Intensification of Pseudocapacitance by Nanopore Engineering on Waste-Bamboo-Derived Carbon as a Positive Electrode for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of NE-C-AEMs
2.2. Characterization
2.3. Electrochemical Characterization
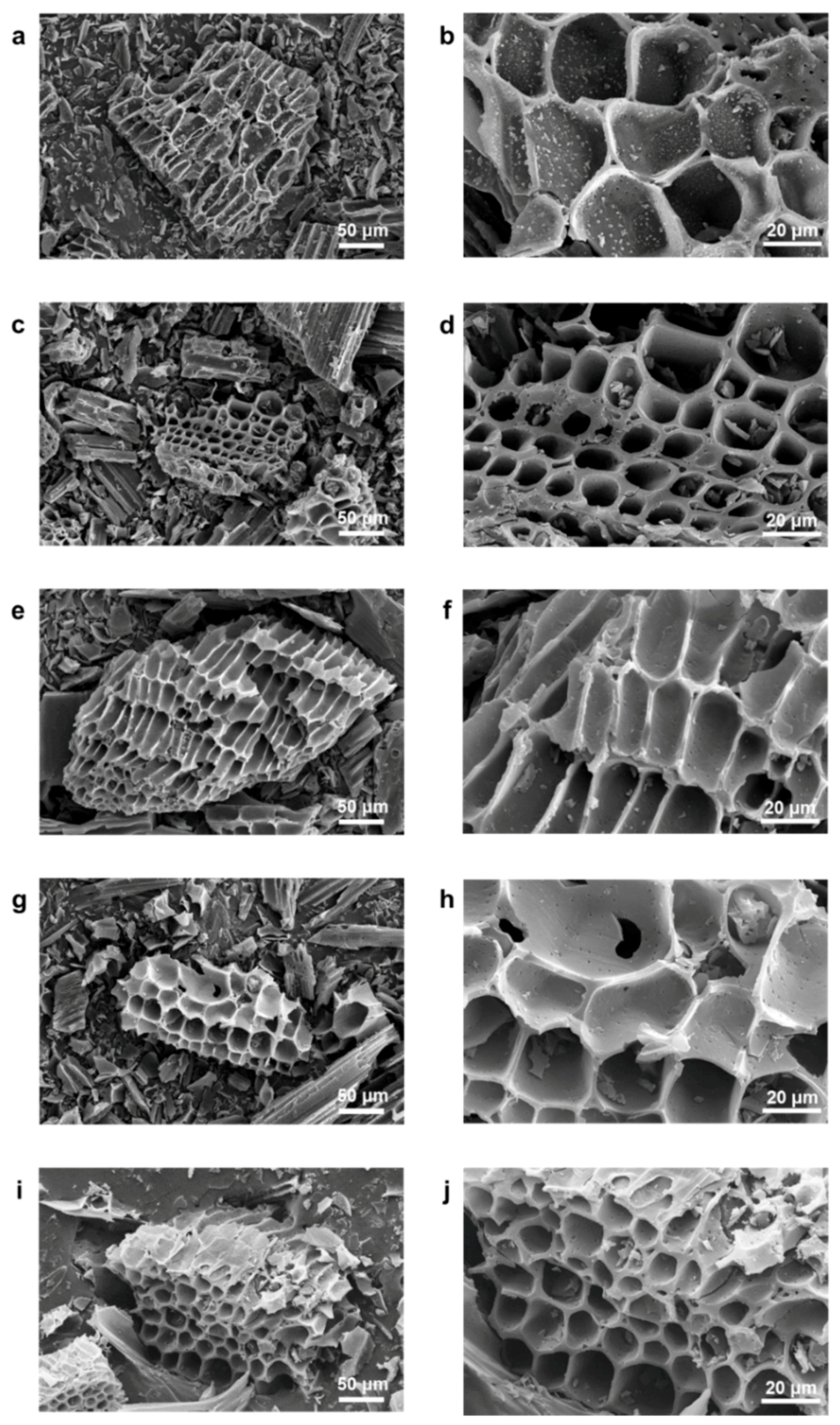
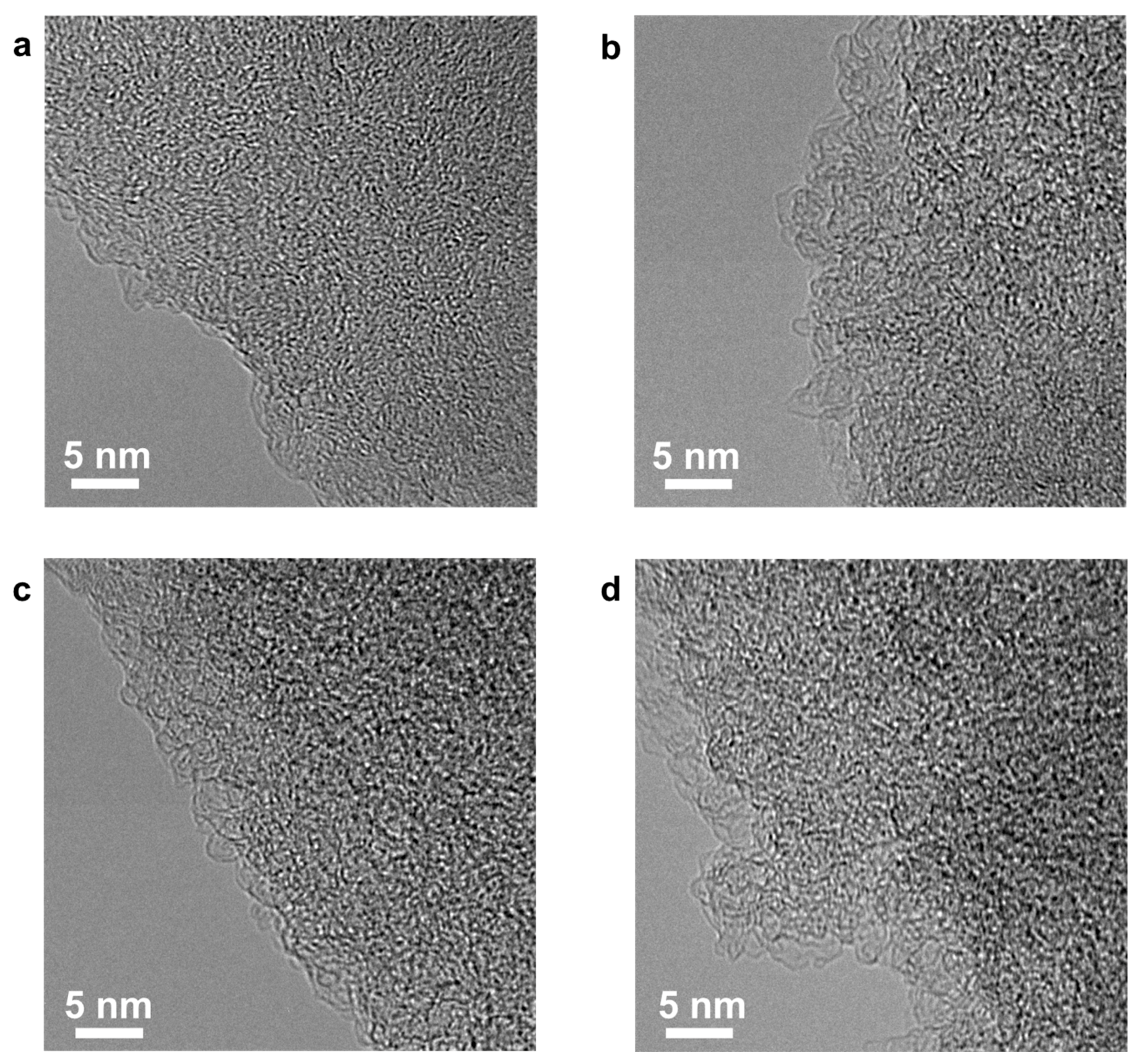
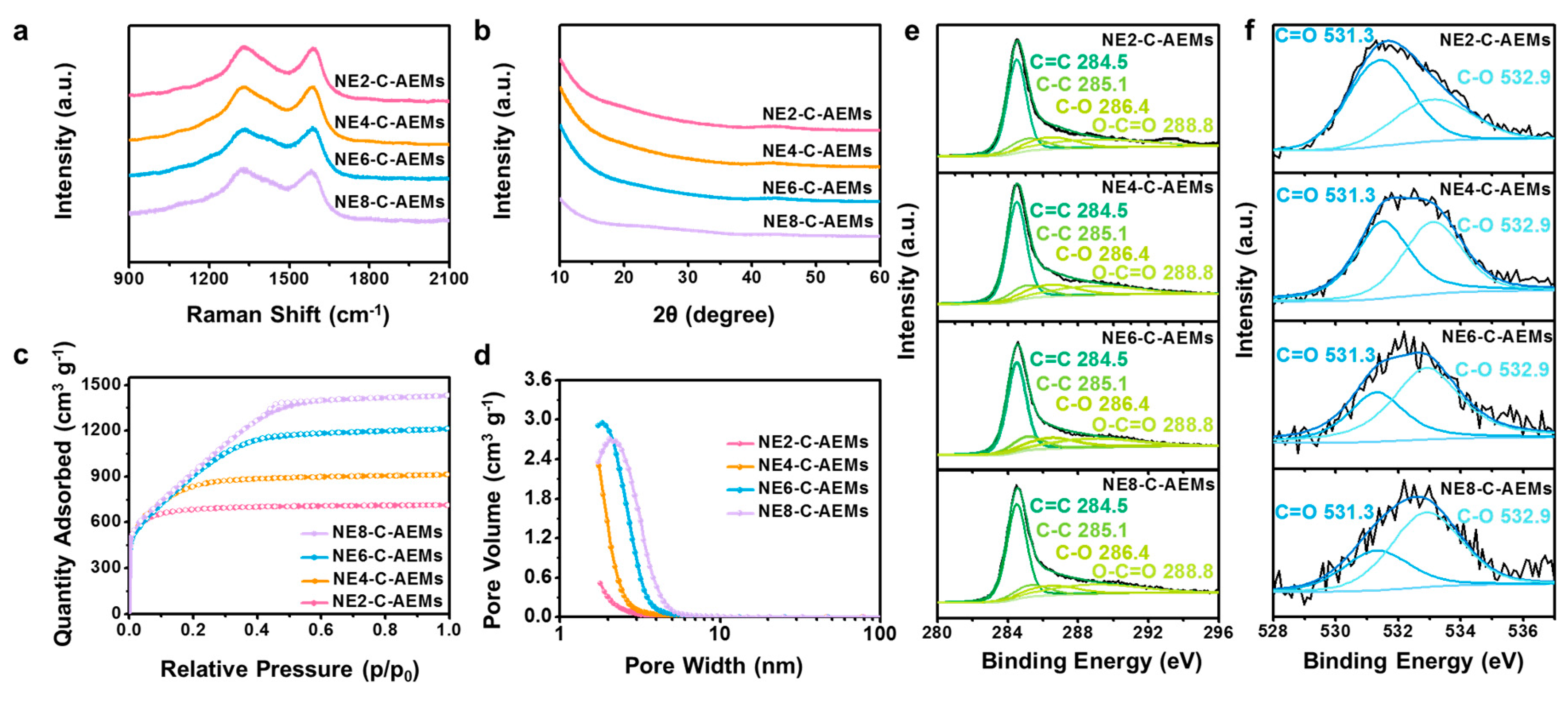
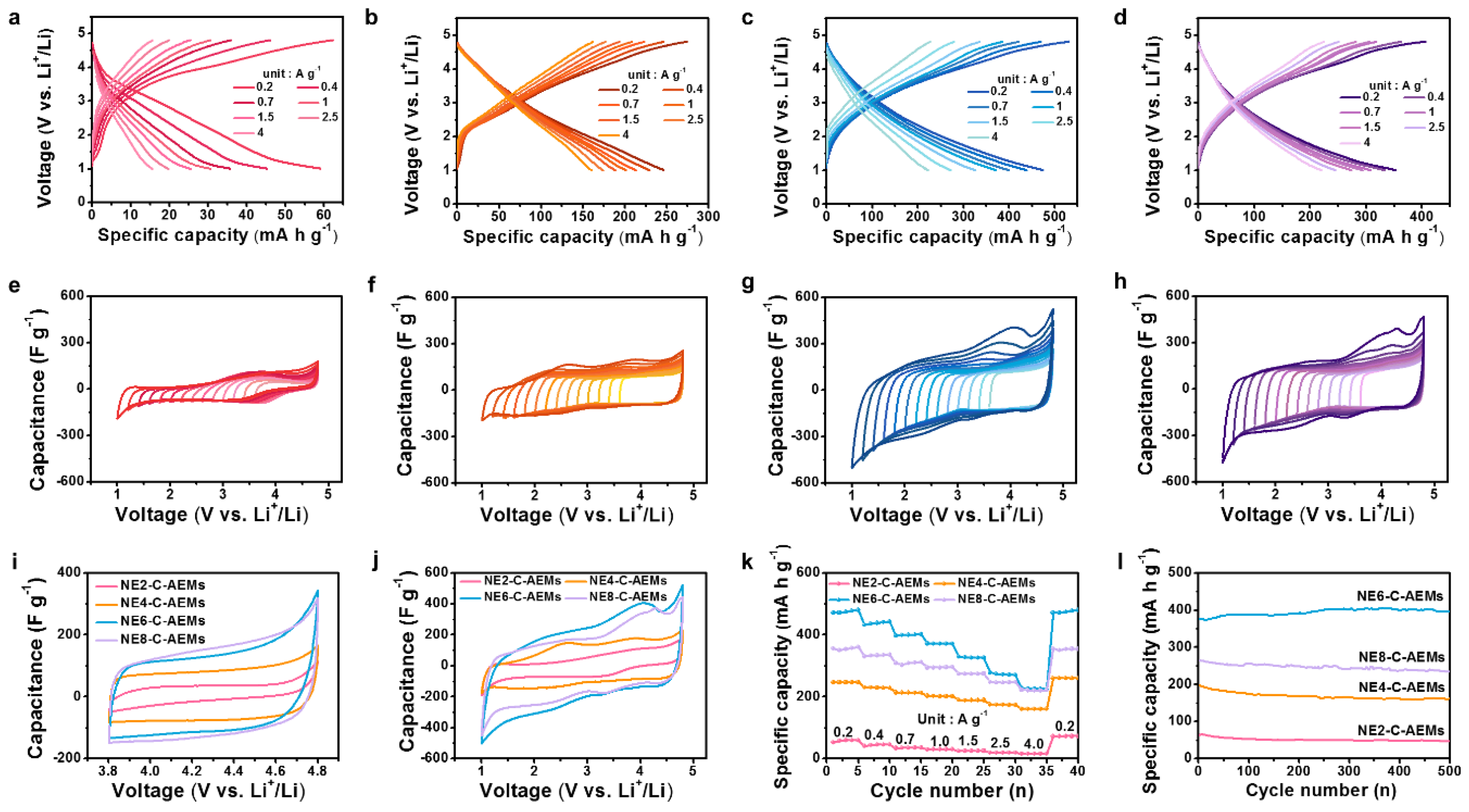
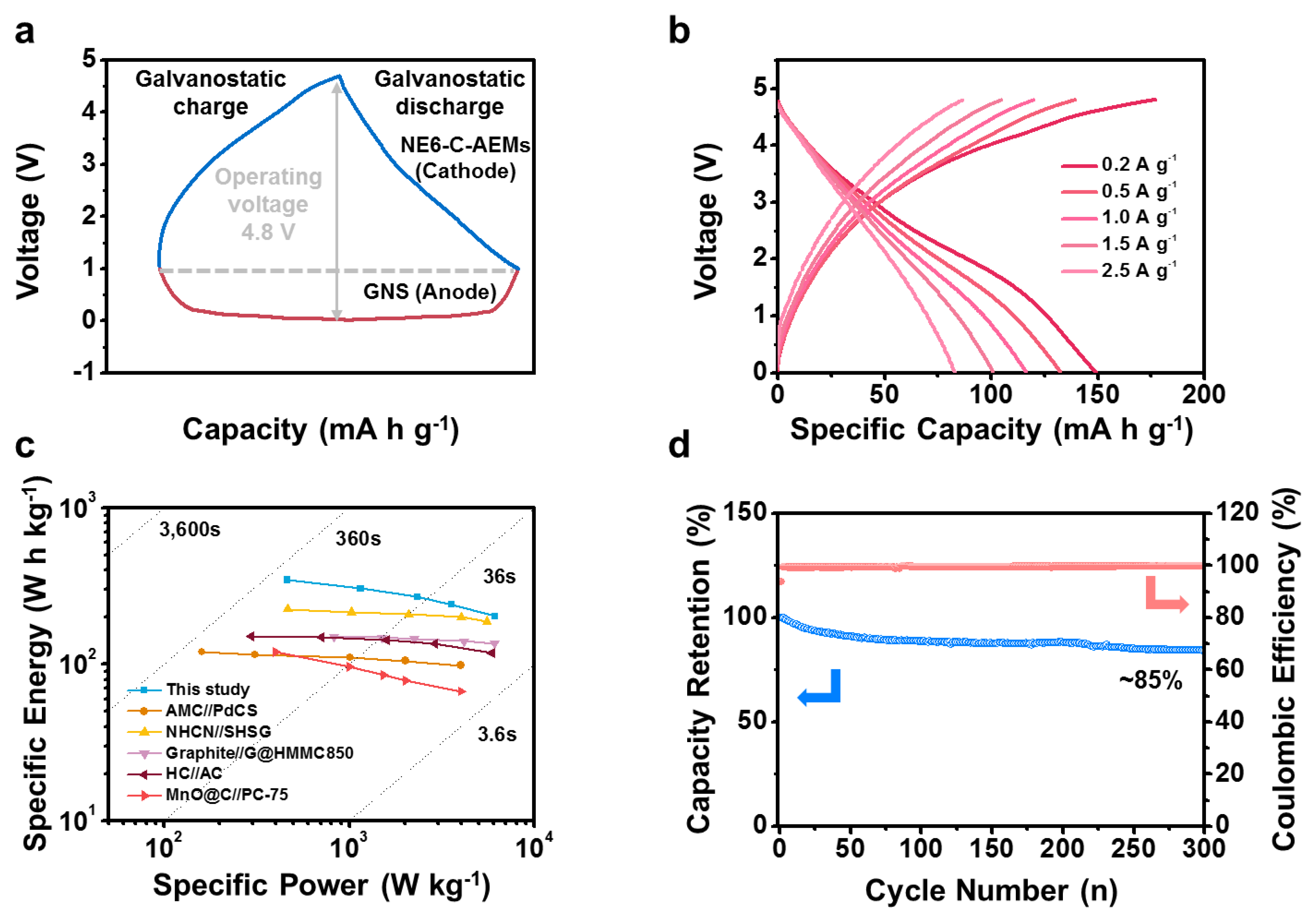
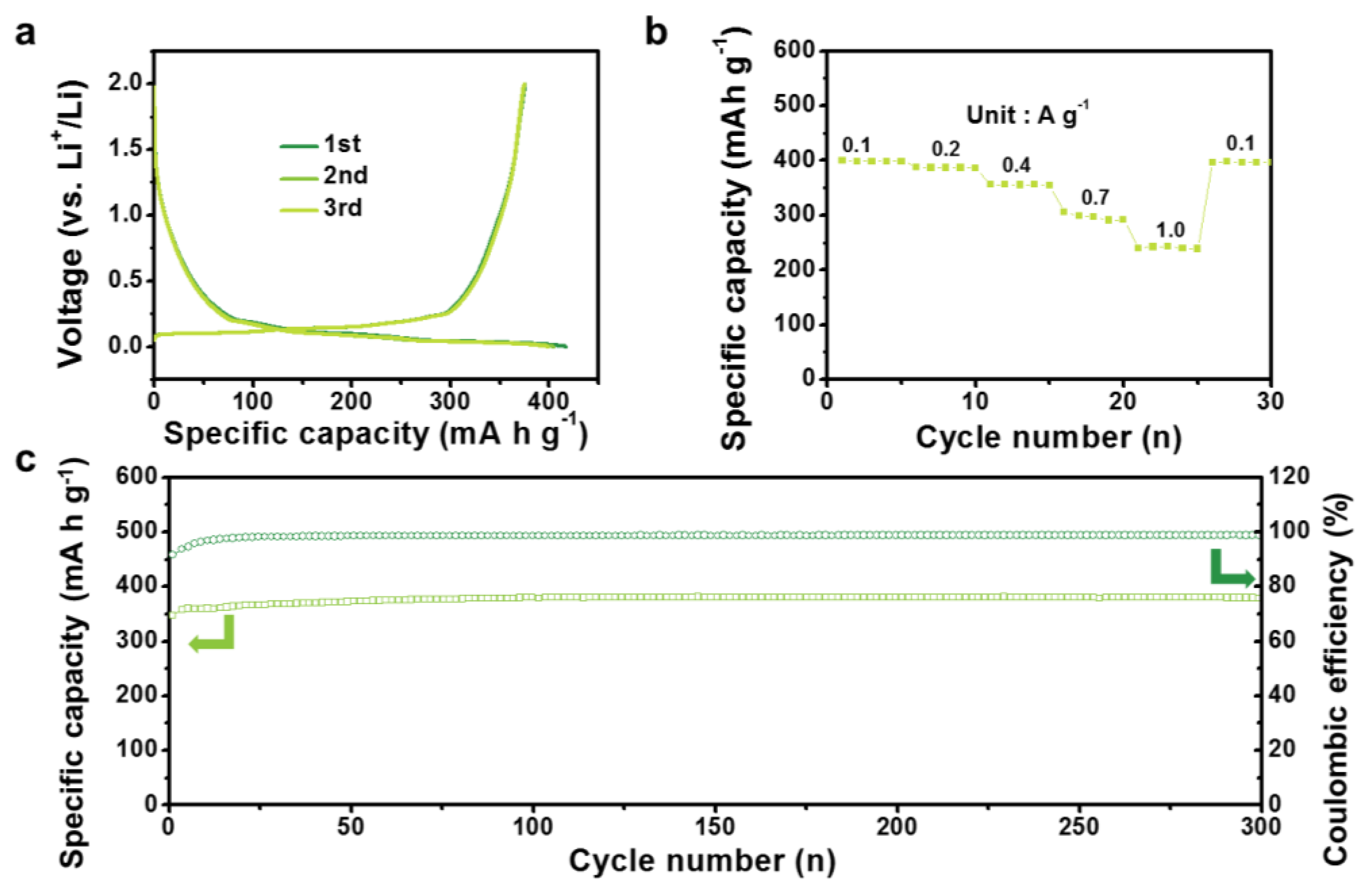
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zubi, G.; Dufo-López, R.; Carvalho, M.; Pasaoglu, G. The lithium ion battery: State of the art and future perspectives. Renew. Sustain. Energy Rev. 2018, 89, 292–308. [Google Scholar] [CrossRef]
- Trappe, W.; Howard, R.; Moore, R.S. Low-energy security: Limits and opportunities in the internet of things. IEEE Secur. Priv. 2015, 13, 14–21. [Google Scholar] [CrossRef]
- Pu, X.; Li, L.; Song, H.; Du, C.; Zhao, Z.; Jiang, C.; Cao, G.; Hu, W.; Wang, Z.L. A self-charging power unit by integration of a textile triboelectric nanogenerator and a flexible lithium-ion battery for wearable electronics. Adv. Mater. 2015, 27, 2472–2478. [Google Scholar] [CrossRef] [PubMed]
