Water Uptake and Transport Properties of La1−xCaxScO3−α Proton-Conducting Oxides
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
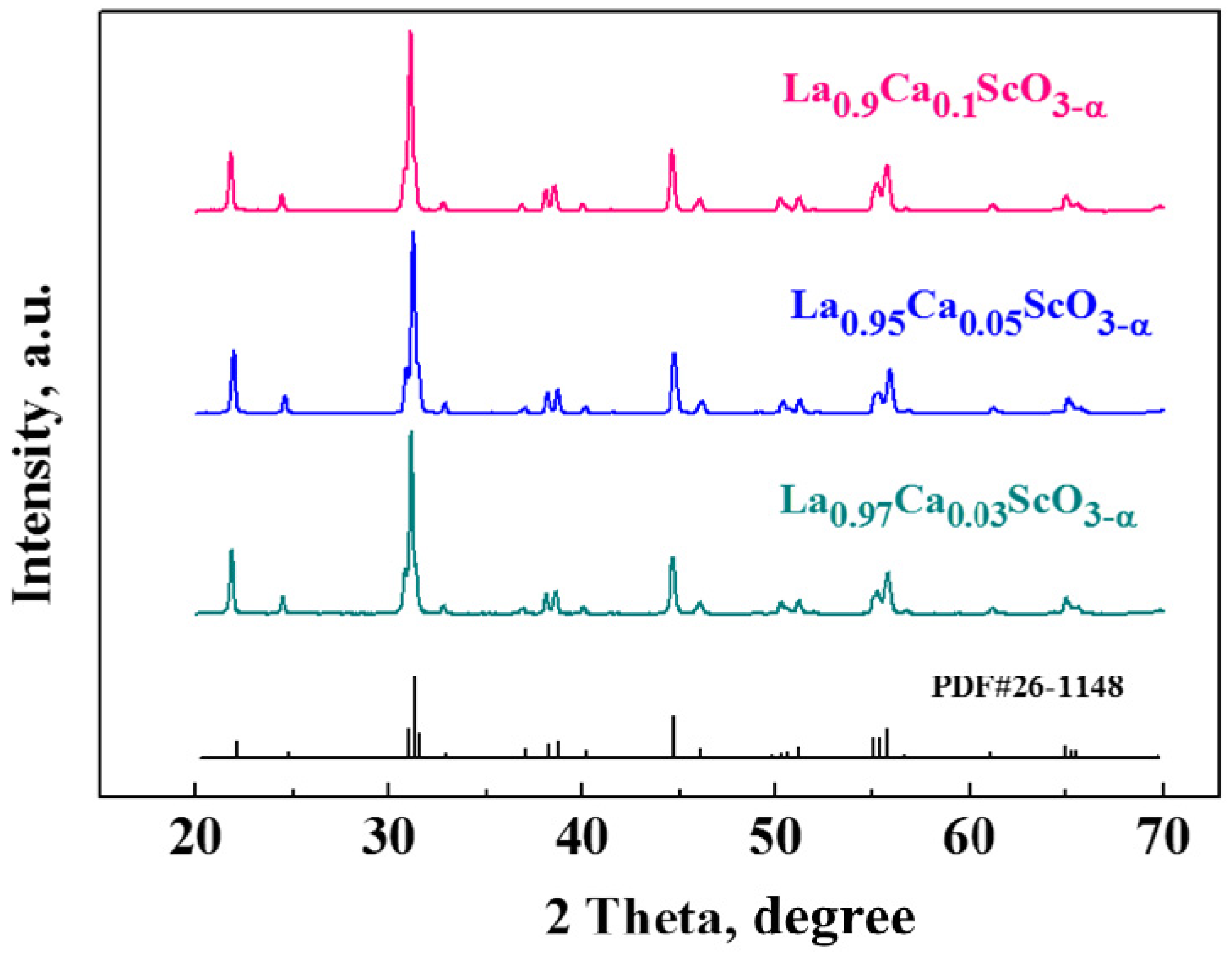
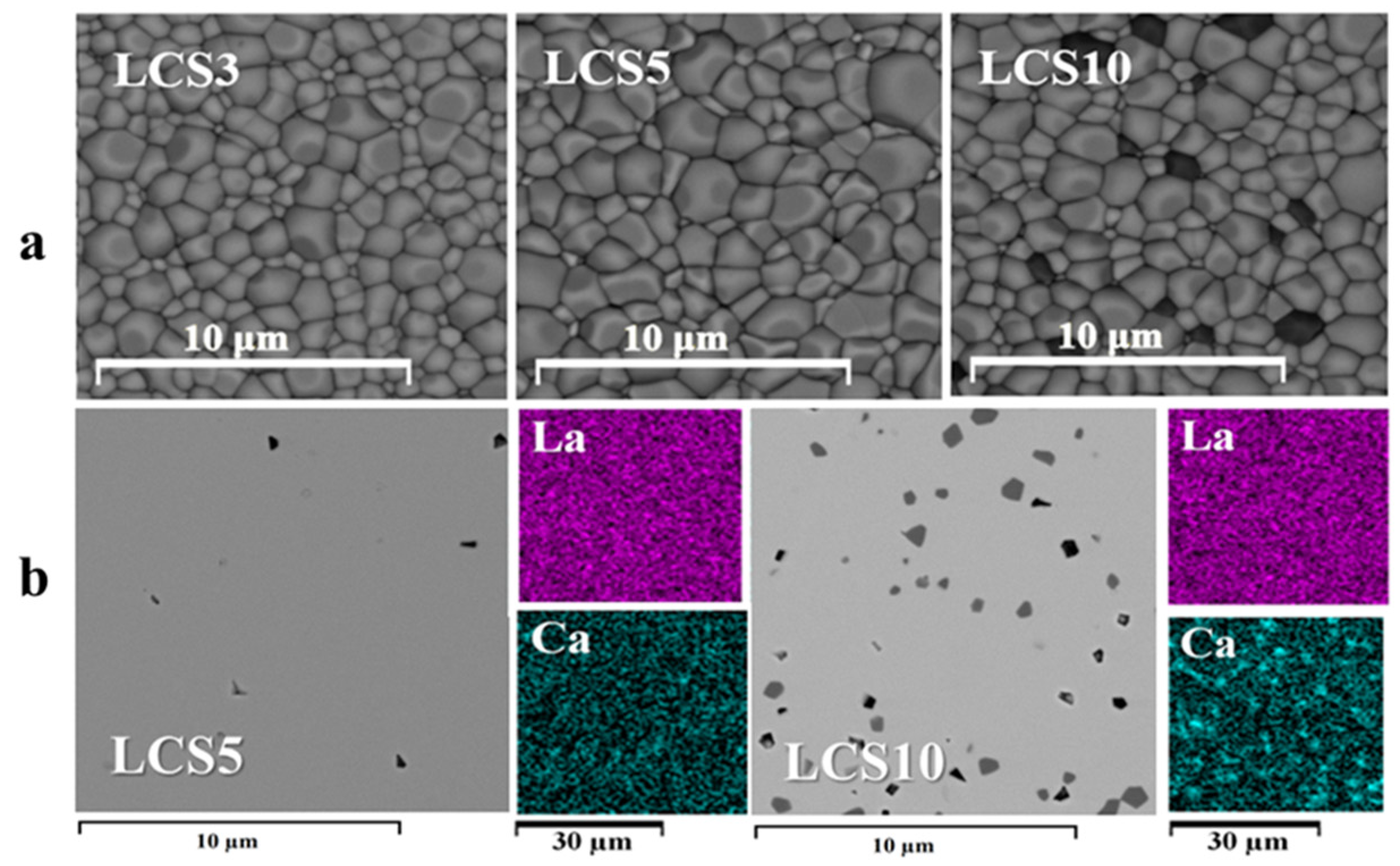
3.1. Ceramics Characterization
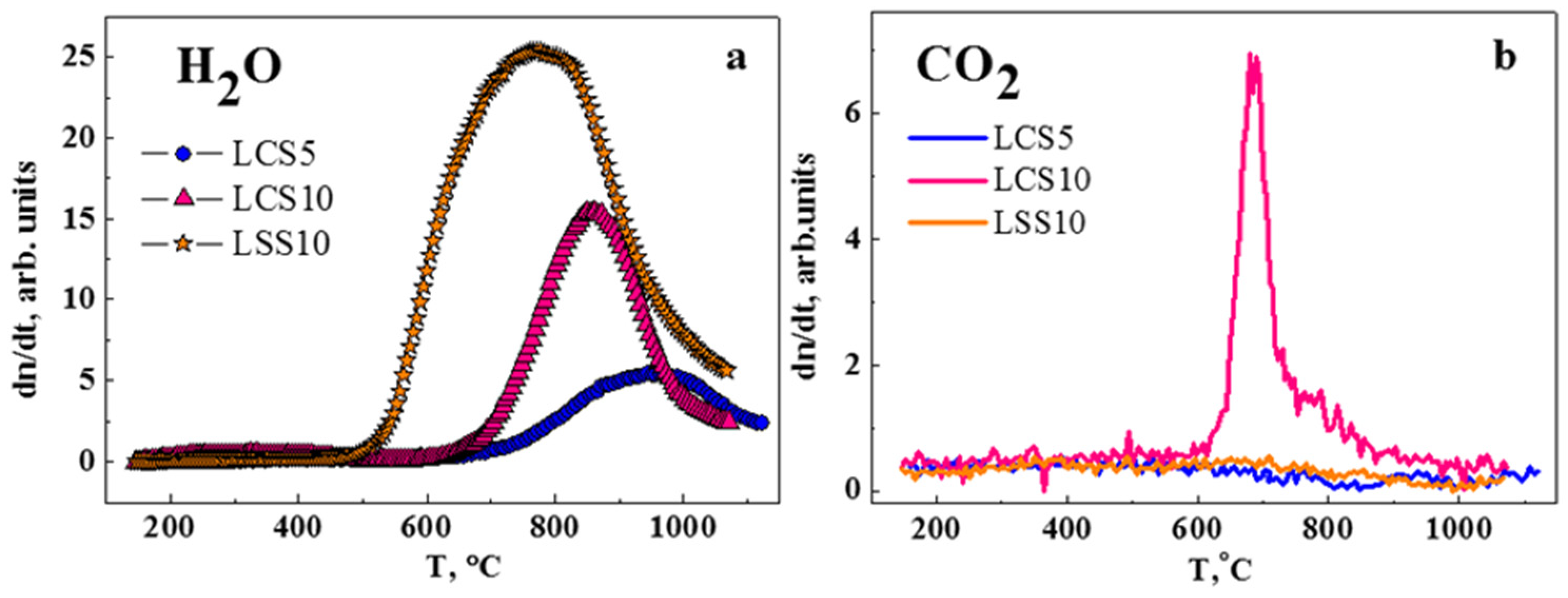
3.2. Thermodesorption Spectroscopy
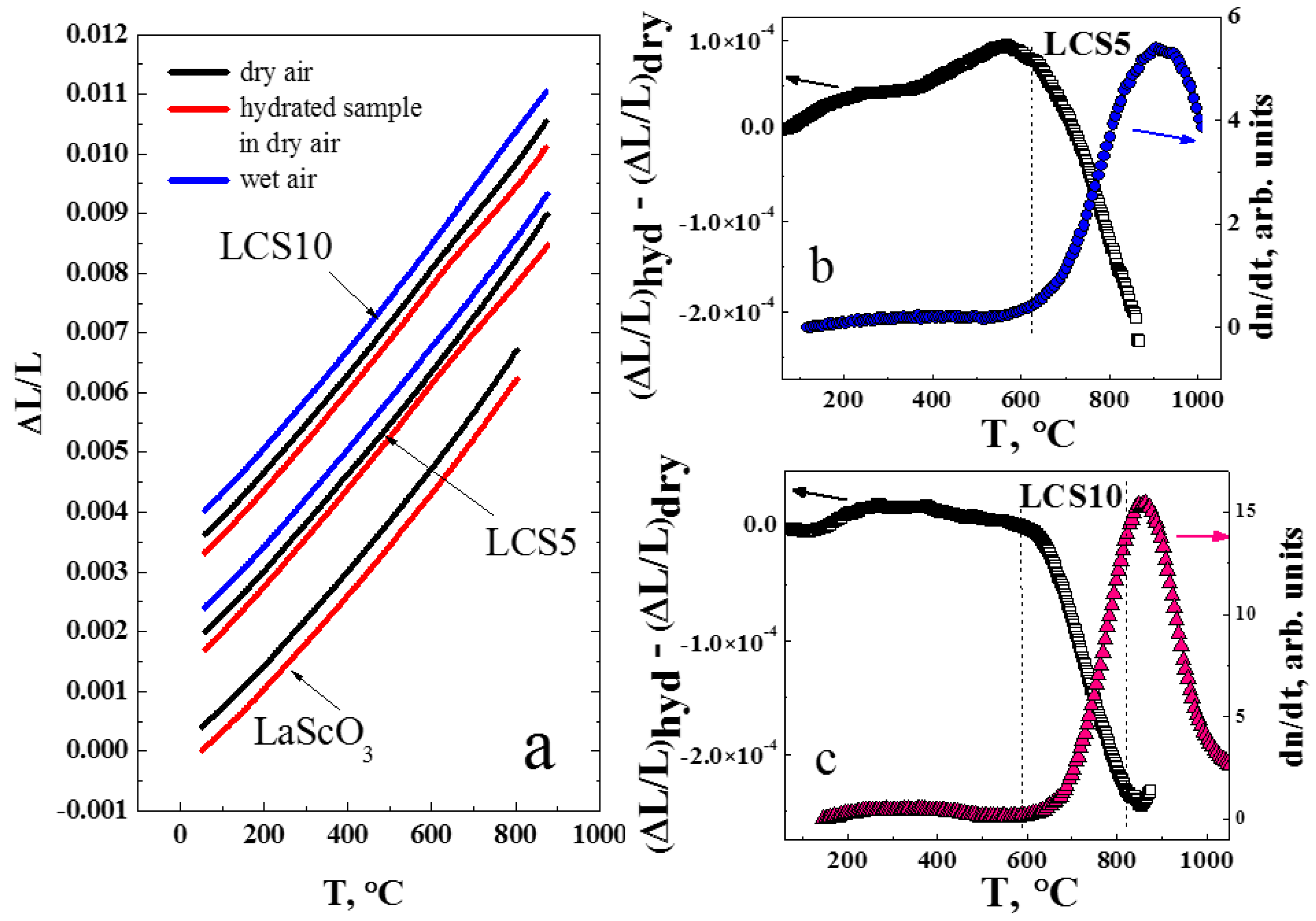
3.3. Thermal Expansion
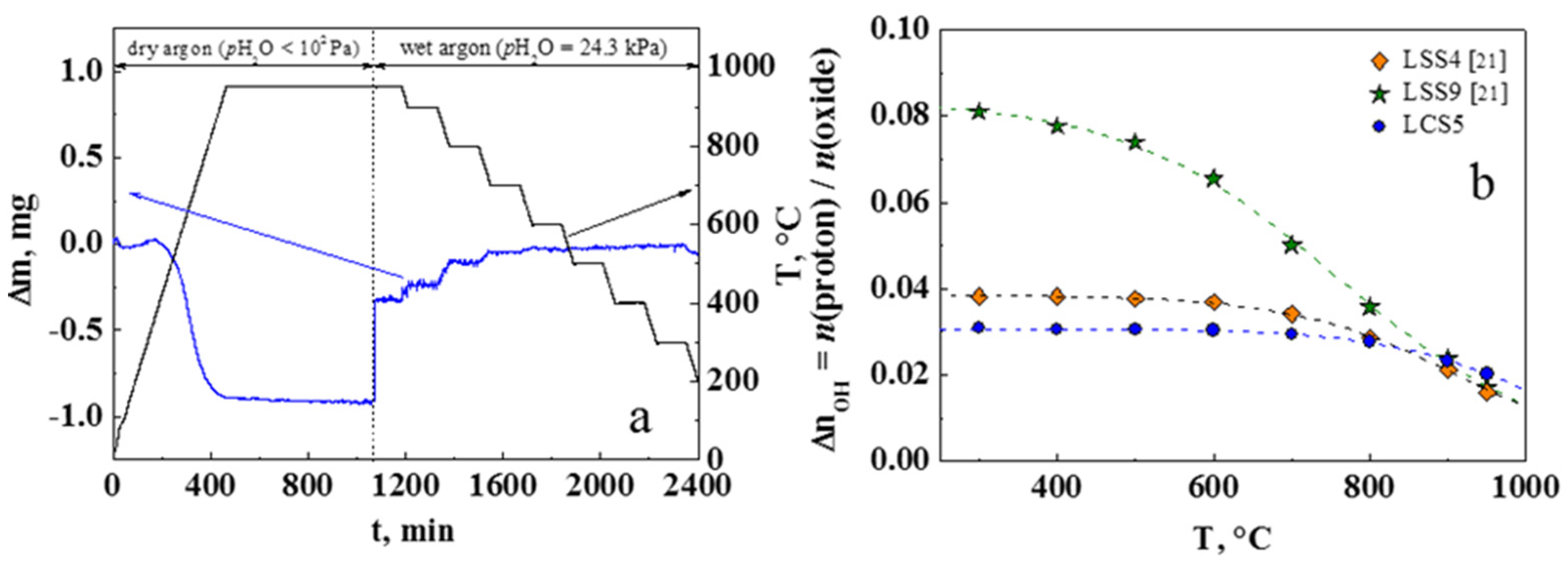
3.4. Water Uptake
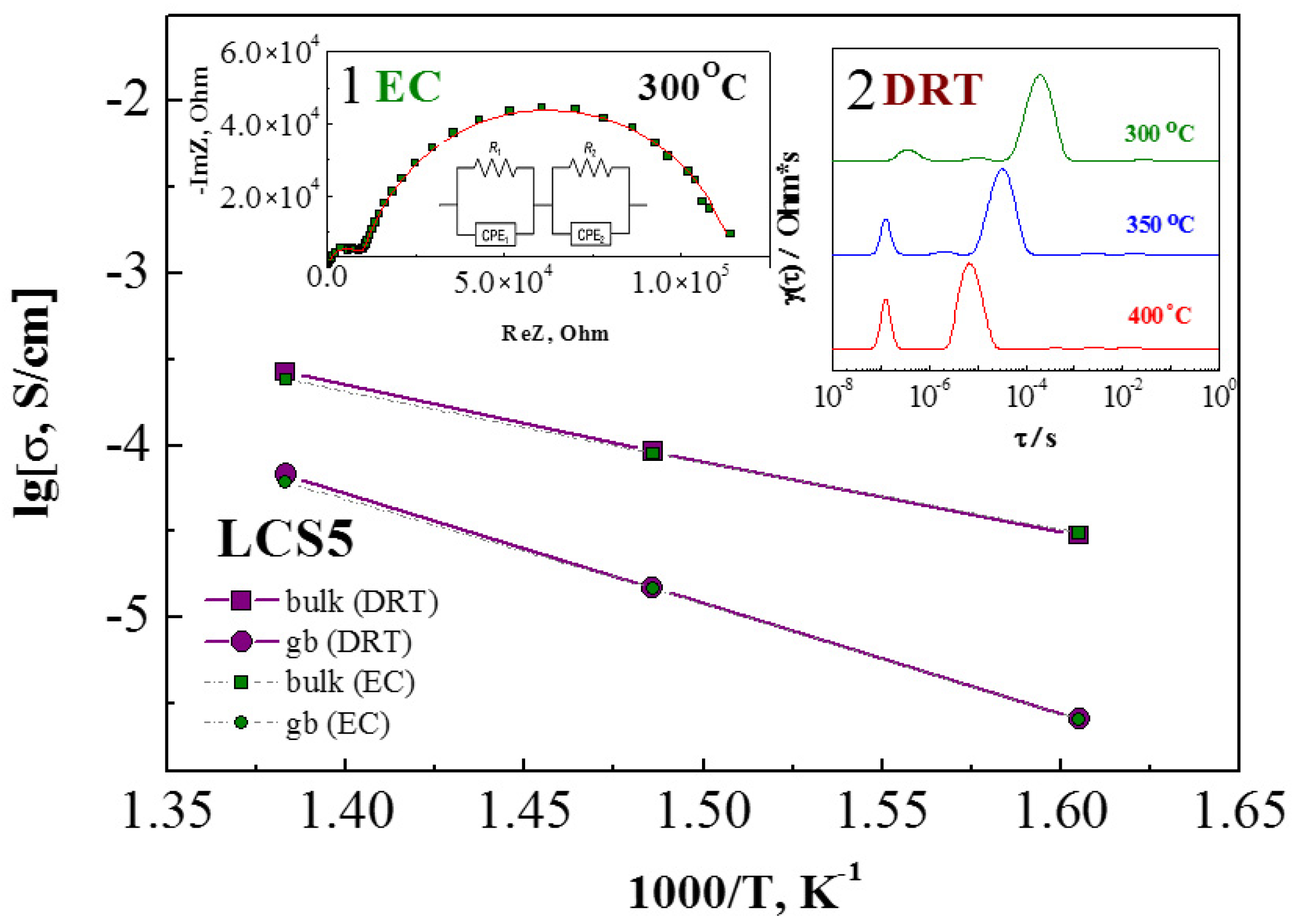
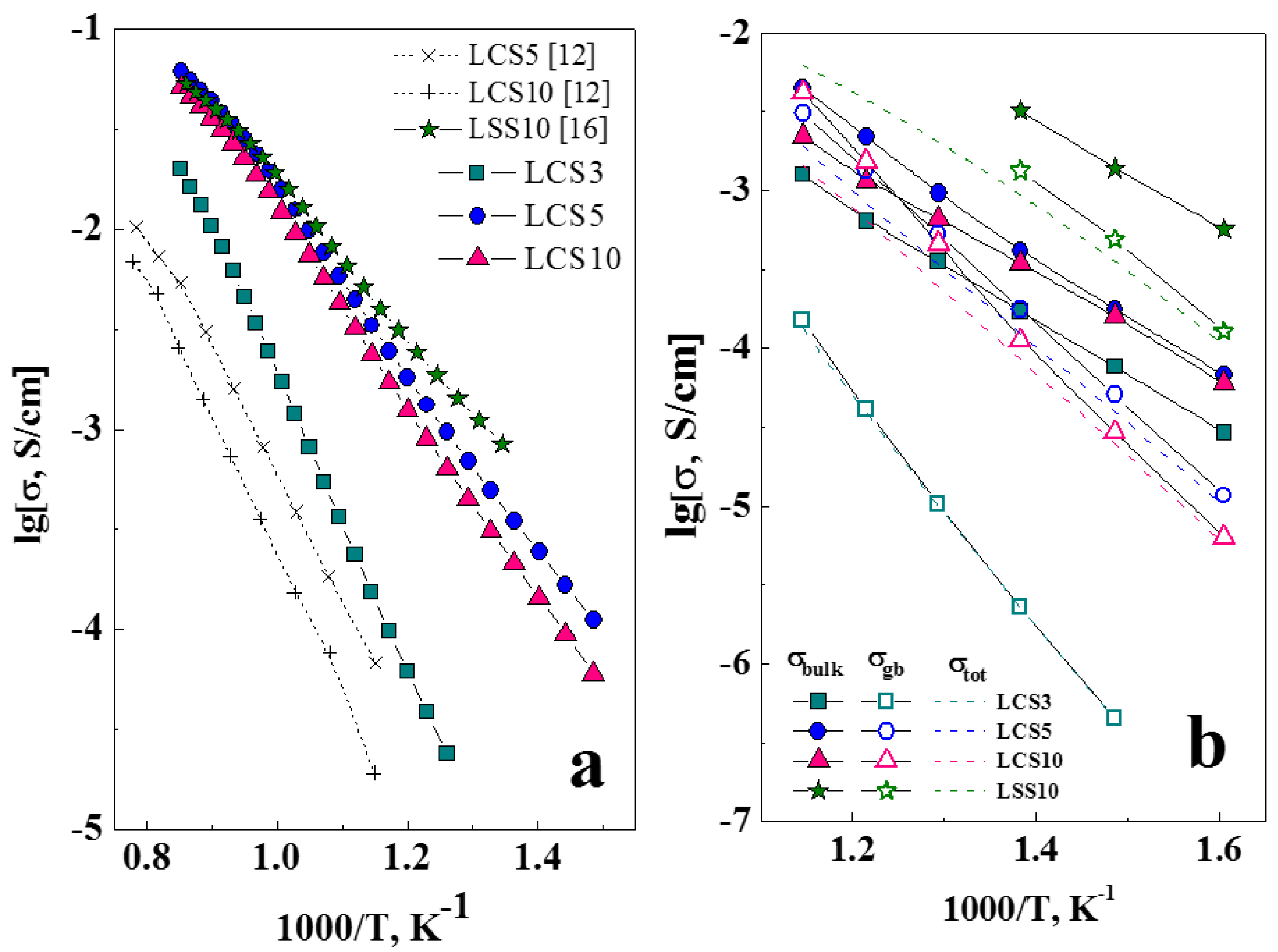
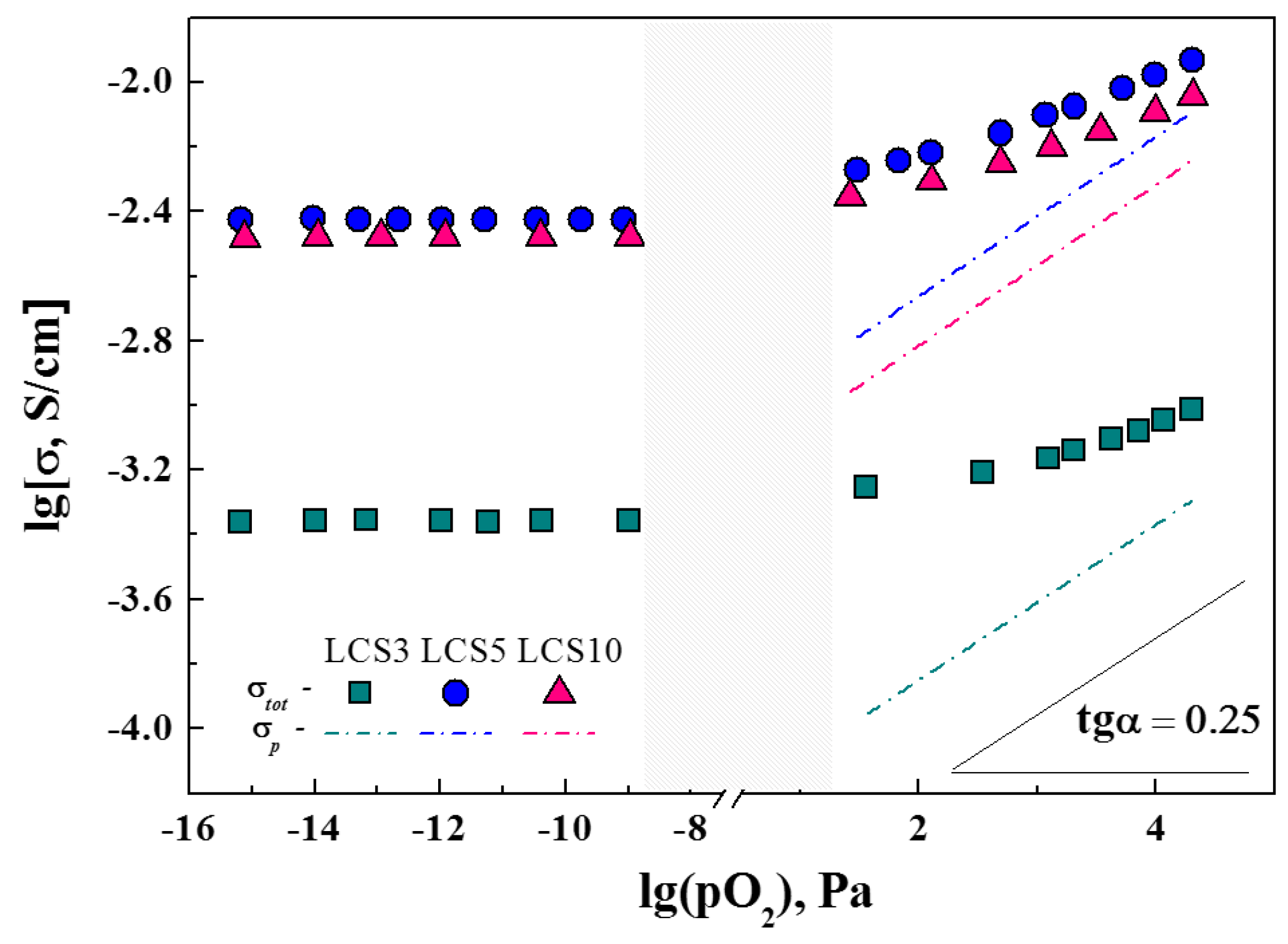
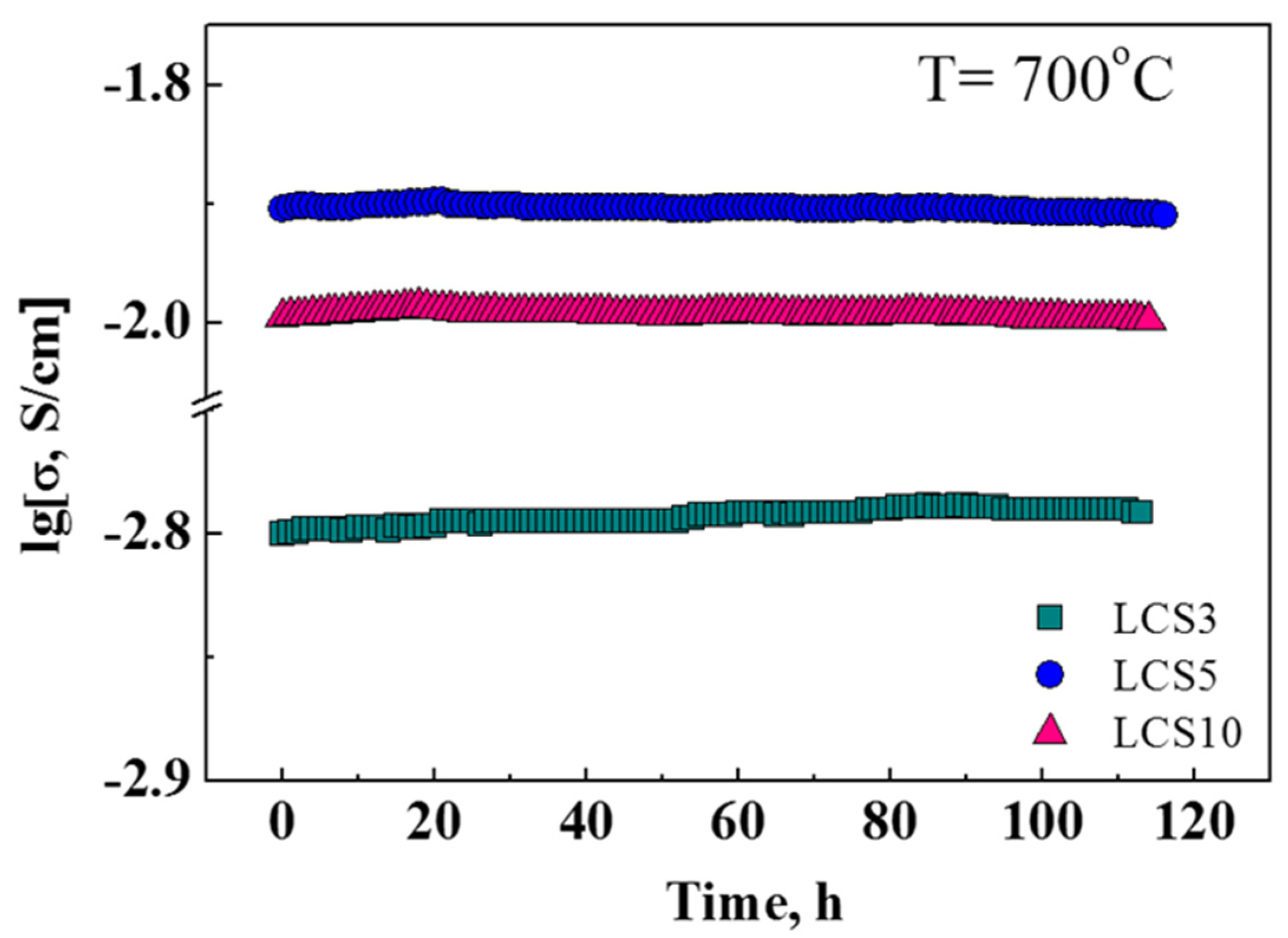
3.5. Transport Properties
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iwahara, H.; Esaka, T.; Uchida, H.; Maeda, N. Proton conduction in sintered oxides and its application to steam electrolysis for hydrogen production. Solid State Ion. 1981, 3, 359–363. [Google Scholar] [CrossRef]
- Norby, T. Solid-state protonic conductors: Principles, properties, progress and prospects. Solid State Ion. 1999, 125, 1–11. [Google Scholar] [CrossRef]
- Yugami, H.; Kato, H.; Iguchi, F. Protonic SOFCs using perovskite-type conductors. Adv. Sci. Technol. 2014, 95, 66–71. [Google Scholar] [CrossRef]
