3.1. Changes in Phase Composition
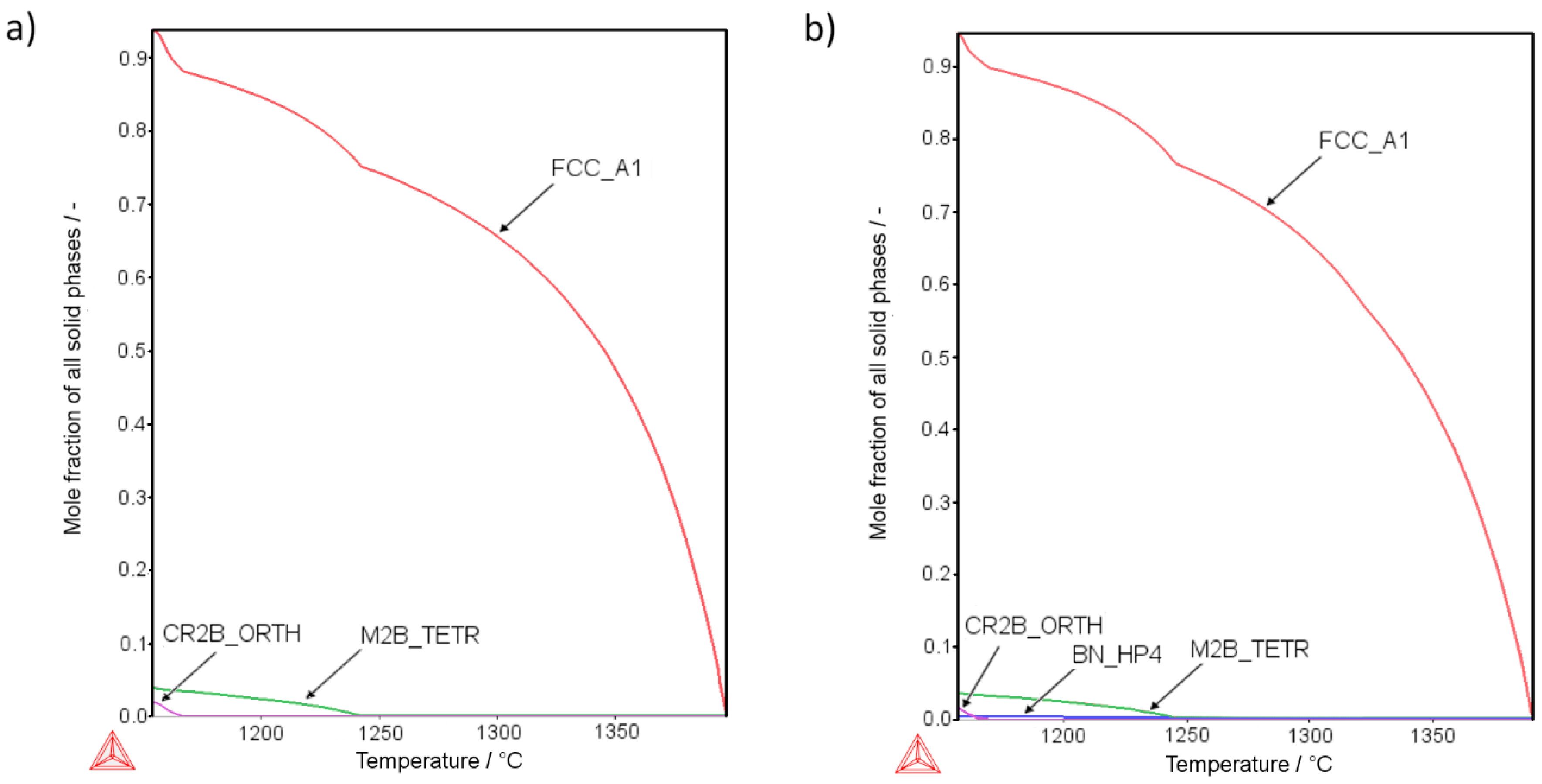
Figure 1 presents the thermodynamic calculations for the AISI 316L phase composition changes along the decreasing temperature curve using the Scheil–Gulliver model with back-diffusion of boron. The calculations show the results for a sample modified with the highest addition of boron (Sample 4-0,
Figure 1a) and also for the highest additions of both boron and silicon nitride (Sample 4-8,
Figure 1b). The calculations showed that introducing even small amounts of silicon nitride could result in the formation of BN instead of metal borides (
Figure 1a,b). According to the calculation results, the more boron was added to the base material, the more borides (in terms of volume) were created. Also, the more silicon nitride was added, the more BN phase appeared, reducing the amount of borides. Detailed numerical and graphical results for all samples are available in the
Supplementary Data: Figures S1–S19 and in Table S1.
According to the calculations, the solidification process of the liquid phase in sample 4-0 results in the creation of two types of borides: (i) Cr
2B with an orthorhombic crystallographic structure, and (ii) M
2B with a tetragonal crystallographic structure. At room temperature, their molar fraction in the microstructure was equal to 0.0378 (3.78%) and 0.0202 (2.02%), respectively (
Figure 1a). In accordance with the simulation, the introduction of both boron and the silicon nitride (sample 4-8) results in the reaction of boron with nitrogen (
Figure 1b). Consequently, the refractory boron nitride is created, which is solid at the sintering temperature. As a result of the sintering process, the total volume of borides present at room temperature should be approximately 5.08 vol.% (3.45 vol.% M
2B-type borides, 1.56 vol % Cr
2B-type borides). Apart from this, the 0.49 vol.% boron nitride is also expected, which should boost the mechanical properties of a sinter by improving the contact surface among the grains. This calculation remains coherent with findings of Soyama et al. [
2], who also found h-BN created in situ.
Detailed calculations show that, at room temperature, two kinds of borides should be present, tetragonal crystallographic M
2B boride and orthorhombic crystallographic Cr
2B. Both borides show a similar composition in both samples (4-0 and 4-8) irrespective of the silicon nitride addition (
Supplementary Data: Figures S20–S23). Interestingly, calculations indicate that when the Si
3N
4 is added, BN is created mostly at the expense of Cr
2B boride. This effect may lead to a complete disappearance of the Cr
2B boride, e.g., sample 1-8 (
Table S1 in Supplementary Data).
