Effect of Thermal Treatments on Sn-Alloyed Al-Mg-Si Alloys
Abstract
1. Introduction
2. Materials and Methods
3. Results
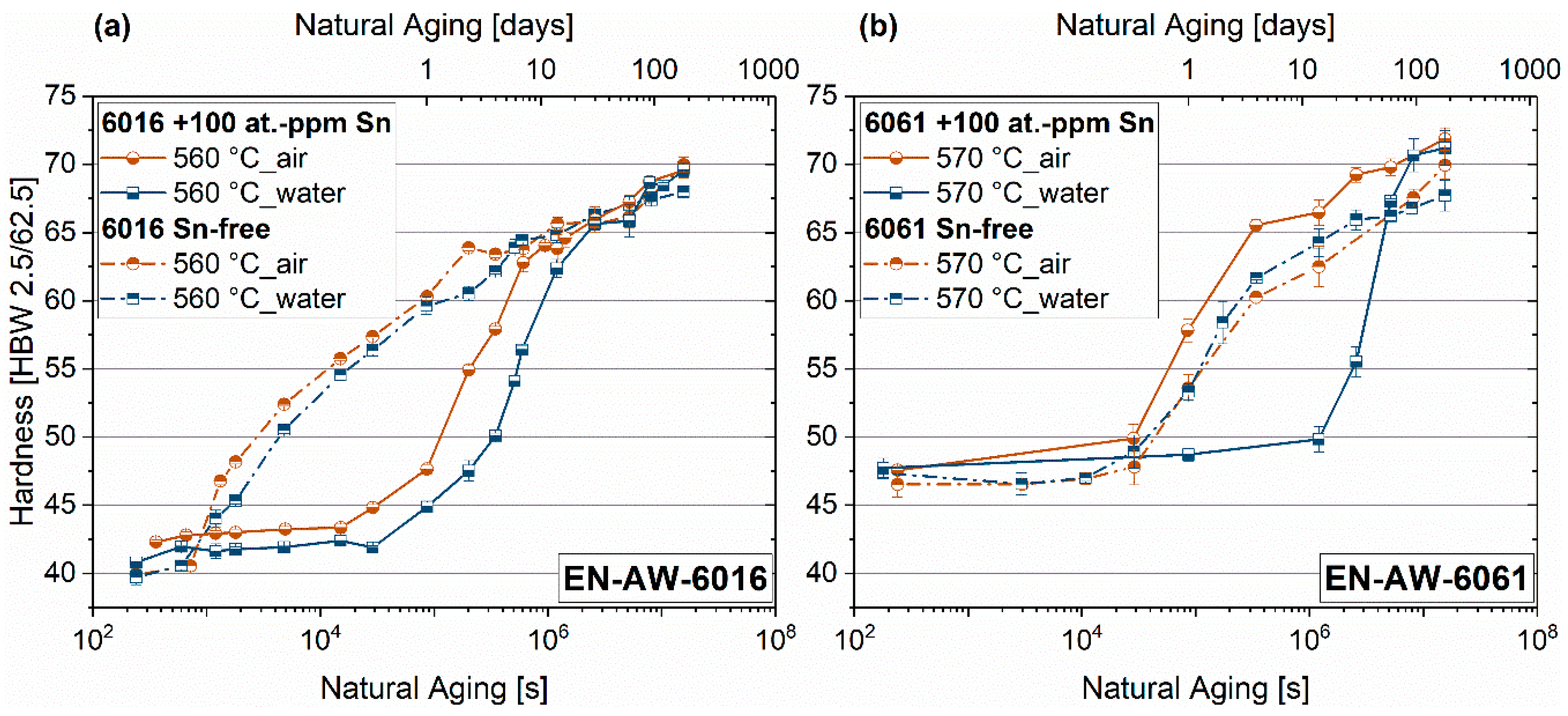
3.1. Quench Sensitivity
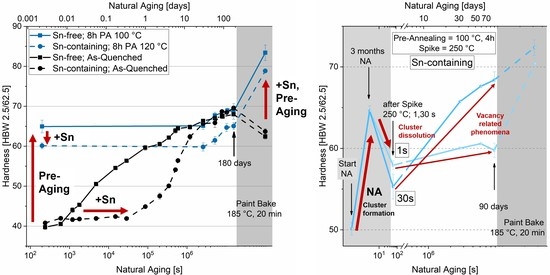
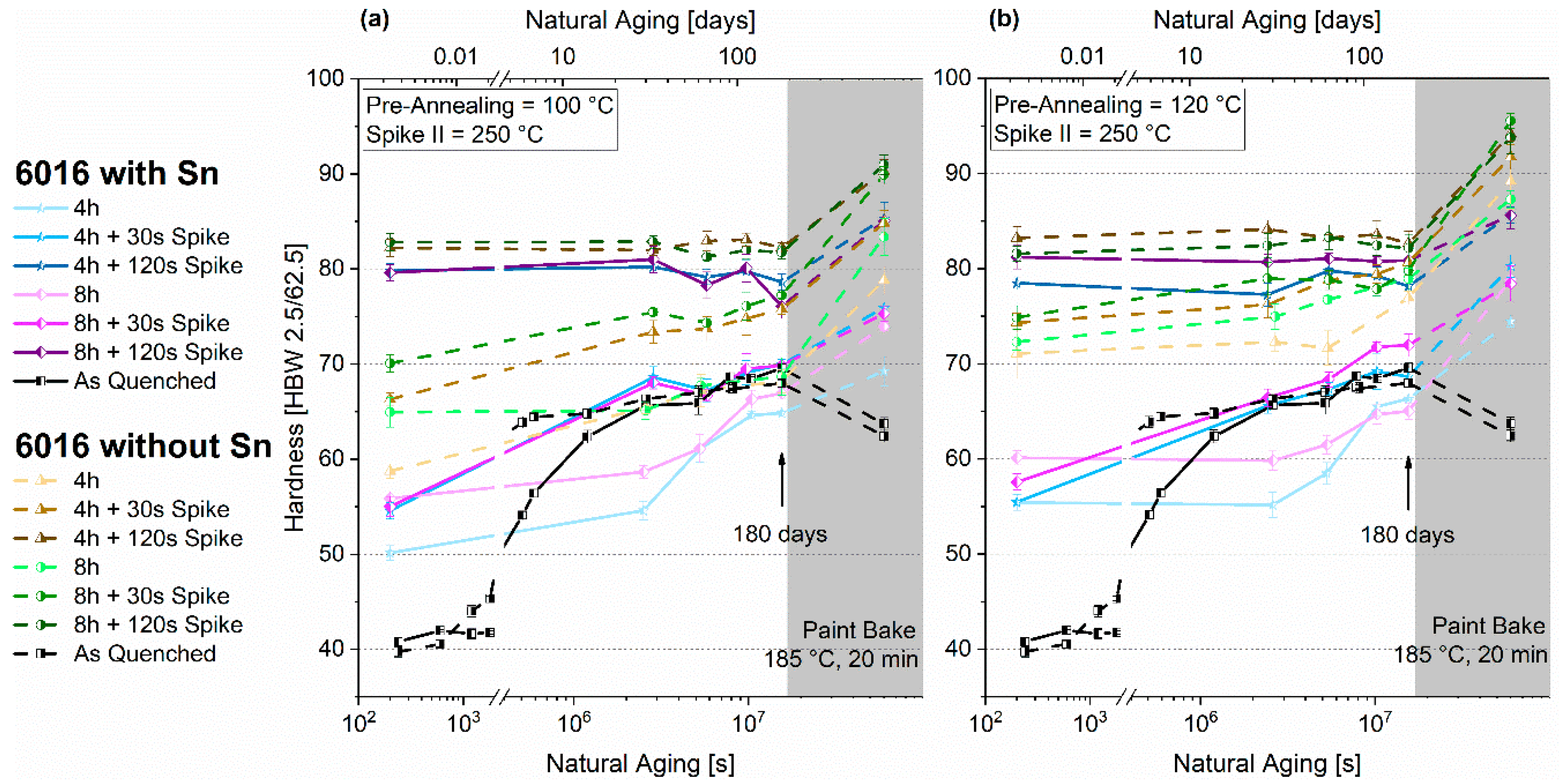
3.2. Pre-Aging 1
3.2.1. Natural Aging
3.2.2. Paint-Bake Simulation
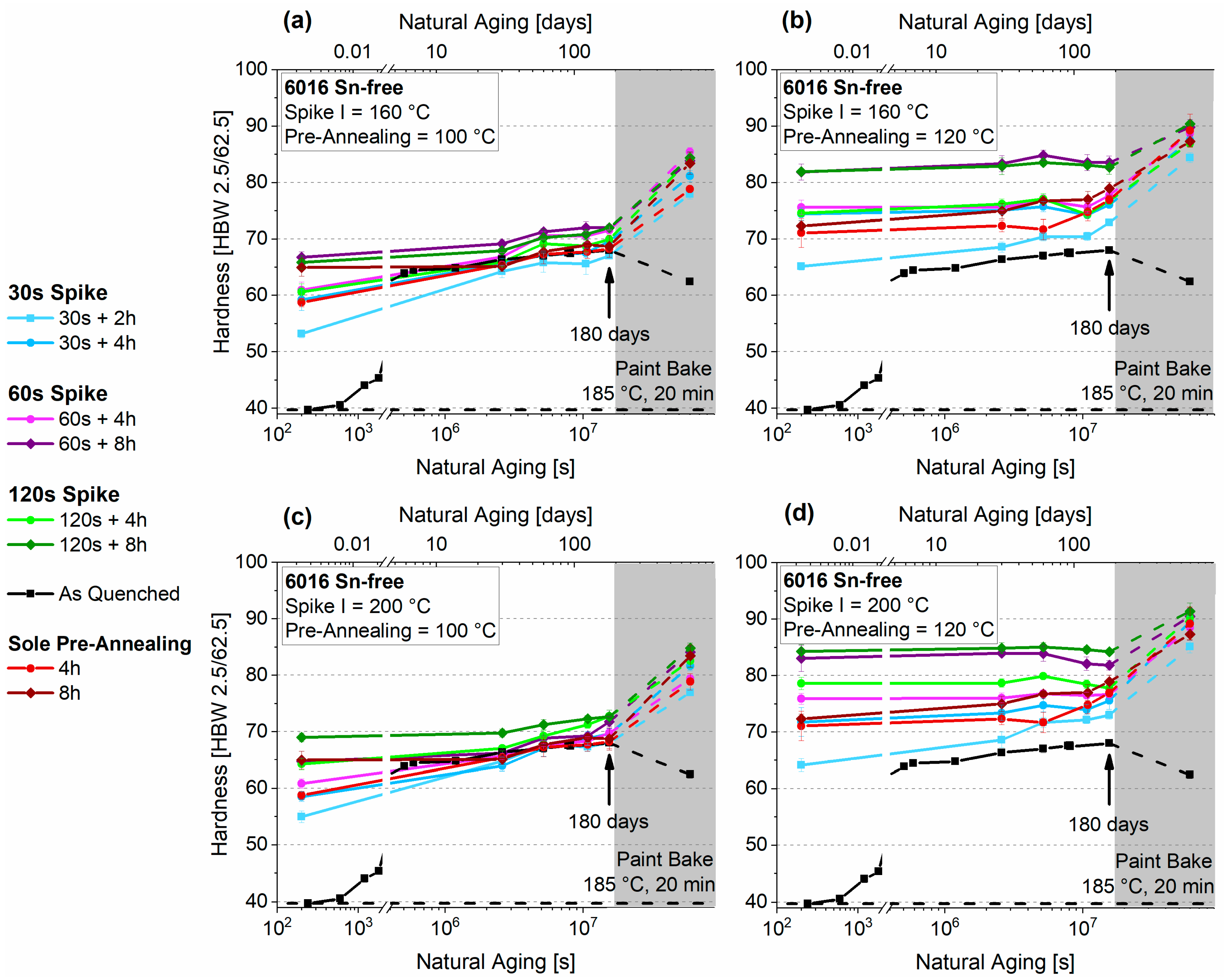
