Evolution of Oxygen–Ion and Proton Conductivity in Ca-Doped Ln2Zr2O7 (Ln = Sm, Gd), Located Near Pyrochlore–Fluorite Phase Boundary
Abstract
1. Introduction
2. Experimental Part
3. Results and Discussion
3.1. Structure of Sm2−xCaxZr2O7−x/2 (x = 0, 0.05, 0.1) and Gd2−xCaxZr2O7- x/2 (x = 0.05, 0.1) Studied by XRD with Rietveld Refinement
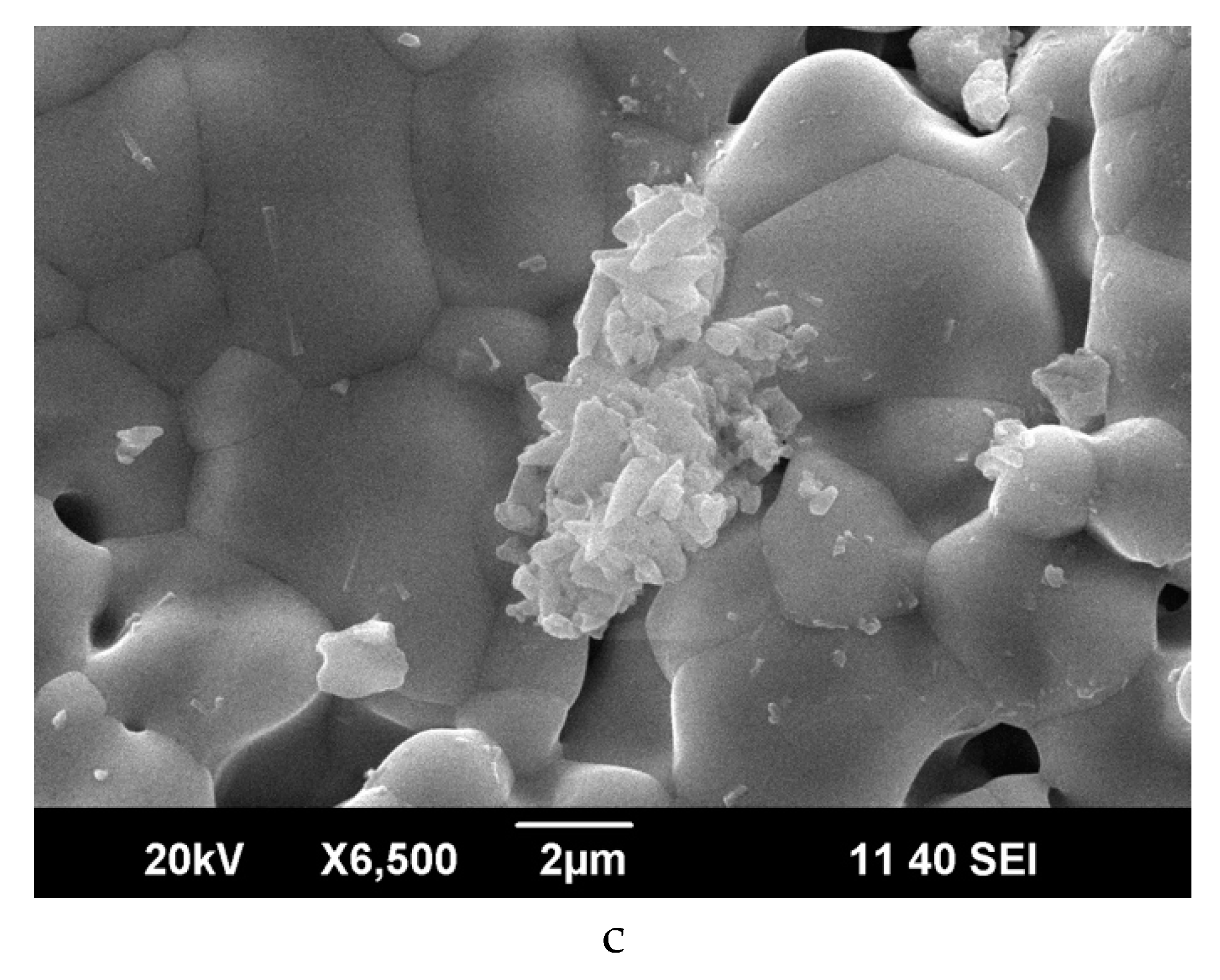
3.2. Microstructure of Sm2−xCaxZr2O7−x/2 (x = 0, 0.1)
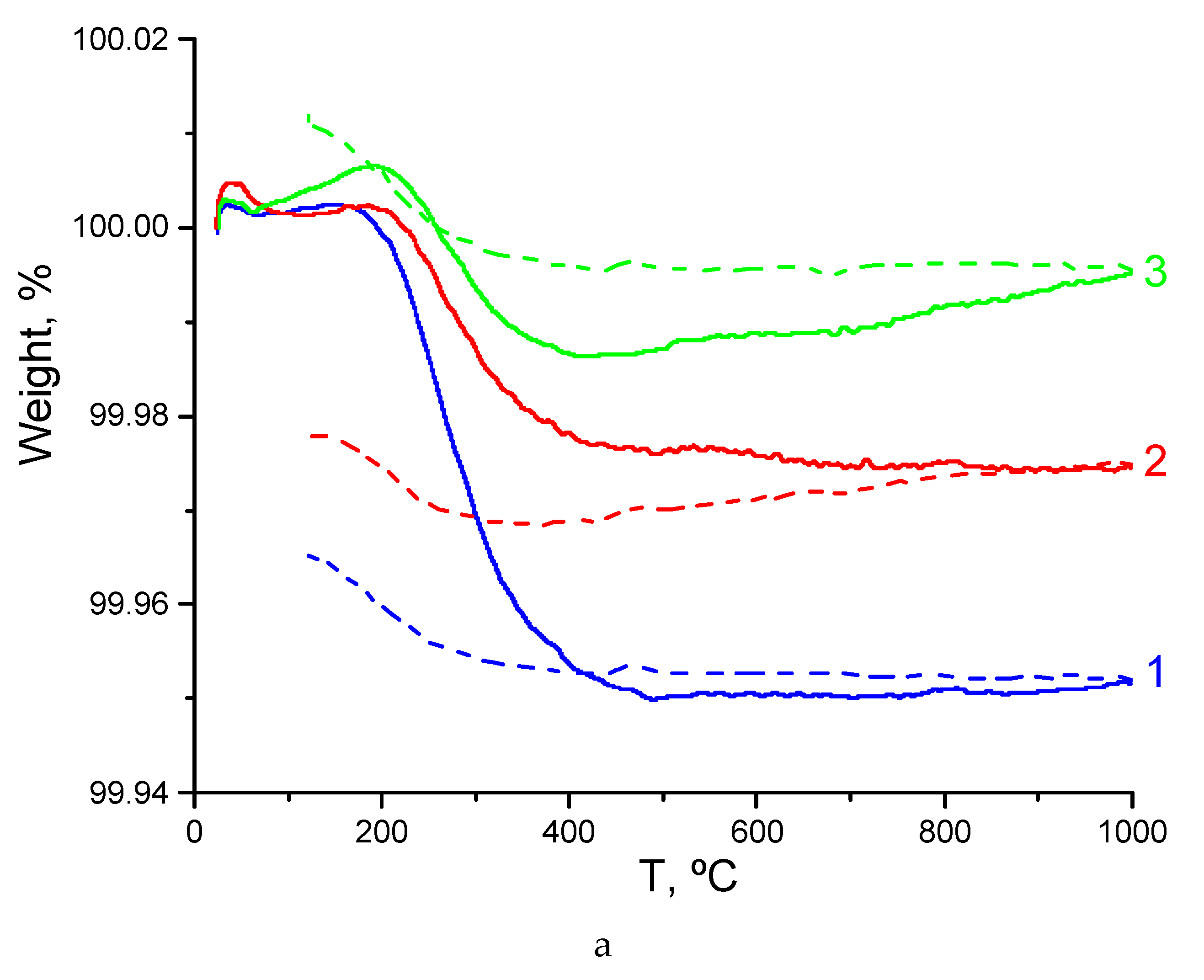
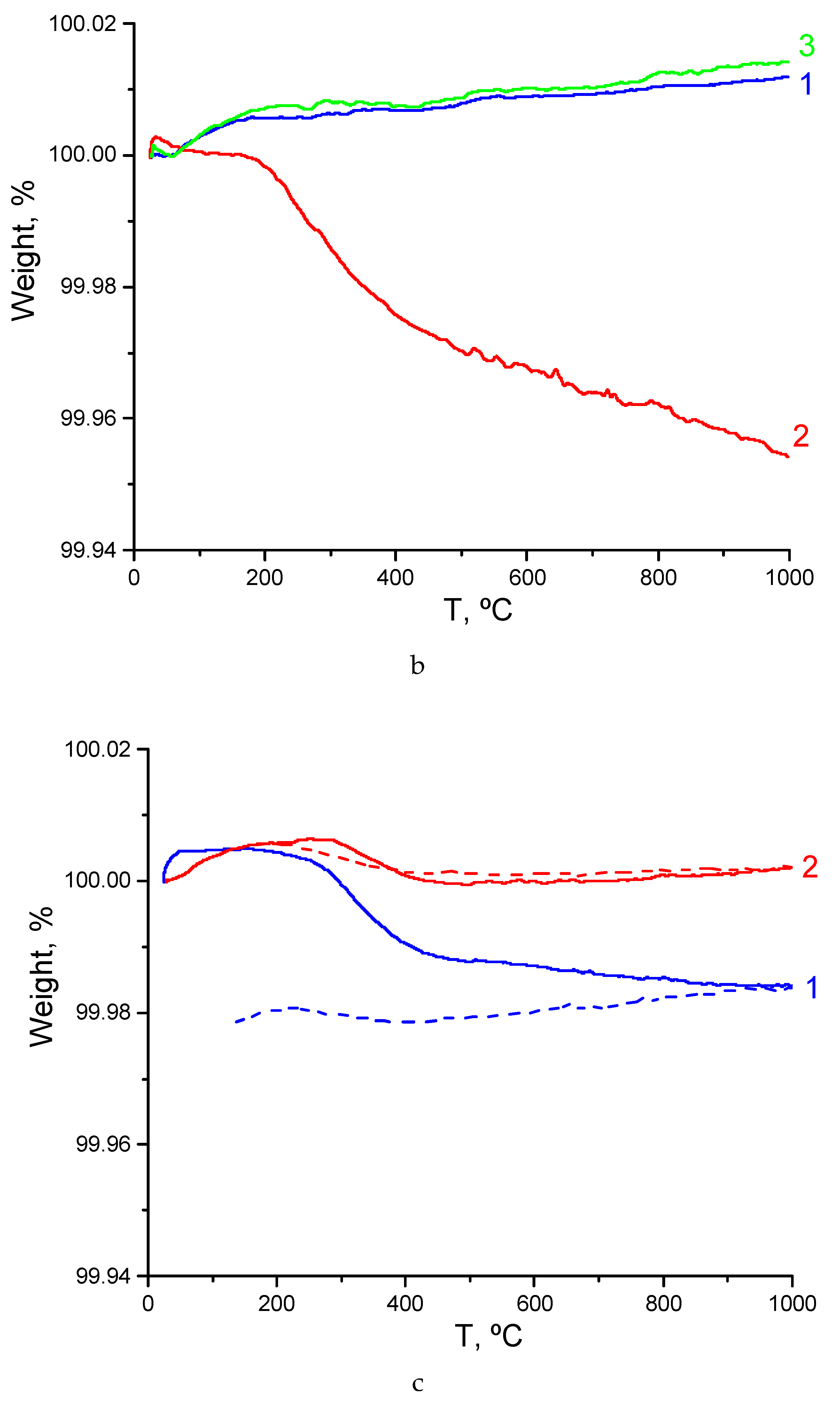
3.3. TG Characterization of Sm2−xCaxZr2O7- x/2 (x = 0, 0.1) and Gd2−xCaxZr2O7- x/2 (x = 0.1)
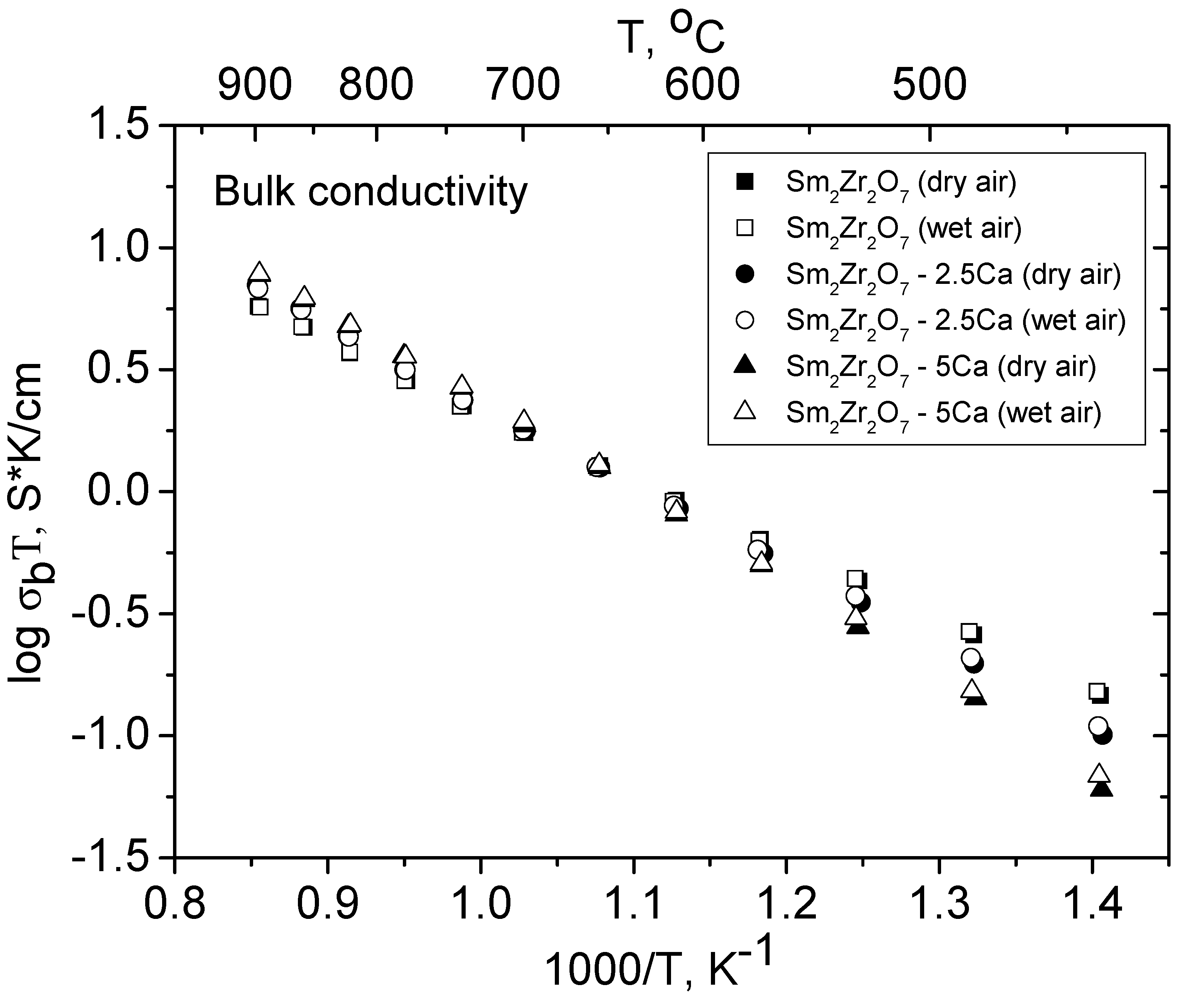
3.4. Conductivity of the Sm2−xCaxZr2O7- x/2 (x = 0, 0.05, 0.1) Solid Solutions in Dry and Wet Air
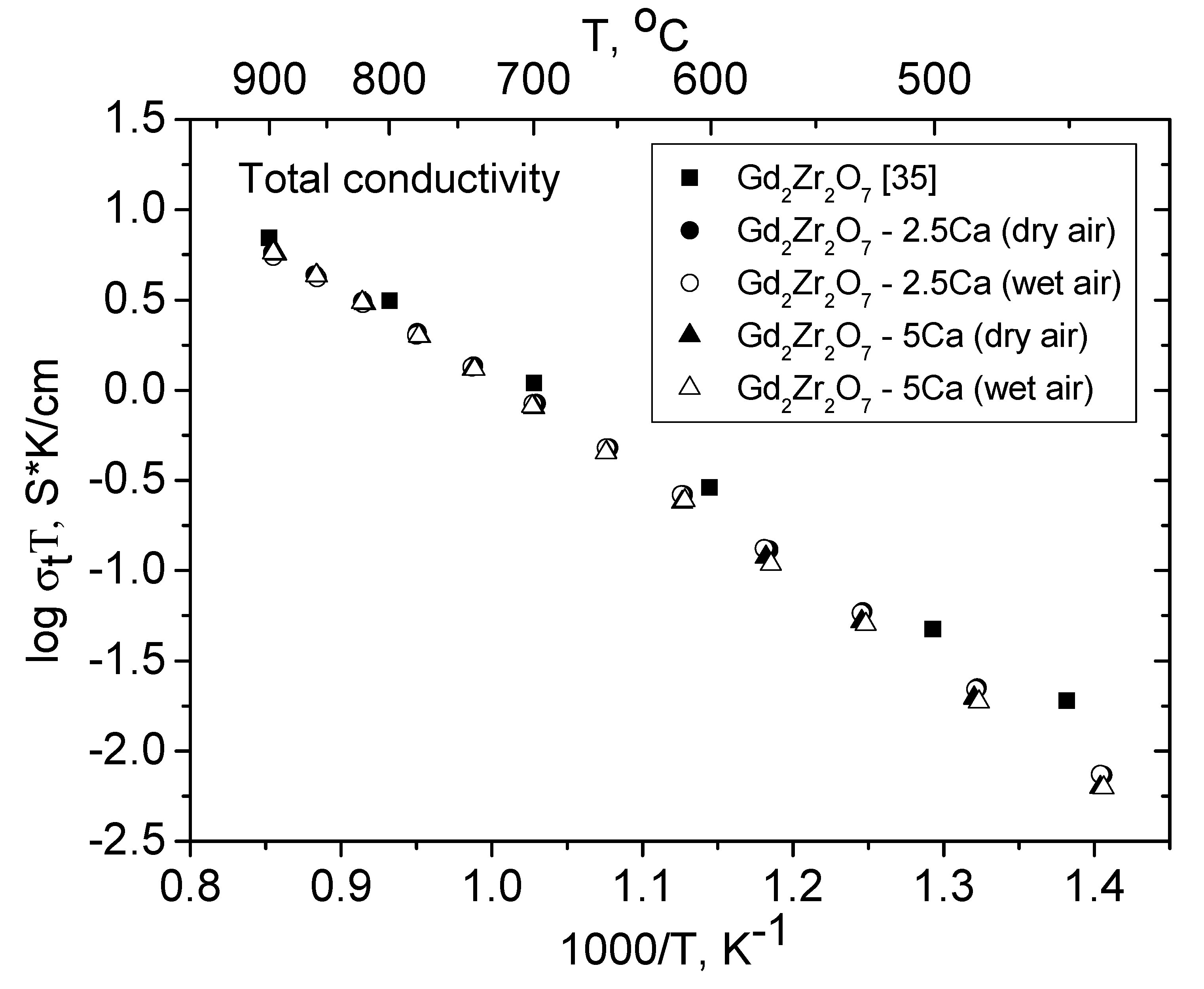
3.5. Conductivity of the Gd2−xCaxZr2O7−x/2 (x = 0.05, 0.1) Solid Solutions in Dry and Wet Air
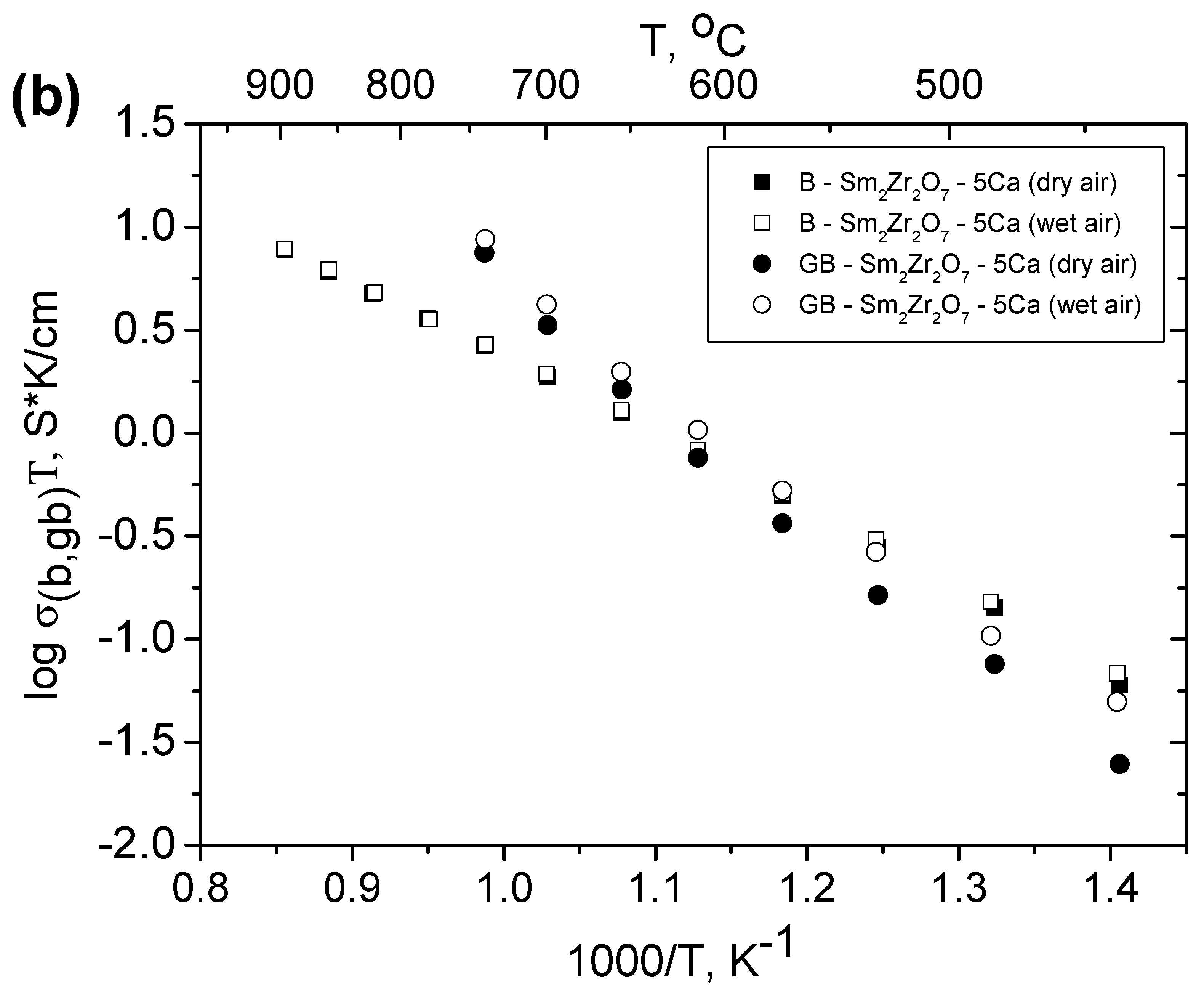
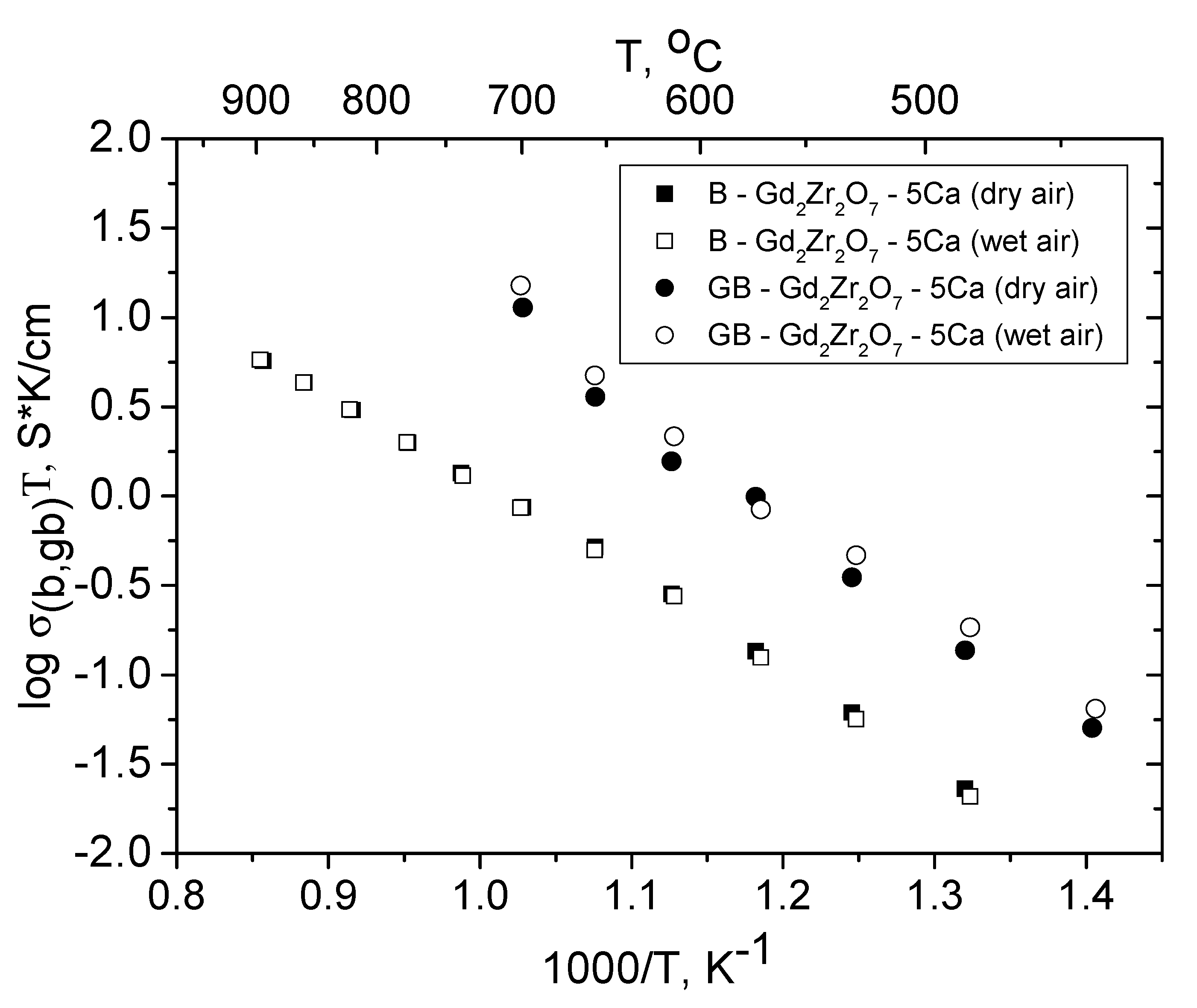
3.6. Relationship between the Grain Boundary and Bulk Conductivities of Ordered and Disordered Pyrochlores
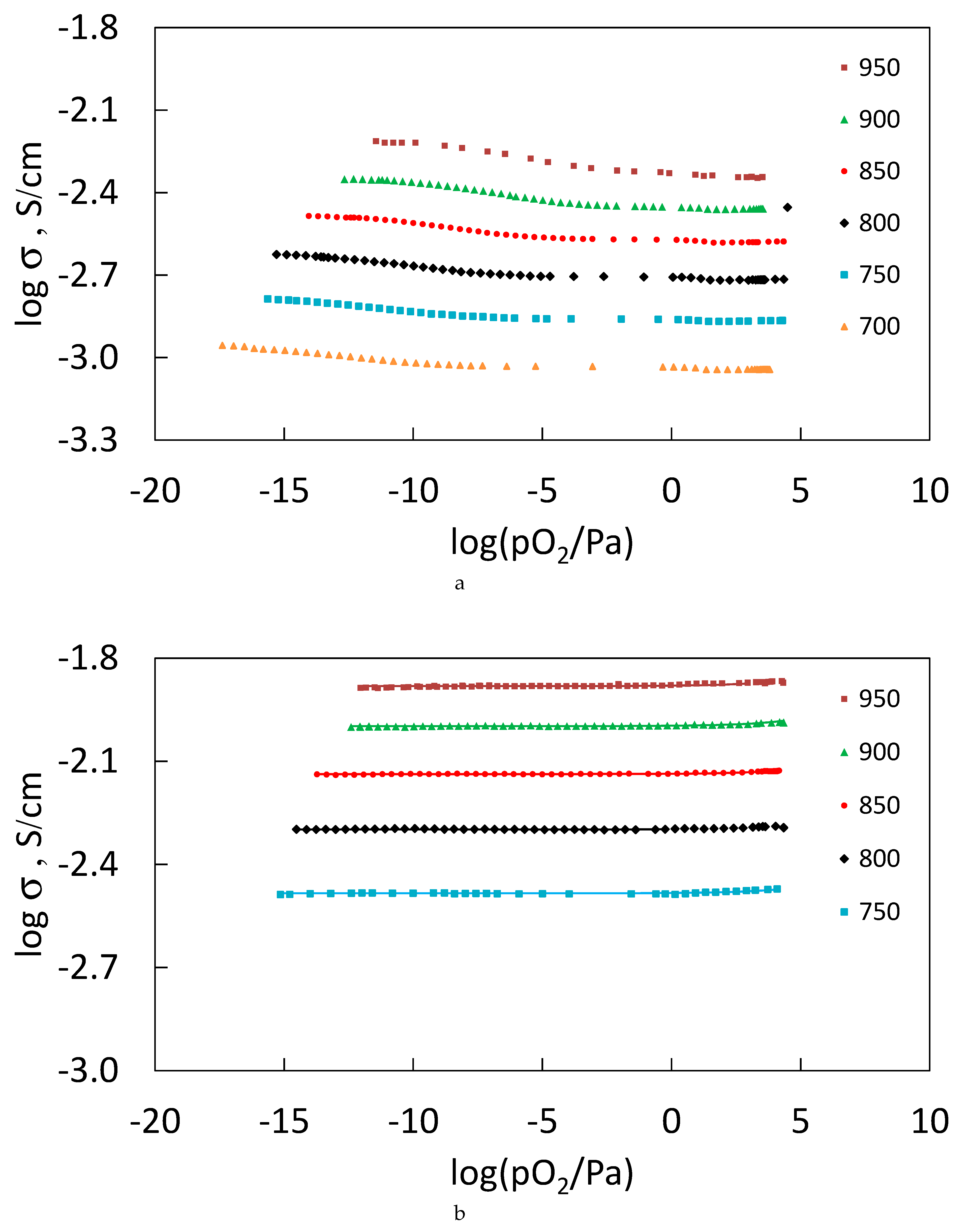
3.7. Oxygen–Ion Conductivity of the Sm2−xCaxZr2O7−x/2 (x = 0.05, 0.1), Gd2−xCaxZr2O7−x/2 (x = 0.1) Solid Solutions in the 700 ≤ T≤ 950 °C Temperature Interval
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yamazaki, Y.; Hernandez-Sanchez, R.; Haile, S.M. Cation non-stoichiometry in yttrium-doped barium zirconate: phase behavior, microstructure, and proton conductivity. J. Mater. Chem. 2010, 20, 8158–8166. [Google Scholar] [CrossRef]
- Wuensch, B.J.; Eberman, K.W.; Heremans, C.; Ku, E.M.; Onnerud, P.; Yeo, E.M.E.; Haile, S.; Stalik, J.K.; Jorgensen, J.D. Connection between oxygen-ion conductivity of pyrochlore fuel-cell materials and structural change with composition and temperature. Solid State Ion. 2000, 129, 111–133. [Google Scholar] [CrossRef]
- Moon, P.K.; Tuller, H.L. Ionic conduction in the Gd2Ti2O7−Gd2Zr2O7 system. Solid State Ion. 1988, 28–30, 470–474. [Google Scholar] [CrossRef]
- Tuller, H.L. Oxygen ion conduction and structural disorder in conductive oxides. J. Phys. Chem. Solids 1994, 55, 1393–1404. [Google Scholar] [CrossRef]
- Heremans, C.; Wuensch, B.J.; Stalick, J.K.; Prince, E. Fast-Ion Conducting Y2(ZryTi1−y)2O7 pyrochlores: Neutron rietveld analysis of disorder induced by Zr substitution. Solid State Chem. 1995, 117, 108–121. [Google Scholar] [CrossRef]
- Yamamura, H.; Nishino, H.; Kakinuma, K.; Nomura, K. Electrical conductivity anomaly around fluorite-pyrochlore phase boundary. Solid State Ion. 2003, 158, 359–365. [Google Scholar] [CrossRef]
