Comprehensive In Vitro Testing of Calcium Phosphate-Based Bioceramics with Orthopedic and Dentistry Applications
Abstract
1. Introduction
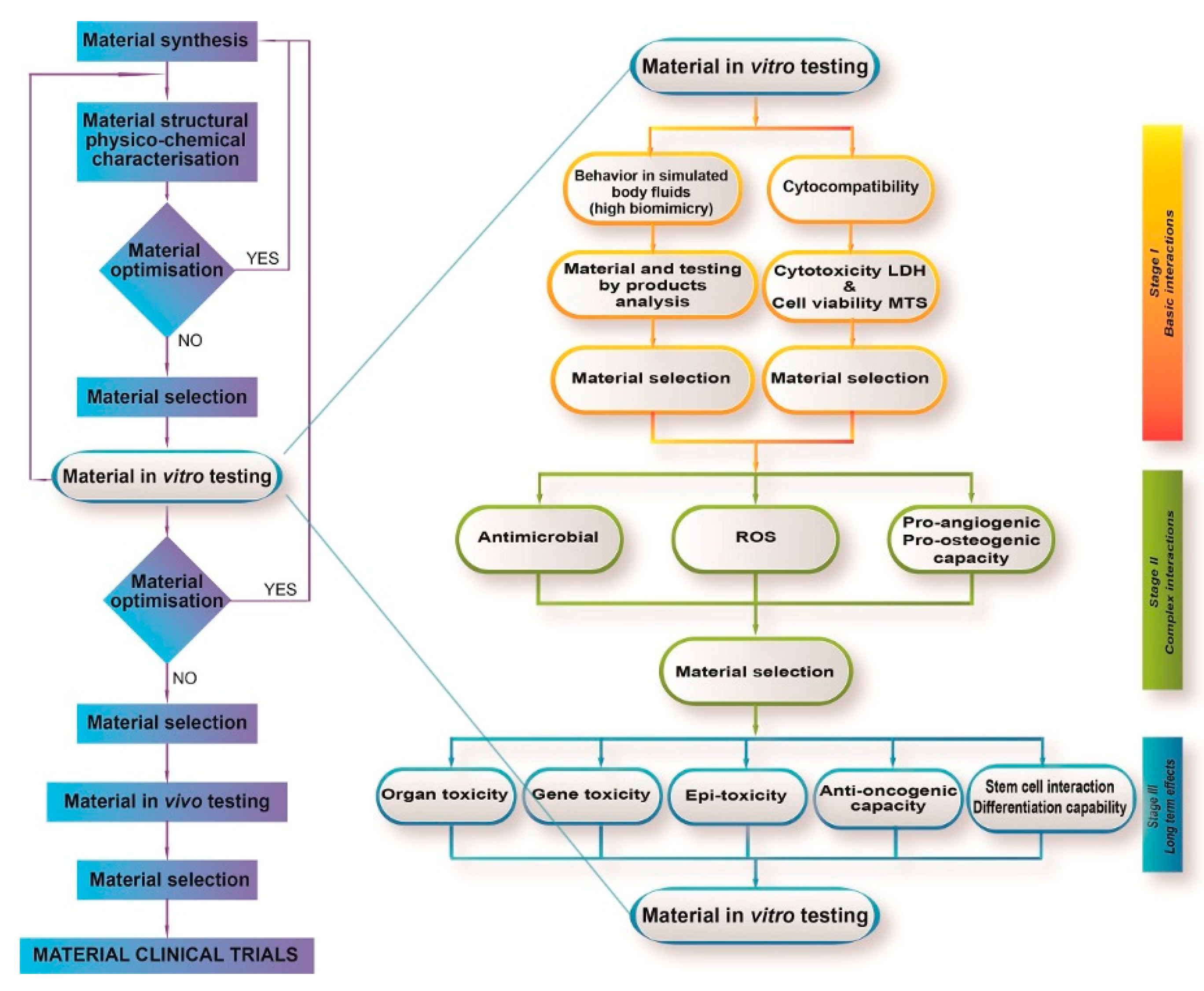
2. Biocompatibility Assessment of CaP-Based Bioceramics
2.1. Regulatory Aspects
2.2. Cell Viability and Cytotoxicity
2.3. Genotoxicity
Gene Mutation Tests
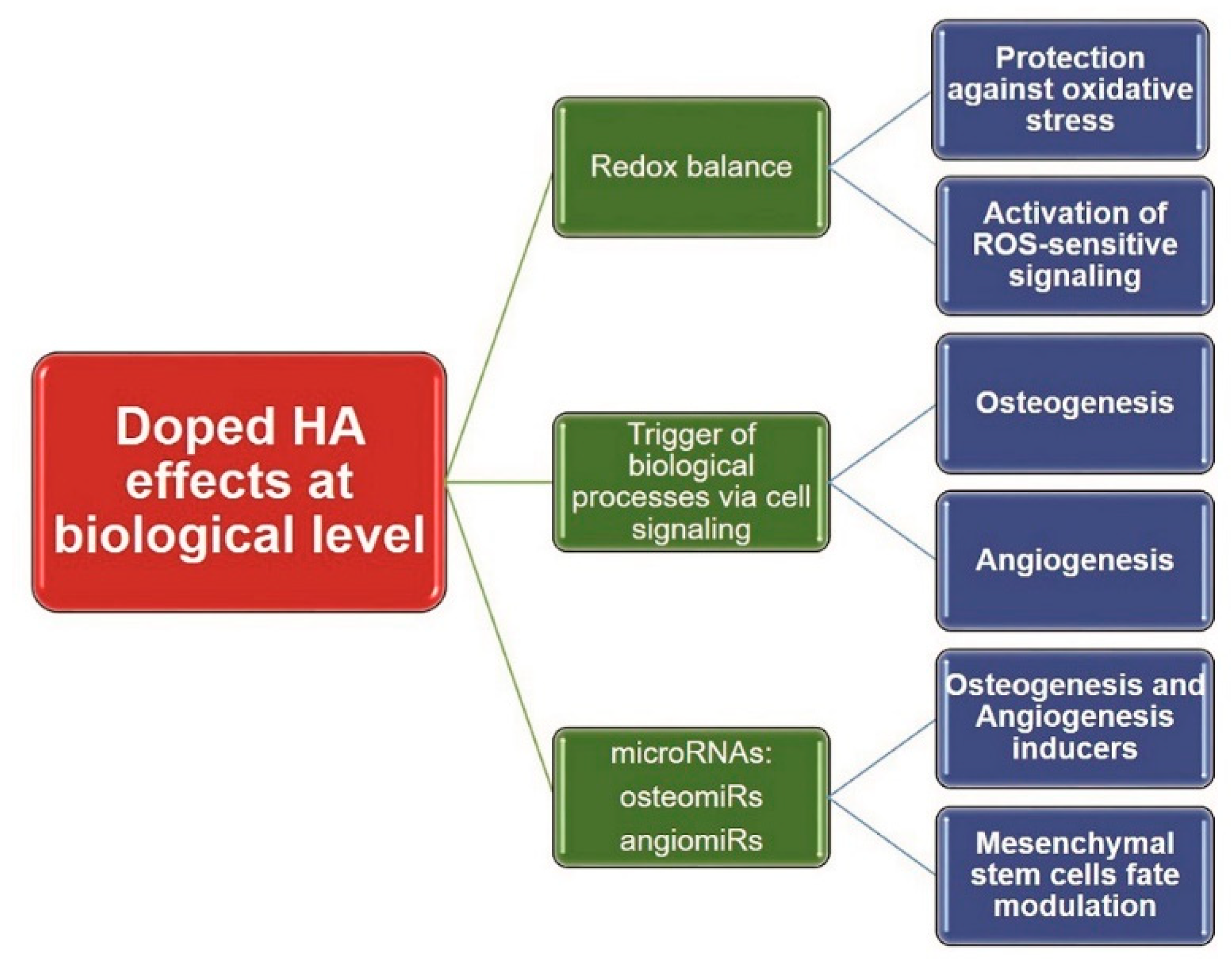
2.4. Oxidative Stress
2.5. Methods to Assess Oxidative Stress
3. Efficacy Evaluation of CaP-Based Bioceramics
3.1. Assessment of Osteogenic Effects
3.2. In Vitro Models of Osteogenesis
3.3. Assessment of Angiogenic Effects
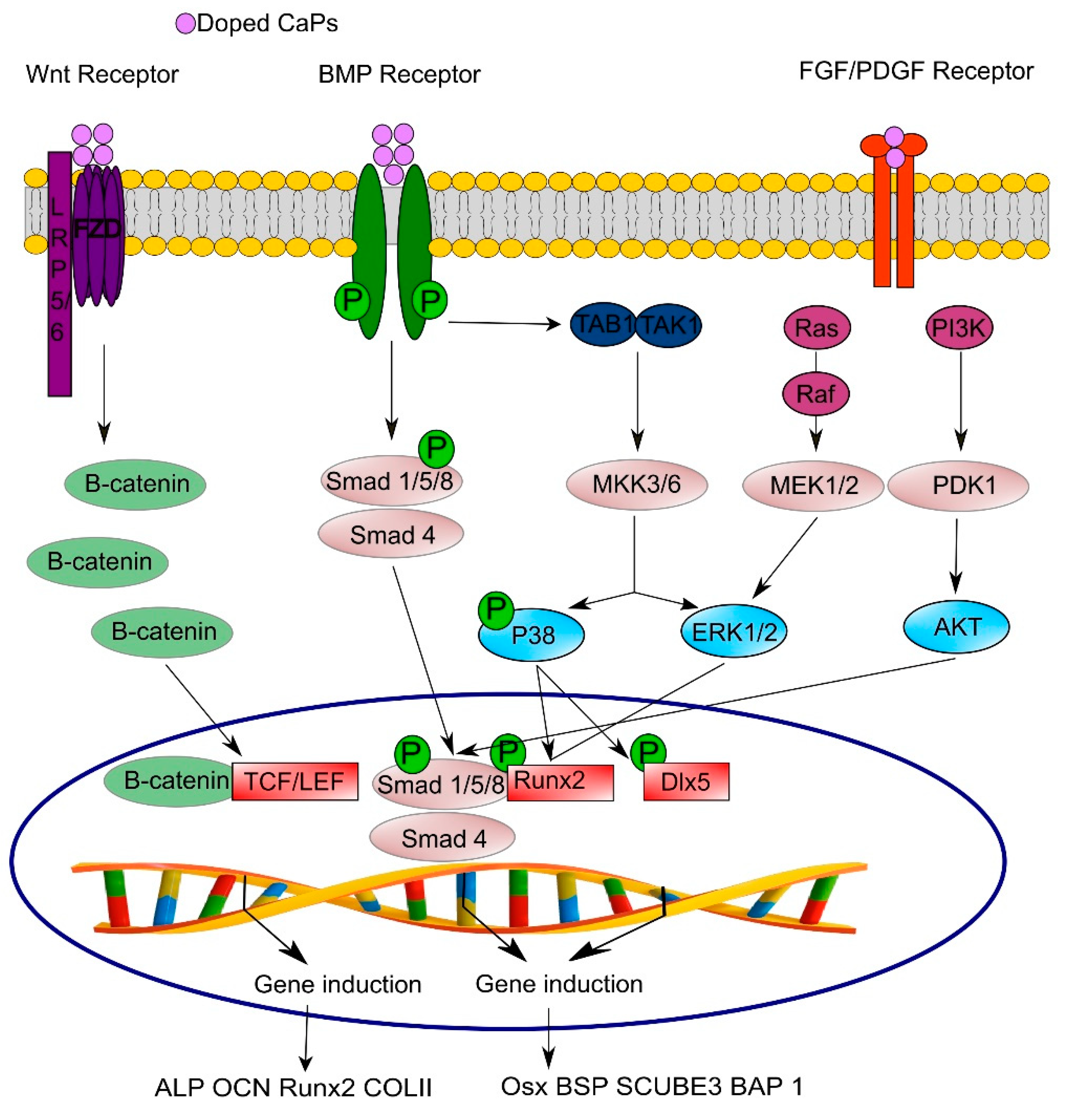
3.4. Signaling Pathways Involved in Osteo- and Angio-genesis
3.5. MicroRNAs Involved in Osteo- and Angiogenesis
4. Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tite, T.; Popa, A.C.; Balescu, L.M.; Bogdan, I.M.; Pasuk, I.; Ferreira, J.M.F.; Stan, G.E. Cationic substitutions in hydroxyapatite: Current status of the derived biofunctional effects and their in vitro interrogation methods. Materials 2018, 11, 2081. [Google Scholar] [CrossRef] [PubMed]
- Popa, A.C.; Stan, G.E.; Enculescu, M.; Tanase, C.; Tulyaganov, D.U.; Ferreira, J.M. Superior biofunctionality of dental implant fixtures uniformly coated with durable bioglass films by magnetron sputtering. J. Mech. Behav. Biomed. Mater. 2015, 51, 313–327. [Google Scholar] [CrossRef] [PubMed]
- Miculescu, F.; Maidaniuc, A.; Voicu, S.I.; Thakur, V.K.; Stan, G.E.; Ciocan, L.T. Progress in hydroxyapatite–starch based sustainable biomaterials for biomedical bone substitution applications. ACS Sustain. Chem. Eng. 2017, 5, 8491–8512. [Google Scholar] [CrossRef]
- Antoniac, I.V. Handbook of Bioceramics and Biocomposites; Springer: Berlin, Germany, 2016. [Google Scholar]
- Mucalo, M. Hydroxyapatite (HAp) for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Vichery, C.; Nedelec, J.M. Bioactive Glass Nanoparticles: From Synthesis to Materials Design for Biomedical Applications. Materials 2016, 9, 288. [Google Scholar] [CrossRef] [PubMed]
- Combes, C.; Cazalbou, S.; Rey, C. Apatite Biominerals. Minerals 2016, 6, 34. [Google Scholar] [CrossRef]
- Miculescu, F.; Mocanu, A.C.; Stan, G.E.; Miculescu, M.; Maidaniuc, A.; Cîmpean, A.; Mitran, V.; Voicu, S.I.; Machedon-Pisu, T.; Ciocan, L.T. Influence of the modulated two-step synthesis of biogenic hydroxyapatite on biomimetic products’ surface. Appl. Surf. Sci. 2018, 438, 147–157. [Google Scholar] [CrossRef]
- Io, O.; Og, A.; Og, O.; Ao, B.; Mo, P. Non-synthetic sources for the development of hydroxyapatite. J. Appl. Biotechnol. Bioeng. 2018, 5. [Google Scholar] [CrossRef]
- Jaber, H.L.; Hammood, A.S.; Parvin, N. Synthesis and characterization of hydroxyapatite powder from natural Camelus bone. J. Aust. Ceram. Soc. 2017, 54, 1–10. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef]
- Šupová, M. Substituted hydroxyapatites for biomedical applications: A review. Ceram. Int. 2015, 41, 9203–9231. [Google Scholar] [CrossRef]
- Deng, L.; Li, D.; Yang, Z.; Xie, X.; Kang, P. Repair of the calvarial defect in goat model using magnesium-doped porous hydroxyapatite combined with recombinant human bone morphogenetic protein-2. Biomed. Mater. Eng. 2017, 28, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Habibovic, P.; Barralet, J.E. Bioinorganics and biomaterials: Bone repair. Acta Biomater. 2011, 7, 3013–3026. [Google Scholar] [CrossRef] [PubMed]
- Vargas, G.E.; Haro Durand, L.A.; Cadena, V.; Romero, M.; Mesones, R.V.; Mackovic, M.; Spallek, S.; Spiecker, E.; Boccaccini, A.R.; Gorustovich, A.A. Effect of nano-sized bioactive glass particles on the angiogenic properties of collagen based composites. J. Mater. Sci. Mater. Med. 2013, 24, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yu, J.; Ke, Q.; Yang, Q.; Zhu, D.; Gao, Y.; Guo, Y.; Zhang, C. La-Doped biomimetic scaffolds facilitate bone remodelling by synchronizing osteointegration and phagocytic activity of macrophages. J. Mater. Chem. B 2019, 7, 3066–3074. [Google Scholar] [CrossRef]
- Luo, Y.; Li, D.; Zhao, J.; Yang, Z.; Kang, P. In vivo evaluation of porous lithium-doped hydroxyapatite scaffolds for the treatment of bone defect. Bio-Med. Mater. Eng. 2018, 29, 699–721. [Google Scholar] [CrossRef]
- Pina, S.; Canadas, R.F.; Jimenez, G.; Peran, M.; Marchal, J.A.; Reis, R.L.; Oliveira, J.M. Biofunctional Ionic-Doped Calcium Phosphates: Silk Fibroin Composites for Bone Tissue Engineering Scaffolding. Cells Tissues Organs 2017, 204, 150–163. [Google Scholar] [CrossRef]
- Graziani, G.; Boi, M.; Bianchi, M. A Review on ionic substitutions in hydroxyapatite thin films: Towards complete biomimetism. Coatings 2018, 8, 269. [Google Scholar] [CrossRef]
- Wu, V.M.; Tang, S.; Uskokovic, V. Calcium phosphate nanoparticles as intrinsic inorganic antimicrobials: The antibacterial effect. ACS Appl. Mater. Interfaces 2018, 10, 34013–34028. [Google Scholar] [CrossRef]
- Epple, M.; Ganesan, K.; Heumann, R.; Klesing, J.; Kovtun, A.; Neumann, S.; Sokolova, V. Application of calcium phosphatenanoparticles in biomedicine. J. Mater. Chem. 2010, 20, 18–23. [Google Scholar] [CrossRef]
- Banik, M.; Basu, T. Calcium phosphate nanoparticles: A study of their synthesis, characterization and mode of interaction with salmon testis DNA. Dalton Trans. 2014, 43, 3244–3259. [Google Scholar] [CrossRef]
- Sadat-Shojai, M.; Atai, M.; Nodehi, A.; Khanlar, L.N. Hydroxyapatite nanorods as novel fillers for improving the properties of dental adhesives: Synthesis and application. Dent. Mater. 2010, 26, 471–482. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.-W.; Zhu, Y.-J.; Chen, F.; Chen, F.-F.; Jiang, Y.-Y.; Zhang, Y.-G.; Wu, J. Ultralong hydroxyapatite nanowires/collagen scaffolds with hierarchical porous structure, enhanced mechanical properties and excellent cellular attachment. Ceram. Int. 2017, 43, 15747–15754. [Google Scholar] [CrossRef]
- Chandanshive, B.B.; Rai, P.; Rossi, A.L.; Ersen, O.; Khushalani, D. Synthesis of hydroxyapatite nanotubes for biomedical applications. Mater. Sci. Eng. C 2013, 33, 2981–2986. [Google Scholar] [CrossRef] [PubMed]
- Nejati, E.; Firouzdor, V.; Eslaminejad, M.B.; Bagheri, F. Needle-like nano hydroxyapatite/poly(l-lactide acid) composite scaffold for bone tissue engineering application. Mater. Sci. Eng. C 2009, 29, 942–949. [Google Scholar] [CrossRef]
- Kozuma, W.; Kon, K.; Kawakami, S.; Bobothike, A.; Iijima, H.; Shiota, M.; Kasugai, S. Osteoconductive potential of a hydroxyapatite fiber material with magnesium: In vitro and in vivo studies. Dent. Mater. J. 2019. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, K.; Huang, F.; Wang, C. Interaction of hydroxyapatite nanoparticles with endothelial cells: Internalization and inhibition of angiogenesis in vitro through the PI3K/Akt pathway. Int. J. Nanomed. 2017, 12, 5781–5795. [Google Scholar] [CrossRef]
- Wang, P.; Zhao, L.; Liu, J.; Weir, M.D.; Zhou, X.; Xu, H.H. Bone tissue engineering via nanostructured calcium phosphate biomaterials and stem cells. Bone Res. 2014, 2, 14017. [Google Scholar] [CrossRef]
