Drug Release Kinetics of Electrospun PHB Meshes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PHB Electrospun Films
2.2. Characterization
2.2.1. Determination of Dynamic Viscosity
2.2.2. Morphological Analysis
2.2.3. Fourier Transform Infrared Spectroscopy (FT-IR)
2.3. Drug in Vitro Release Studies
2.4. Antimicrobial Tests
3. Results and Discussion
3.1. Morphology of PHB Electrospun Meshes
3.2. Entrapment Efficiency and Drug Release Kinetics
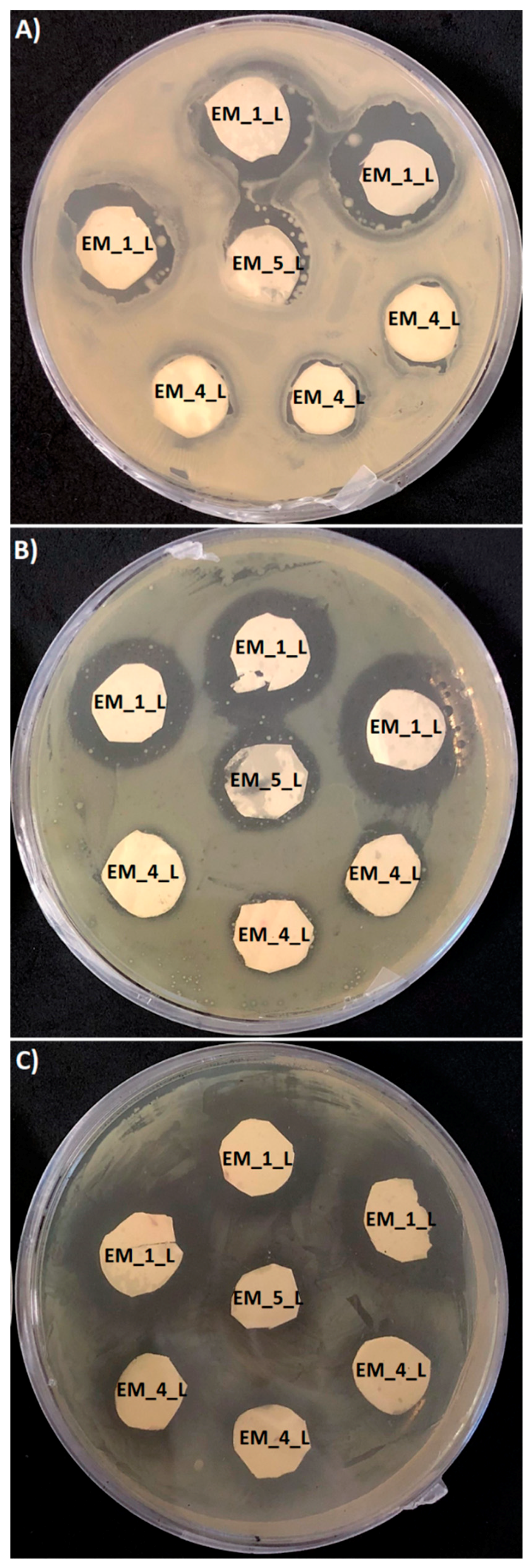
3.3. The Antimicrobial Susceptibility of PHB Loaded Levofloxacin Electrospun Meshes
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sudhakar, Y.; Jayaveera, K.N. Novel Drug Delivery Systems and Regulatory Affairs; SCHAND & Company Limited: New Delhi, India, 2014. [Google Scholar]
- Langer, R.; Peppas, N.A. Advances in biomaterials, drug delivery, and bionanotechnology. AIChE J. 2003, 49, 2990–3006. [Google Scholar] [CrossRef]
- Forbes, D.C.; Peppas, N.A. Oral delivery of small RNA and DNA. J. Control. Release 2012, 162, 438–445. [Google Scholar] [CrossRef]
- Acar, H.; Ting, J.M.; Srivastava, S.; La Belle, J.L.; Tirrell, M.V. Molecular engineering solutions for therapeutic peptide delivery. Chem. Soc. Rev. 2017, 46, 6553–6569. [Google Scholar] [CrossRef]
- Shi, J.; Votruba, A.R.; Farokhzad, O.C.; Langer, R. Nanotechnology in Drug Delivery and Tissue Engineering: From Discovery to Applications. Nano Lett. 2010, 10, 3223–3230. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Fan, C.; Pei, H.; Shi, J.; Huang, Q. Smart Drug Delivery Nanocarriers with Self-Assembled DNA Nanostructures. Adv. Mater. 2013, 25, 4386–4396. [Google Scholar] [CrossRef]
