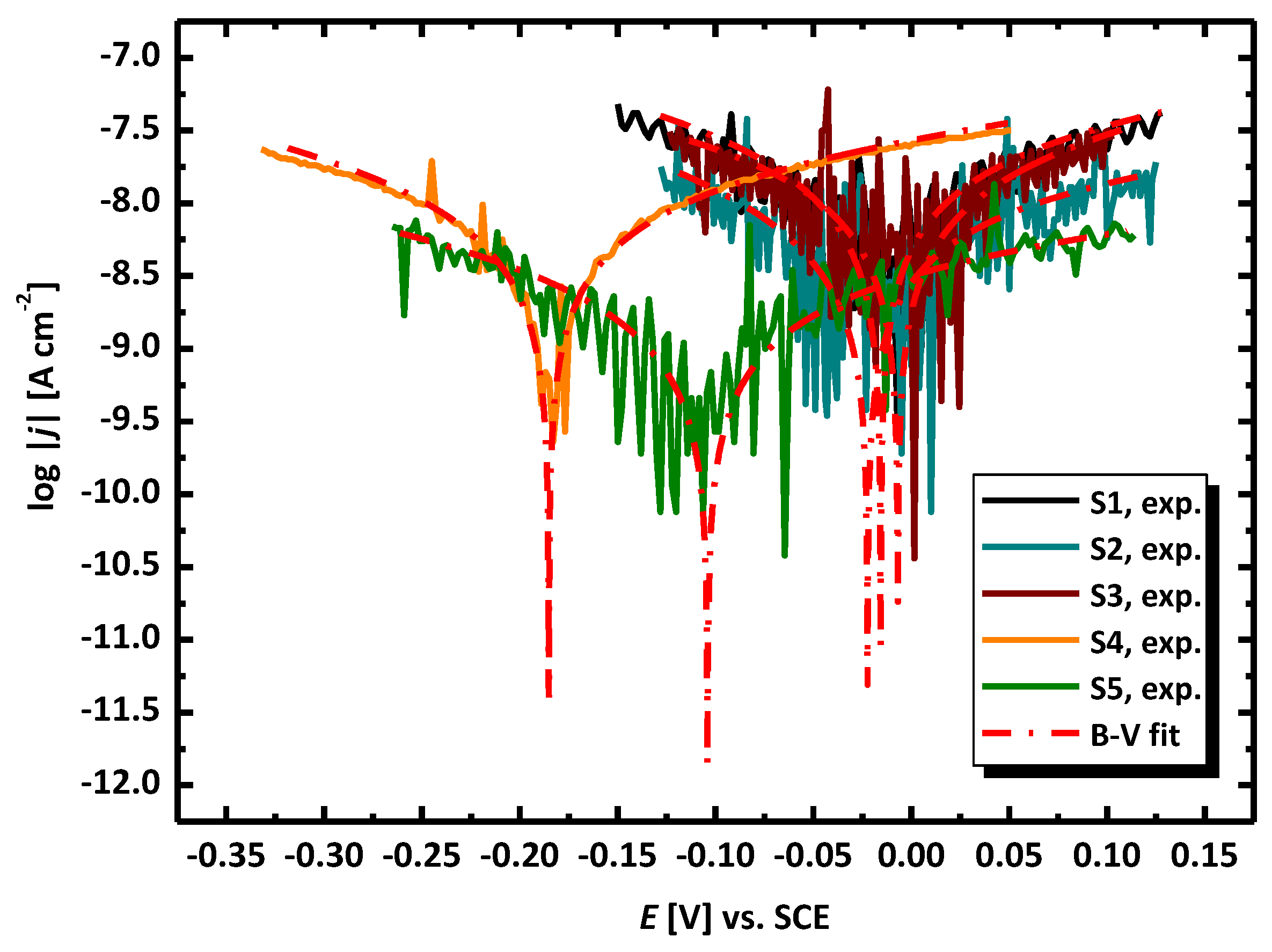
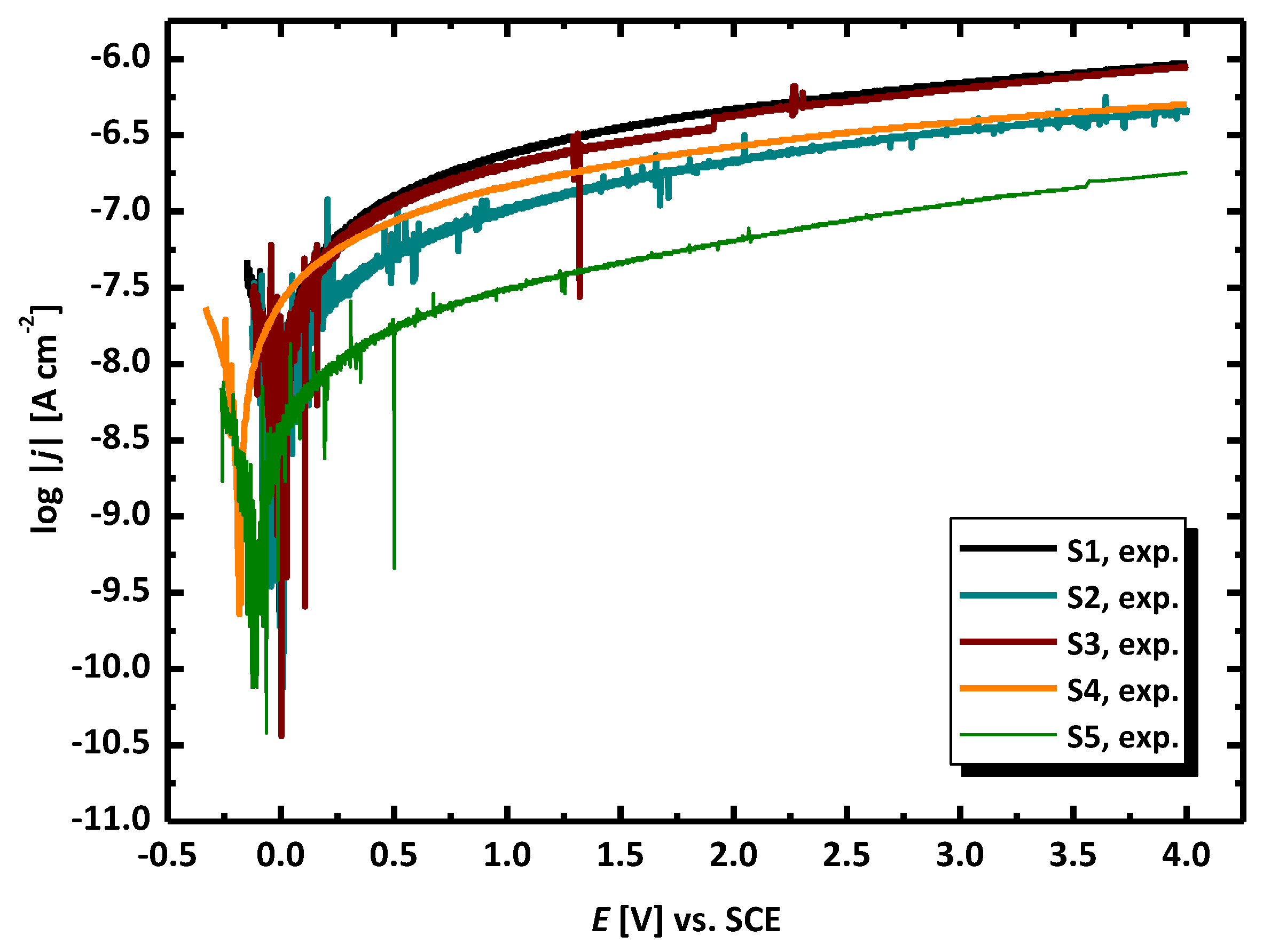
3.2. Electrochemical Methods for Corrosion Assessment
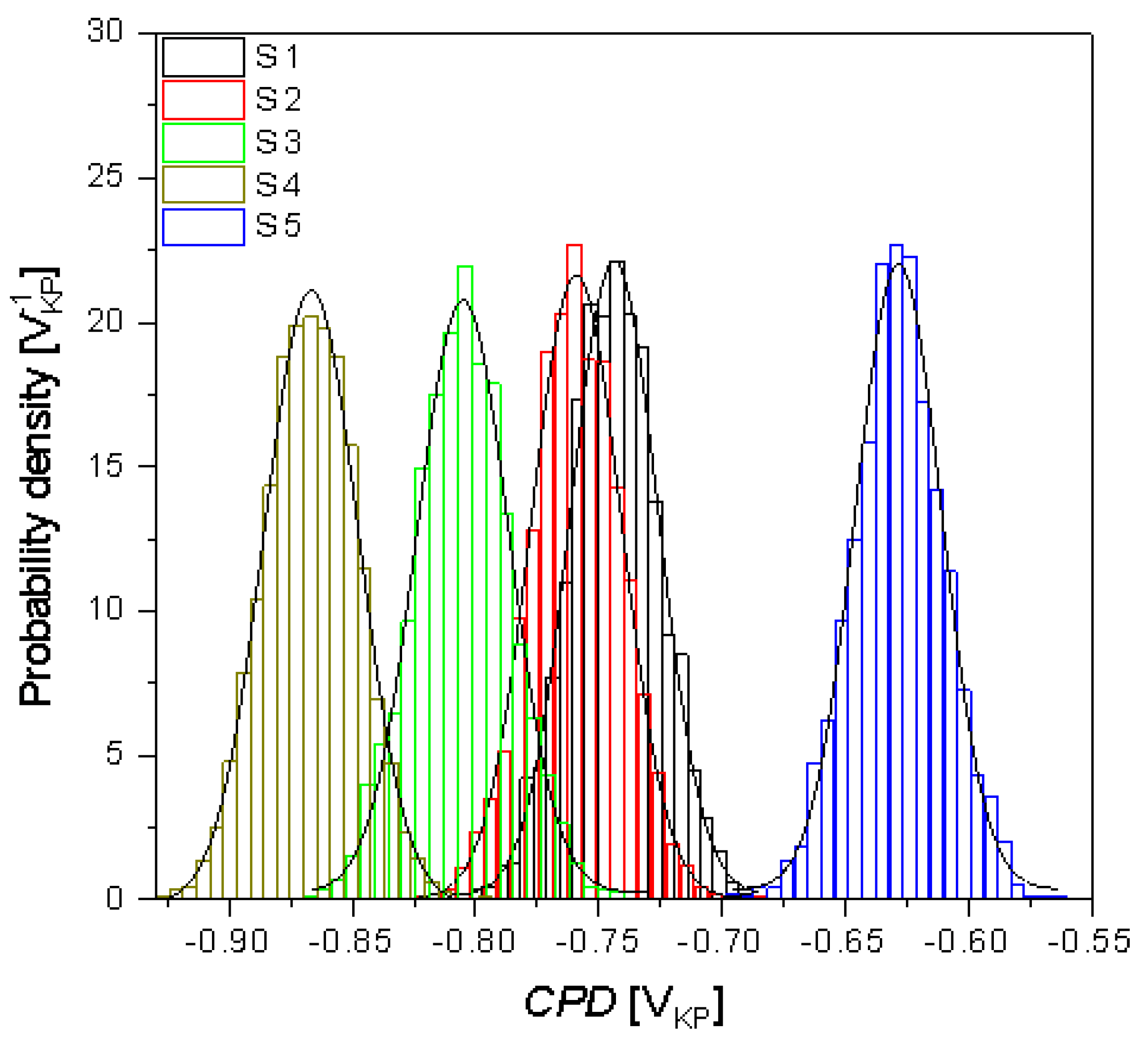
3.3. Surface Corrosion Scanning
4. Conclusions
- The real-time corrosion monitoring with the metal surface mapping procedures were combined together in the pilot-case workflow for the exhaustive assessment of the sulfolane corrosive potential against AISI 1010 carbon steel. Several aspects of corrosion evaluation of the general and localized corrosion modes were examined using a dedicated testing vessel (our own design).
