In-Liquid Plasma Process for Size- and Shape-Controlled Synthesis of Silver Nanoparticles by Controlling Gas Bubbles in Water
Abstract
:1. Introduction
2. Materials and Methods
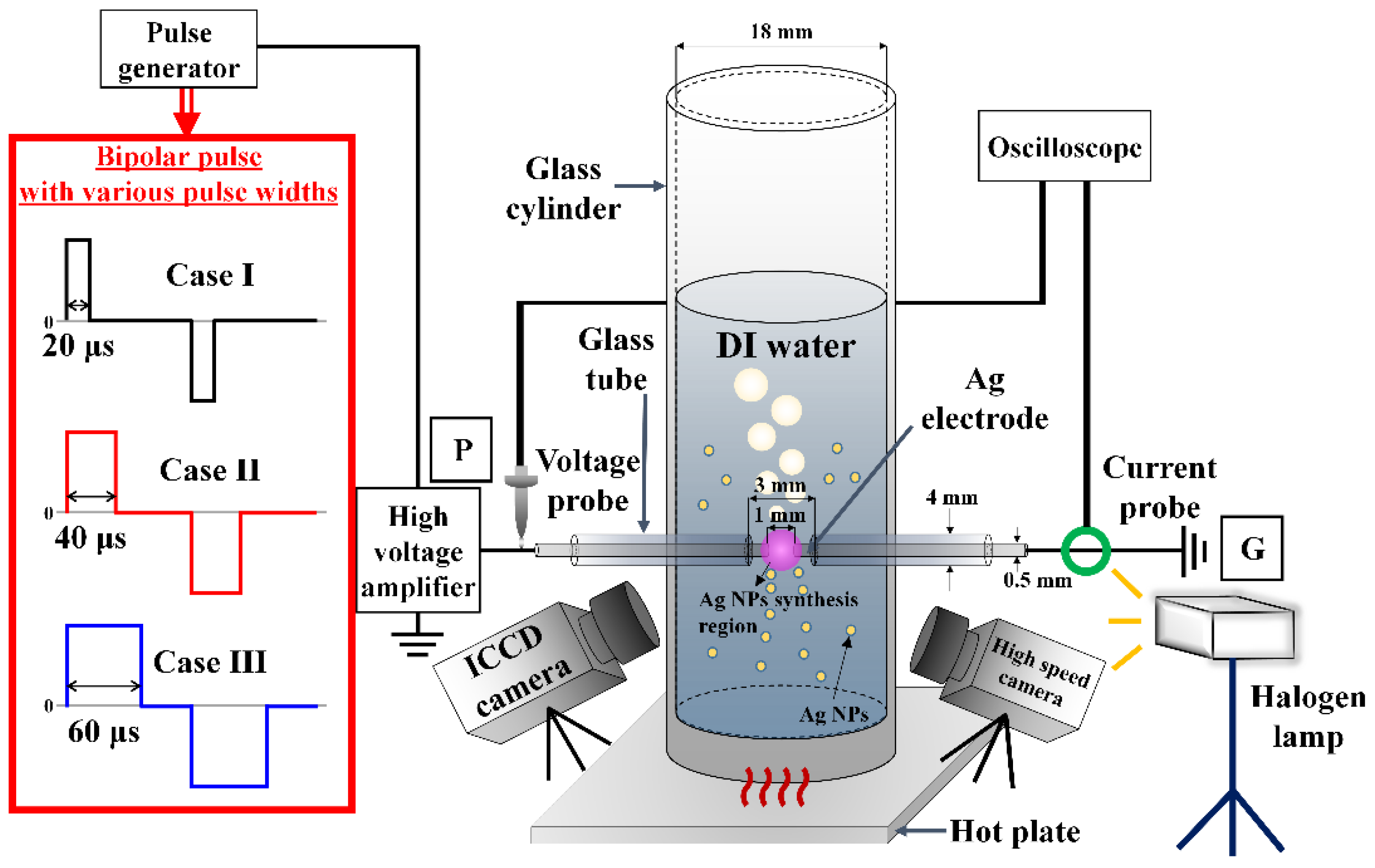
2.1. Liquid Plasma Reactor and Measurement Setup
2.2. Pulsing Method for