- Liu, D.; Yang, F.; Xiong, F.; Gu, N. The smart drug delivery system and its clinical potential. Theranostics 2016, 6, 1306–1323. [Google Scholar] [CrossRef]
- Ramasamy, T.; Ruttala, H.B.; Gupta, B.; Poudel, B.K.; Choi, H.-G.; Yong, C.S.; Kim, J.O. Smart chemistry-based nanosized drug delivery systems for systemic applications: A comprehensive review. J. Control. Release 2017, 258, 226–253. [Google Scholar] [CrossRef]
- Rezaie, H.R.; Bakhtiari, L.; Öchsner, A. A Review of Biomaterials and their Applications in Drug Delivery; Springer: Singapore, 2018. [Google Scholar]
- Park, K. Controlled drug delivery systems: Past forward and future back. J. Control. Release 2014, 190, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Rancan, F.; Papakostas, D.; Hadam, S.; Hackbarth, S.; Delair, T.; Primard, C.; Verrier, B.; Sterry, W.; Blume-Peytavi, U.; Vogt, A. Investigation of Polylactic Acid (PLA) Nanoparticles as Drug Delivery Systems for Local Dermatotherapy. Pharm. Res. 2009, 26, 2027–2036. [Google Scholar] [CrossRef]
- Pellis, A.; Silvestrini, L.; Scaini, D.; Coburn, J.M.; Gardossi, L.; Kaplan, D.L.; Herrero Acero, E.; Guebitz, G.M. Enzyme-catalyzed functionalization of poly(L-lactic acid) for drug delivery applications. Process. Biochem. 2017, 59 Pt A, 77–83. [Google Scholar] [CrossRef]
- Fukuzaki, H.; Yoshida, M.; Asano, M.; Kumakura, M.; Mashimo, T.; Yuasa, H.; Imai, K.; Yamanaka, H. In vivo characteristics of high molecular weight copoly(L-lactide/glycolide) with S-type degradation pattern for application in drug delivery systems. Biomaterials 1991, 12, 433–437. [Google Scholar] [CrossRef]