- In fact, a noticeable influence of water (even at low temperature) on sulfolane corrosivity was observed; an increase in water concentration accelerates sulfolane degradation, as indicated by elevated corrosion rate and an increase in the suspended dark deposits. On the other hand, no detectable impact on carbon steel localized corrosion propensity was noted in the water-sulfolane mixtures.
- The evaluation of the corrosion resistance of AISI 1010 carbon steel after the experiment in sulfolane with the addition of 1–3 vol.% of water at 95 °C in the absence and in the presence of the layer of corrosion products was conducted in pure sulfolane at 25 °C using the open circuit potential method and the potentiodynamic measurements. The estimation of the corrosion rate, polarization resistance, and Stern–Geary constant were made according to the ASTM G102 - 89(2015)e1. Based on the determined parameters of the corrosion resistance it was noticed that the increase in the water content (1–3 vol.%) in sulfolane affects the decrease in the corrosion resistance of AISI 1010 carbon steel on uniform and pitting corrosion due to higher conductance of the electrolyte.
- It was found that the highest corrosion resistance among all tested electrodes revealed the electrode after previous experiment in sulfolane at 95 °C without etching of the layer of corrosion products with barrier properties. The evaluation of the corrosion damage for this electrode revealed the lowest value of the corrosion degree determined using the numerical analysis of sample surface images obtained with SEM, and the highest value of average contact potential difference specified using the scanning Kelvin probe technique.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Attaway, H.; Gooding, C.H.; Schmidt, M.G. Biodegradation of BTEX vapors in a silicone membrane bioreactor system. J. Ind. Microbiol. Biot. 2001, 26, 316–325. [Google Scholar] [CrossRef]
- Curtis, L.; Rea, W.; Smith-Willis, P.; Fenyves, E.; Pan, Y. Adverse health effects of outdoor air pollutants. Environ. Int. 2006, 32, 815–830. [Google Scholar] [CrossRef] [PubMed]
- Kasanke, C.P.; Leight, M.B. Factors limiting sulfolane biodegradation in contaminated subarctic aquifer substrate. PLoS ONE 2017, 12, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jalili, A.H.; Shokouhi, M.; Samani, F.; Hosseini-Jenab, M. Measuring the solubility of CO2 and H2S in sulfolane and the density and viscosity of saturated liquid binary mixtures of (sulfolane + CO2) and (sulfolane + H2S). J. Chem. Thermodyn. 2015, 85, 13–25. [Google Scholar] [CrossRef]
- Angaji, M.T.; Ghanbarabadi, H.; Gohari, F.K.Z. Optimizations of sulfolane concentration in propose Sulfinol-M solvent instead of MDEA solvent in the refineries of Sarakhs. J. Nat. Gas Sci. Eng. 2013, 15, 22–26. [Google Scholar] [CrossRef]
- Tilstam, U. Sulfolane: A versatile dipolar aprotic solvent. Org. Process Res. Dev. 2012, 16, 1273–1278. [Google Scholar] [CrossRef]
- Brown, V.K.H.; Ferrigan, L.W.; Stevenson, D.E. Acute toxicity and skin irritant properties of sulfolane. Br. J. Industr. Med. 1966, 23, 302–304. [Google Scholar] [CrossRef] [PubMed]
- Zaretskii, M.I.; Rusak, V.V.; Chartov, E.M. Sulfolane and dimethyl sulfoxide as extractants. Coke Chem. 2013, 56, 266–268. [Google Scholar] [CrossRef]
- Zaretskii, M.I.; Rusak, V.V.; Chartov, E.M. Extractive rectification by means of sulfolane in chemical technology: A review. Coke Chem. 2011, 54, 299–301. [Google Scholar] [CrossRef]
- Zaretskii, M.I.; Rusak, V.V.; Chartov, E.M. Sulfolane in liquid extraction: A review. Coke Chem. 2011, 54, 211–214. [Google Scholar] [CrossRef]
- Reaxys. Available online: https://www.reaxys.com (accessed on 23 April 2018).
