The Changes in the Morphology of Bi–Sb System under Centrifugal Force at Room Temperature
Abstract
:1. Introduction
2. Experimental
3. Results
4. Discussion and Conclusions
- -
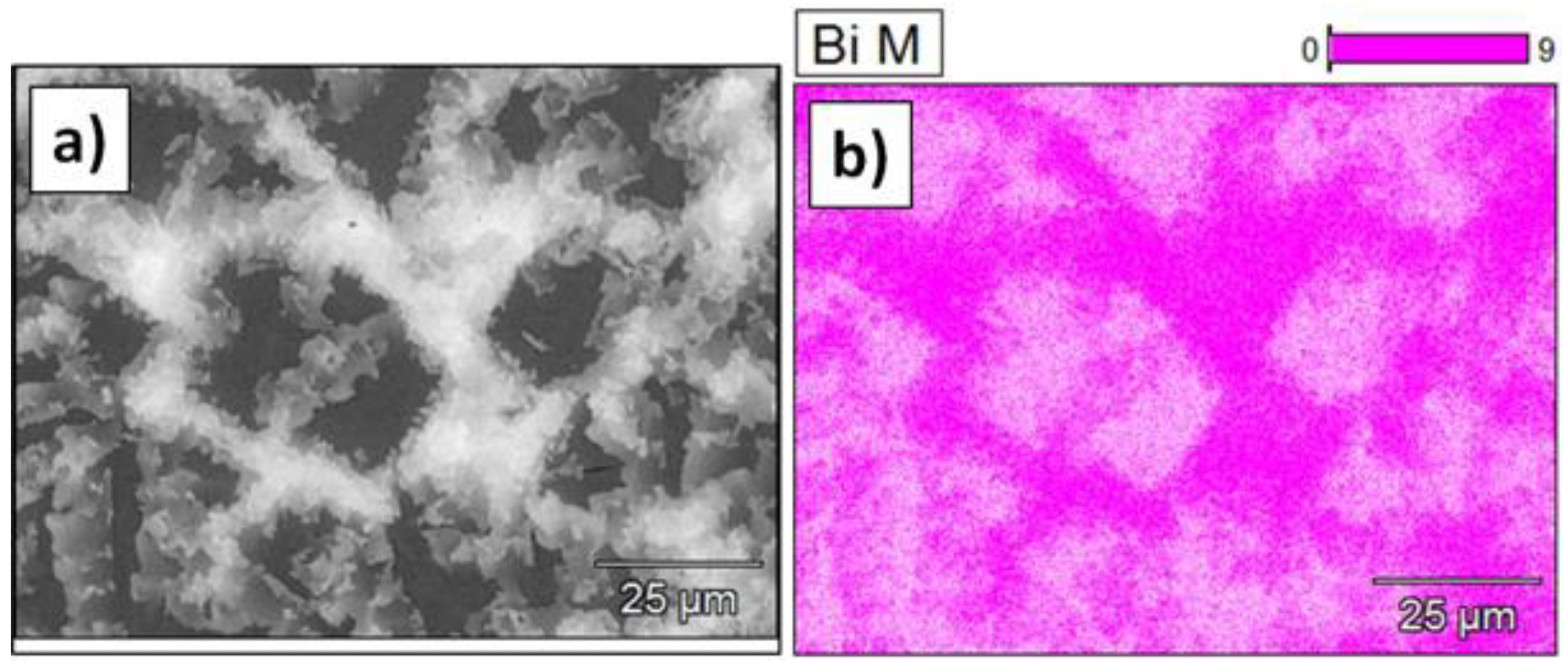
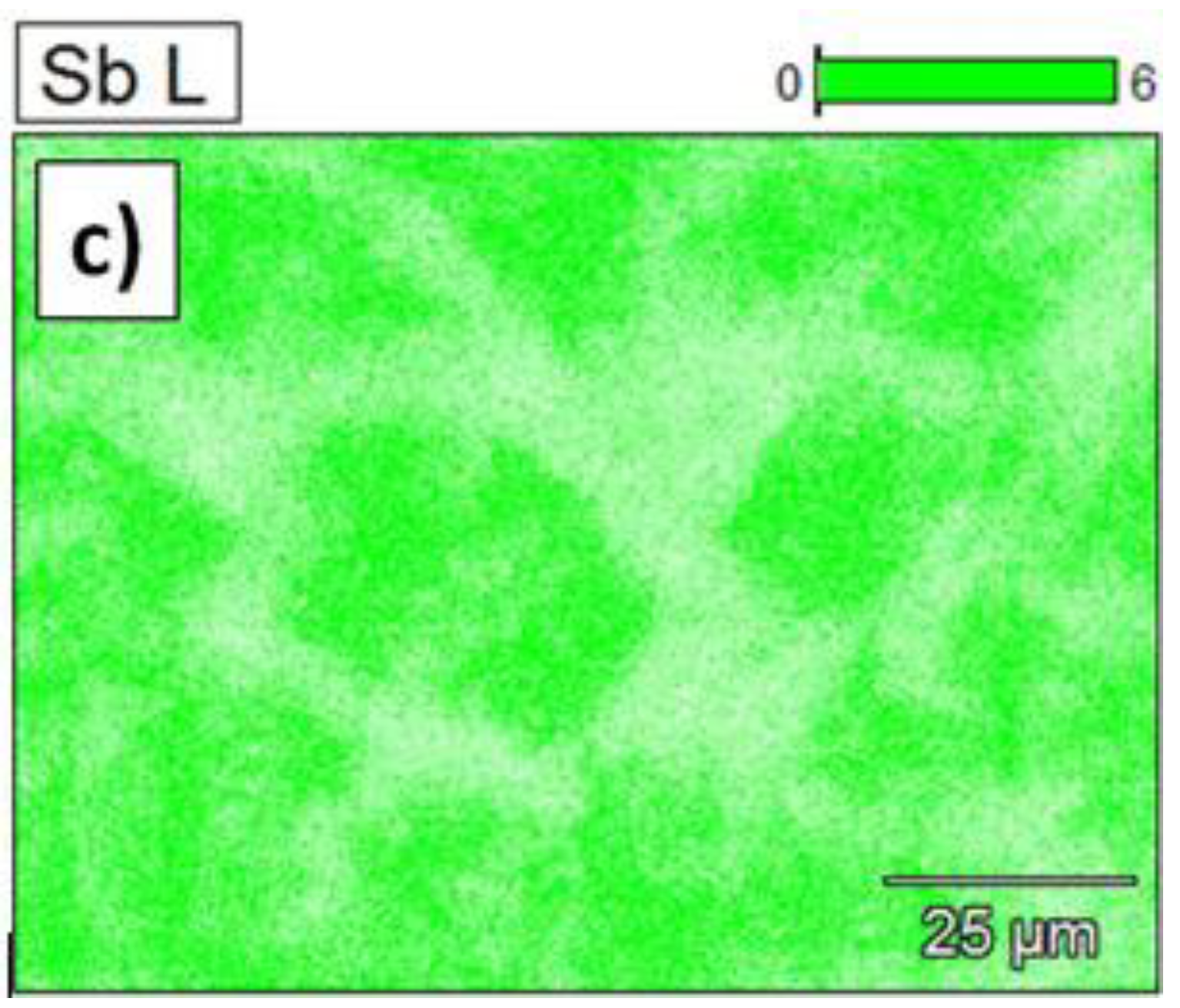
- The binary Bi–Sb alloy showed the formation of two phases: one enriched in Bi and the second enriched in