Aggregation of Gold Nanoparticles Caused in Two Different Ways Involved in 4-Mercaptophenylboronic Acidand Hydrogen Peroxide
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Gold Nanoparticles
2.3. Reaction Conditions Involved in the Investigation
2.4. Characterization Techniques
3. Results and Discussion
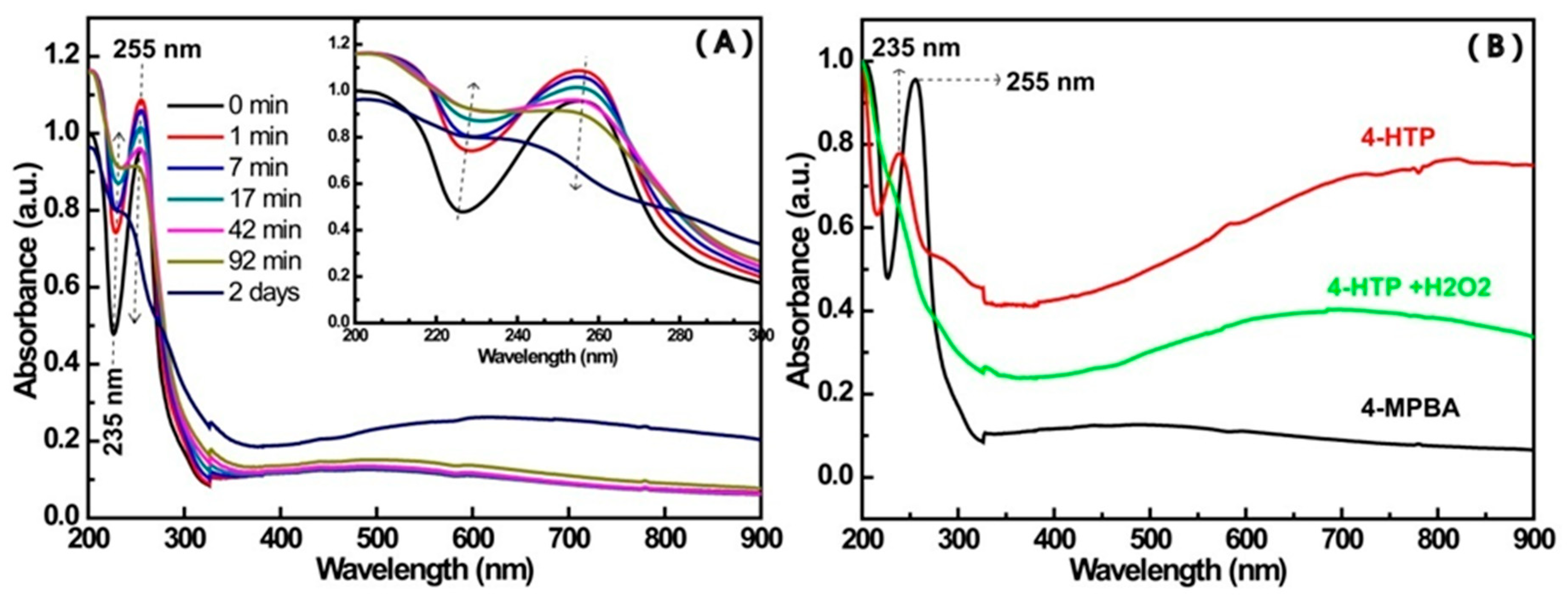
3.1. The UV-Vis Spectroscopy for the Interaction between 4-MPBA and the Citrate-Capped AuNPs
3.2. The UV-Vis Spectroscopy of AuNP Solutions Containing 4-MPBA and H2O2
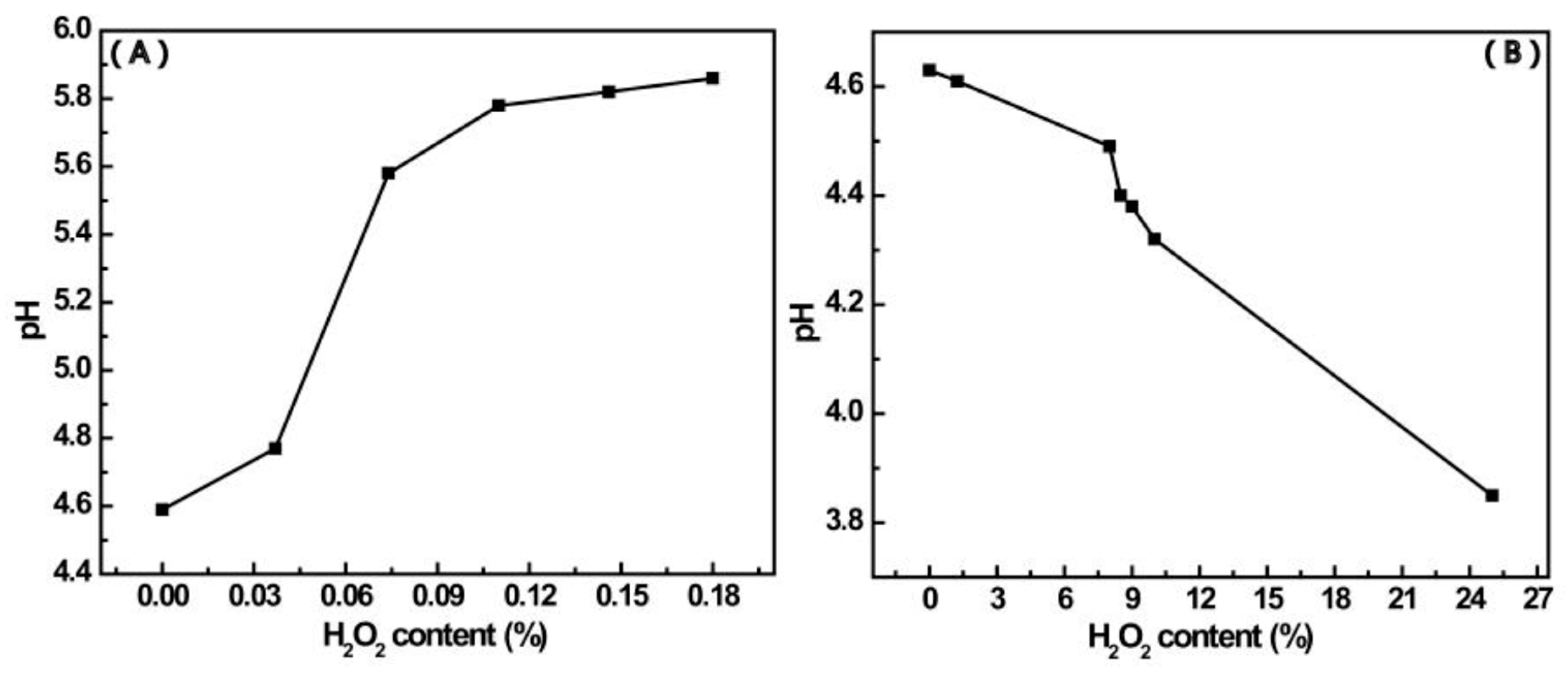
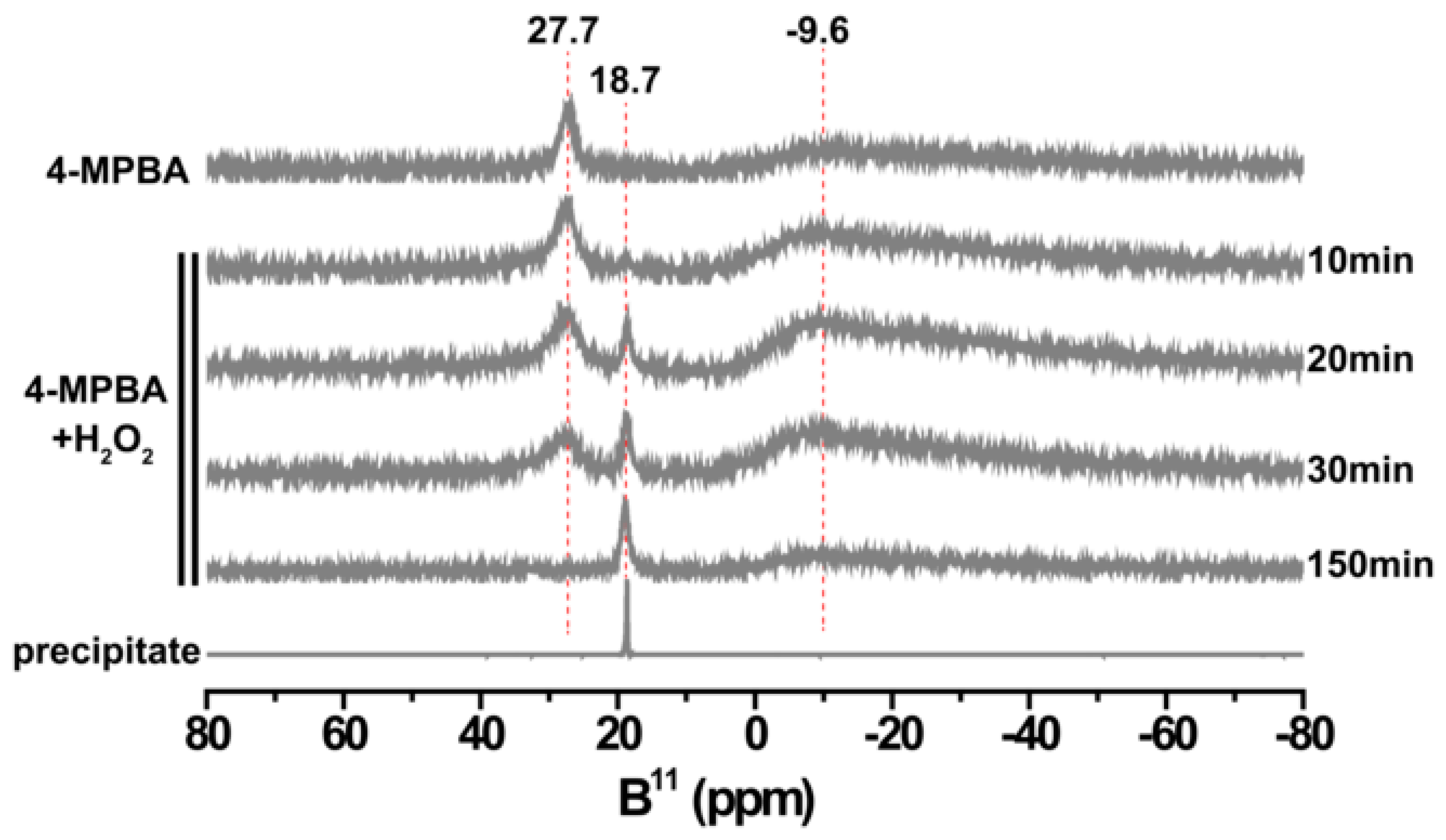
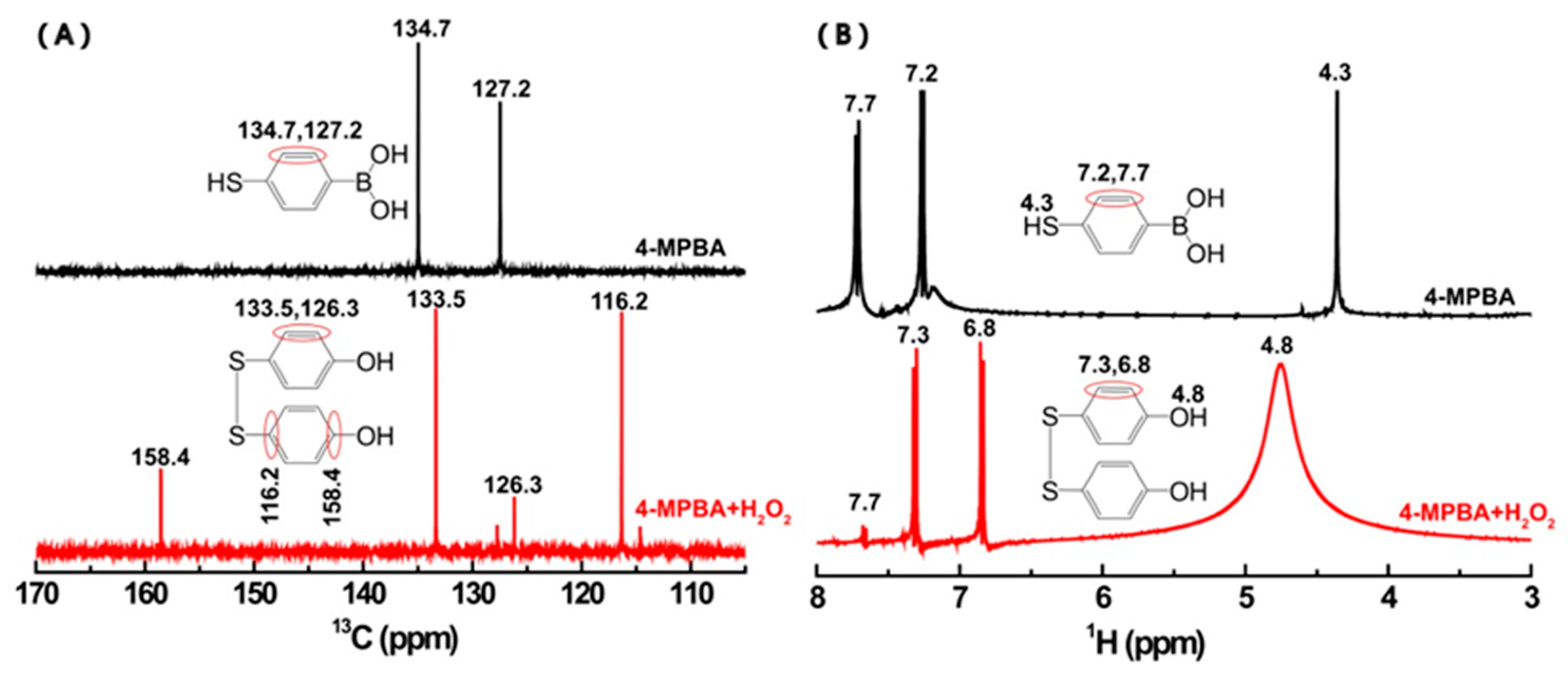
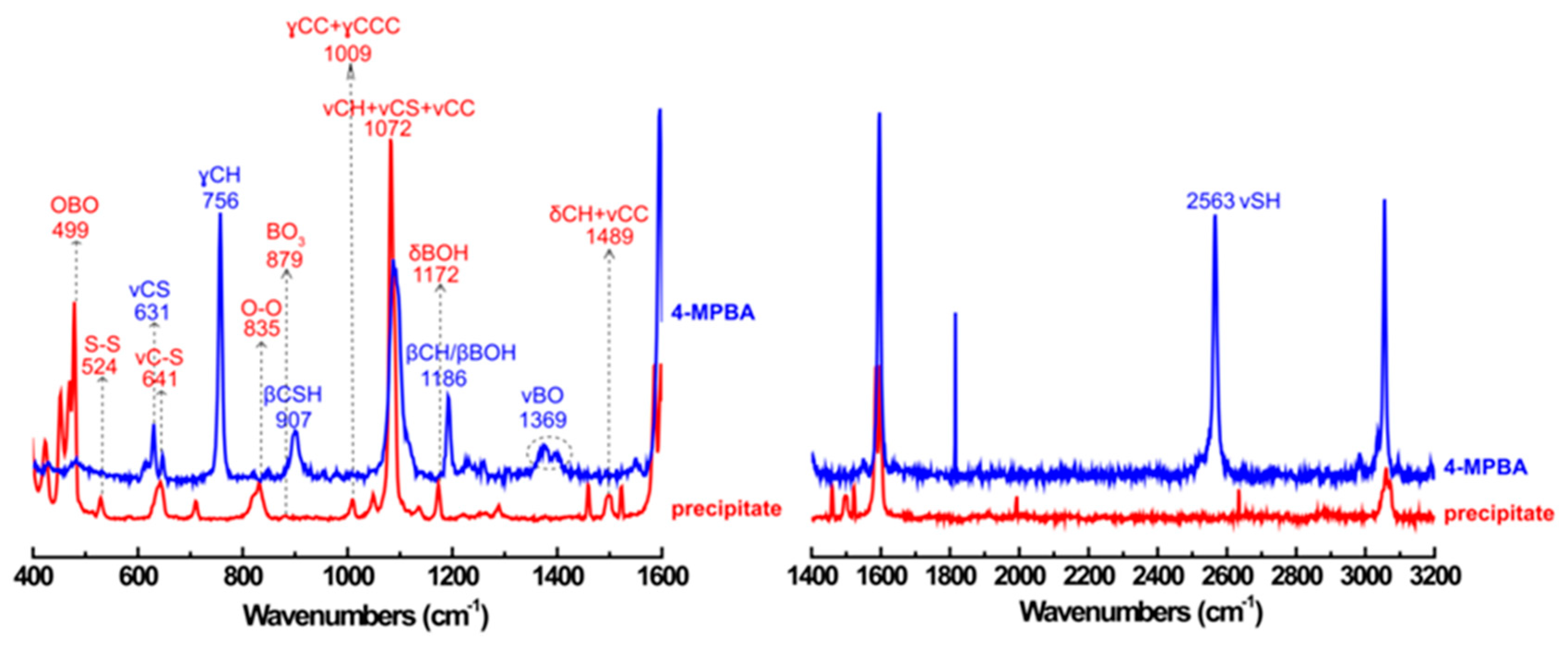
3.3. The Products Formed in the Reaction between 4-MPBA and H2O2
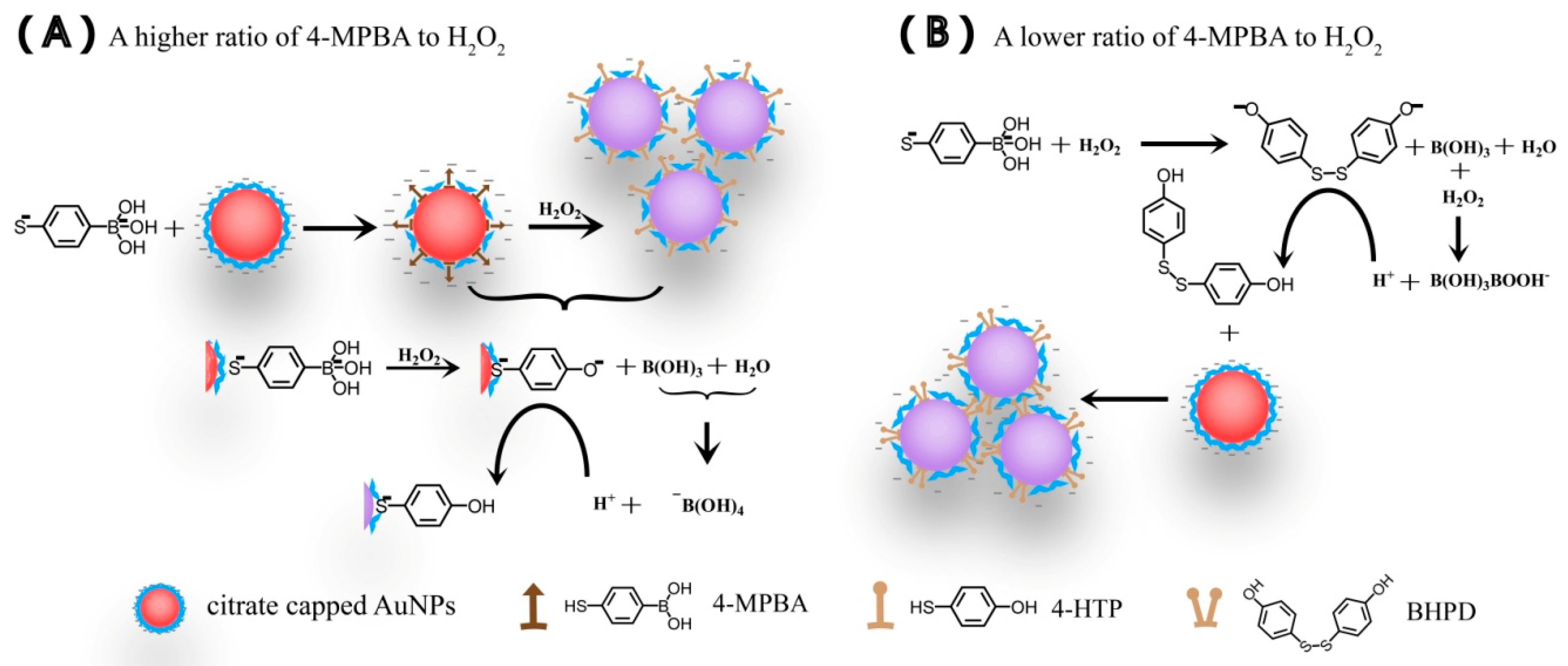
3.4. The Aggregation of AuNPs Caused by the Interaction of 4-MPBA and H2O2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Elghanian, R.; Storhoff, J.J.; Mucic, R.C.; Letsinger, R.L.; Mirkin, C.A. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 1997, 277, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Pal, T. Interparticle coupling effect on the surface plasmon resonance of gold nanoparticles: from theory to applications. Chem. Rev. 2007, 107, 4797–4862. [Google Scholar] [CrossRef]
- Cambre, J.N.; Sumerlin, B.S. Biomedical applications of boronic acid polymers. Polymer 2011, 52, 4631–4643. [Google Scholar] [CrossRef]
- Fang, H.; Kaur, G.; Wang, B. Progress in boronic acid-based fluorescent glucose sensors. J. Fluoresc. 2004, 14, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Springsteen, G.; Wang, B. A detailed examination of boronic acid-diol complexation. Tetrahedron 2002, 58, 5291–5300. [Google Scholar] [CrossRef]
- Yan, J.; Fang, H.; Wang, B. Boronolectins and fluorescent boronolectins: An examination of the detailed chemistry issues important for the design. Med. Res. Rev. 2005, 25, 490–520. [Google Scholar] [CrossRef] [PubMed]