- Stolaroff, J.K.; Samaras, C.; O’ Neill, E.R.; Lubers, A.; Mitchell, A.S.; Ceperley, D. Energy use and life cycle greenhouse gas emissions of drones for commercial package delivery. Nat. Commun. 2018, 9, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Cano, Z.P.; Banham, D.; Ye, S.; Hintennach, A.; Lu, J.; Fowler, M.; Chen, Z. Batteries and fuel cells for emerging electric vehicle markets. Nat. Energy 2018, 3, 279–289. [Google Scholar] [CrossRef]
- Blomgren, G.E. The development and future of lithium ion batteries. J. Electrochem. Soc. 2017, 164, A5019–A5025. [Google Scholar] [CrossRef]
- Thackeray, M.M.; Wolverton, C.; Isaacs, E.D. Electrical energy storage for transportation-approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ. Sci. 2012, 5, 7854–7863. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Cho, S.Y.; Kang, M.; Choi, J.; Lee, M.E.; Yoon, H.J.; Kim, H.J.; Leal, C.; Lee, S.; Jin, H.J.; Yun, Y.S. Pyrolytic carbon nanosheets for ultrafast and ultrastable sodium-ion storage. Small 2018, 14, 1703043. [Google Scholar] [CrossRef]
- Yun, Y.S.; Lee, S.; Kim, N.R.; Kang, M.; Leal, C.; Park, K.Y.; Kang, K.; Jin, H.J. High and rapid alkali cation storage in ultramicroporous carbonaceous materials. J. Power Sources 2016, 313, 142–151. [Google Scholar] [CrossRef]
