Minor Review: An Overview of a Synthetic Nanophase Bone Substitute
Abstract
1. Introduction
2. Material Designed around Interaction with Osteoclasts
3. Material Fabrication
4. In Vitro Material Characterization
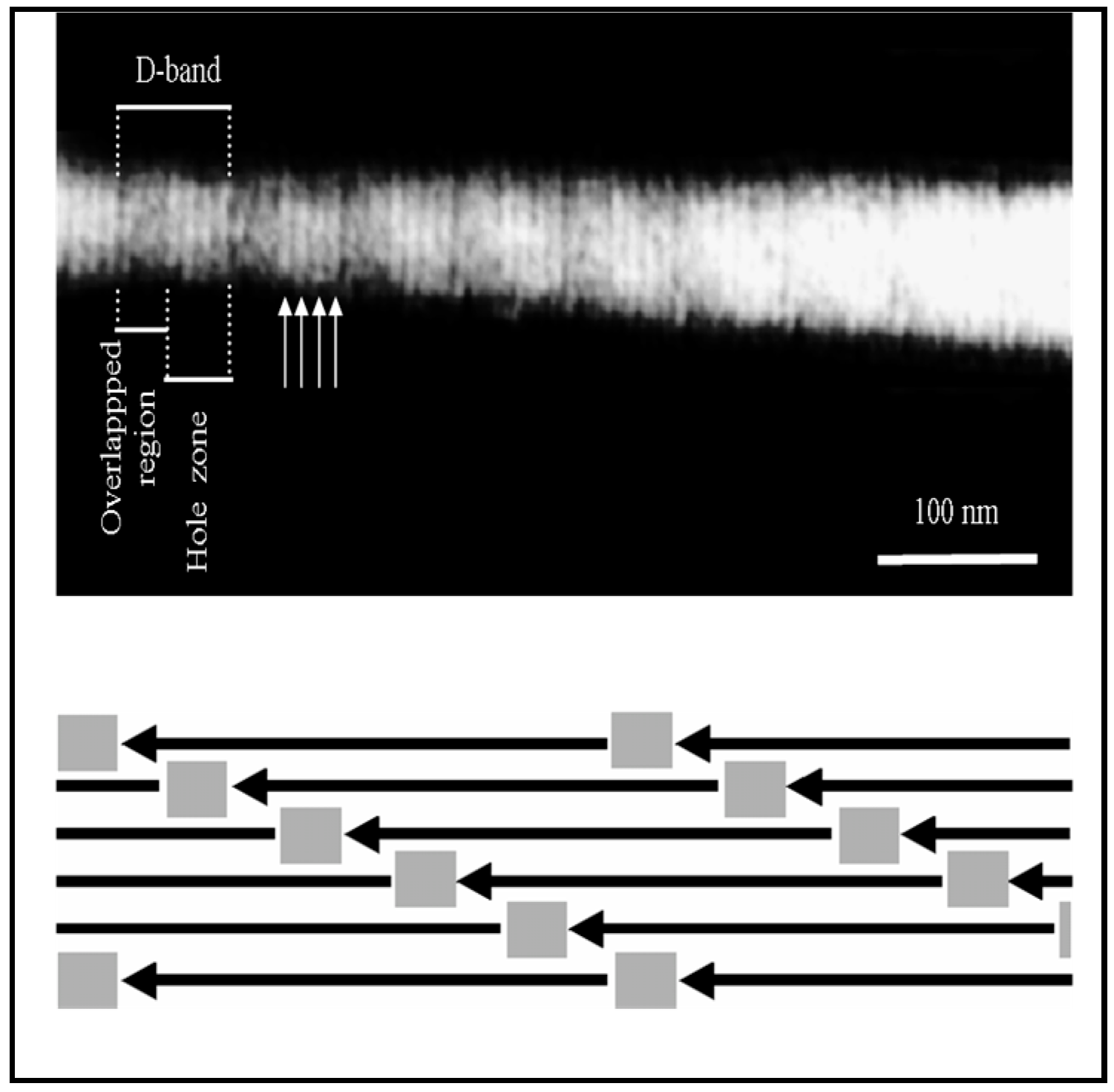
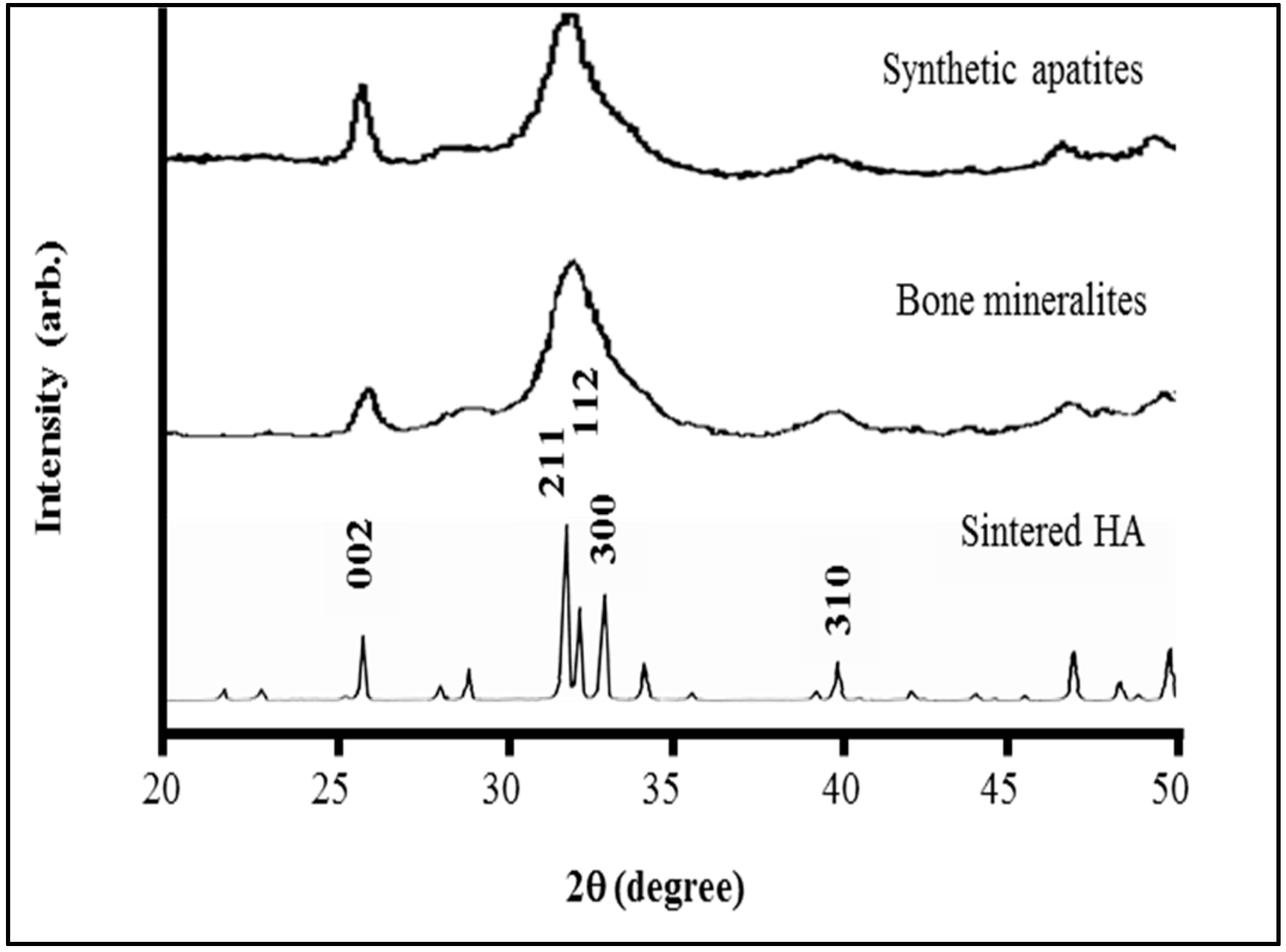
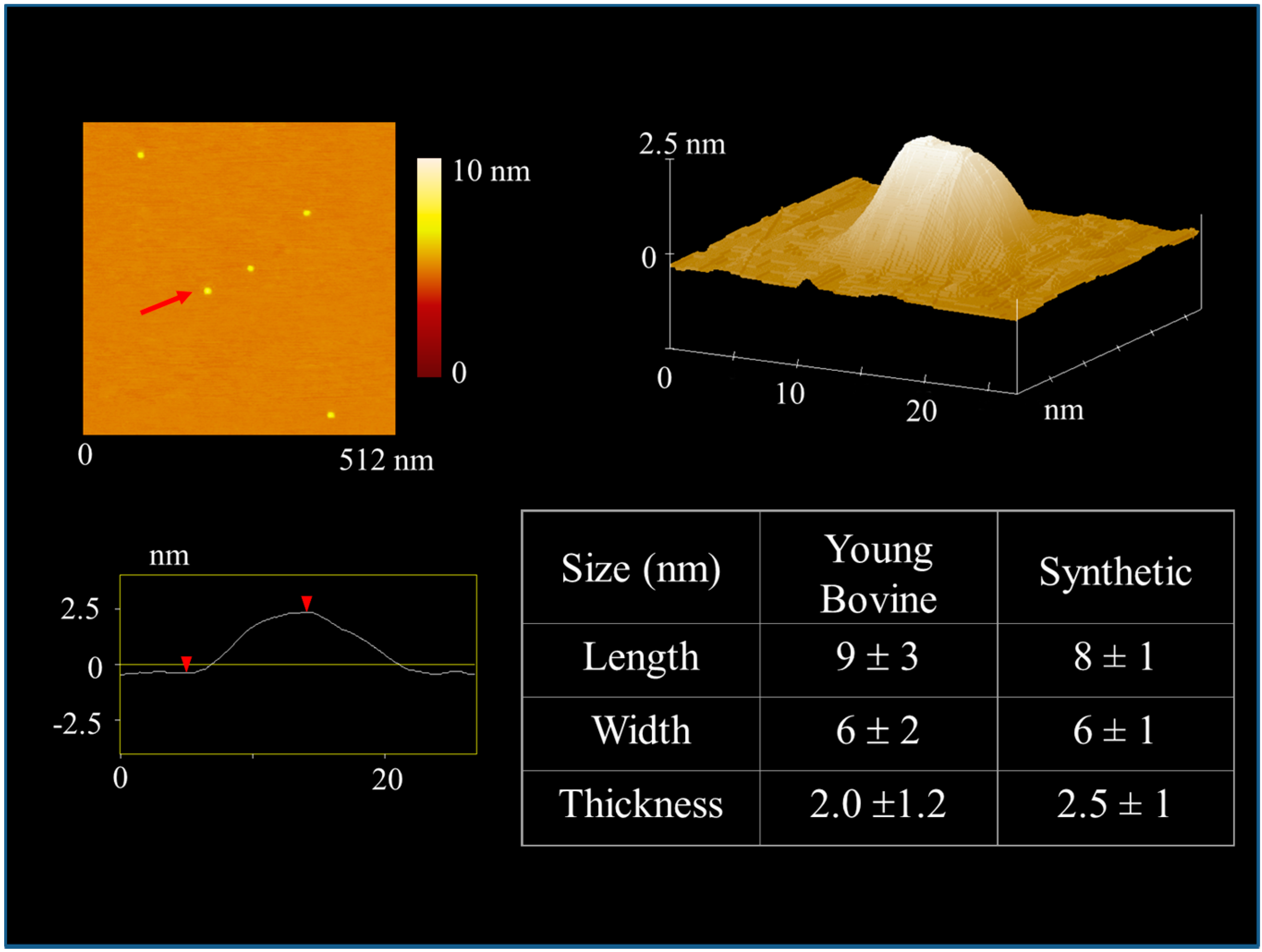
4.1. Sub-µm Structure and Chemistry of Our Material
4.2. BMP-2 Release
4.3. Cellular Response
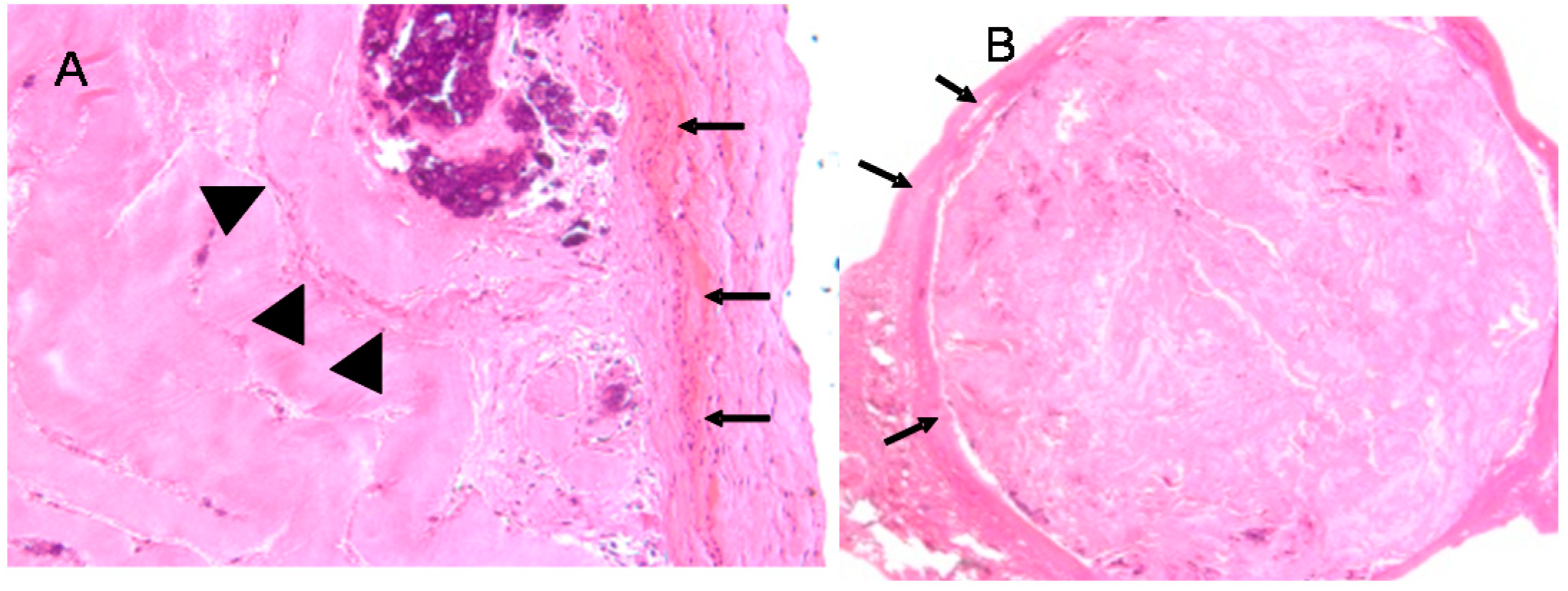
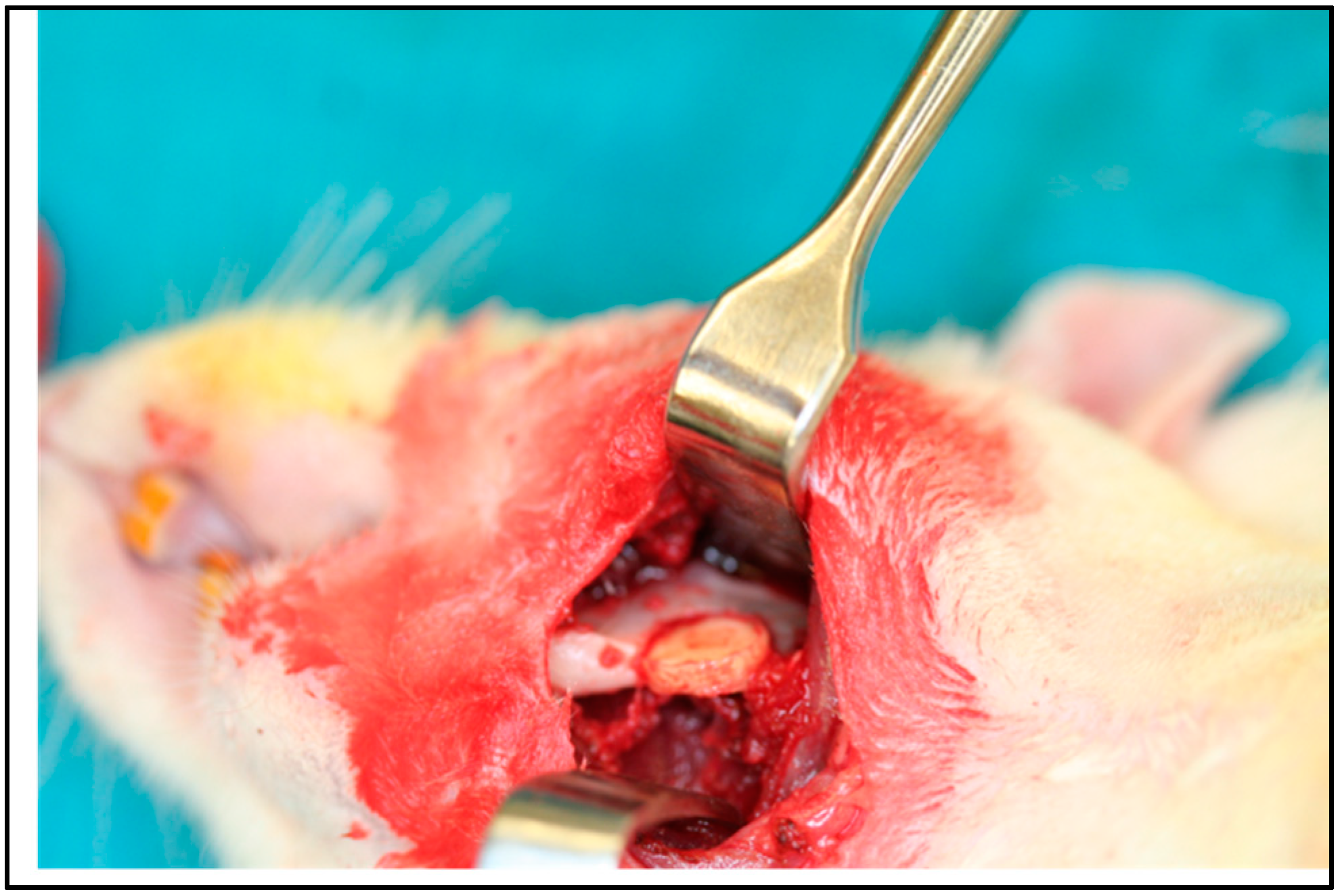
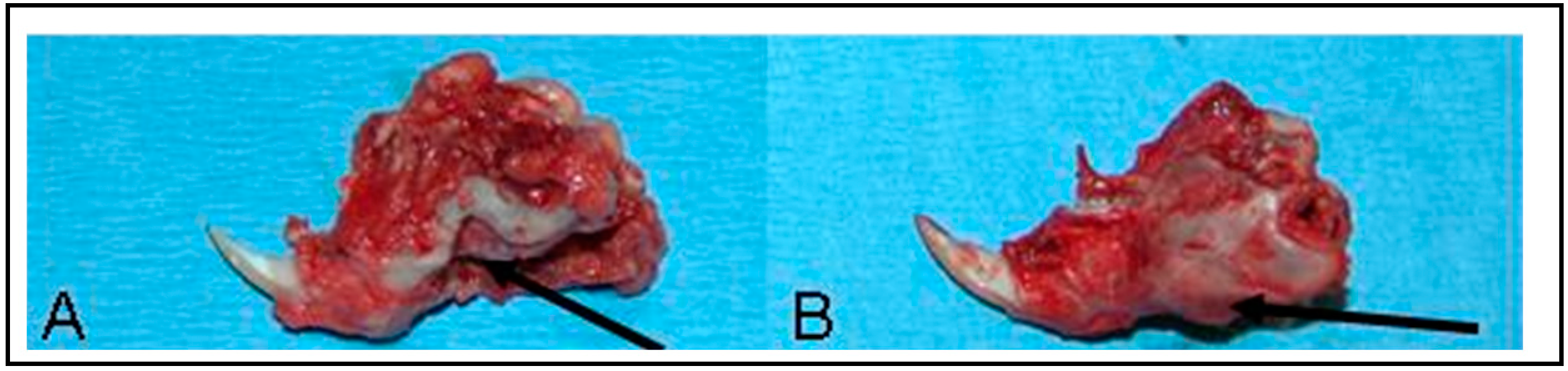
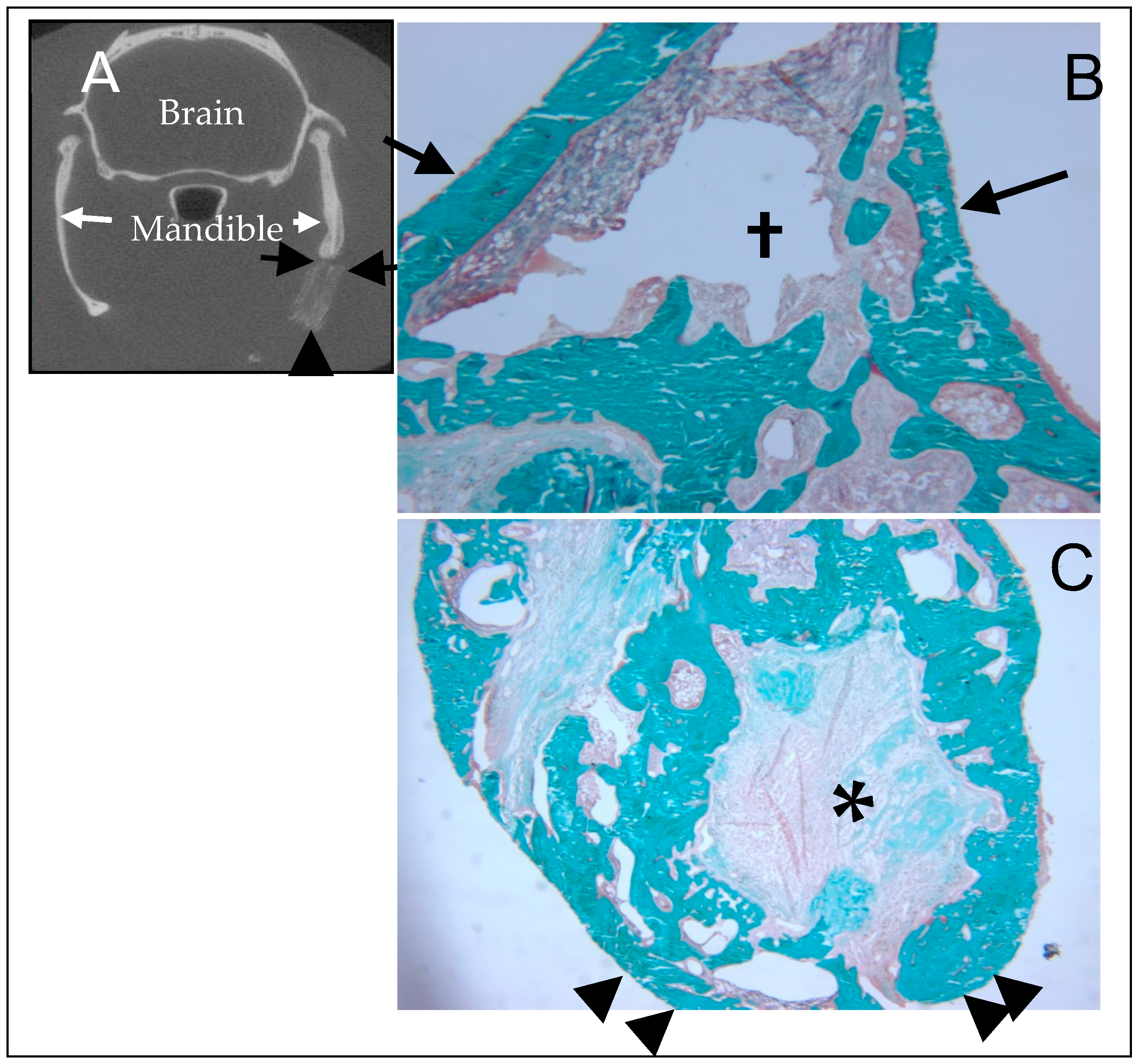
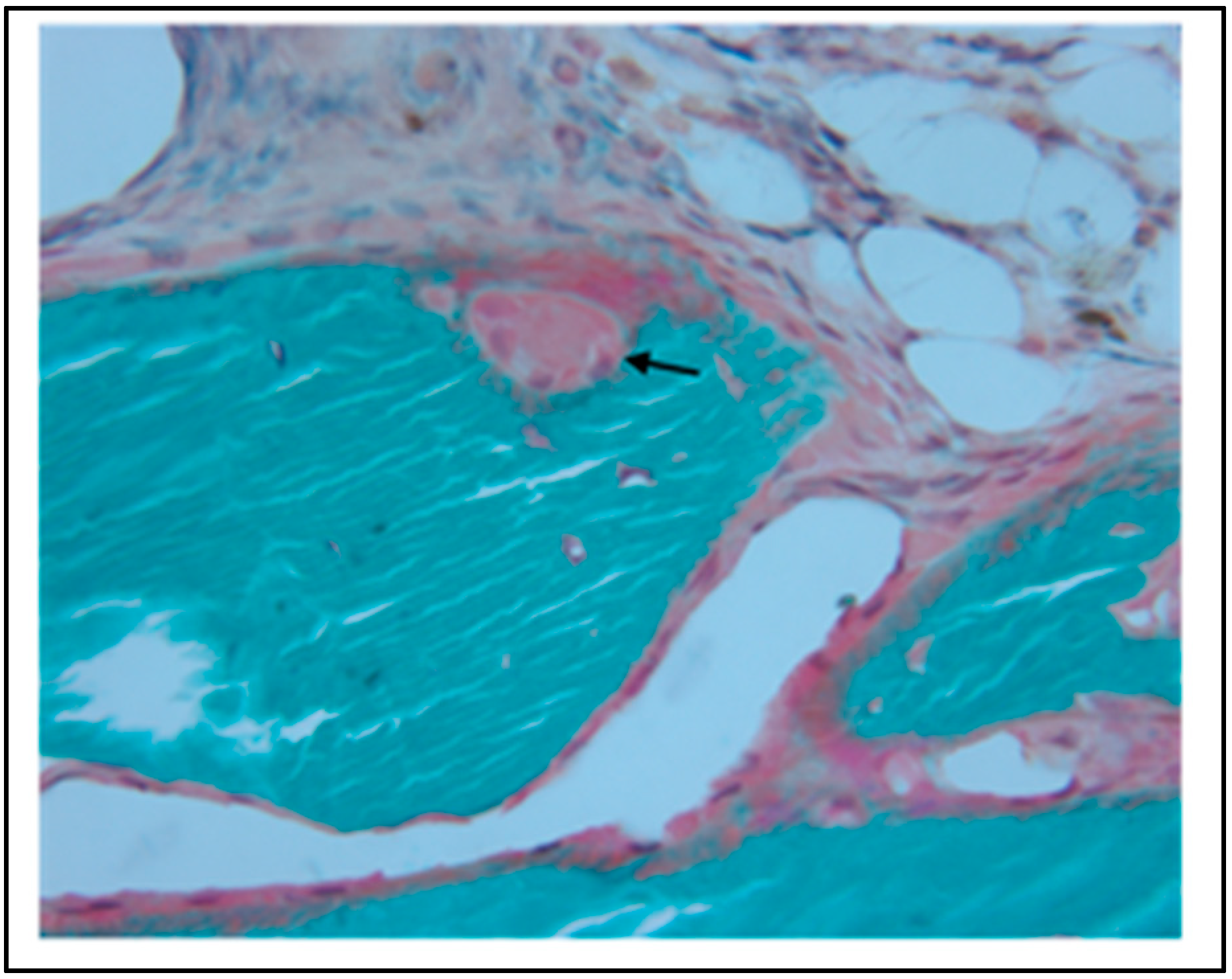
5. In Vivo Testing
6. Rational for the Rodent Mandibular Model
7. Nanocomposite Material as a Bone Substitute and Drug Delivery Vehicle
8. Summary
Funding
Conflicts of Interest
Abbreviations
| BMP-2 | Bone morphogenetic protein 2 |
| Ca/P | Calcium/phosphate |
| CSD | Critical size defect |
| BM | Demineralized bone matrix |
| DI | Deionized |
| EDX | Energy dispersive x-ray analysis |
| ELISA | Enzyme-linked immunosorbent assay |
| FBGC | Foreign body giant cell |
| HA | Hydroxyapatite |
| H&E | Hematoxylin and eosin |
| IACUC | Institutional animal care and use committee |
| NBS | Nanophase bone substitute |
| PLA/PGA | Polylactic acid/polyglycolic acid |
| SEM | Scanning electron microscopy |
| TCP | Tricalcium phosphate |
| TEM | Transmission electron microscopy |
| TRAP | Tartrate resistant acid phosphatase |
| UP | Uniaxially pressed |
References
- Bauer, T.W.; Muschler, G.F. Bone graft materials: An overview of the basic science. Clin. Orthop. Relat. Res. 2000, 371, 10–27. [Google Scholar] [CrossRef]
- Enneking, W.F.; Mindell, E.R. Observations on massive retrieved human allografts. J. Bone Jt. Surg. Am. 1991, 73, 1123–1142. [Google Scholar] [CrossRef]
- Carr, C.R.; Hyatt, G.W. Clinical evaluation of freeze-dried bone grafts. J. Bone Jt. Surg. Am. 1955, 37, 549–566. [Google Scholar] [CrossRef]
