Synthesis of Mesoporous γ-Al2O3 with Spongy Structure: In-Situ Conversion of Metal-Organic Frameworks and Improved Performance as Catalyst Support in Hydrodesulfurization
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Al-MOFs
2.2. Synthesis of Al2O3 Support
2.3. Preparation of CoMo/Al2O3 Catalyst
2.4. Characterization of Materials and Catalysts
2.5. Hydrodesulfurization Catalytic Activity Assessment
3. Results and Discussion
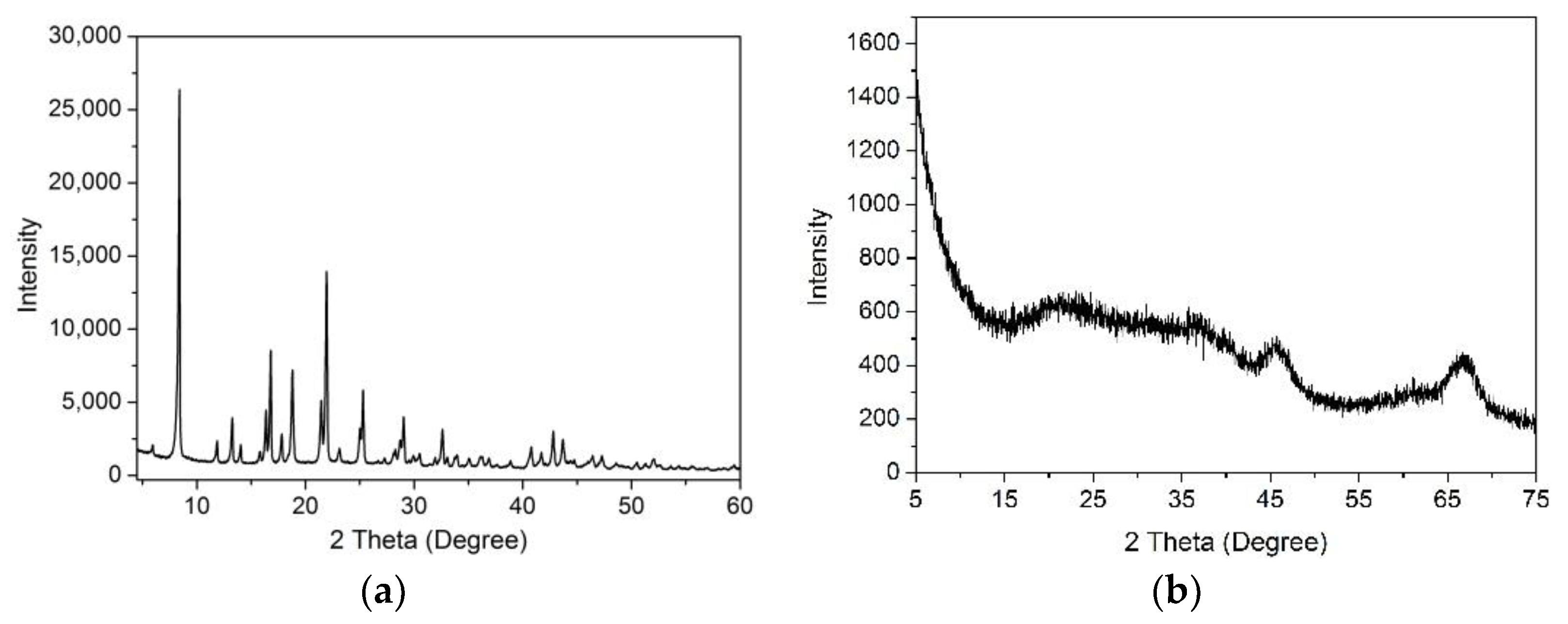
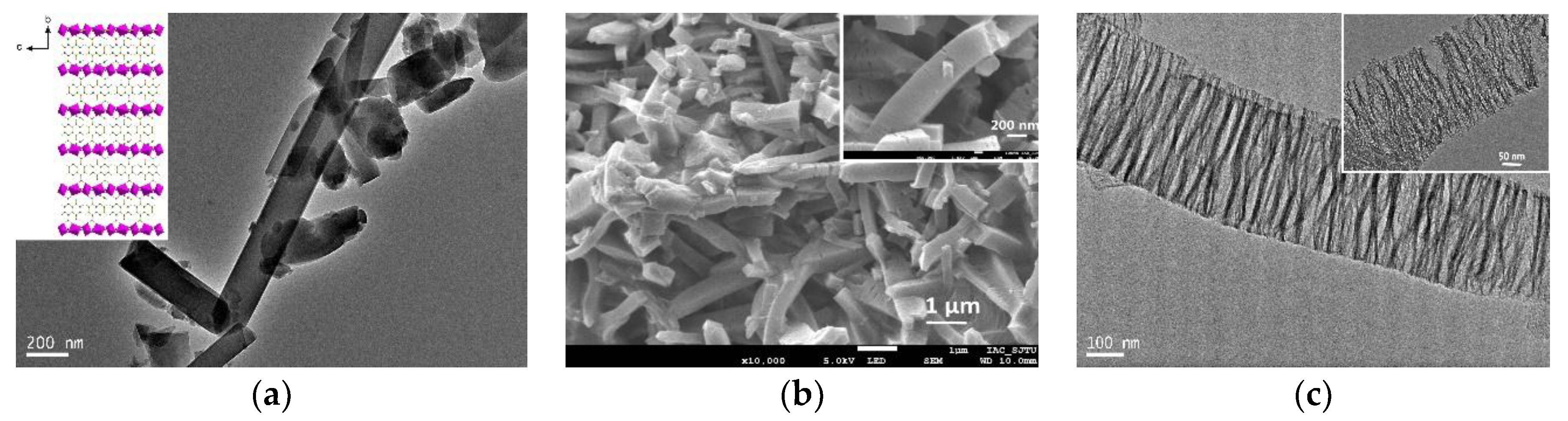
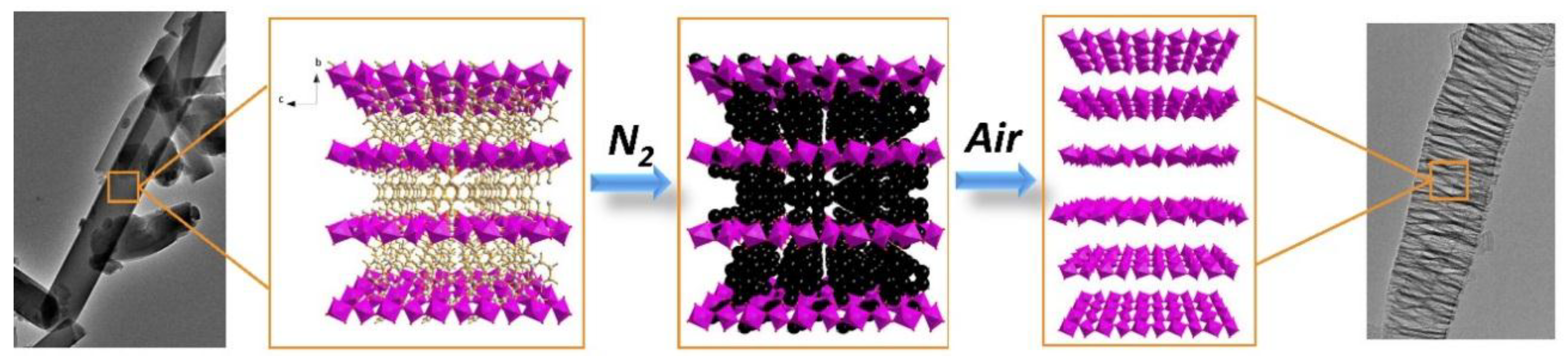
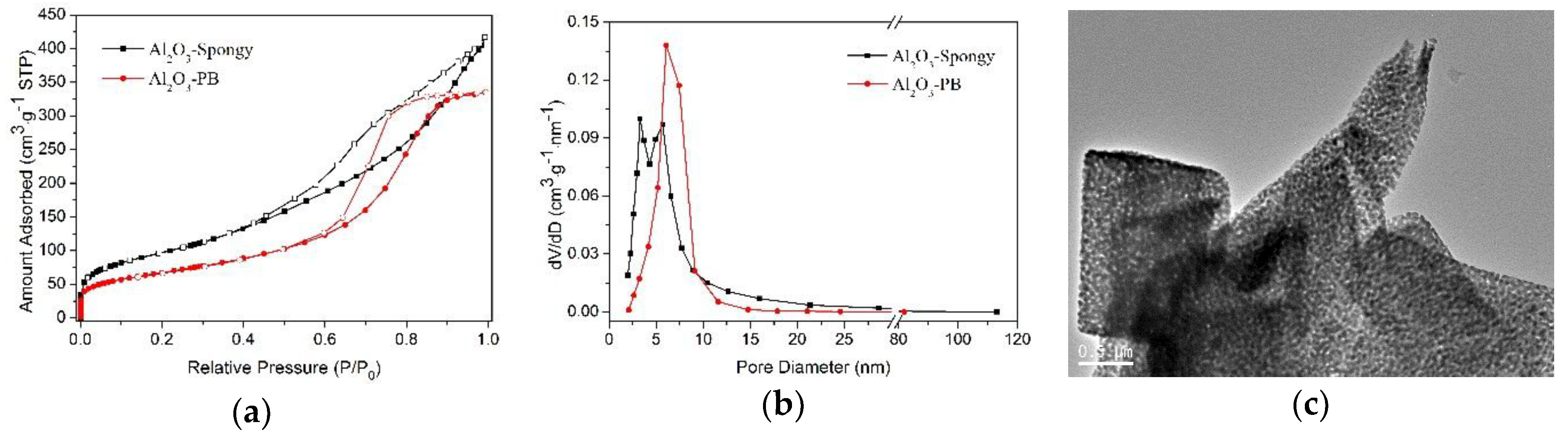
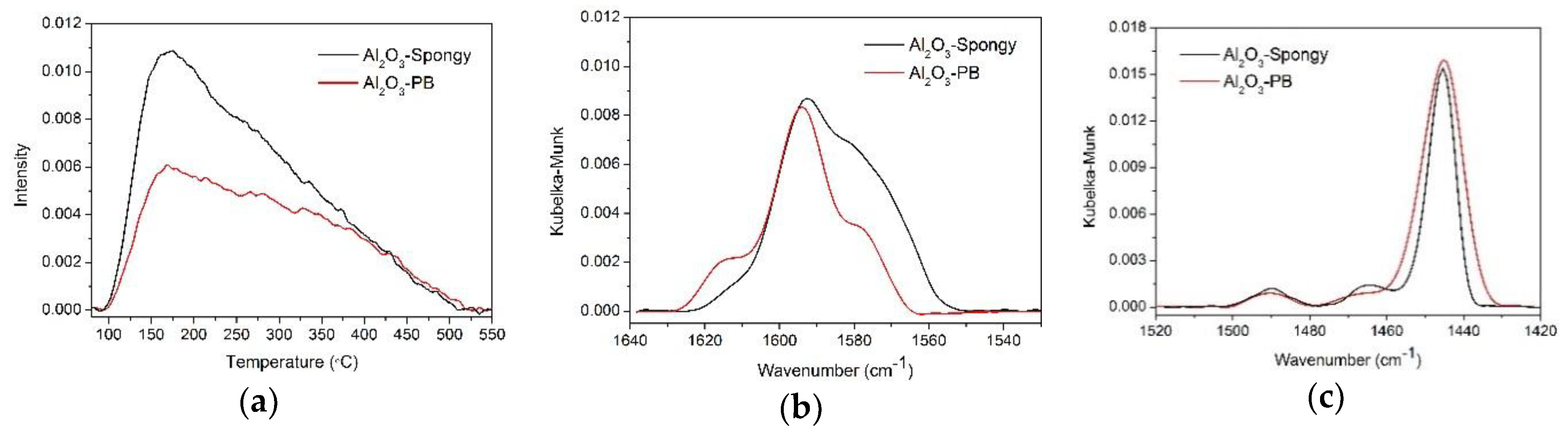
3.1. Characterization of γ-Al2O3 Synthesized from Al-MOFs
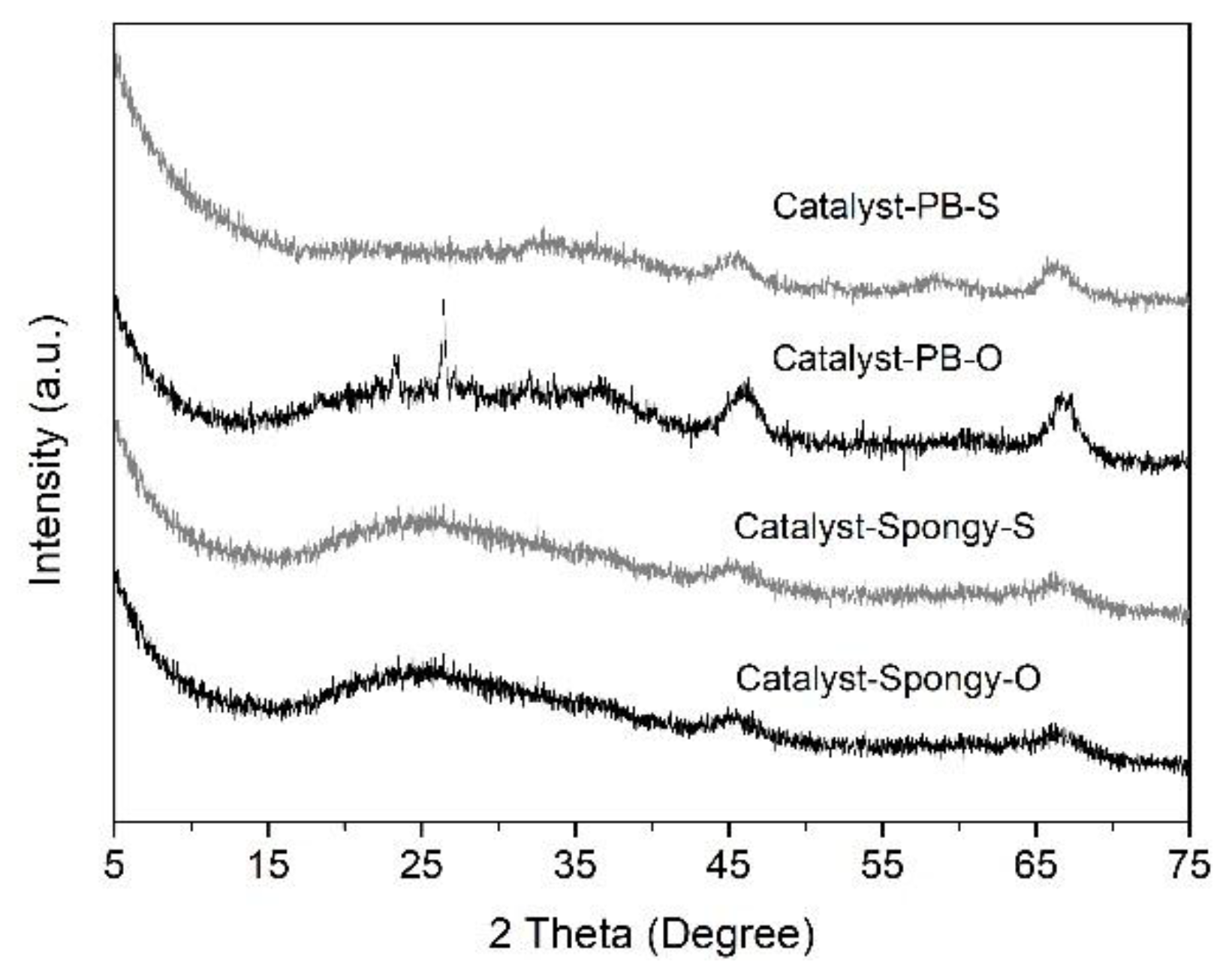
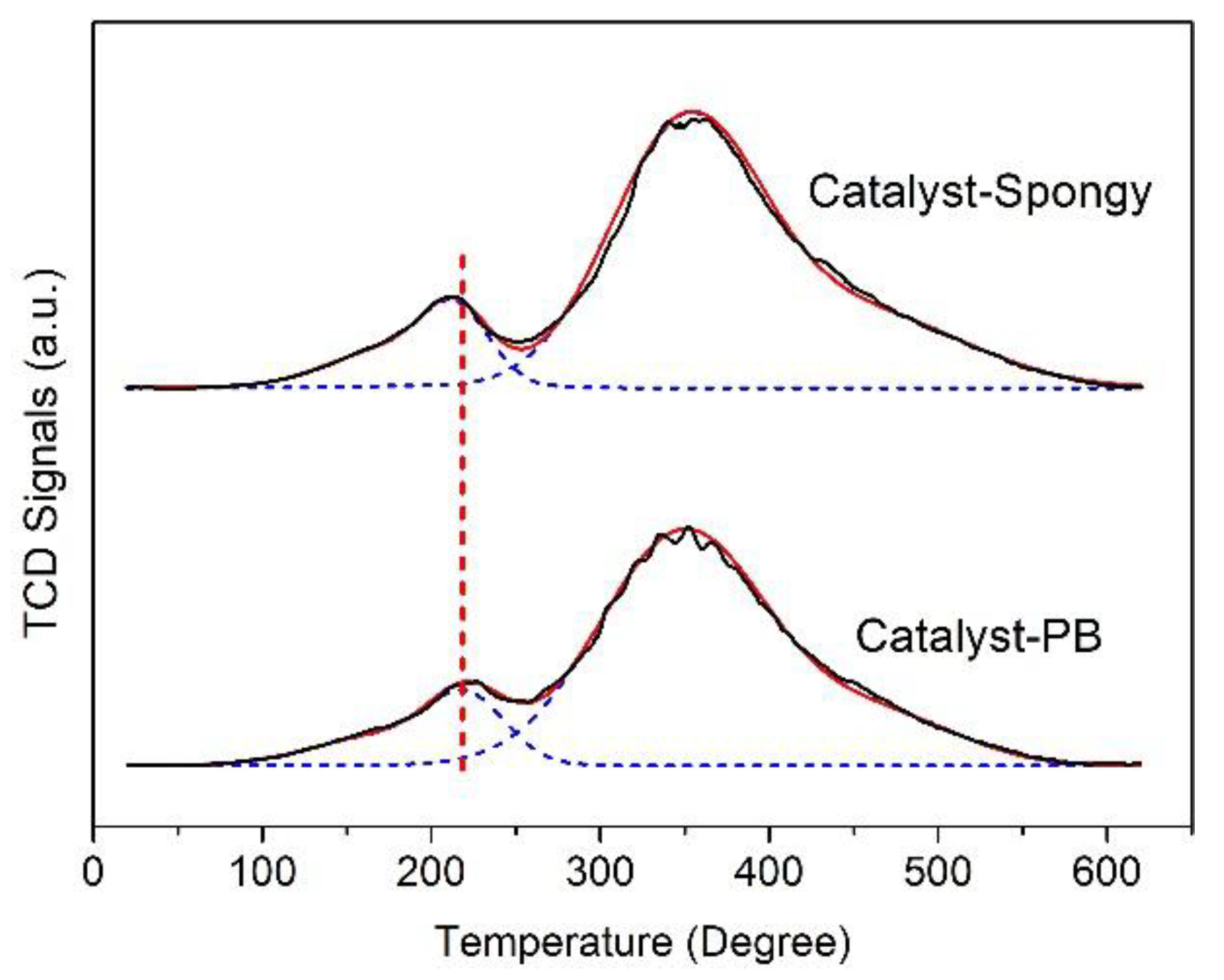
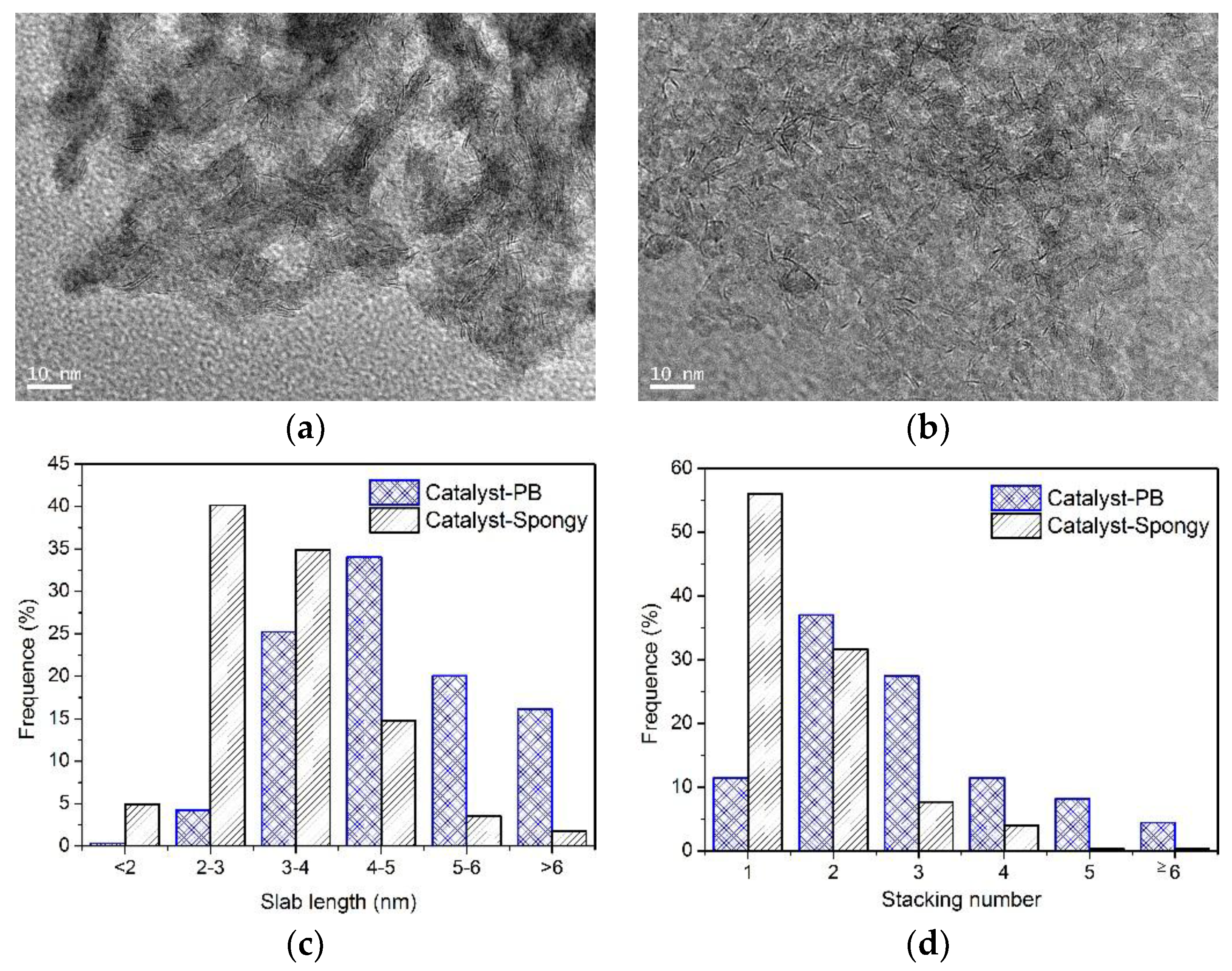
3.2. Characterization of CoMo/Al2O3 Catalysts
3.3. Hydrodesulfurization Activity
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Song, C.; Ma, X. New design approaches to ultra-clean diesel fuels by deep desulfurization and deep dearomatization. Appl. Catal. B Environ. 2003, 41, 207–238. [Google Scholar] [CrossRef]
- Gao, D.; Duan, A.; Zhang, X.; Zhao, Z.; Hong, E.; Li, J.; Wang, H. Synthesis of NiMo catalysts supported on mesoporous Al-SBA-15 with different morphologies and their catalytic performance of DBT HDS. Appl. Catal. B Environ. 2015, 165, 269–284. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z.; Zheng, P.; Chen, Z.; Duan, A.; Xu, C.; Jiao, J.; Zhang, H.; Cao, Z.; Ge, B. Synthesis of NiMo catalysts supported on mesoporous Al2O3 with different crystal forms and superior catalytic performance for the hydrodesulfurization of dibenzothiophene and 4,6-dimethyldibenzothiophene. J. Catal. 