Solid-State Transformations in Inner Coordination Sphere of [Co(NH3)6][Fe(C2O4)3]∙3H2O as a Route to Access Catalytically Active Co-Fe Materials
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
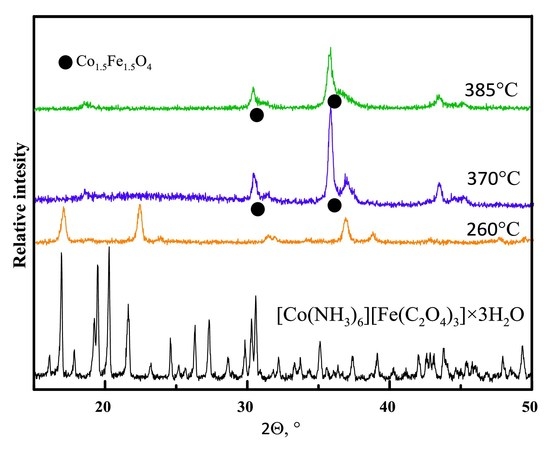
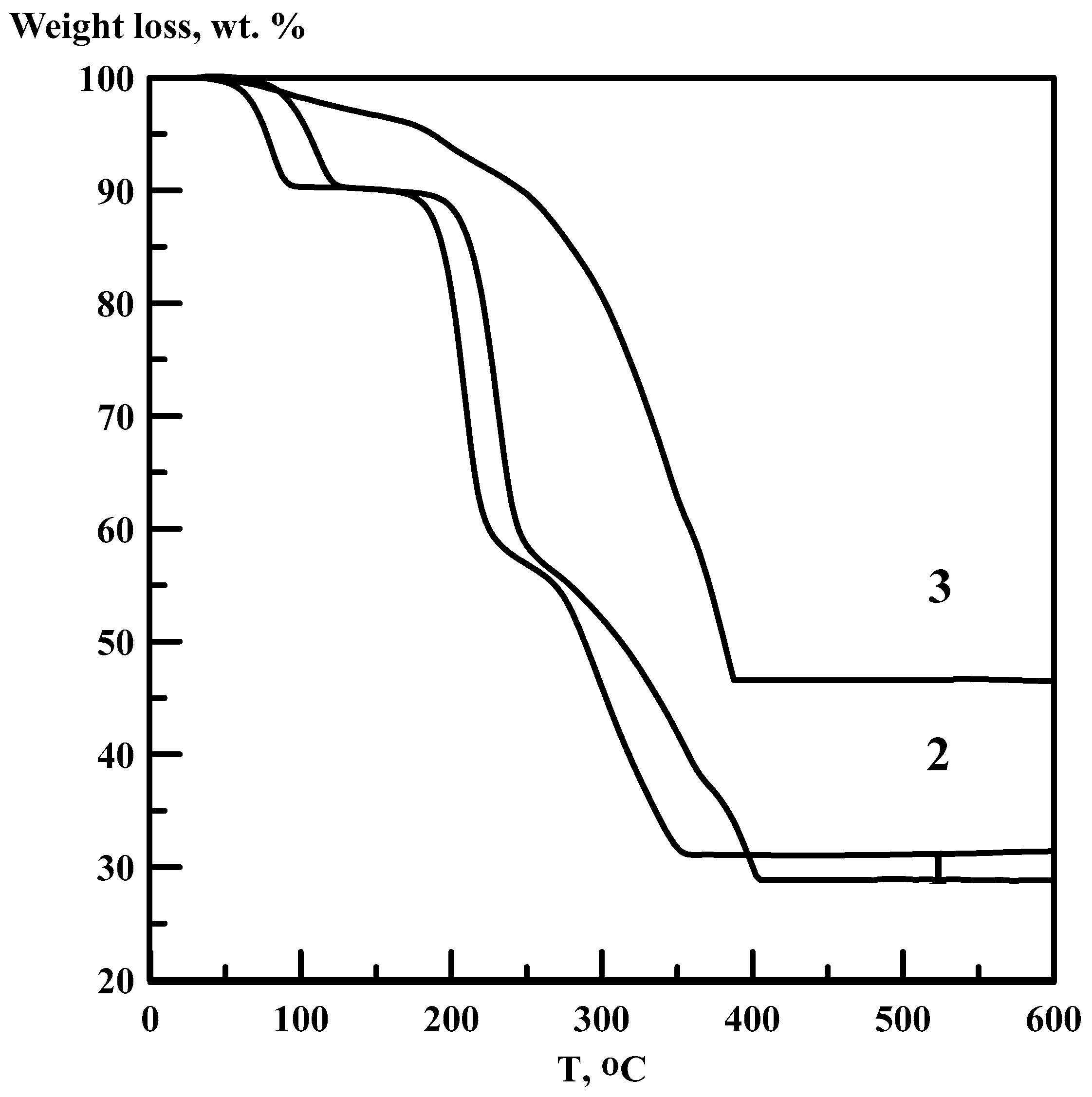
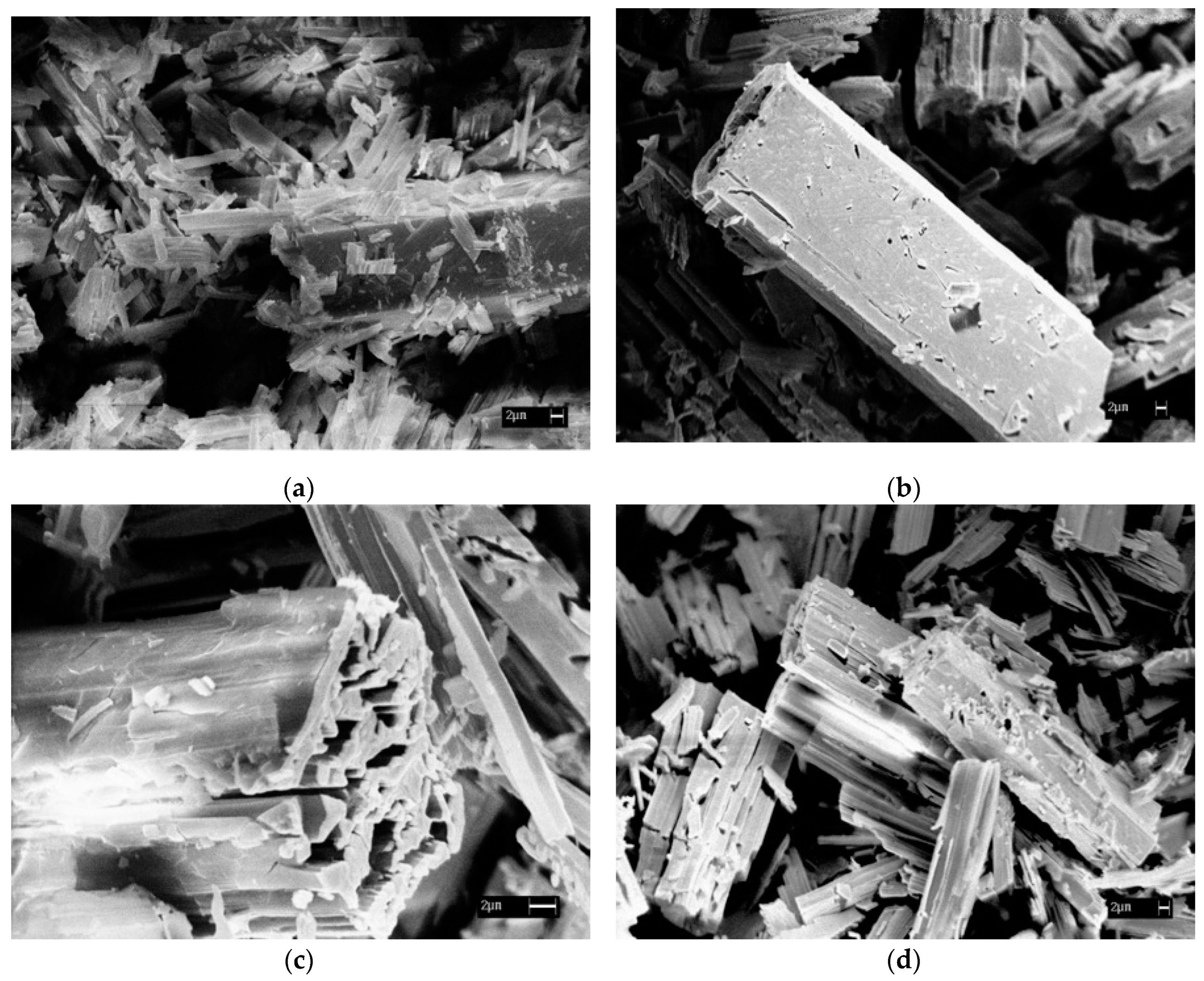
3.1. Thermal Decomposition Products
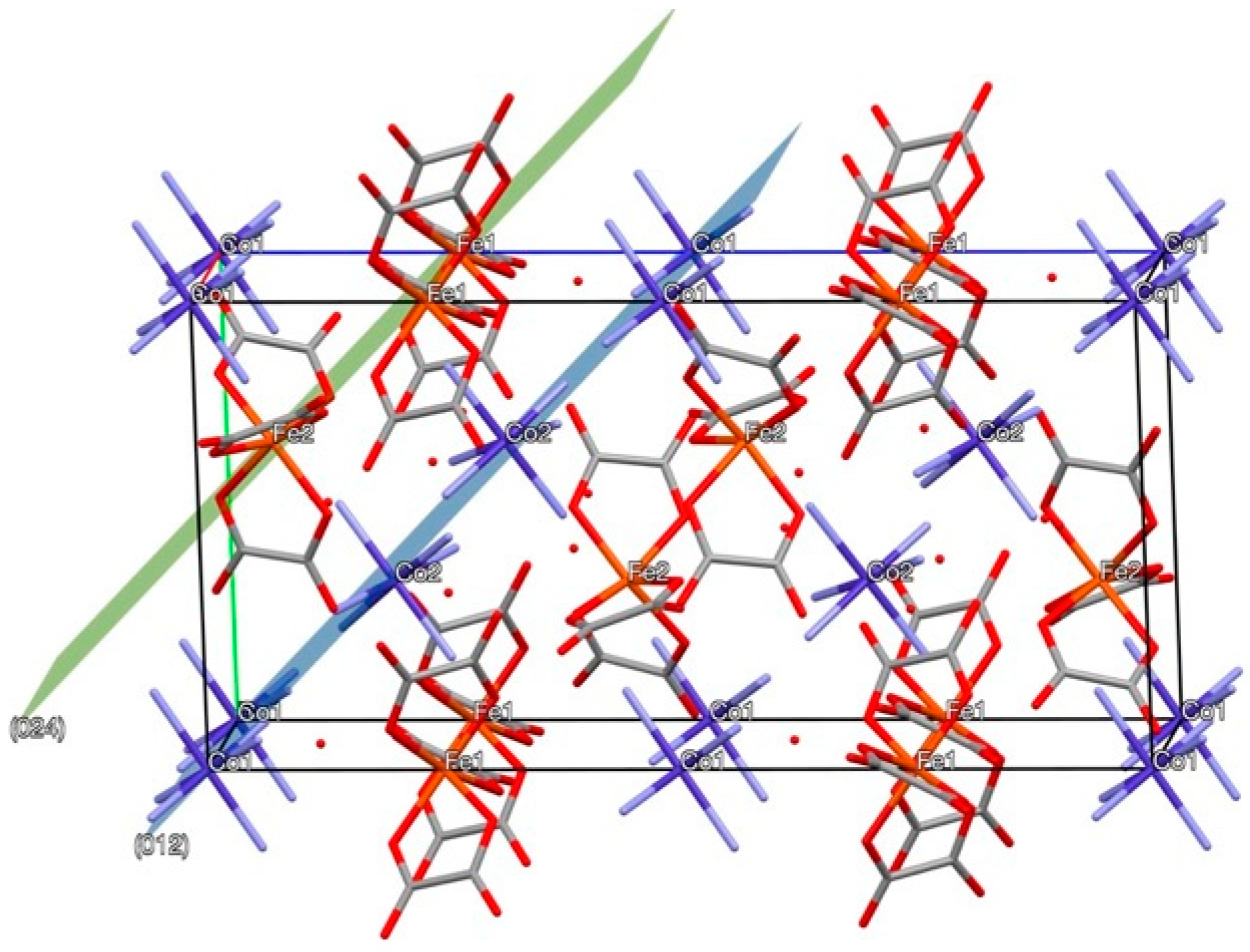
3.2. Insight into the [Co(NH3)6][Fe(C2O4)3]·3H2O Thermal Decomposition
3.3. Catalytic Tests
4. Conclusions
- Thermal decomposition of [Co(NH3)6][Fe(C2O4)3]·3H2O in argon flow corresponds to four clearly distinguishable stages: (a) Dehydration of the initial complex; (b) anhydrous [Co(NH3)6][Fe(C2O4)3] is stable from 100 to 190 °C; (c) further heating results in a formation of CoFe(NH3)2(C2O4)2 intermediate stable in the temperature interval of 230–270 °C; (d) intermediate phase completely decomposes upon heating below 350 °C.
- The initial complex, when heated to 370–420 °C, forms a pyrophoric CoFe alloy, which easily forms Co1.5Fe1.5O4 upon air expose and contains <1 wt % of carbon.
- Under the same conditions, the intermediate forms a mixture of 80 wt % metals and metal oxides.
- The final products of thermolysis are catalytically inactive in the decomposition reaction of H2O2, which we explain by the small specific surfaces and the absence of residual carbon.