Investigating the Effects of Polyaluminum Chloride on the Properties of Ordinary Portland Cement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experiments Methods
3. Results and Discussion
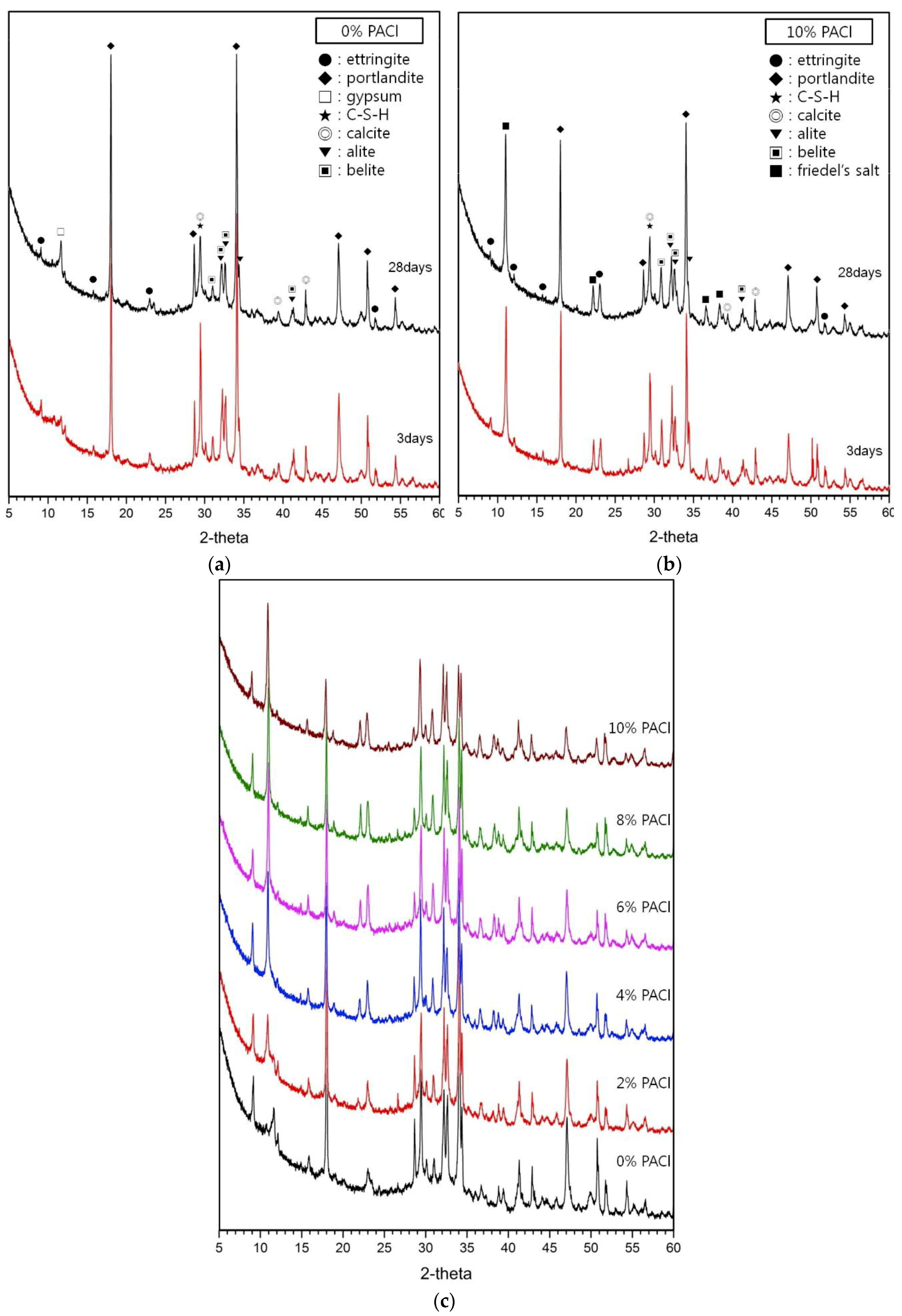
3.1. Hydration Products
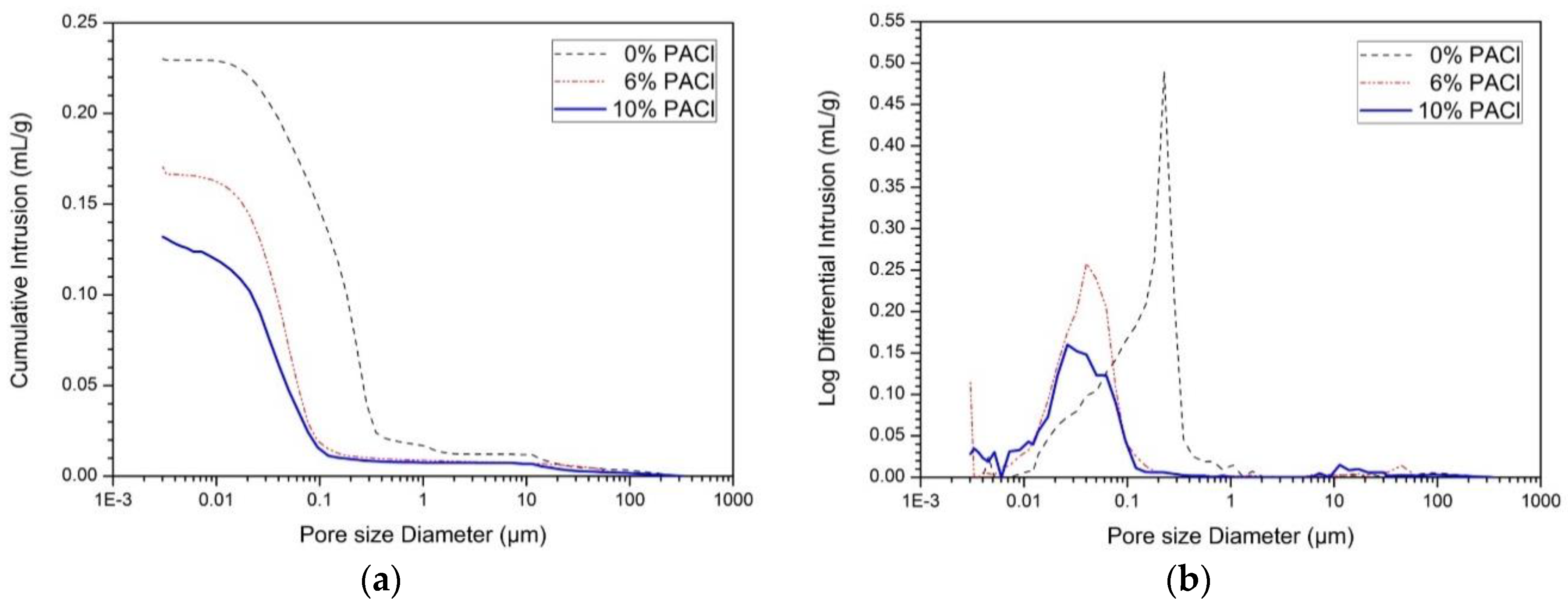
3.2. Pore Structure
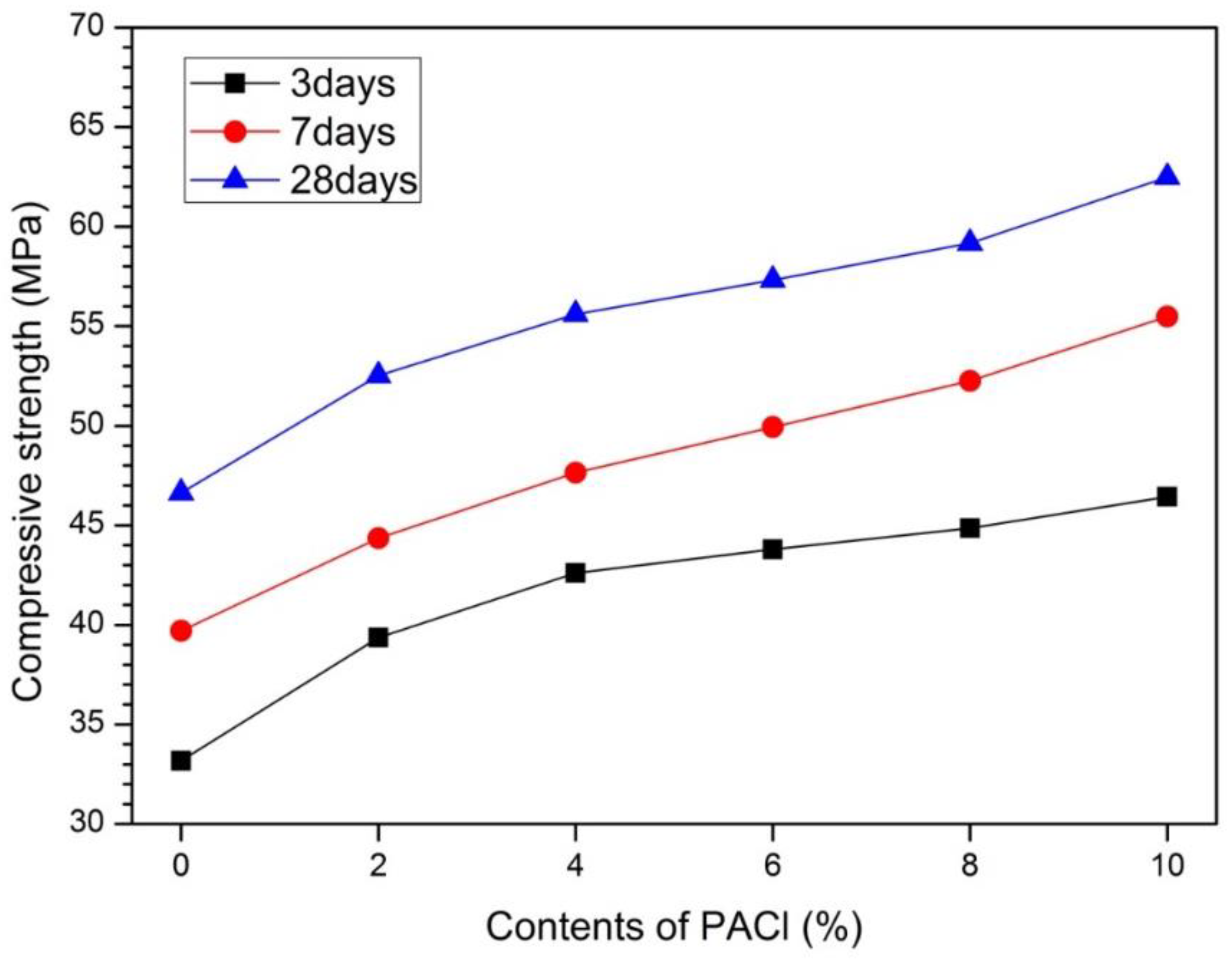
3.3. Compressive Strength
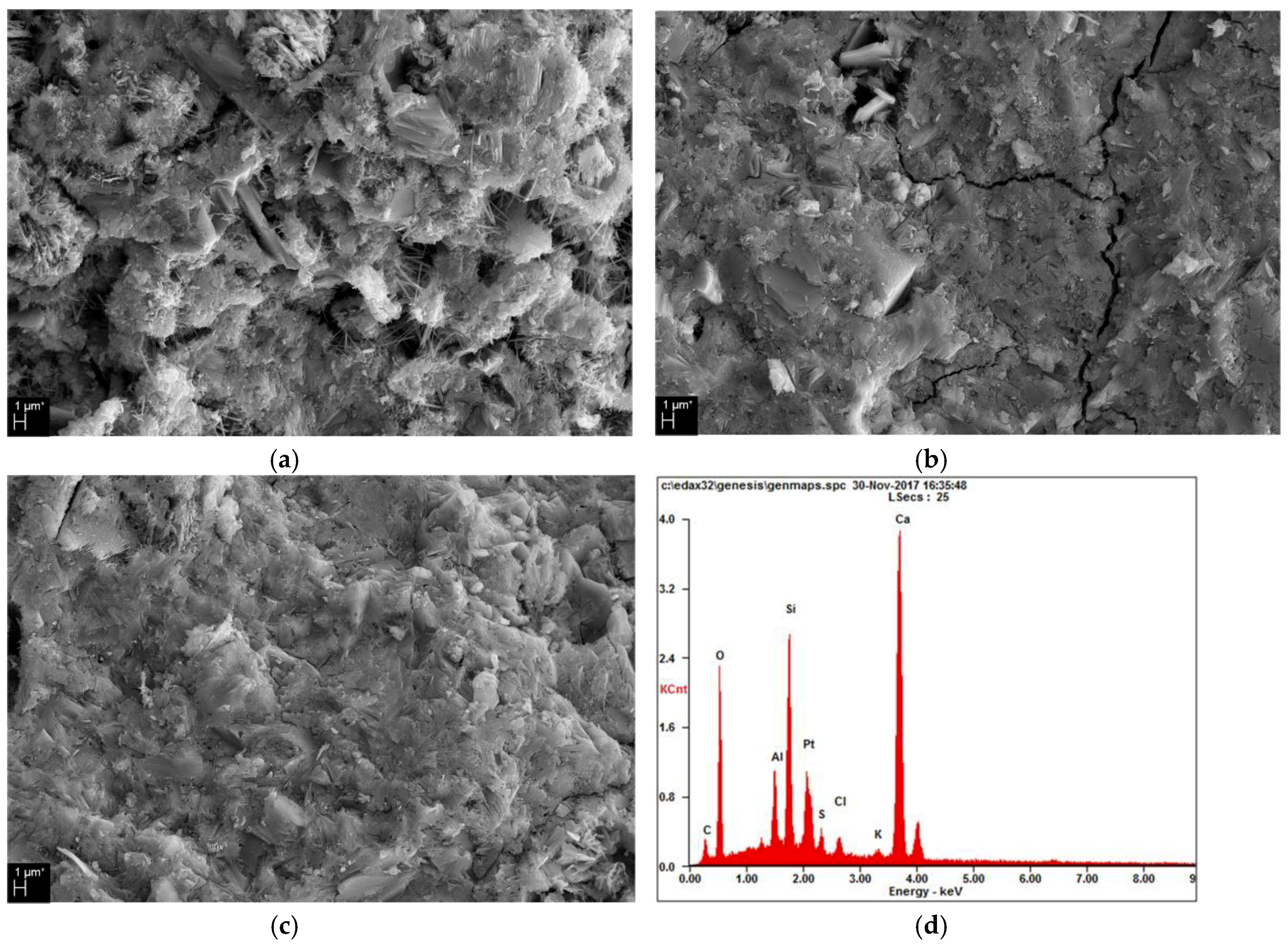
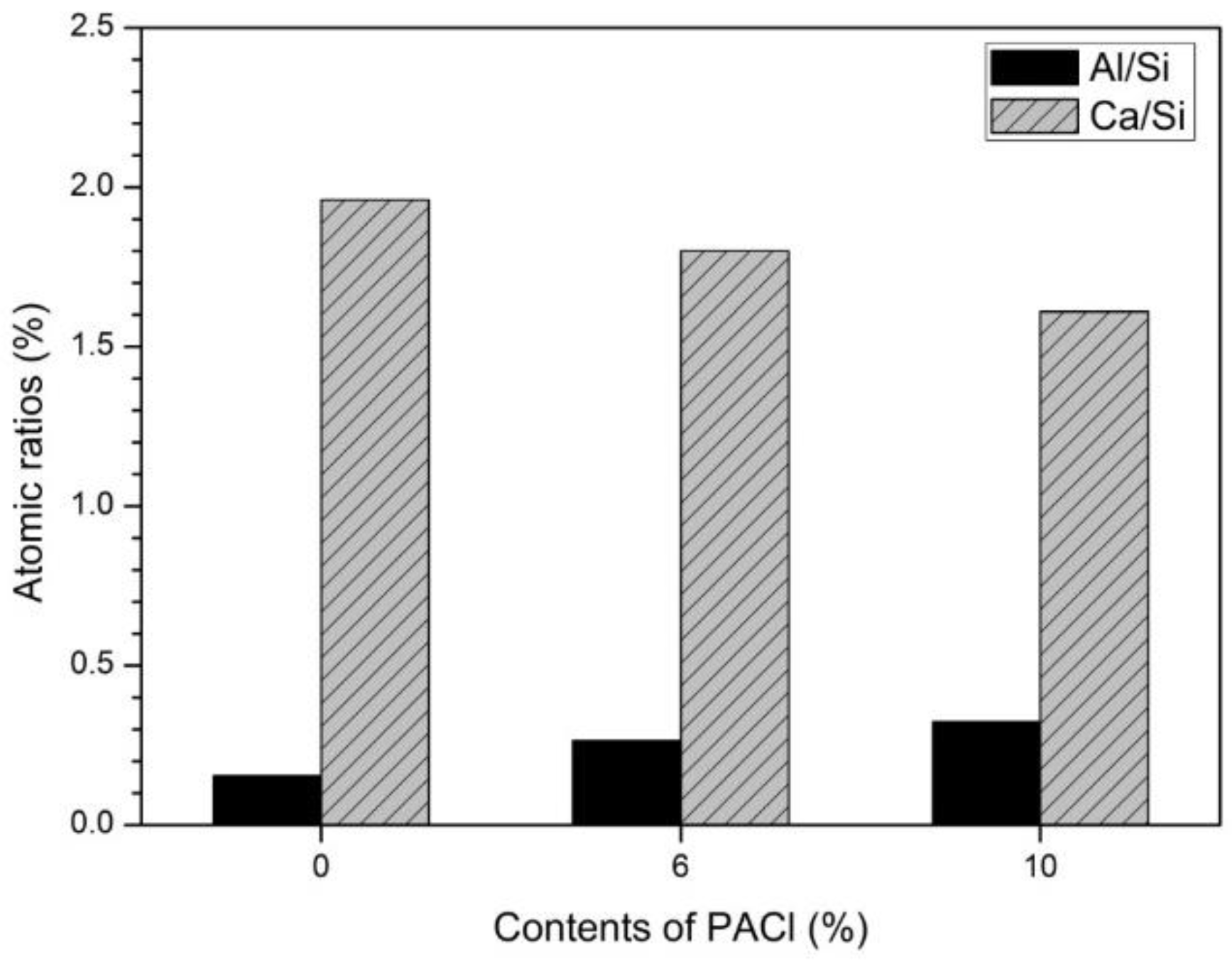
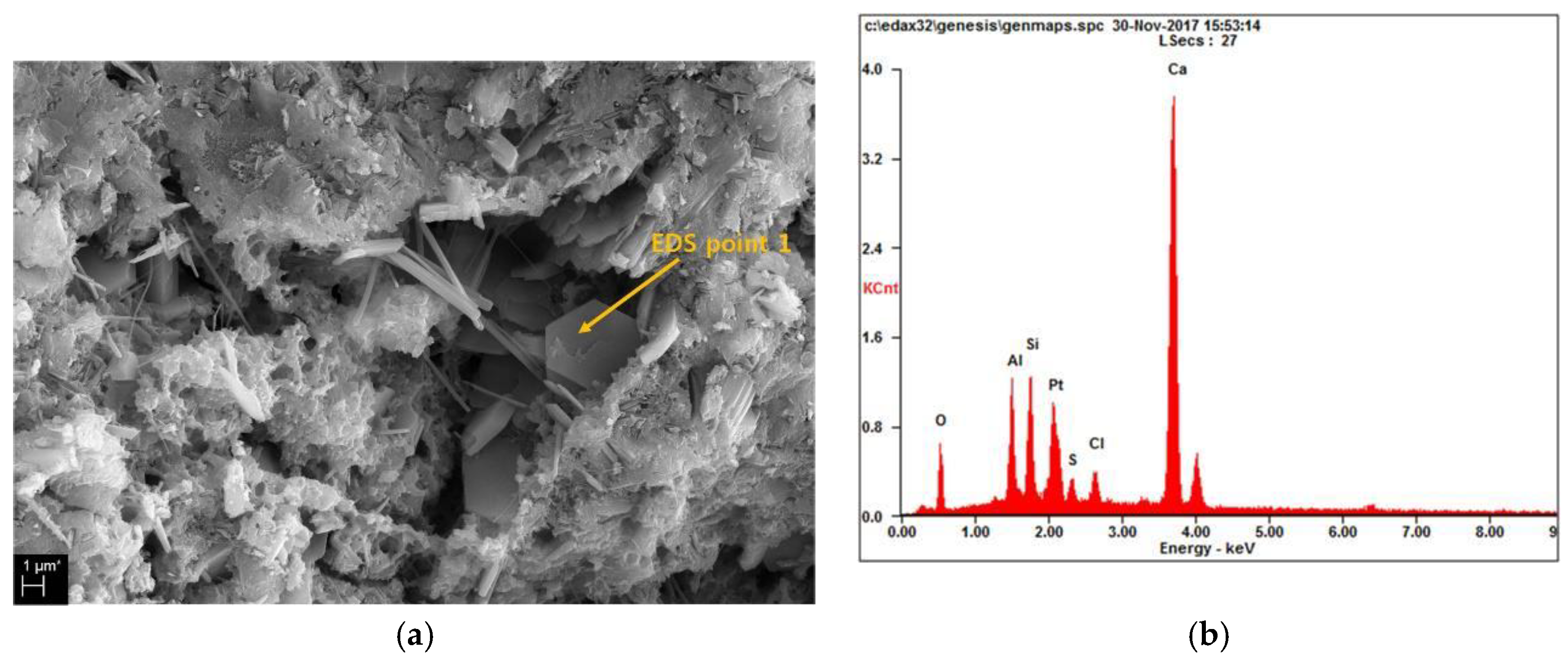
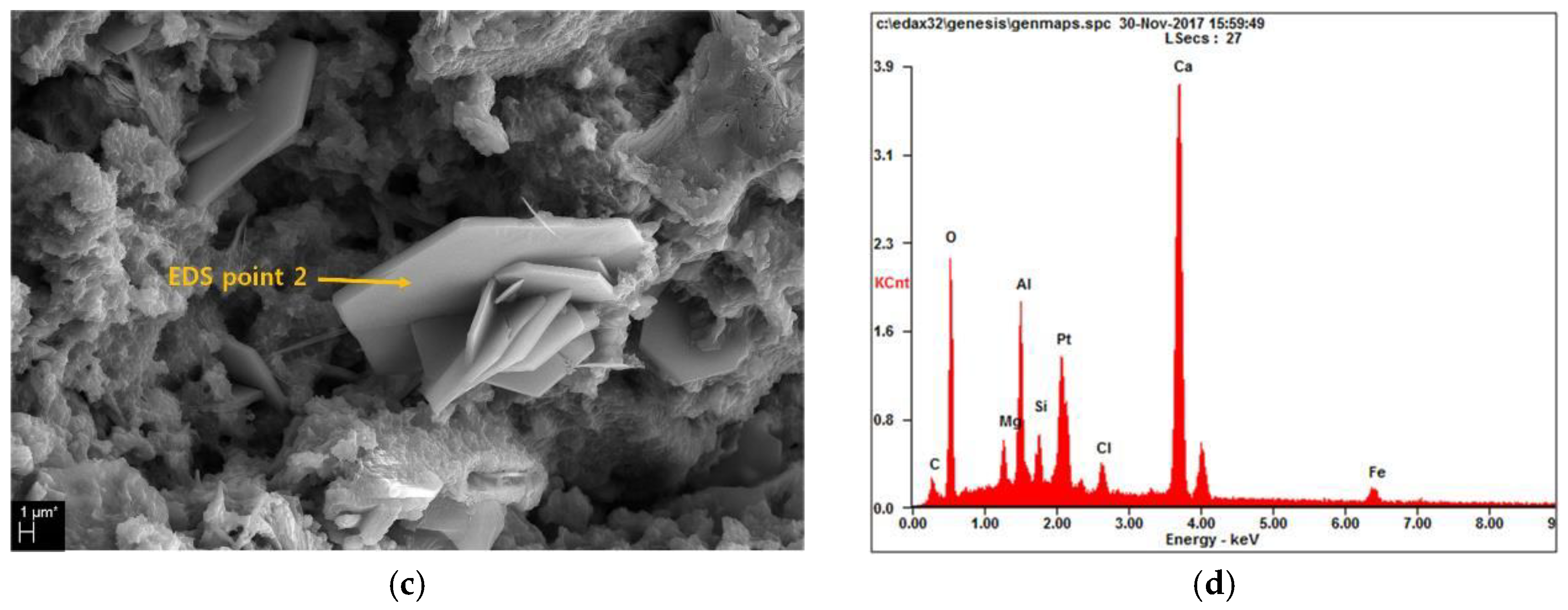
3.4. Microstructure
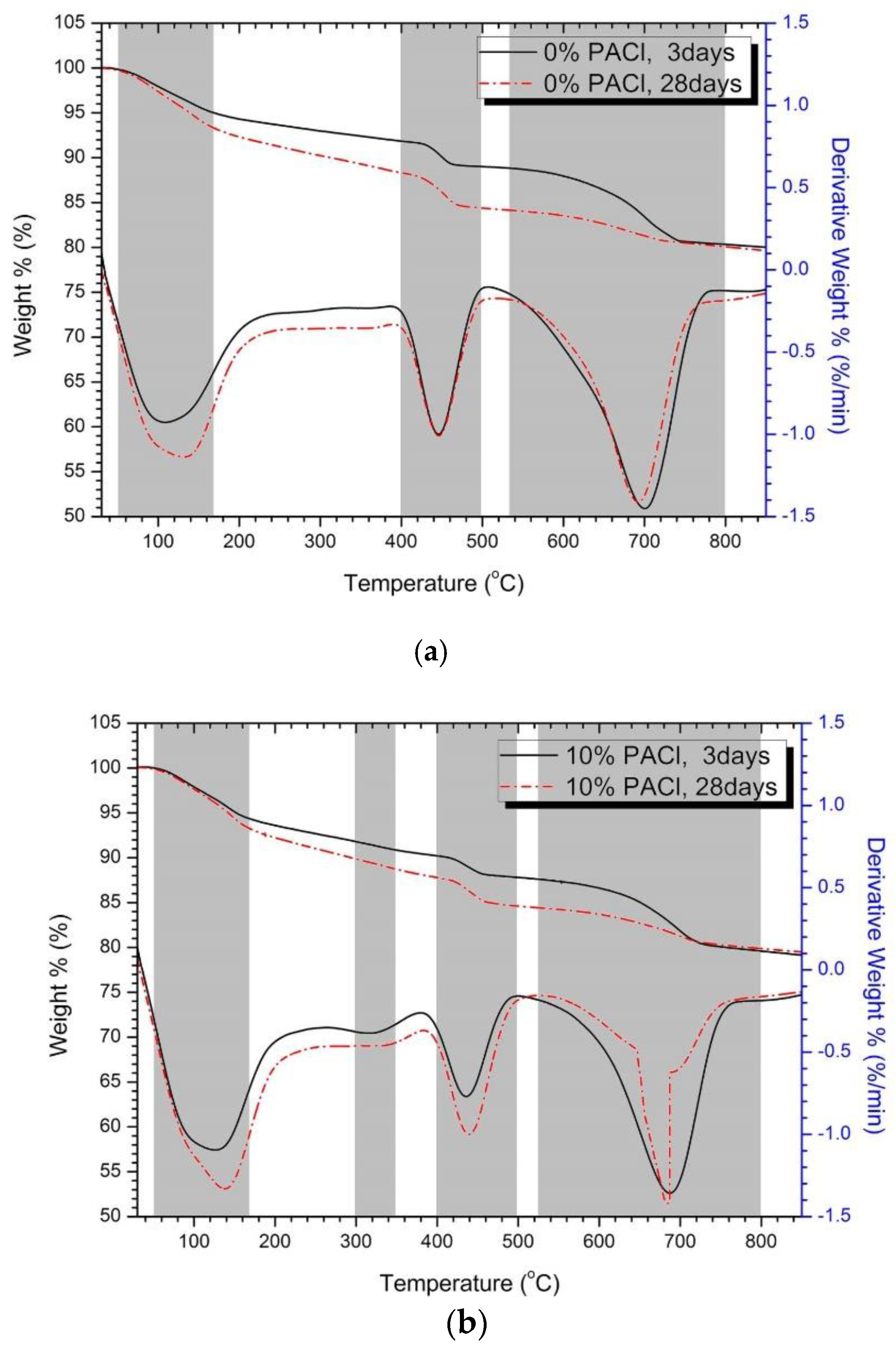
3.5. Thermal Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Z.; Wang, J.; Liu, D.; Li, J.; Wang, X.; Song, B.; Yue, B.; Zhao, K.; Song, Y. Hydrolysis of polyaluminum chloride prior to coagulation: Effects on coagulation behavior and implications for improving coagulation performance. J. Environ. Sci. 2017, 57, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Xiao, F.; Wang, D. Speciation, stability, and coagulation mechanisms of hydroxyl aluminum clusters formed by PACl and alum: A critical review. Adv. Colloid Interface Sci. 2015, 226, 78–85. [Google Scholar] [CrossRef] [PubMed]
- Wei, N.; Zhang, Z.; Liu, D.; Wu, Y.; Wang, J.; Wang, Q. Coagulation behavior of polyaluminum chloride: Effects of pH and coagulant dosage. Chin. J. Chem. Eng. 2015, 23, 1041–1046. [Google Scholar] [CrossRef]
- Bottero, J.Y.; Case, J.M.; Fiessinger, F.; Poivier, J.E. Studies of hydrolyzed aluminium chloride solutions. I. Nature of aluminium species and composition of aqueous solutions. J. Phys. Chem. 1980, 84, 2933–2939. [Google Scholar] [CrossRef]