Different Bubble Formations
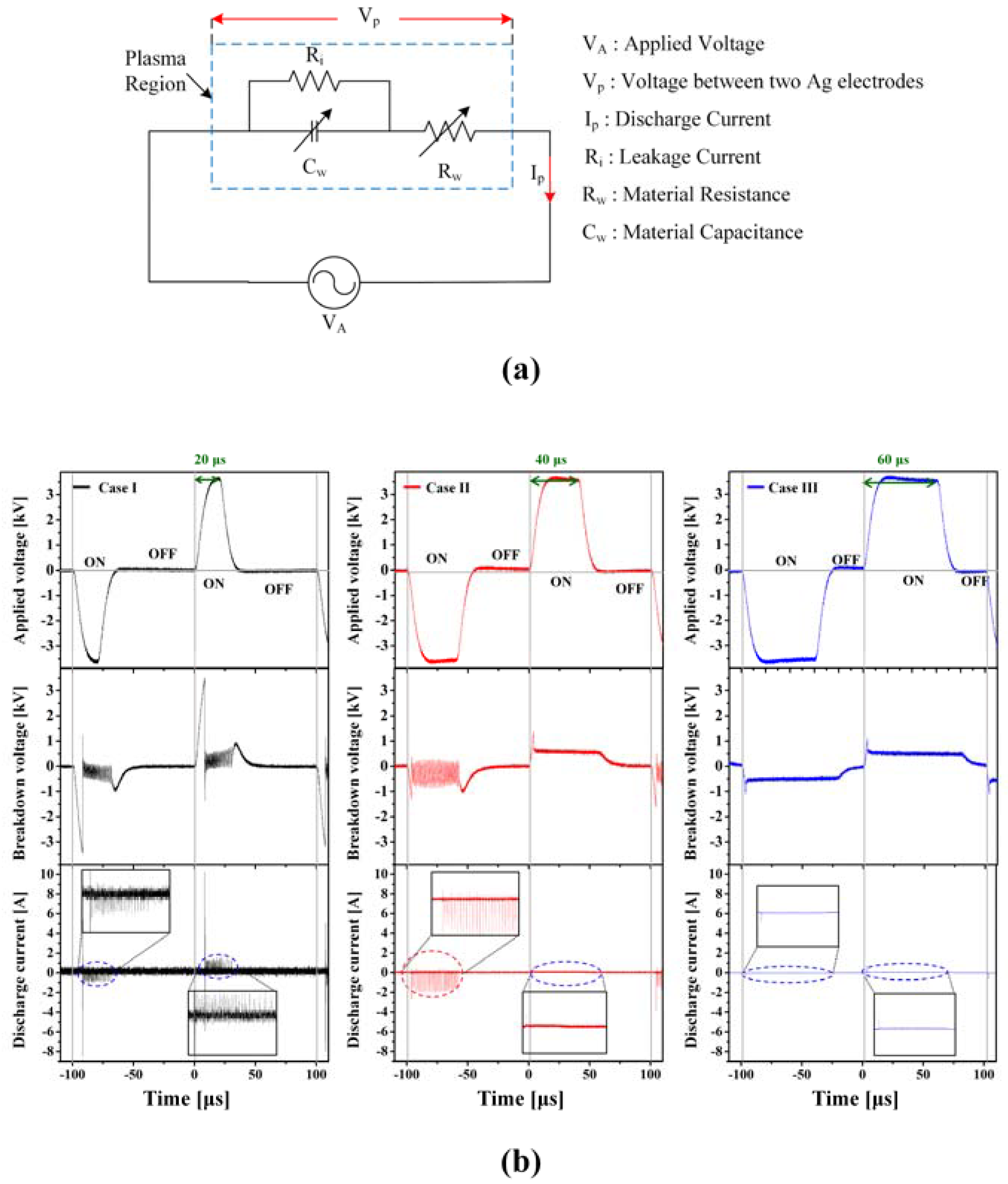
2.3. Voltage-Current Measurement
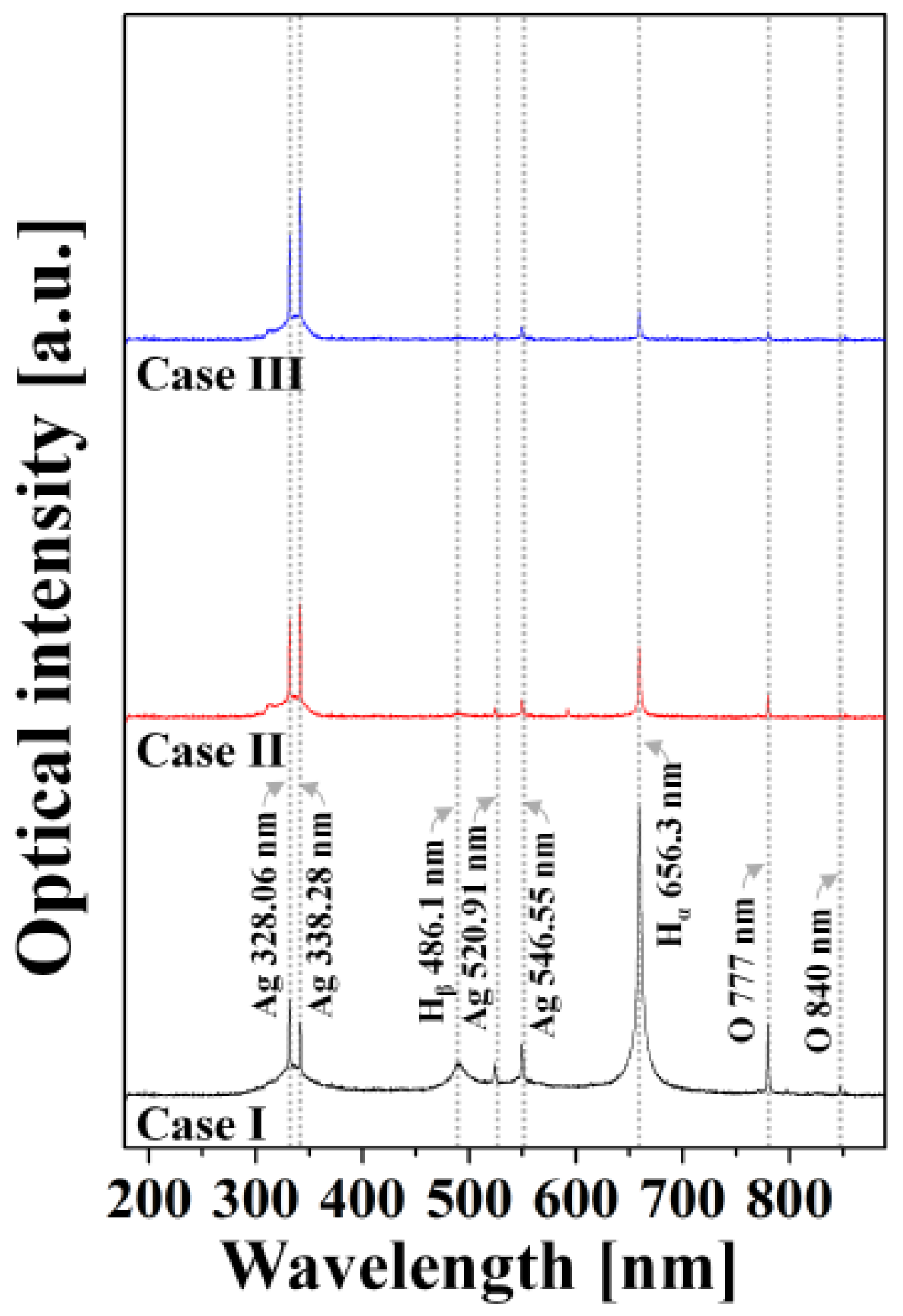