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Borshch, V.N.; Artjuk, V.A.; Zhuk, S.N. Intermetallics synthesized from a mechanized mixture of metals as precursors of oxidation and reduction catalysts. In Proceedings of the International Conference Dedicated to the 50th Anniversary of Self-Propagating High Temperature Synthesis (SHS-50), Chernogolovka, Russia, 20–21 November 2017; pp. 79–81, ISBN 978-5-91845-080-2. [Google Scholar]
- Borshch, V.N.; Pugacheva, E.V.; Zhuk, S.N.; Andreev, D.E.; Sanin, V.N.; Yukhvid, V.I. SHS-intermetallic compounds as promising materials for the synthesis of highly effective polymetallic catalysts. In Proceedings of the International Conference Dedicated to the 50th Anniversary of Self-Propagating High Temperature Synthesis (SHS-50), Chernogolovka, Russia, 20–21 November 2017; pp. 82–85, ISBN 978-5-91845-080-2. [Google Scholar]
- Korenev, S.V.; Venediktov, A.B.; Shubin, Y.V.; Gromilov, S.A.; Yusenko, K.V. Synthesis and structure of binary complexes of platinum group metals—Precursors of metallic materials. J. Struct. Chem. 2003, 44, 46–59. [Google Scholar] [CrossRef]
- Domonov, D.P.; Pechenyuk, S.I.; Gosteva, A.N. Products of binary complex compounds thermolysis: Catalysts for hydrogen peroxide decomposition. Russ. J. Phys. Chem. 2014, 88, 913–918. [Google Scholar] [CrossRef]
- Pechenyuk, S.I.; Semushina, Y.P.; Kuz’mich, L.P.; Ivanov, Y.V. Acid−base and catalytic properties of the products of oxidative thermolysis of double complex compounds. Russ. J. Phys. Chem. 2016, 90, 31–36. [Google Scholar] [CrossRef]