- Liljenqvist, U.; O’Brien, J.P.; Renton, P. Simultaneous combined anterior and posterior lumbar fusion with femoral cortical allograft. Eur. Spine J. 1998, 7, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.L. Grafton demineralized bone matrix: Performance consistency, utility and value. Tissue Eng. 2000, 6, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Yovich, S.; Seydel, U.; Papadimitriou, J.M.; Nicholson, G.C.; Wood, D.J.; Zheng, M.H. Evidence that failure of osteoid bone matrix resorption is caused by perturbation of osteoclast polarization. Histochem. J. 1998, 30, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Chakkalakal, D.A.; Strates, B.S.; Garvin, K.L.; Novak, J.R.; Fritz, E.D.; Mollner, T.J.; McGuire, M.H. Demineralized bone matrix as a biological scaffold for bone repair. Tissue Eng. 2001, 7, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Takikawa, S.; Bauer, T.W.; Kambic, H.; Togawa, D. Comparative evaluation of the osteoinductivity of two formulations of human demineralized bone matrix. J. Biomed. Mater. Res. A 2003, 65, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.E.; Hagler, H.K. Porous hydroxyapatite as a bone graft substitute in cranial reconstruction: A histometric study. Plast. Reconstr. Surg. 1988, 81, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Descamps, M.; Dejou, J.; Koubi, G.; Hardouin, P.; Lemaitre, J.; Proust, J.P. The biodegradation mechanism of calcium phosphate biomaterials in bone. J. Biomed. Mater. Res. 2002, 63, 408–412. [Google Scholar] [CrossRef] [PubMed]
- Metsger, D.S.; DePhilip, R.M.; Hayes, T.G. An autoradiographic study of calcium phosphate ceramic bone implants in turkeys. Clin. Orthop. Relat. Res. 1993, 283–294. [Google Scholar]
- Renooij, W.; Hoogendoorn, H.A.; Visser, W.J.; Lentferink, R.H.; Schmitz, M.G.; Van Ieperen, H.; Oldenburg, S.J.; Janssen, W.M.; Akkermans, L.M.; Wittebol, P. Bioresorption of ceramic strontium-85-labeled calcium phosphate implants in dog femora. A pilot study to quantitate bioresorption of ceramic implants of hydroxyapatite and tricalcium orthophosphate in vivo. Clin. Orthop. Relat. Res. 1985, 272–285. [Google Scholar]
- Wenisch, S.; Stahl, J.P.; Horas, U.; Heiss, C.; Kilian, O.; Trinkaus, K.; Hild, A.; Schnettler, R. In vivo mechanisms of hydroxyapatite ceramic degradation by osteoclasts: Fine structural microscopy. J. Biomed. Mater. Res. A 2003, 67, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Glimcher, M.J. Molecular biology of mineralized tissues with particular reference to bone. Rev. Mod. Phys. 1959, 31, 359–393. [Google Scholar] [CrossRef]