- U.S. Energy Information Administration. Available online: https://www.eia.gov (accessed on 28 May 2019).
- Wagh, R.B.; Gund, S.H.; Nagarkar, J.M. An eco-friendly oxidation of sulfide compounds. J. Chem. Sci. 2016, 128, 1321–1325. [Google Scholar] [CrossRef]
- European Chemical Agency. Available online: https://echa.europa.eu/pl/substance-information/-/substanceinfo/100.004.349 (accessed on 9 July 2018).
- Izadifard, M.; Achari, G.; Langford, C.H. Mineralization of sulfolane in aqueous solutions by Ozone/CaO2 and Ozone/CaO with potential for field application. Chemosphere 2018, 197, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Mingy, L.; Zhong, J.; Xujiang, S. Cause of equipment corrosion and counter measures in the sulfolane recycling system of aromatics extraction unit. Pet. Process Petrochem. 2005, 36, 30–33. [Google Scholar]
- Kus, S.; Srinivasan, S.; Yap, K.M.; Li, H.; Kozik, V.; Bak, A.; Dybal, P. On-Line, real time electrochemical corrosion monitoring in low conductive fluids–sulfolane aromatic extraction. In Proceedings of the CORROSION 2018, Phoenix, AZ, USA, 15–18 April 2018; pp. 2642–2657. [Google Scholar]
- Bosch, R.W.; Hubrecht, J.; Bogaerts, W.F.; Syrett, B.C. Electrochemical frequency modulation: A new electrochemical technique for online corrosion monitoring. Corrosion 2001, 57, 60–70. [Google Scholar] [CrossRef]
- Anderko, A.; Sridhar, N.; Yang, L.T.; Grise, S.L.; Saldanha, B.J.; Dorsey, M.H. Validation of localised corrosion model using real time corrosion monitoring in a chemical plant. Corros. Eng. Sci. Technol. 2005, 40, 33–42. [Google Scholar] [CrossRef]
- Linjewile, T.M.; Valentine, J.; Davis, K.A.; Harding, N.S.; Cox, W.M. Prediction and real-time monitoring techniques for corrosion characterisation in furnaces. Mater. High Temp. 2003, 20, 175–183. [Google Scholar] [CrossRef]