- Yun, Y.S.; Yun, G.; Park, M.; Cho, S.Y.; Lim, H.D.; Kim, H.; Park, Y.W.; Kim, B.H.; Kang, K.; Jin, H.J. Restoration of thermally reduced graphene oxide by atomic-level selenium doping. NPG Asia Mater. 2016, 8, e338. [Google Scholar] [CrossRef]
- Yun, Y.S.; Park, K.Y.; Lee, B.; Cho, S.Y.; Park, Y.U.; Hong, S.J.; Kim, B.H.; Gwon, H.; Kim, H.; Lee, S.; et al. Sodium-ion storage in pyroprotein-based carbon nanoplates. Adv. Mater. 2015, 27, 6914–6921. [Google Scholar] [CrossRef] [PubMed]
- Forse, A.C.; Merlet, C.; Griffin, J.M.; Grey, C.P. New perspectives on the charging mechanisms of supercapacitors. J. Am. Chem. Soc. 2016, 138, 5731–5744. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Forse, A.C.; Griffin, J.M.; Trease, N.M.; Trognko, L.; Taberna, P.L.; Simon, P.; Grey, C.P. In situ NMR spectroscopy of supercapacitors: Insight into the charge storage mechanisms. J. Am. Chem. Soc. 2013, 135, 18968–18980. [Google Scholar] [CrossRef] [PubMed]
- Kondrat, S.; Kornyshev, A. Charging dynamics and optimization of nanoporous supercapacitors. J. Phys. Chem. C 2013, 117, 12399–12406. [Google Scholar] [CrossRef]
- Shi, K.; Ren, M.; Zhitomirsky, I. Activated carbon-coated carbon nanotubes for energy storage in supercapacitors and capacitive water purification. ACS Sustain. Chem. Eng. 2014, 2, 1289–1298. [Google Scholar] [CrossRef]