- Bonanos, N.; Knight, K.; Ellis, B. Perovskite solid electrolytes: Structure, transport properties and fuel cell application. Solid State Ion. 1995, 79, 161–170. [Google Scholar] [CrossRef]
- Malavasi, L.; Fisher, C.A.J.; Islam, M.S. Oxide-ion and proton conducting electrolyte materials for clean energy applications: Structural and mechanistic features. Chem. Soc. Rev. 2010, 39, 70–87. [Google Scholar] [CrossRef]
- Kochetova, N.; Animitsa, I.; Medvedev, D.; Demin, A.; Tsiakaras, P. Recent activity in the development of proton conducting oxides for high-temperature applications. RSC Adv. 2016, 6, 73222–73268. [Google Scholar] [CrossRef]
- Inaba, H.; Hayashi, H.; Suzuki, M. Structural phase transition of perovskite oxides LaMO3 and La0.9Sr0.1MO3 with different size of B-site ions. Solid State Ion. 2001, 144, 99–108. [Google Scholar] [CrossRef]
- Hayashi, H.; Inaba, H.; Matsuyama, M.; Lan, N.G.; Dokiya, M.; Tagawa, H. Structural consideration on the ionic conductivity of perovskite-type oxides. Solid State Ion. 1999, 122, 1–15. [Google Scholar] [CrossRef]