- Keshvari, F.; Bahram, M.; Farhadi, K. A selective, sensitive and label-free visual assay of fructose using anti-aggregation of gold nanoparticles as a colorimetric probe. Chin. Chem. Lett. 2016, 27, 847–851. [Google Scholar] [CrossRef]
- Sankoh, S.; Thammakhet, C.; Numnuam, A.; Limbut, W.; Kanatharana, P.; Thavarungkul, P. 4-Mercaptophenylboronic acid functionalized gold nanoparticles for colorimetric sialic acid detection. Biosens. Bioelectron. 2016, 85, 743–750. [Google Scholar] [CrossRef]
- Keshvari, F.; Bahram, M. Selective, sensitive and reliable colorimetric sensor for catechol detection based on anti-aggregation of unmodified gold nanoparticles utilizing boronic acid–diol reaction: optimization by experimental design methodology. J. Iran. Chem. Soc. 2017, 14, 977–984. [Google Scholar] [CrossRef]
- Zhou, Y.; Dong, H.; Liu, L.; Li, M.; Xiao, K.; Xu, M. Selective and sensitive colorimetric sensor of mercury (II) based on gold nanoparticles and 4-mercaptophenylboronic acid. Sens. Actuat. B 2014, 196, 106–111. [Google Scholar] [CrossRef]
- Kong, Y.; Shen, J.; Fan, A. Colorimetric method for the detection of mercury ions based on gold nanoparticles and mercaptophenyl boronic acid. Anal. Sci. 2017, 33, 925–930. [Google Scholar] [CrossRef]
- Jiang, G.; Zhu, W.; Shen, X.; Xu, L.; Li, X.; Wang, R.; Liu, C.; Zhou, X. Colorimetric and visual determination of adenosine triphosphate using a boronic acidas the recognition element, and based on the deaggregation of gold nanoparticles. Microchim. Acta 2017, 184, 4305–4312. [Google Scholar] [CrossRef]
- Kong, B.; Zhu, A.; Luo, Y.; Tian, Y.; Yu, Y.; Shi, G. Sensitive and selective colorimetric visualization of cerebral dopamine based on double molecular recognition. Angew. Chem. Int. Ed. 2011, 50, 1837–1840. [Google Scholar] [CrossRef]
- Wallace, G.Q.; Tabatabaei, M.; Zuin, M.S.; Workentin, M.S.; Lagugné-labarthet, F. A nanoaggregate-on-mirror platform for molecular and biomolecular detection by surface-enhanced Raman spectroscopy. Anal. Bioanal. Chem. 2016, 408, 609–618. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.; Wu, M.; Liu, Y.; Shi, D.; Liu, B. A novel SERS nanoprobe for the ratiometric imaging of hydrogen peroxide in living cells. Chem. Commun. 2016, 52, 8553–8556. [Google Scholar]
