Almond Shell as a Microporous Carbon Source for Sustainable Cathodes in Lithium–Sulfur Batteries
Abstract
1. Introduction
2. Materials and Methods
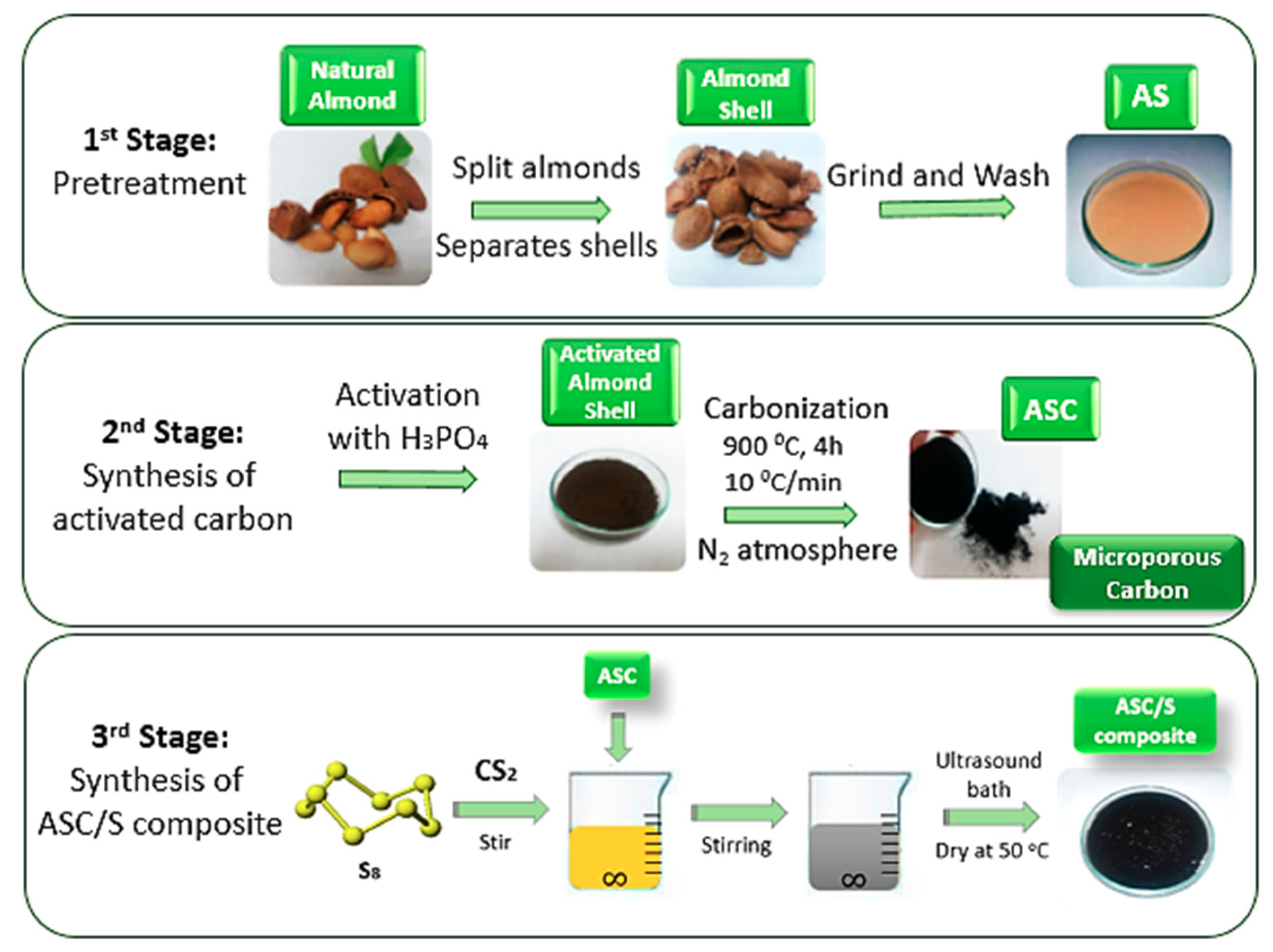
2.1. Synthesis of Activated Carbon (ASC) and Sulfur Composite (ASC/S)
2.2. Carbon and Composite Characterization
2.3. Cathode Preparation and Electrochemical Measurements
3. Results and Discussion
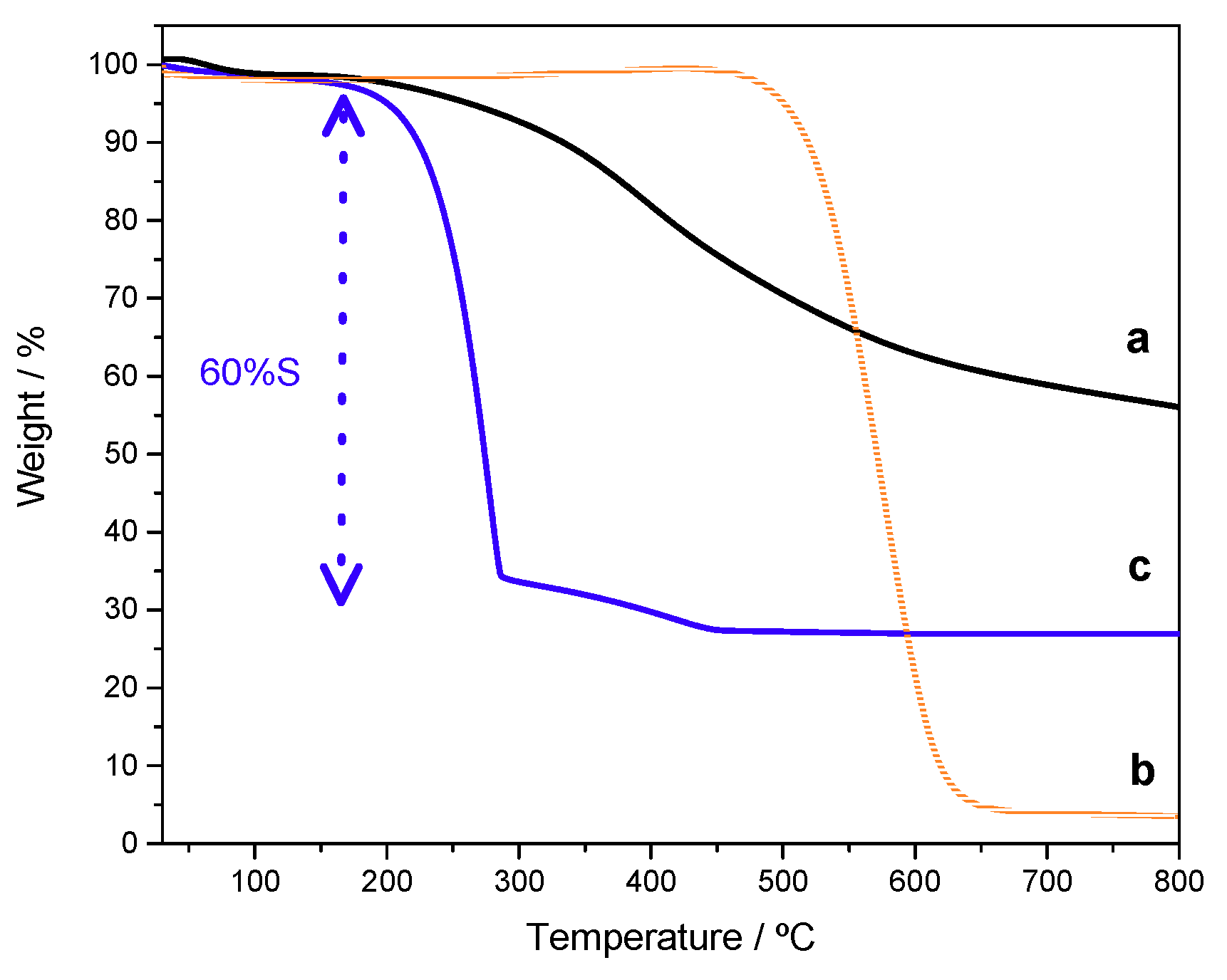
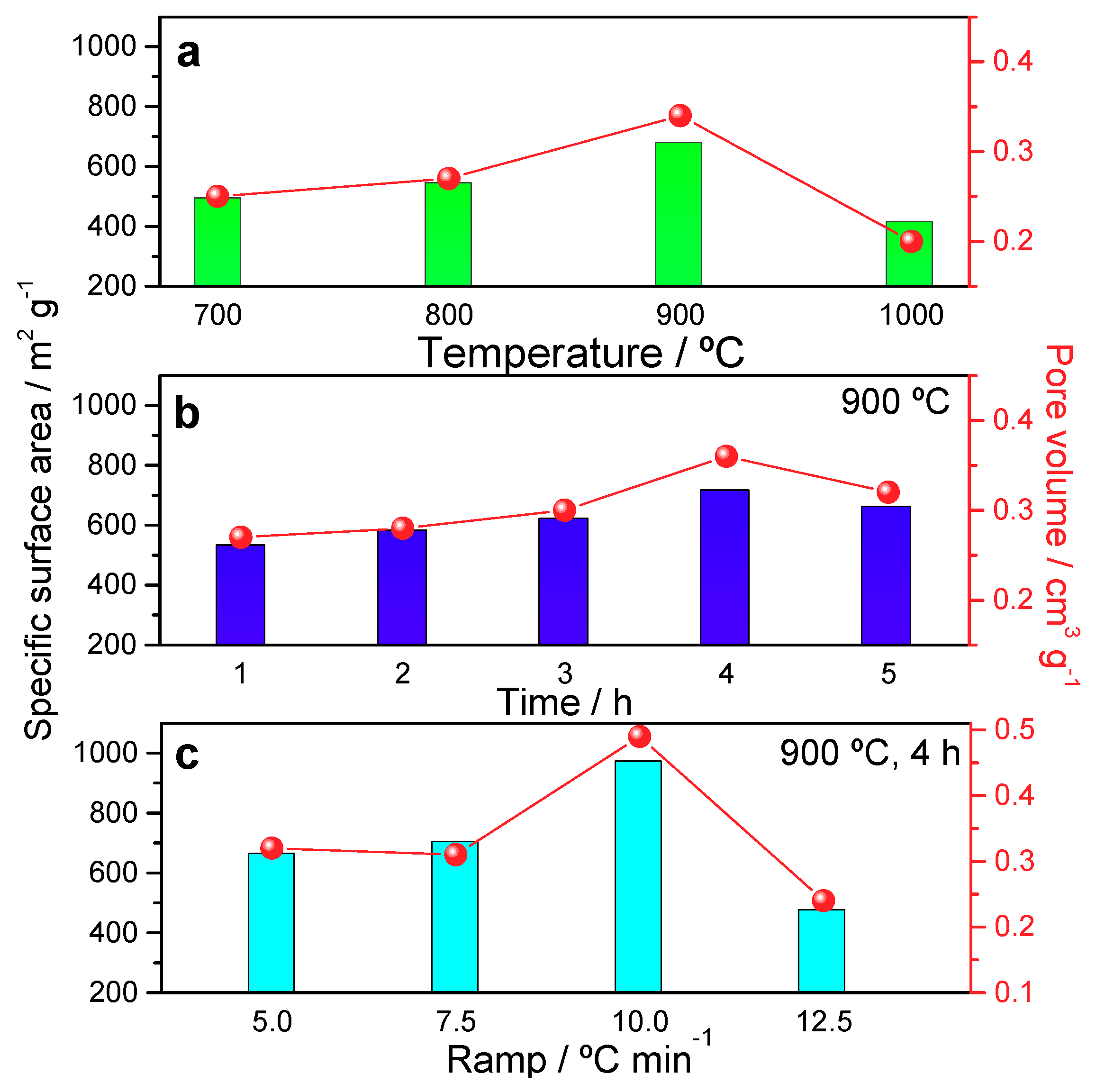
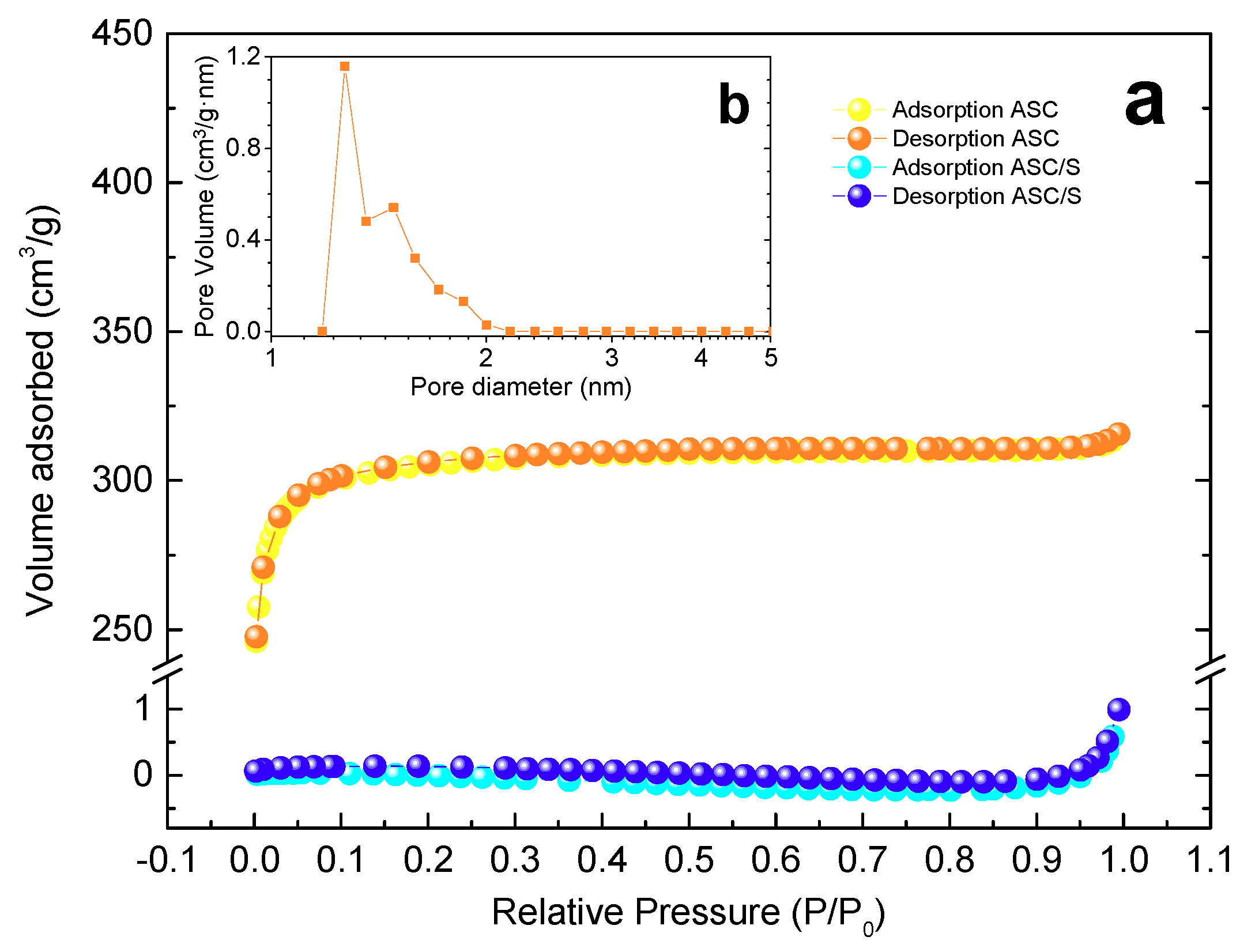
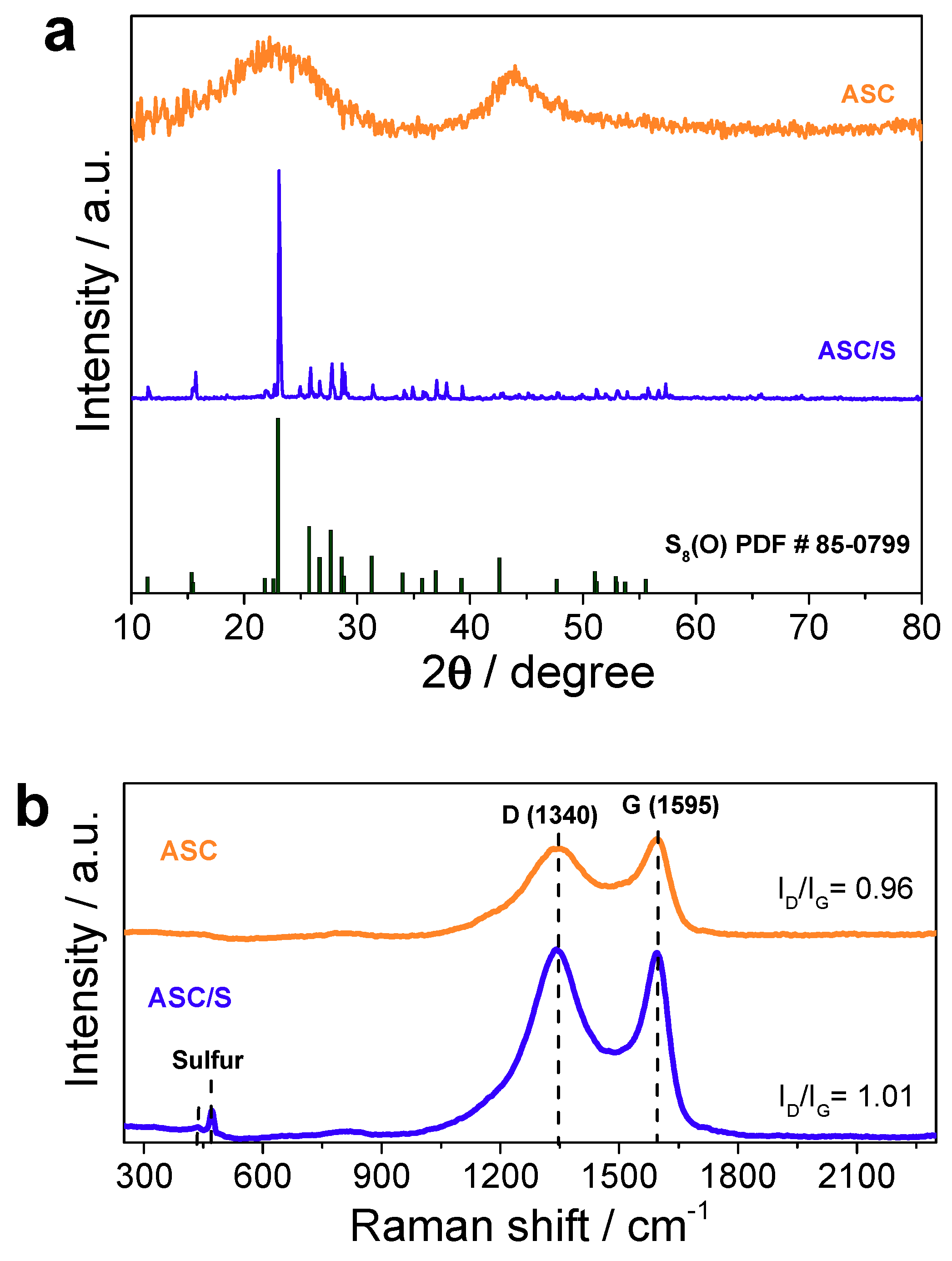
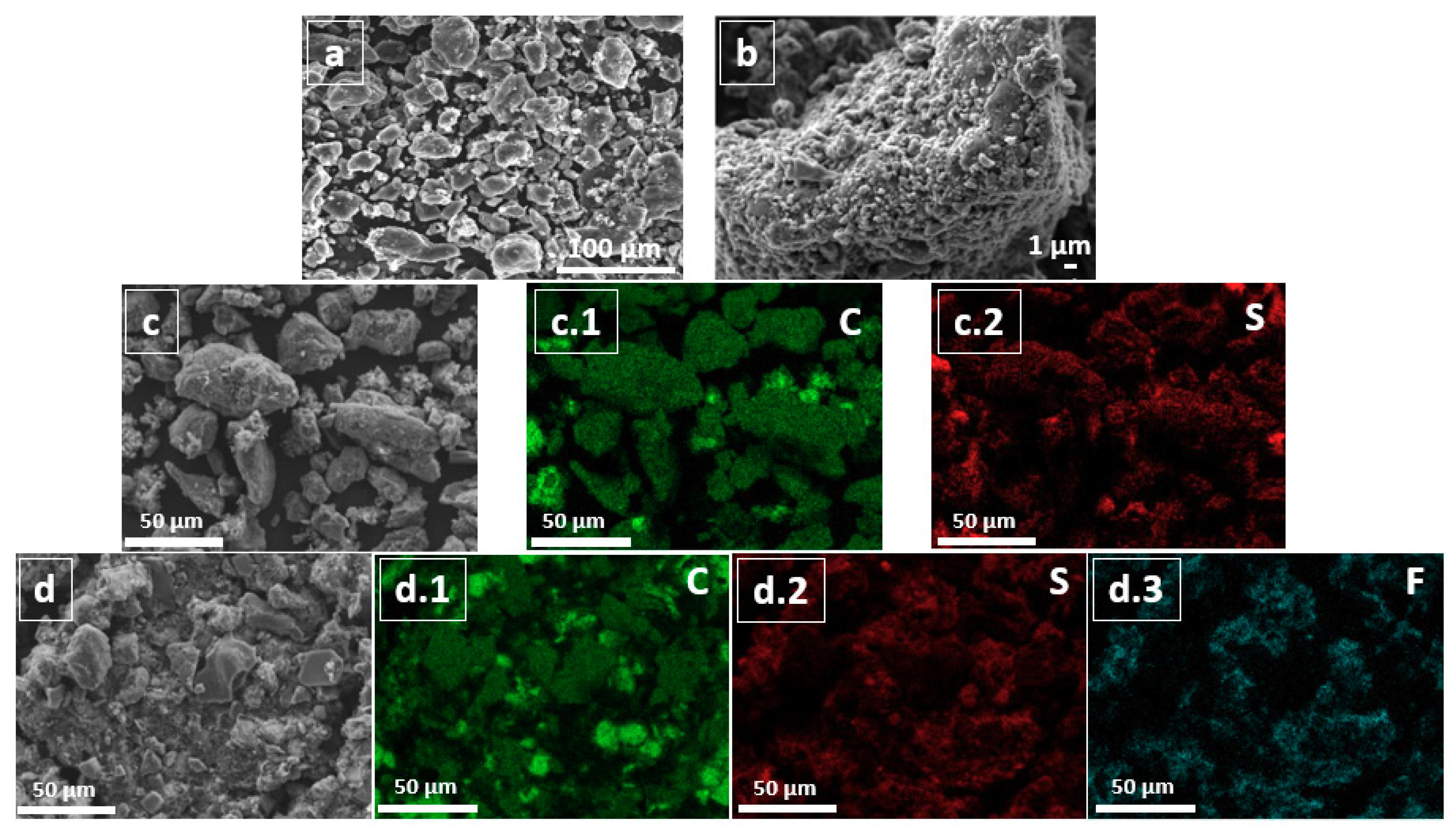
3.1. Structural and Textural Properties
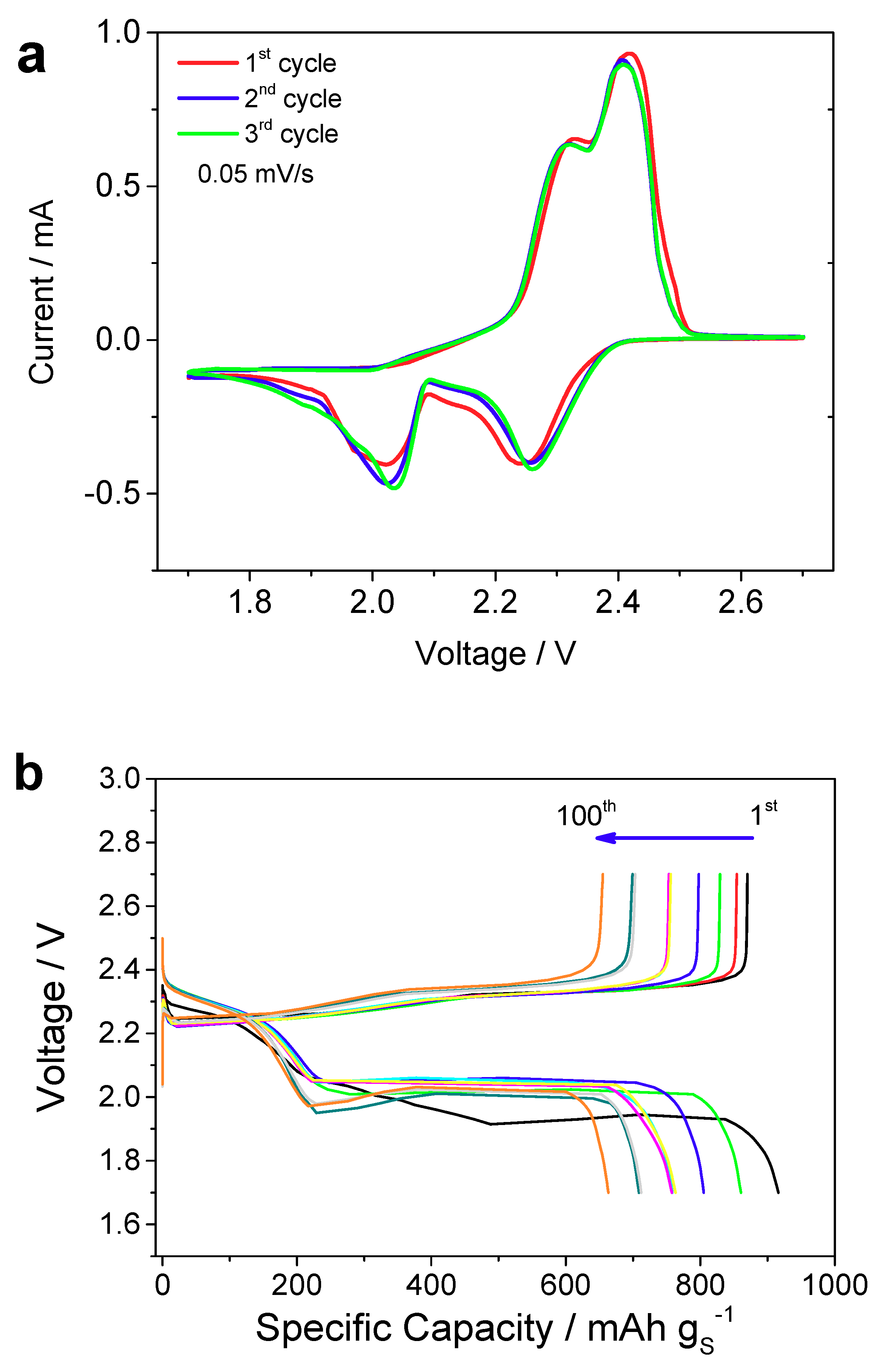
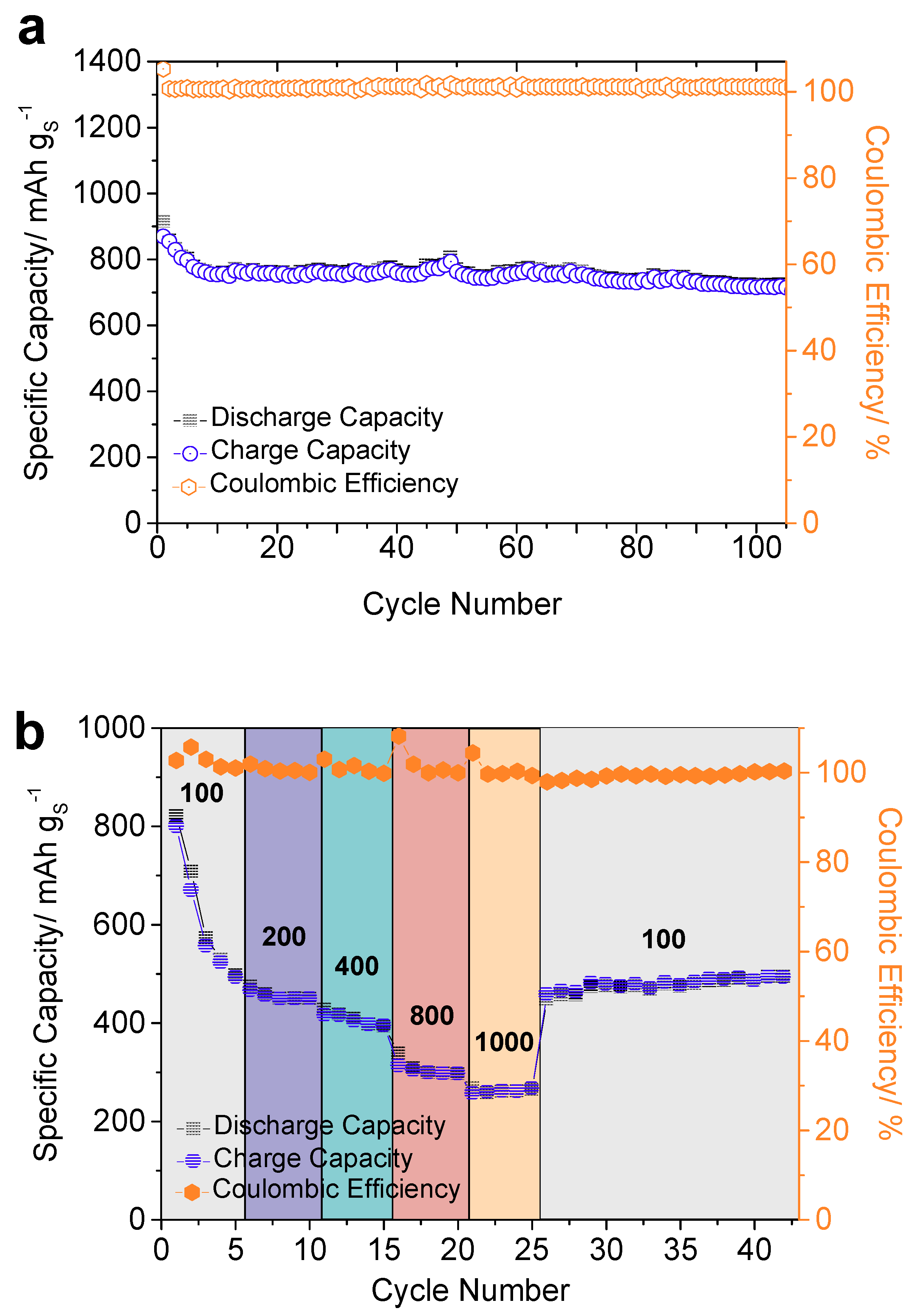
3.2. Electrochemical Properties
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Bresser, D.; Passerini, S.; Scrosati, B. Recent progress and remaining challenges in sulfur-based lithium secondary batteries—A review. Chem. Commun. 2013, 49, 10545–10562. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, M.M.; Wolverton, C.; Isaacs, E.D. Electrical energy storage for transportation—Approaching the limits of, and going beyond, lithium–ion batteries. Energy Environ. Sci. 2012, 5, 7854–7863. [Google Scholar] [CrossRef]
- Wang, J.; Chew, S.Y.; Zhao, Z.W.; Ashraf, S.; Wexler, D.; Chen, J.; Ng, S.H.; Chou, S.L.; Liu, H.K. Sulfur–mesoporous carbon composites in conjunction with a novel ionic liquid electrolyte for lithium rechargeable batteries. Carbon 2008, 46, 229–235. [Google Scholar] [CrossRef]
- Cheon, S.E.; Ko, K.S.; Cho, J.H.; Kim, S.W.; Chin, E.Y.; Kim, H.T. Rechargeable Lithium Sulfur Battery—I. Structural change of sulfur cathode during discharge and charge. J. Electrochem. Soc. 2003, 150, A796–A799. [Google Scholar] [CrossRef]
- Nelson, J.; Misra, S.; Yang, Y.; Jackson, A.; Liu, Y.; Wang, H.; Dai, H.; Andrews, J.C.; Cui, Y.; Toney, M.F. In operando X-ray diffraction and transmission X-ray microscopy of lithium sulfur batteries. J. Am. Chem. Soc. 2012, 134, 6337–6343. [Google Scholar] [CrossRef] [PubMed]