2.4. Optical Emission Spectroscopy
2.5. ICCD Camera
2.6. High-Speed Camera
2.7. Scanning Electron Microscopy
2.8. Transmission Electron Microscopy
2.9. Dynamic Light Scattering
2.10. Ultraviolet-Visible Light Spectroscopy
3. Results and Discussion
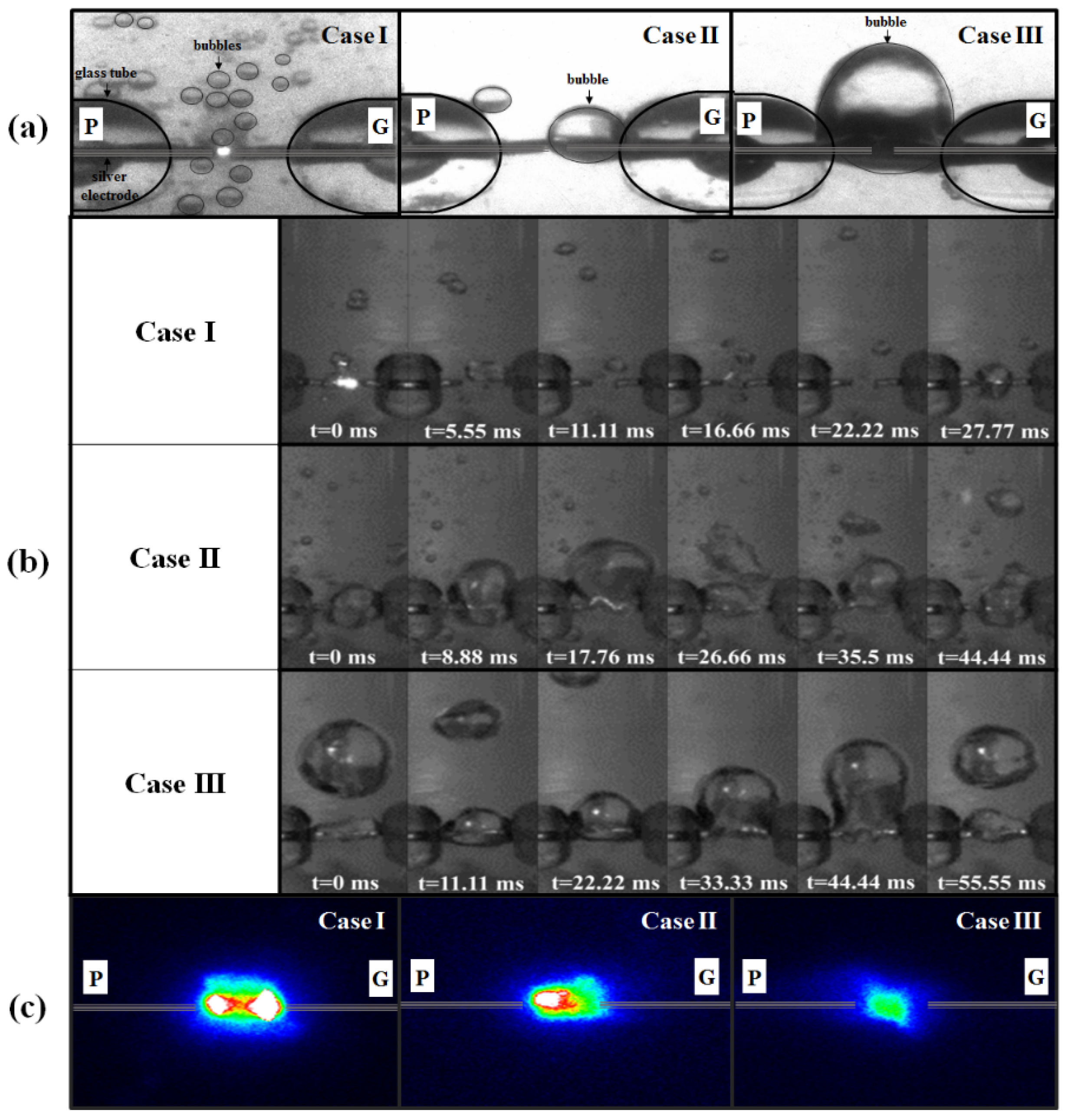