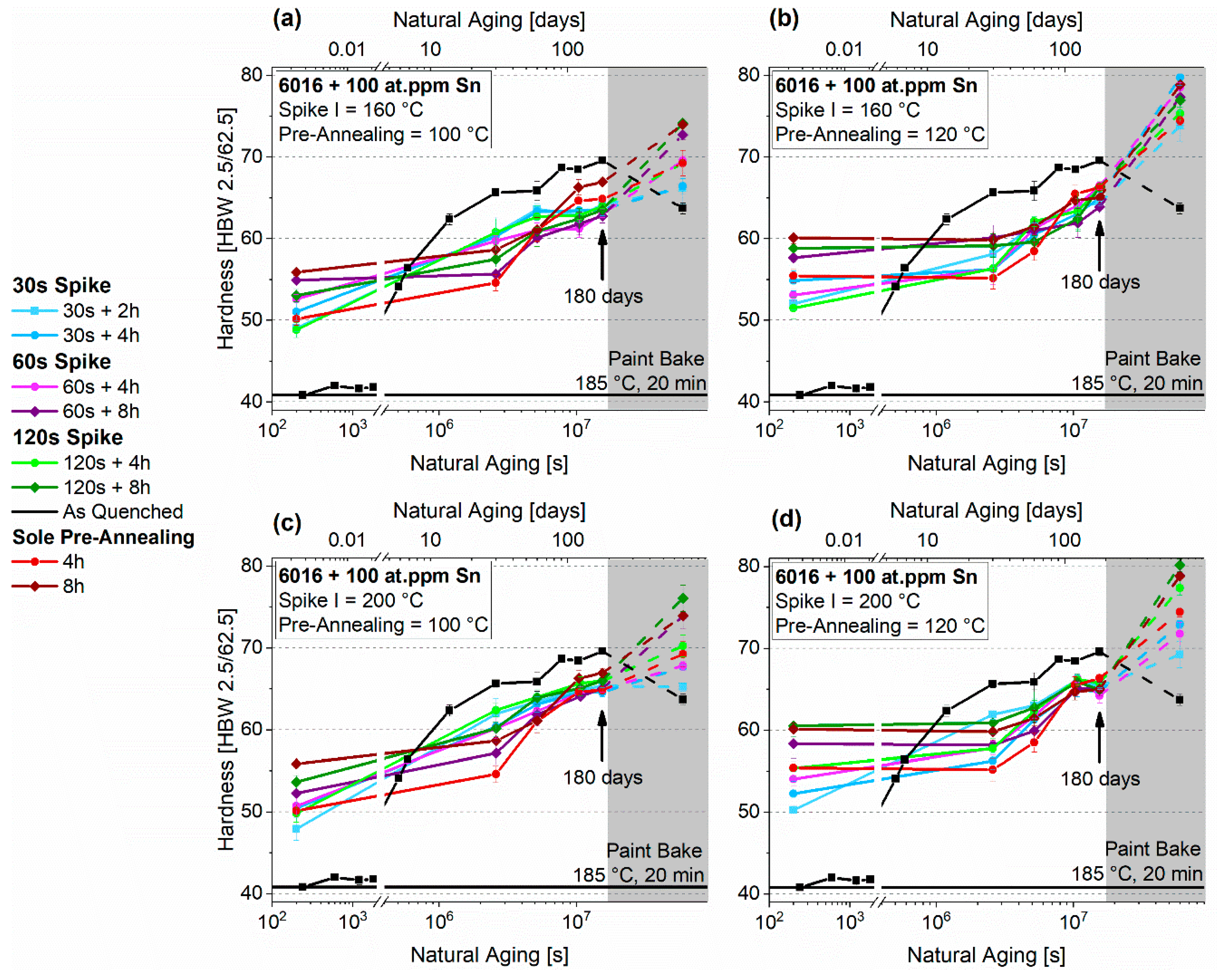
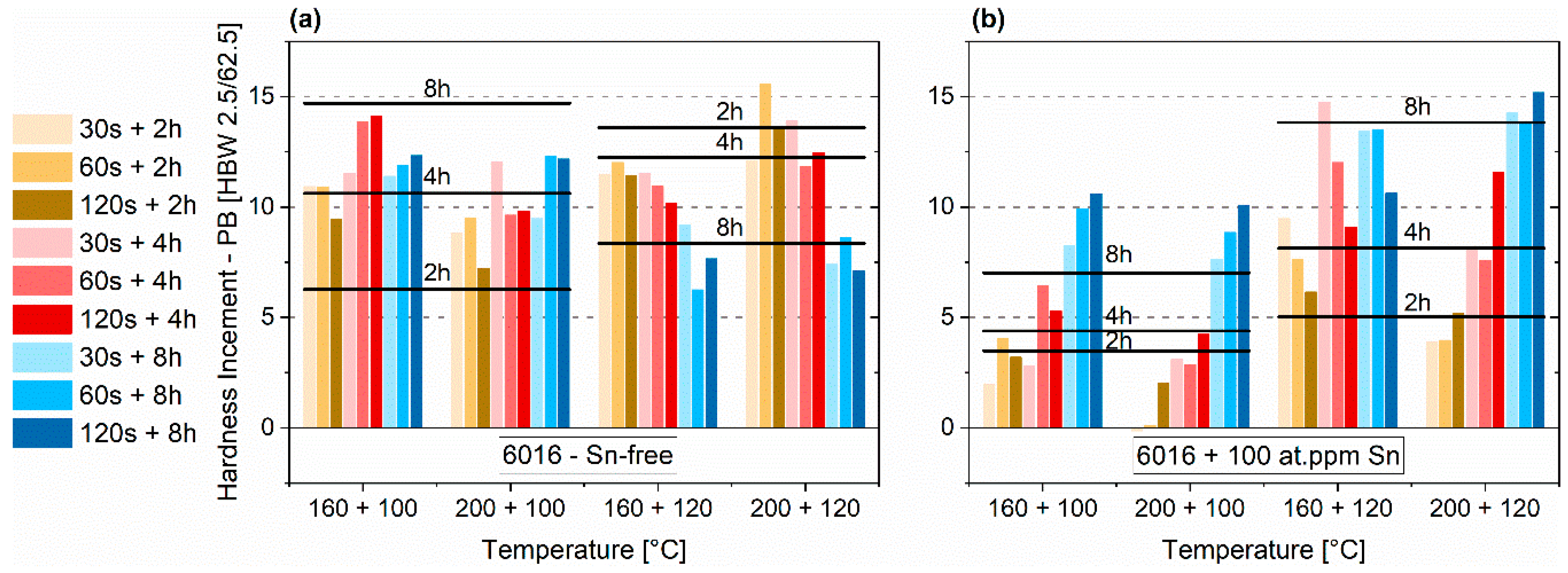
3.3. Pre-Aging 2
3.3.1. Natural Aging
3.3.2. Paint-Bake Simulation
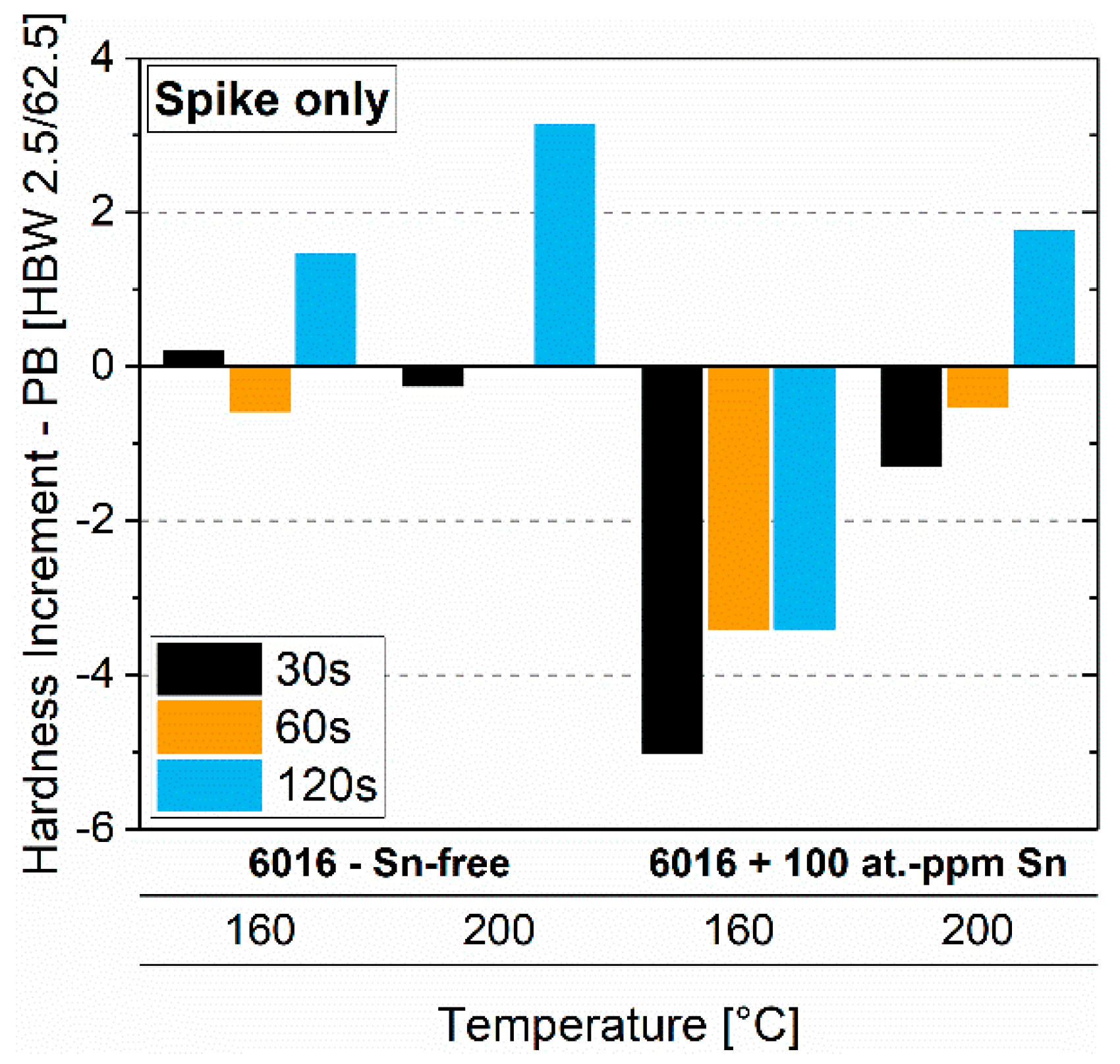
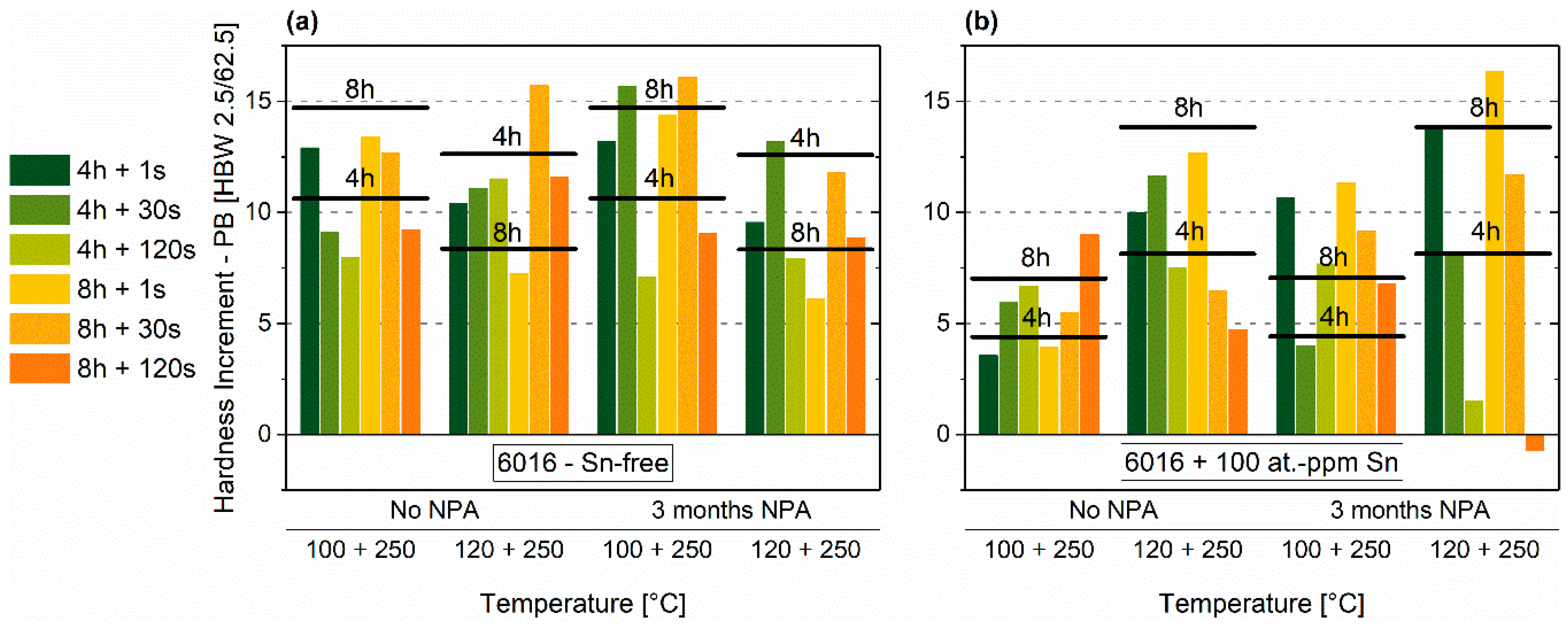
3.4. Pre-Aging 3
3.4.1. Natural Aging
3.4.2. Paint-Bake Simulation
4. Discussion
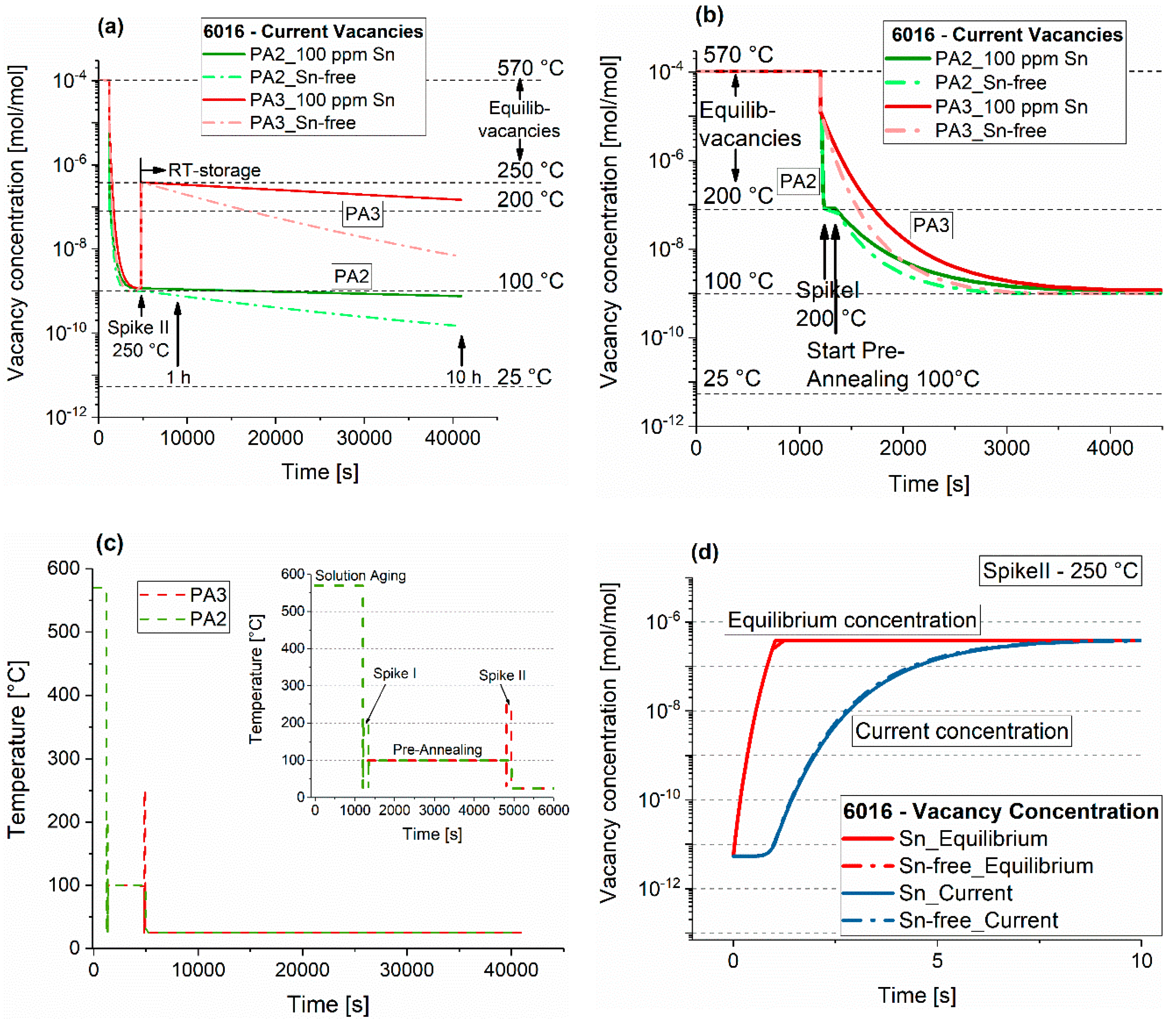
4.1. Quench Sensitivity
4.2. Conventional Pre-Aging (PA1)
4.3. Thermal Spike Plus Pre-Annealing (PA2)
4.4. Pre-Annealing Plus Subsequent Thermal Spike (PA3)
5. Conclusions
- Sn-containing alloys are sensitive to the quenching technique where there are large numbers of incoherent particles in the material. If the quenching rate is too slow, Sn forms precipitates and is no longer active as a vacancy trap.
- Sn decreases clustering kinetics during pre-aging. Therefore, the temperature must be increased by at least 20 °C from 100 to 120 °C to obtain a hardness increase similar to that of Sn-free alloys. In this way, natural aging is suppressed and a comparable hardness increase during the paint-bake simulation can be realized.
- A high-temperature spike (250 °C) alters the natural aging behavior. Applied after three months of natural aging of pre-annealed material, it can lead to a reversion and will either accelerate or suppress subsequent natural aging.
- A spike of a few seconds can only dissolve clusters. More than 8 s are required to reach the equilibrium vacancy concentration at 250 °C, which accelerates subsequent natural aging.