- Dissanayaka, W.L.; Zhu, L.; Hargreaves, K.M.; Jin, L.; Zhang, C. Scaffold-free prevascularized microtissue spheroids for pulp regeneration. J. Dent. Res. 2014, 93, 1296–1303. [Google Scholar] [CrossRef]
- Gomez, S.; Vlad, M.D.; Lopez, J.; Fernandez, E. Design and properties of 3D scaffolds for bone tissue engineering. Acta Biomater. 2016, 42, 341–350. [Google Scholar] [CrossRef]
- Hosseinkhani, M.; Mehrabani, D.; Karimfar, M.H.; Bakhtiyari, S.; Manafi, A.; Shirazi, R. Tissue engineered scaffolds in regenerative medicine. World J. Plastic Surg. 2014, 3, 3–7. [Google Scholar]
- Bose, S.; Fielding, G.; Tarafder, S.; Bandyopadhyay, A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013, 31, 594–605. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Yamaguchi, S. Simulated body fluid and the novel bioactive materials derived from it. J. Biomed. Mater. Res. Part. A 2019, 107, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Popa, A.-C.; Stan, G.; Husanu, M.-A.; Mercioniu, I.; Santos, L.; Fernandes, H.; Ferreira, J. Bioglass implant-coating interactions in synthetic physiological fluids with varying degrees of biomimicry. Int. J. Nanomed. 2017, 12, 683–707. [Google Scholar] [CrossRef]
- ISO. 10993-1:2018—Biological Evaluation of Medical Devices; ISO: Geneve, Switzerland, 2018. [Google Scholar]
- Popa, A.C.; Marques, V.M.F.; Stan, G.E.; Husanu, M.A.; Galca, A.C.; Ghica, C.; Tulyaganov, D.U.; Lemos, A.F.; Ferreira, J.M.F. Nanomechanical characterization of bioglass films synthesized by magnetron sputtering. Thin Solid Films 2014, 553, 166–172. [Google Scholar] [CrossRef]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant Denti. 2019, 5, 10. [Google Scholar] [CrossRef]
- ISO. 10993-5:2009 Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; ISO: Geneve, Switzerland, 2009; p. 34. [Google Scholar]
- Coelho, F.; Cavicchioli, M.; Specian, S.S.; Scarel-Caminaga, R.M.; Penteado, L.A.; Medeiros, A.I.; Ribeiro, S.J.L.; Capote, T.S.O. Bacterial cellulose membrane functionalized with hydroxiapatite and anti-bone morphogenetic protein 2: A promising material for bone regeneration. PLoS ONE 2019, 14, e0221286. [Google Scholar] [CrossRef]
- Coelho, C.C.; Grenho, L.; Gomes, P.S.; Quadros, P.A.; Fernandes, M.H. Nano-hydroxyapatite in oral care cosmetics: Characterization and cytotoxicity assessment. Sci. Rep. 2019, 9, 11050. [Google Scholar] [CrossRef]
- Guan, R.G.; Johnson, I.; Cui, T.; Zhao, T.; Zhao, Z.Y.; Li, X.; Liu, H. Electrodeposition of hydroxyapatite coating on Mg-4.0Zn-1.0Ca-0.6Zr alloy and in vitro evaluation of degradation, hemolysis, and cytotoxicity. J. Biomed. Mater. Res. Part. A 2012, 100, 999–1015. [Google Scholar] [CrossRef]
- Singh, S.S.; Roy, A.; Lee, B.E.; Ohodnicki, J.; Loghmanian, A.; Banerjee, I.; Kumta, P.N. A study of strontium doped calcium phosphate coatings on AZ31. Mater. Sci. Eng. C 2014, 40, 357–365. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Shamsuria, O.; Fadilah, A.S.; Asiah, A.B.; Rodiah, M.R.; Suzina, A.H.; Samsudin, A.R. In vitro cytotoxicity evaluation of biomaterials on human osteoblast cells CRL-1543; hydroxyapatite, natural coral and polyhydroxybutarate. Med. J. Malaysia 2004, 59 (Suppl. B), 174–175. [Google Scholar]
- Przekora, A.; Czechowska, J.; Pijocha, D.; Ślósarczyk, A.; Ginalska, G. Do novel cement-type biomaterials reveal ion reactivity that affects cell viability in vitro? Open Life Sci. 2014, 9, s11535–s12013. [Google Scholar] [CrossRef]
- Gradinaru, S.; Popescu, V.; Leasu, C.; Pricopie, S.; Yasin, S.; Ciuluvica, R.; Ungureanu, E. Hydroxyapatite ocular implant and non-integrated implants in eviscerated patients. J. Med. Life 2015, 8, 90–93. [Google Scholar]
- Kattimani, V.S.; Kondaka, S.; Lingamaneni, K.P. Hydroxyapatite–Past, present, and future in bone regeneration. Bone Tissue Reg. Insights 2016, 7, BTRI.S36138. [Google Scholar] [CrossRef]
- Harun, W.S.W.; Asri, R.I.M.; Sulong, A.B.; Ghani, S.A.C.; Ghazalli, Z. Hydroxyapatite-based coating on biomedical implant. Mater. Sci. 2018. [Google Scholar] [CrossRef]
- Hopkins, G.O. Multiple joint tuberculosis presenting as HLA-B27 disease. Postgrad. Med. J. 1983, 59, 113–115. [Google Scholar] [CrossRef][Green Version]
- Santos, C.; Gomes, P.S.; Duarte, J.A.; Franke, R.P.; Almeida, M.M.; Costa, M.E.; Fernandes, M.H. Relevance of the sterilization-induced effects on the properties of different hydroxyapatite nanoparticles and assessment of the osteoblastic cell response. J. R. Soc. Interface 2012, 9, 3397–3410. [Google Scholar] [CrossRef]
- Ciobanu, C.S.; Iconaru, S.L.; Pasuk, I.; Vasile, B.S.; Lupu, A.R.; Hermenean, A.; Dinischiotu, A.; Predoi, D. Structural properties of silver doped hydroxyapatite and their biocompatibility. Mater. Sci. Eng. C 2013, 33, 1395–1402. [Google Scholar] [CrossRef]
- Zhao, X.; Ng, S.; Heng, B.C.; Guo, J.; Ma, L.; Tan, T.T.; Ng, K.W.; Loo, S.C. Cytotoxicity of hydroxyapatite nanoparticles is shape and cell dependent. Arch. Toxicol. 2013, 87, 1037–1052. [Google Scholar] [CrossRef]
- Assay Guidance Manual; Sittampalam, G.S., Grossman, A., Brimacombe, K., Arkin, M., Auld, D., Austin, C., Baell, J., Bejcek, B., Caaveiro, J.M.M., Chung, T.D.Y., et al., Eds.; Bethesda: Rockville, MD, USA, 2004. [Google Scholar]
- Altman, F.P. Tetrazolium salts and formazans. Progress Histochem. Cytochem. 1976, 9, III-51. [Google Scholar] [CrossRef]
- Aslantürk, Ö.S. In Vitro Cytotoxicity and Cell Viability Assays: Principles, Advantages, and Disadvantages; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef]
- Kim, K.M.; Lee, S.B.; Lee, S.H.; Lee, Y.K.; Kim, K.N. Comparison of validity between WST-1 and MTT test in bioceramic materials. Key Eng. Mater. 2005, 284–286, 585–588. [Google Scholar] [CrossRef]
- Menyhart, O.; Harami-Papp, H.; Sukumar, S.; Schafer, R.; Magnani, L.; de Barrios, O.; Gyorffy, B. Guidelines for the selection of functional assays to evaluate the hallmarks of cancer. Biochim. Biophys. Acta 2016, 1866, 300–319. [Google Scholar] [CrossRef] [PubMed]
- Chamchoy, K.; Pakotiprapha, D.; Pumirat, P.; Leartsakulpanich, U.; Boonyuen, U. Application of WST-8 based colorimetric NAD(P)H detection for quantitative dehydrogenase assays. BMC Biochem. 2019, 20, 4. [Google Scholar] [CrossRef]
- Noorani, T.Y.; Luddin, N.; Rahman, I.A.; Masudi, S.M. In vitro cytotoxicity evaluation of novel nano-hydroxyapatite-silica incorporated glass ionomer cement. J. Clin. Diagn. Res. 2017, 11, ZC105–ZC109. [Google Scholar] [CrossRef]
- El Hadad, A.A.; Peon, E.; Garcia-Galvan, F.R.; Barranco, V.; Parra, J.; Jimenez-Morales, A.; Galvan, J.C. Biocompatibility and corrosion protection behaviour of hydroxyapatite sol-gel-derived coatings on Ti6Al4V alloy. Materials 2017, 10, 94. [Google Scholar] [CrossRef]
- Ramis, J.; Coelho, C.; Córdoba, A.; Quadros, P.; Monjo, M. Safety assessment of nano-hydroxyapatite as an oral care ingredient according to the EU cosmetics regulation. Cosmetics 2018, 5, 53. [Google Scholar] [CrossRef]
- Aghaei, H.; Nourbakhsh, A.A.; Karbasi, S.; JavadKalbasi, R.; Rafienia, M.; Nourbakhsh, N.; Bonakdar, S.; Mackenzie, K.J.D. Investigation on bioactivity and cytotoxicity of mesoporous nano-composite MCM-48/hydroxyapatite for ibuprofen drug delivery. Ceram. Int. 2014, 40, 7355–7362. [Google Scholar] [CrossRef]