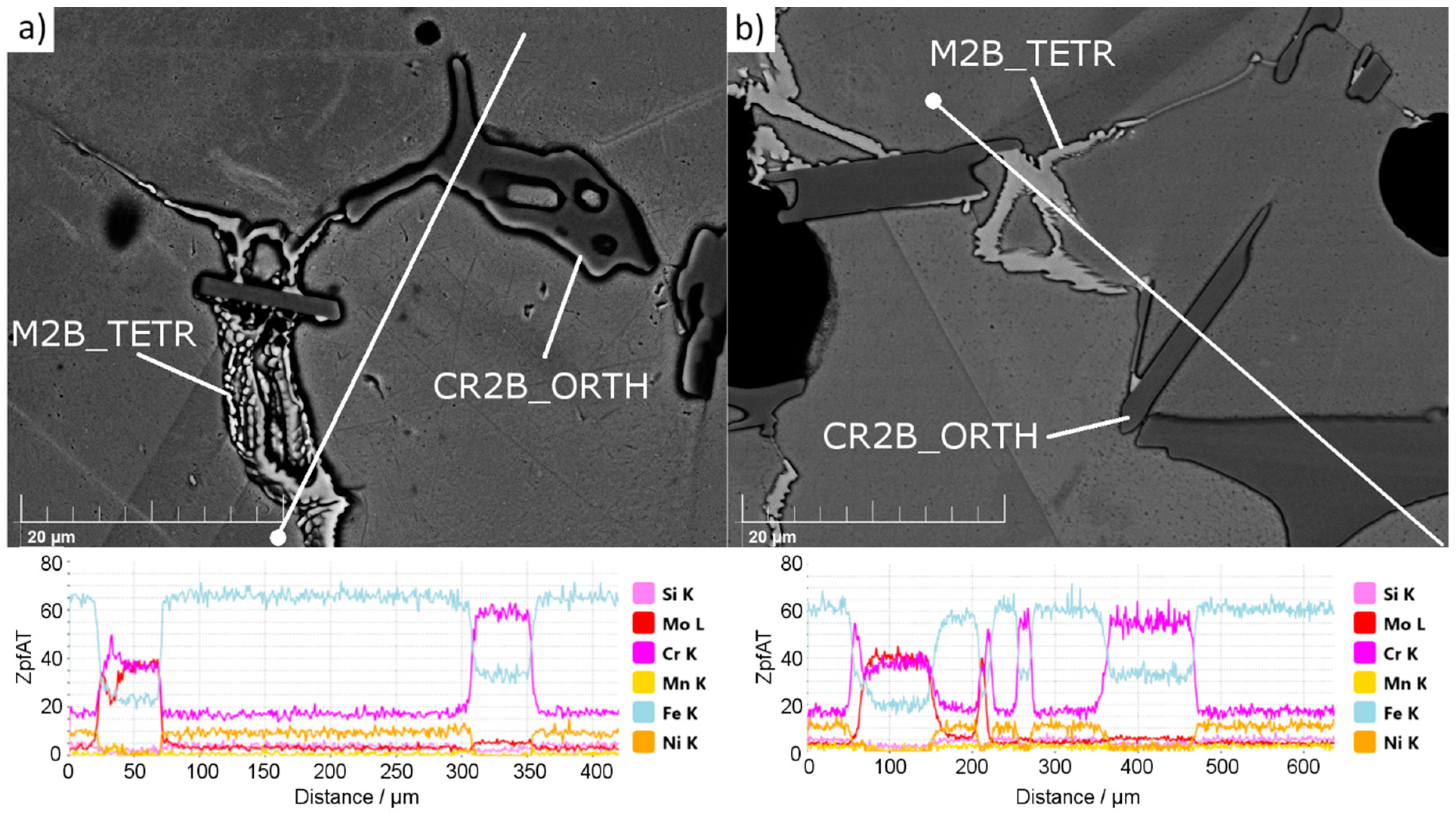
Figure 2 presents the results of an SEM EDX (Scanning Electron Microscope Energy-Dispersive X-ray spectroscopy, MIRA3, TESCAN, Czech Republic) linear scan performed for secondary phases observed in the structure of sample 4-0 (
Figure 2a) and 4-8 (
Figure 2b).
Irrespective of the silicon nitride addition, in all samples only two types of secondary phases were noticed (
Figure 2). SEM EDX observations found no boron nitride or any silicon-rich phase in the microstructure. The observed phases match the complex M
2B borides observed by other researchers investigating steel powders with boron additions [
4,
6,
12,
33,
34,
35]. Two types of borides were noticed. One, with a high concentration of Cr and Fe, is seen as dark agglomerates, and the second, with a high concentration of Cr, Mo, and Fe, is observed as bright “branched” shapes. This observation of chromium, iron, and molybdenum content in borides matches the calculation outcome (
Supplementary Data: Figures S20–S23). When Si
3N
4 was introduced, some of the borides had facets, suggesting that the grain growth process was modified by the presence of Si
3N
4. Borides are the last phase to solidify in the given system; therefore, they occupy places that were left by other phases. This further means that in samples with Si
3N