- Semushina, Y.P.; Pechenyuk, S.I.; Kuzmich, L.F.; Knyazeva, A.I. Relationship between the catalytic properties of the products of the oxidative thermolysis of certain complexes and the porous structures of samples in the oxidation reactions of volatile organic compounds. Russ. J. Phys. Chem. 2017, 91, 26–29. [Google Scholar] [CrossRef]
- Domonov, D.P.; Pechenyuk, S.I. Thermal decomposition of ammonium perchlorate in the presence of bimetallic additives. Rus. Chem. Bull. 2018, 67, 1041–1044. [Google Scholar] [CrossRef]
- Matyukha, V.A.; Zhiganov, A.N. Oxalates of Transition Metals; IzdAT: Moscow, Russia, 2012; p. 592. ISBN 978-5-86656-264-0. (In Russian) [Google Scholar]
- Filatov, E.Y.; Yusenko, K.V.; Vikulova, E.S.; Plyusnin, P.E.; Shubin, Y.V. XRD investigation and thermal properties of [Ir(NH3)6][Co(C2O4)3]·H2O and [Co(NH3)6][Ir(C2O4)3]—Precursors for Co0.50Ir0.5. Z. Kristallogr. Suppl. 2009, 30, 263–268. [Google Scholar] [CrossRef]
- Pechenyuk, S.I.; Domonov, D.P.; Gosteva, A.N. Thermal decomposition of cationic, anionic and double complex compounds of 3d metals. Russ. Chem. J. 2018, 62, 116–140. (In Russian) [Google Scholar]
- Pechenyuk, S.I.; Semushina, Y.P.; Mikhailova, N.L.; Ivanov, Y.V. Binary complexes [Co(A)6][M(C2O4)3] (A = NH3, 1/2C2H8N2, M = Fe, Cr): Synthesis, properties, and thermal decomposition. Russ. J. Coord. Chem. 2015, 41, 175–181. [Google Scholar] [CrossRef]