- Ferreira dos Santos, C.; Gomes, P.S.; Almeida, M.M.; Willinger, M.-G.; Franke, R.-P.; Fernandes, M.H.; Costa, M.E. Gold-dotted hydroxyapatite nanoparticles as multifunctional platforms for medical applications. RSC Adv. 2015, 5, 69184–69195. [Google Scholar] [CrossRef][Green Version]
- Szymonowicz, M.; Korczynski, M.; Dobrzynski, M.; Zawisza, K.; Mikulewicz, M.; Karuga-Kuzniewska, E.; Zywickab, B.; Rybak, Z.; Wiglusz, R.J. Cytotoxicity evaluation of high-temperature annealed nanohydroxyapatite in contact with fibroblast cells. Materials 2017, 10, 590. [Google Scholar] [CrossRef]
- Gao, J.; Feng, Z.; Wang, X.; Zeng, M.; Liu, J.; Han, S.; Xu, J.; Chen, L.; Cao, K.; Long, J.; et al. SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress. Cell Death Diff. 2018, 25, 229–240. [Google Scholar] [CrossRef]
- Guo, L.; Von Dem Bussche, A.; Buechner, M.; Yan, A.; Kane, A.B.; Hurt, R.H. Adsorption of essential micronutrients by carbon nanotubes and the implications for nanotoxicity testing. Small 2008, 4, 721–727. [Google Scholar] [CrossRef]
- Wohlleben, W.; Kolle, S.N.; Hasenkamp, L.-C.; Böser, A.; Vogel, S.; Vacano, B.V.; Ravenzwaay, B.V.; Landsiedel, R. Artifacts by marker enzyme adsorption on nanomaterials in cytotoxicity assays with tissue cultures. J. Phys. Conf. Ser. 2011, 304, 012061. [Google Scholar] [CrossRef]
- Pailleux, M.; Boudard, D.; Pourchez, J.; Forest, V.; Grosseau, P.; Cottier, M. New insight into artifactual phenomena during in vitro toxicity assessment of engineered nanoparticles: Study of TNF-alpha adsorption on alumina oxide nanoparticle. Toxicol. In Vitro 2013, 27, 1049–1056. [Google Scholar] [CrossRef][Green Version]
- Lupu, A.R.; Popescu, T. The noncellular reduction of MTT tetrazolium salt by TiO2 nanoparticles and its implications for cytotoxicity assays. Toxicol. In Vitro 2013, 27, 1445–1450. [Google Scholar] [CrossRef]
- Popescu, T.; Lupu, A.R.; Raditoiu, V.; Purcar, V.; Teodorescu, V.S. On the photocatalytic reduction of MTT tetrazolium salt on the surface of TiO2 nanoparticles: Formazan production kinetics and mechanism. J. Colloid Interface Sci. 2015, 457, 108–120. [Google Scholar] [CrossRef]
- Lee, Y.K.; Choi, E.J.; Webster, T.J.; Kim, S.H.; Khang, D. Effect of the protein corona on nanoparticles for modulating cytotoxicity and immunotoxicity. Int. J. Nanomed. 2015, 10, 97–113. [Google Scholar] [CrossRef]
- Treuel, L.; Docter, D.; Maskos, M.; Stauber, R.H. Protein corona-from molecular adsorption to physiological complexity. Beilstein J. Nanotechnol. 2015, 6, 857–873. [Google Scholar] [CrossRef]
- Nguyen, V.H.; Lee, B.J. Protein corona: A new approach for nanomedicine design. Int. J. Nanomed. 2017, 12, 3137–3151. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Q.; Yang, M.; Zhan, X.; Lan, F.; He, J.; Gu, Z.; Wu, Y. Protein corona of magnetic hydroxyapatite scaffold improves cell proliferation via activation of mitogen-activated protein kinase signaling pathway. ACS Nano 2017, 11, 3690–3704. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, P.; Luo, B.; Lan, F.; He, J.; Wu, Y. Dynamic protein corona influences immune-modulating osteogenesis in magnetic nanoparticle (MNP)-infiltrated bone regeneration scaffolds in vivo. Nanoscale 2019, 11, 6817–6827. [Google Scholar] [CrossRef]
- ISO. 10993-3:2014 Biologicalevaluationof Medicaldevices -Part 3:Tests Forgenotoxicity,Carcinogenicityand Reproductivetoxicity; ISO: Geneve, Switzerland, 2014. [Google Scholar]
- OECD Guidelines. Available online: http://www.oecd.org (accessed on 24 October 2019).
- ISO. 10993-12:2012 Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials; ISO: Geneve, Switzerland, 2012; p. 20. [Google Scholar]
- Lloyd, M.; Kidd, D. The mouse lymphoma assay. Methods Mol. Biol. 2012, 817, 35–54. [Google Scholar] [CrossRef]
- Ab238544. Comet Assay Kit (3-well Slides). Available online: https://www.abcam.com/ps/products/238/ab238544/documents/ab238544%20Comet%20Assay%20Kit%20(3-well%20slides)_v2a%20(website).pdf (accessed on 24 October 2019).
- Wahab, N.; Kannan, T.P.; Mahmood, Z.; Rahman, I.A.; Ismail, H. Genotoxicity assessment of biphasic calcium phosphate of modified porosity on human dental pulp cells using Ames and Comet assays. Toxicol. In Vitro 2018, 47, 207–212. [Google Scholar] [CrossRef]
- Sonmez, E.; Cacciatore, I.; Bakan, F.; Turkez, H.; Mohtar, Y.I.; Togar, B.; Stefano, A.D. Toxicity assessment of hydroxyapatite nanoparticles in rat liver cell model in vitro. Hum. Exp. Toxicol. 2016, 35, 1073–1083. [Google Scholar] [CrossRef]
- Seyedmajidi, S.; Seyedmajidi, M.; Zabihi, E.; Hajian-Tilaki, K. A comparative study on cytotoxicity and genotoxicity of the hydroxyapatite-bioactive glass and fluorapatite-bioactive glass nanocomposite foams as tissue scaffold for bone repair. J. Biomed. Mater. Res. Part A 2018, 106, 2605–2612. [Google Scholar] [CrossRef]
- Kido, H.W.; Ribeiro, D.A.; de Oliveira, P.; Parizotto, N.A.; Camilo, C.C.; Fortulan, C.A.; Marcantonio, E., Jr.; da Silva, V.H.; Renno, A.C. Biocompatibility of a porous alumina ceramic scaffold coated with hydroxyapatite and bioglass. J. Biomed. Mater. Res. Part A 2014, 102, 2072–2078. [Google Scholar] [CrossRef]
- Oledzka, E.; Pachowska, D.; Orlowska, K.; Kolmas, J.; Drobniewska, A.; Figat, R.; Sobczak, M. Pamidronate-conjugated biodegradable branched copolyester carriers: synthesis and characterization. Molecules 2017, 22, 1063. [Google Scholar] [CrossRef]
- Yamamura, H.; da Silva, V.H.P.; Ruiz, P.L.M.; Ussui, V.; Lazar, D.R.R.; Renno, A.C.M.; Ribeiro, D.A. Physico-chemical characterization and biocompatibility of hydroxyapatite derived from fish waste. J. Mech. Behav. Biomed. Mater. 2018, 80, 137–142. [Google Scholar] [CrossRef]
- Sthijns, M.; van Blitterswijk, C.A.; LaPointe, V.L.S. Redox regulation in regenerative medicine and tissue engineering: The paradox of oxygen. J. Tissue Eng. Regen. Med. 2018, 12, 2013–2020. [Google Scholar] [CrossRef]
- Yang, Y.; Karakhanova, S.; Werner, J.; Bazhin, A.V. Reactive oxygen species in cancer biology and anticancer therapy. Curr. Med. Chem. 2013, 20, 3677–3692. [Google Scholar] [CrossRef]
- Sies, H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015, 4, 180–183. [Google Scholar] [CrossRef]
- Mueller, C.F.; Laude, K.; McNally, J.S.; Harrison, D.G. ATVB in focus: Redox mechanisms in blood vessels. Arterioscl. Thromb. Vasc. Biol. 2005, 25, 274–278. [Google Scholar] [CrossRef]
- Sthijns, M.M.; Weseler, A.R.; Bast, A.; Haenen, G.R. Time in redox adaptation processes: from evolution to hormesis. Int. J. Mol. Sci. 2016, 17, 1649. [Google Scholar] [CrossRef]
- Forman, H.J.; Ursini, F.; Maiorino, M. An overview of mechanisms of redox signaling. J. Mol. Cell. Cardiol. 2014, 73, 2–9. [Google Scholar] [CrossRef]
- The Molecular Probes Handbook—A Guide to Fluorescent Probes and Labeling Technologies. Available online: https://www.thermofisher.com/ro/en/home/references/molecular-probes-the-handbook.html (accessed on 24 October 2019).