- Diaz-Guillen, M.R.; Moreno, K.J.; Diaz-Guillen, J.A.; Fuentes, A.F.; Ngai, K.L. Garcia-Barriocanal, J.; Santamaria, J.; Leon, C. Cation size effects in oxygen ion dynamics of highly disordered pyrochlore-type ionic conductors. Phys. Rev. B 2008, 78, 104304. [Google Scholar] [CrossRef]
- Shlyakhtina, A.V.; Shcherbakova, L.G. Polymorphism and high-temperature conductivity of Ln2M2O7 (Ln = Sm-Lu, M= Ti, Zr, Hf) pyrochlores. Solid State Ion. 2011, 192, 200–204. [Google Scholar] [CrossRef]
- Shlyakhtina, A.V.; Shcherbakova, L.G. New solid electrolytes of the pyrochlore family. Russ. J. Electrochem. 2012, 1, 1–25. [Google Scholar] [CrossRef]
- Shukla, R.; Vasundhara, K.; Krishna, P.S.R.; Shinde, A.B.; Sali, S.K.; Kulkarni, N.K.; Achary, S.N.; Tyagi, A.K. High temperature structural and thermal expansion behavior of pyrochlore-type praseodymium zirconate. Int. J. Hydrogen Energy 2015, 40, 15672–15678. [Google Scholar] [CrossRef]
- Gill, J.K.; Pandey, O.P.; Singh, K. Role of sintering temperature on thermal, electrical and structural properties of Y2Ti2O7 pyrochlores. Int. J. Hydrogen Energy 2011, 36, 14943–14947. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, L.; Fang, X.; Cheng, Q.; Liu, W.; Peng, H.; Gao, Z.; Zhou, W.; Wang, X. Ni/Y2B2O7 (B = Ti, Sn, Zr and Ce) catalysts for methane steam reforming: On the effects of B site replacement. Int. J. Hydrogen Energy 2018, 43, 8298–8312. [Google Scholar] [CrossRef]
- Shlyakhtina, A.V.; Abrantes, J.C.C.; Gomes, E.; Shchegolikhin, A.N.; Vorobieva, G.A.; Maslakov, K.I.; Shcherbakova, L.G. Effect of Pr3+/Pr4+ ratio on the oxygen ion transport and thermomechanical properties of the pyrochlore and fluorite phases in the ZrO2–Pr2O3 system. Int. J. Hydrogen Energy 2016, 41, 9982–9992. [Google Scholar] [CrossRef]
- Valdés-Ibarra, M.R.; Díaz-Guillén, J.A.; Padmasree, K.P.; Montemayor, S.M.; Rodríguez-Varela, F.J.; Fuentes, A.F. Oxygen ion conducting pyrochlore oxides prepared by an ultrasound-assisted wet chemistry route: Ca-doped Gd2Ti2O7 nanocrystals. Int. J. Hydrogen Energy 2019, 44, 12515–12524. [Google Scholar] [CrossRef]
- Shlyakhtina, A.V.; Savvin, S.N.; Levchenko, A.V.; Knotko, A.V.; Fedtke, P.; Busch, A.; Barfels, T.; Wienecke, M.; Shcherbakova, L.G. Study of bulk and grain-boundary conductivity of Ln2+xHf2−xO7-δ (Ln = Sm-Gd; x = 0, 0.096) pyrochlores. J. Electroceram. 2010, 24, 300–307. [Google Scholar] [CrossRef]
- Fedtke, P.; Shlyakhtina, A.V.; Busch, A.; Barfels, T.; Wienecke, M.; Shcherbakova, L.G. Effect of oxygen partial pressure on the bulk and grain-boundary components of conductivity in (Yb1-xCax)2Ti 2O7-δ (x = 0, 0.05, 0.1) solid solutions. Mater. Res. Bull. 2013, 48, 2707–2711. [Google Scholar] [CrossRef]
