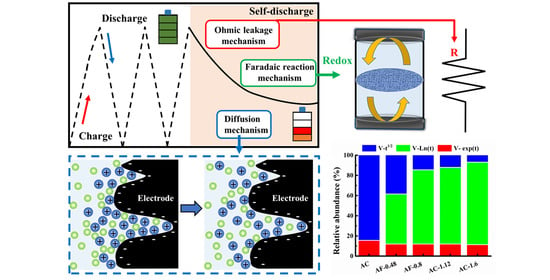
Effects of Fe Impurities on Self-Discharge Performance of Carbon-Based Supercapacitors
Abstract
1. Introduction
2. Experimental
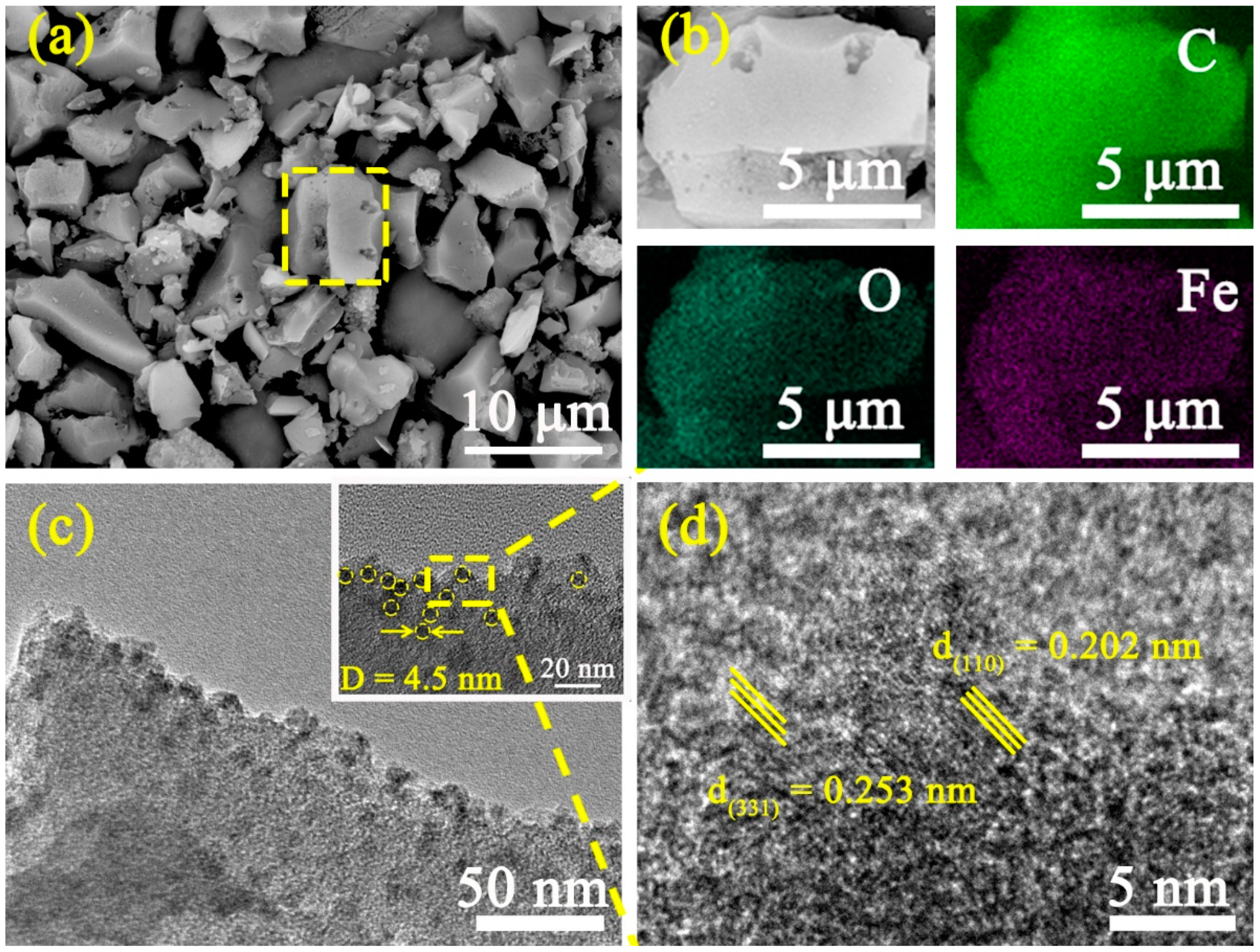
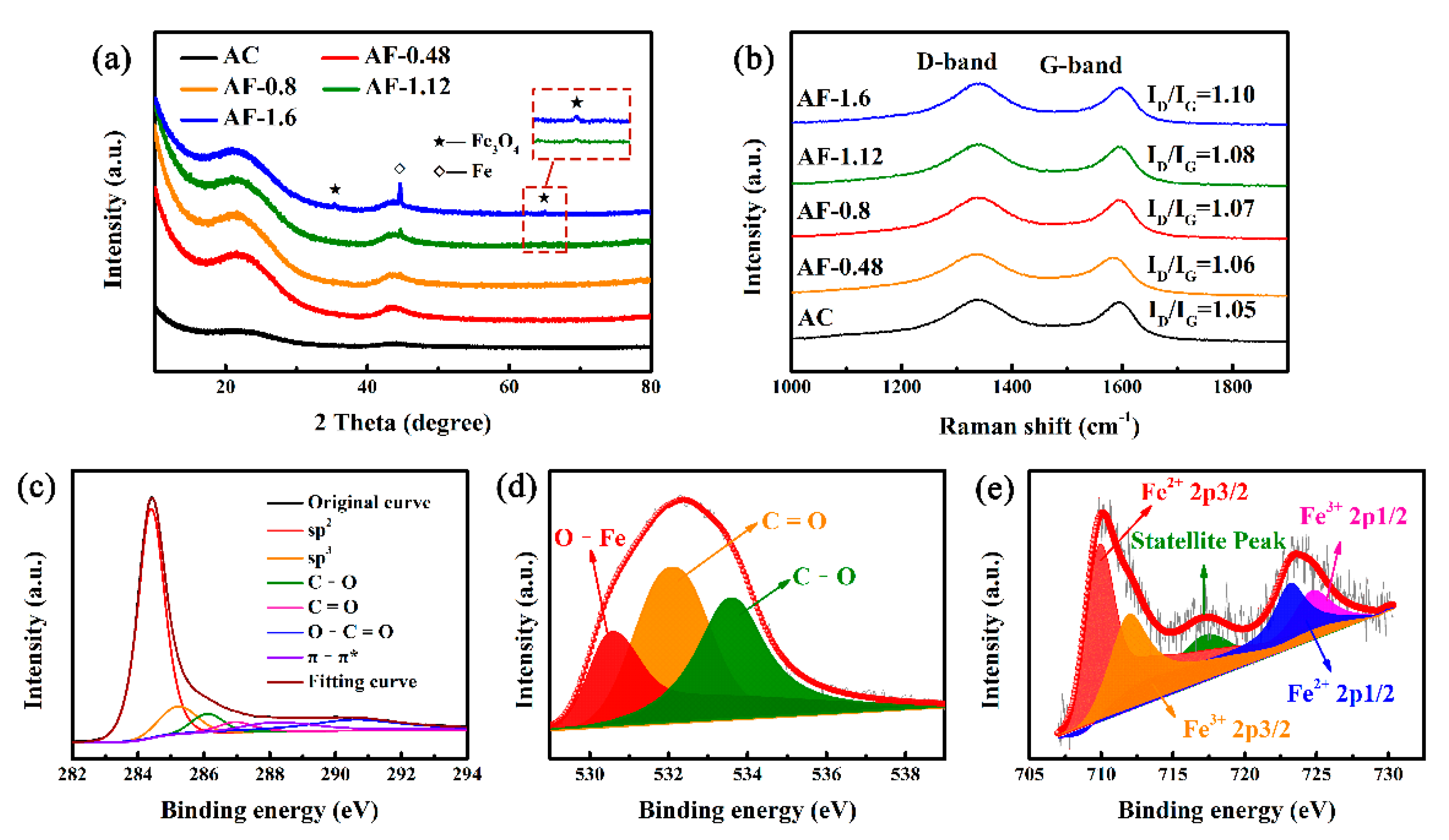
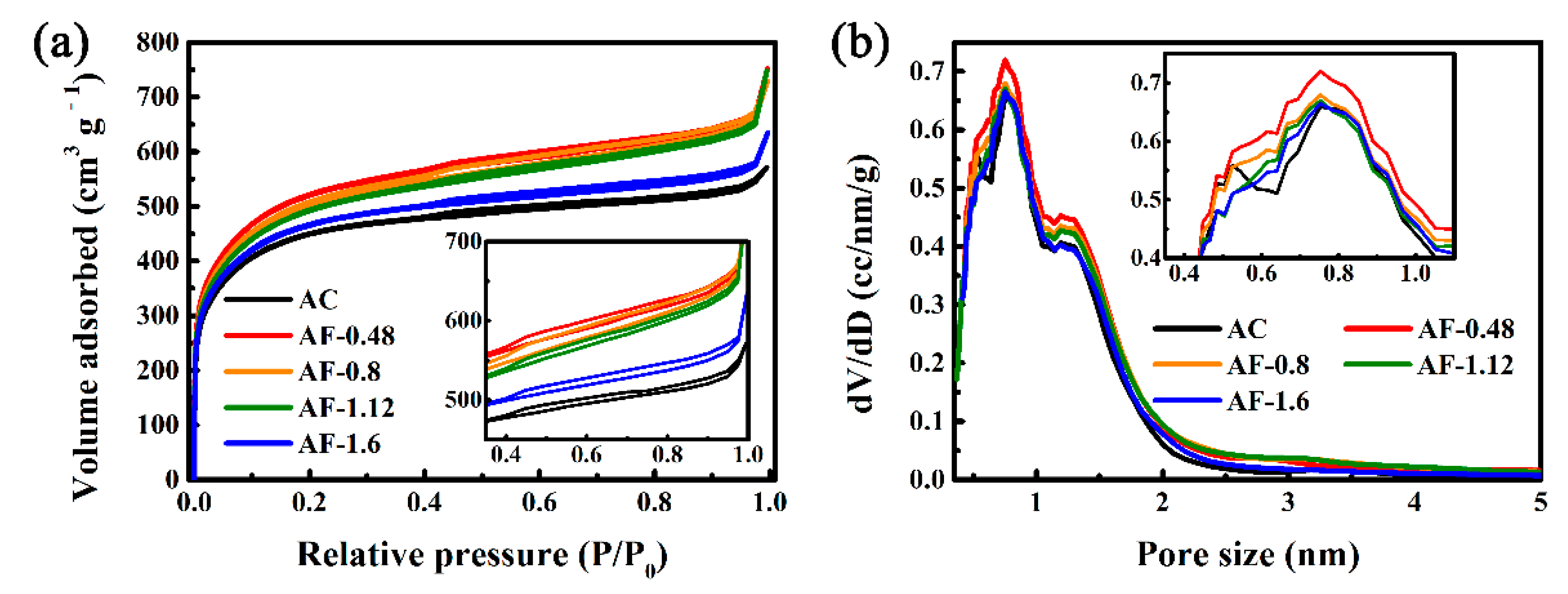
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yuan, S.; Huang, X.; Wang, H.; Xie, L.; Cheng, J.; Kong, Q.; Sun, G.; Chen, C.-M. Structure evolution of oxygen removal from porous carbon for optimizing supercapacitor performance. J. Energy Chem. 2020, 51, 396–404. [Google Scholar] [CrossRef]
- Wang, B.; Wang, C.; Hu, Q.; Zhang, L.; Wang, Z. Modeling the dynamic self-discharge effects of supercapacitors using a con-trolled current source based ladder equivalent circuit. J. Energy Storage 2020, 30, 101473–101490. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, X.; Tong, X.; Chen, Y.; Duan, M.; Shi, J.; Jiang, C.; Hu, L.; Kong, Q.; Zhang, J. High-performance battery-type supercapacitor based on porous biocarbon and biocarbon supported Ni–Co layered double hydroxide. J. Alloys Compd. 2020, 837, 155529. [Google Scholar] [CrossRef]
- Sarno, M.; Baldino, L.; Scudieri, C.; Cardea, S.; Reverchon, E. A one-step SC-CO2 assisted technique to produce compact PVDF-HFP MoS2 supercapacitor device. J. Phys. Chem. Solids 2020, 136, 109132. [Google Scholar] [CrossRef]
- Qian, Y.; Jiang, S.; Li, Y.; Yi, Z.; Zhou, J.; Tian, J.; Lin, N.; Qian, Y. Understanding mesopore volume-enhanced extra-capacity: Optimizing mesoporous carbon for high-rate and long-life potassium-storage. Energy Storage Mater. 2020, 29, 341–349. [Google Scholar] [CrossRef]
- Yong, S.; Owen, J.; Beeby, S. Solid-State Supercapacitor Fabricated in a Single Woven Textile Layer for E-Textiles Applications. Adv. Eng. Mater. 2018, 20, 1700860. [Google Scholar] [CrossRef]
- Lewandowski, A.; Jakobczyk, P.; Galinski, M.; Biegun, M. Self-discharge of electrochemical double layer capacitors. Phys. Chem. Chem. Phys. 2013, 15, 8692–8699. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Y. Self-discharge analysis and characterization of supercapacitors for environmentally powered wireless sensor network applications. J. Power Sources 2011, 196, 8866–8873. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Zhang, P.; Sun, L.; Ren, X.; Mi, H. Hierarchical hollow carbon spheres: Novel synthesis strategy, pore structure engineering and application for micro-supercapacitor. Carbon 2020, 157, 70–79. [Google Scholar] [CrossRef]
- Wu, C.-W.; Unnikrishnan, B.; Chen, I.-W.P.; Harroun, S.G.; Chang, H.-T.; Huang, C.-C. Excellent oxidation resistive MXene aqueous ink for micro-supercapacitor application. Energy Storage Mater. 2020, 25, 563–571. [Google Scholar] [CrossRef]
- Pan, Z.; Yang, J.; Li, L.; Gao, X.; Kang, L.; Zhang, Y.; Zhang, Q.; Kou, Z.; Zhang, T.; Wei, L.; et al. All-in-one stretchable coaxial-fiber strain sensor integrated with high-performing supercapacitor. Energy Storage Mater. 2020, 25, 124–130. [Google Scholar] [CrossRef]
- Wang, Z.; Chu, X.; Xu, Z.; Su, H.; Yan, C.; Liu, F.; Gu, B.; Huang, H.; Xiong, D.; Zhang, H.; et al. Extremely low self-discharge solid-state supercapacitors via the confinement effect of ion transfer. J. Mater. Chem. A 2019, 7, 8633–8640. [Google Scholar] [CrossRef]
- Wang, Y.; Shan, X.; Wang, D.; Cheng, H.; Li, F. Mitigating self-discharge of carbon based electrochemical capacitors by modi-fying their electric double layer to maximize energy efficiency. J. Energy Chem. 2019, 38, 214–218. [Google Scholar] [CrossRef]
- Davis, M.; Andreas, H. Identification and isolation of carbon oxidation and charge redistribution as self-discharge mechanisms in reduced graphene oxide electrochemical capacitor electrodes. Carbon 2018, 139, 299–308. [Google Scholar] [CrossRef]
- Zhu, Z.; Yin, H.; Wang, Y.; Chuang, C.; Xing, L.; Dong, M.; Lu, Y.; Casillas-Garcia, G.; Zheng, Y.; Chen, S.; et al. Coexisting Single-Atomic Fe and Ni Sites on Hierarchically Ordered Porous Carbon as a Highly Efficient ORR Electrocatalyst. Adv. Mater. 2020, 32, 2004670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liao, H.; Liu, X.; Shang, R.; Zhou, Y.; Zhou, Y. Facile synthesis of Fe2O3 nanospheres anchored on oxidized graphitic carbon nitride as a high performance electrode material for supercapacitors. Int. J. Electrochem. Sci. 2020, 15, 2133–2144. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Xu, W.; Wang, Y.; Zhang, B.; Luo, S.; Zhou, X.; Zhang, C.; Gu, X.; Hu, C. Porous Fe2O3 nanospheres anchored on activated carbon cloth for high-performance symmetric supercapacitors. Nano Energy 2019, 57, 379–387. [Google Scholar] [CrossRef]