- Zhao, H.Z.; Liu, C.; Xu, Y.; Ni, J.R. High-concentration polyaluminum chloride: Preparation and effects of the Al concentration on the distribution and transformation of Al species. Chem. Eng. J. 2009, 155, 528–533. [Google Scholar] [CrossRef]
- Abate, C.; Scheetz, B.E. Aqueous Phase Equilibria in the System CaO-Al2O3-CaCl2-H2O: The Significance and Stability of Friedel’s Salt. J. Am. Ceram. Soc. 1995, 78, 939–944. [Google Scholar] [CrossRef]
- Thomas, M.D.A.; Hooton, R.D.; Scott, A.; Zibara, H. The effect of supplementary cementitious materials on chloride binding in hardened cement paste. Cem. Concr. Res. 2012, 42, 1–7. [Google Scholar] [CrossRef]
- Mesbah, A.; Cau-dit-Coumes, C.; Frizon, F.; Leroux, F.; Ravaux, J.; Renaudin, G. A New Investigation of the Cl-CO32-Addition in AFm Phases. J. Am. Ceram. Soc. 2011, 94, 1901–1910. [Google Scholar] [CrossRef]
- Grishchenko, R.O.; Emelina, A.L.; Makarov, P.Y. Thermodynamic properties and thermal behavior of Friedel’s salt. Thermochim. Acta 2013, 570, 74–79. [Google Scholar] [CrossRef]
- Florea, M.V.A.; Brouwers, H.J.H. Chloride binding related to hydration products Part I: Ordinary Portland Cement. Cem. Concr. Res. 2012, 42, 282–290. [Google Scholar] [CrossRef]
- Suryavanshi, A.K.; Scantlebury, J.D.; Lyon, S.B. Mechanism of Friedel’s salt formation in cements rich in tri-calcium aluminate. Cem. Concr. Res. 1996, 26, 717–727. [Google Scholar] [CrossRef]
- Plusquellec, G.; Nonat, A. Interactions between calcium silicate hydrate (C–S–H) and calcium, chloride, bromide and nitrate. Cem. Concr. Res. 2016, 90, 89–96. [Google Scholar] [CrossRef]
- Zhou, Y.; Hou, D.; Jiang, J.; Wang, P. Chloride ions transport and adsorption in the nano-pores of silicate calcium hydrate: Experimental and molecular dynamics studies. Constr. Build. Mater. 2016, 126, 991–1001. [Google Scholar] [CrossRef]
