Microstructure and Properties of Surface-Modified Plates and Their Welded Joints
Abstract
1. Introduction
2. Experimental Procedures and Materials
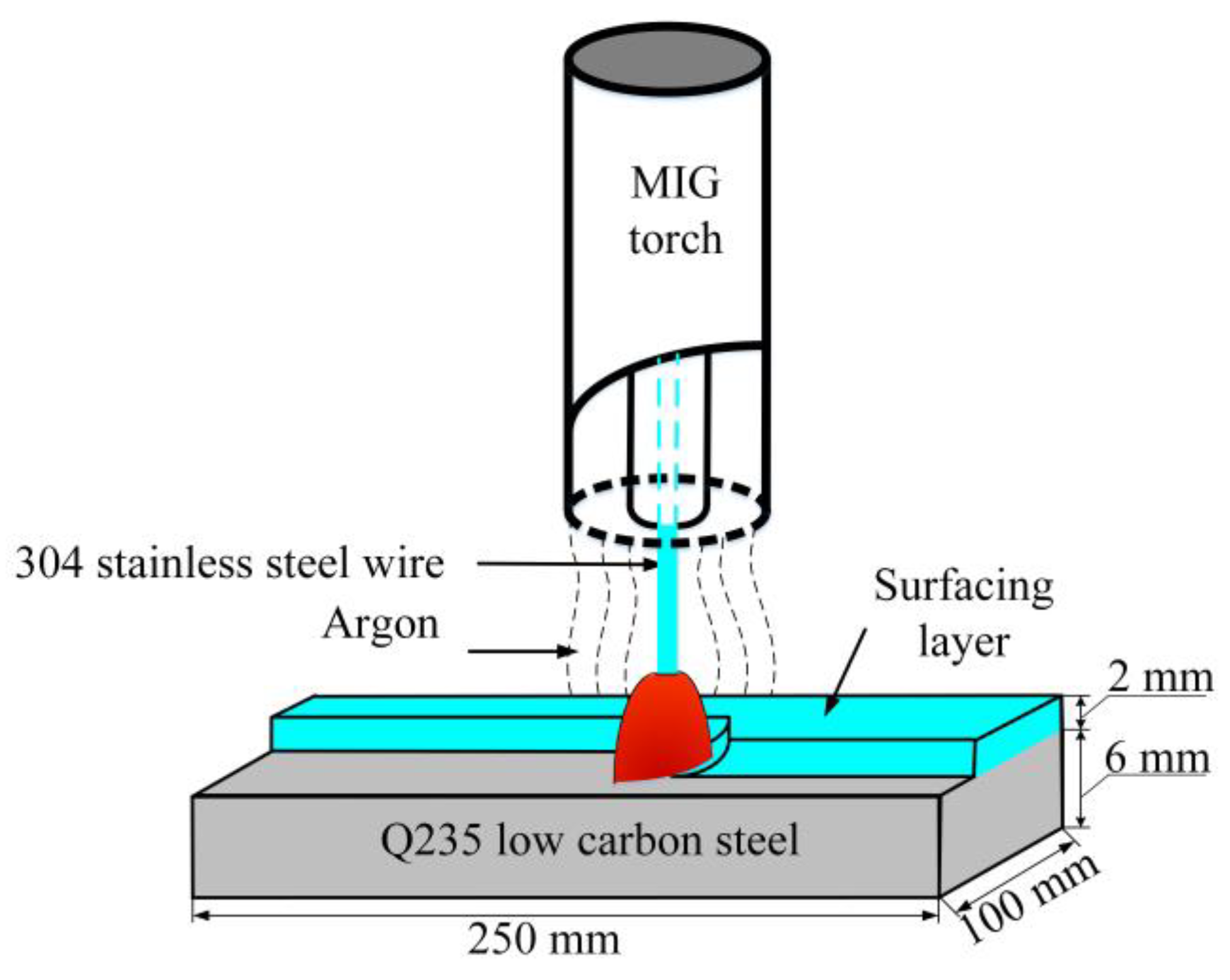
2.1. Fabrication of Surfacing Layer on Low Carbon Steel by MIG Welding
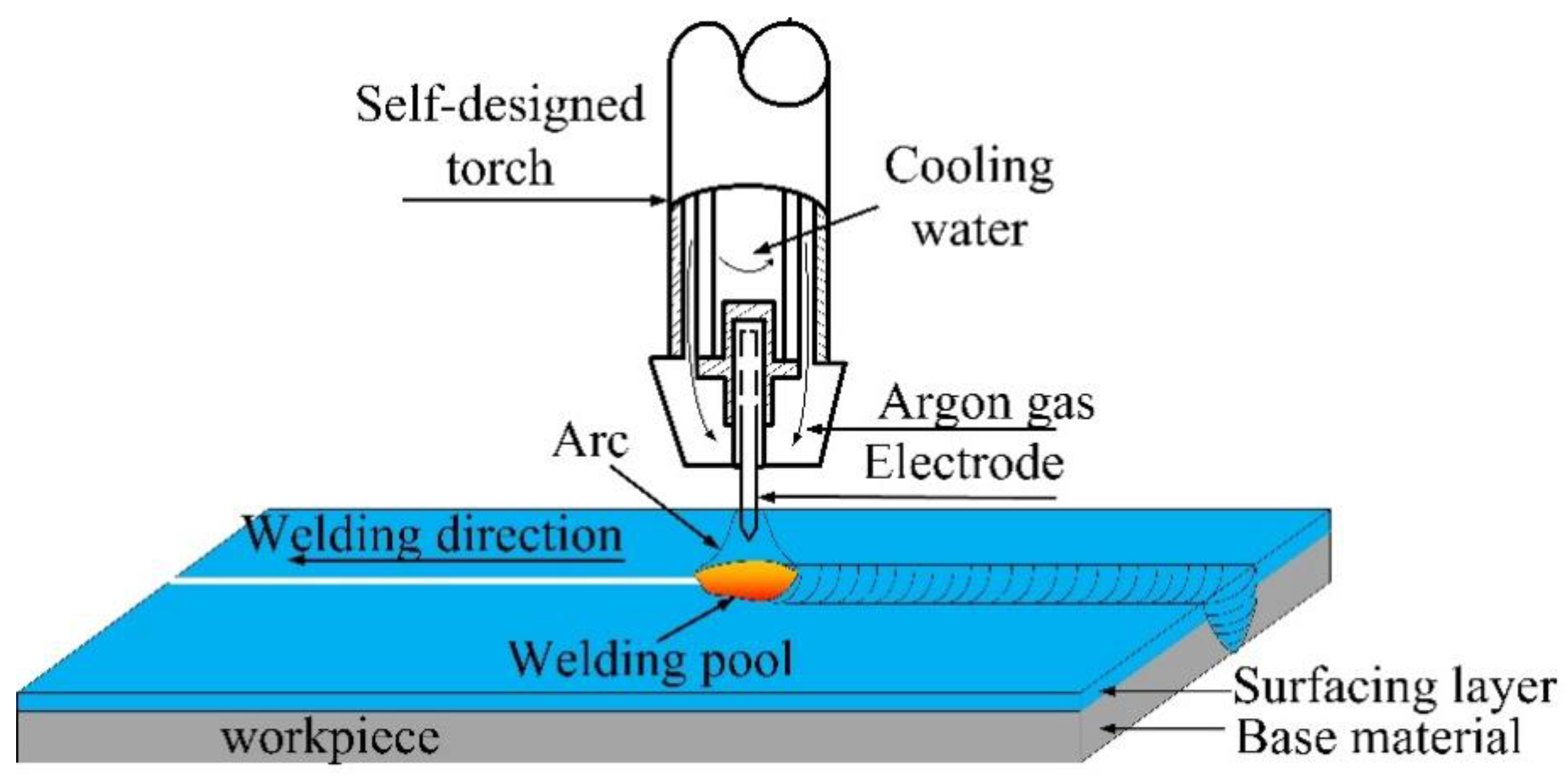
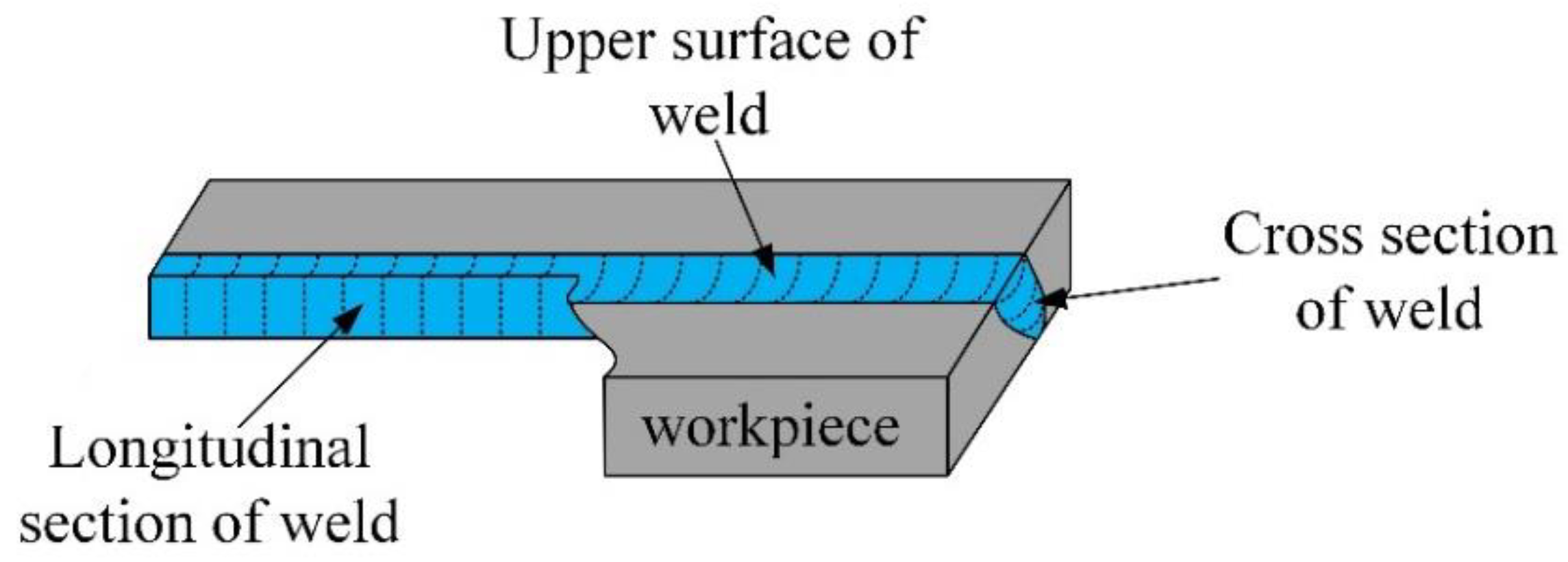
2.2. K-TIG Weld of the Surface-Modified Plates
