Pair Distribution Function Analysis of ZrO2 Nanocrystals and Insights in the Formation of ZrO2-YBa2Cu3O7 Nanocomposites
Abstract
1. Introduction
2. Materials and Methods
2.1. Nanocrystal Synthesis and Stabilization
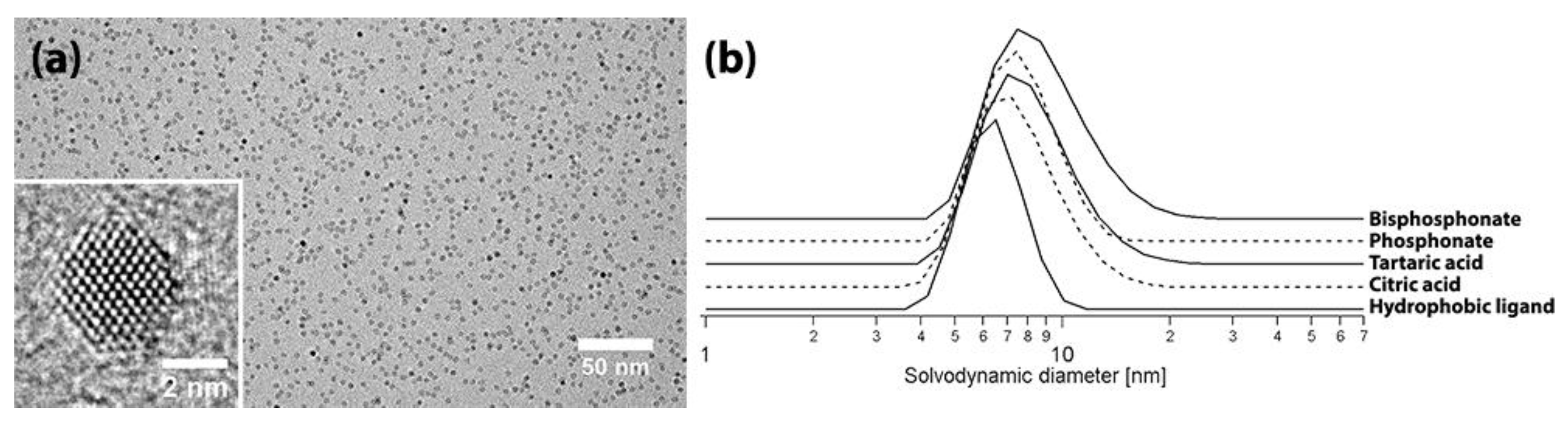
2.2. Nanocrystal Characterization
2.3. Thin Film Deposition and Processing
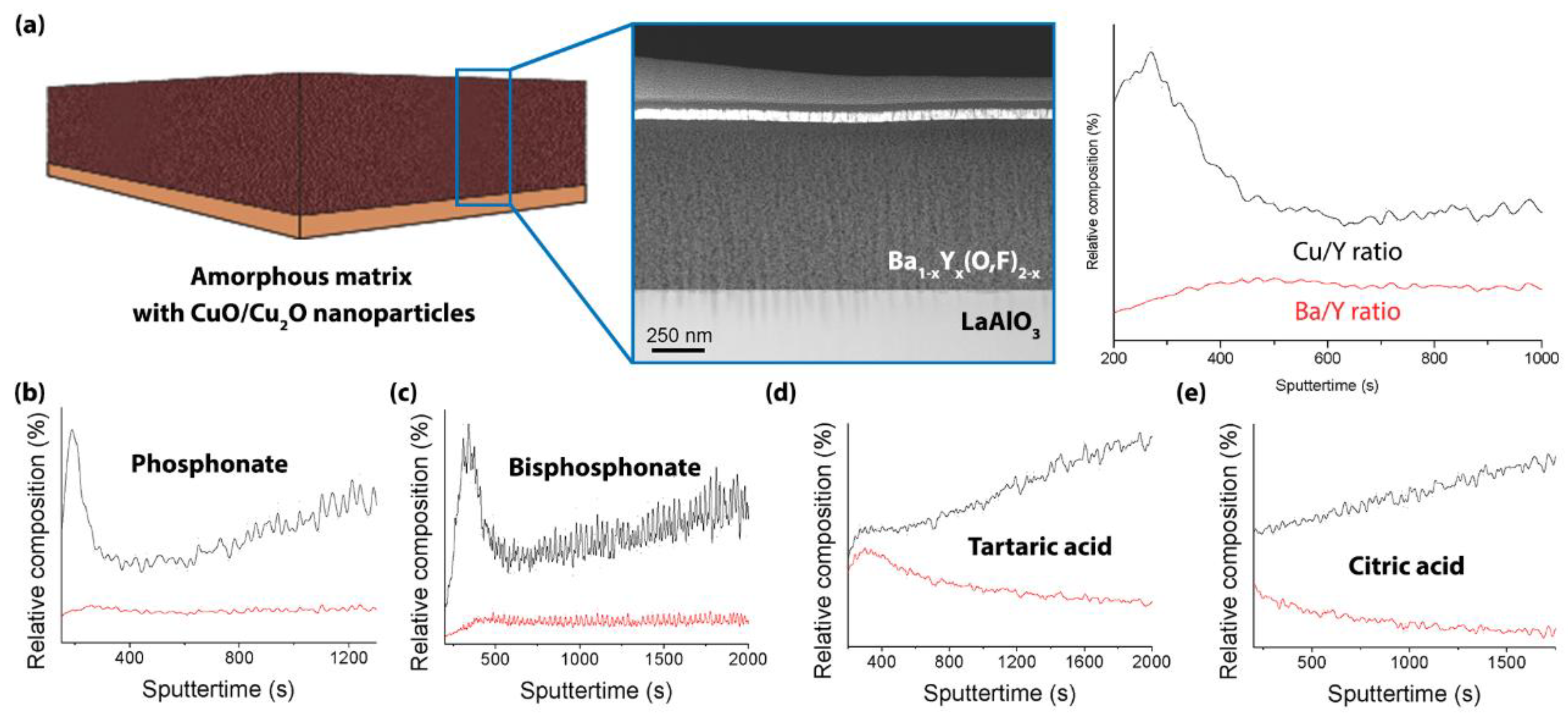
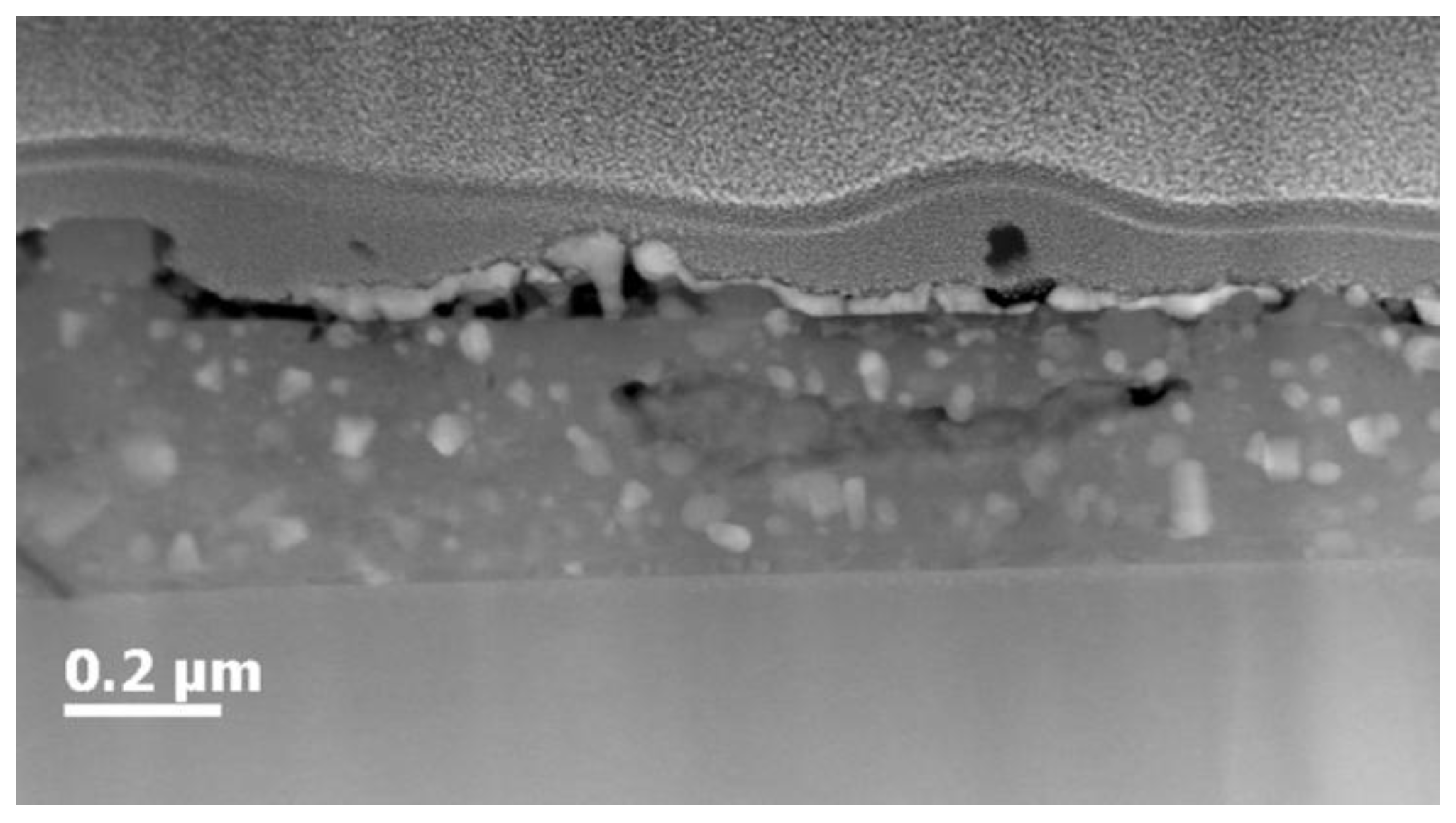
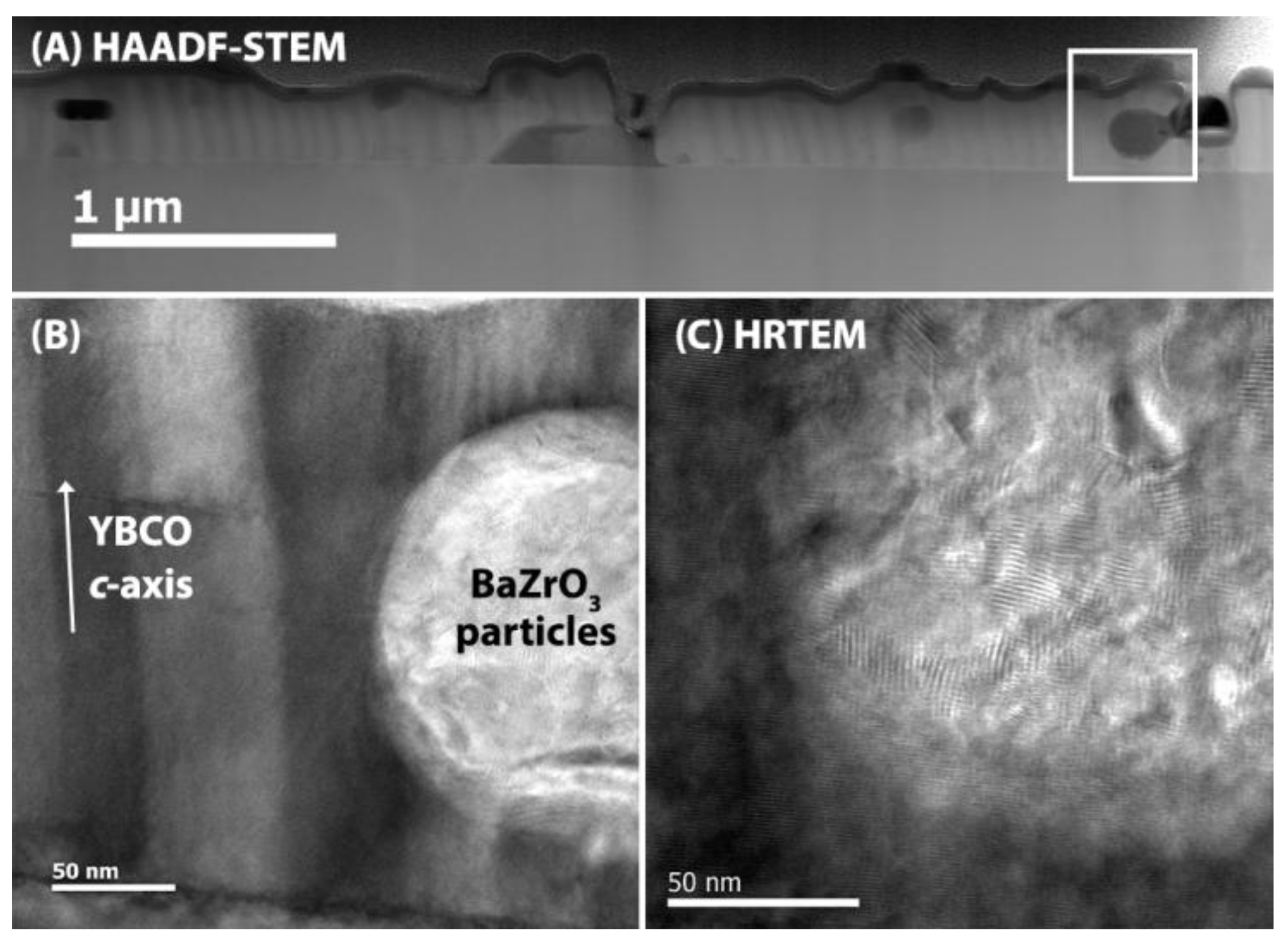
2.4. Microstructural Characterization
2.5. Electrical Characterization
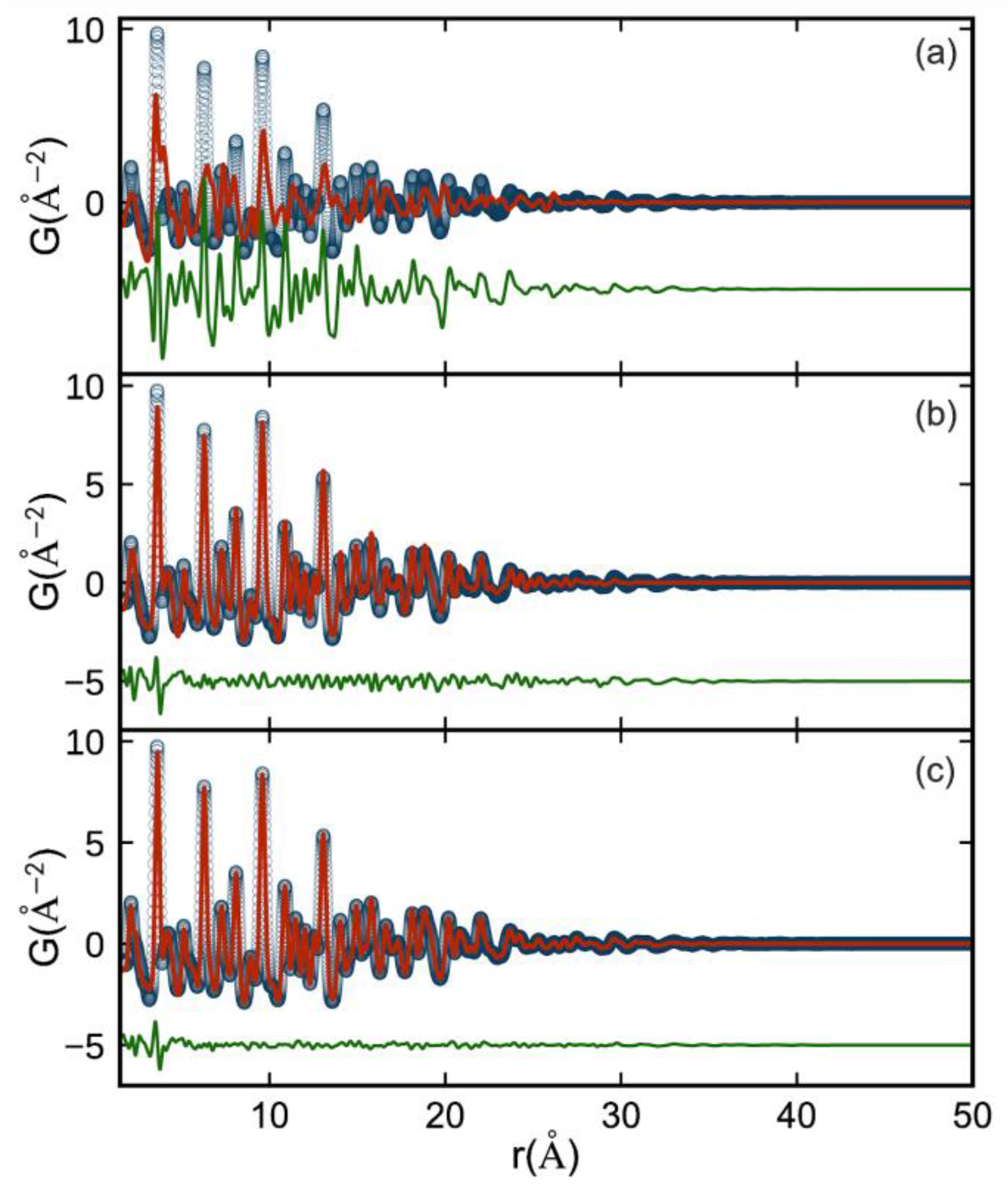
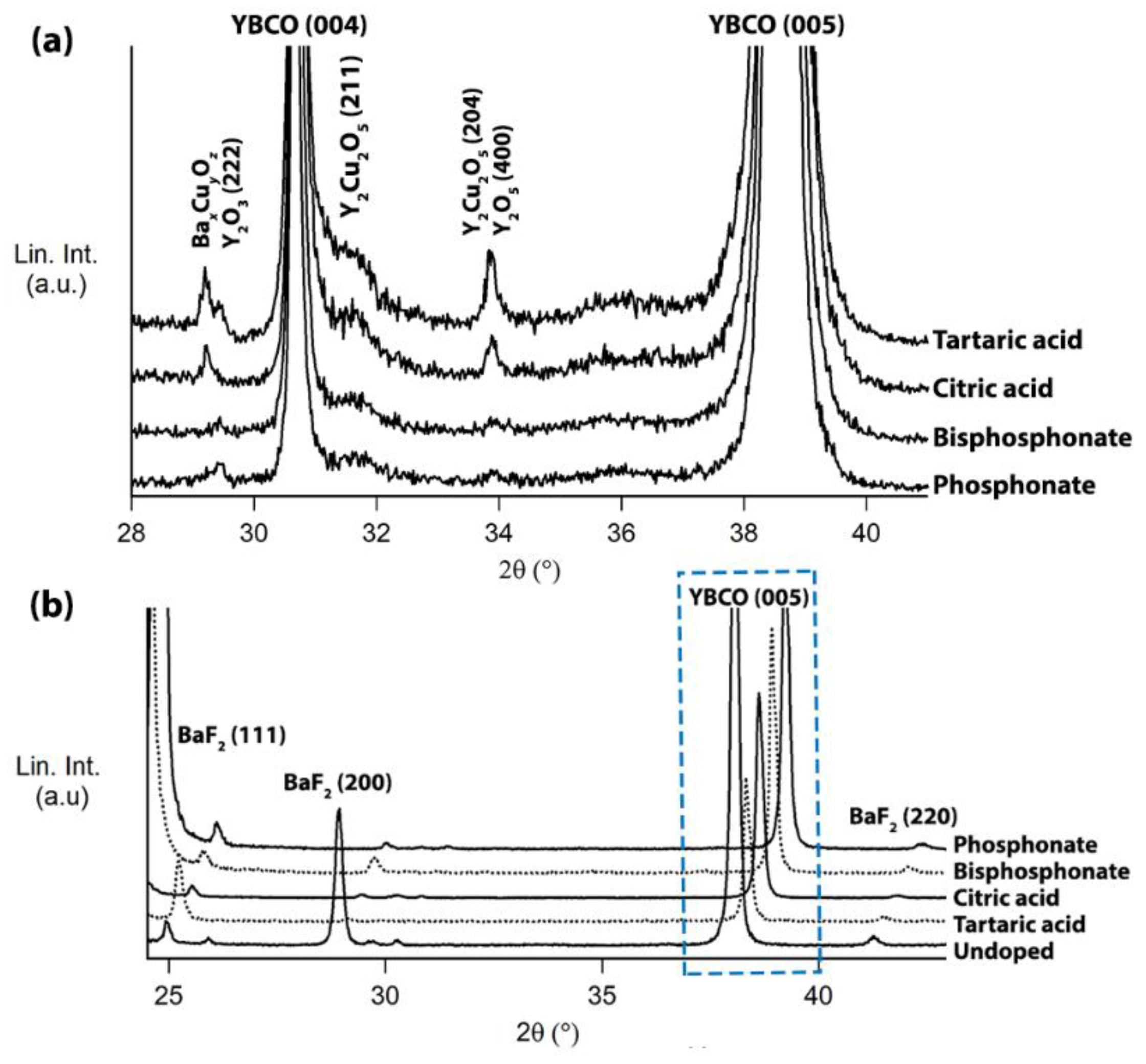
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Larbalestier, D.; Gurevich, A.; Feldmann, D.M.; Polyanskii, A. High-Tc superconducting materials for electric power applications. Nature 2001, 414, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Obradors, X.; Puig, T. Coated conductors for power applications: Materials challenges. Supercond. Sci. Technol. 2014, 27. [Google Scholar] [CrossRef]
- Obradors, X.; Puig, T.; Ricart, S.; Coll, M.; Gazquez, J.; Palau, A.; Granados, X. Growth, nanostructure and vortex pinning in superconducting YBa2Cu3O7 thin films based on trifluoroacetate solutions. Supercond. Sci. Technol. 2012, 25, 123001. [Google Scholar] [CrossRef]
- Hänisch, J.; Cai, C.; Stehr, V.; Hühne, R.; Lyubina, J.; Nenkov, K.; Fuchs, G.; Schultz, L.; Holzapfel, B. Formation and pinning properties of growth-controlled nanoscale precipitates in YBa2Cu3O7−δ/transition metal quasi-multilayers. Supercond. Sci. Technol. 2006, 19, 534–540. [Google Scholar] [CrossRef]