- Shlyakhtina, A.V.; Belov, D.A.; Stefanovich, S.Y.; Nesterova, E.A.; Karyagina, O.K.; Shcherbakova, L.G. Optimization of synthesis conditions for rare-earth titanate based oxygen ion conductors. Solid State Ion. 2013, 230, 52–58. [Google Scholar] [CrossRef]
- Huo, D.; Baldinozzi, G.; Simeone, D.; Khodja, H.; Surble, S. Grain size—Dependent electrical properties of La1.95Sr0.05Zr2O7-δ as potential proton ceramic fuel cell electrolyte. Solid State Ion. 2016, 298, 35–43. [Google Scholar] [CrossRef]
- van Dijk, M.P.; Mijlhoff, F.C.; Burggraaf, A.J. Pyrochlore microdomain formation in fluorite Oxides. J. Solid State Chem. 1986, 62, 377–385. [Google Scholar] [CrossRef]
- Tsipis, E.V.; Shlyakhtina, A.V.; Shcherbakova, L.G.; Kolbanev, I.V.; Kharton, V.V.; Vyshatko, N.P.; Frade, J.R. Mechanically-Activated Synthesis and Mixed Conductivity of TbMO4−δ (M. = Zr, Hf) Ceramics. J. Electroceram. 2003, 10, 153–164. [Google Scholar] [CrossRef]
- Rejith, R.S.; Thomas, J.K.; Solomon, S. Structural, optical and impedance spectroscopic characterizations of RE2Zr2O7 (RE = La, Y) ceramics. Solid State Ion. 2018, 323, 112–122. [Google Scholar] [CrossRef]
- Labrincha, J.A.; Frade, J.R.; Marques, F.M.B. Protonic conduction in La2Zr2O7—Based pyrochlore materials. Solid State Ion. 1997, 99, 33–40. [Google Scholar] [CrossRef]
- Omata, T.; Otsuka-Yao-Matsuo, S. Electrical properties of proton-conducting Ca2+—Doped La2Zr2O7 with a pyrochlore-type structure. J. Electrochem. Soc. 2001, 148, E252–E261. [Google Scholar] [CrossRef]
- Antonova, E.P.; Farlenkov, A.S.; Tropin, E.S.; Eremin, V.A.; Khodimchuk, A.V.; Ananiev, M.V. Oxygen isotope exchange, water uptake and electrical conductivity of Ca-doped lanthanum zirconate. Solid State Ion. 2017, 306, 112–117. [Google Scholar] [CrossRef]
- Omata, T.; Ikeda, K.; Tokashiki, R.; Otsuka-Yao-Matsuo, S. Proton solubility for La2Zr2O7 with a pyrochlore structure doped with a series of alkaline-earth ions. Solid State Ion. 2004, 167, 389–397. [Google Scholar] [CrossRef]
- Fournier, T.; Nots, J.Y.; Muller, J.; Joubert, J.C. Conductivite ionique des phases de type pyrochlore Gd2−xCaxZr2O7−x/2 et Gd2−xCaxSc2O7−x/2. Solid State Ion. 1985, 15, 71–74. [Google Scholar] [CrossRef]
- Moriga, T.; Yoshiasa, A.; Kanamaru, F.; Koto, K.; Yoshimura, M.; Somiya, S. Crystal structure analyses of the pyrochlore and fluorite—Type Zr2Gd2O7 and anti-phase domain structure. Solid State Ion. 1989, 31, 319–328. [Google Scholar] [CrossRef]
- Govindan Kutty, K.V.; Mathews, C.K.; Rao, T.N.; Varadaraju, U.V. Oxide ion conductivity in some substituted rare earth pyrozirconates. Solid State Ion. 1995, 80, 99–110. [Google Scholar] [CrossRef]