- Bak, A.; Kozik, V.; Dybal, P.; Kus, S.; Swietlicka, A.; Jampilek, J. Sulfolane: Magic extractor or bad actor? Pilot–scale study on solvent corrosion potential. Sustainability 2018, 10, 3677. [Google Scholar] [CrossRef]
- Witzaney, A.M.; Fedorak, P.M. A Review of the Characteristics, Analyses and Biodegradability of Sulfolane and Alkanolamines Used in Sour Gas Processing; Department of Biological Sciences, University of Alberta: Edmonton, AB, Canada, 1996. [Google Scholar]
- NACE International. Available online: https://www.nace.org/ (accessed on 19 July 2018).
- Losiewicz, B.; Popczyk, M.; Szklarska, M.; Smolka, A.; Osak, P.; Budniok, A. Application of the scanning kelvin probe technique for characterization of corrosion interfaces. Solid State Phenom. 2015, 228, 369–382. [Google Scholar] [CrossRef]
- Stroz, A.; Goryczka, T.; Losiewicz, B. Electrochemical formation of self-organized nanotubular oxide layers on niobium (Review). Curr. Nanosci. 2019, 15, 42–48. [Google Scholar] [CrossRef]
- Losiewicz, B.; Kubisztal, J. Effect of hydrogen electrosorption on corrosion resistance of Pd80Rh20 alloy in sulfuric acid: EIS and LEIS study. Int. J. Hydrog. Energy 2018, 43, 20004–20010. [Google Scholar] [CrossRef]
- Smolka, A.; Dercz, G.; Rodak, K.; Losiewicz, B. Evaluation of corrosion resistance of nanotubular oxide layers on the ti13zr13nb alloy in physiological saline solution. Arch. Metall. Mater. 2015, 60, 2681–2686. [Google Scholar] [CrossRef]
- Tahara, A.T.; Kodama, T. Potential distribution measurement in galvanic corrosion of Zn/Fe couple by means of Kelvin probe. Corros. Sci. 2000, 42, 655–673. [Google Scholar] [CrossRef]
- Kubisztal, J.; Kubisztal, M.; Haneczok, G. Quantitative characterization of material surface–application to Ni + Mo electrolytic composite coatings. Mater. Charact. 2016, 122, 45–53. [Google Scholar] [CrossRef]