- Kamat, S.; Su, X.; Ballarini, R.; Heuer, A.H. Structural basis for the fracture toughness of the shell of the conch Strombus gigas. Nature 2000, 405, 1036–1040. [Google Scholar] [CrossRef] [PubMed]
- Buehler, M.J. Molecular nanomechanics of nascent bone: Fibrillar toughening by mineralization. Nanotechnology 2007, 18. [Google Scholar] [CrossRef]
- Gao, H.; Ji, B.; Jager, I.L.; Arzt, E.; Fratzl, P. Materials become insensitive to flaws at nanoscale: Lessons from nature. Proc. Natl. Acad. Sci. USA 2003, 100, 5597–5600. [Google Scholar] [CrossRef] [PubMed]
- Ripamonti, U.; Ma, S.; Reddi, A.H. The critical role of geometry of porous hydroxyapatite delivery system in induction of bone by osteogenin, a bone morphogenetic protein. Matrix 1992, 12, 202–212. [Google Scholar] [CrossRef]
- Gower, L.B.; Odom, D.J. Deposition of calcium carbonate films by a polymer-induced liquid-precursor (PILP) process. J. Cryst. Growth 2000, 210, 719–734. [Google Scholar] [CrossRef]
- Olszta, M.J.; Douglas, E.P.; Gower, L.B. Scanning electron microscopic analysis of the mineralization of type I collagen via a polymer-induced liquid-precursor (PILP) process. Calcif. Tissue Int. 2003, 72, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Gower, L.B.; Olszta, M.J. Method for Producing a Mineral Fiber. U.S. Patent 7,455,854, 25 November 2008. [Google Scholar]
- Kenichi, S.; Soichiro, I.; Junzo, T.; Masanori, K. Artificial Pyramid. U.S. Patent 6,887,272, 3 May 2005. [Google Scholar]
- Itoh, S.; Kikuchi, M.; Koyama, Y.; Matumoto, H.N.; Takakuda, K.; Shinomiya, K.; Tanaka, J. Development of a novel biomaterial, hydroxyapatite/collagen (HAp/Col) composite for medical use. Biomed. Med. Mater. Eng. 2005, 15, 29–41. [Google Scholar]
- Itoh, S.; Kikuchi, M.; Koyama, Y.; Takakuda, K.; Shinomiya, K.; Tanaka, J. Development of an artificial vertebral body using a novel biomaterial, hydroxyapatite/collagen composite. Biomaterials 2002, 23, 3919–3926. [Google Scholar] [CrossRef]