2.3. Microstructure Characterization
2.4. Alloying Elements Content and Distribution
2.5. Mechanical Properties
2.6. Corrosion Experiment
3. Results and Discussion
3.1. Microstructures and Properties of the Surface-Modified Plates
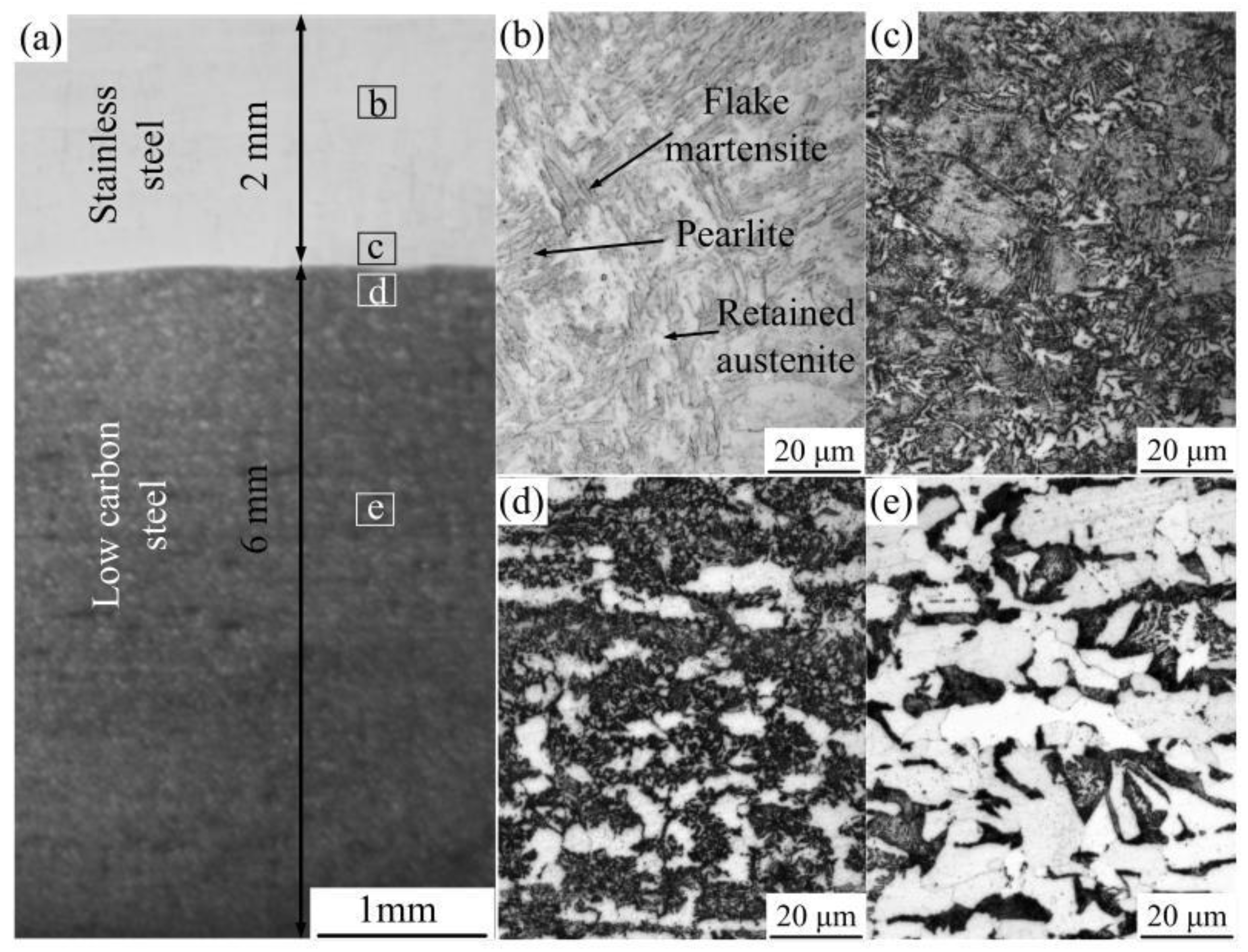
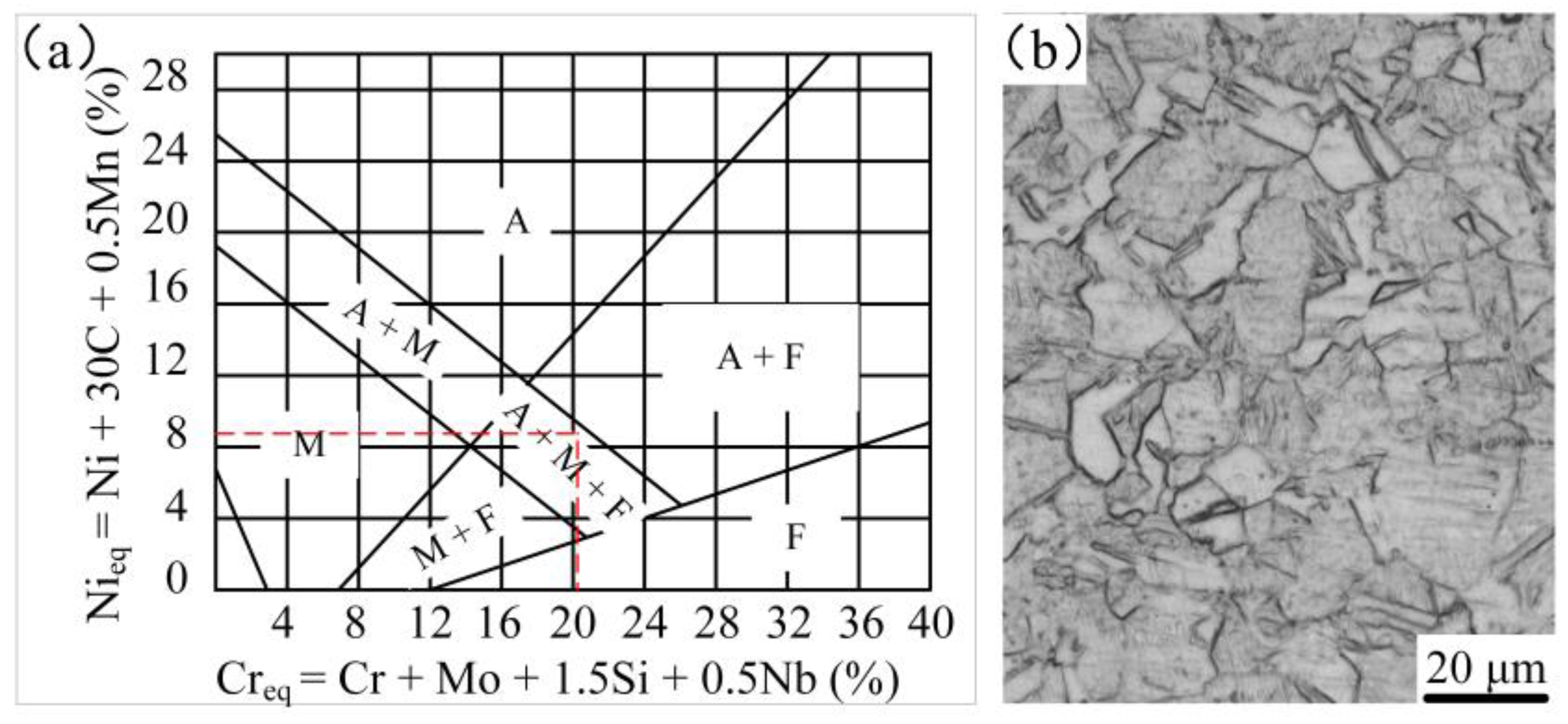
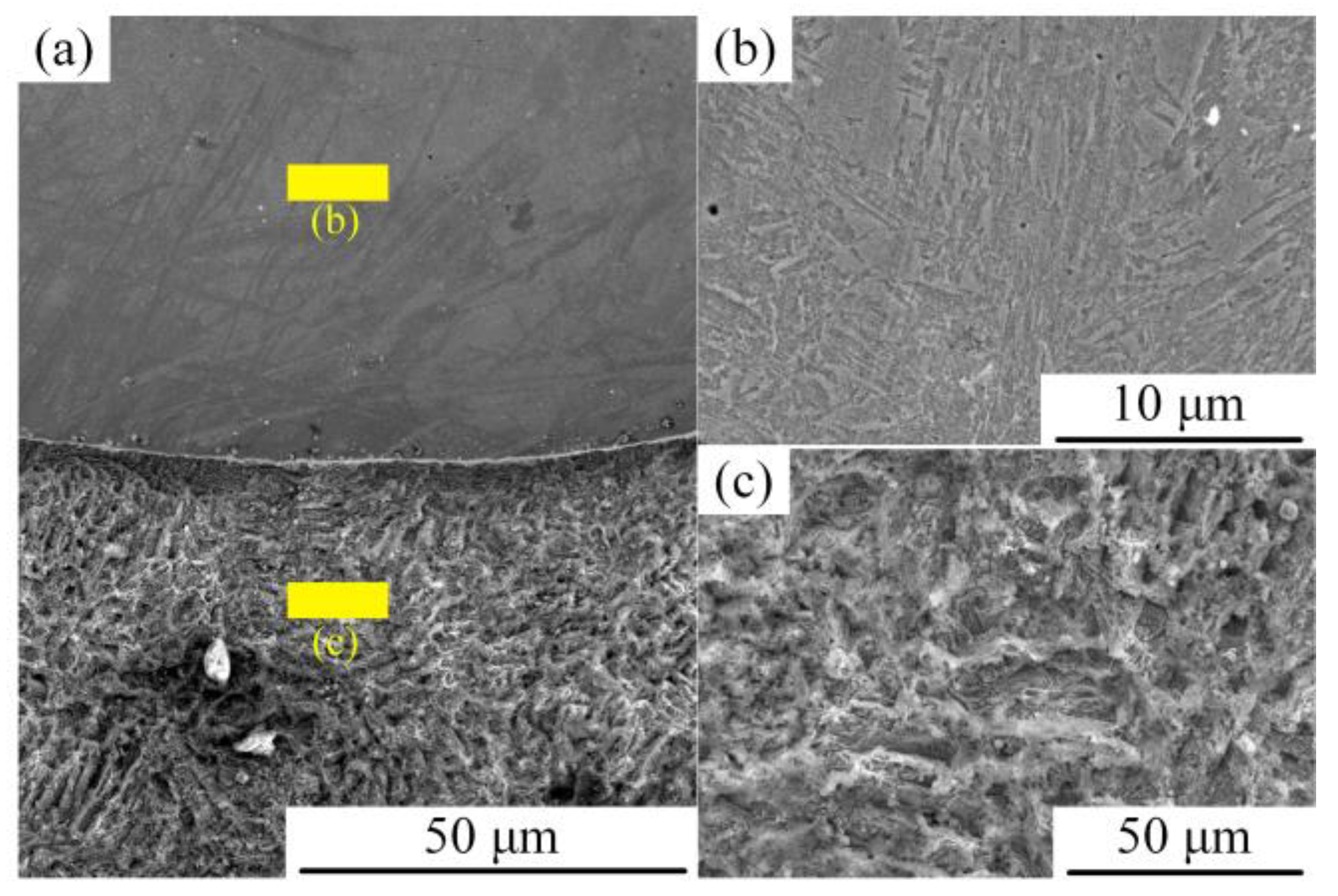
3.1.1. Microstructure of the Surface-Modified Plate