- Shi, K.; Zhitomirsky, I. Asymmetric supercapacitors based on activated-carbon-coated carbon nanotubes. ChemElectroChem 2015, 2, 396–403. [Google Scholar] [CrossRef]
- Shi, K.; Zhitomirsky, I. Influence of chemical structure of dyes on capacitive dye removal from solutions. Electrochim. Acta 2015, 174, 588–595. [Google Scholar] [CrossRef]
- Shi, K.; Zhitomirsky, I. Supercapacitor devices for energy storage and capacitive dye removal from aqueous solutions. RSC Adv. 2015, 5, 320–327. [Google Scholar] [CrossRef]
- Yun, Y.S.; Kim, D.H.; Hong, S.J.; Park, M.H.; Park, Y.W.; Kim, B.H.; Jin, H.J.; Kang, K. Microporous carbon nanosheets with redox-active heteroatoms for pseudocapacitive charge storage. Nanoscale 2015, 7, 15051–15058. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lim, H.D.; Kim, S.W.; Hong, J.; Seo, D.H.; Kim, D.C.; Jeon, S.; Park, S.; Kang, K. Scalable functionalized graphene nano-platelets as tunable cathodes for high-performance lithium rechargeable batteries. Sci. Rep. 2013, 3, 1506. [Google Scholar] [CrossRef]
- Lee, S.W.; Yabuuchi, N.; Gallant, B.M.; Chen, S.; Kim, B.S.; Hammond, P.T.; Shao-Horn, Y. High-power lithium batteries from functionalized carbon-nanotube electrodes. Nat. Nanotechnol. 2010, 5, 531–537. [Google Scholar] [CrossRef]