- Zibara, H.; Hooton, R.D.; Thomas, M.D.A.; Stanish, K. Influence of the C/S and C/A ratios of hydration products on the chloride ion binding capacity of lime-SF and lime-MK mixtures. Cem. Concr. Res. 2008, 38, 422–426. [Google Scholar] [CrossRef]
- Gbozee, M.; Zheng, K.; He, F.; Zhng, X. The influence of aluminum from metakaolin on chemical binding of chloride ions in hydrated cement pastes. Appl. Clay Sci. 2018, 158, 186–194. [Google Scholar] [CrossRef]
- Chen, W.; Li, B.; Li, Q.; Tian, J. Effect of polyaluminum chloride on the properties and hydration of slag-cement paste. Constr. Build. Mater. 2016, 124, 1019–1027. [Google Scholar] [CrossRef]
- Kim, T. The effects of polyaluminum chloride on the mechanical and microstructural properties of alkali-activated slag cement paste. Cem. Concr. Compos. 2019, 96, 46–54. [Google Scholar] [CrossRef]
- ASTM 305. Standard Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortars of Plastic Consistency; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Glasser, F.P.; Kindness, A.; Stronach, S.A. Stability and solubility relationships in AFm phases Part I. Chloride, sulfate and hydroxide. Cem. Concr. Res. 1999, 29, 861–866. [Google Scholar] [CrossRef]
- Paul, G.; Boccaleri, E.; Buzzi, L.; Canonico, F.; Gastaldi, D. Friedel’s salt formation in sulfoaluminate cements: A combined XRD and 27Al MAS NMR study. Cem. Concr. Res. 2015, 67, 93–102. [Google Scholar] [CrossRef]
- Elakneswaran, Y.; Nawa, T.; Kurumisawa, K. Electrokinetic potential of hydrated cement in relation to adsorption of chlorides. Cem. Concr. Res. 2009, 39, 340–344. [Google Scholar] [CrossRef]
- Hirao, H.; Yamada, K.; Takahashi, H.; Zibara, H. Chloride binding of cement estimated by binding isotherms of hydrates. J. Adv. Concr. Technol. 2005, 3, 77–85. [Google Scholar] [CrossRef]