- Nomura, K.; Tanase, S. Electrical behavior in (La0.9Sr0.1) MIIIO3−δ (MIII = Al, Ga, Sc, In, and Lu) perovskites. Solid State Ion. 1997, 98, 229–236. [Google Scholar] [CrossRef]
- Lybye, D.; Poulsen, F.W.; Mogensen, M. Conductivity of A-and B-site doped LaAlO3, LaGaO3, LaScO3 and LaInO3 perovskites. Solid State Ion. 2000, 128, 91–103. [Google Scholar] [CrossRef]
- Gorelov, V.P.; Stroeva, A.Y. Solid proton conducting electrolytes based on LaScO3. Russ. J. Electrochem. 2012, 48, 949–960. [Google Scholar] [CrossRef]
- Fujii, H.; Katayama, Y.; Shimura, T.; Iwahara, H. Protonic conduction in perovskite-type oxide ceramics based on LnScO3 (Ln = La, Nd, Sm or Gd) at high temperature. J. Electroceram. 1998, 2, 119–125. [Google Scholar] [CrossRef]
- Stroeva, A.Y.; Gorelov, V.P.; Kuzmin, A.V.; Antonova, E.P.; Plaksin, S.V. Phase composition and conductivity of La1-xSrxScO3-α (x = 0.01–0.20) under oxidative conditions. Rus. J. Electrochem. 2012, 48, 509–517. [Google Scholar] [CrossRef]
- Stroeva, A.Y.; Gorelov, V.P. Nature of conductivity of perovskites La1-xSrxScO3-α (X = 0.01–0.15) under oxidative and reducing conditions. Rus. J. Electrochem 2012, 48, 1079–1085. [Google Scholar] [CrossRef]