2016, 344, 680–691. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Yan, Z. Facile route to prepare bimodal mesoporous γ-Al2O3 as support for highly active CoMo-based hydrodesulfurization catalyst. Appl. Catal. B Environ. 2012, 121–122, 50–56. [Google Scholar] [CrossRef]
- Yaakob, Z.; Bshish, A.; Ebshish, A.; Tasirin, S.; Alhasan, F. Hydrogen Production by Steam Reforming of Ethanol over Nickel Catalysts Supported on Sol Gel Made Alumina: Influence of Calcination Temperature on Supports. Materials 2013, 6, 2229–2239. [Google Scholar] [CrossRef] [PubMed]
- Mrabet, D.; Vu, M.-H.; Kaliaguine, S.; Do, T.-O. A new route to the shape-controlled synthesis of nano-sized γ-alumina and Ag/γ-alumina for selective catalytic reduction of NO in the presence of propene. J. Colloid Interface Sci. 2017, 485, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, Y.; Lebeau, B.; Valtchev, V. Control of the Morphology and Particle Size of Boehmite Nanoparticles Synthesized under Hydrothermal Conditions. Langmuir 2007, 23, 9435–9442. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.C.; Long, J.R.; Yaghi, O.M. Introduction to metal-organic frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Ferey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Gaab, M.; Trukhan, N.; Maurer, S.; Gummaraju, R.; Müller, U. The progression of Al-based metal-organic frameworks–From academic research to industrial production and applications. Micropor. Mesopor. Mater. 2012, 157, 131–136. [Google Scholar] [CrossRef]