- Akridge, J.R.; Mikhaylik, Y.V.; White, N. Li/S fundamental chemistry and application to high-performance rechargeable batteries. Solid State Ionics 2004, 175, 243–245. [Google Scholar] [CrossRef]
- Zhang, S.S. Liquid electrolyte lithium/sulfur battery: Fundamental chemistry, problems, and solutions. J. Power Sources 2013, 231, 153–162. [Google Scholar] [CrossRef]
- Li, S.; Jin, B.; Zhai, X.; Li, H.; Jiang, Q. Review of carbon materials for Lithium-Sulfur batteries. Chem. Sel. 2018, 3, 2245–2260. [Google Scholar] [CrossRef]
- Long, W.Y.; Fang, B.Z.; Ignaszak, A.; Wu, Z.Z.; Wang, Y.J.; Wilkinson, D. Biomass-derived nanostructured carbons and their composites as anode materials for lithium ion batteries. Chem. Soc. Rev. 2017, 46, 7176–7190. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.; Xia, X.; Deng, S.; Zhan, J.; Fang, R.; Xia, Y.; Wang, X.; Zhang, Q.; Tu, J. Popcorn inspired porous macrocellular carbon: Rapid puffing fabrication from rice and its applications in Lithium–Sulfur batteries. Adv. Energy Mater. 2018, 8, 1701110. [Google Scholar] [CrossRef]
- Sun, Z.J.; Wang, S.J.; Yan, L.L.; Xiao, M.; Han, D.M.; Meng, Y.Z. Mesoporous carbon materials prepared from litchi shell as sulfur encapsulator for lithium–sulfur battery application. J. Power Sources 2016, 324, 547–555. [Google Scholar] [CrossRef]
- Wu, H.L.; Mou, J.R.; Zhou, L.; Zheng, Q.J.; Jiang, N.; Lin, D.M. Cloud cap-like, hierarchically porous carbon derived from mushroom as an excellent host cathode for high performance lithium–sulfur batteries. Electrochim. Acta 2016, 212, 1021–1030. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Y.; Chen, K.; Zhang, N.; Zhao, X.Q.; Zhao, F.H.; Dou, Z.F.; He, X.M.; Wang, L. Biomass-derived activated carbon for rechargeable Lithium–Sulfur batteries. Bioresources 2015, 10, 155–168. [Google Scholar] [CrossRef][Green Version]
- Aditiya, H.B.; Mahlia, T.M.I; Chong, W.T.; Nur, H.; Sebayang, A.H. Second generation bioethanol production: A critical review. Renew. Sustain. Energy Rev. 2016, 66, 631–653. [Google Scholar] [CrossRef]