- Kim, H.; Park, Y.U.; Park, K.Y.; Lim, H.D.; Hong, J.; Kang, K. Novel transition-metal-free cathode for high energy and power sodium rechargeable batteries. Nano Energy 2014, 4, 97–104. [Google Scholar] [CrossRef]
- Kim, N.R.; Lee, S.M.; Kim, M.W.; Yoon, H.J.; Hong, W.G.; Kim, H.J.; Choi, H.J.; Jin, H.J.; Yun, Y.S. Amphicharge-storable pyropolymers containing multitiered nanopores. Adv. Energy Mater. 2017, 7, 1700629. [Google Scholar] [CrossRef]
- Cho, S.Y.; Yoon, H.J.; Kim, N.R.; Yun, Y.S.; Jin, H.J. Sodium-ion supercapacitors based on nanoporous pyroproteins containing redox-active heteroatoms. J. Power Sources 2016, 329, 536–545. [Google Scholar] [CrossRef]
- Han, J.K.; Lee, M.E.; Choi, H.J.; Jin, H.J.; Yun, Y.S. Quantitative characterization of a voltage-dependent pseudocapacitance on heteroatom-enriched nanoporous carbons. Electrochim. Acta 2019, 302, 71–77. [Google Scholar] [CrossRef]
- Cho, S.Y.; Yun, Y.S.; Jang, D.; Jeon, J.W.; Kim, B.H.; Lee, S.; Jin, H.J. Ultra strong pyroprotein fibres with long-range ordering. Nat. Commun. 2017, 8, 74–80. [Google Scholar] [CrossRef]
- Yun, Y.S.; Kim, Y.H.; Song, M.Y.; Kim, N.R.; Ku, K.; An, J.S.; Kang, K.; Choi, H.J.; Jin, H.J. Energy storage capabilities of nitrogen-enriched pyropolymer nanoparticles fabricated through rapid pyrolysis. J. Power Sources 2016, 331, 507–514. [Google Scholar] [CrossRef]