- Volkringer, C.; Popov, D.; Loiseau, T.; Férey, G.R.; Burghammer, M.; Riekel, C.; Haouas, M.; Taulelle, F. Synthesis, Single-Crystal X-ray Microdiffraction, and NMR Characterizations of the Giant Pore Metal-Organic Framework Aluminum Trimesate MIL-100. Chem. Mater. 2009, 21, 5695–5697. [Google Scholar] [CrossRef]
- Volkringer, C.; Popov, D.; Loiseau, T.; Guillou, N.; Ferey, G.; Haouas, M.; Taulelle, F.; Mellot-Draznieks, C.; Burghammer, M.; Riekel, C. A microdiffraction set-up for nanoporous metal-organic-framework-type solids. Nat. Mater. 2007, 6, 760–764. [Google Scholar] [CrossRef] [PubMed]
- Haouas, M.; Volkringer, C.; Loiseau, T.; Férey, G.; Taulelle, F. The Extra-Framework Sub-Lattice of the Metal–Organic Framework MIL-110: A Solid-State NMR Investigation. Chem. Eur. J. 2009, 15, 3139–3146. [Google Scholar] [CrossRef] [PubMed]
- Loiseau, T.; Serre, C.; Huguenard, C.; Fink, G.; Taulelle, F.; Henry, M.; Bataille, T.; Ferey, G. A rationale for the large breathing of the porous aluminum terephthalate (MIL-53) upon hydration. Chem. Eur. J. 2004, 10, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- Volkringer, C.; Loiseau, T.; Haouas, M.; Taulelle, F.; Popov, D.; Burghammer, M.; Riekel, C.; Zlotea, C.; Cuevas, F.; Latroche, M.; et al. Occurrence of Uncommon Infinite Chains Consisting of Edge-Sharing Octahedra in a Porous Metal Organic Framework-Type Aluminum Pyromellitate Al4(OH)8[C10O8H2] (MIL-120): Synthesis, Structure, and Gas Sorption Properties. Chem. Mater. 2009, 21, 5783–5791. [Google Scholar] [CrossRef]