4 at sintering temperature, there was a phase present that mitigated the growth of the austenite grains and resulted in their non-cuboidal shapes.
3.2. Density, Porosity, and Dimensional Precision
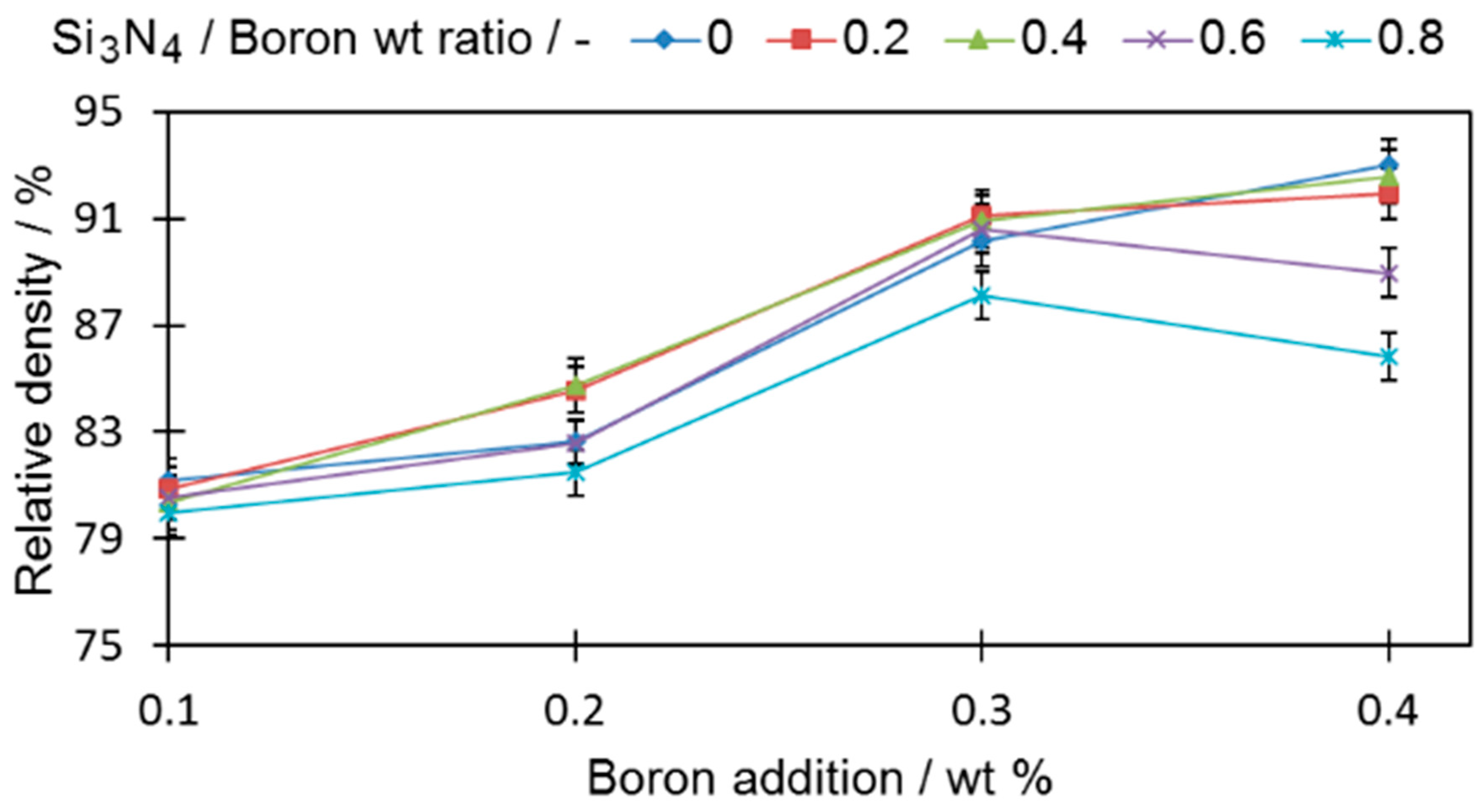
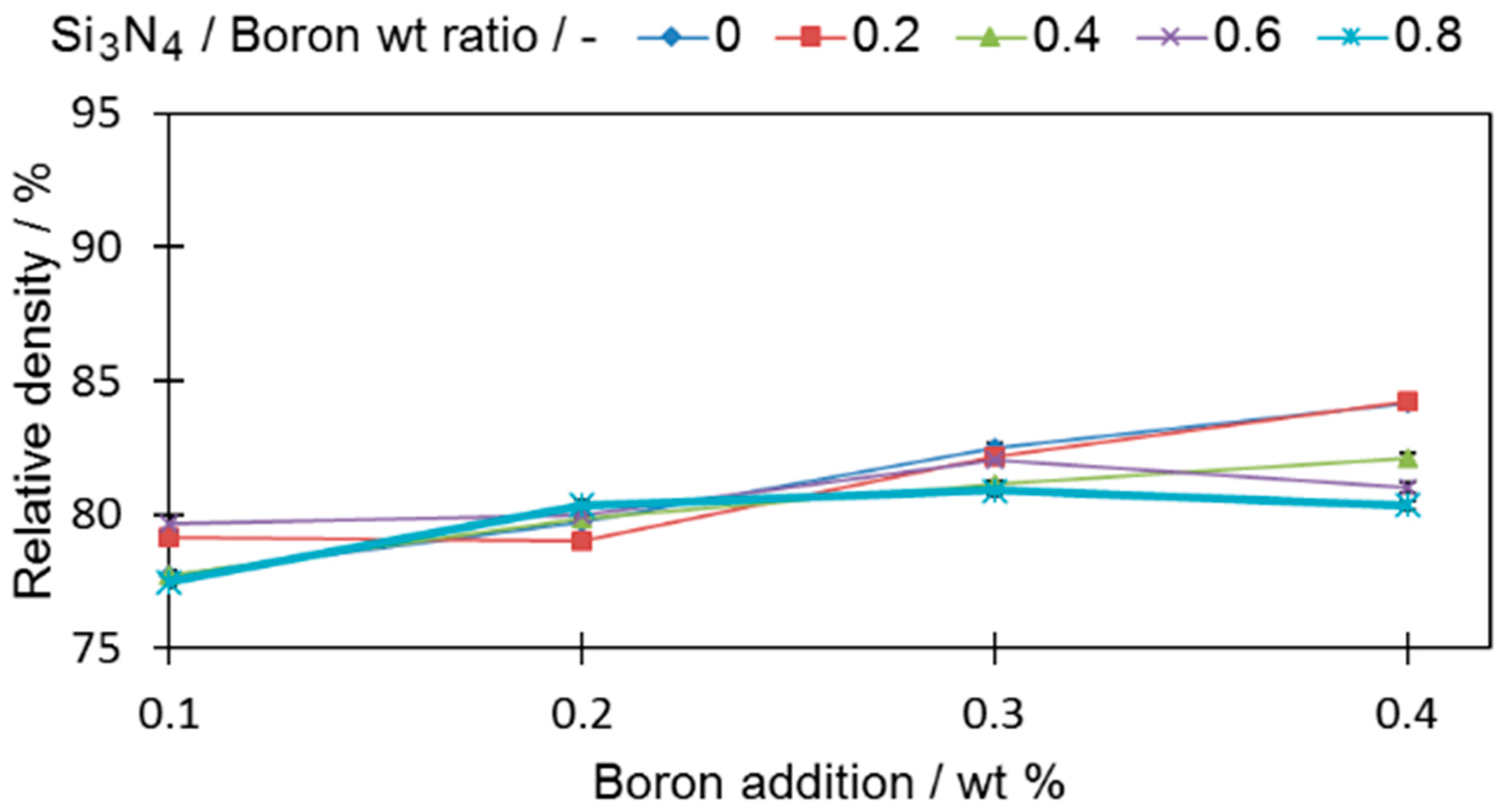
In order to evaluate the impact of boron and silicon nitride on the densification properties, cylindrical samples were analyzed (
Figure 3).
All tested samples reached a higher relative density than the base sample (0-0) (78.95% ± 0.19%). In all samples with only boron added, if more boron was introduced to the sinters, the density became higher, a fact that is in good agreement with the findings of other researchers [
1,
6]. For samples with a ratio of silicon nitride to boron greater than 0.4 (0.6 and 0.8), the density decreases, which permits the expectation that the amount of eutectic liquid that acts as a high-speed route for diffusion was reduced. The extent of this drop was greater when a large amount of boron was introduced. It is expected that this is the result of the increased contact probability for those two compounds in the sinter volume during the sintering process. The density decrease is also in agreement with the observation of [
24], who showed that Si
3N
4, which decomposes at 1127.2 °C, releases free nitrogen that increases porosity in a sinter.