- MacManus-Driscoll, J.; Foltyn, S.; Jia, Q.; Wang, H.; Serquis, A.; Civale, L.; Maiorov, B.; Hawley, M.; Maley, M.; Peterson, D. Strongly enhanced current densities in superconducting coated conductors of YBa2Cu3O7–x+BaZrO3. Nat. Mater. 2004, 3, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Malmivirta, M.; Rijckaert, H.; Paasonen, V.; Huhtinen, H.; Hynninen, T.; Jha, R.; Awana, V.S.; Driessche, I.; Paturi, P. Enhanced flux pinning in YBCO multilayer films with BCO nanodots and segmented BZO nanorods. Sci. Rep. 2017, 1, 14682. [Google Scholar] [CrossRef] [PubMed]
- Feighan, J.; Kursumovic, A.; MacManus-Driscoll, J. Materials design for artificial pinning centres in superconductor PLD coated conductors. Supercond. Sci. Technol. 2017, 30, 123001. [Google Scholar] [CrossRef]
- Haugan, T.; Barnes, P.; Wheeler, R.; Meisenkothen, F.; Sumption, M. Addition of nanoparticle dispersions to enhance flux pinning of the YBa2Cu3O7-x superconductor. Nature 2004, 430, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Opherden, L.; Sieger, M.; Pahlke, P.; Hühne, R.; Schultz, L.; Meledin, A.; Van Tendeloo, G.; Nast, R.; Holzapfel, B.; Bianchetti, M. Large pinning forces and matching effects in YBa2Cu3O7-δ thin films with Ba2Y(Nb/Ta)O6 nano-precipitates. Sci. Rep. 2016, 6, 21188. [Google Scholar] [CrossRef] [PubMed]