- Gu, X.; Wang, H.; Schultz, Z.D.; Camden, J.P. Sensing glucose in urine and serum and hydrogen peroxide in living cells by use of a novel boronate nanoprobe based on Surface-Enhanced Raman Spectroscopy. Anal. Chem. 2016, 88, 7191–7197. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Tseng, W.-L. 1,4-Benzenediboronic-acid-induced aggregation of gold nanoparticles: Application to hydrogen peroxide detection and biotin-avidin-mediated immunoassay with naked-eye detection. Anal. Chem. 2016, 88, 5355–5362. [Google Scholar] [CrossRef]
- Ananthi, A.; Naresh Kumar, T.; Mathiyarasu, J.; Joseph, J.; Phani, K.L.N.; Yegnaraman, V. A novel potentiometric hydrogen peroxide sensor based on pKa changes of vinyl phenylboronic acid membranes. Mater. Lett. 2011, 65, 3563–3565. [Google Scholar] [CrossRef]
- Bastús, N.G.; Comenge, J.; Puntes, V. Kinetically controlled seeded growth synthesis of citrate-stabilized gold nanoparticles of up to 200 nm. Langmuir 2011, 27, 11098–11105. [Google Scholar] [CrossRef]
- Bowey, K.; Tanguay, J.-F.; Sandros, M.G.; Tabrizian, M. Microwave-assisted synthesis of surface-enhanced Raman scattering nanoprobes for celluar sensing. Colloids Surf. B 2014, 122, 617–622. [Google Scholar] [CrossRef]
- Vancoillie, G.; Hoogenboom, R. Synthesis and polymerization of boronic acid containing monomers. Polym. Chem. 2016, 7, 5484–5497. [Google Scholar] [CrossRef]
- Singh, G.; Bremmell, K.E.; Griesser, H.J.; Kingshott, P. Colloid-probe AFM studies of the interaction forces of proteins adsorbed on colloidal crystals. Soft. Matter. 2015, 11, 3188–3197. [Google Scholar] [CrossRef]
- Miao, X.; Ning, X.; Li, Z.; Cheng, Z. Sensitive detection of miRNA by using hybridization chain reaction coupled with positively charged gold nanoparticles. Sci. Rep. 2016, 6, 32358. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, H.; Lin, Y.; Zhu, N.; Ma, Y.; Mao, L. Colorimetric detection of glucose in rat brain using gold nanoparticles. Angew. Chem. Int. Ed. 2010, 49, 4800–4804. [Google Scholar] [CrossRef]