- Xia, X.-L.; Ouyang, J.-H.; Liu, Z.-G. Influence of CaO on structure and electrical conductivity of pyrochlore –type Sm2Zr2O7. J. Powder Sources 2009, 189, 888–893. [Google Scholar] [CrossRef]
- Eurenius, K.E.J.; Ahlberg, E.; Knee, C.S. Role of B-site ion on proton conduction in acceptor-doped Sm2B2O7-δ (B = Ti, Sn, Zr and Ce) pyrochlores and C-type compounds. Dalton Trans. 2011, 40, 3946–3954. [Google Scholar] [CrossRef]
- Shimura, T.; Komori, M.; Iwahara, H. Ionic conduction in pyrochlore—Type oxides containing rare-earth elements at high temperature. Solid State Ion. 1996, 86–88, 685–689. [Google Scholar] [CrossRef]
- Shlyakhtina, A.V.; Pigalskiy, K.S.; Belov, D.A.; Lyskov, N.V.; Kharitonova, E.P.; Kolbanev, I.V.; Borunova, A.B.; Karyagina, O.K.; Sadovskaya, E.M.; Sadykov, V.A.; et al. Proton and oxygen ion conductivity in the pyrochlore/fluorite family of Ln2−xCaxScMO7−δ (Ln = La, Sm, Ho, Yb; M = Nb, Ta; x = 0, 0.05, 0.1) niobates and tantalates. Dalton Trans. 2018, 47, 2376–2392. [Google Scholar] [CrossRef] [PubMed]
- Shenu, A. Structural Analysis and Its Implications for Oxide ion Conductivity of Lanthanide Zirconate Pyrochlores. PhD Thesis, School of Biological and Chemical Sciences, Queen Mary University of London, London, UK, 2018. [Google Scholar]
- Govindan Kutty, K.V.; Mathews, C.K.; Varadaraju, U.V. Effect of aliovalent ion substitution on the oxide ion conductivity in rare-earth pyrohafnates RE2−xSrxHf2O7−δ and RE2Hf2−xAlxO7− δ (RE = Gd and Nd; x = 0, 0.1, and 0.2). Solid State Ion. 1998, 110, 335–340. [Google Scholar]
- Moreno, K.J.; Fuentes, A.F.; Garcia-Barriocanal, J.; Leon, C.; Santamaria, J. Mechanochemical synthesis and ionic conductivity in the Gd2(Sn1−yZry)2O7 (0 ≤ y ≤ 1) solid solution. J. Solid State. Chem. 2006, 179, 323–330. [Google Scholar] [CrossRef]
- Foex, M.; Traverse, J.-P.; Coutures, J. Etude dela structure crystalline des zirconates alcalino-terreux a haute temperature. CR Acad. Sci. Ser. C 1967, 264, 1837–1840. [Google Scholar]
- Subramanian, M.A.; Aravamudan, G.; Subba Rao, G.V. Oxide pyrochlores—A review. Prog. Solid State Chem. 1983, 15, 55–143. [Google Scholar] [CrossRef]
- Zhong, F.; Zhao, J.; Shi, L.; Xiao, Y.; Cai, G.; Zheng, Y.; Long, J. Alkalin-Earth Metals-Doped Pyrochlore Gd2Zr2O7 as oxygen conductors for improved NO2 sensing performance. Sci. Rep. 2017, 7, 4684. [Google Scholar] [CrossRef] [PubMed]
- Kochedykov, V.A.; Zakir’yanova, I.D.; Korzun, I.V. Study of thermal decomposition of products of interaction of REE oxides with the components of the air atmosphere. Anal. Kontrol. 2005, 9, 58–63. [Google Scholar]