- Sieger, M.; Pahlke, P.; Hänisch, J.; Sparing, M.; Bianchetti, M.; MacManus-Driscoll, J.; Lao, M.; Eisterer, M.; Meledin, A.; Van Tendeloo, G. Ba2Y(Nb/Ta)O6–Doped YBCO Films on Biaxially Textured Ni–5at.% W Substrates. IEEE Trans. Appl. Supercond. 2016, 26, 1–5. [Google Scholar] [CrossRef]
- Lei, L.; Zhao, G.; Xu, H.; Wu, N.; Chen, Y. Influences of Y2O3 nanoparticle additions on the microstructure and superconductivity of YBCO films derived from low-fluorine solution. Mater. Chem. Phys. 2011, 127, 91–94. [Google Scholar] [CrossRef]
- Ye, S.; Suo, H.; Wu, Z.; Liu, M.; Xu, Y.; Ma, L.; Zhou, M. Preparation of solution-based YBCO films with BaSnO3 particles. Physica C 2011, 471, 265–269. [Google Scholar] [CrossRef]
- Erbe, M.; Hänisch, J.; Hühne, R.; Freudenberg, T.; Kirchner, A.; Molina-Luna, L.; Damm, C.; Van Tendeloo, G.; Kaskel, S.; Schultz, L. BaHfO3 artificial pinning centres in TFA-MOD-derived YBCO and GdBCO thin films. Supercond. Sci. Technol. 2015, 28, 114002. [Google Scholar] [CrossRef]
- Ding, F.; Gu, H.; Zhang, T.; Wang, H.; Qu, F.; Qiu, Q.; Dai, S.; Peng, X.; Cao, J.-L. Strong enhancement flux pinning in MOD-YBa2Cu3O7−x films with self-assembled BaTiO3 nanocolumns. Appl. Surf. Sci. 2014, 314, 622–627. [Google Scholar] [CrossRef]
- Gutierrez, J.; Llordes, A.; Gazquez, J.; Gibert, M.; Roma, N.; Ricart, S.; Pomar, A.; Sandiumenge, F.; Mestres, N.; Puig, T. Strong isotropic flux pinning in solution-derived YBa2Cu3O7−x nanocomposite superconductor films. Nat. Mater. 