- Among all tested heat treatment combinations, many of them showed promising hardness increases upon paint-bake simulation, which lie on a similar level. Yet, the question arises if other pre-aging treatments could lead to enhanced results. Notably, the Sn-containing alloy exhibited a good paint-bake response, even though the hardness after pre-aging stays at lower values compared to the Sn-free alloy.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ostermann, F. Anwendungstechnologie Aluminium; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Hirsch, J. Aluminium sheet fabrication and processing. In Fundamentals of Aluminium Metallurgy; Elsevier BV: Amsterdam, The Netherlands, 2011; pp. 719–746. [Google Scholar]
- Hirsch, J. Recent development in aluminium for automotive applications. Trans. Nonferrous Met. Soc. China 2014, 24, 1995–2002. [Google Scholar] [CrossRef]
- Banhart, J.; Lay, M.D.H.; Chang, C.S.T.; Hill, A.J. Kinetics of natural aging in Al-Mg-Si alloys studied by positron annihilation lifetime spectroscopy. Phys. B 2011, 83, 83. [Google Scholar] [CrossRef]
- Aruga, Y.; Kozuka, M.; Takaki, Y.; Sato, T. Formation and reversion of clusters during natural aging and subsequent artificial aging in an Al–Mg–Si alloy. Mater. Sci. Eng. A 2015, 631, 86–96. [Google Scholar] [CrossRef]
- Zandbergen, M.; Xu, Q.; Cerezo, A.; Smith, G. Study of precipitation in Al–Mg–Si alloys by Atom Probe Tomography I. Microstructural changes as a function of ageing temperature. Acta Mater. 2015, 101, 136–148. [Google Scholar] [CrossRef]
- Chang, C.; Wieler, I.; Wanderka, N.; Banhart, J. Positive effect of natural pre-ageing on precipitation hardening in Al–0.44at% Mg–0.38at% Si alloy. Ultramicroscopy 2009, 109, 585–592. [Google Scholar] [CrossRef]
- Pogatscher, S.; Antrekowitsch, H.; Ebner, T.; Uggowitzer, P.J. The Role of Co-Clusters in the Artificial Aging of AA6061 and AA6060. In Light Metals 2012; Springer: Berlin, Germany, 2012; pp. 413–420. [Google Scholar]
- Ding, L.; He, Y.; Wen, Z.; Zhao, P.; Jia, Z.; Liu, Q. Optimization of the pre-aging treatment for an AA6022 alloy at various temperatures and holding times. J. Alloy. Compd. 2015, 647, 238–244. [Google Scholar] [CrossRef]
- Birol, Y. Pre-aging to improve bake hardening in a twin-roll cast Al–Mg–Si alloy. Mater. Sci. Eng. A 2005, 391, 175–180. [Google Scholar] [CrossRef]
- Grau, V.N.; Cuniberti, A.; Tolley, A.; Riglos, V.C.; Stipcich, M. Solute clustering behavior between 293K and 373K in a 6082 Aluminum alloy. J. Alloy. Compd. 2016, 684, 481–487. [Google Scholar] [CrossRef]
- De Geuser, F.; Lefebvre, W.; Blavette, D. 3D atom probe study of solute atoms clustering during natural ageing and pre-ageing of an Al-Mg-Si alloy. Philos. Mag. Lett. 2006, 86, 227–234. [Google Scholar] [CrossRef]
- Torsæter, M.; Hasting, H.S.; Lefebvre, W.; Marioara, C.D.; Walmsley, J.C.; Andersen, S.J.; Holmestad, R. The influence of composition and natural aging on clustering during preaging in Al–Mg–Si alloys. J. Appl. Phys. 2010, 108, 73527. [Google Scholar] [CrossRef]
- Aruga, Y.; Kozuka, M.; Takaki, Y.; Sato, T. Evaluation of Solute Clusters Associated with Bake-Hardening Response in Isothermal Aged Al-Mg-Si Alloys Using a Three-Dimensional Atom Probe. Met. Mater. Trans. A 2014, 45, 5906–5913. [Google Scholar] [CrossRef]
- Aruga, Y.; Kozuka, M.; Takaki, Y.; Sato, T. Effects of natural aging after pre-aging on clustering and bake-hardening behavior in an Al–Mg–Si alloy. Scr. Mater. 2016, 116, 82–86. [Google Scholar] [CrossRef]
- Dumitraschkewitz, P.; Gerstl, S.S.A.; Stephenson, L.T.; Uggowitzer, P.J.; Pogatscher, S. Clustering in Age-Hardenable Aluminum Alloys. Adv. Eng. Mater. 2018, 20, 1800255. [Google Scholar] [CrossRef]
- Cao, L.; Rometsch, P.A.; Couper, M.J. Effect of pre-ageing and natural ageing on the paint bake response of alloy AA6181A. Mater. Sci. Eng. A 2013, 571, 77–82. [Google Scholar] [CrossRef]
- Li, H.; Yan, Z.; Cao, L.; Lia, H. Bake hardening behavior and precipitation kinetic of a novel Al-Mg-Si-Cu aluminum alloy for lightweight automotive body. Mater. Sci. Eng. A 2018, 728, 88–94. [Google Scholar] [CrossRef]
- Madanat, M.; Liu, M.; Banhart, J. Reversion of natural ageing in Al-Mg-Si alloys. Acta Mater. 2018, 159, 163–172. [Google Scholar] [CrossRef]
- Gao, G.; Li, Y.; Wang, Z.; Misra, R.; Li, J.; Xu, G. Study of retrogression response in naturally and multi-step aged Al-Mg-Si automotive sheets. J. Alloy. Compd. 2018, 753, 457–464. [Google Scholar] [CrossRef]
- Pogatscher, S.; Antrekowitsch, H.; Werinos, M.; Moszner, F.; Gerstl, S.S.A.; Francis, M.F.; Curtin, W.A.; Löffler, J.F.; Uggowitzer, P.J. Diffusion on Demand to Control Precipitation Aging: Application to Al-Mg-Si Alloys. Phys. Lett. 2014, 112, 225701. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.; Körner, B.; Elsayed, M.; Liang, Z.; Leyvraz, D.; Banhart, J. Effect of Sn and In on the natural ageing kinetics of Al–Mg–Si alloys. Materialia 2019, 6, 100261. [Google Scholar] [CrossRef]
- Werinos, M.; Antrekowitsch, H.; Ebner, T.; Prillhofer, R.; Curtin, W.; Uggowitzer, P.; Pogatscher, S. Design strategy for controlled natural aging in Al–Mg–Si alloys. Acta Mater. 2016, 118, 296–305. [Google Scholar] [CrossRef]
- Werinos, M.; Antrekowitsch, H.; Kozeschnik, E.; Ebner, T.; Moszner, F.; Löffler, J.; Uggowitzer, P.; Pogatscher, S.; Uggowitzer, P. Ultrafast artificial aging of Al–Mg–Si alloys. Scr. Mater. 2016, 112, 148–151. [Google Scholar] [CrossRef]
- Werinos, M.; Antrekowitsch, H.; Fragner, W.; Ebner, T.; Uggowitzer, P.J.; Pogatscher, S. Influence of Sn-solubility on suppression of natural aging in an AA6061 aluminium alloy. In Proceedings of the Materials Science & Technology (MS&T), Pittsburgh, PA, USA, 12–16 October 2014. [Google Scholar]
- MatCalc software package for computer simulation of phase transformation and microstructure evolution in metallic systems. Available online: www.matcalc-engineering.com (accessed on 27 April 2019).