- Harris, P.J.F.; Burian, A.; Duber, S. High-resolution electron microscopy of a microporous carbon. Philos. Mag. Lett. 2012, 80, 381–386. [Google Scholar] [CrossRef]
- Harris, P.J.F. Impact of the discovery of fullerenes on carbon science. Chem. Phys. Carbon 2003, 28, 1–39. [Google Scholar] [CrossRef]
- Lee, W.S.V.; Huang, X.; Tan, T.L.; Xue, J.M. Low li+ insertion barrier carbon for high energy efficient lithium-ion capacitor. ACS Appl. Mater. Interfaces 2018, 10, 1690–1700. [Google Scholar] [CrossRef]
- Yan, D.; Li, S.H.; Guo, L.P.; Dong, X.L.; Chen, Z.Y.; Li, W.C. Hard@soft integrated morning glory like porous carbon as a cathode for a high-energy lithium ion capacitor. ACS Appl. Mater. Interfaces 2018, 10, 43946–43952. [Google Scholar] [CrossRef]
- Li, N.W.; Du, X.; Shi, J.L.; Zhang, X.; Fan, W.; Wang, J.; Zhao, S.; Liu, Y.; Xu, W.; Li, M.; et al. Graphene@hierarchical meso-/microporous carbon for ultrahigh energy density lithium-ion capacitors. Electrochim. Acta 2018, 281, 459–465. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Wang, K.; Sun, X.; Ma, Y. High-power and long-life lithium-ion capacitors constructed from N-doped hierarchical carbon nanolayer cathode and mesoporous graphene anode. Carbon 2018, 140, 237–248. [Google Scholar] [CrossRef]