- Suzen, S.; Gurer-Orhan, H.; Saso, L. Detection of reactive oxygen and nitrogen species by electron paramagnetic resonance (EPR) technique. Molecules 2017, 22, 181. [Google Scholar] [CrossRef]
- Marrocco, I.; Altieri, F.; Peluso, I. Measurement and clinical significance of biomarkers of oxidative stress in humans. Oxid. Med. Cell. Longev. 2017, 2017, 6501046. [Google Scholar] [CrossRef]
- Aranda, A.; Sequedo, L.; Tolosa, L.; Quintas, G.; Burello, E.; Castell, J.V.; Gombau, L. Dichloro-dihydro-fluorescein diacetate (DCFH-DA) assay: A quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol. In Vitro 2013, 27, 954–963. [Google Scholar] [CrossRef]
- Khan, P.; Idrees, D.; Moxley, M.A.; Corbett, J.A.; Ahmad, F.; von Figura, G.; Sly, W.S.; Waheed, A.; Hassan, M.I. Luminol-based chemiluminescent signals: Clinical and non-clinical application and future uses. Appl. Biochem. Biotechnol. 2014, 173, 333–355. [Google Scholar] [CrossRef]
- Yazdani, J.; Ahmadian, E.; Sharifi, S.; Shahi, S.; Maleki Dizaj, S. A short view on nanohydroxyapatite as coating of dental implants. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 105, 553–557. [Google Scholar] [CrossRef]
- Lee, H.; Jang, T.S.; Song, J.; Kim, H.E.; Jung, H.D. The Production of Porous Hydroxyapatite Scaffolds with Graded Porosity by Sequential Freeze-Casting. Materials 2017, 10, 367. [Google Scholar] [CrossRef]
- Pina, S.; Ribeiro, V.P.; Marques, C.F.; Maia, F.R.; Silva, T.H.; Reis, R.L.; Oliveira, J.M. Scaffolding strategies for tissue engineering and regenerative medicine applications. Materials 2019, 12, 1824. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Madler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef]
- Gallud, A.; Kloditz, K.; Ytterberg, J.; Ostberg, N.; Katayama, S.; Skoog, T.; Gogvadze, V.; Chen, Y.Z.; Xue, D.; Moya, S.; et al. Cationic gold nanoparticles elicit mitochondrial dysfunction: A multi-omics study. Sci. Rep. 2019, 9, 4366. [Google Scholar] [CrossRef]
- Culcasi, M.; Benameur, L.; Mercier, A.; Lucchesi, C.; Rahmouni, H.; Asteian, A.; Casano, G.; Botta, A.; Kovacic, H.; Pietri, S. EPR spin trapping evaluation of ROS production in human fibroblasts exposed to cerium oxide nanoparticles: Evidence for NADPH oxidase and mitochondrial stimulation. Chem. Biol. Interact. 2012, 199, 161–176. [Google Scholar] [CrossRef]
- Knaapen, A.M.; Borm, P.J.; Albrecht, C.; Schins, R.P. Inhaled particles and lung cancer. Part A: Mechanisms. Int. J. Cancer 2004, 109, 799–809. [Google Scholar] [CrossRef]
- Khanna, P.; Ong, C.; Bay, B.H.; Baeg, G.H. Nanotoxicity: An interplay of oxidative stress, inflammation and cell death. Nanomaterials 2015, 5, 1163–1180. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Liong, M.; Madler, L.; Gilbert, B.; Shi, H.; Yeh, J.I.; Zink, J.I.; Nel, A.E. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano 2008, 2, 2121–2134. [Google Scholar] [CrossRef]
- Uskokovic, V.; Uskokovic, D.P. Nanosized hydroxyapatite and other calcium phosphates: Chemistry of formation and application as drug and gene delivery agents. J. Biomed. Mater. Res. Part B 2011, 96, 152–191. [Google Scholar] [CrossRef]
- Sun, J.S.; Lin, F.H.; Hung, T.Y.; Tsuang, Y.H.; Chang, W.H.; Liu, H.C. The influence of hydroxyapatite particles on osteoclast cell activities. J. Biomed. Mater. Res. 1999, 45, 311–321. [Google Scholar] [CrossRef]
- Cai, Y.; Liu, Y.; Yan, W.; Hu, Q.; Tao, J.; Zhang, M.; Shi, Z.; Tang, R. Role of hydroxyapatite nanoparticle size in bone cell proliferation. J. Mater. Chem. 2007, 17, 3780. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Liu, X.; Zhang, R.; Feng, Q. In vitro uptake of hydroxyapatite nanoparticles and their effect on osteogenic differentiation of human mesenchymal stem cells. Stem Cells Int. 2018, 2018, 2036176. [Google Scholar] [CrossRef]
- Calabrese, G.; Giuffrida, R.; Forte, S.; Fabbi, C.; Figallo, E.; Salvatorelli, L.; Memeo, L.; Parenti, R.; Gulisano, M.; Gulino, R. Human adipose-derived mesenchymal stem cells seeded into a collagen-hydroxyapatite scaffold promote bone augmentation after implantation in the mouse. Sci. Rep. 2017, 7, 7110. [Google Scholar] [CrossRef]
- Zhang, Y.; Marsboom, G.; Toth, P.T.; Rehman, J. Mitochondrial respiration regulates adipogenic differentiation of human mesenchymal stem cells. PLoS ONE 2013, 8, e77077. [Google Scholar] [CrossRef]
- Chen, L.; McCrate, J.M.; Lee, J.C.; Li, H. The role of surface charge on the uptake and biocompatibility of hydroxyapatite nanoparticles with osteoblast cells. Nanotechnology 2011, 22, 105708. [Google Scholar] [CrossRef]
- Porter, J.R.; Ruckh, T.T.; Popat, K.C. Bone tissue engineering: A review in bone biomimetics and drug delivery strategies. Biotechnol. Prog. 2009, 25, 1539–1560. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, C.; Wei, J.; Sun, J. Effects of four types of hydroxyapatite nanoparticles with different nanocrystal morphologies and sizes on apoptosis in rat osteoblasts. J. Appl. Toxicol. 2012, 32, 429–435. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, X.; Liu, H.; Chen, S.; Gao, C.; Ge, K.; Zhang, C.; Zhang, J. Oxidative stress-induced apoptosis of osteoblastic MC3T3-E1 cells by hydroxyapatite nanoparticles through lysosomal and mitochondrial pathways. RSC Adv. 2017, 7, 13010–13018. [Google Scholar] [CrossRef]
- Werner, S.; Grose, R. Regulation of wound healing by growth factors and cytokines. Physiol. Rev. 2003, 83, 835–870. [Google Scholar] [CrossRef]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants—A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef]
- Mestres, G.; Espanol, M.; Xia, W.; Persson, C.; Ginebra, M.P.; Ott, M.K. Inflammatory response to nano- and microstructured hydroxyapatite. PLoS ONE 2015, 10, e0120381. [Google Scholar] [CrossRef]
- Gittens, R.A.; Olivares-Navarrete, R.; Schwartz, Z.; Boyan, B.D. Implant osseointegration and the role of microroughness and nanostructures: Lessons for spine implants. Acta Biomater. 2014, 10, 3363–3371. [Google Scholar] [CrossRef]
- Koundouros, N.; Poulogiannis, G. Phosphoinositide 3-Kinase/Akt signaling and redox metabolism in cancer. Front. Oncol. 2018, 8, 160. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, Y.; Xiao, C.; Zhao, W.; Wu, H.; Tang, J.; Li, Z.; Yu, S.; Li, X.; Min, L.; et al. Application of hydroxyapatite nanoparticles in tumor-associated bone segmental defect. Sci. Adv. 2019, 5, eaax6946. [Google Scholar] [CrossRef]
- Han, Y.; Li, S.; Cao, X.; Yuan, L.; Wang, Y.; Yin, Y.; Qiu, T.; Dai, H.; Wang, X. Different inhibitory effect and mechanism of hydroxyapatite nanoparticles on normal cells and cancer cells in vitro and in vivo. Sci. Rep. 2014, 4, 7134. [Google Scholar] [CrossRef]
- Qing, F.; Wang, Z.; Hong, Y.; Liu, M.; Guo, B.; Luo, H.; Zhang, X. Selective effects of hydroxyapatite nanoparticles on osteosarcoma cells and osteoblasts. J. Mater. Sci. Mater. Med. 2012, 23, 2245–2251. [Google Scholar] [CrossRef]
- Xu, J.; Xu, P.; Li, Z.; Huang, J.; Yang, Z. Oxidative stress and apoptosis induced by hydroxyapatite nanoparticles in C6 cells. J. Biomed. Mater. Res. Part A 2012, 100, 738–745. [Google Scholar] [CrossRef]
- Zhao, X.; Heng, B.C.; Xiong, S.; Guo, J.; Tan, T.T.; Boey, F.Y.; Ng, K.W.; Loo, J.S. In vitro assessment of cellular responses to rod-shaped hydroxyapatite nanoparticles of varying lengths and surface areas. Nanotoxicology 2011, 5, 182–194. [Google Scholar] [CrossRef]
- Nelson, B.C.; Johnson, M.E.; Walker, M.L.; Riley, K.R.; Sims, C.M. Antioxidant cerium oxide nanoparticles in biology and medicine. Antioxidants 2016, 5, 15. [Google Scholar] [CrossRef]
- Celardo, I.; Pedersen, J.Z.; Traversa, E.; Ghibelli, L. Pharmacological potential of cerium oxide nanoparticles. Nanoscale 2011, 3, 1411–1420. [Google Scholar] [CrossRef]
- Li, C.; Shi, X.; Shen, Q.; Guo, C.; Hou, Z.; Zhang, J. Hot topics and challenges of regenerative nanoceria in application of antioxidant therapy. J. Nanomater. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Xu, C.; Qu, X. Cerium oxide nanoparticle: A remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014, 6, e90. [Google Scholar] [CrossRef]
- Xue, Y.; Luan, Q.; Yang, D.; Yao, X.; Zhou, K. Direct evidence for hydroxyl radical scavenging activity of cerium oxide nanoparticles. J. Phys. Chem. C 2011, 115, 4433–4438. [Google Scholar] [CrossRef]
- Heckert, E.G.; Karakoti, A.S.; Seal, S.; Self, W.T. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials 2008, 29, 2705–2709. [Google Scholar] [CrossRef]
- Pirmohamed, T.; Dowding, J.M.; Singh, S.; Wasserman, B.; Heckert, E.; Karakoti, A.S.; King, J.E.; Seal, S.; Self, W.T. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem. Commun. (Camb.) 2010, 46, 2736–2738. [Google Scholar] [CrossRef]
- Deshpande, S.; Patil, S.; Kuchibhatla, S.V.N.T.; Seal, S. Size dependency variation in lattice parameter and valency states in nanocrystalline cerium oxide. Appl. Phys. Lett. 2005, 87, 133113. [Google Scholar] [CrossRef]
- Farias, I.A.P.; Dos Santos, C.C.L.; Sampaio, F.C. Antimicrobial activity of cerium oxide nanoparticles on opportunistic microorganisms: A systematic review. BioMed Res. Int. 2018, 2018, 1923606. [Google Scholar] [CrossRef]
- Badmaev, V.; Majeed, M.; Passwater, R.A. Selenium: A quest for better understanding. Alt. Ther. Health Med. 1996, 2, 59–62, 65–67. [Google Scholar]
- Steinbrenner, H.; Speckmann, B.; Klotz, L.O. Selenoproteins: Antioxidant selenoenzymes and beyond. Arch. Biochem. Biophys. 2016, 595, 113–119. [Google Scholar] [CrossRef]
- Moreno-Reyes, R.; Egrise, D.; Neve, J.; Pasteels, J.L.; Schoutens, A. Selenium deficiency-induced growth retardation is associated with an impaired bone metabolism and osteopenia. J. Bone Miner. Res. 2001, 16, 1556–1563. [Google Scholar] [CrossRef]
- Uskokovic, V.; Iyer, M.A.; Wu, V.M. One ion to rule them all: Combined antibacterial, osteoinductive and anticancer properties of selenite-incorporated hydroxyapatite. J. Mater. Chem. B 2017, 5, 1430–1445. [Google Scholar] [CrossRef] [PubMed]
- Rajamannan, N.M. Oxidative-mechanical stress signals stem cell niche mediated Lrp5 osteogenesis in eNOS(-/-) null mice. J. Cell. Biochem. 2012, 113, 1623–1634. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.W.; Kim, T.S.; Lee, S.Y.; Lee, S.H.; Choi, Y.; Kim, N.; Min, B.M.; Jeong, D.W.; Kim, I.Y. Selenite-induced apoptosis of osteoclasts mediated by the mitochondrial pathway. Toxicol. Lett. 2006, 160, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Role of Nrf2 in oxidative stress and toxicity. Ann. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Zheng, F.; Li, H. Evaluation of Nrf2 with exposure to nanoparticles. Methods Mol. Biol 2019, 1894, 229–246. [Google Scholar] [CrossRef]
- Sun, Y.X.; Xu, A.H.; Yang, Y.; Li, J. Role of Nrf2 in bone metabolism. J. Biomed. Sci. 2015, 22, 101. [Google Scholar] [CrossRef]
- Nuss, K.M.R.; Rechenberg, B.V. Biocompatibility issues with modern implants in bone—A review for clinical orthopedics. Open Orthop. J. 2008, 2, 66–78. [Google Scholar] [CrossRef]