- Reinsch, H.; Feyand, M.; Ahnfeldt, T.; Stock, N. CAU-3: A new family of porous MOFs with a novel Al-based brick: [Al2(OCH3)4(O2C-X-CO2)] (X = aryl). Dalton Trans. 2012, 41, 4164–4171. [Google Scholar] [CrossRef] [PubMed]
- Ahnfeldt, T.; Guillou, N.; Gunzelmann, D.; Margiolaki, I.; Loiseau, T.; Ferey, G.; Senker, J.; Stock, N. [Al4(OH)2(OCH3)4(H2N-bdc)3]x·xH2O: A 12-connected porous metal-organic framework with an unprecedented aluminum-containing brick. Angew. Chem. Int. Ed. Engl. 2009, 48, 5163–5166. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Dai, F.; Tang, Z.; Liu, Y.; Liu, C. The structure-directed effect of Al-based metal–organic frameworks on fabrication of alumina by thermal treatment. Mater. Res. Bull. 2015, 65, 287–292. [Google Scholar] [CrossRef]
- Liu, D.; Dai, F.; Li, X.; Liang, J.; Liu, Y.; Liu, C. A non-template approach to fabricate mesoporous alumina with predefined morphology by solid-state transformation of Al-based metal-organic frameworks. RSC Adv. 2015, 5, 15182–15186. [Google Scholar] [CrossRef]
- Liu, D.; Dai, F.; Liu, H.; Liu, Y.; Liu, C. An investigation of the transformation of Al-based metal-organic frameworks to mesoporous Al2O3 with core-shell and nanoporous structure. Mater. Lett. 2015, 139, 7–11. [Google Scholar] [CrossRef]
- Comotti, A.; Bracco, S.; Sozzani, P.; Horike, S.; Matsuda, R.; Chen, J.; Takata, M.; Kubota, Y.; Kitagawa, S. Nanochannels of Two Distinct Cross-Sections in a Porous Al-Based Coordination Polymer. J. Am. Chem. Soc. 2008, 130, 13664–13672. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Wu, M.; Wei, P.; Zhao, J.; Huang, H.; Li, C.; Lu, Y.; Liu, Y.; Liu, C. Efficient hydrodesulfurization catalysts derived from Strandberg PMoNi polyoxometalates. J. Catal. 2018, 358, 155–167. [Google Scholar] [CrossRef]