- Kuzmin, A.V.; Stroeva, A.Y.; Plekhanov, M.S.; Gorelov, V.P.; Farlenkov, A.S. Chemical solution deposition and characterization of the La1-xSrxScO3-a thin films on La1-xSrxMnO3-a substrate. Int. J. Hydr. Energy 2018, 43, 19206–19212. [Google Scholar] [CrossRef]
- Kuzmin, A.V.; Stroeva, A.Y.; Gorelov, V.P.; Novikova, Y.V.; Lesnichyova, A.S.; Farlenkov, A.S.; Khodimchuk, A.V. Synthesis and characterization of dense proton-conducting La1-xSrxScO3-α ceramics. Int. J. Hydr. Energy 2019, 44, 1130–1138. [Google Scholar] [CrossRef]
- Aksenova, T.I.; Khromushin, I.V.; Zhotabaev, Z.R.; Bukenov, K.D.; Berdauletov, A.K.; Medvedeva, Z.V. Thermodesorption study of barium and strontium cerates. Solid State Ion. 2003, 162, 31–36. [Google Scholar] [CrossRef]
- Kuzmin, A.V.; Gorelov, V.P.; Vaganov, E.G.; Korzun, I.V.; Kazantsev, V.A.; Aksenova, T.I.; Khromushin, I.V. Phase transitions, thermal desorption of gases, and electroconduction in BaCeO3, a base for high-temperature protonic conductors. Rus. J. Electrochem. 2005, 41, 544–549. [Google Scholar] [CrossRef]