- Colomban, P. Proton and Protonic Species: The Hidden Face of Solid State Chemistry. How to Measure H-Content in Materials? Fuel Cells 2013, 13, 6–18. [Google Scholar] [CrossRef]
- López-Vergara, A.; Porras-Vázquez, J.M.; Infantes-Molina, A.; Canales-Vázquez, J.; Cabeza, A.; Losilla, E.R.; Marrero-López, D. Effect of preparation conditions on the polymorphism and transport properties of La6−xMoO12−δ (0 ≤ x ≤ 0.8). Chem. Mater. 2017, 29, 6966–6975. [Google Scholar] [CrossRef]
- Shlyakhtina, A.V.; Knotko, A.V.; Boguslavskii, M.V.; Stefanovich, S.Y.; Kolbanev, I.V.; Larina, L.L.; Shcherbakova, L.G. Effect of non-stoichiometry and synthesis temperature on the structure and conductivity of Ln2+xM2−xO7−x/2(Ln = Sm-Gd; M = Zr, Hf; x = 0–0.286). Solid State Ion. 2007, 178, 59–66. [Google Scholar] [CrossRef]
- Shlyakhtina, A.V.; Belov, D.A.; Knotko, A.V.; Avdeev, M.; Kolbanev, I.V.; Vorobieva, G.A.; Karyagina, O.K.; Shcherbakova, L.G. Oxide ion transport in (Nd2−xZrx)Zr2O7+δ electrolytes by an interstitial mechanism. J. Alloys Compd. 2014, 603, 274–281. [Google Scholar] [CrossRef]
- Shlyakhtina, A.V.; Belov, D.A.; Knotko, A.V.; Kolbanev, I.V.; Streletskii, A.N.; Karyagina, O.K.; Shcherbakova, L.G. Oxygen interstitial and vacancy conduction in symmetric Ln2±xZr−± xO7± x/2 (Ln—Nd, Sm) solid solutions. Inorg. Mater. 2014, 50, 1035–1049. [Google Scholar] [CrossRef]
- Shlyakhtina, A.V.; Belov, D.A.; Knotko, A.V.; Kolbanev, I.V.; Streletskii, A.N.; Shcherbakova, L.G. Interstitial oxide ion conduction in (Sm2−xZrx)Zr2O7+δ. Solid State Ion. 2014, 262, 543–547. [Google Scholar] [CrossRef]
- Besikiotis, V.; Ricote, S.; Jensen, M.H.; Norby, T.; Haugsrud, R. Conductivity and hydration trends in disordered fluorite and pyrochlore oxides: A study on lanthanum cerate-zirconate based compounds. Solid State Ion. 2012, 229, 26–32. [Google Scholar] [CrossRef]
- Belov, D.A.; Shlyakhtina, A.V.; Stefanovich, S.Y.; Shchergolikhin, A.N.; Knotko, A.V.; Karyagina, O.K.; Shcherbakova, L.G. Antiferroelectric phase transition in pyrochlore-like (Dy1−xCax)2Ti2O7−δ (x = 0, 0.01) high-temperature conductors. Solid State Ion. 2011, 192, 188–194. [Google Scholar] [CrossRef]
- Du, Y.; Jin, Z.; Huang, P. Calculation of the Zirconia-Calcia System. J. Am. Ceram. Soc. 2005, 75, 3040–3048. [Google Scholar] [CrossRef]
- Hwang, S.C.; Choi, G.M. The effect of cation nonstoichiometry on the electrical conductivity of acceptor-doped CaZrO3. Solid State Ion. 2006, 177, 3099–3103. [Google Scholar] [CrossRef]
- Iwahara, H.; Yajima, T.; Hibino, T.; Ozaki, K.; Suzuki, H. Proton conduction in calcium, strontium, barium zirconates. Solid State Ion. 1993, 61, 65–69. [Google Scholar] [CrossRef]