- Itoh, S.; Kikuchi, M.; Koyama, Y.; Takakuda, K.; Shinomiya, K.; Tanaka, J. Development of a hydroxyapatite/collagen nanocomposite as a medical device. Cell Transplant. 2004, 13, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Tong, W. Synthesis of Nanometer-sized Apatites Using Highly Ordered Collagen Structure. Ph.D. Thesis, Case Western Reserve University, Cleveland, OH, USA, 2001. [Google Scholar]
- Baskin, J.Z.; Eppell, S.J.; Tong, W. Biomaterial Implants. U.S. Patent 8,491,924, 23 July 2013. [Google Scholar]
- Tomson, M.B.; Nancollas, G.H. Mineralization kinetics: A constant composition approach. Science 1978, 200, 1059–1060. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, M.H.; Nesbitt, S.A.; Lakkakorpi, P.T.; Barnes, M.J.; Bodary, S.C.; Shankar, G.; Mason, W.T.; Mendrick, D.L.; Vaananen, H.K.; Horton, M.A. Beta 1 integrins and osteoclast function: Involvement in collagen recognition and bone resorption. Bone 1996, 19, 317–328. [Google Scholar] [CrossRef]
- Horton, M.A.; Dorey, E.L.; Nesbitt, S.A.; Samanen, J.; Ali, F.E.; Stadel, J.M.; Nichols, A.; Greig, R.; Helfrich, M.H. Modulation of vitronectin receptor-mediated osteoclast adhesion by Arg-Gly-Asp peptide analogs: A structure-function analysis. J. Bone Min. Res. 1993, 8, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Lakkakorpi, P.T.; Horton, M.A.; Helfrich, M.H.; Karhukorpi, E.K.; Vaananen, H.K. Vitronectin receptor has a role in bone resorption but does not mediate tight sealing zone attachment of osteoclasts to the bone surface. J. Cell Biol. 1991, 115, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Kameda, T.; Mano, H.; Yamada, Y.; Takai, H.; Amizuka, N.; Kobori, M.; Izumi, N.; Kawashima, H.; Ozawa, H.; Ikeda, K.; et al. Calcium-sensing receptor in mature osteoclasts, which are bone resorbing cells. Biochem. Biophys. Res. Commun. 1998, 245, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Malgaroli, A.; Meldolesi, J.; Zallone, A.Z.; Teti, A. Control of cytosolic free calcium in rat and chicken osteoclasts. The role of extracellular calcium and calcitonin. J. Biol. Chem. 1989, 264, 14342–14347. [Google Scholar] [PubMed]