- Liu, L.; Chang, Y.; Xia, N.; Peng, P.; Zhang, L.; Jiang, M.; Zhang, J.; Liu, L. Simple, sensitive and label–free electrochemical detection of microRNAs based on the in situ formation of silver nanoparticles aggregates for signal amplification. Biosens. Bioelectron. 2017, 94, 235–242. [Google Scholar] [CrossRef]
- Xia, N.; Cheng, C.; Liu, L.; Peng, P.; Liu, C.; Chen, J. Electrochemical glycoprotein aptasensors based on the in-situ aggregation of silver nanoparticles induced by 4-mercaptophenylboronic acid. Microchim. Acta 2017, 184, 4393–4400. [Google Scholar] [CrossRef]
- Liu, L.; Sun, T.; Ren, H. Electrochemical detection of hydrogen peroxide by inhibiting the p-benzenediboronic acid-triggered assembly of citrate-capped Au/Ag nanoparticles on electrode surface. Materials 2017, 10, 40. [Google Scholar] [CrossRef]
- Zhang, C.; Liang, X.; You, T.; Yang, N.; Gao, Y.; Yin, P. An ultrasensitive “turn-off” SERS sensor for quantitatively detecting heparin based on 4-mercaptobenzoic acid functionalized gold nanoparticles. Anal. Methods 2017, 9, 2517–2522. [Google Scholar] [CrossRef]
- Zhang, J.; He, L.; Zhang, X.; Wang, J.; Yang, L.; Liu, B.; Jiang, C.; Zhang, Z. Chemical colorimetric and SERS dual-readout for assaying alkaline phosphatase activity by ascorbic acid induced aggregation of Ag coated Au nanoparticles. Sens. Actuators B 2017, 253, 839–845. [Google Scholar] [CrossRef]
- Chen, S. 4-Hydroxythiophenol-protected gold nanoclusters in aqueous media. Langmuir 1999, 15, 7551–7557. [Google Scholar] [CrossRef]
- Buytendyk, A.M.; Graham, J.D.; Collins, K.D.; Bowen, K.H.; Wu, C.-H.; Wu, J.I. The hydrogen bond strength of the phenol – phenolate anionic complex: A computational and photoelectron spectroscopic study. Phys. Chem. Chem. Phys. 2015, 17, 25109–25113. [Google Scholar] [CrossRef]