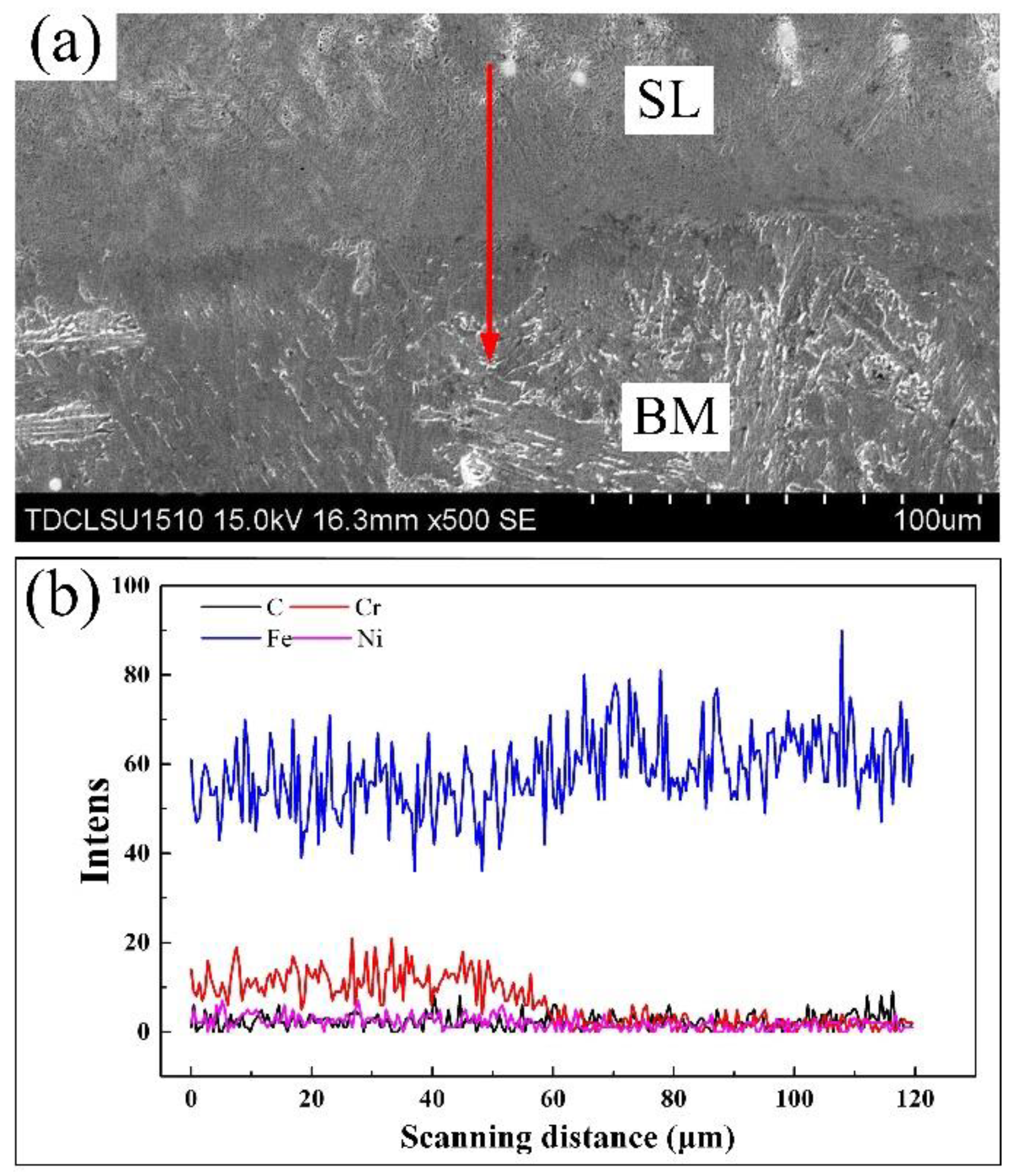
3.1.2. Distribution of Alloying Elements in the Surface Modified Plate
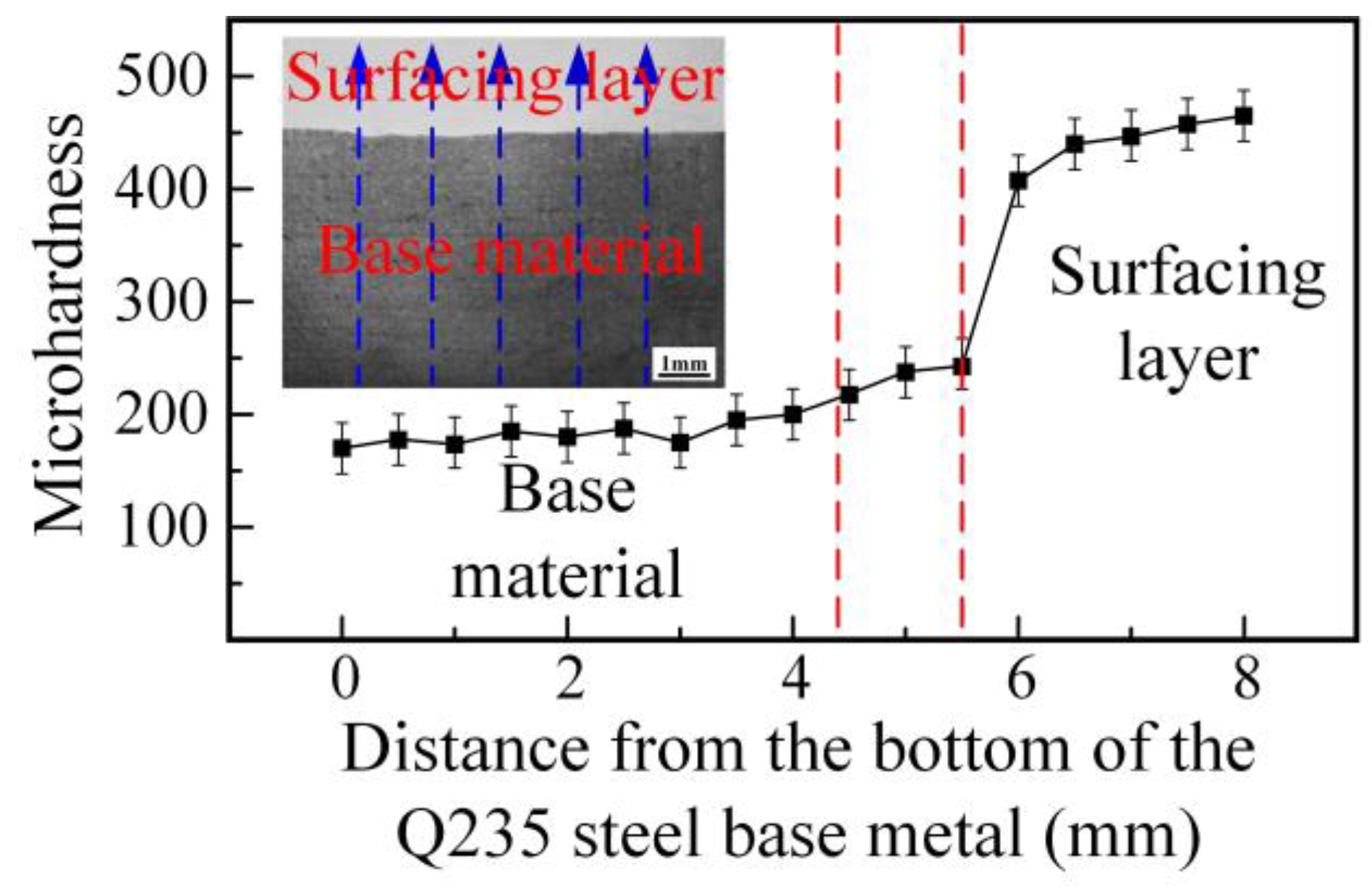
3.1.3. Hardness Characteristics of the Surface Modified Plate
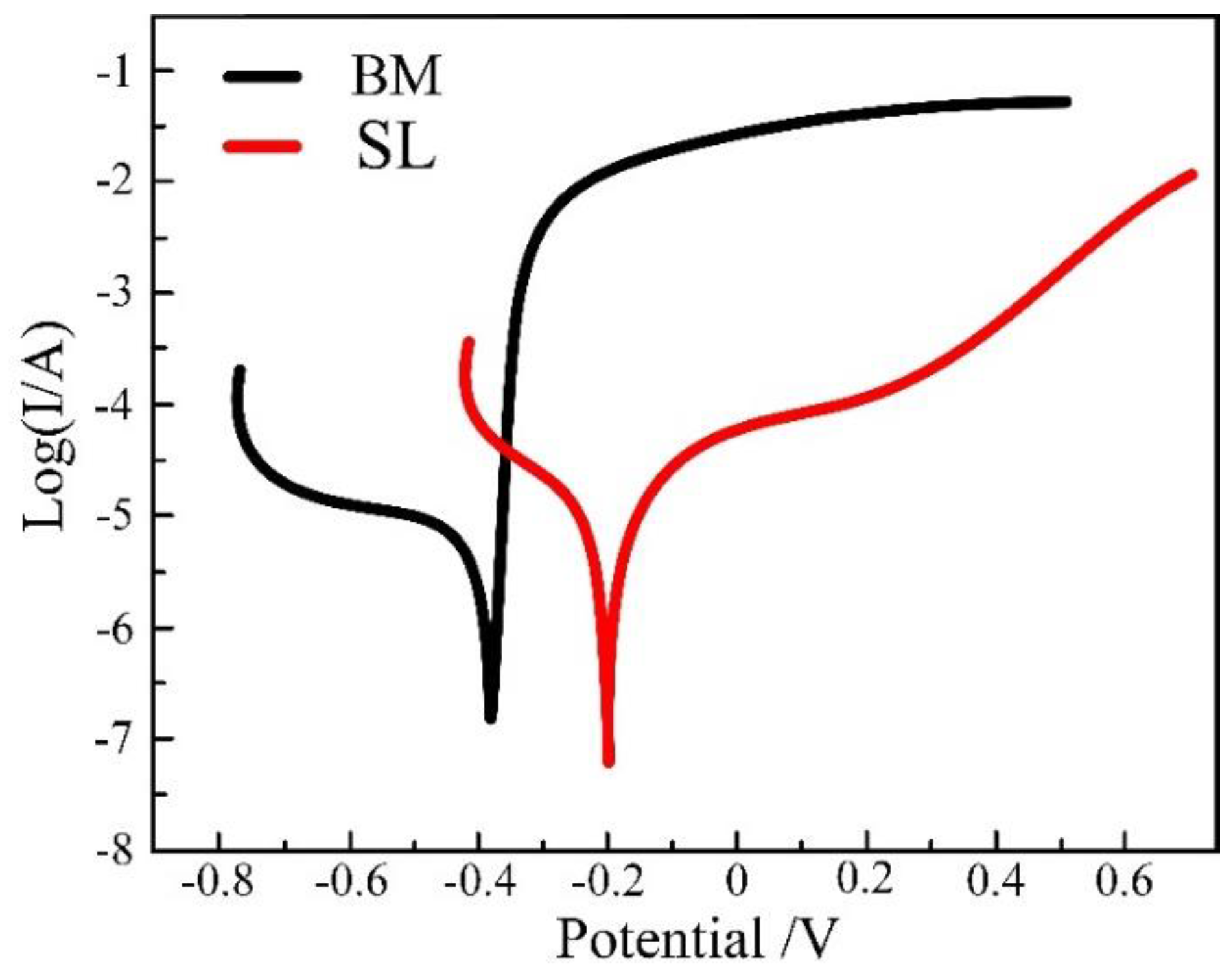
3.1.4. Corrosion Resistance of the Surfacing Layer and Base Material
3.2. Microstructures and Properties of Weld Joints
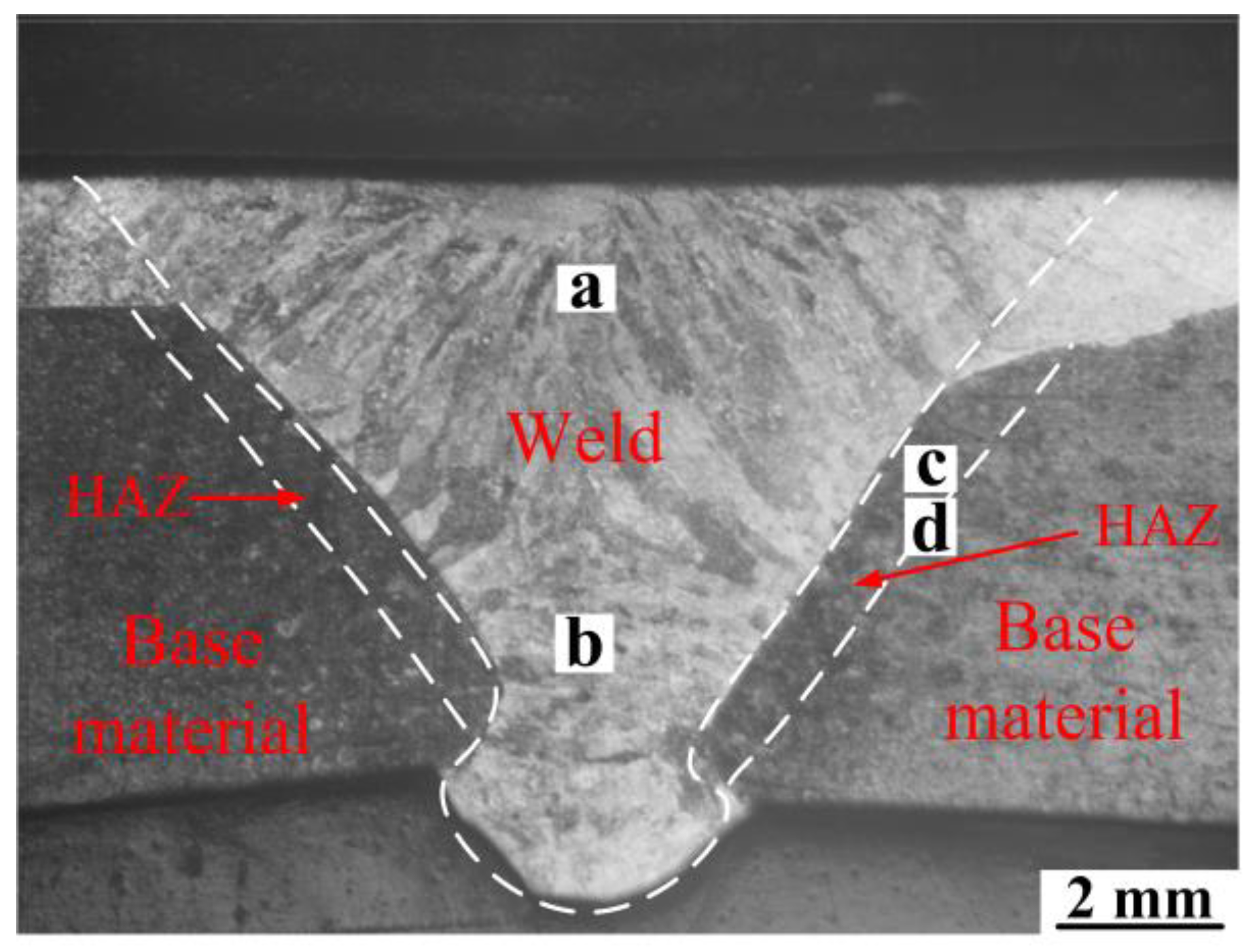