2007, 6, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Coll, M.; Guzman, R.; Garcés, P.; Gazquez, J.; Rouco, V.; Palau, A.; Ye, S.; Magen, C.; Suo, H.; Castro, H.; et al. Size-controlled spontaneously segregated Ba2YTaO6 nanoparticles in YBa2Cu3O7 nanocomposites obtained by chemical solution deposition. Supercond. Sci. Technol. 2014, 27, 044008. [Google Scholar] [CrossRef]
- Rijckaert, H.; Pollefeyt, G.; Sieger, M.; Hänisch, J.; Bennewitz, J.; De Keukeleere, K.; De Roo, J.; Hühne, R.; Bäcker, M.; Paturi, P.; et al. Optimizing Nanocomposites through Nanocrystal Surface Chemistry: Superconducting YBa2Cu3O7 Thin Films via Low-Fluorine Metal Organic Deposition and Preformed Metal Oxide Nanocrystals. Chem. Mater. 2017, 29, 6104–6113. [Google Scholar] [CrossRef]
- De Keukeleere, K.; Cayado, P.; Meledin, A.; Vallès, F.; De Roo, J.; Rijckaert, H.; Pollefeyt, G.; Bruneel, E.; Palau, A.; Coll, M.; et al. Superconducting YBa2Cu3O7–δ Nanocomposites Using Preformed ZrO2 Nanocrystals: Growth Mechanisms and Vortex Pinning Properties. Adv. Electr. Mater. 2016, 2, 1600161. [Google Scholar] [CrossRef]
- Cayado, P.; De Keukeleere, K.; Garzón, A.; Perez-Mirabet, L.; Meledin, A.; De Roo, J.; Vallés, F.; Mundet, B.; Rijckaert, H.; Pollefeyt, G.; et al. Epitaxial YBa2Cu3O7−x nanocomposite thin films from colloidal solutions. Supercond. Sci. Technol. 2015, 28, 124007. [Google Scholar] [CrossRef]
- De Keukeleere, K.; Coucke, S.; De Canck, E.; Van Der Voort, P.; Delpech, F.; Coppel, Y.; Hens, Z.; Van Driessche, I.; Owen, J.S.; De Roo, J. Stabilization of Colloidal Ti, Zr, and Hf Oxide Nanocrystals by Protonated Tri-n-octylphosphine Oxide (TOPO) and Its Decomposition Products. Chem. Mater. 2017, 29, 10233–10242. [Google Scholar] [CrossRef]