- Shi, Z.; Geiker, M.R.; Weerdt, K.D.; Østnor, T.A.; Lothenbach, B.; Winnefeld, F.; Skibsted, J. Role of calcium on chloride binding in hydrated Portland cement-metakaolin-limestone blends. Cem. Concr. Res. 2017, 95, 205–216. [Google Scholar] [CrossRef]
- L’Hôpital, E.; Lothenbach, B.; Kulik, D.A.; Scrivener, K. Influence of calcium to silica ratio on aluminium uptake in calcium silicate hydrate. Cem. Concr. Res. 2016, 85, 111–121. [Google Scholar] [CrossRef]
- L’Hôpital, E.; Lothenbach, B.; Le Saout, G.; Kulik, D.; Scrivener, K. Incorporation of aluminium in calcium-silicate-hydrates. Cem. Concr. Res. 2015, 75, 91–103. [Google Scholar] [CrossRef]
- Lothenbach, B.; Nonat, A. Calcium silicate hydrates: Solid and liquid phase composition. Cem. Concr. Res. 2015, 78, 57–70. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; Separovic, F.; van Deventer, J.S.J. Effect of Alkali Cations on Aluminum Incorporation in Geopolymeric Gels. Ind. Eng. Chem. Res. 2005, 44, 832–839. [Google Scholar] [CrossRef]
- Talero, R.; Trusilewicz, L.; Delgado, A.; Pedrajas, C.; Lannegrand, R.; Rahhal, V.; Mejía, R.; Delvasto, S.; Ramírez, F.A. Comparative and semi-quantitative XRD analysis of Friedel’s salt originating from pozzolan and Portland cement. Constr. Build. Mater. 2011, 25, 2370–2380. [Google Scholar] [CrossRef]
- Talero, R. Synergic effect of Friedel’s salt from pozzolan and from OPC co-precipitating in a chloride solution. Constr. Build. Mater. 2012, 33, 164–180. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, Y.; Mu, S.; Chang, H.; Sun, W.; Jiang, J. Improving the chloride binding capacity of cement paste by adding nano-Al2O3. Constr. Build. Mater. 2019, 195, 415–422. [Google Scholar] [CrossRef]