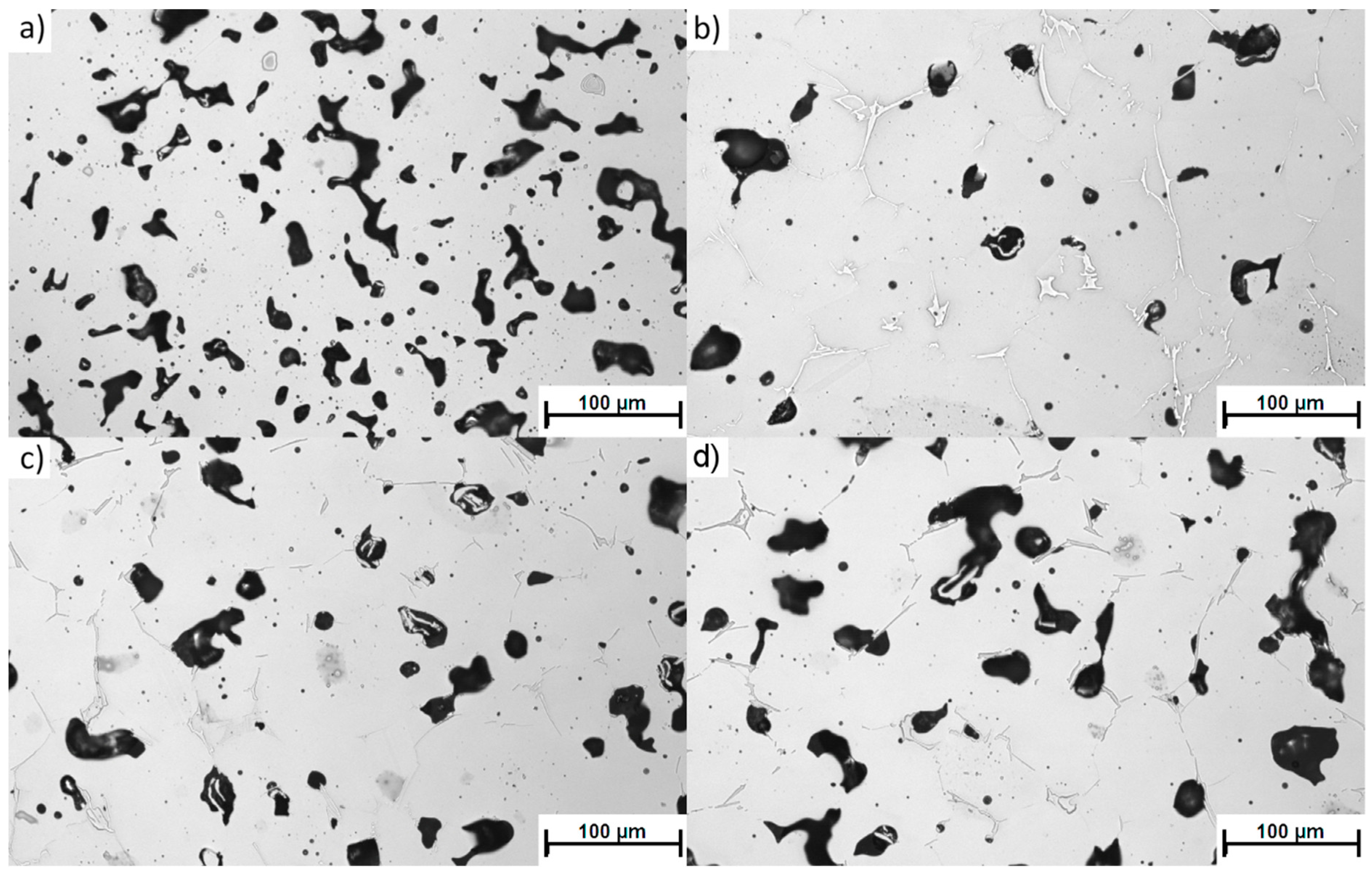
The most detrimental sinter microstructure features that affect its properties the most are the porosity and the shape. It is well known that the addition of boron causes a rounding of the pores (sample 4-0,
Figure 4b) compared to non-modified 316L (sample 0-0,
Figure 4a). This effect was observed in all samples with boron addition—the more boron added, the more pronounced the effect. On the other hand, increasing the silicon nitride addition resulted in an overall increase in porosity (sample 4-4 in
Figure 4c, sample 4-8 in
Figure 4d) and caused less rounded pore shapes (
Figure 5). In this case, the more Si
3N
4 added, the more pronounced the effect.
This observation suggests that, as expected, the amount of eutectic liquid under sintering conditions was reduced by the presence of silicon nitride.
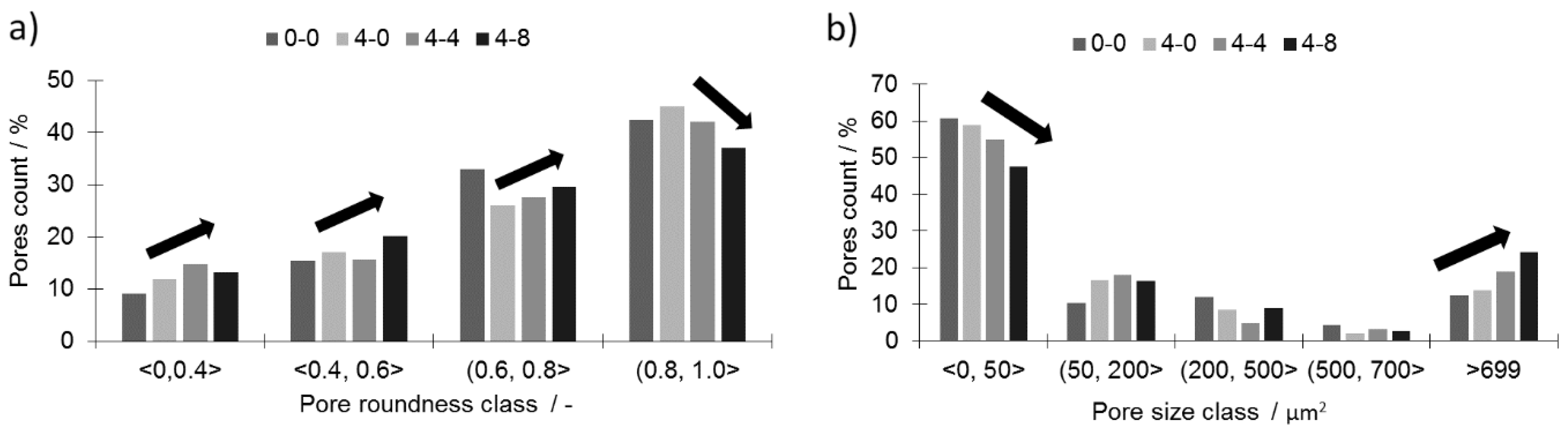
All three samples with a 0.4 wt % addition of boron (4-0, 4-4, and 4-8) and an increasing addition of silicon nitride are characterized by a considerably lower porosity than the reference sample (0-0). The histograms of the pore roundness and area are plotted in
Figure 5 to estimate the qualitative influence of silicon nitride addition on the sinters. In the tested samples with boron addition (4-0, 4-4, and 4-8), the addition of silicon nitride reduced the fraction of well-rounded pores (0.81 < round < 1.0) while simultaneously increasing the fraction of less-rounded pores (
Figure 5a). At the same time, the large pores fraction (>700 µm
2) increased at the cost of the small pores (<50 µm
2), as presented in
Figure 5b. This means the pores grew larger and became less rounded with the addition of silicon nitride. This may lower the mechanical properties of the given sinters. The decreasing pore roundness also indicates a limited influence of the liquid phase. This limitation may be associated with both a lower overall amount of liquid at the sintering temperature and shortened residence time.
Microstructural analysis was performed (
Figure 6a,d). The grain size analysis showed that even large additions of silicon nitride to the samples modified with boron (sample 4-4 and 4-8) did not alter the average grain diameter (
Table 4).
Since large grains grew during liquid phase sintering through dissolution and reprecipitation, one may conclude that the liquid phase was also present when large amounts of silicon nitride were added. Without Si
3N
4 powder addition, abnormal grain growth can be seen along with many twins in the large grains. In contrast, average grain growth, no twins in grains, and grain boundary waving can be seen in the samples with Si
3N
4 addition. These large grains were interpreted as meaning that the residence time was sufficiently long for the liquid to act and cause the extensive grain growth via the reprecipitation process, which constitutes the final step of the liquid phase influence on the microstructure according to liquid phase theory [
8]. Furthermore, this means that a quantity of eutectic liquid was present during the entire sintering cycle, a conclusion that is in good agreement with the observed borides (
Figure 2), which show a footprint of the eutectic phase presence.