- Miyauchi, A.; Hruska, K.A.; Greenfield, E.M.; Duncan, R.; Alvarez, J.; Barattolo, R.; Colucci, S.; Zambonin-Zallone, A.; Teitelbaum, S.L.; Teti, A. Osteoclast cytosolic calcium, regulated by voltage-gated calcium channels and extracellular calcium, controls podosome assembly and bone resorption. J. Cell. Biol. 1990, 111, 2543–2552. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Rey, C.; Glimcher, M.J. Isolation of calcium-phosphate crystals of bone by non-aqueous methods at low temperature. J. Bone Min. Res. 1995, 10, 1589–1601. [Google Scholar] [CrossRef] [PubMed]
- Swedlow, D.B.; Frasca, P.; Harper, R.A.; Katz, J.L. Scanning and transmission electron microscopy of calcified tissues. Biomater. Med. Devices Artif. Organs 1975, 3, 121–153. [Google Scholar] [CrossRef] [PubMed]
- Tong, W.; Glimcher, M.J.; Katz, J.L.; Kuhn, L.; Eppell, S.J. Size and shape of mineralites in young bovine bone measured by atomic force microscopy. Calcif. Tissue Int. 2003, 72, 592–598. [Google Scholar] [PubMed]
- Baskin, J.Z.; Soenjaya, Y.; McMasters, J.; Ko, A.; Vasanji, A.; Morris, N.; Eppell, S.J. Nanophase bone substitute for craniofacial load bearing application: Pilot study in the rodent. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.Z.; Vasanji, A.; McMasters, J.; Soenjaya, Y.; Barbu, A.M.; Eppell, S.J. Nanophase bone substitute in vivo response to subcutaneous implantation. J. Biomed. Mater. Res. A 2012, 100, 2462–2473. [Google Scholar] [CrossRef] [PubMed]
- Holmes, D.F.; Capaldi, M.J.; Chapman, J.A. Reconstitution of collagen fibrils in vitro—The assembly process depends on the initiating procedure. Int. J. Boil. Macromol. 1986, 8, 161–166. [Google Scholar] [CrossRef]
- Oyane, A.; Onuma, K.; Ito, A.; Kim, H.M.; Kokubo, T.; Nakamura, T. Formation and growth of clusters in conventional and new kinds of simulated body fluids. J. Biomed. Mater. Res. Part A 2003, 64A, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.J.; Petruska, J.A. Recent studies with the electron microscope on ordered aggregates of the tropocollagen molecules. In Aspects of Protein Structure; Ramachandran, G.N., Ed.; Academic Press: New York, NY, USA, 1963; pp. 289–300. [Google Scholar]
- Landis, W.J.; Hodgens, K.J.; Arena, J.; Song, M.J.; McEwen, B.F. Structural relations between collagen and mineral in bone as determined by high voltage electron microscopic tomography. Microsc. Res. Tech. 1996, 33, 192–202. [Google Scholar] [CrossRef]