- Fischer, F.; Svoboda, J.; Appel, F.; Kozeschnik, E. Modeling of excess vacancy annihilation at different types of sinks. Acta Mater. 2011, 59, 3463–3472. [Google Scholar] [CrossRef]
- Povoden-Karadeniz, E.; Lang, P.; Warczok, P.; Falahati, A.; Jun, W.; Kozeschnik, E. CALPHAD modeling of metastable phases in the Al–Mg–Si system. Calphad 2013, 43, 94–104. [Google Scholar] [CrossRef]
- Werinos, M.; Antrekowitsch, H.; Ebner, T.; Prillhofer, R.; Uggowitzer, P.; Pogatscher, S. Hardening of Al–Mg–Si alloys: Effect of trace elements and prolonged natural aging. Mater. Des. 2016, 107, 257–268. [Google Scholar] [CrossRef]
- Kuijpers, N.C.W.; Tirel, J.; Hanlon, D.N.; van der Zwaag, S. Characterization of the alpha-Al(FeMn)Si nuclei on beta-AlFeSi intermetallics by laser scanning confocal microscopy. J. Mat. Sci. Lett. 2003, 22, 1385–1387. [Google Scholar] [CrossRef]
- Busby, A.K.; Edwards, L.; Martin, J.W. Effect of aging and dispersoid content on tensile properties of Al–0·6Mg–1 Si alloys. Mater. Sci. Technol. 1986, 2, 363–367. [Google Scholar] [CrossRef]
- Milkereit, B.; Wanderka, N.; Schick, C.; Kessler, O. Continuous cooling precipitation diagrams of Al–Mg–Si alloys. Mater. Sci. Eng. A 2012, 550, 87–96. [Google Scholar] [CrossRef]
- Shishido, H.; Takaki, Y.; Kozuka, M.; Matsumoto, K.; Aruga, Y. Effects of Sn Addition on Clustering and Age-Hardening Behavior in a Pre-Aged Al-Mg-Si Alloy. MSF 2017, 877, 455–460. [Google Scholar] [CrossRef]
- Kaufman, J.G. Parametric analyses of high-temperature data for aluminum alloys; ASM International: Materials Park, OH, USA, 2008. [Google Scholar]
- Starink, M.; Cao, L.; Rometsch, P. A model for the thermodynamics of and strengthening due to co-clusters in Al–Mg–Si-based alloys. Acta Mater. 2012, 60, 4194–4207. [Google Scholar] [CrossRef]
- Pogatscher, S.; Kozeschnik, E.; Antrekowitsch, H.; Werinos, M.; Gerstl, S.; Löffler, J.; Uggowitzer, P.; Uggowitzer, P. Process-controlled suppression of natural aging in an Al–Mg–Si alloy. Scr. Mater. 2014, 89, 53–56. [Google Scholar] [CrossRef]





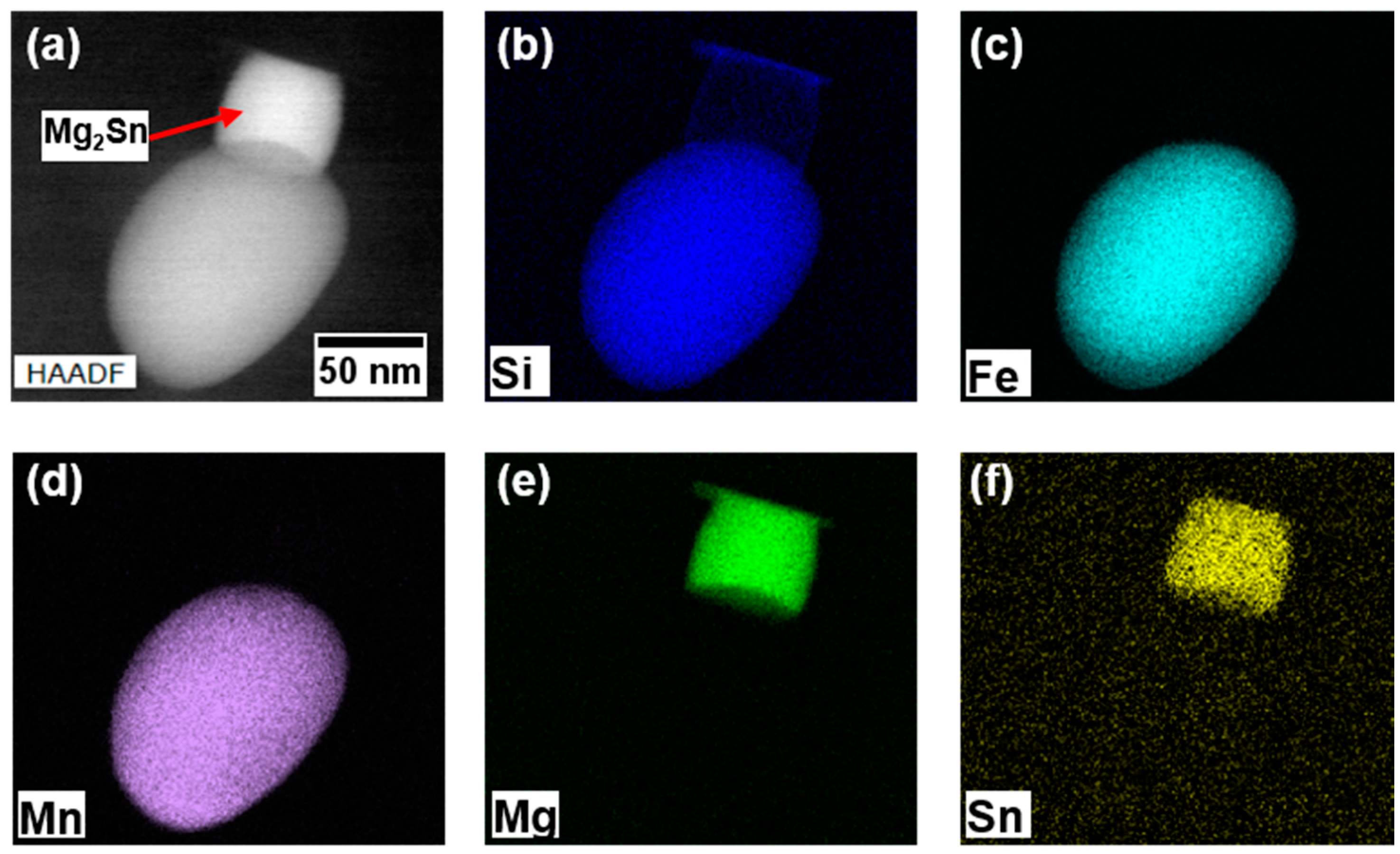
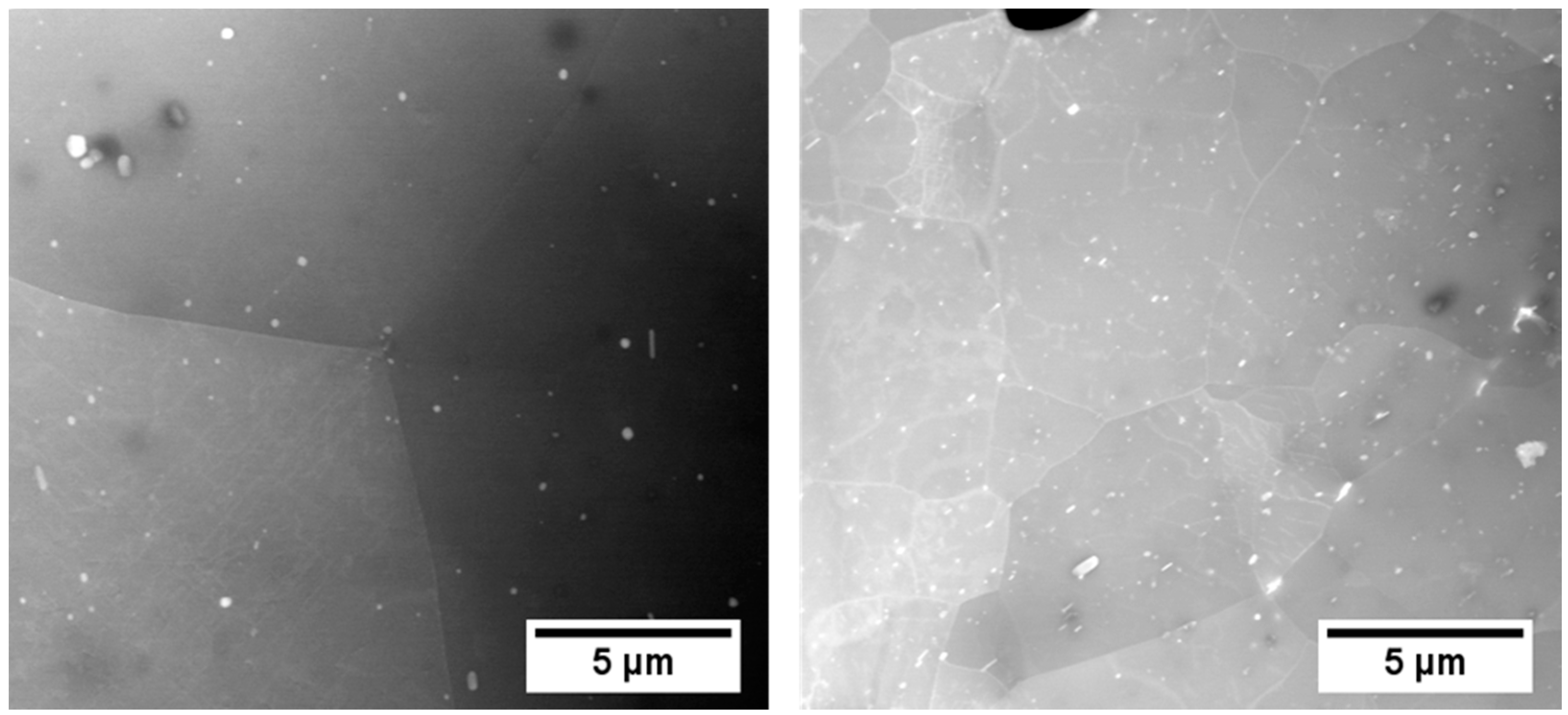