- Lyagaeva, J.; Danilov, N.; Korona, D.; Farlenkov, A.; Medvedev, D.; Demin, A.; Animitsa, I.; Tsiakaras, P. Improved ceramic and electrical properties of CaZrO3-based proton-conducting materials prepared by a new convenient combustion synmethod. Ceram. Int. 2017, 43, 7184–7192. [Google Scholar] [CrossRef]
- Davies, R.A.; Islam, M.S.; Chadwick, A.V.; Rush, G.E. Cation dopant sites in the CaZrO3 proton conductor: A combined EXAFS and computer simulation study. Solid State Ion. 2000, 130, 115–122. [Google Scholar] [CrossRef]
- Perriot, R.; Dholabhai, P.P.; Uberiaga, B.P. Disorder-induced transition from grain boundary to bulk dominated ionic diffusion in pyrochlores. Nanoscale 2017, 9, 6826–6836. [Google Scholar] [CrossRef]
- Belov, D.A.; Shlyakhtina, A.V.; Stefanovich, S.Y.; Kolbanev, I.V.; Belousov, Y.A.; Karyagina, O.K.; Shcherbakova, L.G. Acceptor doping of Ln2Ti2O7 (Ln = Dy, Ho, Yb) pyrochlores with divalent cations (Mg, Ca, Sr, Zn). Mater. Res. Bull. 2009, 44, 1613–1620. [Google Scholar] [CrossRef]
- Shlyakhtina, A.V.; Knotko, A.V.; Boguslavskii, M.V.; Stefanovich, S.Y.; Peryshkov, D.V.; Kolbanev, I.V.; Shcherbakova, L.G. Effect of the synthesis procedure, doping and non-stoichiometry on the order-disorder transformation in Ln2Ti2O7 (Ln = Tm-Lu) oxygen-ion conductors. Solid State Ion. 2005, 176, 2297–2304. [Google Scholar] [CrossRef]

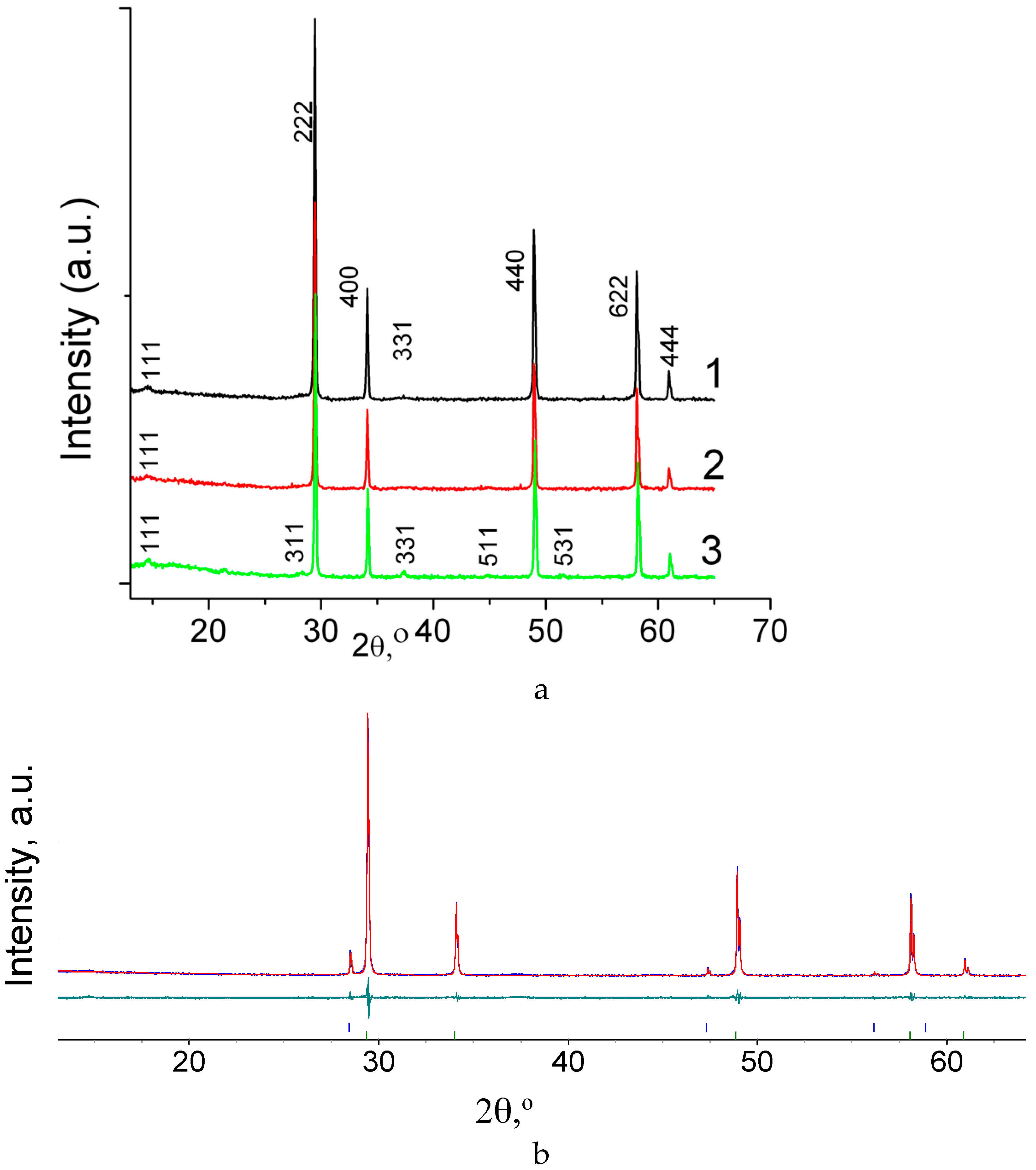




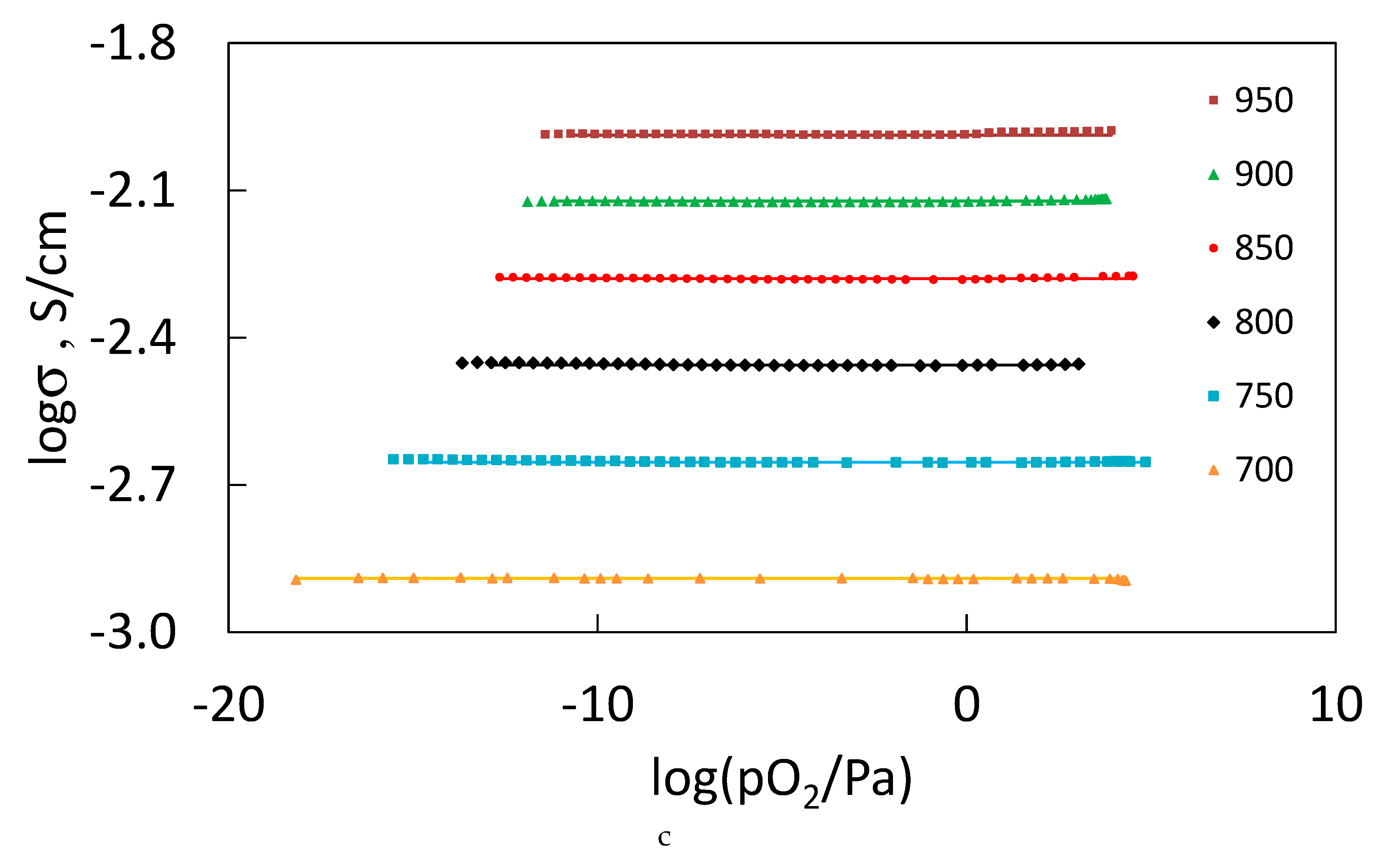
| Sample No. | Formula | Sintering Annealing | Phase Composition According to XRD | Color | Relative Density, % |
|---|---|---|---|---|---|
| 1 | Sm2Zr2O7 | 1600 °C, 10 h | Pyrochlore (P) | Cream | 89 |
| 2 | Sm1.95Ca0.05Zr2O6.975 | 1600 °C, 4 h | Pyrochlore (P) | Cream | 91.6 |
| 3 | Sm1.9Ca0.1Zr2O6.95 | 1600 °C, 4 h | Pyrochlore (P) | Reddish-brown | 92.6 |
| 4 | Gd1.95Ca0.05Zr2O6.975 | 1600 °C, 4 h | Fluorite (F) | Gray | 89.1 |
| 5 | Gd1.9Ca0.1Zr2O6.95 | 1600 °C, 4 h | Fluorite (F) | Gray | 89 |
| Composition | Site | Occupancy | x | y | z | Rexp, % Rwp, % Rp, % GOF | Parameter a, Å |
|---|---|---|---|---|---|---|---|
| Sm2Zr2O7 space group: | SmSm (16d) | 0.905(5) | 0.500 | 0.500 | 0.500 | 3.46 4.16 3.28 1.20 | a =10.5975(1) |
| ZrSm (16d) | 0.095(5) | 0.500 | 0.500 | 0.500 | |||
| ZrZr (16c) | 0.905(5) | 0.000 | 0.000 | 0.000 | |||
| SmZr (16c) | 0.095(5) | 0.000 | 0.000 | 0.000 | |||
| O(1) (8b) | 1 | 0.375 | 0.375 | 0.375 | |||
| O(2) (48f) | 1 | 0.339 | 0.125 | 0.125 | |||
| Sm1.95Ca0.05Zr2O6.975 space group: | SmSm (16d) | 0.975(1) | 0.500 | 0.500 | 0.500 | 3.36 4.49 3.47 1.34 | a = 10.5925(1) |
| ZrSm (16d) | 0 | 0.500 | 0.500 | 0.500 | |||
| CaSm (16d) | 0.025 | 0.500 | 0.500 | 0.500 | |||
| ZrZr (16c) | 1 | 0.000 | 0.000 | 0.000 | |||