- Pizer, R.; Tihal, C. Peroxoborates. Interaction of boric acid and hydrogen peroxide in aqueous solution. Inorg. Chem. 1987, 26, 3639–3642. [Google Scholar] [CrossRef]
- Hiebel, M.-A.; Berteina-raboin, S. Iodine-catalyzed region selective sulfenylation of imidazoheterocycles in PEG400. Green Chem. 2015, 17, 937–944. [Google Scholar] [CrossRef]
- Oae, S.; Yoshihara, M. Polar conjugative effect of aryldithia group on the dissociation and ultraviolet spectra of substituted phenols. Bull. Chem. Soc. Jpn. 1968, 41, 2082–2086. [Google Scholar] [CrossRef]
- Deary, M.E.; Durrant, M.C.; Davies, D.M. A kinetic and theoretical study of the borate catalysed reactions of hydrogen peroxide: The role of dioxaborirane as the catalytic intermediate for a wide range of substrates. Org. Biomol. Chem. 2013, 11, 309–317. [Google Scholar] [CrossRef]
- Biswas, A.; Malferrari, S.; Kalaskar, D.M.; Das, A.K. Arylboronate esters mediated self-healable and biocompatible dynamic G-quadruplex hydrogels as promising 3D-bioinks. Chem. Commun. 2018, 54, 1778–1781. [Google Scholar] [CrossRef]
- Coddington, J.M.; Taylor, M.J. High field 11B and13C NMR investigations of aqueous borate solutions and borate-diol complexes. J. Coord. Chem. 1989, 20, 27–38. [Google Scholar] [CrossRef]
- Brikh, A.; Morin, C. Boronated thiophenols: A preparation of4-mercaptophenylboronic acid and derivatives. J. Organomet. Chem. 1999, 581, 82–86. [Google Scholar] [CrossRef]
- Kawaguchi, A.W.; Sudo, A.; Endo, T. Thio- functionalized 1,3-benzoxazine: preperation and its use as a precursor for highly polymerizable benzoxazine monomers bearing sulfide moiety. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1448–1457. [Google Scholar] [CrossRef]
- Chatterjee, T.; Ranu, B.C. Aerobic oxidation of thiols to disulfides under ballmilling in the absence of any catalyst, solvent, or base. RSC Adv. 2013, 3, 10680–10686. [Google Scholar] [CrossRef]