- Bauerle, J.E. Study of solid electrolyte polarization by a complex admittance method. J. Phys. Chem. Solids 1969, 30, 2657–2670. [Google Scholar] [CrossRef]
- Wan, T.H.; Saccoccio, M.; Chen, C.; Ciucci, F. Influence of the discretization methods on the distribution of relaxation times deconvolution: Implementing radial basis functions with DRTtools. Electrochim. Acta. 2015, 184, 483–499. [Google Scholar] [CrossRef]
- Farlenkov, A.S.; Smolnikov, A.G.; Ananyev, M.V.; Khodimchuk, A.V.; Buzlukov, A.L.; Kuzmin, A.V.; Porotnikova, N.M. Local disorder and water uptake in La1−xSrxScO3−δ. Solid State Ion. 2017, 306, 82–88. [Google Scholar] [CrossRef]
- Kreuer, K.D. Aspects of the formation and mobility of protonic charge carriers and the stability of perovskite-type oxides. Solid State Ion. 1999, 125, 285–302. [Google Scholar] [CrossRef]
- Kreuer, K.D. Proton-conducting oxides. Annu. Rev. Mater. Res. 2003, 33, 333–359. [Google Scholar] [CrossRef]
- Stroeva, A.Y.; Gorelov, V.P.; Antonov, B.D. Electrotransfer in nonstoichiometric lanthanum scandate LaSc1-XO3-α (x = 0–0.1). Rus. J. Electrochem. 2012, 48, 1171–1178. [Google Scholar] [CrossRef]