- Oickle, A.M.; Tom, J.; Andreas, H.A. Carbon oxidation and its influence on self-discharge in aqueous electrochemical capac-itors. Carbon 2016, 110, 232–242. [Google Scholar] [CrossRef]
- Ike, I.S.; Sigalas, I.; Iyuke, S. Understanding performance limitation and suppression of leakage current or self-discharge in electrochemical capacitors: A review. Phys. Chem. Chem. Phys. 2016, 18, 661–680. [Google Scholar] [CrossRef]
- Andreas, H.A. Self-Discharge in Electrochemical Capacitors: A Perspective Article. J. Electrochem. Soc. 2015, 162, A5047–A5053. [Google Scholar] [CrossRef]
- Andreas, H.A.; Black, J.M.; Oickle, A.A. Self-discharge in Manganese Oxide Electrochemical Capacitor Electrodes in Aqueous Electrolytes with Comparisons to Faradaic and Charge Redistribution Models. Electrochim. Acta 2014, 140, 116–124. [Google Scholar] [CrossRef]
- Zhang, Q.; Rong, J.; Wei, B. A divided potential driving self-discharge process for single walled carbon nanotube based su-percapacitors. RSC Adv. 2011, 1, 989–994. [Google Scholar] [CrossRef]
- Anantharaj, S.; Kundu, S.; Noda, S. The Fe Effect: A review unveiling the critical roles of Fe in enhancing OER activity of Ni and Co based catalysts. Nano Energy 2021, 80, 105514. [Google Scholar] [CrossRef]
- Zhang, Q.; Cai, C.; Qin, J.; Wei, B. Tunable self-discharge process of carbon nanotube based supercapacitors. Nano Energy 2014, 4, 14–22. [Google Scholar] [CrossRef]
- Andreas, H.A.; Lussier, K.; Oickle, A.M. Effect of Fe contamination on rate of self-discharge in carbon-based aqueous elec-trochemical capacitors. J. Power Sources 2009, 187, 275–283. [Google Scholar] [CrossRef]
- Hess, L.; Fulik, N.; Röhner, J.; Zhang, E.; Kaskel, S.; Brunner, E.; Balducci, A. The role of diffusion processes in the self-discharge of electrochemical capacitors. Energy Storage Mater. 2021, 37, 501–508. [Google Scholar] [CrossRef]