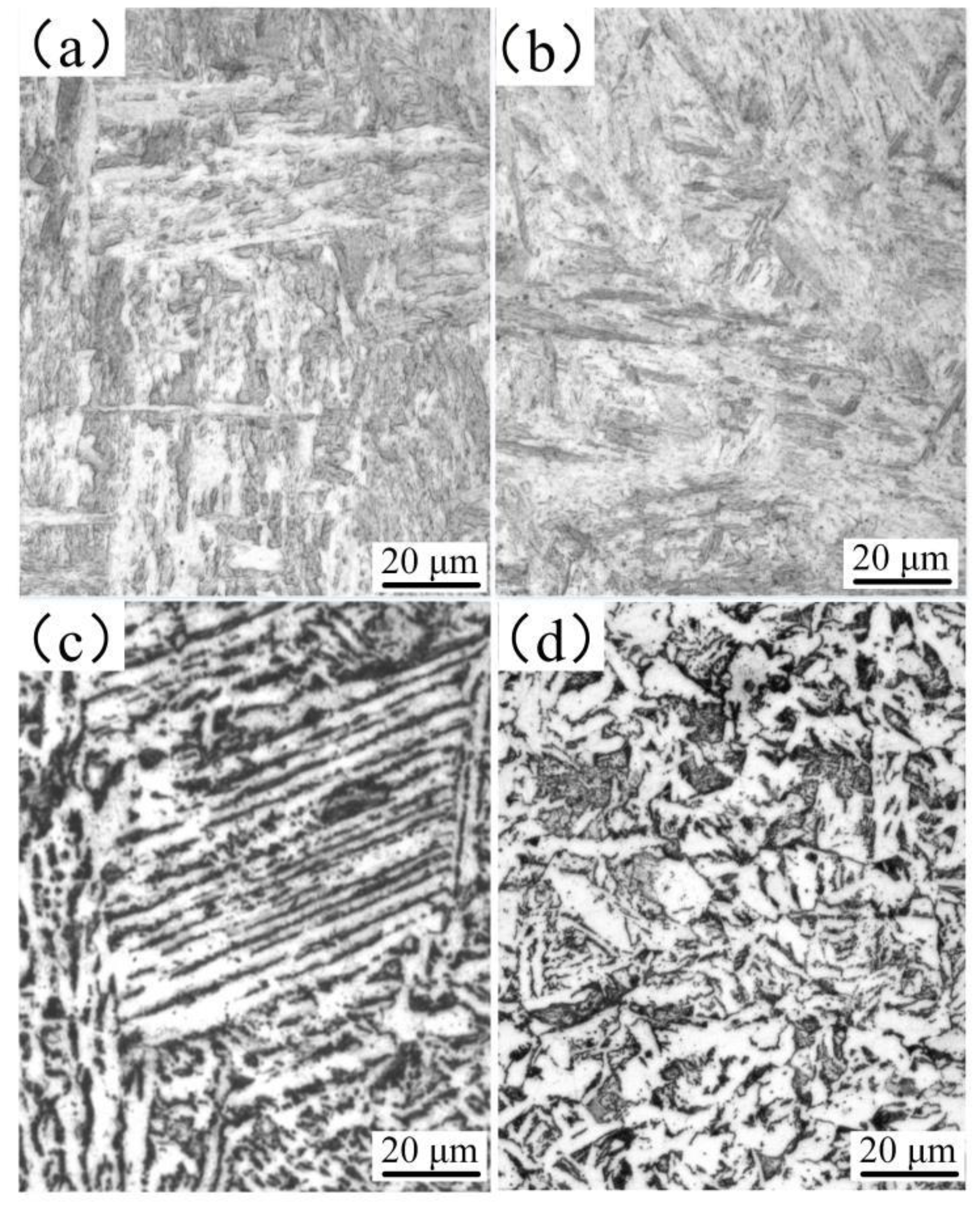
3.2.1. Microstructure of the Welded Joint Obtained by the K-TIG Welding Method
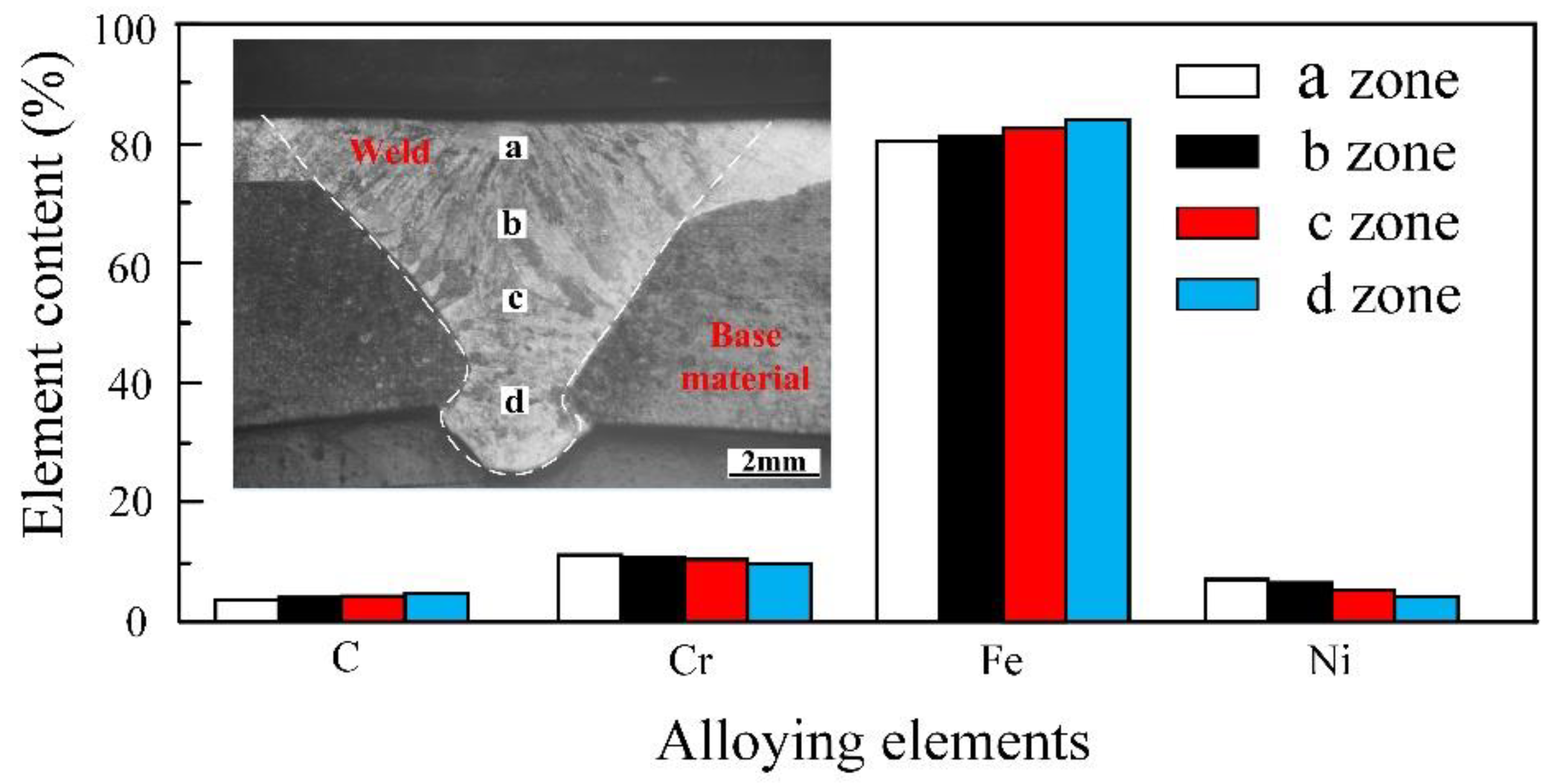
3.2.2. Distribution of Alloying Elements in the Weld
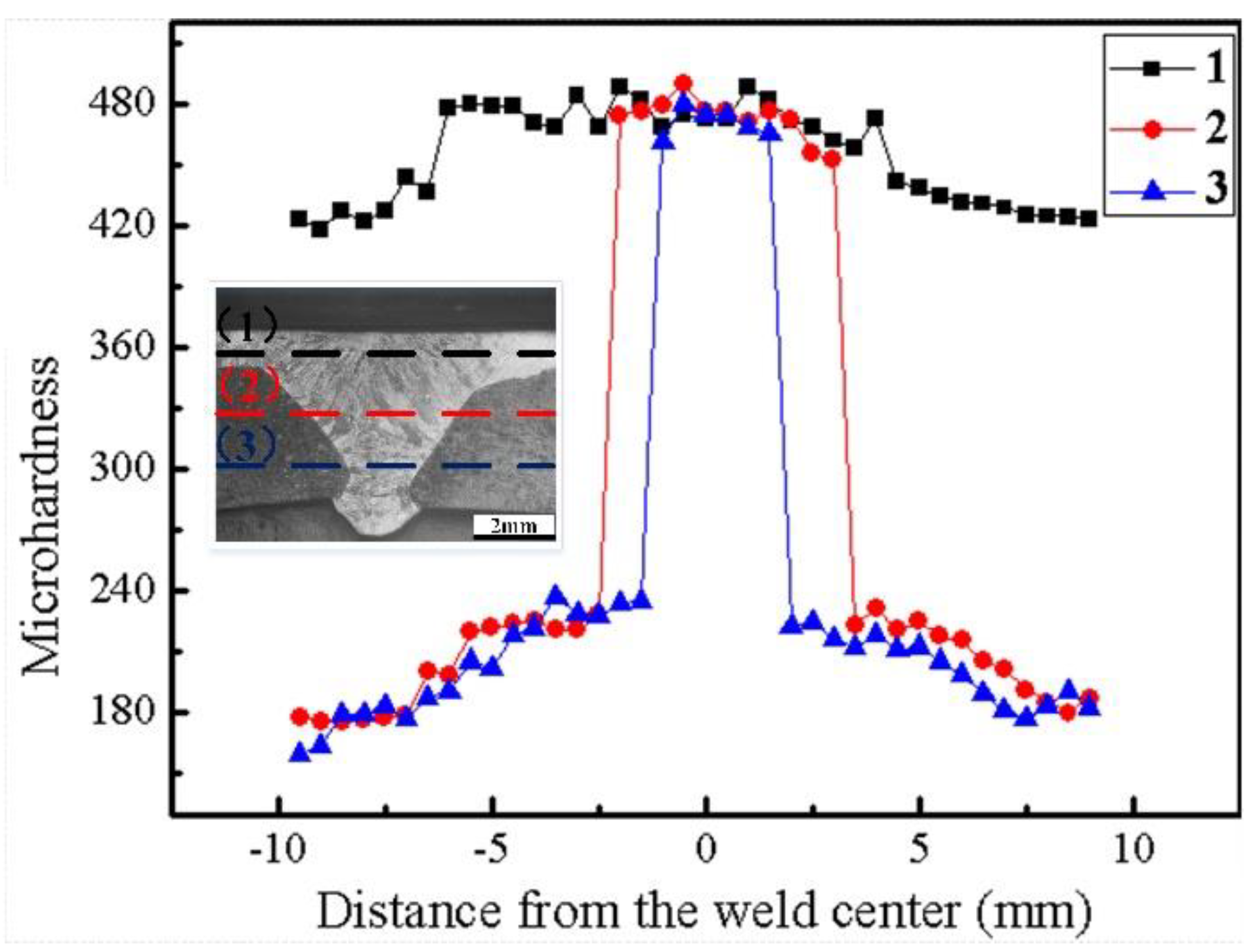
3.2.3. Hardness Distribution of the Weld
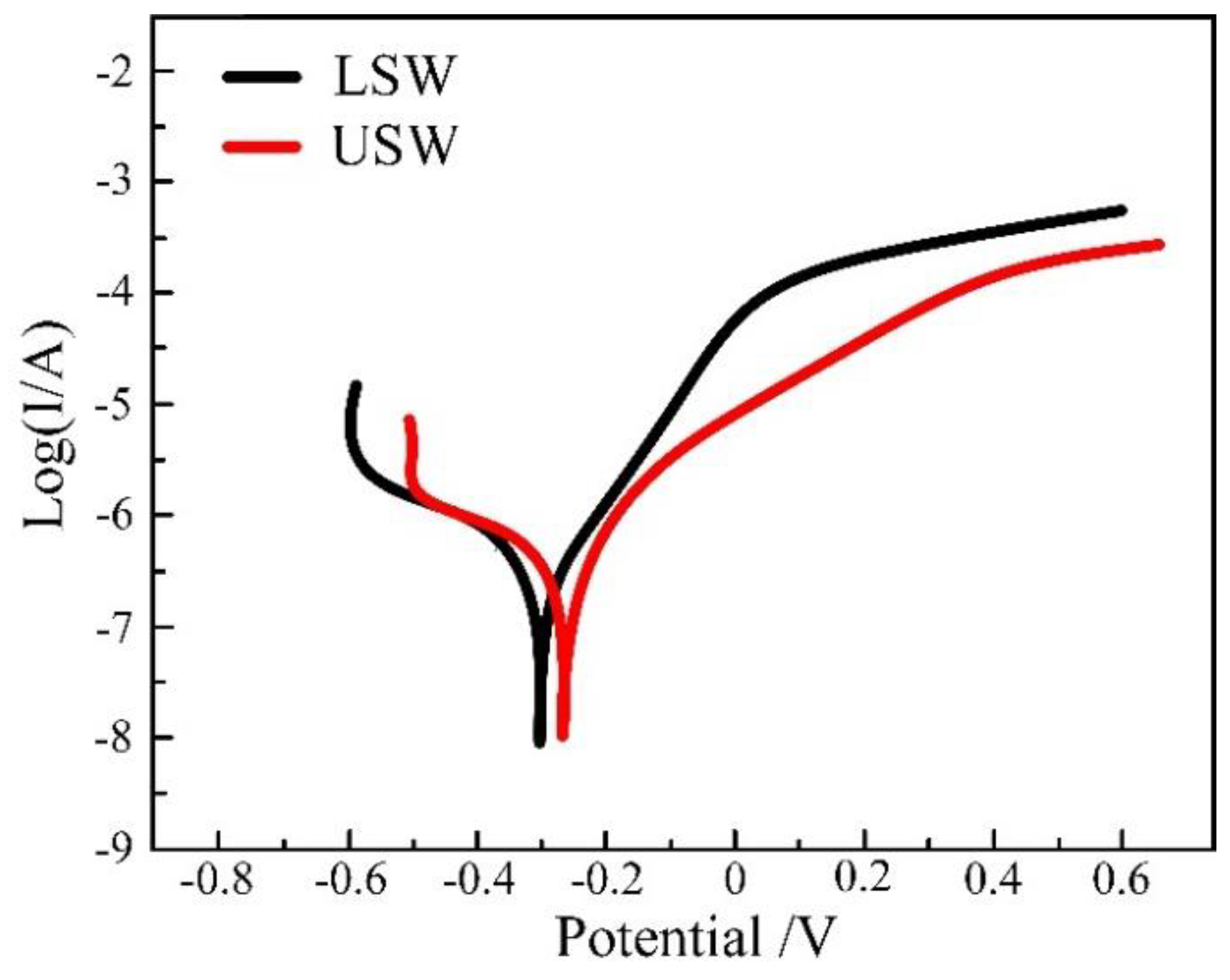
3.2.4. Corrosion Resistance of the Weld
4. Conclusions
- Using suitable welding parameters, MIG surfacing technology can be used to fabricate a defect-free stainless steel surfacing layer on low carbon steel, and the K-TIG welding method can be applied to join the surface-modified plates. There are no macroscopic defects in the surface-modified plate and its welded joint.
- The structure of the stainless steel surfacing layer consists of austenite + martensite + ferrite. Lath martensite appeared at the upper part of the weld and flake martensite at the lower end.
- There are slight differences in the content of alloying elements in the weld. Cr and Ni content was higher at the location close to the surfacing layer, while less-alloying elements were found near the base material at the lower part of the weld. The difference in content of alloying elements affects corrosion resistance, but has little effect on hardness.
- The corrosion morphology and polarization curve concludes that corrosion resistance of the surfacing layer is the best, the corrosion resistance of the upper zone of the weld is slightly higher than that of the lower part of the weld, and the corrosion resistance of the base material is the worst.