- Charisiadis, P.; Kontogianni, V.G.; Tsiafoulis, C.G.; Tzakos, A.G.; Siskos, M.; Gerothanassis, I.P. 1H-NMR as a structural and analytical tool of intra- and intermolecular hydrogen bonds of phenol-containing natural products and model compounds. Molecules 2014, 19, 13643–13682. [Google Scholar] [CrossRef]
- Chaudhari, S.R. Screening and assignment of phenylboronic acid and its anhydride formation by NMR spectroscopy. Chem. Phys. Lett. 2015, 634, 95–97. [Google Scholar] [CrossRef]
- Su, H.; Wang, Y.; Yu, Z.; Liu, Y.; Zhang, X.; Wang, X.; Sui, H.; Sun, C.; Zhao, B. Surface-enhanced Raman spectroscopy study on the structure changes of4-mercaptophenylboronic acid under different pH conditions. Spectrochim. Acta Part A 2017, 185, 336–342. [Google Scholar] [CrossRef]
- Xu, H.; Li, D.; Zhao, Y.; Wang, X.; Li, D.; Wang, Y. Sodium 4- mercaptophenolate capped CdSe/ZnS quantum dots as a fluorescent probe for pH detection in acidic aqueous media. Luminescence 2018, 33, 410–416. [Google Scholar] [CrossRef]
- Cardey, B.; Enescu, M. Selenocysteine versus cysteine reactivity: A theoretical study of their oxidation by hydrogen peroxide. J. Phys. Chem. A. 2007, 111, 673–678. [Google Scholar] [CrossRef]
- Quesada, A.R.; Byrnes, R.W.; Krezoski, S.O.; Petering, D.H. Direct reaction of H2O2 with sulfhydryl groups in HL-60 Cells: Zinc-metallothionein and other sites. Arch. Biochem. Biophys. 1996, 334, 241–250. [Google Scholar] [CrossRef]
- Shiang, Y.-C.; Huang, C.-C.; Chang, H.-T. Gold nanodot-based luminescent sensor for the detection of hydrogen peroxide and glucose. Chem. Commun. 2009, 23, 3437–3439. [Google Scholar] [CrossRef]
- Ruano, J.L.G.; Parra, A.; Alemán, J. Efficient synthesis of disulfides by air oxidation of thiols under sonication. Green Chem. 2008, 10, 706–711. [Google Scholar] [CrossRef]
- Sasaki, S.; Sutoh, K.; Yoshifuji, M. Synthesis, structure, and reactivity of 2, 6-Diaryl-4-hydroxybenzenethiol. Heteroat. Chem. 2014, 25, 442–448. [Google Scholar] [CrossRef]
- Yuan, L.-F.; He, Y.-J.; Zhao, H.; Zhou, Y.; Gu, P. Colorimetric detection of D-amino acids based on anti-aggregation of gold nanoparticles. Chin. Chem. Lett. 2014, 25, 995–1000. [Google Scholar] [CrossRef]