- Mindess, S.; Young, J.F.; Darwin, D. Concrete, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2003. [Google Scholar]
- Barbhuiya, S.; Mukherjee, S.; Nikraz, H. Effects of nano-Al2O3 on early-age microstructural properties of cement paste. Constr. Build. Mater. 2014, 52, 189–193. [Google Scholar] [CrossRef]
- Li, Z.; Wang, H.; He, S.; Lu, Y.; Wang, M. Investigations on the preparation and mechanical properties of the nano-alumina reinforced cement composite. Mater. Lett. 2006, 60, 356–359. [Google Scholar] [CrossRef]
- Lodeiro, G.; Fernández-Jimenez, A.; Palomo, A.; Macphee, D.E. Effect on fresh C-S-H gels of the simultaneous addition of alkali and aluminium. Cem. Concr. Res. 2010, 40, 27–32. [Google Scholar] [CrossRef]
- Goni, S.; Frias, M.; Vigil de la Villa, R.; Garcia, R. Sodium chloride effect on durability of ternary blended cement. Microstructural characterization and strength. Compos. Part B 2013, 54, 163–168. [Google Scholar] [CrossRef]
- Lin, K.L.; Wang, K.S.; Tzeng, B.Y.; Wang, N.F.; Lin, C.Y. Effects of Al2O3 on the hydration activity of municipal solid waste incinerator fly ash slag. Cem. Concr. Res. 2004, 34, 587–592. [Google Scholar] [CrossRef]
- L’ Hôpital, E.; Lothenbach, B.; Scrivener, K.; Kulik, D.A. Alkali uptake in calclium alumina silicate hydrate (C-A-S-H). Cem. Concr. Res. 2016, 85, 122–136. [Google Scholar] [CrossRef]
- Richardson, I.G. The nature of C-S-H in hardened cements. Cem. Concr. Res. 1999, 29, 1131–1147. [Google Scholar] [CrossRef]
- Girão, A.V.; Richardson, I.G.; Taylor, R.; Brydson, R.M.D. Composition, morphology and nanostructure of C–S–H in 70% white Portland cement–30% fly ash blends hydrated at 55 °C. Cem. Concr. Res. 2010, 40, 1350–1359. [Google Scholar] [CrossRef]