Author Contributions
Funding
Conflicts of Interest
References
- Taweejun, N.; Kanchanomai, C. Effects of Carbon and Nitrogen on the Microstructure and Mechanical Properties of Carbonitrided Low-Carbon Steel. J. Mater. Eng. Perform. 2015, 24, 4853–4862. [Google Scholar] [CrossRef]
- Cho, Y.-W.; Kang, Y.-J.; Baek, J.-H.; Woo, J.-H.; Cho, Y.-R. Investigation of Microstructure, Nanohardness and Corrosion Resistance for Oxi-Nitrocarburized Low Carbon Steel. Metals 2019, 9, 190. [Google Scholar] [CrossRef]
- Jin, Y.; Li, R.; Yu, Z.; Wang, Y. Microstructure and Mechanical Properties of Plasma Arc Brazed AISI 304L Stainless Steel and Galvanized Steel Plates. J. Mater. Eng. Perform. 2016, 25, 1327–1335. [Google Scholar] [CrossRef]
- Rafi, H.K.; Babu, N.K.; Phanikumar, G.; Rao, K.P. Microstructural Evolution during Friction Surfacing of Austenitic Stainless Steel AISI 304 on Low Carbon Steel. Metall. Mater. Trans. A 2013, 44, 345–350. [Google Scholar] [CrossRef]
- Gao, X.-L.; Zhang, L.-J.; Liu, J.; Zhang, J.-X. Comparison of Tensile Damage Evolution in Ti6A14V Joints Between laser Beam Welding and Gas Tungsten Arc Welding. J. Mater. Eng. Perform. 2014, 23, 4316–4327. [Google Scholar] [CrossRef]
- Song, G.; Diao, Z.; Lv, X.; Liu, L. TIG and laser–TIG hybrid filler wire welding of casting and wrought dissimilar magnesium alloy. J. Manuf. Process. 2018, 34, 204–214. [Google Scholar] [CrossRef]
- Li, H.; Qin, W.; Galloway, A.; Toumpis, A. Friction Surfacing of Aluminium Alloy 5083 on DH36 Steel Plate. Metals 2019, 9, 479. [Google Scholar] [CrossRef]
- Dhib, Z.; Guermazi, N.; Gaspérini, M.; Haddar, N. Cladding of low-carbon steel to austenitic stainless steel by hot-roll bonding: Microstructure and mechanical properties properties before and after welding. Mater. Sci. Eng. A 2016, 656, 130–141. [Google Scholar] [CrossRef]
- Nam, T.H.; An, E.; Kim, B.J.; Shin, S.; Ko, W.S.; Park, N.; Kang, N.; Jeon, J.B. Effect of Post Weld Heat Treatment on the Microstructure and Mechanical Properties of a Submerged-Arc-Welded 304 Stainless Steel. Metals 2018, 8, 26. [Google Scholar] [CrossRef]
- Vespa, P.; Pinard, P.T.; Gauvin, R. Analysis of WC/Ni-Based Coatings Deposited by Controlled Short-Circuit MIG Welding. J. Mater. Eng. Perform. 2012, 21, 865–876. [Google Scholar] [CrossRef]
- Fei, Z.; Pan, Z.; Cuiuri, D.; Li, H.; Wu, B.; Ding, D.; Su, L.; Gazder, A.A. Investigation into the viability of K-TIG for joining armour grade quenched and tempered steel. J. Manuf. Process. 2018, 32, 482–493. [Google Scholar] [CrossRef]
- Erick, A.G.O.; Régis, H.G.S.; Jair, C.D. Study of keyhole TIG welding by comparative analysis of two high-productivity torches for joining medium-thickness carbon steel plates. Weld. Int. 2017, 31, 337–347. [Google Scholar]
- Liu, Z.M.; Fang, Y.X.; Cui, S.L. Stable keyhole welding process with K-TIG. J. Mater. Process. Technol. 2016, 238, 65–72. [Google Scholar] [CrossRef]
- Rosellini, C.; Jarvis, L. The keyhole TIG welding process: A valid alternative for valuable metal joints. Weld. Int. 2009, 23, 616–621. [Google Scholar] [CrossRef]
- Cui, S.L.; Liu, Z.M.; Fang, Y.X. Keyhole process in K-TIG welding on 4mm thick 304 stainless steel. J. Mater. Process. Technol. 2017, 243, 217–228. [Google Scholar] [CrossRef]
- Xie, Y.; Cai, Y.; Zhang, X.; Luo, Z. Characterization of keyhole gas tungsten arc welded AISI 430 steel and joint performance optimization. Int. J. Adv. Manuf. Technol. 2018, 99, 347–361. [Google Scholar] [CrossRef]
- Fei, Z.; Pan, Z.; Cuiuri, D.; Li, H.; Wu, B.; Su, L. Improving the weld microstructure and material properties of K-TIG welded armour steel joint using filler material. Int. J. Adv. Manuf. Technol. 2018, 100, 1931–1944. [Google Scholar] [CrossRef]
- Liu, R.; Wang, B.; Wu, J.; Xue, W.; Jin, X.; Du, J.; Hua, M. Spectroscopic investigation of plasma electrolytic borocarburizing on q235 low-carbon steel. Appl. Surf. Sci. 2014, 321, 348–352. [Google Scholar] [CrossRef]
- Zhang, H.; Hei, Z.; Liu, G.; Lü, J.; Lu, K. Formation of nanostructured surface layer on AISI 304 stainless steel by means of surface mechanical attrition treatment. Acta Mater. 2003, 51, 1871–1881. [Google Scholar] [CrossRef]
- Feng, Y.Q.; Luo, Z.; Liu, Z.M. Keyhole gas tungsten arc welding of AISI 316L stainless steel. Mater. Des. 2015, 85, 24–31. [Google Scholar] [CrossRef]
- Anke, S.K.; Andree, L.L.; Matthias, M.; Walther, F.F.; Frank, W. Fatigue and Corrosion Fatigue Behaviour of Brazed Stainless Steel Joints AISI 304L/BAu-4 in Synthetic Exhaust Gas Condensate. Materials 2019, 12, 1040. [Google Scholar]
- Wei, Y.J.; Xia, D.H.; Song, S.Z. Detection of SCC of 304 NG stainless steel in an acidic NaCl solution using electrochemical noise based on chaos and wavelet analysis. Russ. J. Electrochem. 2016, 52, 560–575. [Google Scholar] [CrossRef]
- Leban, M.B.; Črt, M.; Tadeja, K.; Boštjan, M.; Janez, K. The Effect of Surface Roughness on the Corrosion Properties of Type AISI 304 Stainless Steel in Diluted NaCl and Urban Rain Solution. J. Mater. Eng. Perform. 2014, 23, 1695–1702. [Google Scholar] [CrossRef]
- Ezuber, H.; Alshater, A.; Nisar, S.O. Effect of Surface Finish on the Pitting Corrosion Behavior of Sensitized AISI 304 Austenitic Stainless Steel Alloys in 3.5% NaCl Solutions. Sur. Eng. Appl. Electrochem. 2018, 54, 73–80. [Google Scholar] [CrossRef]
- Kianersi, D.; Mostafaei, A.; Amadeh, A.A. Resistance spot welding joints of AISI 316L austenitic stainless steel sheets: Phase transformations, mechanical properties and microstructure characterizations. Mater. Des. 2014, 61, 251–263. [Google Scholar] [CrossRef]
- Khuenkaew, T.; Kanlayasiri, K. Resistance Spot Welding of SUS316L Austenitic/SUS425 Ferritic Stainless Steels: Weldment Characteristics, Mechanical Properties, Phase Transformation and Solidification. Metals 2019, 9, 710. [Google Scholar] [CrossRef]


| Materials | C | Si | Mn | S | P | Al | Cr | Ni | Fe |
|---|---|---|---|---|---|---|---|---|---|
| Q235 | 0.16 | 0.3 | 0.65 | 0.004 | 0.01 | 0.033 | – | – | Bal |
| 304 | 0.05 | 1.0 | 1.5 | 0.03 | 0.035 | – | 21 | 9 | Bal |
| Current (A) | Voltage (V) | Welding Speed (mm/s) | Shielding Gas | Gas Flow (L/min) |
|---|---|---|---|---|
| 150 | 22 | 5 | Ar | 25 |
| Samples | Ecorr (V) | Icorr (µA/cm2) |
|---|---|---|
| Low carbon steel | −0.4 | −5.762 |
| Surfacing layer | −0.2 | −5.122 |
| Samples | Ecorr (V) | Icorr (µA/cm2) |
|---|---|---|
| Longitudinal section of weld | −0.29 | −6.114 |
| Upper surface of weld | −0.23 | −5.887 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Ao, S.S.; Manladan, S.M.; Cai, Y.C.; Luo, Z. Microstructure and Properties of Surface-Modified Plates and Their Welded Joints. Materials 2019, 12, 2883. https://doi.org/10.3390/ma12182883
Wang T, Ao SS, Manladan SM, Cai YC, Luo Z. Microstructure and Properties of Surface-Modified Plates and Their Welded Joints. Materials. 2019; 12(18):2883. https://doi.org/10.3390/ma12182883
Chicago/Turabian StyleWang, Tai, San San Ao, S. M. Manladan, Yang Chuan Cai, and Zhen Luo. 2019. "Microstructure and Properties of Surface-Modified Plates and Their Welded Joints" Materials 12, no. 18: 2883. https://doi.org/10.3390/ma12182883
APA StyleWang, T., Ao, S. S., Manladan, S. M., Cai, Y. C., & Luo, Z. (2019). Microstructure and Properties of Surface-Modified Plates and Their Welded Joints. Materials, 12(18), 2883. https://doi.org/10.3390/ma12182883