| SmZr (16c) | 0 | 0.000 | 0.000 | 0.000 | |||
| CaZr (16c) | 0 | 0.000 | 0.000 | 0.000 | |||
| O(1) (8b) | 1 | 0.375 | 0.375 | 0.375 | |||
| O(2) (48f) | 1 | 0.339 | 0.125 | 0.125 | |||
| Sm1.9Ca0.1Zr2O6.95 space group: | SmSm (16d) | 0.930(2) | 0.500 | 0.500 | 0.500 | 3.51 4.94 3.79 1.41 | a = 10.5923(1) |
| ZrSm (16d) | 0.030(2) | 0.500 | 0.500 | 0.500 | |||
| CaSm (16d) | 0.04 | 0.500 | 0.500 | 0.500 | |||
| ZrZr (16c) | 0.960(2) | 0.000 | 0.000 | 0.000 | |||
| SmZr (16c) | 0.030(2) | 0.000 | 0.000 | 0.000 | |||
| CaZr (16c) | 0.01 | 0.000 | 0.000 | 0.000 | |||
| O(1) (8b) | 1 | 0.375 | 0.375 | 0.375 | |||
| O(2) (48f) | 1 | 0.339 | 0.125 | 0.125 | |||
| Gd1.95Ca0.05Zr2O6.975 space group: | GdGd (16d) | 0.585(40) | 0.500 | 0.500 | 0.500 | 2.92 3.72 2.88 1.27 | a = 10.5326(1) |
| ZrGd (16d) | 0.400(40) | 0.500 | 0.500 | 0.500 | |||
| CaGd (16d) | 0.015 | 0.500 | 0.500 | 0.500 | |||
| ZrZr (16c) | 0.600(40) | 0.000 | 0.000 | 0.000 | |||
| GdZr (16c) | 0.390(40) | 0.000 | 0.000 | 0.000 | |||
| CaZr (16c) | 0.01 | 0.000 | 0.000 | 0.000 | |||
| O(1) (8b) | 1 | 0.375 | 0.375 | 0.375 | |||
| O(2) (48f) | 1 | 0.339 | 0.125 | 0.125 | |||
| Gd1.95Ca0.05Zr2O6.975 space group: | GdGd (4a) | 0.4875(1) | 0.000 | 0.000 | 0.000 | 2.92 3.67 2.82 1.26 | a = 5.2663(1) |
| ZrGd (4a) | 0.5000(1) | 0.000 | 0.000 | 0.000 | |||
| CaGd (4a) | 0.0125(1) | 0.000 | 0.000 | 0.000 | |||
| O(1) (8c) | 0.8750 | 0.250 | 0.250 | 0.250 | |||
| Gd1.9Ca0.1Zr2O6.95 space group: | GdGd (16d) | 0.580(40) | 0.500 | 0.500 | 0.500 | 2.80 3.66 2.83 1.31 | a = 10.5321(1) |
| ZrGd (16d) | 0.390(40) | 0.500 | 0.500 | 0.500 | |||
| CaGd (16d) | 0.03 | 0.500 | 0.500 | 0.500 | |||
| ZrZr (16c) | 0.610(40) | 0.000 | 0.000 | 0.000 | |||
| GdZr (16c) | 0.370(40) | 0.000 | 0.000 | 0.000 | |||
| CaZr (16c) | 0.02 | 0.000 | 0.000 | 0.000 | |||
| O(1) (8b) | 1 | 0.375 | 0.375 | 0.375 | |||
| O(2) (48f) | 1 | 0.339 | 0.125 | 0.125 | |||
| Gd1.9Ca0.1Zr2O6.95 space group: | GdGd (4a) | 0.475(1) | 0.000 | 0.000 | 0.000 | 2.80 | a = 5.2661(1) |
| ZrGd (4a) | 0.500(1) | 0.000 | 0.000 | 0.000 | 3.62 | ||
| CaGd (4a) | 0.025(1) | 0.000 | 0.000 | 0.000 | 2.81 | ||
| O(1) (8c) | 0.875 | 0.250 | 0.250 | 0.250 | 1.29 |
| Analyzing Area | Concentration, at.% | Ratio | ||||
|---|---|---|---|---|---|---|
| Sm | Ca | Zr | [Zr]/[Ca] | [Sm]/[Ca] | [Zr]/[Sm] | |
| Nominal stoichiometry | 47.5 | 2.5 | 50.0 | 20.0 | 19.0 | 1.1 |
| SEM/EDX point analysis | ||||||
| Micron-size grain(s) only (well co-sintered) | 55.2 | 1.7 | 43.1 | 26.5 | 33.9 | 0.8 |
| 51.5 | 2.5 | 46.0 | 18.5 | 20.7 | 0.9 | |
| Grain boundary | 57.5 | 1.6 | 40.9 | 25.7 | 36.1 | 0.7 |
| 57.6 | 1.6 | 40.8 | 25.5 | 36.0 | 0.7 | |