- Kubisztal, J.; Kubisztal, M.; Stach, S.; Haneczok, G. Corrosion resistance of anodic coatings studied by scanning microscopy and electrochemical methods. Surf. Coat. Tech. 2018, 350, 419–427. [Google Scholar] [CrossRef]
- Bockris, J.; Reddy, A.; Gamboa-Aldeco, M. Modern Electrochemistry 2A. Fundamentals of Electrodics, 2nd ed.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2000; p. 1083. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods. Fundamentals and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001. [Google Scholar]
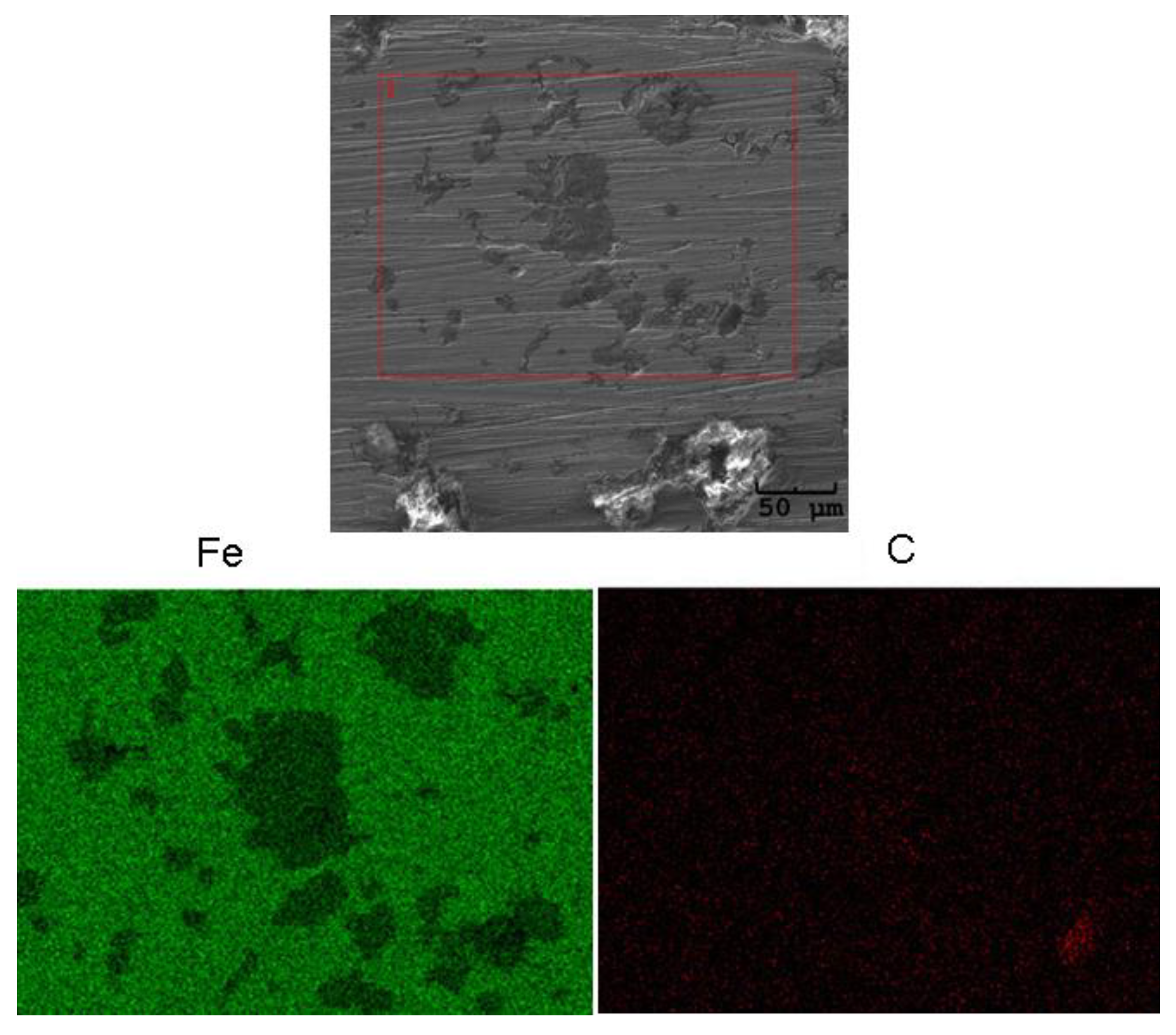
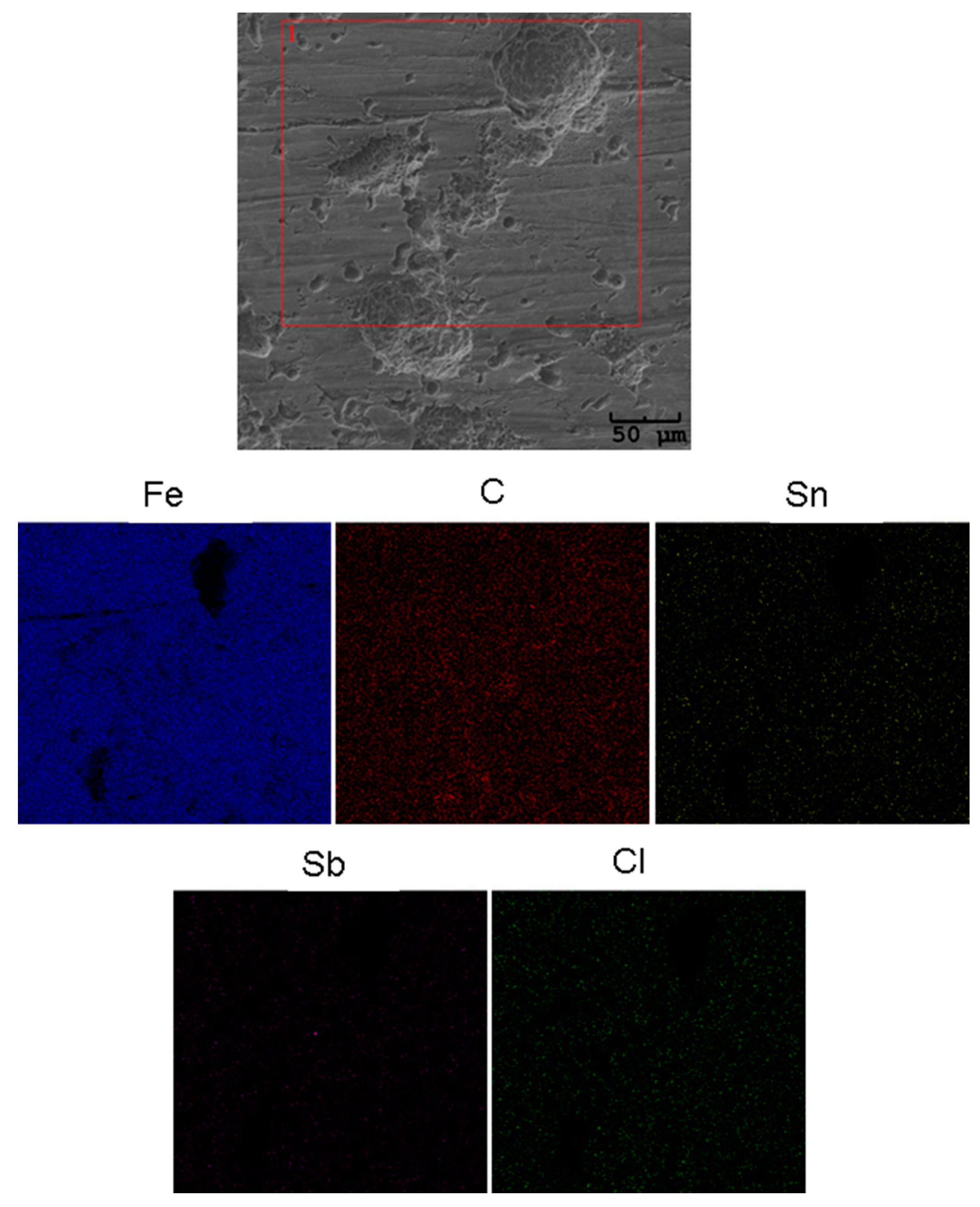
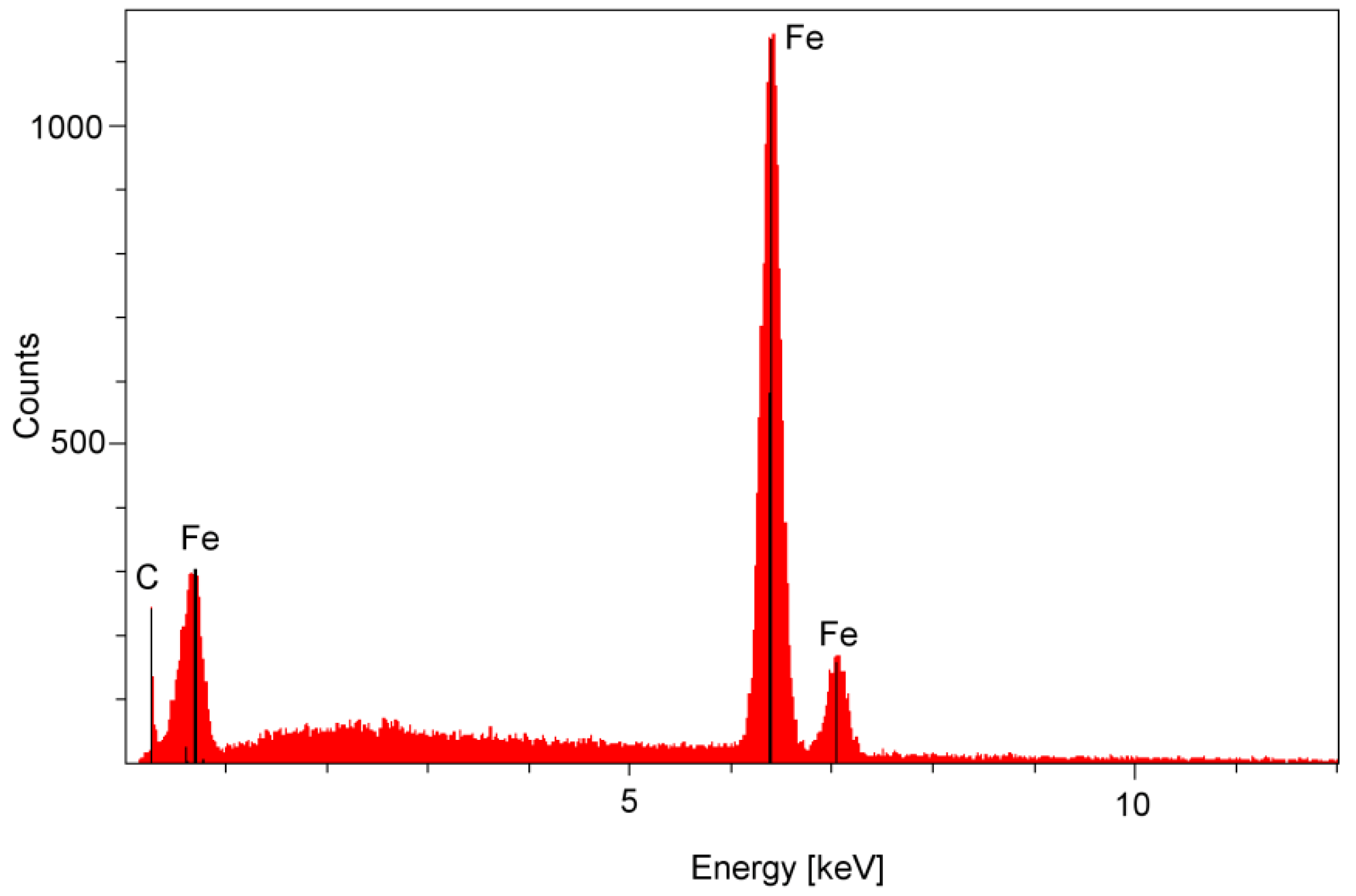
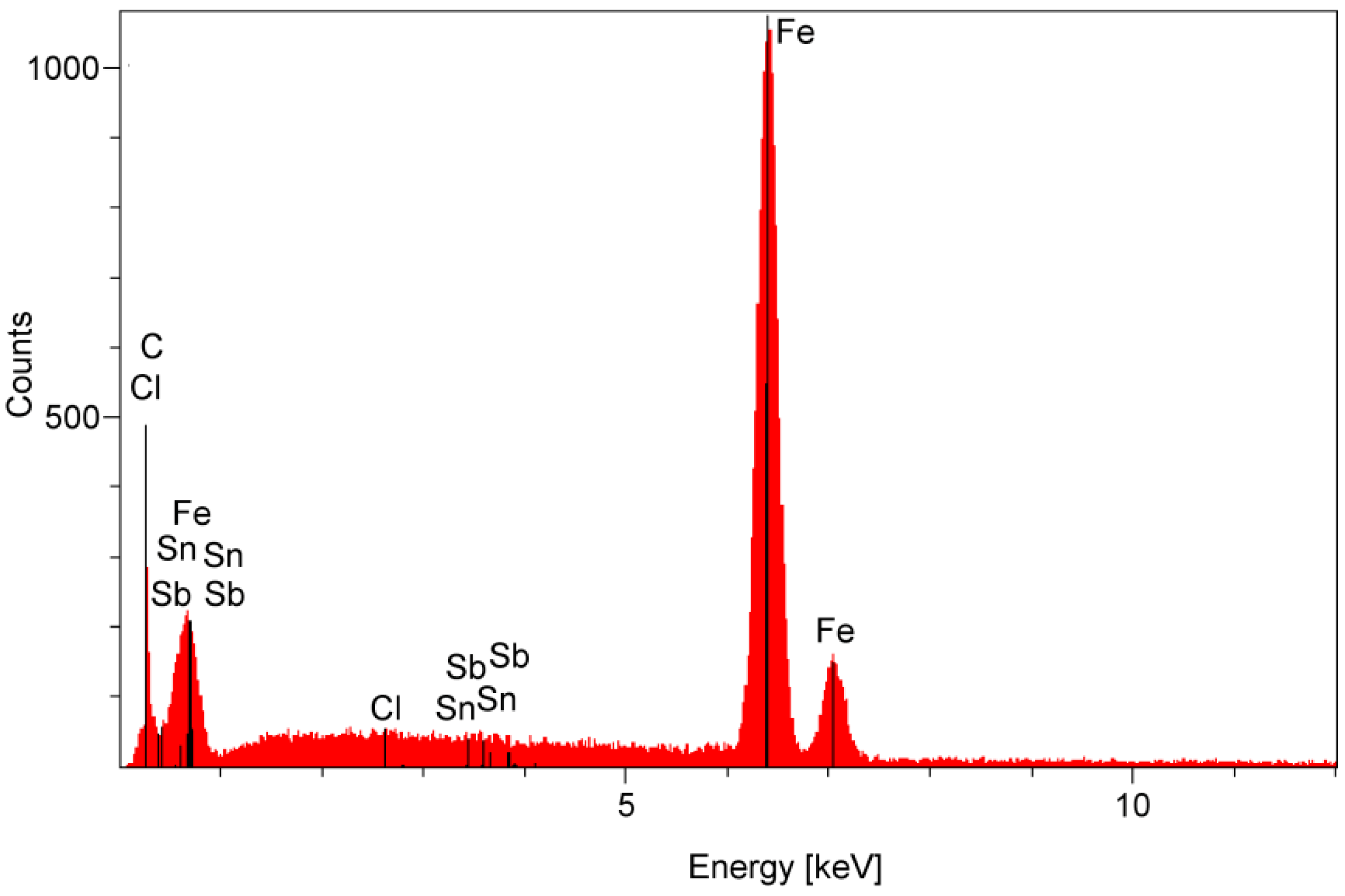
| Parameter | Definition |
|---|---|
| General corrosion rate (PV) | Measurement of the real part of the Low Frequency Impedance (LFI) of the working electrode. SmartCET uses Linear Polarization Resistance (LPR) technique to calculate the General Corrosion Rate that is usually the prime variable of interest, because it reflects the overall rate of metallic corrosion. Corrosion may be directly related to operational parameters, e.g., temperatures, flow, chemical composition. |
| Pitting Factor (PF) | Ratio of the depth of the deepest pit (point or small area, that takes the form of cavities) resulting from corrosion divided by the average penetration as calculated from weight loss. It is a measure of the overall stability of the corrosion process obtained from a measurement of the intrinsic current noise of the working electrode and comparing this measurement to the general corrosion obtained from the LPR measurement. |