- Semushina, Y.P.; Plyusnin, P.E.; Shubin, Y.V.; Pechenyuk, S.I.; Ivanov, Y.V. Thermal decomposition of [Co(a)6][M(Ox)3]·3H2O in an inert and reducing atmosphere. Russ. Chem. Bull. 2015, 64, 1963–1966. [Google Scholar] [CrossRef]
- Handbuch der Präparativen Anorganischen Chemie (in drei Banden); Brauer, G.F. (Ed.) Enke: Stuttgart, Germany, 1978. [Google Scholar]
- Pechenyuk, S.I.; Domonov, D.P.; Shimkin, A.A.; Semushina, Y.P.; Ivanov, Y.V. Thermal behaviour of binary complex compounds containing hexacyanoferrate-anion. Russ. J. Gen. Chem. 2017, 87, 2212–2223. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; John Wiley & Sons: New York, NY, USA, 1986; 245p. [Google Scholar]
- Venkataraman, A.; Sastry, N.V.; Ray, A. Studies on the mechanism of thermal dehydration of cobalt oxalate dihydrate. J. Phys. Chem. Solids 1992, 53, 681–685. [Google Scholar] [CrossRef]
- Mazzi, F.; Garavelli, C. The structure of oxalate FeC2O4∙2(H2O). Periodico di Mineralogia. 1957, 26, 269–303. [Google Scholar]
- Zheng, X.; Li, P.; Zheng, S.; Zhang, Y. Thermal decomposition of ammonium perchlorate in the presence of Cu(OH)2·2Cr(OH)3 nanoparticles. Powder Technol. 2014, 268, 446–451. [Google Scholar] [CrossRef]
- Sharma, J.K.; Srivastava, P.; Singh, G.; Akhtar, M.S.; Ameen, S. Catalytic thermal decomposition of ammonium perchlorate and combustion of composite solid propellants over green synthesized CuO nanoparticles. Thermochim. Acta 2015, 614, 110. [Google Scholar] [CrossRef]
- Kapoor, I.P.S.; Srivastava, P.; Singh, G. Nanocrystalline Transition Metal Oxides as Cataysts in the Thermal Decomposition of Ammonium Perchlorate. Propellants Explos. Pyrotech. 2009, 34, 351–356. [Google Scholar] [CrossRef]