- Landis, W.J.; Hodgens, K.J.; Song, M.J.; Arena, J.; Kiyonaga, S.; Marko, M.; Owen, C.; McEwen, B.F. Mineralization of collagen may occur on fibril surfaces: Evidence from conventional and high-voltage electron microscopy and three-dimensional imaging. J. Struct. Boil. 1996, 117, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Hattersley, G.; Chambers, T.J. Generation of osteoclasts from hemopoietic cells and a multipotential cell line in vitro. J. Cell. Physiol. 1989, 140, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Akatsu, T.; Tamura, T.; Takahashi, N.; Udagawa, N.; Tanaka, S.; Sasaki, T.; Yamaguchi, A.; Nagata, N.; Suda, T. Preparation and characterization of a mouse osteoclast-like multinucleated cell population. J. Bone Min. Res. 1992, 7, 1297–1306. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.J.; Boyde, A.; Ali, N.N. The resorption of biological and non-biological substrates by cultured avian and mammalian osteoclasts. Anat. Embryol. 1984, 170, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Pitaru, S.; Noff, M.; Blok, L.; Nir, E.; Pitaru, S.; Goldlust, A.; Savion, N. Long-term efficacy of a novel ribose-cross-linked collagen dermal filler: A histologic and histomorphometric study in an animal model. Dermatol. Surg. 2007, 33, 1045–1054. [Google Scholar] [CrossRef] [PubMed]
- Hollinger, J.O.; Kleinschmidt, J.C. The critical size defect as an experimental model to test bone repair materials. J. Craniofac. Surg. 1990, 1, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Le Guehennec, L.; Goyenvalle, E.; Aguado, E.; Houchmand-Cuny, M.; Enkel, B.; Pilet, P.; Daculsi, G.; Layrolle, P. Small-animal models for testing macroporous ceramic bone substitutes. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 72, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Siegel, M.I.; Mooney, M.P. Appropriate animal models for craniofacial biology. Cleft Palate J. 1990, 27, 18–25. [Google Scholar] [CrossRef]