- Xu, P.; Gullotti, E.; Tong, L.; Highley, C.B.; Errabelli, D.R.; Hasan, T.; Cheng, J.-X.; Kohane, D.S.; Yeo, Y. Intracellular Drug Delivery by Poly(lactic-co-glycolic acid) Nanoparticles, Revisited. Mol. Pharm. 2009, 6, 190–201. [Google Scholar] [CrossRef]
- Xiao, Y.; Yuan, M.; Zhang, J.; Yan, J.; Lang, M. Functional Poly(ε-caprolactone) Based Materials: Preparation, Self-assembly and Application in Drug Delivery. Curr. Top. Med. Chem. 2014, 14, 781–818. [Google Scholar] [CrossRef]
- Michalak, M.; Kurcok, P.; Hakkarainen, M. Polyhydroxyalkanoate-based drug delivery systems. Polym. Int. 2017, 66, 617–622. [Google Scholar] [CrossRef]
- Shrivastav, A.; Kim, H.-Y.; Kim, Y.-R. Advances in the applications of polyhydroxyalkanoate nanoparticles for novel drug delivery system. BioMed Res. Int. 2013, 2013, 581684. [Google Scholar] [CrossRef]
- Merli, D.; Profumo, A.; Quadrelli, P.; Arciola, C.R.; Visai, L. Drug Delivery Systems for Chemotherapeutics through Selected Polysaccharidic Vehicles. Curr. Org. Chem. 2018, 22, 1157–1192. [Google Scholar] [CrossRef] [Green Version]
- Salehi Dashtebayaz, M.S.; Nourbakhsh, M.S. Interpenetrating networks hydrogels based on hyaluronic acid for drug delivery and tissue engineering. Int. J. Polym. Mater. Polym. Biomater. 2019, 68, 442–451. [Google Scholar] [CrossRef]
- Siepmann, J.; Siegel, R.A.; Rathbone, M.J. Fundamentals and Applications of Controlled Release Drug Delivery; Springer: New York, NY, USA, 2011; p. 594. [Google Scholar]
- Thakur, V.K.; Thakur, M.K. Handbook of Polymers for Pharmaceutical Technologies, Biodegradable Polymers; Wiley: Hoboken, NJ, USA, 2015; p. 608. [Google Scholar]