- Chen, W.; Long, X.; Li, M.; Nie, H.; Li, D. Influence of active phase structure of CoMo/Al2O3 catalyst on the selectivity of hydrodesulfurization and hydrodearomatization. Catal. Today 2017, 292, 97–109. [Google Scholar] [CrossRef]
- Kasztelan, S.; Toulhoat, H.; Grimblot, J.; Bonnelle, J.P. A geometrical model of the active phase of hydrotreating catalysts. Appl. Catal. 1984, 13, 127–159. [Google Scholar] [CrossRef]
- Klimov, O.V.; Leonova, K.A.; Koryakina, G.I.; Gerasimov, E.Y.; Prosvirin, I.P.; Cherepanova, S.V.; Budukva, S.V.; Pereyma, V.Y.; Dik, P.P.; Parakhin, O.A.; et al. Supported on alumina Co-Mo hydrotreating catalysts: Dependence of catalytic and strength characteristics on the initial AlOOH particle morphology. Catal. Today 2014, 220–222, 66–77. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, Y.; Wang, Y.; Luo, G. Preparation of Large-Pore-Volume γ-Alumina Nanofibers with a Narrow Pore Size Distribution in a Membrane Dispersion Microreactor. Ind. Eng. Chem. Res. 2017, 56, 8888–8894. [Google Scholar] [CrossRef]
- Breysse, M.; Afanasiev, P.; Geantet, C.; Vrinat, M. Overview of support effects in hydrotreating catalysts. Catal. Today 2003, 86, 5–16. [Google Scholar] [CrossRef]
- Shakhtakhtinskaya, A.T.; Mamedova, Z.M.; Mutallibova, S.F.; Alieva, S.Z.; Mardzhanova, R.G. TPD study of catalyst surface acidity. React. Kinet. Catal. Lett. 1989, 39, 137–140. [Google Scholar] [CrossRef]