- Xiao, X.; Peng, B.; Cai, L.; Liu, S.; Wang, Y. The high efficient catalytic properties for thermal decomposition of ammonium perchlorate using mesoporous ZnCo2O4 rods synthesized by oxalate co-precipitation method. Sci. Rep. 2018, 8, 7571. [Google Scholar] [CrossRef]

| Temperature | Residue, wt % | Residual Carbon, wt % | Total Carbon, wt % | SBET m2/g | V∑, cm3/g | Comment |
|---|---|---|---|---|---|---|
| RT | 100 | 13.46 | 100 | - | - | Precursor |
| 300 | 52.4 | 13.14 | 50 | 29 | 0.05 | tubular crystals |
| 350 | 41.6 | 10.3 | 33 | 62 | 0.16 | |
| 385 | 26.1 | 0.18 | <1 | 45 | ||
| 400 | 24.8 | 0.15 | <1 | 28 | 0.15 | Air stable |
| 400 | 29.2 | 0.12 | <1 | 17 | 0.08 | Pyrophoric |
| 450 | 25.05 | 0.095 | <1 | 14.5 | 0.08 | Air stable |
| Sample | T, °C | Yield, wt % | M.M. Cp. | Content of Elements, at.% | The Released Gas Products, mol/mol DCC | SBET m2/g | Composition and Properties | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Co | Fe | C | NH3 | CO2 | ||||||
| I-1 | 250 | 60.0 | 321 | 18.4 | 17.0 | 14.9 | 3.6 | 0.78 | 16.8 | Light brown, X-ray amorphous Co:Fe:C = 1.03:1:4.08 |
| I-2 | 260 | 56.9 | 304 | 19.7 | 19.1 | 14.5 | 4.1 | 0.74 | 36.5 | Light brown Co:Fe:C = 0.98:1:3.53 |
| II-1 | 420 | 37.5 | 120.4 | 48.1 | 45.6 | 0.06 | 1.1 | 1.4 | 11.2 | Black, loose Co:Fe:O = 1:1:0.5 |
| II-2 | 385 | 42.5 | 129.2 | 46.6 | 46.0 | 0.14 | 2.3 | 2.0 | 17.8 | Black Co:Fe:O = 1:1.04:0.53 |