- Hernández-Rentero, C.; Córdoba, R.; Moreno, N.; Caballero, A.; Morales, J.; Olivares-Marín, M.; Gómez-Serrano, V. Low-cost disordered carbons for Li/S batteries: A high-performance carbon with dual porosity derived from cherry pits. J. Nano Res. 2018, 11, 89–100. [Google Scholar] [CrossRef]
- Moreno, N.; Caballero, A.; Hernan, L.; Morales, J. Lithium–sulfur batteries with activated carbons derived from olive stones. Carbon 2014, 70, 241–248. [Google Scholar] [CrossRef]
- Zhang, J.; Xiang, J.; Dong, Z.; Liu, Y.; Wu, Y.; Xu, C.; Du, G. Biomass derived activated carbon with 3D connected architecture for rechargeable lithium−sulfur batteries. Electrochim. Acta 2014, 116, 146–151. [Google Scholar] [CrossRef]
- Cheng, Y.; Ji, S.; Xu, X.; Liu, J. Wheat straw carbon matrix wrapped sulfur composites as a superior cathode for Li–S batteries. RSC Adv. 2015, 5, 100089–100096. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.; Jiang, F.; Zhao, S.; Su, Q.; Du, G. Microporous carbon nanosheets derived from corncobs for lithium–sulfur batteries. Electrochim. Acta 2015, 176, 853–860. [Google Scholar] [CrossRef]
- Geng, Z.; Xiao, Q.F.; Wang, D.B.; Yi, G.H.; Xu, Z.G.; Li, B.; Zhang, C.M. Improved Electrochemical performance of biomass-derived nanoporous carbon/sulfur composites cathode for Lithium–Sulfur batteries by nitrogen doping. Electrochim. Acta 2016, 202, 131–139. [Google Scholar] [CrossRef]
- Yuan, G.; Yin, F.; Zhao, Y.; Bakenov, Z.; Wang, G.; Zhang, Y. Corn stalk-derived activated carbon with a stacking sheet-like structure as sulfur cathode supporter for lithium/sulfur batteries. Ionics 2016, 22, 63–69. [Google Scholar] [CrossRef]
- Balakumar, K.; Sathish, R.; Kalaiselvi, N. Exploration of microporous bio-carbon scaffold for efficient utilization of sulfur in lithium–sulfur system. Electrochim. Acta 2016, 209, 171–182. [Google Scholar]