- Koller, M.; Marsalek, L.; de Sousa Dias, M.M.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017, 37 Pt A, 24–38. [Google Scholar] [CrossRef]
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174. [Google Scholar] [CrossRef]
- Ray, S.; Kalia, V.C. Biomedical Applications of Polyhydroxyalkanoates. Indian J. Microbiol. 2017, 57, 261–269. [Google Scholar] [CrossRef]
- Kovalcik, A.; Meixner, K.; Mihalic, M.; Zeilinger, W.; Fritz, I.; Fuchs, W.; Kucharczyk, P.; Stelzer, F.; Drosg, B. Characterization of polyhydroxyalkanoates produced by Synechocystis salina from digestate supernatant. Int. J. Biol. Macromol. 2017, 102, 497–504. [Google Scholar] [CrossRef]
- Pouton, C.W.; Akhtar, S. Biosynthetic polyhydroxyalkanoates and their potential in drug delivery. Adv. Drug Deliv. Rev. 1996, 18, 133–162. [Google Scholar] [CrossRef]
- Atkins, T.W.; Peacock, S.J. In vitro biodegradation of polyhydroxybutyrate-hydroxyvalerate microcapsules exposed to Hank’s buffer, newborn calf serum, pancreatin and synthetic gastric juice. J. Microencapsul. 1997, 14, 35–49. [Google Scholar] [CrossRef]
- Yu, J.; Plackett, D.; Chen, L.X.L. Kinetics and mechanism of the monomeric products from abiotic hydrolysis of poly[(R)-3-hydroxybutyrate] under acidic and alkaline conditions. Polym. Degrad. Stabil. 2005, 89, 289–299. [Google Scholar] [CrossRef]
- Gogolewski, S.; Jovanovic, M.; Perren, S.M.; Dillon, J.G.; Hughes, M.K. Tissue response and in vivo degradation of selected polyhydroxyacids: Polylactides (PLA), poly(3-hydroxybutyrate) (PHB), and poly(3-hydroxybutyrate-co-3-hydroxyvalerate) (PHB/VA). J. Biomed. Mater. Res. 1993, 27, 1135–1148. [Google Scholar] [CrossRef]