- Okuyama, Y.; Kozai, T.; Ikeda, S.; Matsuka, M.; Sakaid, T.; Matsumoto, H. Incorporation and conduction of proton in Sr-doped LaMO3 (M = Al, Sc, In, Yb, Y). Electrochim. Acta. 2014, 125, 443–449. [Google Scholar] [CrossRef]
- Farlenkov, A.S.; Putilov, L.P.; Ananyev, M.V.; Antonova, E.P.; Eremina, V.A.; Stroeva, A.Y.; Sherstobitova, E.A.; Voronin, V.I.; Berger, I.F.; Tsidilkovski, V.I.; et al. Water uptake, ionic and hole transport in La0.9Sr0.1ScO3−δ. Solid State Ion. 2017, 306, 126–136. [Google Scholar] [CrossRef]
- Nomura, K.; Kageyama, H. Neutron diffraction study of LaScO3-based proton conductor. Solid State Ion. 2014, 262, 841–844. [Google Scholar] [CrossRef]
- Bohn, H.G.; Schober, T. Electrical conductivity of the high-temperature proton conductor BaZr0.9Y0.1O2.95. J. Am. Ceram. Soc. 2000, 83, 768–772. [Google Scholar] [CrossRef]
- Danilov, N.; Pikalova, E.; Lyagaeva, J.; Antonov, B.; Medvedev, D.; Demin, A.; Tsiakaras, P. Grain and grain boundary transport in BaCe0.5Zr0.3Ln0.2O3-δ (Ln-Y or lanthanide) electrolytes attractive for protonic ceramic fuel cells application. J. Power Sources 2017, 366, 161–168. [Google Scholar] [CrossRef]