| Dynamic B value | Corrosion constant also known as Stern–Geary constant. It is an essential part of the corrosion rate calculation that is directly proportional to the corrosion rate value. It represents a correction factor constant determined by the mechanism/kinetics of the corrosion process. In a dynamic process the B value is not constant. The knowledge of the B value enables to refine the LPR-generated corrosion rate estimation, since the uncertainty regarding the standard (default) B value is removed. The B value is directly related to the mechanistic properties of the component anodic and cathodic corrosion processes. |
| Corrosion mechanism indicator (CMI) | The CMI is a qualitative indicator of a surface film presence. If there is no film and only corrosion is present, the CMI will have an intermediate value. Inorganic scale, or thick passive oxide films with little or no conductivity, will show a low CMI value. |
| Shape | Material | Temperature | Electrode | Surface Corrosion Sampling |
|---|---|---|---|---|
| 3 × flat coupon electrode 89 mm × 20 mm × 2 mm | AISI 1010 carbon steel | 95 °C | S1 | The corrosion test and etching |
| S2 | The corrosion test with the addition of 1 vol.% of water and etching | |||
| S3 | The corrosion test with the addition of 2 vol.% of water and etching | |||
| S4 | The corrosion test with the addition of 3 vol.% of water and etching | |||
| S5 | The corrosion test without etching of corrosion products |
| Electrode No. | Ecor (V) | jcor (A cm−2) | ba (V dec−1) | bc (V dec−1) | B (V) | Rp (cm2) | CR at Ecor (mpy) |
|---|---|---|---|---|---|---|---|
| S1 | −0.016 ± 5.20 × 10−4 | 3.10 × 10−8 ± 4.40 × 10−9 | 0.562 ± 0.084 | 0.398 ± 0.048 | 0.101 | 3.26 × 106 | 0.014 |
| S2 | −0.022 ± 5.54 ×10−4 | 1.16 × 10−8 ± 1.59 × 10−9 | 0.626 ± 0.111 | 0.298 ± 0.031 | 0.088 | 7.56 × 106 | 0.005 |