- Cacciotti, I. Cationic and anionic substitutions in hydroxyapatite. In Handbook of Bioceramics and Biocomposites, 1st ed.; Antoniac, I., Ed.; Springer: Basel, Switzerland, 2016; pp. 145–211. ISBN 978-3-319-12459-9. [Google Scholar]
- Ashton Acton, Q. Apatites—Advances in Research and Application; 2012 Edition; Scholarly Editions: Atlanta, GA, USA, 2012. [Google Scholar]
- ISO. 10993-6:2016 Biological Evaluation of Medical Devices—Part 6: Tests for Local Effects AFTER Implantation; ISO: Geneve, Switzerland, 2016; p. 29. [Google Scholar]
- Hung, B.P.; Hutton, D.L.; Kozielski, K.L.; Bishop, C.J.; Naved, B.; Green, J.J.; Caplan, A.I.; Gimble, J.M.; Dorafshar, A.H.; Grayson, W.L. Platelet-derived growth factor BB enhances osteogenesis of adipose-derived but not bone marrow-derived mesenchymal stromal/stem cells. Stem Cells 2015, 33, 2773–2784. [Google Scholar] [CrossRef]
- Sun, X.; Su, W.; Ma, X.; Zhang, H.; Sun, Z.; Li, X. Comparison of the osteogenic capability of rat bone mesenchymal stem cells on collagen, collagen/hydroxyapatite, hydroxyapatite and biphasic calcium phosphate. Regen. Biomater. 2018, 5, 93–103. [Google Scholar] [CrossRef]
- Schumacher, M.; Lode, A.; Helth, A.; Gelinsky, M. A novel strontium(II)-modified calcium phosphate bone cement stimulates human-bone-marrow-derived mesenchymal stem cell proliferation and osteogenic differentiation in vitro. Acta Biomater. 2013, 9, 9547–9557. [Google Scholar] [CrossRef] [PubMed]
- Mencia Castano, I.; Curtin, C.M.; Duffy, G.P.; O’Brien, F.J. Next generation bone tissue engineering: Non-viral miR-133a inhibition using collagen-nanohydroxyapatite scaffolds rapidly enhances osteogenesis. Sci. Rep. 2016, 6, 27941. [Google Scholar] [CrossRef] [PubMed]
- Klontzas, M.E.; Vernardis, S.I.; Heliotis, M.; Tsiridis, E.; Mantalaris, A. Metabolomics analysis of the osteogenic differentiation of umbilical cord blood mesenchymal stem cells reveals differential sensitivity to osteogenic agents. Stem Cells Dev. 2017, 26, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Liu, N.; Zhang, Q.; Tian, T.; Ma, Q.; Zhang, T.; Cai, X. Effect of tetrahedral DNA nanostructures on proliferation and osteogenic differentiation of human periodontal ligament stem cells. Cell Prolif. 2019, 52, e12566. [Google Scholar] [CrossRef] [PubMed]
- Puchtler, H.; Meloan, S.N.; Terry, M.S. On the history and mechanism of alizarin and alizarin red S stains for calcium. J. Histochem. Cytochem. 1969, 17, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Bonewald, L.F.; Harris, S.E.; Rosser, J.; Dallas, M.R.; Dallas, S.L.; Camacho, N.P.; Boyan, B.; Boskey, A. von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation. Calcified Tissue Int. 2003, 72, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Xiong, W.; Fang, Z.; Guan, H.; Wu, W.; Li, Y.; Zhang, Y.; Alvarez, M.M.; Gao, B.; Huo, K.; et al. Strontium (Sr) and silver (Ag) loaded nanotubular structures with combined osteoinductive and antimicrobial activities. Acta Biomater. 2016, 31, 388–400. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, Z.; Zhang, X.; Chang, X.; Zhang, X.; Li, Y.; Ye, T.; Han, R.; Han, S.; Gao, Y.; et al. Nanotube-formed Ti substrates coated with silicate/silver co-doped hydroxyapatite as prospective materials for bone implants. J. Alloy. Comp. 2017, 697, 182–199. [Google Scholar] [CrossRef]
- Ilmer, M.; Karow, M.; Geissler, C.; Jochum, M.; Neth, P. Human osteoblast–derived factors induce early osteogenic markers in human mesenchymal stem cells. Tissue Eng. Part. A 2009, 15, 2397–2409. [Google Scholar] [CrossRef]
- Komori, T. Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res. 2010, 339, 189–195. [Google Scholar] [CrossRef]
- Li, Y.L.; Xiao, Z.S. Advances in Runx2 regulation and its isoforms. Med. Hypotheses 2007, 68, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, A.; Srinaath, N.; Rohini, M.; Selvamurugan, N. Regulation of Runx2 by MicroRNAs in osteoblast differentiation. Life Sci. 2019, 232, 116676. [Google Scholar] [CrossRef] [PubMed]
- Fakhry, M.; Hamade, E.; Badran, B.; Buchet, R.; Magne, D. Molecular mechanisms of mesenchymal stem cell differentiation towards osteoblasts. World J. Stem Cells 2013, 5, 136–148. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Hilton, M.J.; Tu, X.; Yu, K.; Ornitz, D.M.; Long, F. Sequential roles of Hedgehog and Wnt signaling in osteoblast development. Development 2005, 132, 49–60. [Google Scholar] [CrossRef]
- Glass, D.A., 2nd; Bialek, P.; Ahn, J.D.; Starbuck, M.; Patel, M.S.; Clevers, H.; Taketo, M.M.; Long, F.; McMahon, A.P.; Lang, R.A.; et al. Canonical Wnt signaling in differentiated osteoblasts controls osteoclast differentiation. Dev. Cell 2005, 8, 751–764. [Google Scholar] [CrossRef]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef]
- Nakashima, K.; Zhou, X.; Kunkel, G.; Zhang, Z.; Deng, J.M.; Behringer, R.R.; de Crombrugghe, B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 2002, 108, 17–29. [Google Scholar] [CrossRef]
- Kulanthaivel, S.; Mishra, U.; Agarwal, T.; Giri, S.; Pal, K.; Pramanik, K.; Banerjee, I. Improving the osteogenic and angiogenic properties of synthetic hydroxyapatite by dual doping of bivalent cobalt and magnesium ion. Ceram. Int. 2015, 41, 11323–11333. [Google Scholar] [CrossRef]
- Won, J.E.; Yun, Y.R.; Jang, J.H.; Yang, S.H.; Kim, J.H.; Chrzanowski, W.; Wall, I.B.; Knowles, J.C.; Kim, H.W. Multifunctional and stable bone mimic proteinaceous matrix for bone tissue engineering. Biomaterials 2015, 56, 46–57. [Google Scholar] [CrossRef]
- Curtin, C.M.; Cunniffe, G.M.; Lyons, F.G.; Bessho, K.; Dickson, G.R.; Duffy, G.P.; O’Brien, F.J. Innovative collagen nano-hydroxyapatite scaffolds offer a highly efficient non-viral gene delivery platform for stem cell-mediated bone formation. Adv. Mater. 2012, 24, 749–754. [Google Scholar] [CrossRef]
- Rana, A.A.; Karim, M.M.; Gafur, M.A.; Hossan, M.J. Mechanical properties of Gelatin–Hydroxyapatite composite for bone tissue engineering. Bangladesh J. Sci. Ind. Res. 2015, 50, 15–20. [Google Scholar] [CrossRef]
- Nieto-Suarez, M.; Lopez-Quintela, M.A.; Lazzari, M. Preparation and characterization of crosslinked chitosan/gelatin scaffolds by ice segregation induced self-assembly. Carbohydr. Polym. 2016, 141, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fang, H.; Zhang, K.; Yin, J. Homologous sodium alginate/chitosan-based scaffolds, but contrasting effect on stem cell shape and osteogenesis. ACS Appl. Mater. Interfaces 2018, 10, 6930–6941. [Google Scholar] [CrossRef] [PubMed]
- Cunniffe, G.M.; O’Brien, F.J.; Partap, S.; Levingstone, T.J.; Stanton, K.T.; Dickson, G.R. The synthesis and characterization of nanophase hydroxyapatite using a novel dispersant-aided precipitation method. J. Biomed. Mater. Res. Part A 2010, 95, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Tierney, E.G.; Duffy, G.P.; Hibbitts, A.J.; Cryan, S.A.; O’Brien, F.J. The development of non-viral gene-activated matrices for bone regeneration using polyethyleneimine (PEI) and collagen-based scaffolds. J. Control. Release 2012, 158, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, S.K.; Li, L.; Qin, L.; Wang, X.L.; Lai, Y.X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Trans. 2015, 3, 95–104. [Google Scholar] [CrossRef]
- Kim, J.M.; Kim, J.H.; Lee, B.H.; Choi, S.H. Vertical Bone Augmentation Using Three-dimensionally Printed Cap in the Rat Calvarial Partial Defect. In Vivo 2018, 32, 1111–1117. [Google Scholar] [CrossRef]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef]
- Saulacic, N.; Nakahara, K.; Iizuka, T.; Haga-Tsujimura, M.; Hofstetter, W.; Scolozzi, P. Comparison of two protocols of periosteal distraction osteogenesis in a rabbit calvaria model. J. Biomed. Mater. Res. Part B 2016, 104, 1121–1131. [Google Scholar] [CrossRef]
- Fujio, M.; Osawa, Y.; Matsushita, M.; Ogisu, K.; Tsuchiya, S.; Kitoh, H.; Hibi, H. A Mouse Distraction Osteogenesis Model. J. Vis. Exp. 2018. [Google Scholar] [CrossRef]
- Osawa, Y.; Matsushita, M.; Hasegawa, S.; Esaki, R.; Fujio, M.; Ohkawara, B.; Ishiguro, N.; Ohno, K.; Kitoh, H. Activated FGFR3 promotes bone formation via accelerating endochondral ossification in mouse model of distraction osteogenesis. Bone 2017, 105, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Carey, E.G.; Deshpande, S.S.; Zheutlin, A.R.; Nelson, N.S.; Donneys, A.; Kang, S.Y.; Gallagher, K.K.; Felice, P.A.; Tchanque-Fossuo, C.N.; Buchman, S.R. A Comparison of vascularity, bone mineral density distribution, and histomorphometrics in an isogenic versus an outbred murine model of mandibular distraction osteogenesis. J. Oral Maxillofac. Surg. 2016, 74, 2055–2065. [Google Scholar] [CrossRef] [PubMed]
- Zheutlin, A.R.; Deshpande, S.S.; Nelson, N.S.; Kang, S.Y.; Gallagher, K.K.; Polyatskaya, Y.; Rodriguez, J.J.; Donneys, A.; Ranganathan, K.; Buchman, S.R. Bone marrow stem cells assuage radiation-induced damage in a murine model of distraction osteogenesis: A histomorphometric evaluation. Cytotherapy 2016, 18, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Wang, C.; Zhang, C.; Xu, J.; Chai, Y.; Jia, Y.; Han, P.; Wen, G. All-trans retinoic acid promotes osteogenic differentiation and bone consolidation in a rat distraction osteogenesis model. Calcif. Tissue Int. 2019, 104, 320–330. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.M.; Morse, A.; Birke, O.; Yu, N.Y.C.; Mikulec, K.; Peacock, L.; Schindeler, A.; Liu, M.; Ke, H.Z.; Little, D.G. Sclerostin antibody enhances bone formation in a rat model of distraction osteogenesis. J. Orthop. Res. 2018, 36, 1106–1113. [Google Scholar] [CrossRef]
- Tchanque-Fossuo, C.N.; Donneys, A.; Deshpande, S.S.; Sarhaddi, D.; Nelson, N.S.; Monson, L.A.; Dahle, S.E.; Goldstein, S.A.; Buchman, S.R. Radioprotection with amifostine enhances bone strength and regeneration and bony union in a rat model of mandibular distraction osteogenesis. Ann. Plast. Surg. 2018, 80, 176–180. [Google Scholar] [CrossRef]
- Pithioux, M.; Roseren, F.; Jalain, C.; Launay, F.; Charpiot, P.; Chabrand, P.; Roffino, S.; Lamy, E. An efficient and reproducible protocol for distraction osteogenesis in a rat model leading to a functional regenerated femur. J. Vis. Exp. 2017. [Google Scholar] [CrossRef]
- Jiang, X.; Zou, S.; Ye, B.; Zhu, S.; Liu, Y.; Hu, J. bFGF-Modified BMMSCs enhance bone regeneration following distraction osteogenesis in rabbits. Bone 2010, 46, 1156–1161. [Google Scholar] [CrossRef]
- Yassine, K.A.; Mokhtar, B.; Houari, H.; Karim, A.; Mohamed, M. Repair of segmental radial defect with autologous bone marrow aspirate and hydroxyapatite in rabbit radius: A clinical and radiographic evaluation. Vet. World 2017, 10, 752–757. [Google Scholar] [CrossRef]
- Montes-Medina, L.; Hernandez-Fernandez, A.; Gutierrez-Rivera, A.; Ripalda-Cemborain, P.; Bitarte, N.; Perez-Lopez, V.; Granero-Molto, F.; Prosper, F.; Izeta, A. Effect of bone marrow stromal cells in combination with biomaterials in early phases of distraction osteogenesis: An experimental study in a rabbit femur model. Injury 2018, 49, 1979–1986. [Google Scholar] [CrossRef]