- Arnaiz, M.; Nair, V.; Mitra, S.; Ajuria, J. Furfuryl alcohol derived high-end carbons for ultrafast dual carbon lithium ion capacitors. Electrochim. Acta 2019, 304, 437–446. [Google Scholar] [CrossRef]
- Holtstiege, F.; Bärmann, P.; Nölle, R.; Winter, M.; Placke, T. Pre-lithiation strategies for rechargeable energy storage technologies: Concepts, promises and challenges. Batteries 2018, 4, 4. [Google Scholar] [CrossRef]
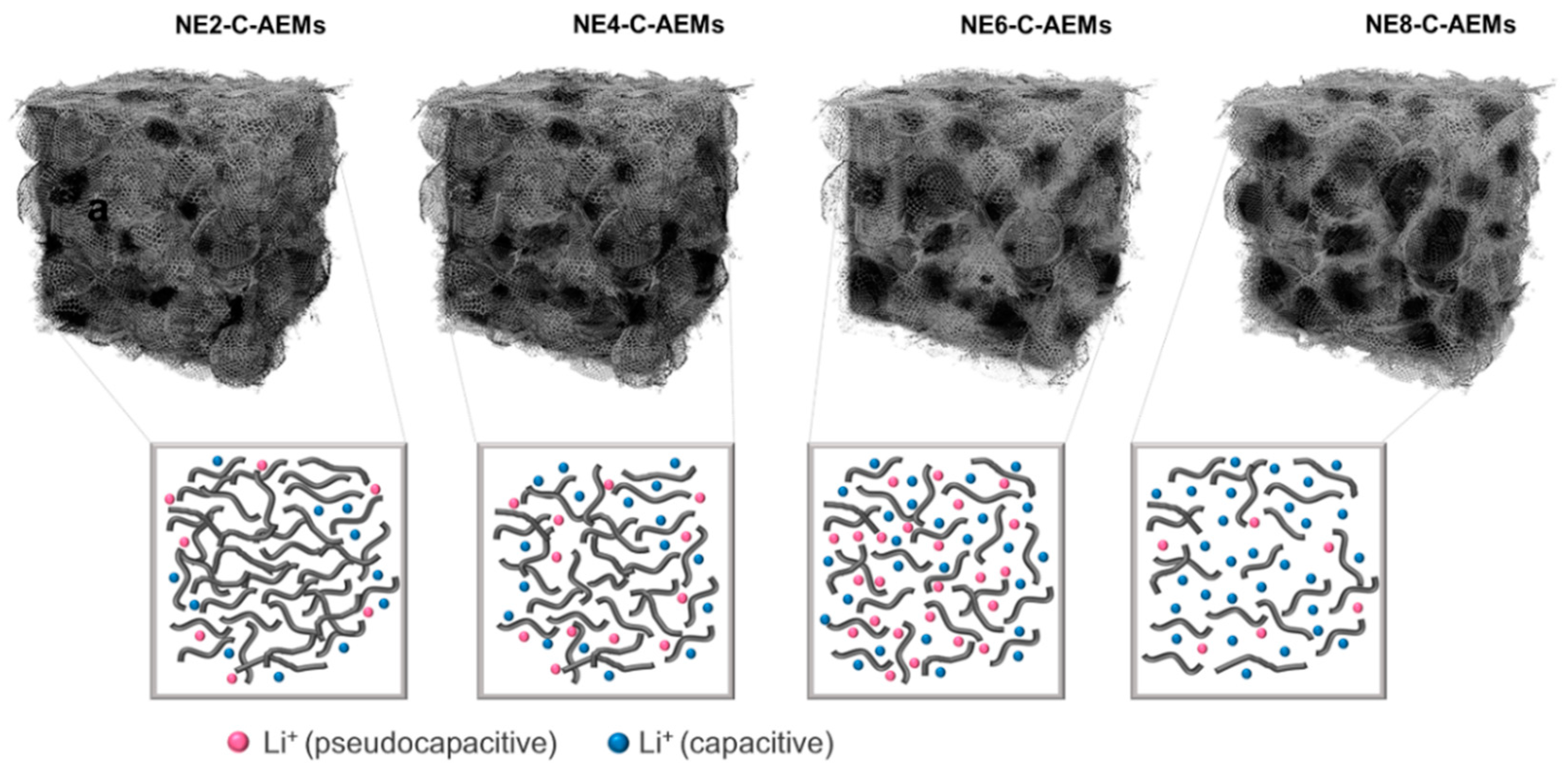
| Sample | a SBET | b VTot | c SMic | d SMeso | e VMic | f APS | g APR |
|---|---|---|---|---|---|---|---|
| NE2-C-AEMs | 2566.2 | 1.09 | 2320.7 | 245.5 | 0.94 | 23.38 | 3.65 |
| NE4-C-AEMs | 2910 | 1.4 | 2317 | 593 | 0.98 | 20.61 | 4.64 |
| NE6-C-AEMs | 3396.2 | 1.86 | 2143.3 | 1252.9 | 0.91 | 17.66 | 5.84 |
| NE8-C-AEMs | 3651.5 | 2.20 | 2317.5 | 1334 | 1.0 | 16.43 | 6.40 |
| Sample Name | XPS (at.%) | Elemental Analysis (wt.%) | ||||
|---|---|---|---|---|---|---|
| C | O | C/O Ratio | C | O | C/O Ratio | |
| NE2-C-AEMs | 85.6 | 14.4 | 5.9 | 83.3 | 16.2 | 5.1 |
| NE4-C-AEMs | 91.4 | 8.6 | 10.6 | 89.0 | 10.6 | 8.4 |
| NE6-C-AEMs | 95.4 | 6.4 | 15.1 | 91.2 | 8.4 | 10.9 |