- Chen, F.; Yang, J.; Bai, T.; Long, B.; Zhou, X. Biomass waste-derived honeycomb-like nitrogen and oxygen dual-doped porous carbon for high performance lithium–sulfur batteries. Electrochim. Acta 2016, 192, 99–109. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, G.; Zhang, X.; Wang, S.; Li, C.; Wang, G. Hierarchical porous carbon derived from soybean hulls as a cathode matrix for lithium–sulfur batteries. J. Alloys Compd. 2017, 695, 2246–2252. [Google Scholar] [CrossRef]
- Chulliyote, R.; Hareendrakrishnakumar, H.; Raja, M.; Gladis, J.M.; Stephan, A.M. Sulfur-immobilized nitrogen and oxygen co–doped hierarchically porous biomass carbon for Lithium-Sulfur batteries: Influence of sulfur content and distribution on its performance. Chem. Sel. 2017, 2, 10484–10495. [Google Scholar] [CrossRef]
- Chen, Z.H.; Du, X.L.; He, J.B.; Li, F.; Wang, Y.; Li, Y.L.; Li, B.; Xin, S. Porous coconut shell carbon offering high retention and deep lithiation of sulfur for Lithium–Sulfur batteries. ACS Appl. Mater. Interfaces 2017, 9, 33855–33862. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, B.; Wang, C.; Huang, Z.; Hu, L.; Ke, X.; Liu, L.; Shi, Z.; Guo, Z. Walnut shell–Derived activated carbon: Synthesis and its application in the sulfur cathode for lithium–sulfur batteries. J. Alloys Compd. 2017, 718, 373–378. [Google Scholar] [CrossRef]
- Li, F.; Qin, F.; Zhang, K.; Fang, J.; Lai, Y.; Li, J. Hierarchically porous carbon derived from banana peel for lithium sulfur battery with high areal and gravimetric sulfur loading. J. Power Sources 2017, 362, 160–167. [Google Scholar] [CrossRef]
- Yang, K.; Gao, Q.; Tan, Y.; Tian, W.; Qian, W.; Zhu, L.; Yang, C. Biomass-derived porous carbon with micropores and small mesopores for high-performance Lithium–Sulfur batteries. Chem. Eur. J. 2016, 22, 3239–3244. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Xue, D. Multiple functional biomass-derived activated carbon materials for aqueous supercapacitors, Lithium–ion capacitors and Lithium–Sulfur batteries. Chin. J. Chem. 2017, 35, 861–866. [Google Scholar] [CrossRef]
- Rybarczyk, M.K.; Peng, H.J.; Tang, C.; Lieder, M.; Zhang, Q.; Titirici, M.M. Porous carbon derived from rice husks as sustainable bioresources: Insights into the role of micro-/mesoporous hierarchy in hosting active species for lithium–sulphur batteries. Green Chem. 2016, 18, 5169–5179. [Google Scholar] [CrossRef]