- Asiri, A.M.; Mohammad, A. Applications of Nanocomposite Materials in Drug Delivery; Elsevier Science: Cambridge, UK, 2018. [Google Scholar]
- Cao, K.; Liu, Y.; Olkhov, A.; Siracusa, V.; Iordanskii, A. PLLA-PHB fiber membranes obtained by solvent-free electrospinning for short-time drug delivery. Drug Deliv. Transl. Res. 2018, 8, 291–302. [Google Scholar] [CrossRef]
- Acevedo, F.; Villegas, P.; Urtuvia, V.; Seeger, M.; Hermosilla, J.; Navia, R. Bacterial polyhydroxybutyrate for electrospun fiber production. Int. J. Biol. Macromol. 2018, 106, 692–697. [Google Scholar] [CrossRef]
- Ding, Y.; Li, W.; Zhang, F.; Liu, Z.; Zanjanizadeh Ezazi, N.; Liu, D.; Santos, H.A. Electrospun Fibrous Architectures for Drug Delivery, Tissue Engineering and Cancer Therapy. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Koller, M.; Koller, M. Biodegradable and Biocompatible Polyhydroxy-alkanoates (PHA): Auspicious Microbial Macromolecules for Pharmaceutical and Therapeutic Applications. Molecules 2018, 23, 362. [Google Scholar] [CrossRef]
- Fernandes, J.G.; Correia, D.M.; Botelho, G.; Padrao, J.; Dourado, F.; Ribeiro, C.; Lanceros-Mendez, S.; Sencadas, V. PHB-PEO electrospun fiber membranes containing chlorhexidine for drug delivery applications. Polym. Test. 2014, 34, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Yan, K.-W.; Lin, Y.-D.; Hsieh, P.C.H. Biodegradable Core/Shell Fibers by Coaxial Electrospinning: Processing, Fiber Characterization, and Its Application in Sustained Drug Release. Macromolecules 2010, 43, 6389–6397. [Google Scholar] [CrossRef]