- Nurpeissova, Z.A.; Alimkhanova, S.G.; Mangazbayeva, R.A.; Shaikhutdinov, Y.M.; Mun, G.A.; Khutoryanskiy, V.V. Redox- and glucose-responsive hydrogels from poly(vinyl alcohol) and 4-mercaptophenylboronic acid. Eur. Polym. J. 2015, 69, 132–139. [Google Scholar] [CrossRef]
- Li, S.; Zhou, Q.; Chu, W.; Zhao, W.; Zheng, J. Surface-enhanced Raman scattering behaviour of 4-mercaptophenyl boronic acid on assembled silver nanoparticles. Phys. Chem. Chem. Phys. 2015, 17, 17638–17645. [Google Scholar] [CrossRef]
- Li, R.; Ji, W.; Chen, L.; Lv, H.; Cheng, J.; Zhao, B. Vibrational spectroscopy and density functional theory study of 4-mercaptophenol. Spectrochim. Acta A 2014, 122, 698–703. [Google Scholar] [CrossRef]
- Ji, W.; Xue, X.; Ruan, W.; Wang, C.; Ji, N.; Chen, L.; Li, Z.; Song, W.; Zhao, B.; Lombardi, J.R. Scanned chemical enhancement of surface-enhanced Raman scattering using a charge-transfer complex. Chem. Commun. 2011, 47, 2426–2428. [Google Scholar] [CrossRef]
- Price, R.C.; Whetten, R.L. Raman spectroscopy of benzenethiolates on nanometer-scale gold clusters. J. Phys. Chem. B 2006, 110, 22166–22171. [Google Scholar] [CrossRef]
- Reid, D.G.; Smith, G.C. The X-ray photoelectron spectroscopy of surface films formed during the ASTM D-130/ ISO 2160 copper corrosion test. Pet. Sci. Technol. 2014, 32, 387–394. [Google Scholar] [CrossRef][Green Version]
- Yamauchi, S.; Doi, S. Raman spectroscopic study on the behavior of boric acidin wood. J. Wood Sci. 2003, 49, 227–234. [Google Scholar] [CrossRef]
- Krishnan, K. The Raman spectrum of boric acid. Proc. Indian Acad. Sci. 1963, 57, 103–108. [Google Scholar] [CrossRef]
- Ahijado, M.; Braun, T. Rhodium derivatives of peroxoboronic acids and peroxoboricacid: Formation of metallatrioxaborolanes from an η2 -peroxo complex. Angew. Chem. Int. Ed. 2008, 120, 2996–3000. [Google Scholar] [CrossRef]