- Hammersley, A.P. FIT2D V12. 012 Reference Manual V6.0; ESRF Internal Report ESRF98HA01T; ESRF: Grenoble, France, 2004. [Google Scholar]
- Juhás, P.; Davis, T.; Farrow, C.L.; Billinge, S.J. PDFgetX3: A rapid and highly automatable program for processing powder diffraction data into total scattering pair distribution functions. J. Appl. Crystallogr. 2013, 46, 560–566. [Google Scholar] [CrossRef]
- Yang, X.; Juhas, P.; Farrow, C.L.; Billinge, S.J. xPDFsuite: An end-to-end software solution for high throughput pair distribution function transformation, visualization and analysis. arXiv 2014, arXiv:1402.3163. [Google Scholar]
- Farrow, C.; Juhas, P.; Liu, J.; Bryndin, D.; Božin, E.; Bloch, J.; Proffen, T.; Billinge, S. PDFfit2 and PDFgui: Computer programs for studying nanostructure in crystals. J. Phys. Condens. Matter 2007, 19, 335219. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.K.; Newkirk, W. The crystal structure of baddeleyite (monoclinic ZrO2) and its relation to the polymorphism of ZrO2. Acta Crystallogr. 1965, 18, 983–991. [Google Scholar] [CrossRef]
- Howard, C.J.; Kisi, E.H.; Roberts, R.B.; Hill, R.J. Neutron Diffraction Studies of Phase Transformations between Tetragonal and Orthorhombic Zirconia in Magnesia-Partially-Stabilized Zirconia. J. Am. Ceram. Soc. 1990, 73, 2828–2833. [Google Scholar] [CrossRef]