- Doi, Y.; Kanesawa, Y.; Kunioka, M.; Saito, T. Biodegradation of microbial copolyesters: Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) and poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Macromolecules 1990, 23, 26–31. [Google Scholar] [CrossRef]
- Lee, Y.-F.; Sridewi, N.; Ramanathan, S.; Sudesh, K. The influence of electrospinning parameters and drug loading on polyhydroxyalkanoate (PHA) nanofibers for drug delivery. Int. J. Biotechnol. Wellness Ind. 2015, 4, 103–113. [Google Scholar]
- Mahaling, B.; Katti, D.S. Fabrication of micro-structures of poly [(R)-3-hydroxybutyric acid] by electro-spraying/-spinning: Understanding the influence of polymer concentration and solvent type. J. Mater. Sci. 2014, 49, 4246–4260. [Google Scholar] [CrossRef]
- Mhlanga, N.; Ray, S.S. Kinetic models for the release of the anticancer drug doxorubicin from biodegradable polylactide/metal oxide-based hybrids. Int. J. Biol. Macromol. 2015, 72, 1301–1307. [Google Scholar] [CrossRef]
- Naveen, N.; Kumar, R.; Balaji, S.; Uma, T.S.; Natrajan, T.S.; Sehgal, P.K. Synthesis of Nonwoven Nanofibers by Electrospinning—A Promising Biomaterial for Tissue Engineering and Drug Delivery. Adv. Eng. Mater. 2010, 12, B380–B387. [Google Scholar] [CrossRef]
- Levofloxacin. Available online: https://www.drugbank.ca/drugs/DB01137 (accessed on 29 April 2019).
- Correia, D.M.; Ribeiro, C.; Ferreira, J.C.; Botelho, G.; Ribelles, J.L.G.; Lanceros-Méndez, S.; Sencadas, V. Influence of electrospinning parameters on poly (hydroxybutyrate) electrospun membranes fiber size and distribution. Polym. Eng. Sci. 2014, 54, 1608–1617. [Google Scholar] [CrossRef]