- Ling, X.Y.; Yan, R.; Lo, S.; Hoang, D.T.; Liu, C.; Fardy, M.A.; Khan, S.B.; Asiri, A.M.; Bawaked, S.M.; Yang, P. Alumina coated Agnanocrystal monolayer as surface-enhanced Raman spectroscopy platforms for direct spectroscopic detection of water splitting reaction intermediates. Nano Res. 2013, 7, 132–143. [Google Scholar] [CrossRef]
- Bosch, L.I.; Fyles, T.M.; James, T.D. Binary and ternary phenylboronic acid complexes with saccharides and Lewis bases. Tetrahedron 2004, 60, 11175–11190. [Google Scholar] [CrossRef]
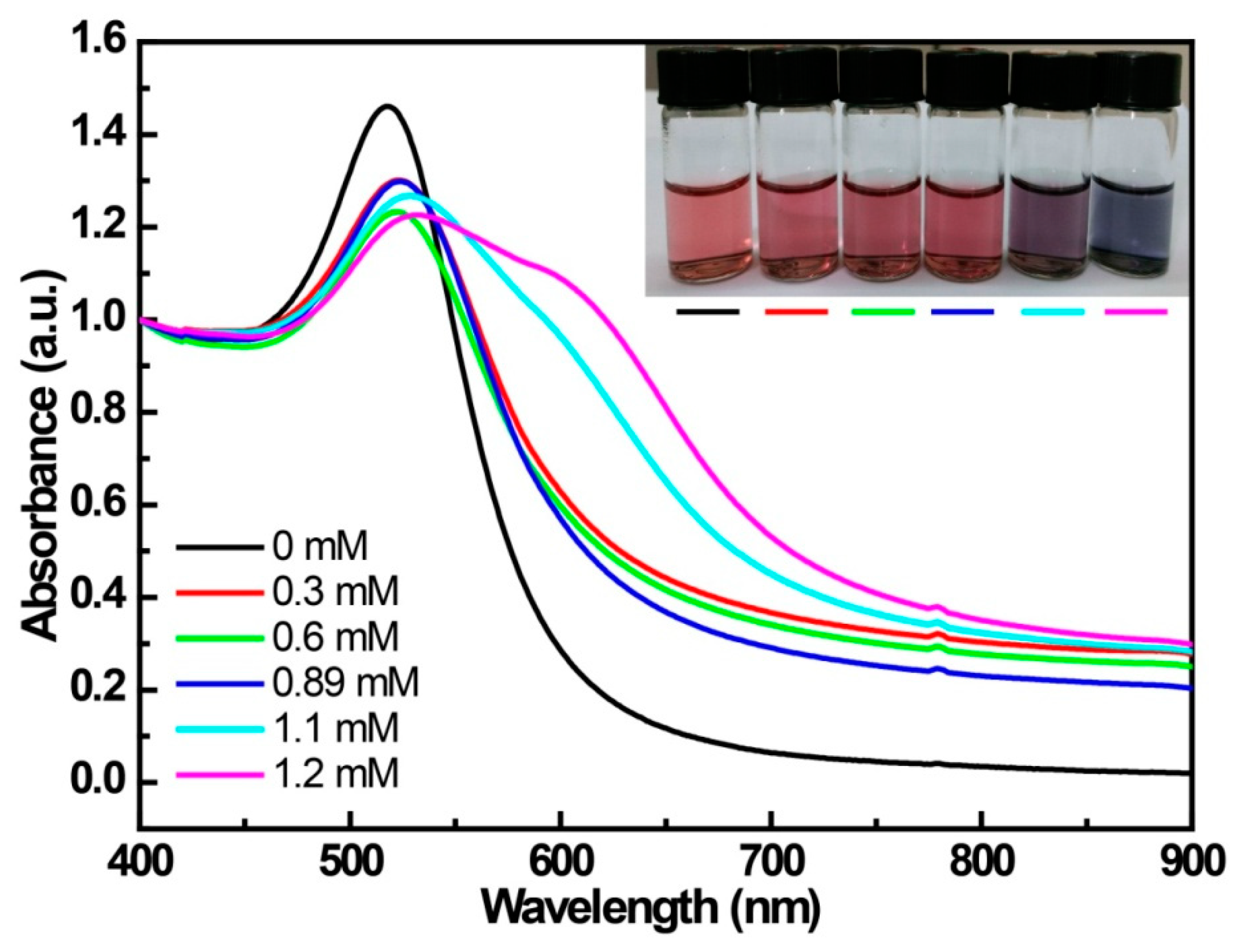
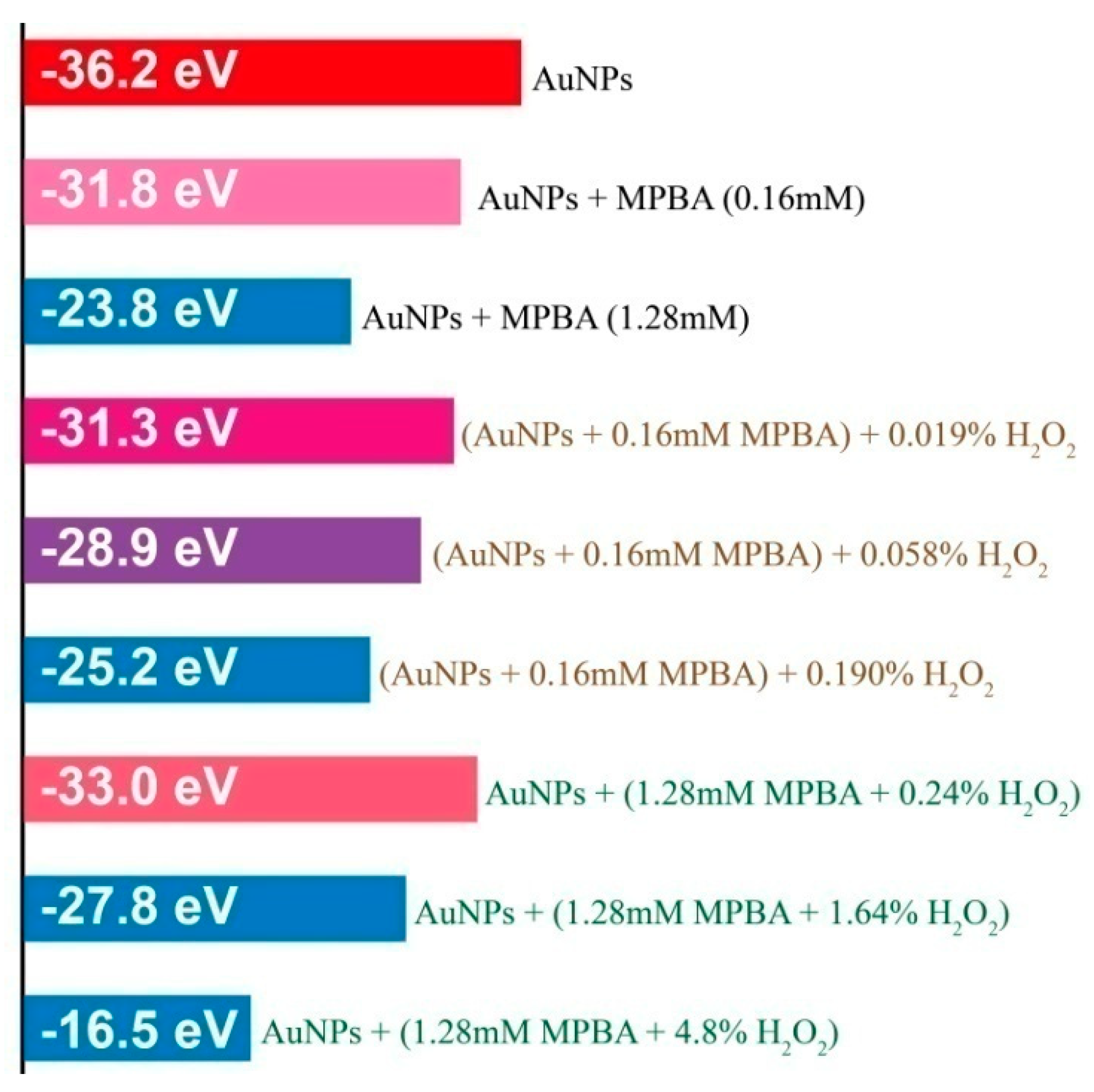

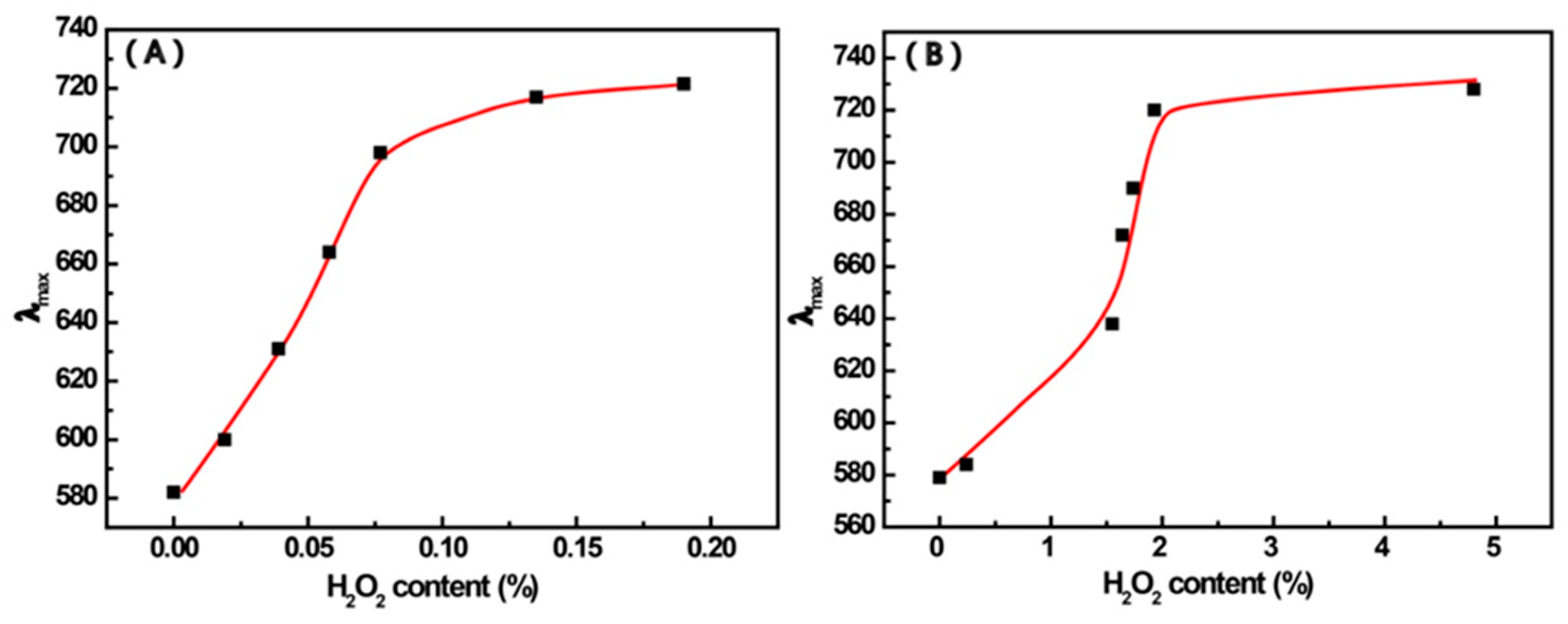
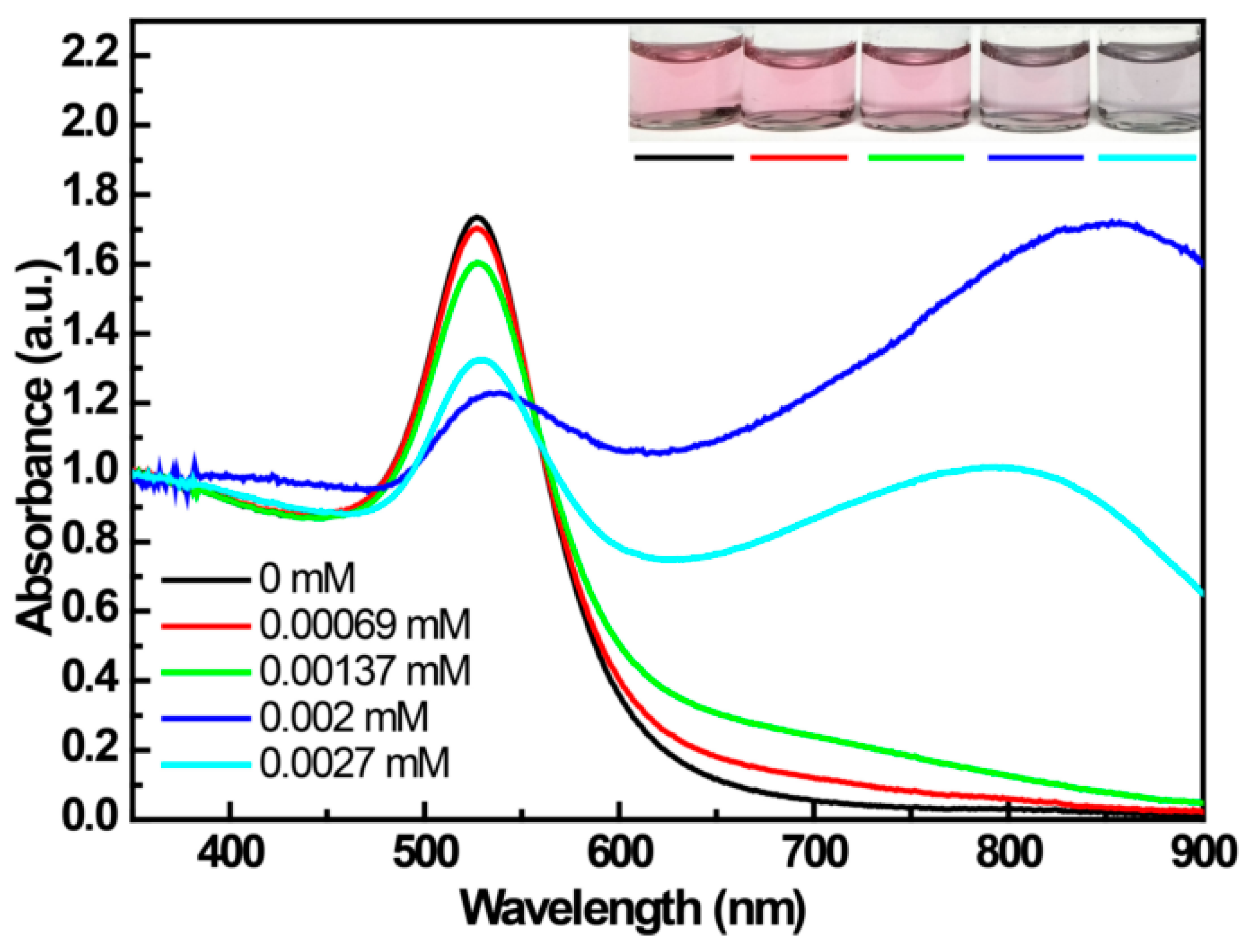
| Reactants | Mixt.1 a | Mixt.2 a | Mixt.3 | Mixt.4 b,d | Mixt.5 |
|---|---|---|---|---|---|
| 4-MPBA (mM) | 0.16 | 1.28 | 0.15 | --- | --- |
| 4-HTP (mM) | --- | --- | --- | 0~0.0027 | 0.07 |
| H2O2 (%) | 0~0.19% | 0~4.8% | 0.012% | ---- | ---- |
| H2O2 (2 mL) | --- | --- | --- | ---- | 0.012% |
| AuNPs (3 × 1012 NPs/mL) c | 500 μL | 500 μL | --- | 1 mL | --- |
| Reaction time (min) | 10 | 10 | 0~2 days | 5 | 20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, R.; Gu, X.; Liang, X.; Hou, S.; Hu, D. Aggregation of Gold Nanoparticles Caused in Two Different Ways Involved in 4-Mercaptophenylboronic Acidand Hydrogen Peroxide. Materials 2019, 12, 1802. https://doi.org/10.3390/ma12111802
Li R, Gu X, Liang X, Hou S, Hu D. Aggregation of Gold Nanoparticles Caused in Two Different Ways Involved in 4-Mercaptophenylboronic Acidand Hydrogen Peroxide. Materials. 2019; 12(11):1802. https://doi.org/10.3390/ma12111802
Chicago/Turabian StyleLi, Runmei, Xuefan Gu, Xingtang Liang, Shi Hou, and Daodao Hu. 2019. "Aggregation of Gold Nanoparticles Caused in Two Different Ways Involved in 4-Mercaptophenylboronic Acidand Hydrogen Peroxide" Materials 12, no. 11: 1802. https://doi.org/10.3390/ma12111802
APA StyleLi, R., Gu, X., Liang, X., Hou, S., & Hu, D. (2019). Aggregation of Gold Nanoparticles Caused in Two Different Ways Involved in 4-Mercaptophenylboronic Acidand Hydrogen Peroxide. Materials, 12(11), 1802. https://doi.org/10.3390/ma12111802