- Morterra, C.; Magnacca, G. A case study: Surface chemistry and surface structure of catalytic aluminas, as studied by vibrational spectroscopy of adsorbed species. Catal. Today 1996, 27, 497–532. [Google Scholar] [CrossRef]
- Kline, C.H.; Turkevich, J. The Vibrational Spectrum of Pyridine and the Thermodynamic Properties of Pyridine Vapors. J. Chem. Phys. 1944, 12, 300–309. [Google Scholar] [CrossRef]
- Scheffer, B.; Dekker, N.J.J.; Mangnus, P.J.; Moulijn, J.A. A temperature-programmed reduction study of sulfided Co-Mo/Al2O3 hydrodesulfurization catalysts. J. Catal. 1990, 121, 31–46. [Google Scholar] [CrossRef]
- Byskov, L.S.; Nørskov, J.K.; Clausen, B.S.; Topsøe, H. DFT Calculations of Unpromoted and Promoted MoS2-Based Hydrodesulfurization Catalysts. J. Catal. 1999, 187, 109–122. [Google Scholar] [CrossRef]
- Yasuda, H.; Higo, M.; Yoshitomi, S.; Sato, T.; Imamura, M.; Matsubayashi, H.; Shimada, H.; Nishijima, A.; Yoshimura, Y. Hydrogenation of tetralin over sulfided nickel-tungstate/alumina and nickel-molybdate/alumina catalysts. Catal. Today 1997, 39, 77–87. [Google Scholar] [CrossRef]
- Lauritsen, J.V.; Besenbacher, F. Atom-resolved scanning tunneling microscopy investigations of molecular adsorption on MoS2 and CoMoS hydrodesulfurization catalysts. J. Catal. 2015, 328, 49–58. [Google Scholar] [CrossRef]
- Gutiérrez, O.Y.; Klimova, T. Effect of the support on the high activity of the (Ni)Mo/ZrO2–SBA-15 catalyst in the simultaneous hydrodesulfurization of DBT and 4,6-DMDBT. J. Catal. 2011, 281, 50–62. [Google Scholar] [CrossRef]