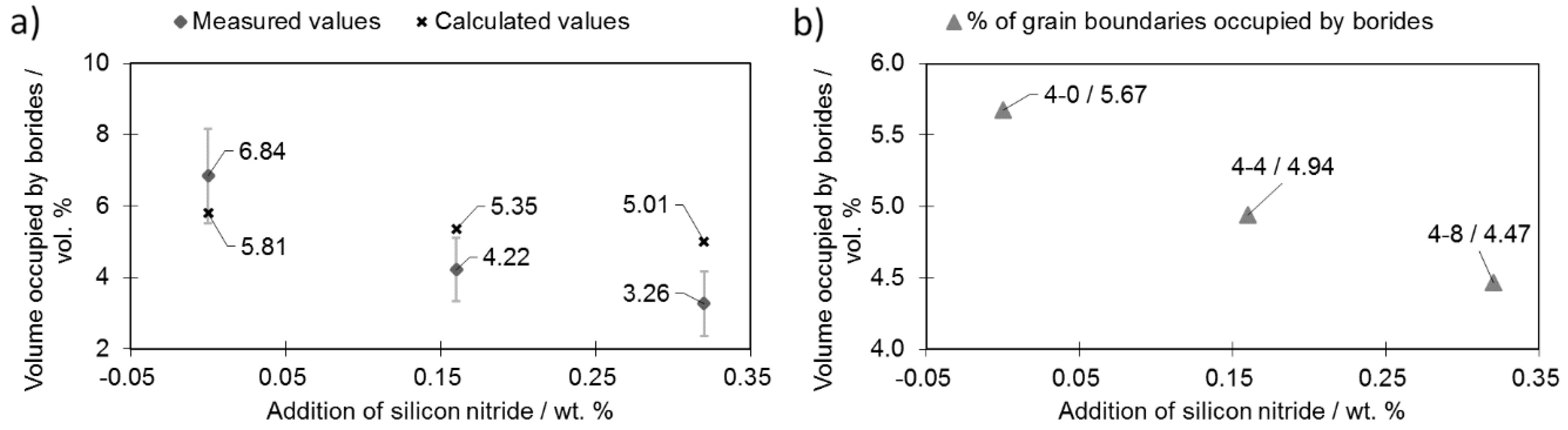
Figure 7a presents the total amounts of borides observed in selected samples (containing 0.4 wt% boron) during silicon nitride addition.
Analysis of the microstructure with a focus on the boride distribution confirmed the simulation results (
Figure 1)—as more silicon nitride was added, fewer borides were observed (
Figure 7a). The measured boride values roughly match the calculated ones. The experimental relationship between the volume occupied by bodies and the Si
3N
4 addition deviated from the linear relationship obtained by calculation. This is the effect of the eutectic liquid behavior: When no Si
3N
4 is added, the eutectic liquid tends to flow towards the center of the sample [
17]. On the other hand, when Si
3N
4 is added, flow towards the sample core is impeded and fewer borides are observed in this area and therefore fewer borides are observed in the investigated area.
The data obtained were used to calculate the total fraction of grain boundaries occupied by borides (
Figure 7b). This calculation was performed on the assumption that the grains have a regular 20-hedron shape with diameters equal to the mean grain diameter. Based on 100 random boride thickness observations (placed on the grain boundaries), the mean thickness was calculated as 16.5 µm. Porosity was subtracted from the sample volume.
Due to the increasing porosity contribution (
Figure 3) along the increasing silicon nitride addition curve, the percentage grain boundaries occupied by borides did not decrease significantly when more silicon nitride was introduced (sample 4-8). Nevertheless, the addition of 0.32 wt % of Si
3N
4 (sample 4-8) was enough to reduce the percentage of grain boundaries occupied by borides from 5.67% (sample 4-0) down to 4.47% (sample 4-8).
The microstructural changes caused by additions of boron and silicon nitride (
Figure 6) may have a detrimental influence on corrosion resistance behavior (which in this case is one of the most crucial features of the tested material). Corrosion tests were therefore performed only on selected samples (
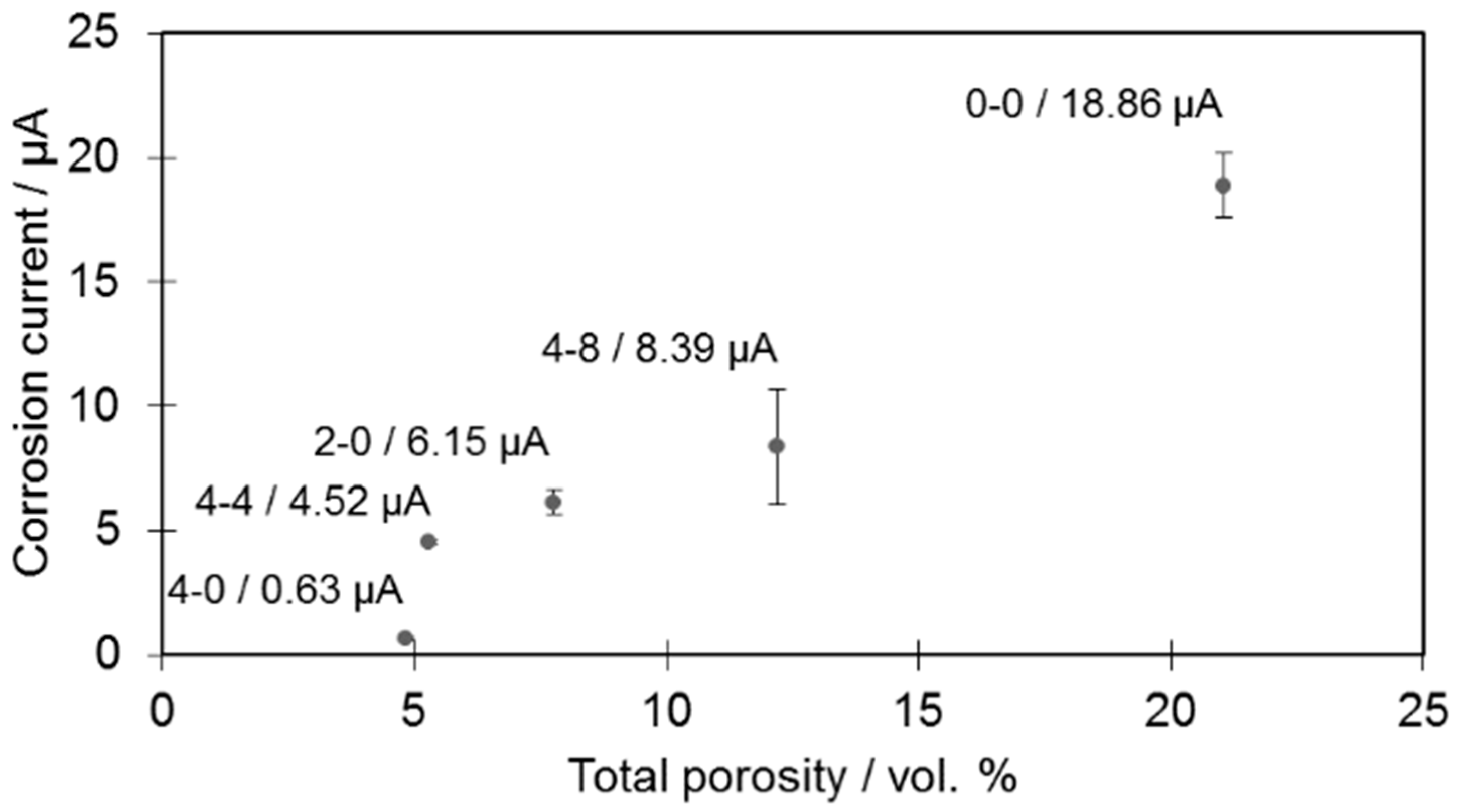
Figure 8).