- Półrolniczak, P.; Nowicki, P.; Wasiński, K.; Pietrzak, R.; Walkowiak, M. Biomass-derived hierarchical carbon as sulfur cathode stabilizing agent for lithium–sulfur batteries. Solid State Ionics 2016, 297, 59–63. [Google Scholar] [CrossRef]
- Lee, J.T.; Zhao, Y.; Thieme, S.; Kim, H.; Oschatz, M.; Borchardt, L.; Magasinski, A.; Cho, W.I.; Kaskel, S.; Yushin, G. Sulfur-infiltrated micro- and mesoporous silicon carbide-derived carbon cathode for high-performance Lithium Sulfur batteries. Adv. Mater. 2013, 25, 4573–4579. [Google Scholar] [CrossRef] [PubMed]
- Yushin, G.; Dash, R.; Jagiello, J.; Fischer, J.E.; Gogotsi, Y. Carbide-derived carbons: Effect of pore size on hydrogen uptake and heat of adsorption. Adv. Funct. Mater. 2006, 16, 2288–2293. [Google Scholar] [CrossRef]
- Ji, L.; Rao, M.; Zheng, H.; Zhang, L.; Li, Y.; Duan, W.; Guo, J.; Cairns, E.J.; Zhang, Y. Graphene oxide as a sulfur immobilizer in high performance Lithium/Sulfur cells. J. Am. Chem. Soc. 2011, 133, 18522–18525. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Cao, Y.; Xiao, J.; Schwenzer, B.; Engelhard, M.H.; Saraf, L.V.; Nie, Z.; Exarhos, G.J.; Liu, J. A soft approach to encapsulate sulfur: Polyaniline nanotubes for Lithium-Sulfur batteries with long cycle life. Adv. Mater. 2012, 24, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, F.; Jagtoyen, M.; Thwaites, M. Porosity in Carbons: Characterization and Applications; Patrick, J.W., Edward, A., Eds.; Wiley: London, UK, 1995; Chapter 9; pp. 227–252. [Google Scholar]
- Srinivasakannan, C.; Bakar, M.Z.A. Production of activated carbon from rubber wood sawdust. Biomass Bioenergy 2004, 27, 89–96. [Google Scholar] [CrossRef]
- Haimour, N.M.; Emeish, S. Utilization of date stones for production of activated carbon using phosphoric acid. Waste Manag. 2006, 26, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Plaza, M.G.; Pevida, C.; Martín, C.F.; Fermoso, J.; Pis, J.J.; Rubiera, F. Developing almond shell-derived activated carbons as CO2 adsorbents. Sep. Purif. Technol. 2010, 71, 102–106. [Google Scholar] [CrossRef]