- Omata, T.; Matsuo, O.Y. Electrical properties of proton-conducting Ca2+-doped La2Zr2O7 with a pyrochlore-type structure. J. Electrochem. Soc. 2001, 6, 252–261. [Google Scholar] [CrossRef]

| Sample | V, Å3 | Estimated Density, g/cm3 | Relative Density, % |
|---|---|---|---|
| LCS3 | 265.83 | 5.58 ± 0.2 | 97 |
| LCS5 | 266.12 | 5.49 ± 0.2 | 97 |
| LCS10 | 267.88 | 5.45 ± 0.2 | 98 |
| Element | La0.97Ca0.03ScO3−α | La0.95Ca0.05ScO3−α | La0.90Ca0.10ScO3−α |
|---|---|---|---|
| La | 49.1 ± 0.4 | 47.8 ± 0.4 | 45.7 ± 0.3 |
| Ca | 1.2 ± 0.1 | 2.2 ± 0.1 | 4.0 ± 0.2 |
| Sc | 49.7 ± 0.4 | 50.0 ± 0.5 | 50.3 ± 0.5 |
| Sample | Effective Activation Energies, eV | |||||
|---|---|---|---|---|---|---|
| Air | Hydrogen | |||||
| pH2O = 4.5 kPa | pH2O < 0.1 kPa | pH2O = 4.5 kPa | ||||
| HT | LT | HT | LT | HT | LT | |
| LCS3 | 1.44 | 1.54 | 1.34 | 1.85 | 0.82 | 1.17 |
| LCS5 | 0.87 | 0.96 | 0.72 | 1.39 | 0.33 | 0.70 |
| LCS10 | 0.91 | 1.02 | 0.76 | 1.45 | 0.34 | 0.78 |
| LSS5 [12] | 0.69 | 0.79 | 0.66 | 0.92 | 0.33 | 0.53 |