In the case of the tested samples, it was established that the higher the porosity value, the higher the corrosion current, which is consistent with observations from other researchers [
6]. The increase was observed irrespective of the changing amounts of borides present among the samples. This means that the introduced powder modifications do not alter the general characteristics of the material and that pores are mainly responsible for the increased corrosion rate by increasing the contact surface for a corrosive medium.
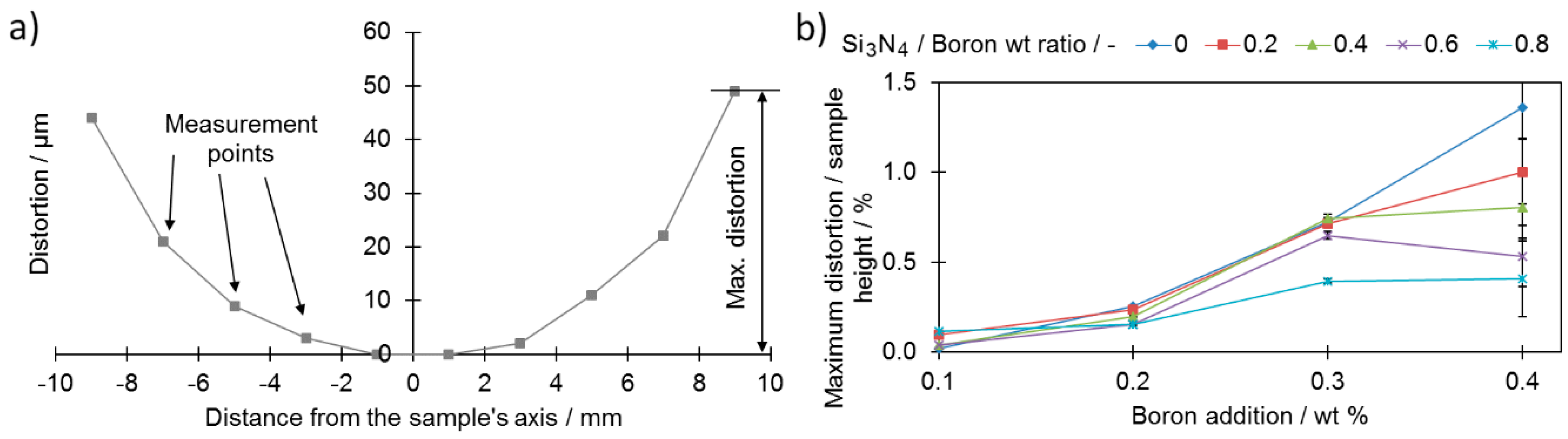
Another feature, which was almost not investigated, is the extent of the dimensional distortions of the sinter—the lower they, are the better. Dimensional distortions are typical for samples with a large boron addition and sintered at a temperature exceeding the eutectic reaction temperature of boron and iron (approximately 1177 °C). This is caused mainly by the eutectic liquid volume, which may lead to separation of neighboring grains by a thin layer of liquid when it exceeds 20% to 25%, and is a consequence of the relative motion of these grains [
6,
7,
35]. The distortions were measured on the pressing side of the samples. A measurement schematic is presented in
Figure 9a.
As presented in
Figure 9b, all samples, except sample 1-0, were found to present higher maximum dimensional distortions after sintering compared to the base sample (0-0) (0.04% ± 0.002% of the sample height). For samples with a high boron addition (0.4 wt %), the introduction of the silicon nitride resulted in improved dimensional precision (
Figure 9b). On the other hand, for samples with a lower addition of boron (0.1 and 0.2 wt %), no significant improvement was noticed along the increased silicon nitride addition curve. This provides information for the case of samples with high boron additions for which the addition of silicon nitride prevented full separation of the neighboring grains responsible for grain reorganization.
When comparing the density values of the cylindrical samples with maximum distortions, a high relative density (
Figure 3) may be obtained with relatively low distortions, e.g., sample 4-6.
In the next step, the TRS samples (30 mm × 12 mm × 6 mm) were tested for density. As presented in
Figure 10, noticeably lower densities were obtained for prismatic samples in comparison to cylindrical ones.
This results from the lower compatibility of prismatic samples due to the presence of corners and relatively large outline surfaces compared to the cross-section. Both of these features impede the compaction process and, therefore, decrease the green density. The average green density of the cylindrical sample was 6.3 g*cm
−1, while the value for the prismatic sample was 5.95 g*cm
−1 (
Table S4 in the Supplementary Data). Despite the altered sample geometry and lower densities, the general sintering mechanisms remained similar. Furthermore, the greater the additions of both boron and Si
3N
4, the lower the green density of samples. The same liquid volume was thus not able to fill the whole internal porosity potential and to act with the same efficiency as in cylindrical samples. The density values are presented in relation to fully dense wrought 316L steel.