- Floerkemeier, T.; Thorey, F.; Wellmann, M.; Hurschler, C.; Budde, S.; Windhagen, H. alphaBSM failed as a carrier of rhBMP-2 to enhance bone consolidation in a sheep model of distraction osteogenesis. Acta Bioeng. Biomech. 2017, 19, 55–62. [Google Scholar] [PubMed]
- Andreasen, C.M.; Henriksen, S.S.; Ding, M.; Theilgaard, N.; Andersen, T.L.; Overgaard, S. The efficacy of poly-d,l-lactic acid- and hyaluronic acid-coated bone substitutes on implant fixation in sheep. J. Orthop. Trans. 2017, 8, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Fellah, B.H.; Gauthier, O.; Weiss, P.; Chappard, D.; Layrolle, P. Osteogenicity of biphasic calcium phosphate ceramics and bone autograft in a goat model. Biomaterials 2008, 29, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Eom, T.G.; Jeon, G.R.; Jeong, C.M.; Kim, Y.K.; Kim, S.G.; Cho, I.H.; Cho, Y.S.; Oh, J.S. Experimental study of bone response to hydroxyapatite coating implants: Bone-implant contact and removal torque test. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, 411–418. [Google Scholar] [CrossRef]
- Lukaszewska-Kuska, M.; Krawczyk, P.; Martyla, A.; Hedzelek, W.; Dorocka-Bobkowska, B. Effects of a hydroxyapatite coating on the stability of endosseous implants in rabbit tibiae. Dent. Med. Probl. 2019, 56, 123–129. [Google Scholar] [CrossRef]
- Komori, T. Animal models for osteoporosis. Eur. J. Pharmacol. 2015, 759, 287–294. [Google Scholar] [CrossRef]
- Calciolari, E.; Donos, N.; Mardas, N. Osteoporotic Animal models of bone healing: Advantages and pitfalls. J. Investig. Surg. 2016, 30, 342–350. [Google Scholar] [CrossRef]
- Xu, J.; Gong, H.; Lu, S.; Deasey, M.J.; Cui, Q. Animal models of steroid-induced osteonecrosis of the femoral head-a comprehensive research review up to 2018. Int. Orthop. 2018, 42, 1729–1737. [Google Scholar] [CrossRef]
- O’Brien, C.A.; Morello, R. Modeling rare bone diseases in animals. Curr. Osteoporosis Rep. 2018, 16, 458–465. [Google Scholar] [CrossRef]
- Ewald, A.; Kreczy, D.; Bruckner, T.; Gbureck, U.; Bengel, M.; Hoess, A.; Nies, B.; Bator, J.; Klammert, U.; Fuchs, A. Development and bone regeneration capacity of premixed magnesium phosphate cement pastes. Materials 2019, 12, 2119. [Google Scholar] [CrossRef]
- Tian, Q.; Lin, J.; Rivera-Castaneda, L.; Tsanhani, A.; Dunn, Z.S.; Rodriguez, A.; Aslani, A.; Liu, H. Nano-to-submicron hydroxyapatite coatings for magnesium-based bioresorbable implants—deposition, characterization, degradation, mechanical properties, and cytocompatibility. Sci. Rep. 2019, 9, 810. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Gao, P.; Zhang, H.; Guo, Z.; Zheng, Y.; Han, Y. Osteoimmunomodulation, osseointegration, and in vivo mechanical integrity of pure Mg coated with HA nanorod/pore-sealed MgO bilayer. Biomater. Sci. 2018, 6, 3202–3218. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Qu, X.; Lin, W.; Wang, C.; Zhu, D.; Dai, K.; Zheng, Y. In vitro and in vivo studies on zinc-hydroxyapatite composites as novel biodegradable metal matrix composite for orthopedic applications. Acta Biomater. 2018, 71, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Suruagy, A.A.; Alves, A.T.; Sartoretto, S.C.; Calasans-Maia, J.A.; Granjeiro, J.M.; Calasans-Maia, M.D. Physico-chemical and histomorphometric evaluation of zinc-containing hydroxyapatite in rabbits calvaria. Braz. Dent. J. 2016, 27, 717–726. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, S.; Xiao, W.; Cui, X.; Huang, W.; Rahaman, M.N.; Zhang, C.; Wang, D. Three-dimensional zinc incorporated borosilicate bioactive glass scaffolds for rodent critical-sized calvarial defects repair and regeneration. Colloids Surf. B Biointerf. 2015, 130, 149–156. [Google Scholar] [CrossRef]
- Glenske, K.; Donkiewicz, P.; Kowitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R.; et al. Applications of metals for bone regeneration. Int. J. Mol. Sci. 2018, 19, 826. [Google Scholar] [CrossRef]
- Li, D.; Huifang, L.; Zhao, J.; Yang, Z.; Xie, X.; Wei, Z.; Kang, P. Porous lithium-doped hydroxyapatite scaffold seeded with hypoxia-preconditioned bone-marrow mesenchymal stem cells for bone-tissue regeneration. Biomed. Mater. 2018, 13, 055002. [Google Scholar] [CrossRef]
- Li, D.; Xie, X.; Yang, Z.; Wang, C.; Wei, Z.; Kang, P. Enhanced bone defect repairing effects in glucocorticoid-induced osteonecrosis of the femoral head using a porous nano-lithium-hydroxyapatite/gelatin microsphere/erythropoietin composite scaffold. Biomater. Sci. 2018, 6, 519–537. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, S.; Shi, Y.; Ma, J. 3D printing of strontium-doped hydroxyapatite based composite scaffolds for repairing critical-sized rabbit calvarial defects. Biomed. Mater. 2018, 13, 065004. [Google Scholar] [CrossRef]
- Chandran, S.; Babu, S.S.; Vs, H.K.; Varma, H.K.; John, A. Osteogenic efficacy of strontium hydroxyapatite micro-granules in osteoporotic rat model. J. Biomater. Appl. 2016, 31, 499–509. [Google Scholar] [CrossRef]
- Li, Y.; Shui, X.; Zhang, L.; Hu, J. Cancellous bone healing around strontium-doped hydroxyapatite in osteoporotic rats previously treated with zoledronic acid. J. Biomed. Mater. Res. Part B 2016, 104, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luo, E.; Zhu, S.; Li, J.; Zhang, L.; Hu, J. Cancellous bone response to strontium-doped hydroxyapatite in osteoporotic rats. J. Appl. Biomater. Funct. Mater. 2015, 13, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Barba, A.; Maazouz, Y.; Diez-Escudero, A.; Rappe, K.; Espanol, M.; Montufar, E.B.; Ohman-Magi, C.; Persson, C.; Fontecha, P.; Manzanares, M.C.; et al. Osteogenesis by foamed and 3D-printed nanostructured calcium phosphate scaffolds: Effect of pore architecture. Acta Biomater. 2018, 79, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Ke, X.; Liu, A.; Sun, M.; He, Y.; Yang, X.; Fu, J.; Liu, Y.; Zhang, L.; Yang, G.; et al. Bone regeneration in 3D printing bioactive ceramic scaffolds with improved tissue/material interface pore architecture in thin-wall bone defect. Biofabrication 2017, 9, 025003. [Google Scholar] [CrossRef]
- Barba, A.; Diez-Escudero, A.; Espanol, M.; Bonany, M.; Sadowska, J.M.; Guillem-Marti, J.; Ohman-Magi, C.; Persson, C.; Manzanares, M.C.; Franch, J.; et al. Impact of biomimicry in the design of osteoinductive bone substitutes: Nanoscale matters. ACS Appl. Mater. Interfaces 2019, 11, 8818–8830. [Google Scholar] [CrossRef]
- Diao, J.; OuYang, J.; Deng, T.; Liu, X.; Feng, Y.; Zhao, N.; Mao, C.; Wang, Y. 3D-plotted beta-tricalcium phosphate scaffolds with smaller pore sizes improve in vivo bone regeneration and biomechanical properties in a critical-sized calvarial defect rat model. Adv. Healthc. Mater. 2018, 7, e1800441. [Google Scholar] [CrossRef]
- Kubasiewicz-Ross, P.; Hadzik, J.; Seeliger, J.; Kozak, K.; Jurczyszyn, K.; Gerber, H.; Dominiak, M.; Kunert-Keil, C. New nano-hydroxyapatite in bone defect regeneration: A histological study in rats. Ann. Anat. 2017, 213, 83–90. [Google Scholar] [CrossRef]
- Elrayah, A.; Zhi, W.; Feng, S.; Al-Ezzi, S.; Lei, H.; Weng, J. Preparation of micro/nano-structure copper-substituted hydroxyapatite scaffolds with improved angiogenesis capacity for bone regeneration. Materials 2018, 11, 1516. [Google Scholar] [CrossRef]
- Handoll, H.H.; Watts, A.C. Bone grafts and bone substitutes for treating distal radial fractures in adults. Cochrane Database Syst. Rev. 2008, CD006836. [Google Scholar] [CrossRef]
- Buser, Z.; Brodke, D.S.; Youssef, J.A.; Meisel, H.J.; Myhre, S.L.; Hashimoto, R.; Park, J.B.; Tim Yoon, S.; Wang, J.C. Synthetic bone graft versus autograft or allograft for spinal fusion: A systematic review. J. Neurosurg. Spine 2016, 25, 509–516. [Google Scholar] [CrossRef]
- Kaiser, M.G.; Groff, M.W.; Watters, W.C., 3rd; Ghogawala, Z.; Mummaneni, P.V.; Dailey, A.T.; Choudhri, T.F.; Eck, J.C.; Sharan, A.; Wang, J.C.; et al. Guideline update for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 16: Bone graft extenders and substitutes as an adjunct for lumbar fusion. J. Neurosurg. Spine 2014, 21, 106–132. [Google Scholar] [CrossRef] [PubMed]
- Chepelev, L.; Wake, N.; Ryan, J.; Althobaity, W.; Gupta, A.; Arribas, E.; Santiago, L.; Ballard, D.H.; Wang, K.C.; Weadock, W.; et al. Radiological Society of North America (RSNA) 3D printing Special Interest Group (SIG): Guidelines for medical 3D printing and appropriateness for clinical scenarios. 3D Print. Med. 2018, 4, 11. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Lin, K.; Jiang, X.; Fang, B.; Xu, Y.; Liu, J.; Zeng, D.; Zhang, M.; Zhang, X.; Chang, J.; et al. Effect of nano-structured bioceramic surface on osteogenic differentiation of adipose derived stem cells. Biomaterials 2014, 35, 8514–8527. [Google Scholar] [CrossRef] [PubMed]
- Almubarak, S.; Nethercott, H.; Freeberg, M.; Beaudon, C.; Jha, A.; Jackson, W.; Miclau, T.; Healy, K.; Bahney, C. Tissue engineering strategies for promoting vascularized bone regeneration. Bone 2016, 83, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, A.; Habibovic, P. Calcium phosphates and angiogenesis: Implications and advances for bone regeneration. Trends Biotechnol. 2016, 34, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Amini, A.R.; Laurencin, C.T.; Nukavarapu, S.P. Bone tissue engineering: recent advances and challenges. Crit. Rev. Biomed. Eng. 2012, 40, 363–408. [Google Scholar] [CrossRef]
- Levingstone, T.J.; Barron, N.; Ardhaoui, M.; Benyounis, K.; Looney, L.; Stokes, J. Application of response surface methodology in the design of functionally graded plasma sprayed hydroxyapatite coatings. Surf. Coat. Technol. 2017, 313, 307–318. [Google Scholar] [CrossRef]
- Rahmani, A.; Hashemi-Najafabadi, S.; Eslaminejad, M.B.; Bagheri, F.; Sayahpour, F.A. The effect of modified electrospun PCL-nHA-nZnO scaffolds on osteogenesis and angiogenesis. J. Biomed. Mater. Res. Part A 2019, 107, 2040–2052. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, C.; Wang, X.; Shi, M.; Zhu, Y.; Jing, L.; Wu, C.; Chang, J. Stimulation of osteogenesis and angiogenesis by micro/nano hierarchical hydroxyapatite via macrophage immunomodulation. Nanoscale 2019, 11, 17699–17708. [Google Scholar] [CrossRef]
- Son, J.; Kim, J.; Lee, K.; Hwang, J.; Choi, Y.; Seo, Y.; Jeon, H.; Kang, H.C.; Woo, H.-M.; Kang, B.-J.; et al. DNA aptamer immobilized hydroxyapatite for enhancing angiogenesis and bone regeneration. Acta Biomater. 2019. [Google Scholar] [CrossRef]
- Maier, J.A.; Bernardini, D.; Rayssiguier, Y.; Mazur, A. High concentrations of magnesium modulate vascular endothelial cell behaviour in vitro. Biochim. Biophys. Acta 2004, 1689, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Sartori, M.; Pagani, S.; Ferrari, A.; Costa, V.; Carina, V.; Figallo, E.; Maltarello, M.C.; Martini, L.; Fini, M.; Giavaresi, G. A new bi-layered scaffold for osteochondral tissue regeneration: In vitro and in vivo preclinical investigations. Mater. Sci. Eng. C 2017, 70, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Both, S.K.; van Osch, G.J.; Wang, Y.; Jansen, J.A.; Yang, F. Performance of different three-dimensional scaffolds for in vivo endochondral bone generation. Eur. Cells Mater. 2014, 27, 350–364. [Google Scholar] [CrossRef]