- Love, C.A.; Richardson, I.G.; Brough, A.R. Composition and structure of C–S–H in white Portland cement–20% metakaolin pastes hydrated at 25 °C. Cem. Concr. Res. 2007, 37, 109–117. [Google Scholar] [CrossRef]
- Taylor, R.; Richardson, I.G.; Brydson, R.M.D. Composition and microstructure of 20-year-old ordinary Portland cement–ground granulated blast-furnace slag blends containing 0 to 100% slag. Cem. Concr. Res. 2010, 40, 971–983. [Google Scholar] [CrossRef]
- Richardson, I.G. The calcium silicate hydrates. Cem. Concr. Res. 2008, 38, 137–158. [Google Scholar] [CrossRef]
- Richardson, I.G.; Groves, G.W. The incorporation of minor and trace elements into calcium silicate hydrate (C-S-H) gel in hydrened cememt pastes. Cem. Concr. Res. 1993, 23, 131–138. [Google Scholar] [CrossRef]
- Sun, C.K.; Young, J.F.; Kirkpatrick, R.J. The role of Al in C-S-H: NMR, XRD, and compositional results for precipitated samples. Cem. Concr. Res. 2006, 36, 18–29. [Google Scholar] [CrossRef]
- Faucon, P.; Delagrave, A.; Petit, J.C.; Richet, C.; Marchand, J.M.; Zanni, H. Aluminum Incorporation in Calcium Silicate Hydrates (C-S-H) Depending on Their Ca/Si Ratio. J. Phys. Chem. B 1999, 103, 7796–7802. [Google Scholar] [CrossRef]
- Renaudin, G.; Russias, J.; Leroux, F.; Cau-dit-Coumes, C.; Frizon, F. Structural characterization of C–S–H and C–A–S–H samples—Part II: Local environment investigated by spectroscopic analyses. J. Solid State Chem. 2009, 182, 3320–3329. [Google Scholar] [CrossRef]
- Richardson, I.G.; Skibsted, J.; Black, L.; Kirkpatrick, R.J. Characterisation of cement hydrate phases by TEM, NMR and Raman spectroscopy. Adv. Cem. Res. 2010, 22, 233–248. [Google Scholar] [CrossRef]
- Pardal, X.; Pochard, I.; Nonat, A. Experimental study of Si–Al addition in calcium-silicate-hydrate (C-S-H) prepared under equilibrium conditions. Cem. Concr. Res. 2009, 39, 637–643. [Google Scholar] [CrossRef]