| [wt.-%] | Mg | Si | Cu | Sn | Fe | Mn | Cr | Zn | Ti | Al | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6016 | Sn-free | 0.36 | 1.06 | 0.08 | 0.002 | 0.17 | 0.08 | 0.00 | 0.01 | 0.02 | residue |
| +100 at.-ppm Sn | 0.40 | 1.08 | 0.07 | 0.042 | 0.18 | 0.06 | 0.01 | 0.01 | 0.02 | residue | |
| 6061 | Sn-free | 0.83 | 0.48 | 0.37 | 0.002 | 0.46 | 0.11 | 0.16 | 0.05 | 0.01 | residue |
| +100 at.-ppm Sn | 0.81 | 0.59 | 0.23 | 0.040 | 0.46 | 0.11 | 0.16 | 0.05 | 0.05 | residue |
| Solution Aging | Quenching Method | Spike I | Pre-Annealing | Spike II | |||||
|---|---|---|---|---|---|---|---|---|---|
| T [°C] | t [min] | T [°C] | t [s] | T [°C] | t [h] | T [°C] | t [s] | ||
| HT0 | 560/570 | 20 | air/water | - | - | - | |||
| PA1 | 570 | 20 | water | - | 80/100/ 120/140 | 2/4/6/8/10 | - | ||
| PA2 | 570 | 20 | water | 160/200 | 30/60/ 120 | 100/120 | 2/4/8 | - | |
| PA3/PA3’ | 570 | 20 | water | - | 100/120 | 4/8 | 250 | 1/30/ 120 | |
| Pre-Annealing | Spike | ||||||
|---|---|---|---|---|---|---|---|
| T [°C] | T [°C] | ||||||
| 100 | 120 | 160 | 200 | ||||
| t [h] | 2 | 5299 | 5583 | t [s] | 30 | 5120 | 5593 |
| 4 | 5411 | 5701 | 60 | 5251 | 5735 | ||
| 8 | 5524 | 5820 | 120 | 5381 | 5878 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmid, F.; Uggowitzer, P.J.; Schäublin, R.; Werinos, M.; Ebner, T.; Pogatscher, S. Effect of Thermal Treatments on Sn-Alloyed Al-Mg-Si Alloys. Materials 2019, 12, 1801. https://doi.org/10.3390/ma12111801
Schmid F, Uggowitzer PJ, Schäublin R, Werinos M, Ebner T, Pogatscher S. Effect of Thermal Treatments on Sn-Alloyed Al-Mg-Si Alloys. Materials. 2019; 12(11):1801. https://doi.org/10.3390/ma12111801
Chicago/Turabian StyleSchmid, Florian, Peter J. Uggowitzer, Robin Schäublin, Marion Werinos, Thomas Ebner, and Stefan Pogatscher. 2019. "Effect of Thermal Treatments on Sn-Alloyed Al-Mg-Si Alloys" Materials 12, no. 11: 1801. https://doi.org/10.3390/ma12111801
APA StyleSchmid, F., Uggowitzer, P. J., Schäublin, R., Werinos, M., Ebner, T., & Pogatscher, S. (2019). Effect of Thermal Treatments on Sn-Alloyed Al-Mg-Si Alloys. Materials, 12(11), 1801. https://doi.org/10.3390/ma12111801