- Rodríguez-Reinoso, F.; López-González, J.D.; Berenguer, C. Activated carbons from almond shells—I: Preparation and characterization by nitrogen adsorption. Carbon 1982, 20, 513–518. [Google Scholar] [CrossRef]
- Li, W.; Yao, H.; Yan, K.; Zhen, G.; Liang, Z.; Chiang, Y.M.; Cui, Y. The synergetic effect of lithium polysulfide and lithium nitrate to prevent lithium dendrite growth. Nat. Commun. 2015, 6, 7436. [Google Scholar] [CrossRef] [PubMed]
- Carbone, L.; Greenbaum, S.G.; Hassoun, J. Lithium sulfur and lithium oxygen batteries: New frontiers of sustainable energy storage. Sustain. Energy Fuels 2017, 1, 228–247. [Google Scholar] [CrossRef]
- Nayak, A.; Bhushan, B.; Gupta, V.; Sharma, P. Chemically activated carbon from lignocellulosic wastes for heavy metal wastewater remediation: Effect of activation conditions. J. Colloid Interface Sci. 2017, 493, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, M.; Al-Swaidan, H.M. Ramp rate influence on synthesis of sliced porous activated carbon from date palm tree by physical activation method. Asian J. Chem. 2014, 26, 5295–5297. [Google Scholar]
- Olivares-Marín, M.; Fernández-González, C.; Macías-García, A.; Gómez-Serrano, V. Porous structure of activated carbon prepared from cherry stones by chemical activation with phosphoric acid. Energy Fuels 2007, 21, 2942–2949. [Google Scholar] [CrossRef]
- Li, Z.; Yuan, L.X.; Yi, Z.Q.; Sun, Y.M.; Liu, Y.; Jiang, Y.; Shen, Y.; Xin, Y.; Zhang, Z.L.; Huang, Y.H. Insight into the electrode mechanism in Lithium-Sulfur batteries with ordered microporous carbon confined sulfur as the cathode. Adv. Energy Mater. 2014, 4, 1301473. [Google Scholar] [CrossRef]
- Tao, X.; Zhang, J.; Xia, Y.; Huang, H.; Du, J.; Xiao, H.; Zhang, W.; Gan, Y. Bio-inspired fabrication of carbon nanotiles for high performance cathode of Li–S batteries. J. Mater. Chem. A 2014, 2, 2290–2296. [Google Scholar] [CrossRef]