3.3. Mechanical Properties
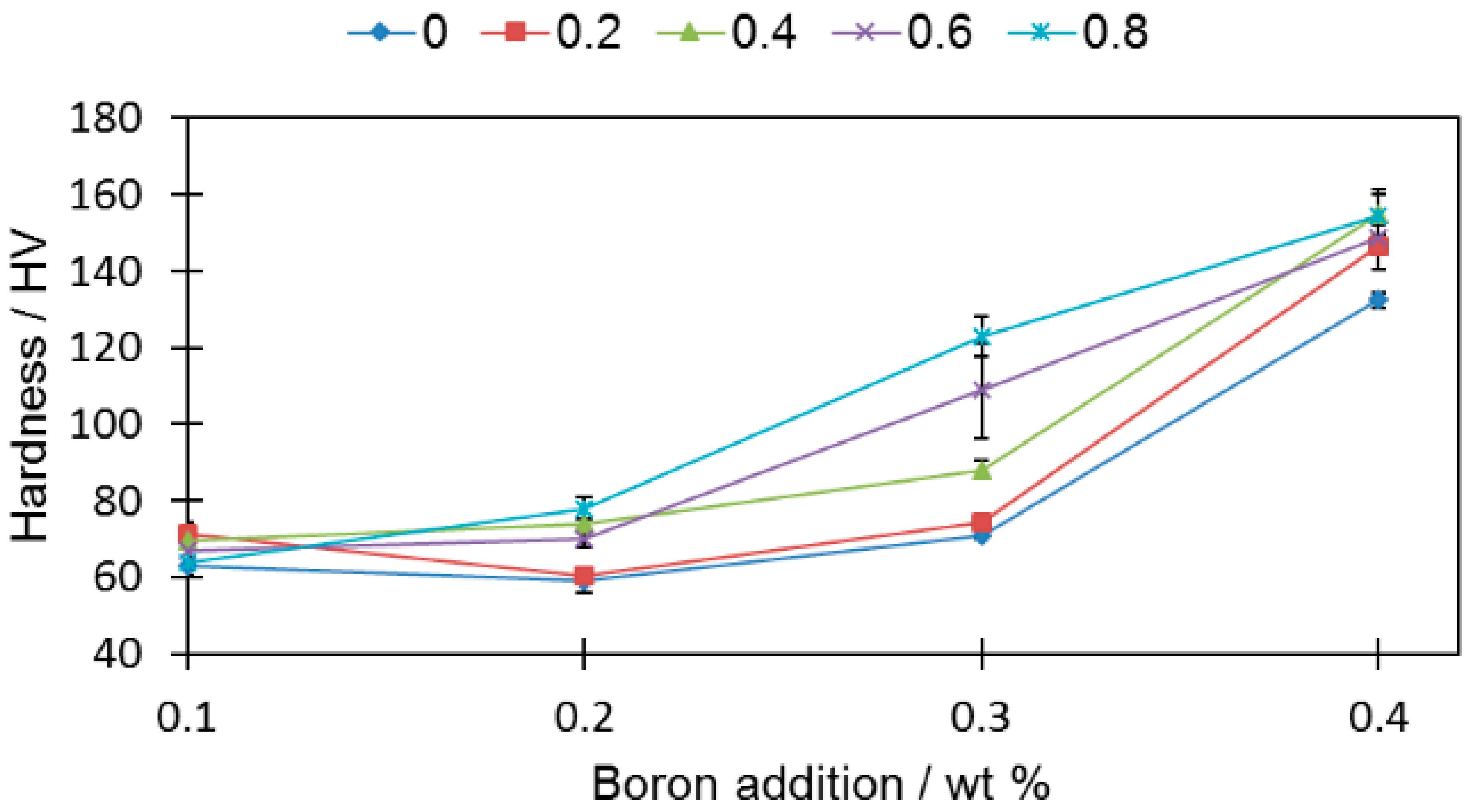
Despite having a deteriorating influence on the density (
Figure 10), the silicon nitride addition resulted in increased hardness (
Figure 11). In general, all the tested samples (aside from samples 2-0 and 2-2) were characterized with a higher hardness than the reference sample (0-0) (HV 60.8 ± 1.2).
For the rest of the samples, the hardness increase was independent of the boron addition. It was also irrespective of the porosity, i.e., hardness grows even the when porosity drops. Sample 4-8 had an over two times higher (HV 154 ± 6.9) hardness value than sample 0-0 (HV 60.8 ± 1.2), despite comparable relative densities (80.35% ± 0.18% and 79.62% ± 0.19%, respectively).
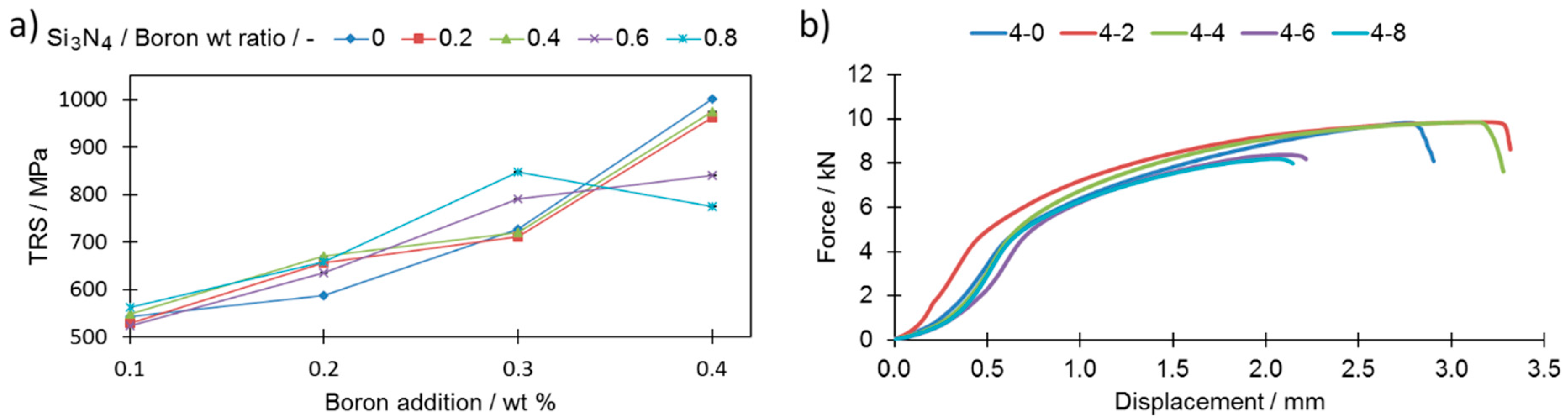
The combined influence of the porosity change and the reduced number of secondary phases, together with the varying additions of boron and silicon nitride, were checked by performing standardized transverse rupture strength (TRS) tests.
As a general rule, the stronger the interconnections among neighboring grains, the higher the TRS. Although the highest TRS value was registered for sample 4-0, this was severely distorted; this powder composition is thus considered to be technologically useless. By comparing samples from extreme cases, i.e., 0-0 and 4-8, which had roughly similar relative densities (79.62 and 80.34), respectively, one can observe a significant increase in the TRS value, from 457 MPa for sample 0-0 to 775 MPa for sample 4-8 (
Figure 12a,b). As presented in
Figure 12a, the addition of silicon nitride to the boron-modified samples increases the TRS value only when the boron addition does not exceed 0.2 wt% and if the Si
3N
4 to boron ratio is below 0.4. When higher amounts of boron and/or Si
3N
4 are added, then this effect is not visible anymore. For higher Si
3N
4 additions (Si
3N
4 to boron ratio = 0.6), the TRS value is always higher than for samples without Si
3N
4. When the ratio reaches 0.8, one may observe a drastic drop of the TRS. The same effect was observed in terms of displacement during the TRS test (
Figure 9b).
A change of this kind was possible due to a percentage drop in the grain boundaries occupied by borides as presented in
Figure 7b. This increase was achieved despite an unfavorable decrease in the pore roundness value and an increase in their volume together with an increasing Si
3N
4 addition (
Figure 5a,b).