- Swennen, G.; Dempf, R.; Schliephake, H. Cranio-facial distraction osteogenesis: A review of the literature. Part II: Experimental studies. Int. J. Oral Maxillofac. Surg. 2002, 31, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Uludag, H.; Friess, W.; Williams, D.; Porter, T.; Timony, G.; D’Augusta, D.; Blake, C.; Palmer, R.; Biron, B.; Wozney, J. rhBMP-collagen sponges as osteoinductive devices: Effects of in vitro sponge characteristics and protein pI on in vivo rhBMP pharmacokinetics. Ann. N. Y. Acad. Sci. 1999, 875, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Magit, D.P.; Maak, T.; Trioano, N.; Raphael, B.; Hamouria, Q.; Polzhofer, G.; Drespe, I.; Albert, T.J.; Grauer, J.N. Healos/recombinant human growth and differentiation factor-5 induces posterolateral lumbar fusion in a New Zealand white rabbit model. Spine 2006, 31, 2180–2188. [Google Scholar] [CrossRef] [PubMed]
- Ohura, K.; Hamanishi, C.; Tanaka, S.; Matsuda, N. Healing of segmental bone defects in rats induced by a beta-TCP-MCPM cement combined with rhBMP-2. J. Biomed. Mater. Res. 1999, 44, 168–175. [Google Scholar] [CrossRef]
- Spiro, R.C.; Liu, L.; Heidaran, M.A.; Thompson, A.Y.; Ng, C.K.; Pohl, J.; Poser, J.W. Inductive activity of recombinant human growth and differentiation factor-5. Biochem. Soc. Trans. 2000, 28, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Uludag, H.; D’Augusta, D.; Palmer, R.; Timony, G.; Wozney, J. Characterization of rhBMP-2 pharmacokinetics implanted with biomaterial carriers in the rat ectopic model. J. Biomed. Mater. Res. 1999, 46, 193–202. [Google Scholar] [CrossRef]
- Ziegler, J.; Mayr-Wohlfart, U.; Kessler, S.; Breitig, D.; Gunther, K.P. Adsorption and release properties of growth factors from biodegradable implants. J. Biomed. Mater. Res. 2002, 59, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Bodalia, P.N.; Balaji, V.; Kaila, R.; Wilson, L. Effectiveness and safety of recombinant human bone morphogenetic protein-2 for adults with lumbar spine pseudarthrosis following spinal fusion surgery: A systematic review. Bone Jt. Res. 2016, 5, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Fu, R.; Selph, S.; McDonagh, M.; Peterson, K.; Tiwari, A.; Chou, R.; Helfand, M. Effectiveness and harms of recombinant human bone morphogenetic protein-2 in spine fusion: A systematic review and meta-analysis. Ann. Intern. Med. 2013, 158, 890–902. [Google Scholar] [CrossRef] [PubMed]