Sb. Formation of the two phases most likely originate due to the rapid cooling during alloy casting;
- -
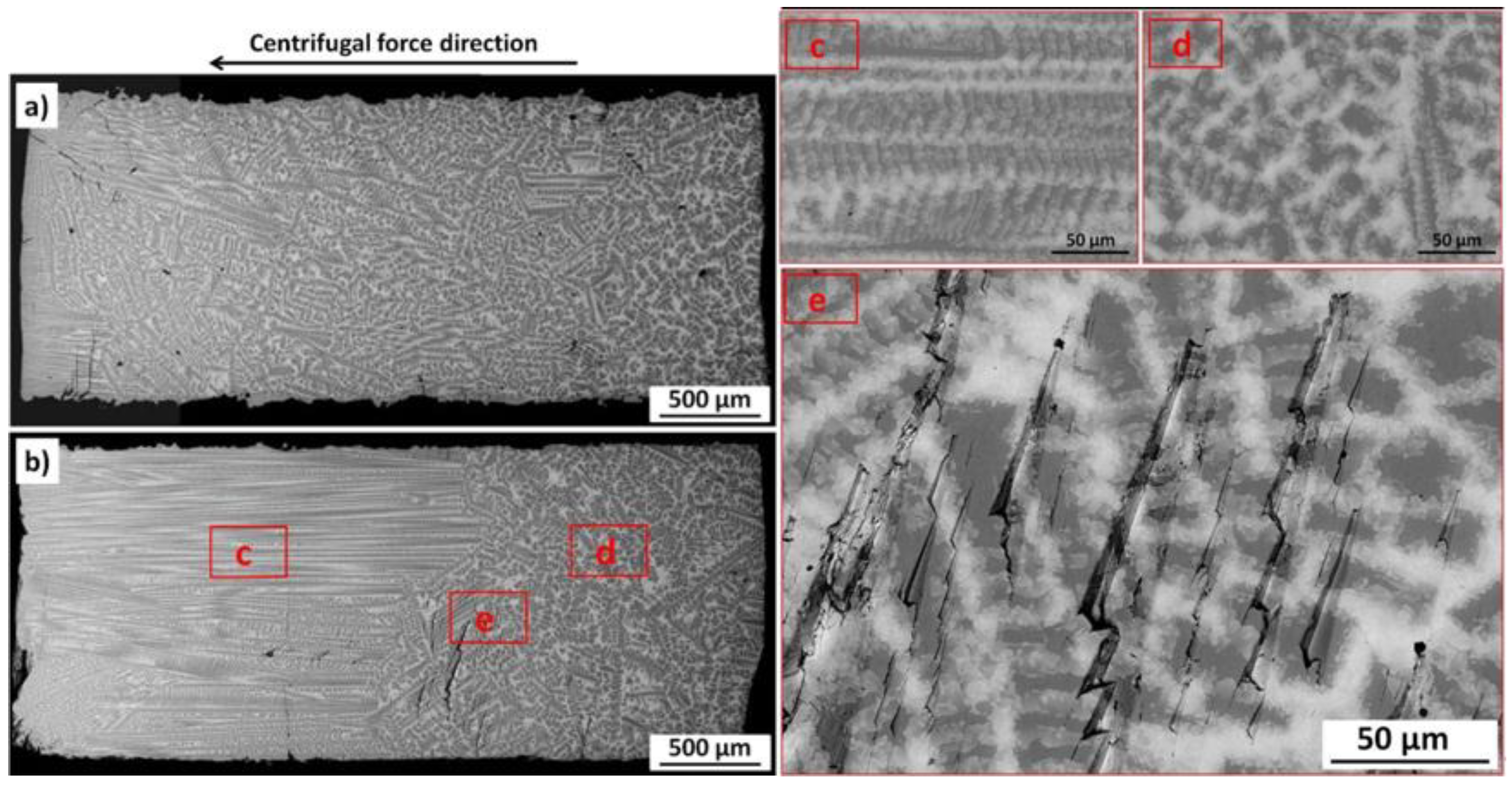
- Under centrifugal force the phases become oriented in the direction parallel to the direction of the centrifugal force;
- -
- Ordering of the phases results in void movement in the direction opposite to the centrifugal force direction. The movements of such voids result in their accumulation and formation of cracks at the interface between ordered and disordered parts of the sample;
- -
- Changes in phase composition after centrifugation was observed as well. Namely, more peaks of Sb phase are observed as well as higher intensities of the peaks from Bi enriched phases, e.g., Bi0.85–Sb0.15.