3.4. Summary
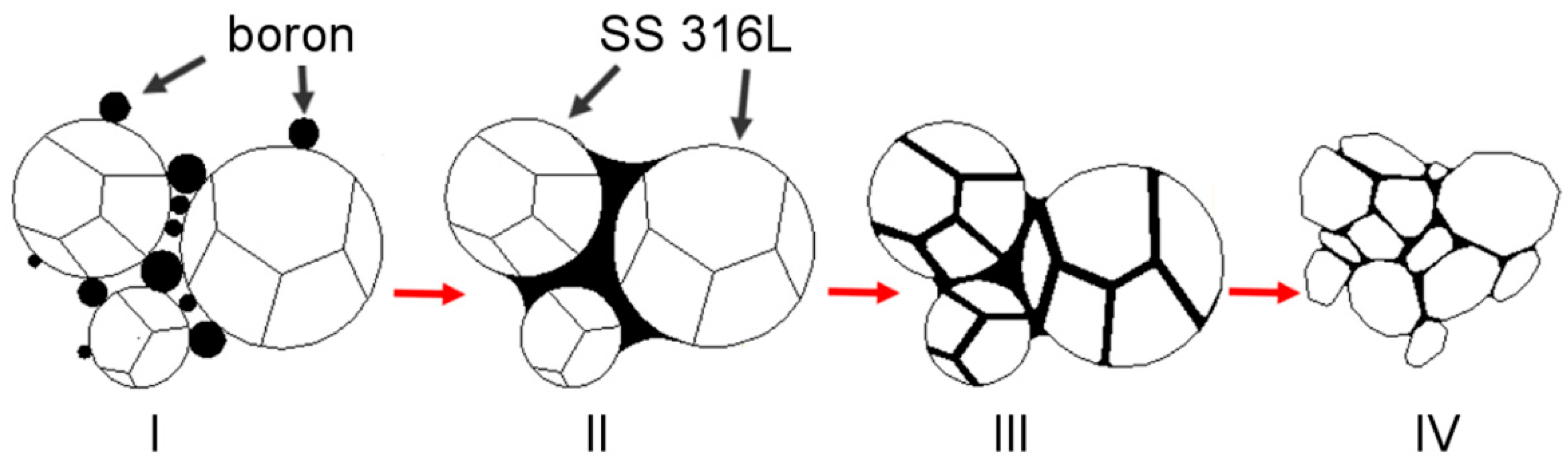
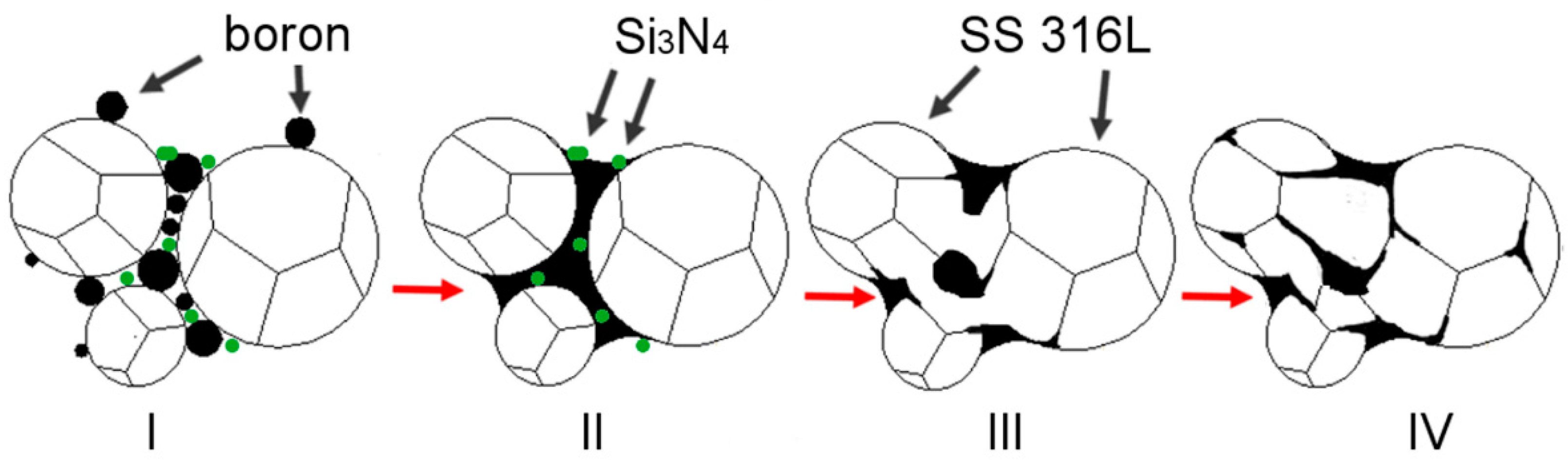
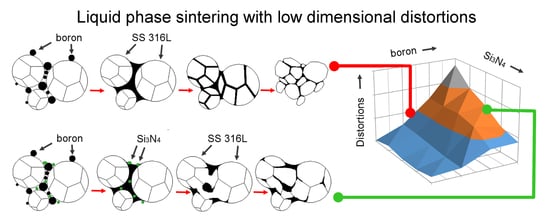
A general explanation is proposed and described in detail in a subsequent part of this work to explain the observed influence of silicon nitride addition on samples modified with boron addition. When boron is added to the ferrous metal powder and heated up, a eutectic reaction takes place. As a result, a eutectic liquid phase is created and, due to the insolubility of boron in the matrix, the liquid persists as long as the temperature is higher than its solidus temperature. A generally accepted microstructural impact of a persistent liquid phase on green compact, which was described in
Figure 13 [
6,
7,
35], is, in this case, altered by the presence of silicon nitride (
Figure 14).
The presence of the eutectic liquid at the sintering temperature depends to a significant extent on the boron concentration along the liquidus lines surrounding the eutectic point. When a nitrogen atom combines with a boron atom from E(M
2B + M) (where M = Fe, Cr, Mo), an in-situ h-BN molecule is created, leading to the expulsion of three selected metal atoms: Cr, Fe, and/or Mo [
2]. All of these also solidify on the neighboring grains due to the lack of boron and create mutual interconnections without borides.
This effect may be seen in
Figure 7b, where the total percentage of grain boundaries occupied by borides drops from 5.67% (sample 4-0) to 4.47% (sample 4-8). This means that enhanced interconnections among grains were created on over 1.2% of all grain boundaries (a relative increase of 21%). These interconnections create solid joints among the grains, preventing them from reorganization in the initial sintering step, which in turn decreases distortions during sintering. This mechanism was present in all tested samples to various extents depending on the amounts of added boron and Si
3N
4.
Equation (2) describes a general mechanism of boride formation in AISI 316L steel modified with boron; this is in good agreement with the microstructural observations (
Figure 7a,b). Based on the agreement between the decreasing borides amounts and the amount calculations presented in
Figure 7a,b, one may conclude that Equation (3) is no longer valid when silicon nitride is added. An interaction between the eutectic liquid and the Si
3N
4 occurs as proposed in Equation (3):
where
M is Fe, Cr, or Mo and
T is temperature (°C).
Opposite observations were presented in Grądzka-Dahlke et al. (2007) [
36], where Co-Cr-Mo powder was modified with B
4C and Si
3N
4 additions were added independently. Neither of these compounds resulted in an increased sinter density during the sintering process. This result was due to the high thermodynamic stability of B
4C, due to which the boron could not interact with the matrix and, as a result, no eutectic reaction occurred. In the case of a separate Si
3N
4 addition, the density was also not increased due to lack of chemical interaction with the matrix. In both cases, large interconnected pores were spotted in the microstructure, which hindered the diffusion process.