- Egami, T.; Billinge, S.J. Underneath the Bragg Peaks: Structural Analysis of Complex Materials; Elsevier: New York, NY, USA, 2003. [Google Scholar]
- Golovchanskiy, I.; Pan, A.; Shcherbakova, O.; Fedoseev, S. Rectifying differences in transport, dynamic, and quasi-equilibrium measurements of critical current density. J. Appl. Phys. 2013, 114, 163910. [Google Scholar] [CrossRef]
- Gateshki, M.; Petkov, V.; Williams, G.; Pradhan, S.; Ren, Y. Atomic-scale structure of nanocrystalline ZrO2 prepared by high-energy ball milling. Phys. Rev. B 2005, 71, 224107. [Google Scholar] [CrossRef]
- Gateshki, M.; Petkov, V.; Hyeon, T.; Joo, J.; Niederberger, M.; Ren, Y. Interplay between the local structural disorder and the length of structural coherence in stabilizing the cubic phase in nanocrystalline ZrO2. Solid State Commun. 2006, 138, 279–284. [Google Scholar] [CrossRef]
- Dippel, A.-C.; Jensen, K.; Tyrsted, C.; Bremholm, M.; Bøjesen, E.D.; Saha, D.; Birgisson, S.; Christensen, M.; Billinge, S.J.; Iversen, B.B. Towards atomistic understanding of polymorphism in the solvothermal synthesis of ZrO2 nanoparticles. Acta Crystallogr. 2016, 72, 645–650. [Google Scholar] [CrossRef]
- De Roo, J.; Coucke, S.; Rijckaert, H.; De Keukeleere, K.; Sinnaeve, D.; Hens, Z.; Martins, J.C.; Van Driessche, I. Amino Acid-Based Stabilization of Oxide Nanocrystals in Polar Media: From Insight in Ligand Exchange to Solution 1H NMR Probing of Short-Chained Adsorbates. Langmuir 2016, 32, 1962–1970. [Google Scholar] [CrossRef] [PubMed]