- Chung, J.; Park, H.; Jung, C. Electropolymerizable isocyanate-based electrolytic additive to mitigate diffusion-controlled self-discharge for highly stable and capacitive activated carbon supercapacitors. Electrochim. Acta 2021, 369, 137698. [Google Scholar] [CrossRef]
- Wang, S.; Liu, X.; Duan, H.; Deng, Y.; Chen, G. Fe3C/Fe nanoparticles embedded in N-doped porous carbon nanosheets and graphene: A thin functional interlayer for PP separator to boost performance of Li-S batteries. Chem. Eng. J. 2021, 415, 129001. [Google Scholar] [CrossRef]
- Liao, B.; Li, H.; Xu, M.; Xing, L.; Liao, Y.; Ren, X.; Fan, W.; Yu, L.; Xu, K.; Li, W. Designing Low Impedance Interface Films Simultaneously on Anode and Cathode for High Energy Batteries. Adv. Energy Mater. 2018, 8, 1800802. [Google Scholar] [CrossRef]
- Luo, X.; Chen, S.; Hu, T.; Chen, Y.; Li, F. Renewable biomass-derived carbons for electrochemical capacitor applications. Sustain. Mater. 2021. [Google Scholar] [CrossRef]
- Deng, Y.; Zheng, Y.; Zhang, D.; Han, C.; Cheng, A.; Shen, J.; Zeng, G.; Zhang, H. A novel and facile to synthesize three di-mensional honeycomb like nano-Fe3O4@C composite: Electromagnetic wave absorption with wide bandwidth. Carbon 2020, 169, 118–128. [Google Scholar] [CrossRef]
- Li, Z.; Lin, H.; Ding, S.; Ling, H.; Wang, T.; Miao, Z.; Zhang, M.; Meng, A.; Li, Q. Synthesis and enhanced electromagnetic wave absorption performances of Fe3O4@C decorated walnut shell-derived porous carbon. Carbon 2020, 167, 148–159. [Google Scholar] [CrossRef]
- Meng, X.; Liu, Y.; Han, G.; Yang, W.; Yu, Y. Three dimensional (Fe3O4/ZnO)@C Double core@shell porous nanocomposites with enhanced broadband microwave absorption. Carbon 2020, 162, 356–364. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Zhou, C.; Wang, C.; Ba, X.; Li, Y.; Huang, X.; Liu, J. Carbon-stabilized high-capacity ferroferric oxide nanorod array for flexible solid-state alkaline battery-supercapacitor hybrid device with high environmental suitability. Adv. Funct. Mater. 2015, 25, 5384–5394. [Google Scholar] [CrossRef]