| LSS10 [12] | 0.63 | 0.69 | 0.67 | 0.72 | 0.39 | 0.44 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lesnichyova, A.; Stroeva, A.; Belyakov, S.; Farlenkov, A.; Shevyrev, N.; Plekhanov, M.; Khromushin, I.; Aksenova, T.; Ananyev, M.; Kuzmin, A. Water Uptake and Transport Properties of La1−xCaxScO3−α Proton-Conducting Oxides. Materials 2019, 12, 2219. https://doi.org/10.3390/ma12142219
Lesnichyova A, Stroeva A, Belyakov S, Farlenkov A, Shevyrev N, Plekhanov M, Khromushin I, Aksenova T, Ananyev M, Kuzmin A. Water Uptake and Transport Properties of La1−xCaxScO3−α Proton-Conducting Oxides. Materials. 2019; 12(14):2219. https://doi.org/10.3390/ma12142219
Chicago/Turabian StyleLesnichyova, Alyona, Anna Stroeva, Semyon Belyakov, Andrey Farlenkov, Nikita Shevyrev, Maksim Plekhanov, Igor Khromushin, Tatyana Aksenova, Maxim Ananyev, and Anton Kuzmin. 2019. "Water Uptake and Transport Properties of La1−xCaxScO3−α Proton-Conducting Oxides" Materials 12, no. 14: 2219. https://doi.org/10.3390/ma12142219
APA StyleLesnichyova, A., Stroeva, A., Belyakov, S., Farlenkov, A., Shevyrev, N., Plekhanov, M., Khromushin, I., Aksenova, T., Ananyev, M., & Kuzmin, A. (2019). Water Uptake and Transport Properties of La1−xCaxScO3−α Proton-Conducting Oxides. Materials, 12(14), 2219. https://doi.org/10.3390/ma12142219