- Lykissas, M.; Gkiatas, I. Use of recombinant human bone morphogenetic protein-2 in spine surgery. World J. Orthop. 2017, 8, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Zellin, G.; Linde, A. Importance of delivery systems for growth-stimulatory factors in combination with osteopromotive membranes. An experimental study using rhBMP-2 in rat mandibular defects. J. Biomed. Mater. Res. 1997, 35, 181–190. [Google Scholar] [CrossRef]
- Baskin, J.Z.; Eppell, S.J. Development of a nanophase biomimetic material as a load bearing cortical bone substitute. In Proceedings of the 9th International Symposium of Facial Plastic Surgery, Las Vegas, NV, USA, 1–4 May 2006. [Google Scholar]
- Hollinger, J.O.; Uludag, H.; Winn, S.R. Sustained release emphasizing recombinant human bone morphogenetic protein-2. Adv. Drug Deliv. Rev. 1998, 31, 303–318. [Google Scholar] [PubMed]
- Logeart-Avramoglou, D.; Anagnostou, F.; Bizios, R.; Petite, H. Engineering bone: Challenges and obstacles. J. Cell. Mol. Med. 2005, 9, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Scheufler, C.; Sebald, W.; Hulsmeyer, M. Crystal structure of human bone morphogenetic protein-2 at 2.7 A resolution. J. Mol. Biol. 1999, 287, 103–115. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eppell, S.J.; Tong, W.; McMasters, J.; Soenjaya, Y.; Barbu, A.M.; Ko, A.; Baskin, J.Z. Minor Review: An Overview of a Synthetic Nanophase Bone Substitute. Materials 2018, 11, 1556. https://doi.org/10.3390/ma11091556
Eppell SJ, Tong W, McMasters J, Soenjaya Y, Barbu AM, Ko A, Baskin JZ. Minor Review: An Overview of a Synthetic Nanophase Bone Substitute. Materials. 2018; 11(9):1556. https://doi.org/10.3390/ma11091556
Chicago/Turabian StyleEppell, Steven J., Weidong Tong, James McMasters, Yohannes Soenjaya, Anca M. Barbu, Alvin Ko, and Jonathan Z. Baskin. 2018. "Minor Review: An Overview of a Synthetic Nanophase Bone Substitute" Materials 11, no. 9: 1556. https://doi.org/10.3390/ma11091556
APA StyleEppell, S. J., Tong, W., McMasters, J., Soenjaya, Y., Barbu, A. M., Ko, A., & Baskin, J. Z. (2018). Minor Review: An Overview of a Synthetic Nanophase Bone Substitute. Materials, 11(9), 1556. https://doi.org/10.3390/ma11091556