- Canullo, L.; Heinemann, F.; Gedrange, T.; Biffar, R.; Kunert-Keil, C. Histological evaluation at different times after augmentation of extraction sites grafted with a magnesium-enriched hydroxyapatite: Double-blinded randomized controlled trial. Clin. Oral Implants Res. 2013, 24, 398–406. [Google Scholar] [CrossRef]
- Frank, P.G.; Woodman, S.E.; Park, D.S.; Lisanti, M.P. Caveolin, caveolae, and endothelial cell function. Arterioscler. Thromb. Vasc. Biol. 2003, 2, 1161–1168. [Google Scholar] [CrossRef]
- Woodman, S.E.; Ashton, A.W.; Schubert, W.; Lee, H.; Williams, T.M.; Medina, F.A.; Wyckoff, J.B.; Combs, T.P.; Lisanti, M.P. Caveolin-1 knockout mice show an impaired angiogenic response to exogenous stimuli. Am. J. Pathol. 2003, 162, 2059–2068. [Google Scholar] [CrossRef]
- Sun, T.W.; Yu, W.L.; Zhu, Y.J.; Yang, R.L.; Shen, Y.Q.; Chen, D.Y.; He, Y.H.; Chen, F. Hydroxyapatite nanowire@magnesium silicate core-shell hierarchical nanocomposite: Synthesis and application in bone regeneration. ACS Appl. Mater. Interfaces 2017, 9, 16435–16447. [Google Scholar] [CrossRef]
- Calabrese, G.; Giuffrida, R.; Forte, S.; Salvatorelli, L.; Fabbi, C.; Figallo, E.; Gulisano, M.; Parenti, R.; Magro, G.; Colarossi, C.; et al. Bone augmentation after ectopic implantation of a cell-free collagen-hydroxyapatite scaffold in the mouse. Sci. Rep. 2016, 6, 36399. [Google Scholar] [CrossRef]
- Saghiri, M.A.; Asatourian, A.; Orangi, J.; Sorenson, C.M.; Sheibani, N. Functional role of inorganic trace elements in angiogenesis – Part II: Cr, Si, Zn, Cu, and S. Crit. Rev. Oncol. Hematol. 2015, 96, 143–155. [Google Scholar] [CrossRef]
- Barralet, J.; Gbureck, U.; Habibovic, P.; Vorndran, E.; Gerard, C.; Doillon, C.J. Angiogenesis in calcium phosphate scaffolds by inorganic copper ion release. Tissue Eng. Part A 2009, 15, 1601–1609. [Google Scholar] [CrossRef]
- Imrie, F.E.; Skakle, J.M.S. Preparation of copper-doped hydroxyapatite with varying x in the composition Ca10(PO4)6CuxOyHz. Bioceram. Dev. Appl. 2013, 3, S1:005. [Google Scholar] [CrossRef]
- Kargozar, S.; Lotfibakhshaiesh, N.; Ai, J.; Mozafari, M.; Brouki Milan, P.; Hamzehlou, S. Strontium- and cobalt-substituted bioactive glasses seeded with human umbilical cord perivascular cells to promote bone regeneration via enhanced osteogenic and angiogenic activities. Acta Biomater. 2017, 58, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Xie, H.; Li, L.; Zhang, X.; Liu, F.; Yu, X. Application of strontium-doped calcium polyphosphate scaffold on angiogenesis for bone tissue engineering. J. Mater. Sci. Mater. Med. 2013, 24, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Ehret, C.; Aid-Launais, R.; Sagardoy, T.; Siadous, R.; Bareille, R.; Rey, S.; Pechev, S.; Etienne, L.; Kalisky, J.; de Mones, E.; et al. Strontium-doped hydroxyapatite polysaccharide materials effect on ectopic bone formation. PLoS ONE 2017, 12, e0184663. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi Birgani, Z.; Fennema, E.; Gijbels, M.J.; de Boer, J.; van Blitterswijk, C.A.; Habibovic, P. Stimulatory effect of cobalt ions incorporated into calcium phosphate coatings on neovascularization in an in vivo intramuscular model in goats. Acta Biomater. 2016, 36, 267–276. [Google Scholar] [CrossRef]
- Zamani, A.; Omrani, G.R.; Nasab, M.M. Lithium’s effect on bone mineral density. Bone 2009, 44, 331–334. [Google Scholar] [CrossRef]
- Li, J.; Khavandgar, Z.; Lin, S.H.; Murshed, M. Lithium chloride attenuates BMP-2 signaling and inhibits osteogenic differentiation through a novel WNT/GSK3- independent mechanism. Bone 2011, 48, 321–331. [Google Scholar] [CrossRef]
- Nakatsu, M.N.; Ding, Z.; Ng, M.Y.; Truong, T.T.; Yu, F.; Deng, S.X. Wnt/beta-catenin signaling regulates proliferation of human cornea epithelial stem/progenitor cells. Investig. Ophthalmol. Visual Sci. 2011, 52, 4734–4741. [Google Scholar] [CrossRef]
- James, A.W.; LaChaud, G.; Shen, J.; Asatrian, G.; Nguyen, V.; Zhang, X.; Ting, K.; Soo, C. A Review of the clinical side effects of Bone Morphogenetic Protein-2. Tissue Eng. Part B 2016, 22, 284–297. [Google Scholar] [CrossRef]
- Cecchi, S.; Bennet, S.J.; Arora, M. Bone morphogenetic protein-7: Review of signalling and efficacy in fracture healing. J. Orthop. Trans. 2016, 4, 28–34. [Google Scholar] [CrossRef]
- Garrison, K.R.; Shemilt, I.; Donell, S.; Ryder, J.J.; Mugford, M.; Harvey, I.; Song, F.; Alt, V. Bone morphogenetic protein (BMP) for fracture healing in adults. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef] [PubMed]
- Begam, H.; Nandi, S.K.; Kundu, B.; Chanda, A. Strategies for delivering bone morphogenetic protein for bone healing. Mater. Sci. Eng. C 2017, 70, 856–869. [Google Scholar] [CrossRef] [PubMed]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef] [PubMed]
- Son, S.R.; Sarkar, S.K.; Nguyen-Thuy, B.L.; Padalhin, A.R.; Kim, B.R.; Jung, H.I.; Lee, B.T. Platelet-rich plasma encapsulation in hyaluronic acid/gelatin-BCP hydrogel for growth factor delivery in BCP sponge scaffold for bone regeneration. J. Biomater. Appl. 2015, 29, 988–1002. [Google Scholar] [CrossRef]
- Gemini-Piperni, S.; Milani, R.; Bertazzo, S.; Peppelenbosch, M.; Takamori, E.R.; Granjeiro, J.M.; Ferreira, C.V.; Teti, A.; Zambuzzi, W. Kinome profiling of osteoblasts on hydroxyapatite opens new avenues on biomaterial cell signaling. Biotechnol. Bioeng. 2014, 111, 1900–1905. [Google Scholar] [CrossRef]
- Liang, H.; Xu, X.; Feng, X.; Ma, L.; Deng, X.; Wu, S.; Liu, X.; Yang, C. Gold nanoparticles-loaded hydroxyapatite composites guide osteogenic differentiation of human mesenchymal stem cells through Wnt/beta-catenin signaling pathway. Int. J. Nanomed. 2019, 14, 6151–6163. [Google Scholar] [CrossRef]
- Ou, L.; Lan, Y.; Feng, Z.; Feng, L.; Yang, J.; Liu, Y.; Bian, L.; Tan, J.; Lai, R.; Guo, R. Functionalization of SF/HAP scaffold with GO-PEI-miRNA inhibitor complexes to enhance bone regeneration through activating Transcription Factor 4. Theranostics 2019, 9, 4525–4541. [Google Scholar] [CrossRef]
- Gizer, M.; Kose, S.; Karaosmanoglu, B.; Taskiran, E.Z.; Berkkan, A.; Timucin, M.; Korkusuz, F.; Korkusuz, P. The Effect of boron-containing nano-hydroxyapatite on bone cells. Biol. Trace Elem. Res. 2019. [Google Scholar] [CrossRef]
- Li, K.; Shen, Q.; Xie, Y.; You, M.; Huang, L.; Zheng, X. Incorporation of cerium oxide into hydroxyapatite coating protects bone marrow stromal cells against H2O2-induced inhibition of osteogenic differentiation. Biol. Trace Elem. Res. 2018, 182, 91–104. [Google Scholar] [CrossRef]
- Yang, W.; Han, W.; He, W.; Li, J.; Wang, J.; Feng, H.; Qian, Y. Surface topography of hydroxyapatite promotes osteogenic differentiation of human bone marrow mesenchymal stem cells. Mater. Sci. Eng. C 2016, 60, 45–53. [Google Scholar] [CrossRef]
- Hankenson, K.D.; Dishowitz, M.; Gray, C.; Schenker, M. Angiogenesis in bone regeneration. Injury 2011, 42, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Pensa, N.W.; Curry, A.S.; Reddy, M.S.; Bellis, S.L. The addition of a polyglutamate domain to the angiogenic QK peptide improves peptide coupling to bone graft materials leading to enhanced endothelial cell activation. PLoS ONE 2019, 14, e0213592. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Hariya, R.; Matsumoto, M.; Aizawa, M. Acceleration of osteogenesis via stimulation of angiogenesis by combination with scaffold and connective tissue growth factor. Materials 2019, 12, 2068. [Google Scholar] [CrossRef] [PubMed]
- Wenz, A.; Tjoeng, I.; Schneider, I.; Kluger, P.J.; Borchers, K. Improved vasculogenesis and bone matrix formation through coculture of endothelial cells and stem cells in tissue-specific methacryloyl gelatin-based hydrogels. Biotechnol. Bioeng. 2018, 115, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Liu, Y.; Du, Z.; Weng, Z.; Yao, W.; Zhang, X.; Huang, X.; Yao, X.; Crawford, R.; Hang, R.; et al. Differential effect of hydroxyapatite nano-particle versus nano-rod decorated titanium micro-surface on osseointegration. Acta Biomater. 2018, 76, 344–358. [Google Scholar] [CrossRef] [PubMed]
- Catalanotto, C.; Cogoni, C.; Zardo, G. MicroRNA in control of gene expression: An overview of nuclear functions. Int. J. Mol. Sci. 2016, 17, 1712. [Google Scholar] [CrossRef] [PubMed]
- Shivdasani, R.A. MicroRNAs: Regulators of gene expression and cell differentiation. Blood 2006, 108, 3646–3653. [Google Scholar] [CrossRef]
- Lian, J.B.; Stein, G.S.; van Wijnen, A.J.; Stein, J.L.; Hassan, M.Q.; Gaur, T.; Zhang, Y. MicroRNA control of bone formation and homeostasis. Nat. Rev. Endocrinol. 2012, 8, 212–227. [Google Scholar] [CrossRef]
- Papaioannou, G.; Mirzamohammadi, F.; Kobayashi, T. MicroRNAs involved in bone formation. Cell. Mol. Life Sci. CMLS 2014, 71, 4747–4761. [Google Scholar] [CrossRef]
- Nakasa, T.; Yoshizuka, M.; Andry Usman, M.; Elbadry Mahmoud, E.; Ochi, M. MicroRNAs and bone regeneration. Curr. Genomics 2015, 16, 441–452. [Google Scholar] [CrossRef]
- Schubert, T.; Xhema, D.; Veriter, S.; Schubert, M.; Behets, C.; Delloye, C.; Gianello, P.; Dufrane, D. The enhanced performance of bone allografts using osteogenic-differentiated adipose-derived mesenchymal stem cells. Biomaterials 2011, 32, 8880–8891. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Rodriguez, P.; Sanchez, M.; Landin, M. Drug-loaded biomimetic ceramics for tissue engineering. Pharmaceutics 2018, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Gennari, L.; Bianciardi, S.; Merlotti, D. MicroRNAs in bone diseases. Osteoporosis Int. 2017, 28, 1191–1213. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Gao, D.; Gao, C.; Wei, P.; Niu, M.; Shuai, C. MicroRNAs regulate signaling pathways in osteogenic differentiation of mesenchymal stem cells (Review). Mol. Med. Rep. 2016, 14, 623–629. [Google Scholar] [CrossRef]
- Frohlich, L.F. Micrornas at the Interface between osteogenesis and angiogenesis as targets for bone regeneration. Cells 2019, 8, 121. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, M.; Dou, C.; Cao, Z.; Dong, S. MicroRNAs in bone balance and osteoporosis. Drug Dev. Res. 2015, 76, 235–245. [Google Scholar] [CrossRef]
- Deng, Y.; Wu, S.; Zhou, H.; Bi, X.; Wang, Y.; Hu, Y.; Gu, P.; Fan, X. Effects of a miR-31, Runx2, and Satb2 regulatory loop on the osteogenic differentiation of bone mesenchymal stem cells. Stem Cells Dev. 2013, 22, 2278–2286. [Google Scholar] [CrossRef]
- Zhang, J.F.; Fu, W.M.; He, M.L.; Xie, W.D.; Lv, Q.; Wan, G.; Li, G.; Wang, H.; Lu, G.; Hu, X.; et al. MiRNA-20a promotes osteogenic differentiation of human mesenchymal stem cells by co-regulating BMP signaling. RNA Biol. 2011, 8, 829–838. [Google Scholar] [CrossRef]
- Qu, J.; Lu, D.; Guo, H.; Miao, W.; Wu, G.; Zhou, M. MicroRNA-9 regulates osteoblast differentiation and angiogenesis via the AMPK signaling pathway. Mol. Cell. Biochem. 2016, 411, 23–33. [Google Scholar] [CrossRef]
- Vo, T.N.; Kasper, F.K.; Mikos, A.G. Strategies for controlled delivery of growth factors and cells for bone regeneration. Adv. Drug Deliv. Rev. 2012, 64, 1292–1309. [Google Scholar] [CrossRef]