- Sofokleous, P.; Stride, E.; Edirisinghe, M. Preparation, characterization, and release of amoxicillin from electrospun fibrous wound dressing patches. Pharm. Res. 2013, 30, 1926–1938. [Google Scholar] [CrossRef]
- Fan, X.; Jiang, Q.; Sun, Z.; Li, G.; Ren, X.; Liang, J.; Huang, T. Preparation and characterization of electrospun antimicrobial fibrous membranes based on polyhydroxybutyrate (PHB). Fibers Polym. 2015, 16, 1751–1758. [Google Scholar] [CrossRef]
- Soares, G.M.S.; Figueiredo, L.C.; Faveri, M.; Cortelli, S.C.; Duarte, P.M.; Feres, M. Mechanisms of action of systemic antibiotics used in periodontal treatment and mechanisms of bacterial resistance to these drugs. J. Appl. Oral. Sci. 2012, 20, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, G.; Saigal, S.; Elongavan, A. Action and resistance mechanisms of antibiotics: A guide for clinicians. J. Anaesthesiol. Clin. Pharmacol. 2017, 33, 300–305. [Google Scholar] [CrossRef]
- Percival, S.L.; Suleman, L.; Vuotto, C.; Donelli, G. Healthcare-associated infections, medical devices and biofilms: Risk, tolerance and control. J. Med. Microbiol. 2015, 64, 323–334. [Google Scholar] [CrossRef]
- Chen, S.; Liu, B.; Carlson, M.A.; Gombart, A.F.; Reilly, D.A.; Xie, J. Recent advances in electrospun nanofibers for wound healing. Nanomedicine 2017, 12, 1335–1352. [Google Scholar] [CrossRef]
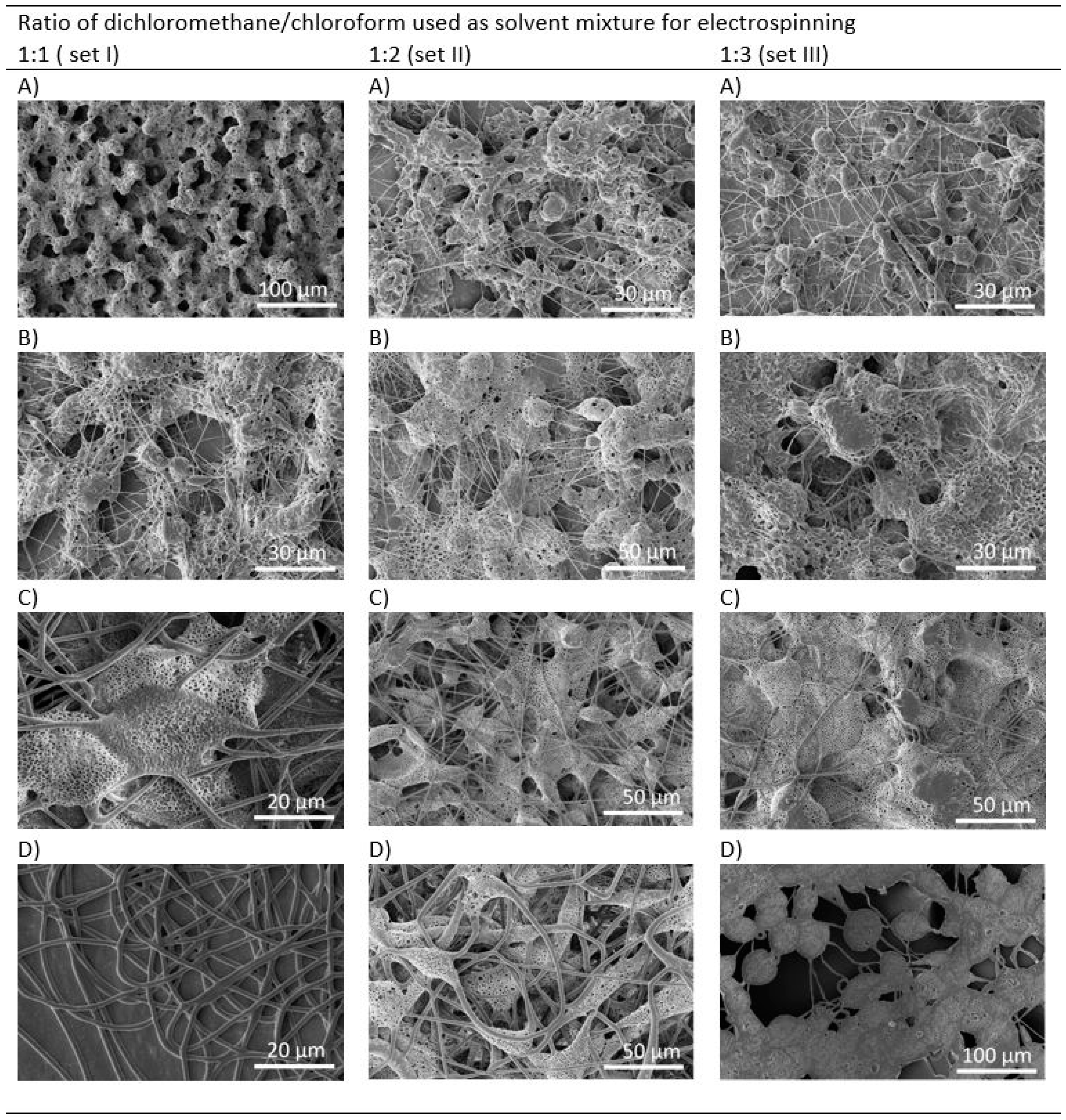
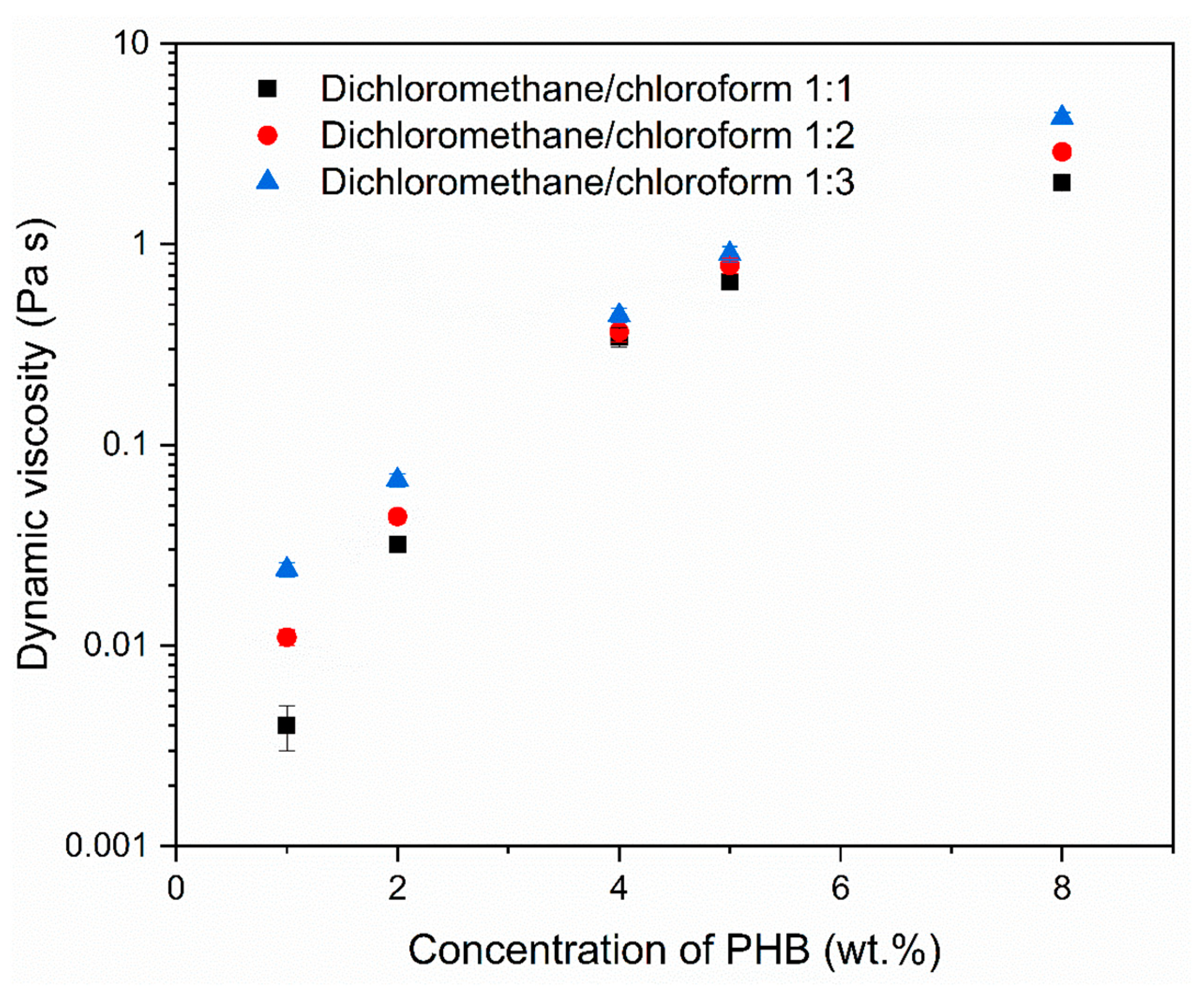
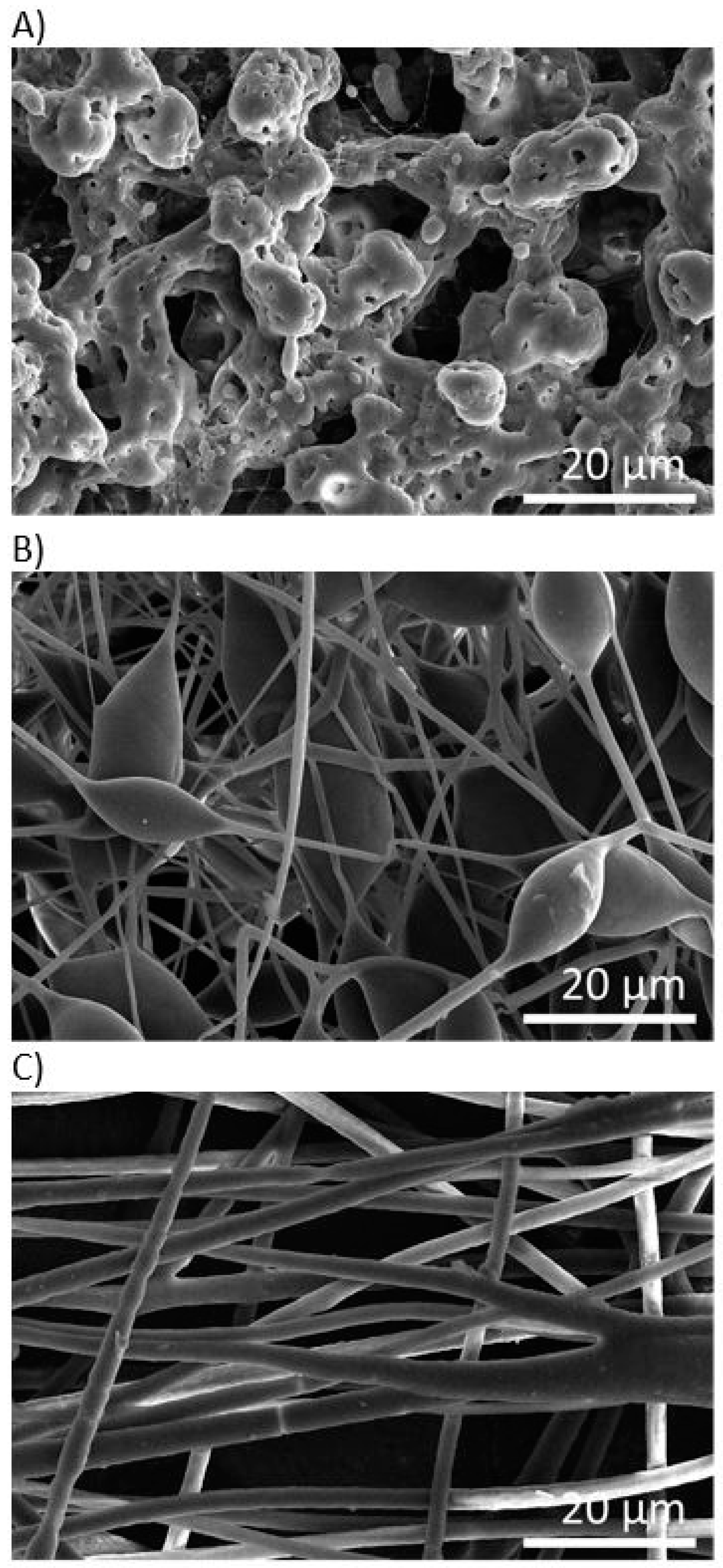
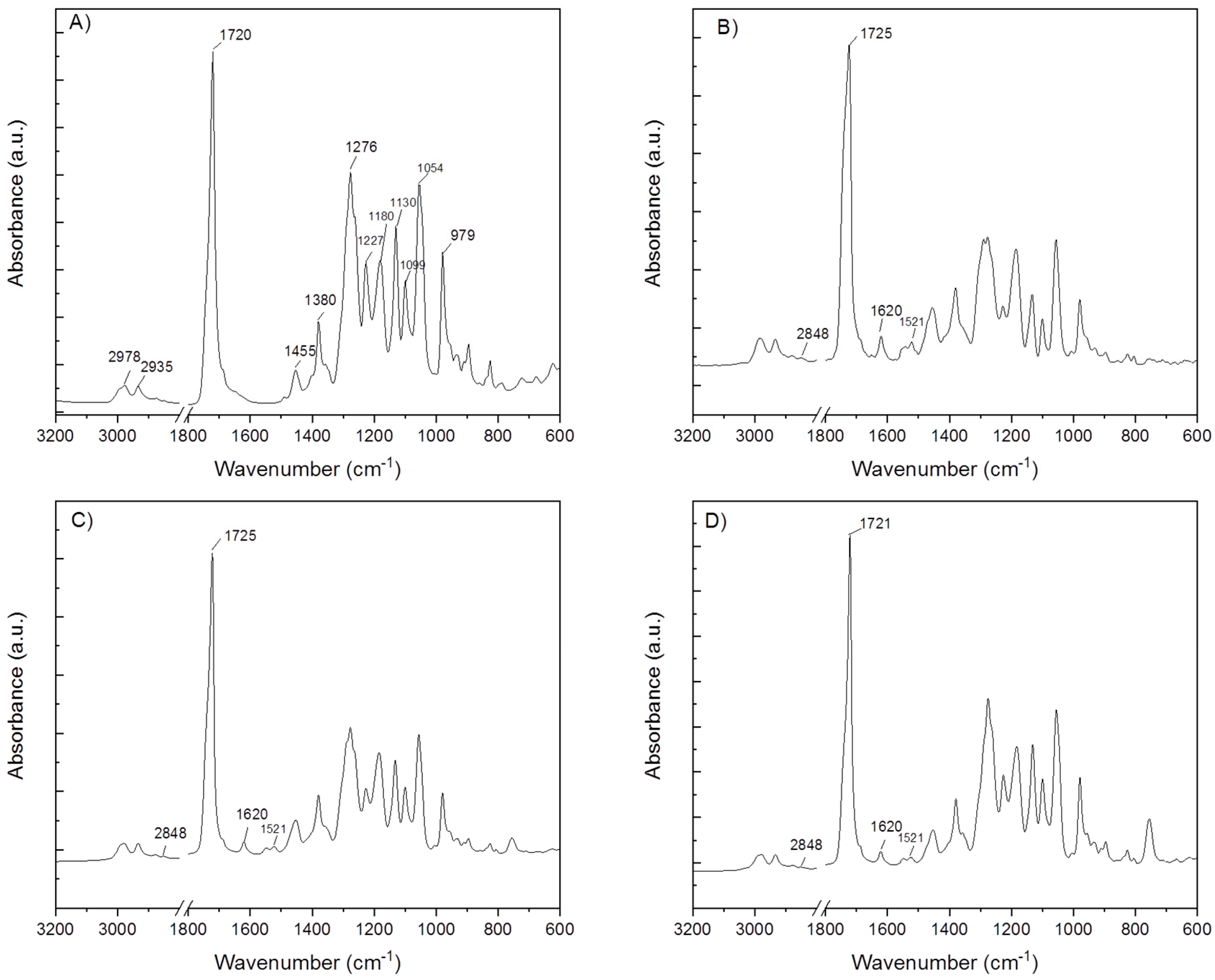

| Electrode Distance (mm) | Syringe Diameter (mm) | Needle Diameter (mm) | Voltage Difference (kV) | Intensity of Electrical Field (kV/mm) | Feed Rate (µL/min) |
|---|---|---|---|---|---|