- Fu, Y.; Su, Y.S; Manthiram, A. Sulfur-Polypyrrole composite cathodes for Lithium–Sulfur batteries. J. Electrochem. Soc. 2012, 159, A1420–A1424. [Google Scholar] [CrossRef]
- Guo, J.; Xu, Y.; Wang, C. Sulfur-impregnated disordered carbon nanotubes cathode for Lithium–Sulfur batteries. Nano Lett. 2011, 11, 4288–4294. [Google Scholar] [CrossRef] [PubMed]
- Song, M.K.; Cairns, E.J.; Zhang, Y.G. Lithium/sulfur batteries with high specific energy: Old challenges and new opportunities. Nanoscale 2013, 5, 2186–2204. [Google Scholar] [CrossRef] [PubMed]
- Xi, K.; Kidambi, P.R.; Chen, R.; Gao, C.; Peng, X.; Ducati, C.; Hofmann, S.; Kumar, R.V. Binder free three-dimensional sulphur/few-layer graphene foam cathode with enhanced high-rate capability for rechargeable lithium sulphur batteries. Nanoscale 2014, 6, 5746–5753. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.; Gu, X.; Khan, K.; Mahmood, N.; Yang, W.; Huang, X.; Guo, S.; Hou, Y. 3D vertically aligned and interconnected porous carbon nanosheets as sulfur immobilizers for high performance Lithium–Sulfur batteries. Adv. Energy Mater. 2016, 6, 1502518. [Google Scholar] [CrossRef]
- Li, G.; Sun, J.; Hou, W.; Jiang, S.; Huang, Y.; Geng, J. Three-dimensional porous carbon composites containing high sulfur nanoparticle content for high-performance lithium–sulfur batteries. Nat. Commun. 2016, 7, 10601. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.P.; Soin, N.; Basak, S.; Sachdeva, S.; Roy, S.S.; Zanderbergen, H.W.; McLaughlin, J.A.; Huijben, M.; Wagemaker, M. 3-D vertically aligned few layer graphene-partially reduced graphene oxide/sulfur electrodes for high performance lithium–sulfur batteries. Sustain. Energy Fuels 2017, 1, 1516–1523. [Google Scholar] [CrossRef]
- Chen, K.; Cao, J.; Lu, Q.; Wang, Q.; Yao, M.; Han, M.; Niu, Z.; Chen, J. Sulfur nanoparticles encapsulated in reduced graphene oxide nanotubes for flexible lithium–sulfur batteries. Nano Res. 2018, 11, 1345–1357. [Google Scholar] [CrossRef]
| Carbon Source | SBET (m2 g−1) | Vpore (cm3 g−1) | Specific Capacity [a]/Rate [b] | Ref. |