- Peng, Z.; Hu, Y.; Wang, J.; Liu, S.; Li, C.; Jiang, Q.; Lu, J.; Zeng, X.; Peng, P.; Li, F. Fullerene-based in situ doping of N and Fe into a 3D cross-like hierarchical carbon composite for high-performance supercapacitors. Adv. Energy Mater. 2019, 9, 1802928–1802938. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Lee, D.; Yoon, M. Bioinspired 2D-Carbon flakes and Fe3O4 nanoparticles composite for arsenite removal. ACS Appl. Mater. Interfaces 2016, 8, 23876–23885. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, X.; Zhang, Y.X.; Qian, X. Fe2O3/RGO/Fe3O4 composite in situ grown on Fe foil for high performance su-percapacitors. ACS Appl. Mater. Interfaces 2016, 8, 30133–30142. [Google Scholar] [CrossRef]
- Ren, Y.-L.; Wu, H.-Y.; Lu, M.-M.; Chen, Y.-J.; Zhu, C.-L.; Gao, P.; Cao, M.-S.; Li, C.-Y.; Ouyang, Q.-Y. Quaternary Nanocomposites Consisting of Graphene, Fe3O4@Fe Core@Shell, and ZnO Nanoparticles: Synthesis and Excellent Electromagnetic Absorption Properties. ACS Appl. Mater. Interfaces 2012, 4, 6436–6442. [Google Scholar] [CrossRef]
- Niu, Y.; Li, X.; Dong, W.; Zhang, C.; Zhao, K.; Wang, F.; Wang, H. Synthesis of N-doped carbon with embedded Fe/Fe3C particles for microwave absorption. J. Mater. Sci. 2020, 55, 11970–11983. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, C.; Zhang, M.; Feng, A.; Qu, S.L.; Zhang, Y.; Liu, X.; Jia, Z.; Wu, G. Synthesis of Fe3O4/carbon foams com-posites with broadened bandwidth and excellent electromagnetic wave absorption performance. Compos. Part A Appl. Sci. Manuf. 2019, 127, 105627. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y.; Wu, H.; Qian, X.; Chen, L.; Dan, Y.; Yu, Q. Porous Fe3O4/C nanoaggregates by the carbon polyhedrons as tem-plates derived from metal organic framework as battery type materials for supercapacitors. Electrochim. Acta 2020, 337, 135818. [Google Scholar] [CrossRef]
- Cao, Z.; Ma, X. Encapsulated Fe3O4 into tubular mesoporous carbon as a superior performance anode material for lithium-ion batteries. J. Alloys Compd. 2020, 815, 152542. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, C.; Niu, T.; Huang, R.; Li, S.; Zhang, J.Z.; Chen, J.G. Controlled Synthesis of Fe3O4 Nanospheres Coated with Nitrogen-Doped Carbon for High Performance Supercapacitors. ACS Appl. Energy Mater. 2018, 1, 4599–4605. [Google Scholar] [CrossRef]