- Li, X.; Guo, L.; Liu, Y.; Su, Y.; Xie, Y.; Du, J.; Zhou, J.; Ding, G.; Wang, H.; Bai, Y. MicroRNA-21 promotes osteogenesis of bone marrow mesenchymal stem cells via the Smad7-Smad1/5/8-Runx2 pathway. Biochem. Biophys. Res. Commun. 2017, 493, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, G.; Feng, Z.; Bai, S.; Dong, Y.; Wu, G.; Zhao, Y. Microarc-oxidized titanium surfaces functionalized with microRNA-21-loaded chitosan/hyaluronic acid nanoparticles promote the osteogenic differentiation of human bone marrow mesenchymal stem cells. Int. J. Nanomed. 2015, 10, 6675–6687. [Google Scholar] [CrossRef]
- Pizzicannella, J.; Cavalcanti, M.; Trubiani, O.; Diomede, F. MicroRNA 210 mediates VEGF upregulation in human periodontal ligament stem cells cultured on 3Dhydroxyapatite ceramic scaffold. Int. J. Mol. Sci. 2018, 19, 3916. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S.; Saravanan, S.; Vairamani, M.; Gopalakrishnan, C.; Sastry, T.P.; Selvamurugan, N. A Combinatorial effect of carboxymethyl cellulose based scaffold and microRNA-15b on osteoblast differentiation. Int. J. Biol. Macromol. 2016, 93, 1457–1464. [Google Scholar] [CrossRef]
- Dhivya, S.; Saravanan, S.; Sastry, T.P.; Selvamurugan, N. Nanohydroxyapatite-reinforced chitosan composite hydrogel for bone tissue repair in vitro and in vivo. J. Nanobiotechnol. 2015, 13, 40. [Google Scholar] [CrossRef]
- Sierra, M.I.; Valdes, A.; Fernandez, A.F.; Torrecillas, R.; Fraga, M.F. The effect of exposure to nanoparticles and nanomaterials on the mammalian epigenome. Int. J. Nanomed. 2016, 11, 6297–6306. [Google Scholar] [CrossRef]
- Larsson, L.; Pilipchuk, S.P.; Giannobile, W.V.; Castilho, R.M. When epigenetics meets bioengineering-A material characteristics and surface topography perspective. J. Biomed. Mater. Res. Part B 2018, 106, 2065–2071. [Google Scholar] [CrossRef]
- Ha, S.W.; Jang, H.L.; Nam, K.T.; Beck, G.R., Jr. Nano-hydroxyapatite modulates osteoblast lineage commitment by stimulation of DNA methylation and regulation of gene expression. Biomaterials 2015, 65, 32–42. [Google Scholar] [CrossRef]
- Moorthi, A.; Vimalraj, S.; Avani, C.; He, Z.; Partridge, N.C.; Selvamurugan, N. Expression of microRNA-30c and its target genes in human osteoblastic cells by nano-bioglass ceramic-treatment. Int. J. Biol. Macromol. 2013, 56, 181–185. [Google Scholar] [CrossRef]
| Type of CaP | Type of Cells | Methodological Approach | Main Effects | References |
|---|---|---|---|---|
| Cell Viability | ||||
| HA nanoparticles produced via wet chemical synthesis (37 °C) and hydrothermal synthesis (180 °C) | MG63 osteoblast-like cells | MTS cell proliferation assay | Neither particle, in doses lower than 0.5 mg/mL, affected cell viability and proliferation. For concentrations between 0.5 and 2 mg/mL, the inhibition of cell proliferation was time-dependent, with slightly higher values corresponding to chemically synthesized HA when compared with hydrothermally synthesized HA. | [52] |
| Nano-HA–silica-incorporated glass ionomer cement (HA–SiO2–GIC) | human Dental Pulp Stem Cells (DPSC) | MTT assay | HA–SiO2–GIC showed cytotoxic effects for all tested concentrations (3.125–200 mg/mL). | [61] |
| HA coatings prepared by a sol–gel method on Ti6Al4V | human fetal osteoblasts, subcultures 4–6 | MTT assay | HA sol–gel-derived coatings showed low toxicity in osteoblast cell culture after 3 days (due to poor adhesion of the cells). Subsequently, cell viability increased in cells treated for 7 and 14 days with HA. | [62] |
| HA nanoparticles (HA NPs) | Reconstructed human gingival epithelium (HGE) | MTT test; LDH assay | 3.1% HA NP solution did not induce cell death after 10 min, 1 h, and 3 h of incubation. | [63] |
| HA composite with the mesoporous silicate MCM-48 | MG68 cells | MTT assay | MTT results showed the biocompatibility of the new material and supported its possible use as drug carrier. | [64] |
| HA–Au nanoparticles | Human mesenchymal stem cells | MTS test; LDH assay | When compared with controls, the MTS assay showed no significant differences in the cell viability of cells exposed to 1–100 μg/mL HA–Au nanoparticles. LDH results indicated minimal damage to the cell membranes. | [65] |
| High-temperature annealed nano-HA obtained via wet chemistry at 800 °C, 900 °C, and 1000 °C | L929 (NCTC clone 929) mouse fibroblast cells | MTT assay | All tested samples slightly decreased the viability of cells treated with 2.5, 5, 10, and 20 g/mL nanoparticle suspensions. | [66] |
| Type of CaP | Biological Samples | Methodological Approach | Main Effects | References |
|---|---|---|---|---|
| Collagen/HA, HA, biphasic calcium phosphate | Rat MSCs | Cell proliferation (MTT) qRT-PCR | Rapid increase of osteogenic marker gene expression; increased expression of ALP | [154] |
| Sr-doped CaP | Human MSCs | Cell proliferation (LDH) ALP activity qRT-PCR of BSP II | Increased proliferation; enhanced ALP activity; increased expression of BSP II | [155] |
| Collagen–nano-HA scaffolds functionalized with microRNA (miRNA) | Human MSCs | qRT-PCR Mineral deposits quantification Calcium deposition IFA | Increased osteogenic markers; mineral deposition | [156] |
| Ag-doped hydroxyapatite/calcium silicate coating nano-Ti substrates | Mouse preosteobasts (MC3T3-E1 cells) | Cell proliferation (MTT) ALP activity ELISA | Enhanced proliferation; enhanced ALP activity; increased OCN expression | [162] |
| Co2+- and Mg2+-doped HA | MG-63 osteoblasts | Flow cytometry IFA | Similar cell-cycle profile as control cells; induced RUNX2 expression | [172] |
| Biphasic calcium phosphate ceramics | Animal tissue | Histological analysis | Mineralized bone formation | [197] |
| HA-coated implants | Animal tissue | Removal torque test Histological analysis | Higher removal torque value for HA group; new bone formation with increased density | [198] |
| HA-coated titanium implants | Animal tissue | Implant stability test | HA-favorable effect on osseointegration | [199] |
| Ca-doped MgP, HA | Animal tissue | Histological analysis | Bone healing results with complete osseointegration | [204] |
| Nano-to-submicron hydroxyapatite coatings | MSCs | Cell count and morphology analysis | Reduced cell adhesion | [205] |
| Sr-doped HA | MC3T3-E1 Animal tissue | Cell proliferation ALP activity Histological analysis | Enhanced proliferation and ALP activity; new bone formation | [213] |
| Sr-doped HA | Animal tissue | Histological analysis | Higher regeneration efficacy of Sr-doped HA compared to HA and control | [214] |
| Sr-doped HA | Animal tissue | Micro-CT assessment Histological analysis | Increased bone density around Sr-HA implants; improved trabecular microarchitecture compared to HA | [215,216] |
| Nanostructured HA scaffolds | Animal tissue | Histological analysis Micro-CT | Superior osteogenic capacity of foamed scaffolds compared to 3D-printed structures | [217] |
| β-TCP scaffolds | MSCs Animal tissue | Cell proliferation (CCK-8), Micro-CT Histological analysis | Smaller pore sizes; improved bone regeneration | [220] |
| Nano-HA | Animal tissue | Histological analysis | Bone regeneration similar to commercially available materials | [221] |
| Nano-HA scaffolds | MSCs Animal tissue | Cell proliferation (MTT) ALP activity qRT-PCR Western blot Micro-CT Histological analysis | Nanostructured HA surfaces promote cell attachment, proliferation, and osteogenic differentiation; enhanced osteo- and angiogenesis in vivo | [227] |
| Type of CaP | Biological Samples | Methodological Approach | Main Effects | References |
|---|---|---|---|---|
| Mg-doped HA | Co-culture model of HUVECs and MG63 | ELISA PCR | Significant effects on bone formation and angiogenesis; Increasing VEGF | [13,238] |
| - | IHC | Early angiogenesis followed by early osteogenesis | ||
| Bi-layered scaffold (type I collagen and Mg/HA) | hMSCs | IHC | Stimulating proliferation and differentiation of hMSCs for tissue growth and neo-angiogenesis | [236] |
| 3D scaffold (HA/TCP, PU, PLGA/PCL and collagen I gel. | - | IHC | Stimulating blood vessel formation | [237] |
| HANW@MS/CS (Magnesium Silicate) | rBMMSCs | SEM RT-qPCR analysis | Mg and Si elements contribute to angiogenic induction, bone formation, and blood vessel formation | [241] |
| Cu-doped HA | Animal tissue | IHC | CaP scaffold doped with low doses of copper sulfate led to the formation of micro-vessels | [244] |
| Animal tissue | SEM | The micro/nano-structure of the Cu5–HA scaffold resulted in more angiogenesis, which formed the new blood vessels | [222] | |
| Sr-doped CaP scaffold | Co-culture model of HUVEC and osteoblasts Animal tissue | Phase-contrast microscopy IHC | Formation of tube-like structure and the expression of platelet endothelial cell adhesion molecule in co-cultured model was better in SCPP scaffold Potential to promote the formation of angiogenesis | [247] |
| Animal tissue | IHC | New vessel formation in Matrix-50Sr-HA explants, mainly after 4 weeks of implantations, suggested a positive effect of Sr on angiogenesis | [248] | |
| Co-doped HA | Animal tissue | IHC | Enhanced vascularization in vivo; large blood vessels were predominantly found in Co-doped HA | [249] |
| Zn-doped HA | - | CAM assay | The number of vessel branches in the modified scaffolds with n-ZnO was significantly higher compared to the modified scaffolds without n-ZnO | [232] |
| Li-doped HA | BMMSCs (bone-marrow mesenchymal stem cells) | Western blot analysis IHC and IF | HIF-1α and VEGF immunohistochemistry indicated that the hypoxia BMMSCs group had significantly more positive cells than the other three groups | [211] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Albulescu, R.; Popa, A.-C.; Enciu, A.-M.; Albulescu, L.; Dudau, M.; Popescu, I.D.; Mihai, S.; Codrici, E.; Pop, S.; Lupu, A.-R.; et al. Comprehensive In Vitro Testing of Calcium Phosphate-Based Bioceramics with Orthopedic and Dentistry Applications. Materials 2019, 12, 3704. https://doi.org/10.3390/ma12223704
Albulescu R, Popa A-C, Enciu A-M, Albulescu L, Dudau M, Popescu ID, Mihai S, Codrici E, Pop S, Lupu A-R, et al. Comprehensive In Vitro Testing of Calcium Phosphate-Based Bioceramics with Orthopedic and Dentistry Applications. Materials. 2019; 12(22):3704. https://doi.org/10.3390/ma12223704
Chicago/Turabian StyleAlbulescu, Radu, Adrian-Claudiu Popa, Ana-Maria Enciu, Lucian Albulescu, Maria Dudau, Ionela Daniela Popescu, Simona Mihai, Elena Codrici, Sevinci Pop, Andreea-Roxana Lupu, and et al. 2019. "Comprehensive In Vitro Testing of Calcium Phosphate-Based Bioceramics with Orthopedic and Dentistry Applications" Materials 12, no. 22: 3704. https://doi.org/10.3390/ma12223704
APA StyleAlbulescu, R., Popa, A.-C., Enciu, A.-M., Albulescu, L., Dudau, M., Popescu, I. D., Mihai, S., Codrici, E., Pop, S., Lupu, A.-R., Stan, G. E., Manda, G., & Tanase, C. (2019). Comprehensive In Vitro Testing of Calcium Phosphate-Based Bioceramics with Orthopedic and Dentistry Applications. Materials, 12(22), 3704. https://doi.org/10.3390/ma12223704