| S3 | −0.0 07 ± 7.76 × 10−4 | 2.92 × 10−8 ± 1.21 × 10−8 | 0.478 ± 0.168 | 0.584 ± 0.268 | 0.114 | 3.91 × 106 | 0.013 |
| S4 | −0.185 ± 4.98 × 10−4 | 1.46 × 10−8 ± 6.45 × 10−10 | 0.548 ± 0.021 | 0.387 ± 0.016 | 0.098 | 6.75 × 106 | 0.007 |
| S5 | −0.104 ± 3.64 × 10−4 | 4.21 × 10−9 ± 2.30 × 10−10 | 0.777 ± 0.046 | 0.485 ± 0.022 | 0.130 | 3.08 × 107 | 0.002 |
| Parameter | S1 | S2 | S3 | S4 | S5 |
|---|---|---|---|---|---|
| CPDav (mVKP) | −743.7(4) | −759.1(4) | −805.1(4) | −866.7(2) | −628.3(4) |
| CPDq (mVKP) | 17.8(1) | 18.3(9) | 18.7(9) | 19.2(7) | 17.5(1) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bak, A.; Losiewicz, B.; Kozik, V.; Kubisztal, J.; Dybal, P.; Swietlicka, A.; Barbusinski, K.; Kus, S.; Howaniec, N.; Jampilek, J. Real-Time Corrosion Monitoring of AISI 1010 Carbon Steel with Metal Surface Mapping in Sulfolane. Materials 2019, 12, 3276. https://doi.org/10.3390/ma12193276
Bak A, Losiewicz B, Kozik V, Kubisztal J, Dybal P, Swietlicka A, Barbusinski K, Kus S, Howaniec N, Jampilek J. Real-Time Corrosion Monitoring of AISI 1010 Carbon Steel with Metal Surface Mapping in Sulfolane. Materials. 2019; 12(19):3276. https://doi.org/10.3390/ma12193276
Chicago/Turabian StyleBak, Andrzej, Bozena Losiewicz, Violetta Kozik, Julian Kubisztal, Paulina Dybal, Aleksandra Swietlicka, Krzysztof Barbusinski, Slawomir Kus, Natalia Howaniec, and Josef Jampilek. 2019. "Real-Time Corrosion Monitoring of AISI 1010 Carbon Steel with Metal Surface Mapping in Sulfolane" Materials 12, no. 19: 3276. https://doi.org/10.3390/ma12193276
APA StyleBak, A., Losiewicz, B., Kozik, V., Kubisztal, J., Dybal, P., Swietlicka, A., Barbusinski, K., Kus, S., Howaniec, N., & Jampilek, J. (2019). Real-Time Corrosion Monitoring of AISI 1010 Carbon Steel with Metal Surface Mapping in Sulfolane. Materials, 12(19), 3276. https://doi.org/10.3390/ma12193276