Author Contributions
Funding
Conflicts of Interest
References
- Barr, L.W.; Smith, F.A. Observations on the equilibrium distribution of gold diffusing in solid potassium in a centrifugal field. Philos. Mag. 1969, 20, 1293–1294. [Google Scholar] [CrossRef]
- Anthony, T.R. Sedimentation in the solid state, sedimentation a l’etat solide sedimentation in festkörpern. Acta Metall. 1970, 18, 877–880. [Google Scholar] [CrossRef]
- Mashimo, T.; Okazaki, S.; Shibazaki, S. Ultracentrifuge apparatus to generate a strong acceleration field of over 1,000,000 g at high temperature in condensed matter. Rev. Sci. Instrum. 1996, 67, 3170–3174. [Google Scholar] [CrossRef]
- Huang, X.S.; Ono, M.; Ueno, H.; Iguchi, Y.; Tomita, T.; Okayasu, S.; Mashimo, T.J. Formation of atomic-scale graded structure in Se-Te semiconductor under strong gravitational field. J. Appl. Phys. 2007, 101, 113502. [Google Scholar] [CrossRef]
- Mashimo, T. Self-consistent approach to the diffusion induced by a centrifugal field in condensed matter: Sedimentation. Phys. Rev. A 1988, 38, 4149–4154. [Google Scholar] [CrossRef]
- Huang, X.; Mashimo, T.; Ono, M.; Tomita, T.; Sawai, T.; Osakabe, T. Effects of ultrastrong gravitational field on the crystalline state of a Bi-Sb alloy. J. Appl. Phys. 2004, 96, 1336–1340. [Google Scholar] [CrossRef]
- Mashimo, T.; Ono, M.; Huang, X.S.; Iguchi, Y.; Okayasu, S.; Kobayashi, K.; Nakamura, E. Gravity-induced diffusion of isotope atoms in monoatomic solid Se. EPL 2008, 81, 56002. [Google Scholar] [CrossRef]
- Mashimo, T.; Okazaki, S.; Tashiro, S. Sedimentation of Atoms in Solid under a Strong Acceleration Field of around 1 Million g. Jpn. J. Appl. Phys. 1997, 36, L498–L500. [Google Scholar] [CrossRef]
- Mashimo, T.; Ikeda, T.; Minato, I. Atomic-scale graded structure formed by sedimentation of substitutional atoms in a Bi–Sb alloy. J. Appl. Phys. 2001, 90, 741–744. [Google Scholar] [CrossRef]
- Ono, M.; Mashimo, T. Sedimentation process for atoms in Bi-Sb system alloy under the strong gravitational field: A new type of diffusion of substitutional solutes. Philos. Mag. A 2002, 82, 591–600. [Google Scholar] [CrossRef]
- Mashimo, T.; Ono, M.; Huang, X.; Iguchiet, Y.; Okayasu, S.; Kobayashi, K.; Nakamura, E. Sedimentation of isotope atoms in monatomic liquid Se. Appl. Phys. Lett. 2007, 91, 231917. [Google Scholar] [CrossRef]
- Iguchi, Y.; Mashimo, T.; Ono, M.; Okayasu, S. Deformation twinning of Bi–Sb solid alloy formed under a strong gravitational field. Philos. Mag. Lett. 2010, 90, 513–518. [Google Scholar] [CrossRef]
- Mashimo, T. Atomic-Scale Materials Processing under Strong Gravitational Field. Defect Diffus. Forum 2012, 323, 517–522. [Google Scholar] [CrossRef]
- Ogata, Y.; Iguchi, Y.; Tokuda, M.; Januszko, K.; Islam Khandaker, J.; Ono, M.; Mashimo, T. Diffusion phenomenon at the interface of Cu-brass under a strong gravitational field. J. Appl. Phys. 2015, 117, 125902. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wierzba, B.; Nowak, W.J. The Changes in the Morphology of Bi–Sb System under Centrifugal Force at Room Temperature. Materials 2018, 11, 1065. https://doi.org/10.3390/ma11071065
Wierzba B, Nowak WJ. The Changes in the Morphology of Bi–Sb System under Centrifugal Force at Room Temperature. Materials. 2018; 11(7):1065. https://doi.org/10.3390/ma11071065
Chicago/Turabian StyleWierzba, Bartek, and Wojciech J. Nowak. 2018. "The Changes in the Morphology of Bi–Sb System under Centrifugal Force at Room Temperature" Materials 11, no. 7: 1065. https://doi.org/10.3390/ma11071065
APA StyleWierzba, B., & Nowak, W. J. (2018). The Changes in the Morphology of Bi–Sb System under Centrifugal Force at Room Temperature. Materials, 11(7), 1065. https://doi.org/10.3390/ma11071065