| 51.2 | 2.0 | 46.8 | 22.9 | 25.1 | 0.9 | |
| 100–300 nm grains on the surface of micron-size well co-sintered grains | 57.5 | 2.0 | 40.5 | 20.7 | 29.3 | 0.7 |
| 51.8 | 3.1 | 45.1 | 14.4 | 16.6 | 0.9 | |
| Individual grains with the size of 0.6–1.1 mkm | 73.6 | 2.9 | 23.5 | 8.1 | 25.2 | 0.3 |
| 49.1 | 4.9 | 46.0 | 9.4 | 10.1 | 0.9 | |
| 58.0 | 3.7 | 38.3 | 10.3 | 15.6 | 0.7 | |
| Micron-size agglomerate formed from small grains, Figure 3c | 26.0 | 54.5 | 19.5 | 0.4 | 0.5 | 0.7 |
| 20.3 | 66.7 | 13.0 | 0.2 | 0.3 | 0.6 | |
| Compound | Structure (XRD Data) | Temperature, °С | Activation Energy for Total Conductivity, eV (Dry Air) | Activation Energy for Total Conductivity, eV (Wet Air) |
|---|---|---|---|---|
| Sm2Zr2O7 | Pyrochlore | Above 500 °С | 0.7 | 0.7 |
| Below 500 °С | 0.83 | 0.73 | ||
| Sm1.95Ca0.05Zr2O6.975 | Pyrochlore | 300–900 °С | 0.82 | 0.79 |
| Sm1.9Ca0.1Zr2O6.975 | Pyrochlore | 300–900 °С | 0.95 | 0.89 |
| Gd1.95Ca0.05Zr2O6.975 | Fluorite | Above 580 °С | 0.99 | 0.99 |
| Below 580 °С | 1.15 | 1.15 | ||
| Gd1.9Ca0.1Zr2O6.95 | Fluorite | Above 580 °С | 1.03 | 1.03 |
| Below 580 °С | 1.17 | 1.17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shlyakhtina, A.V.; Abrantes, J.C.C.; Gomes, E.; Lyskov, N.V.; Konysheva, E.Y.; Chernyak, S.A.; Kharitonova, E.P.; Karyagina, O.K.; Kolbanev, I.V.; Shcherbakova, L.G. Evolution of Oxygen–Ion and Proton Conductivity in Ca-Doped Ln2Zr2O7 (Ln = Sm, Gd), Located Near Pyrochlore–Fluorite Phase Boundary. Materials 2019, 12, 2452. https://doi.org/10.3390/ma12152452
Shlyakhtina AV, Abrantes JCC, Gomes E, Lyskov NV, Konysheva EY, Chernyak SA, Kharitonova EP, Karyagina OK, Kolbanev IV, Shcherbakova LG. Evolution of Oxygen–Ion and Proton Conductivity in Ca-Doped Ln2Zr2O7 (Ln = Sm, Gd), Located Near Pyrochlore–Fluorite Phase Boundary. Materials. 2019; 12(15):2452. https://doi.org/10.3390/ma12152452
Chicago/Turabian StyleShlyakhtina, A.V., J.C.C. Abrantes, E. Gomes, N.V. Lyskov, E.Yu. Konysheva, S.A. Chernyak, E.P. Kharitonova, O.K. Karyagina, I.V. Kolbanev, and L.G. Shcherbakova. 2019. "Evolution of Oxygen–Ion and Proton Conductivity in Ca-Doped Ln2Zr2O7 (Ln = Sm, Gd), Located Near Pyrochlore–Fluorite Phase Boundary" Materials 12, no. 15: 2452. https://doi.org/10.3390/ma12152452
APA StyleShlyakhtina, A. V., Abrantes, J. C. C., Gomes, E., Lyskov, N. V., Konysheva, E. Y., Chernyak, S. A., Kharitonova, E. P., Karyagina, O. K., Kolbanev, I. V., & Shcherbakova, L. G. (2019). Evolution of Oxygen–Ion and Proton Conductivity in Ca-Doped Ln2Zr2O7 (Ln = Sm, Gd), Located Near Pyrochlore–Fluorite Phase Boundary. Materials, 12(15), 2452. https://doi.org/10.3390/ma12152452