| NE8-C-AEMs | 95.7 | 3.4 | 28.1 | 95.4 | 4.2 | 22.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hyun, J.C.; Kwak, J.H.; Lee, M.E.; Choi, J.; Kim, J.; Kim, S.-S.; Yun, Y.S. Intensification of Pseudocapacitance by Nanopore Engineering on Waste-Bamboo-Derived Carbon as a Positive Electrode for Lithium-Ion Batteries. Materials 2019, 12, 2733. https://doi.org/10.3390/ma12172733
Hyun JC, Kwak JH, Lee ME, Choi J, Kim J, Kim S-S, Yun YS. Intensification of Pseudocapacitance by Nanopore Engineering on Waste-Bamboo-Derived Carbon as a Positive Electrode for Lithium-Ion Batteries. Materials. 2019; 12(17):2733. https://doi.org/10.3390/ma12172733
Chicago/Turabian StyleHyun, Jong Chan, Jin Hwan Kwak, Min Eui Lee, Jaewon Choi, Jinsoo Kim, Seung-Soo Kim, and Young Soo Yun. 2019. "Intensification of Pseudocapacitance by Nanopore Engineering on Waste-Bamboo-Derived Carbon as a Positive Electrode for Lithium-Ion Batteries" Materials 12, no. 17: 2733. https://doi.org/10.3390/ma12172733
APA StyleHyun, J. C., Kwak, J. H., Lee, M. E., Choi, J., Kim, J., Kim, S.-S., & Yun, Y. S. (2019). Intensification of Pseudocapacitance by Nanopore Engineering on Waste-Bamboo-Derived Carbon as a Positive Electrode for Lithium-Ion Batteries. Materials, 12(17), 2733. https://doi.org/10.3390/ma12172733