- Nikulshin, P.A.; Salnikov, V.A.; Mozhaev, A.V.; Minaev, P.P.; Kogan, V.M.; Pimerzin, A.A. Relationship between active phase morphology and catalytic properties of the carbon–alumina-supported Co(Ni)Mo catalysts in HDS and HYD reactions. J. Catal. 2014, 309, 386–396. [Google Scholar] [CrossRef]
- Daage, M.; Chianelli, R.R. Structure-Function Relations in Molybdenum Sulfide Catalysts: The “Rim-Edge” Model. J. Catal. 1994, 149, 414–427. [Google Scholar] [CrossRef]
- Berhault, G.; Rosa, M.P.; Mehta, A.; Yacaman, M.J.; Chianelli, R.R. The single-layered morphology of supported MoS2-based catalysts-The role of the cobalt promoter and its effects in the hydrodesulfurization of dibenzothiophene. Appl. Catal. A Gen. 2008, 345, 80–88. [Google Scholar] [CrossRef]
| Sample | Al2O3 Content/% | Contents of Impurity (%) | ||
|---|---|---|---|---|
| Na2O | Fe2O3 | SiO2 | ||
| Al2O3-Spongy | >99.9 | 0.026 | 0.002 | 0.009 |
| Al2O3-PB | 99.67 | 0.304 | 0.009 | 0.016 |
| Sample | Specific Surface Area [m2 g−1] | Pore Volume [cm3 g−1] | Average Pore Size [nm] |
|---|---|---|---|
| Al2O3-Spongy | 350 | 0.63 | 7.2 |
| Al2O3-PB | 240 | 0.52 | 8.6 |
| Sample | [nm] | fMo | |
|---|---|---|---|
| Catalyst-PB-S | 4.78 | 2.81 | 0.25 |
| Catalyst-Spongy-S | 3.29 | 1.62 | 0.35 |
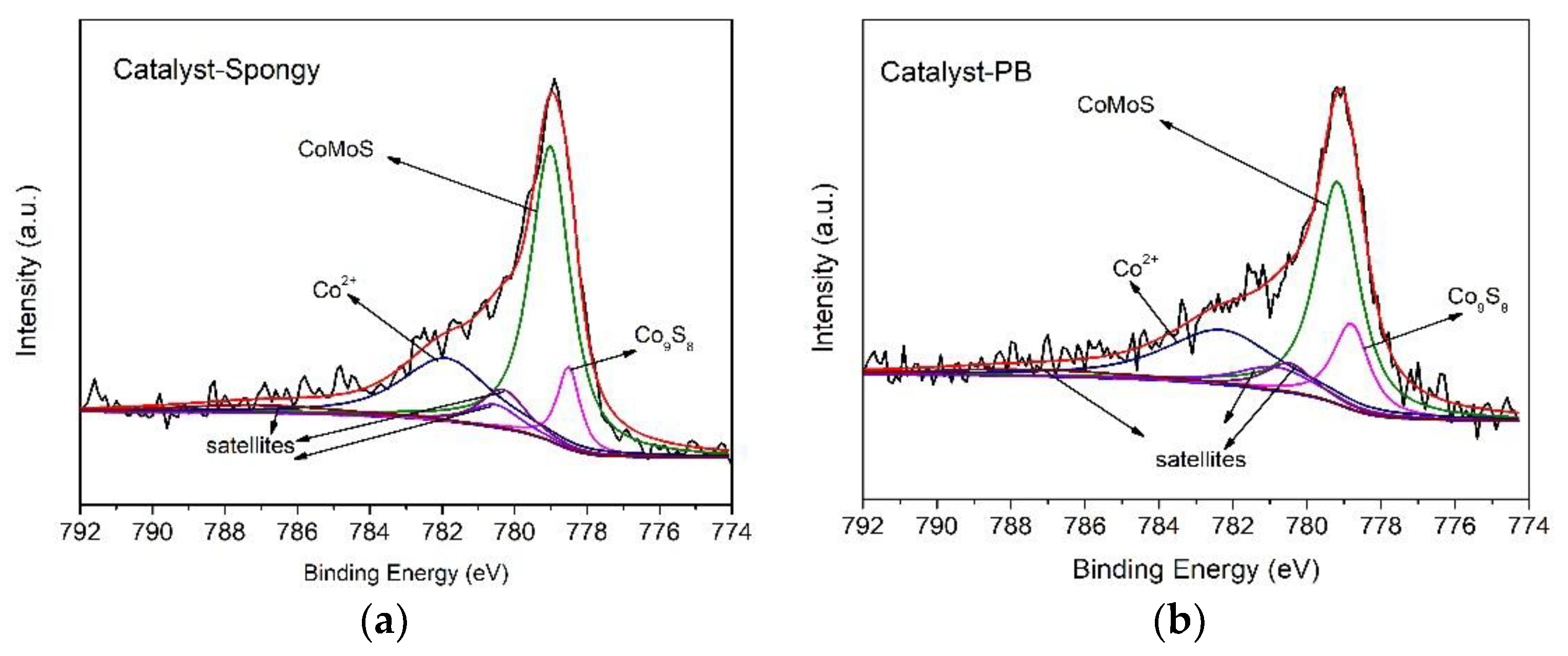
| Sample | CoMoS | Co9S8 | Co2+ | |||
|---|---|---|---|---|---|---|
| B.E. (eV) | At % | B.E. (eV) | At % | B.E. (eV) | At % | |
| Catalyst- Spongy-S | 779.0 | 61.1 | 778.5 | 9.7 | 781.9 | 29.2 |
| Catalyst-PB-S | 779.2 | 50.7 | 778.8 | 16.4 | 782.3 | 32.9 |
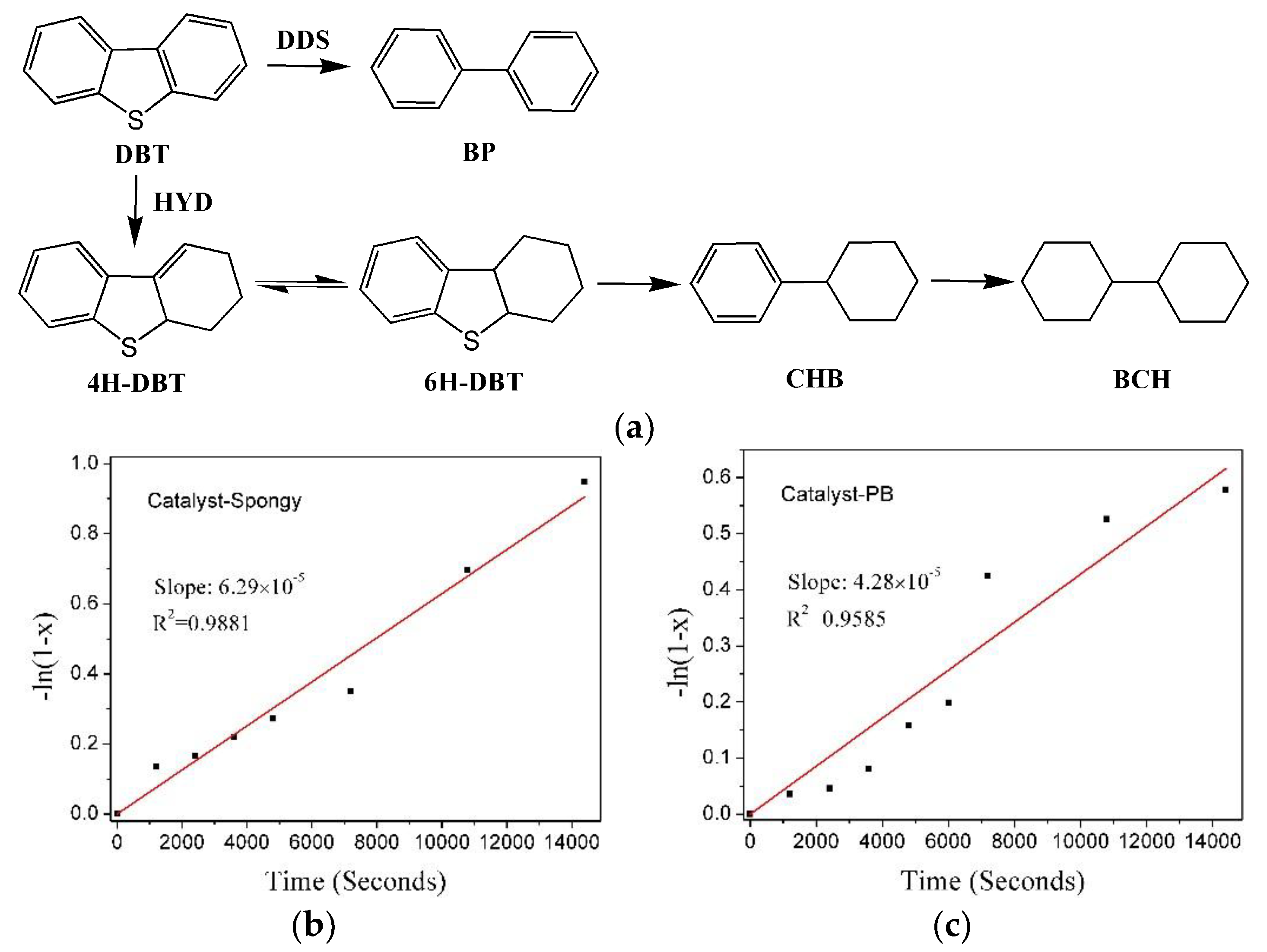
| Sample | Conversion 1 [%] | kHDS 2/[10−5 mol Kgcat−1 s−1] | TOF 3 [h−1] | Product Selectivity 4 [%] | HYD/DDS Selectivity 4 | ||