- Lee, J.S.; Shin, D.H.; Jun, J.; Lee, C.; Jang, J. Fe3O4/Carbon Hybrid Nanoparticle Electrodes for High-Capacity Electrochemical Capacitors. ChemSusChem 2014, 7, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.-Y.; Chen, Y.; Mo, Y. A review of charge storage in porous carbon-based supercapacitors. New Carbon Mater. 2021, 36, 49–68. [Google Scholar] [CrossRef]
- Kang, N.; Ji, L.; Zhao, J.; Zhou, X.; Weng, X.; Li, H.; Zhang, X.; Yang, F. Uniform growth of Fe3O4 nanocubes on the single-walled carbon nanotubes as an electrosensor of organic dyes and the study on its catalytic mechanism. J. Electroanal. Chem. 2019, 833, 70–78. [Google Scholar] [CrossRef]
- Liu, X.; Tian, J.; Li, Y.; Sun, N.; Mi, S.; Xie, Y.; Chen, Z. Enhanced dyes adsorption from wastewater via Fe3O4 nanopar-ticles functionalized activated carbon. J. Hazard. Mater. 2019, 373, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jia, M.; Zhu, Q.; Cao, B.; Chen, R.; Wang, Y.; Wu, F.; Xu, B. 3D-0D Graphene-Fe3O4 Quantum Dot Hybrids as High-Performance Anode Materials for Sodium-Ion Batteries. ACS Appl. Mater. Interfaces 2016, 8, 26878–26885. [Google Scholar] [CrossRef]
- Wang, L.; Yu, J.; Dong, X.; Li, X.; Xie, Y.; Chen, S.; Li, P.; Hou, H.; Song, Y. Three-Dimensional Macroporous Carbon/Fe3O4-Doped Porous Carbon Nanorods for High-Performance Supercapacitor. ACS Sustain. Chem. Eng. 2015, 4, 1531–1537. [Google Scholar] [CrossRef]
- Yan, J.; Leng, Y.C.; Guo, Y.; Wang, G.; Gong, H.; Guo, P.; Tan, P.; Long, Y.Z.; Liu, X.; Han, W. Highly conductive graphene paper with vertically aligned reduced graphene oxide sheets fabricated by improved electrospray deposition technique. ACS Appl. Mater. Interfaces 2019, 11, 10810–10817. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Wang, H.; Qu, Z.; Wang, K.; Wang, L.; Gao, J.; Gao, J.; Liu, S.; Lu, Y. Carboxyl dominant oxygen rich carbon for im-proved sodium ion storage: Synergistic enhancement of adsorption and intercalation mechanisms. ACS Appl. Eenergy Mater. 2021, 11, 2002981. [Google Scholar]