- Rijckaert, H.; De Roo, J.; Roeleveld, K.; Pollefeyt, G.; Bennewitz, J.; Bäcker, M.; Lynen, F.; De Keukeleere, K.; Van Driessche, I. Microwave-assisted YBa2Cu3O7 precursors: A fast and reliable method towards chemical precursors for superconducting films. J. Am. Ceram. Soc. 2017, 100, 2407–2418. [Google Scholar] [CrossRef]
- Paturi, P.; Malmivirta, M.; Palonen, H.; Huhtinen, H. Dopant Diameter Dependence of J(c)(B) in Doped YBCO Films. IEEE Trans. Appl. Supercond. 2016, 26. [Google Scholar] [CrossRef]
| P21/c | Fm-3m | P42/nmc-I | P42/nmc-II | |
|---|---|---|---|---|
| a (Å) | 5.200 | 5.125 | 3.603 | 3.603 |
| b (Å) | 5.231 | 5.125 | 3.603 | 3.603 |
| c (Å) | 5.617 | 5.125 | 5.188 | 5.186 |
| β | 94.8 | 90.0 | 90.0 | 90.0 |
| Zr-Uiso (Å) | 0.008 | 0.010 | 0.008 | 0.009 |
| O-Uiso (Å) | 0.046 | 0.072 | 0.072 | 0.041 |
| Crystallite size (Å) | 36.9 | 34.2 | 38.8 | 39.1 |
| z(O1) (f.c.) | – | 0.25 | 0.50 | 0.45 |
| Rw | 0.737 | 0.151 | 0.120 | 0.098 |
| Ligands | Thickness (nm) | Critical Current, Ic (A) |
|---|---|---|
| Undoped | 275 ± 14 | 139 ± 25 |
| Phosphonate | 280 ± 10 | 144 ± 17 |
| Bisphosphonate | 282 ± 17 | 129 ± 14 |
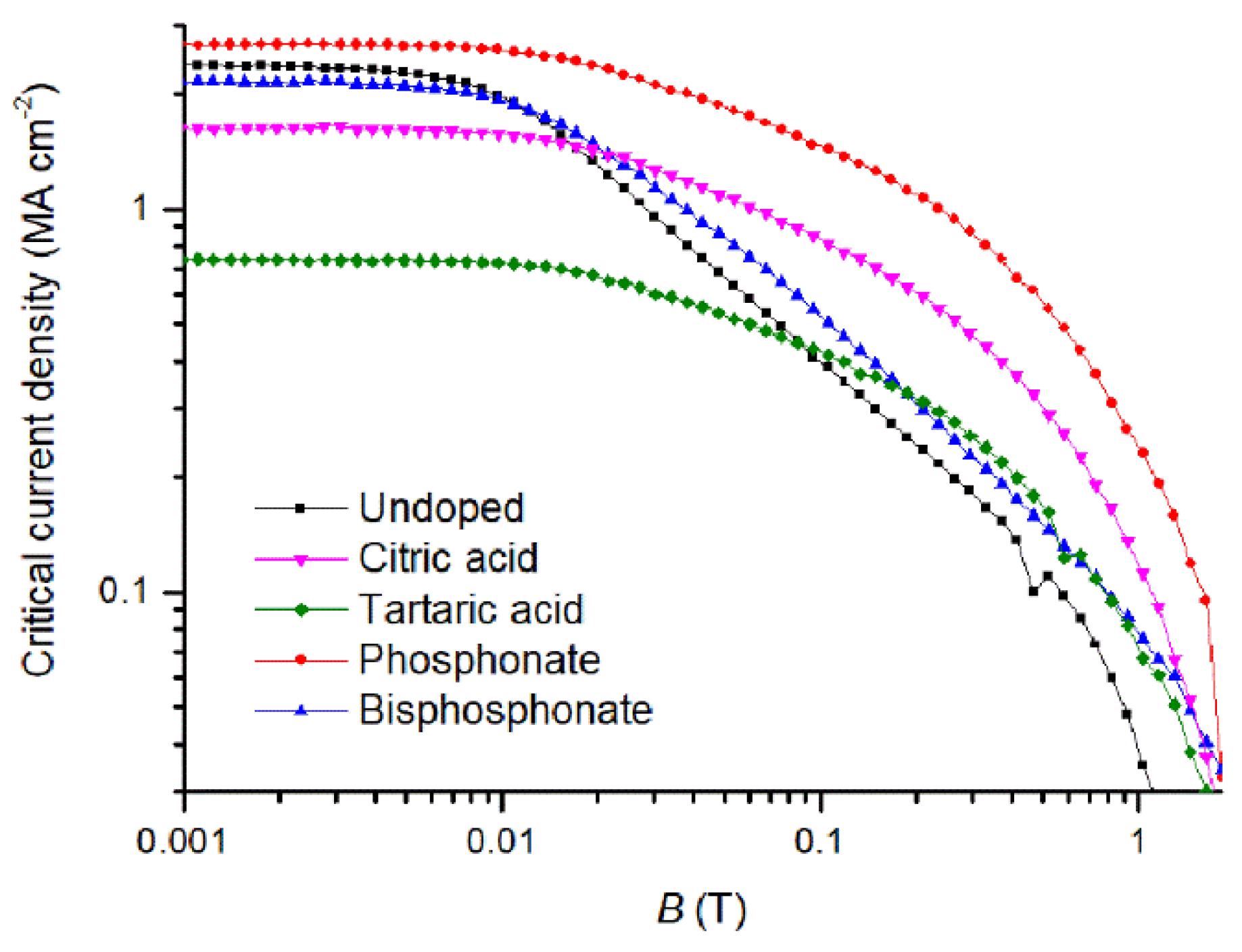
| Ligands | Tc (K) | ΔTc (K) | Jc,mag (0 T) (MA cm−2) | Jc,mag (1 T) (kA cm−2) | B* (mT) | α |
|---|---|---|---|---|---|---|
| Undoped | 90.0 | 1.1 | 2.37 | 41.54 | 7.62 | 0.68 |
| Phosphonate | 90.5 | 1.5 | 2.68 | 237.00 | 17.02 | 0.39 |
| Bisphosphonate | 91.5 | 1.6 | 2.14 | 79.05 | 9.85 | 0.58 |
| Citric acid | 90.5 | 2.2 | 1.65 | 120.32 | 15.52 | 0.40 |