- Haas, J.; Nonat, A. From C–S–H to C–A–S–H: Experimental study and thermodynamic modelling. Cem. Concr. Res. 2015, 68, 124–138. [Google Scholar] [CrossRef]
- Pardal, X.; Brunet, F.; Charpentie, R.T.; Pochard, I.; Nonat, A. 27Al and 29Si solid-state NMR characterization of calcium–aluminosilicate–hydrate. Inorg. Chem. 2012, 51, 1827–1836. [Google Scholar] [CrossRef]
- Soyer-Uzun, S.; Chae, S.R.; Benmore, C.J.; Wenk, H.R.; Monteiro, P.J.M. Compositional Evolution of Calcium Silicate Hydrate (C–S–H) Structures by Total X-Ray Scattering. J. Am. Ceram. Soc. 2012, 95, 793–798. [Google Scholar] [CrossRef]
- Birnin-Yauri, U.A.; Glasser, F.P. Friedel’s salt, Ca2Al(OH)6(Cl,OH)·2H2O: Its solid solutions and their role in chloride binding. Cem. Concr. Res. 1998, 28, 1713–1723. [Google Scholar] [CrossRef]
- Zhu, Q.; Jiang, L.; Chen, Y.; Xu, J.; Mo, L. Effect of chloride salt type on chloride binding behavior of concrete. Constr. Build. Mater. 2012, 37, 512–517. [Google Scholar] [CrossRef]
- Trauchessec, R.; Mechling, J.M.; Lecomte, A.; Roux, A.; Le Rolland, B. Hydration of ordinary Portland cement and calcium sulfoaluminate cement blends. Cem. Concr. Compos. 2015, 56, 106–114. [Google Scholar] [CrossRef]
- Wang, S.D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements—Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- Bizzozero, J.; Gosselin, C.; Scrivener, K.L. Expansion mechanisms in calcium aluminate and sulfoaluminate systems with calcium sulfate. Cem. Concr. Res. 2014, 56, 190–202. [Google Scholar] [CrossRef]
- Garbev, K.; Bornefeld, M.; Beuchle, G.; Stemmermann, P. Cell Dimensions and Composition of Nanocrystalline Calcium Silicate Hydrate Solid Solutions. Part 2 Synchrotron-Based X-Ray Diffraction. J. Am. Ceram. Soc. 2008, 91, 3015–3023. [Google Scholar] [CrossRef]