- Sun, F.; Qu, Z.; Gao, J.; Wu, H.B.; Liu, F.; Han, R.; Wang, L.; Pei, T.; Zhao, G.; Lu, Y. In situ doping boron atoms into porous carbon nanoparticles with increased oxygen graft enhances both affinity and durability toward electrolyte for greatly improved super-capacitive performance. Adv. Funct. Mater. 2018, 28, 1804190–1804200. [Google Scholar] [CrossRef]
- Li, Z.N.; Gadipelli, S.; Li, H.C.; Howard, C.A.; Brett, D.J.L.; Shearing, P.R.; Guo, Z.; Parkin, I.P.; Li, F. Tuning the interlayer spacing of graphene laminate films for efficient pore utilization towards compact capacitive energy storage. Nat. Energy 2020, 5, 160–168. [Google Scholar] [CrossRef]
- Li, X.R.; Jiang, Y.H.; Wang, P.Z.; Mo, Y.; Lai, W.D.; Li, Z.J.; Yu, R.J.; Du, Y.T.; Zhang, X.R.; Chen, Y. Effect of the oxygen functional groups of activated carbon on its electrochemical performance for supercapacitors. N. Carbon Mater. 2020, 35, 232–243. [Google Scholar] [CrossRef]
- Fan, H.; Niu, R.; Duan, J.; Liu, W.; Shen, W. Fe3O4@Carbon Nanosheets for All-Solid-State Supercapacitor Electrodes. ACS Appl. Mater. Interfaces 2016, 8, 19475–19483. [Google Scholar] [CrossRef] [PubMed]
- Nawwar, M.; Poon, R.; Chen, R.; Sahu, R.P.; Puri, I.K.; Zhitomirsky, I. High areal capacitance of Fe3O4-decorated carbon nanotubes for supercapacitor electrodes. Carbon Energy 2019, 1, 124–133. [Google Scholar] [CrossRef]
- Sun, J.; Zan, P.; Yang, X.; Ye, L.; Zhao, L. Room-temperature synthesis of Fe3O4/Fe-carbon nanocomposites with Fe-carbon double conductive network as supercapacitor. Electrochim. Acta 2016, 215, 483–491. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, R.K.; Vaz, A.R.; Savu, R.; Moshkalev, S.A. Self-Assembled and One-Step Synthesis of Interconnected 3D Network of Fe3O4/Reduced Graphene Oxide Nanosheets Hybrid for High-Performance Supercapacitor Electrode. ACS Appl. Mater. Interfaces 2017, 9, 8880–8890. [Google Scholar] [CrossRef]