| Tartaric acid | 89.0 | 2.5 | 0.74 | 72.76 | 20.06 | 0.40 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rijckaert, H.; De Roo, J.; Van Zele, M.; Banerjee, S.; Huhtinen, H.; Paturi, P.; Bennewitz, J.; Billinge, S.J.L.; Bäcker, M.; De Buysser, K.; et al. Pair Distribution Function Analysis of ZrO2 Nanocrystals and Insights in the Formation of ZrO2-YBa2Cu3O7 Nanocomposites. Materials 2018, 11, 1066. https://doi.org/10.3390/ma11071066
Rijckaert H, De Roo J, Van Zele M, Banerjee S, Huhtinen H, Paturi P, Bennewitz J, Billinge SJL, Bäcker M, De Buysser K, et al. Pair Distribution Function Analysis of ZrO2 Nanocrystals and Insights in the Formation of ZrO2-YBa2Cu3O7 Nanocomposites. Materials. 2018; 11(7):1066. https://doi.org/10.3390/ma11071066
Chicago/Turabian StyleRijckaert, Hannes, Jonathan De Roo, Matthias Van Zele, Soham Banerjee, Hannu Huhtinen, Petriina Paturi, Jan Bennewitz, Simon J. L. Billinge, Michael Bäcker, Klaartje De Buysser, and et al. 2018. "Pair Distribution Function Analysis of ZrO2 Nanocrystals and Insights in the Formation of ZrO2-YBa2Cu3O7 Nanocomposites" Materials 11, no. 7: 1066. https://doi.org/10.3390/ma11071066
APA StyleRijckaert, H., De Roo, J., Van Zele, M., Banerjee, S., Huhtinen, H., Paturi, P., Bennewitz, J., Billinge, S. J. L., Bäcker, M., De Buysser, K., & Van Driessche, I. (2018). Pair Distribution Function Analysis of ZrO2 Nanocrystals and Insights in the Formation of ZrO2-YBa2Cu3O7 Nanocomposites. Materials, 11(7), 1066. https://doi.org/10.3390/ma11071066