| 200 ± 1 | 18.0 | 1.0 | 20.0 ± 0.1 | 1 | 100 |
| Solvent | Boiling Point (°C) | Dynamic Viscosity at 20 °C (MPa) | Vapour Pressure at 20 °C (kPa) | Dielectric Constant at 20 °C | Surface Tension at 20 °C (mN·m−1) |
|---|---|---|---|---|---|
| Dichloromethane | 40 | 0.43 | 47 | 9.1 | 28.1 |
| Chloroform | 61 | 0.58 | 21 | 4.8 | 27.2 |
| Sample | Growth Inhibition Halo (mm) | |||||
|---|---|---|---|---|---|---|
| Micrococcus Luteus | Serratia Marcescens | Escherichia Coli | ||||
| a Measured Value | b Corrected Value | Measured Value | Corrected Value | Measured Value | Corrected Value | |
| EM_1_L | 3.7 ± 1.1 | 3.7 ± 1.1 | 4.1 ± 1.1 | 4.1 ± 1.1 | 5.1 ± 1.1 | 5.1 ± 1.1 |
| EM_4_L | 0.8 ± 1.0 | 0.75 ± 0.9 | 1.2 ± 1.2 | 1.1 ± 1.1 | 2.0 ± 1.1 | 1.9 ± 1.0 |
| EM_5_L | 2.1 ± 1.2 | 0.65 ± 0.4 | 2.2 ± 1.1 | 0.68 ± 0.3 | 3.0 ± 1.0 | 0.93 ± 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kundrat, V.; Cernekova, N.; Kovalcik, A.; Enev, V.; Marova, I. Drug Release Kinetics of Electrospun PHB Meshes. Materials 2019, 12, 1924. https://doi.org/10.3390/ma12121924
Kundrat V, Cernekova N, Kovalcik A, Enev V, Marova I. Drug Release Kinetics of Electrospun PHB Meshes. Materials. 2019; 12(12):1924. https://doi.org/10.3390/ma12121924
Chicago/Turabian StyleKundrat, Vojtech, Nicole Cernekova, Adriana Kovalcik, Vojtech Enev, and Ivana Marova. 2019. "Drug Release Kinetics of Electrospun PHB Meshes" Materials 12, no. 12: 1924. https://doi.org/10.3390/ma12121924
APA StyleKundrat, V., Cernekova, N., Kovalcik, A., Enev, V., & Marova, I. (2019). Drug Release Kinetics of Electrospun PHB Meshes. Materials, 12(12), 1924. https://doi.org/10.3390/ma12121924