|---|---|---|---|---|---|---|---|
| BP | CHB | 4H-DBT | |||||
| Catalyst-PB-S | 82.2 | 12.8 | 1.10 | 81.4 | 17.6 | 1.0 | 0.229 |
| Catalyst-Spongy-S | 93.5 | 18.8 | 3.07 | 71.6 | 28.0 | 0.4 | 0.397 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Zhu, H.; Zhao, J.; Pan, L.; Dai, P.; Gu, X.; Li, L.; Liu, Y.; Zhao, X. Synthesis of Mesoporous γ-Al2O3 with Spongy Structure: In-Situ Conversion of Metal-Organic Frameworks and Improved Performance as Catalyst Support in Hydrodesulfurization. Materials 2018, 11, 1067. https://doi.org/10.3390/ma11071067
Liu D, Zhu H, Zhao J, Pan L, Dai P, Gu X, Li L, Liu Y, Zhao X. Synthesis of Mesoporous γ-Al2O3 with Spongy Structure: In-Situ Conversion of Metal-Organic Frameworks and Improved Performance as Catalyst Support in Hydrodesulfurization. Materials. 2018; 11(7):1067. https://doi.org/10.3390/ma11071067
Chicago/Turabian StyleLiu, Dandan, Hongwei Zhu, Jinchong Zhao, Longjun Pan, Pengcheng Dai, Xin Gu, Liangjun Li, Yunqi Liu, and Xuebo Zhao. 2018. "Synthesis of Mesoporous γ-Al2O3 with Spongy Structure: In-Situ Conversion of Metal-Organic Frameworks and Improved Performance as Catalyst Support in Hydrodesulfurization" Materials 11, no. 7: 1067. https://doi.org/10.3390/ma11071067
APA StyleLiu, D., Zhu, H., Zhao, J., Pan, L., Dai, P., Gu, X., Li, L., Liu, Y., & Zhao, X. (2018). Synthesis of Mesoporous γ-Al2O3 with Spongy Structure: In-Situ Conversion of Metal-Organic Frameworks and Improved Performance as Catalyst Support in Hydrodesulfurization. Materials, 11(7), 1067. https://doi.org/10.3390/ma11071067