| III | 370 | 30.7 * | 164.2 | 37.4 | 35.2 | 0.06 | 5.4 | 3.36 | 12.1 | Black, pyrophoric, according to X-ray analysis Co1.5Fe1.5O4 Co:Fe:O = 1:1:2.7 |
| IV | 385 | 29.5* | 157.7 | 39.6 | 38.5 | 0.07 | 5.2 | 2.8 | 16.1 | Black, pyrophoric Co:Fe:O = 1:1.03:1.55 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domonov, D.P.; Pechenyuk, S.I.; Semushina, Y.P.; Yusenko, K.V. Solid-State Transformations in Inner Coordination Sphere of [Co(NH3)6][Fe(C2O4)3]∙3H2O as a Route to Access Catalytically Active Co-Fe Materials. Materials 2019, 12, 221. https://doi.org/10.3390/ma12020221
Domonov DP, Pechenyuk SI, Semushina YP, Yusenko KV. Solid-State Transformations in Inner Coordination Sphere of [Co(NH3)6][Fe(C2O4)3]∙3H2O as a Route to Access Catalytically Active Co-Fe Materials. Materials. 2019; 12(2):221. https://doi.org/10.3390/ma12020221
Chicago/Turabian StyleDomonov, Denis P., Sophia I. Pechenyuk, Yulia P. Semushina, and Kirill V. Yusenko. 2019. "Solid-State Transformations in Inner Coordination Sphere of [Co(NH3)6][Fe(C2O4)3]∙3H2O as a Route to Access Catalytically Active Co-Fe Materials" Materials 12, no. 2: 221. https://doi.org/10.3390/ma12020221
APA StyleDomonov, D. P., Pechenyuk, S. I., Semushina, Y. P., & Yusenko, K. V. (2019). Solid-State Transformations in Inner Coordination Sphere of [Co(NH3)6][Fe(C2O4)3]∙3H2O as a Route to Access Catalytically Active Co-Fe Materials. Materials, 12(2), 221. https://doi.org/10.3390/ma12020221