- Jiao, L.; Wan, G.; Zhang, R.; Zhou, H.; Yu, S.; Jiang, H. From Metal organic frameworks to single atom Fe implanted N doped porous carbons: Efficient oxygen reduction in both alkaline and acidic media. Angew. Chem. Int. Ed. 2018, 57, 8525–8529. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, Y.; Pei, Z.; Wu, K.-H.; Tan, C.; Wang, H.; Wei, L.; Mahmood, A.; Yan, C.; Dong, J.; et al. Recent Progress of Carbon-Supported Single-Atom Catalysts for Energy Conversion and Storage. Matter 2020, 3, 1442–1476. [Google Scholar] [CrossRef]
- Li, Q.-K.; Li, X.-F.; Zhang, G.; Jiang, J. Cooperative Spin Transition of Monodispersed FeN3 Sites within Graphene Induced by CO Adsorption. J. Am. Chem. Soc. 2018, 140, 15149–15152. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhang, Y.; Huang, L.-B.; Liu, X.-Z.; Zhang, Q.-H.; He, C.; Wu, Z.-Y.; Zhang, L.-J.; Wu, J.; Yang, W.; et al. Cascade anchoring strategy for general mass production of high-loading single-atomic metal-nitrogen catalysts. Nat. Commun. 2019, 10, 1278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Rong, J.; Peng, M.D.; Wei, B. The governing self-discharge processes in activated carbon fabric based superca-pacitors with different organic electrolytes. Energy Environ. Sci. 2011, 4, 2152. [Google Scholar] [CrossRef]
- Xia, M.; Nie, J.; Zhang, Z.; Lu, X.; Wang, Z.L. Suppressing self-discharge of supercapacitors via electrorheological effect of liquid crystals. Nano Energy 2018, 47, 43–50. [Google Scholar] [CrossRef]
- Ricketts, B.; Ton-That, C. Self-discharge of carbon-based supercapacitors with organic electrolytes. J. Power Sources 2000, 89, 64–69. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Mo, Y.; Chen, Y. Effects of Fe Impurities on Self-Discharge Performance of Carbon-Based Supercapacitors. Materials 2021, 14, 1908. https://doi.org/10.3390/ma14081908
Du Y, Mo Y, Chen Y. Effects of Fe Impurities on Self-Discharge Performance of Carbon-Based Supercapacitors. Materials. 2021; 14(8):1908. https://doi.org/10.3390/ma14081908
Chicago/Turabian StyleDu, Yuting, Yan Mo, and Yong Chen. 2021. "Effects of Fe Impurities on Self-Discharge Performance of Carbon-Based Supercapacitors" Materials 14, no. 8: 1908. https://doi.org/10.3390/ma14081908
APA StyleDu, Y., Mo, Y., & Chen, Y. (2021). Effects of Fe Impurities on Self-Discharge Performance of Carbon-Based Supercapacitors. Materials, 14(8), 1908. https://doi.org/10.3390/ma14081908