| Chemical Components (%) | Density (g/mm3) | Fineness (m2/kg) | LOI (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OPC | SiO2 | Al2O | Fe2O | MgO | CaO | K2O | SO3 | |||
| 21.75 | 4.83 | 3.52 | 4.04 | 62.16 | 0.67 | 2.83 | 0.00315 | 330 | 0.74 | |
| Chemical Components (%) | Density (g/mm3) | pH | Basicity (%) | |||
|---|---|---|---|---|---|---|
| Al2O | Fe2O | SO3 | Cl- | |||
| 17.27 | 0.01 | 0.30 | 21.33 | 0.00137 | 4.01 | 40 |
| Level | 0% PACl | 6% PACl | 10% PACl |
|---|---|---|---|
| Micro pores (>10 µm; %) | 1.1 | 2.7 | 4.0 |
| Large-capillary pores (10–0.05 µm; %) | 81.7 | 35.6 | 28.2 |
| Medium-capillary pores (0.05–0.01 µm; %) | 16.1 | 52.3 | 52.6 |
| Gel pores (<0.01 µm; %) | 1.1 | 9.4 | 15.2 |
| Total porosity (%) | 41.9 | 30.9 | 23.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.; Kang, C.; Hong, S.; Seo, K.-Y. Investigating the Effects of Polyaluminum Chloride on the Properties of Ordinary Portland Cement. Materials 2019, 12, 3290. https://doi.org/10.3390/ma12203290
Kim T, Kang C, Hong S, Seo K-Y. Investigating the Effects of Polyaluminum Chloride on the Properties of Ordinary Portland Cement. Materials. 2019; 12(20):3290. https://doi.org/10.3390/ma12203290
Chicago/Turabian StyleKim, Taewan, Choonghyun Kang, Sungnam Hong, and Ki-Young Seo. 2019. "Investigating the Effects of Polyaluminum Chloride on the Properties of Ordinary Portland Cement" Materials 12, no. 20: 3290. https://doi.org/10.3390/ma12203290
APA StyleKim, T., Kang, C., Hong, S., & Seo, K.-Y. (2019). Investigating the Effects of Polyaluminum Chloride on the Properties of Ordinary Portland Cement. Materials, 12(20), 3290. https://doi.org/10.3390/ma12203290