|---|---|---|---|---|
| Cherry pits | 1662 | 0.97 | 915/100 | [15] |
| 700/837 | ||||
| Olive stone | 587 | 0.33 | 670/100 | [16] |
| Pomelo peels | 1533 | 0.83 | 760/335 | [17] |
| Wheat straw | 1066 | 0.62 | 920/167 | [18] |
| 440/1675 | ||||
| Corncob | 1198 | 0.67 | 600/167 | [19] |
| 2724 | 1.49 | 720/558 | [20] | |
| Corn stalks | 140 | 0.26 | 750/335 | [21] |
| Coir pith | 1952 | 0.86 | 600/167 | [22] |
| 470/837 | ||||
| Soybean residues | 2690 | 1.34 | 750/334 | [23] |
| 400/1675 | ||||
| 1232 | 0.54 | 450/837 | [24] | |
| Pinecone | 2065 | 1.50 | 1260/167 | [25] |
| Coconut shell | 2160 | 0.68 | 1030/837 | [26] |
| 1030/1675 | ||||
| Walnut shell | 2318 | 1.13 | 910/167 | [27] |
| Banana peel | 220 | 0.76 | 700/334 | [28] |
| 2741 | 1.23 | 750/1675 | [29] | |
| Rice husk | 1098 | - | 200/167 | [30] |
| 350/334 | ||||
| 665 | 0.31 | 690/837 | [31] | |
| 580/1675 | ||||
| Mandarin peels | 1077 | 0.57 | 790/83 | [32] |
| Almond shell | 967 | 0.49 | 760/100 | This work |
| 400/400 | ||||
| 300/800 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benítez, A.; González-Tejero, M.; Caballero, Á.; Morales, J. Almond Shell as a Microporous Carbon Source for Sustainable Cathodes in Lithium–Sulfur Batteries. Materials 2018, 11, 1428. https://doi.org/10.3390/ma11081428
Benítez A, González-Tejero M, Caballero Á, Morales J. Almond Shell as a Microporous Carbon Source for Sustainable Cathodes in Lithium–Sulfur Batteries. Materials. 2018; 11(8):1428. https://doi.org/10.3390/ma11081428
Chicago/Turabian StyleBenítez, Almudena, Marcos González-Tejero, Álvaro Caballero, and Julián Morales. 2018. "Almond Shell as a Microporous Carbon Source for Sustainable Cathodes in Lithium–Sulfur Batteries" Materials 11, no. 8: 1428. https://doi.org/10.3390/ma11081428
APA StyleBenítez, A., González-Tejero, M., Caballero, Á., & Morales, J. (2018). Almond Shell as a Microporous Carbon Source for Sustainable Cathodes in Lithium–Sulfur Batteries. Materials, 11(8), 1428. https://doi.org/10.3390/ma11081428