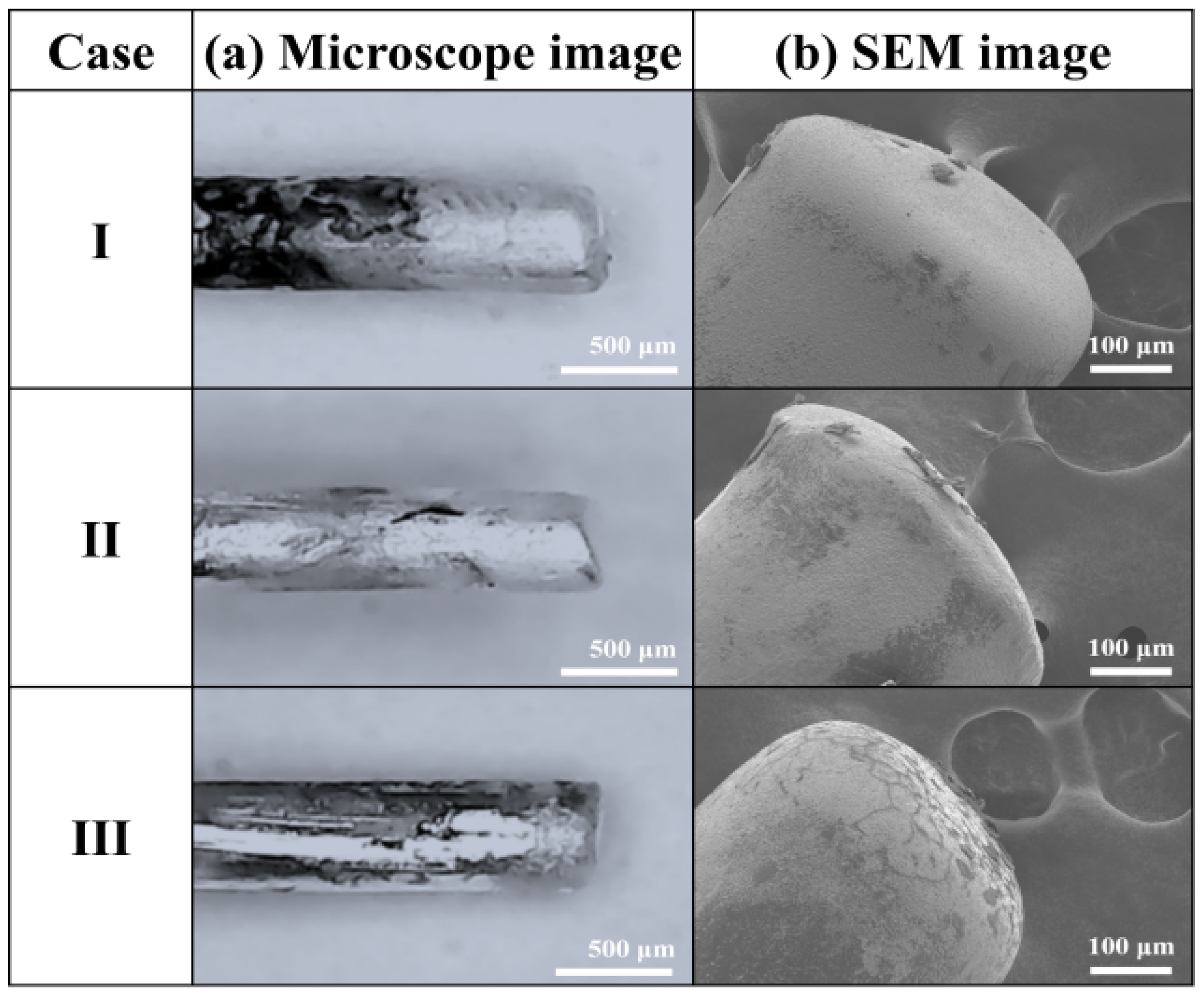
3.1. Variations in Discharge Characteristics According to Bubble Formation
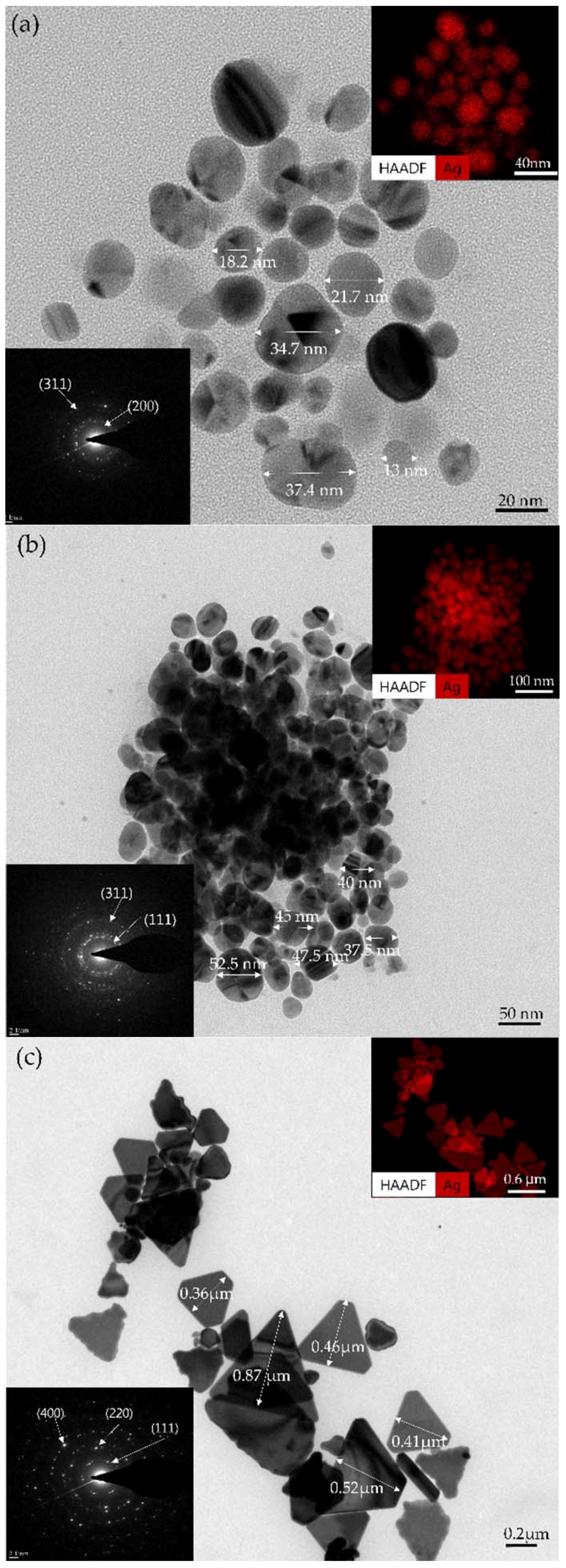
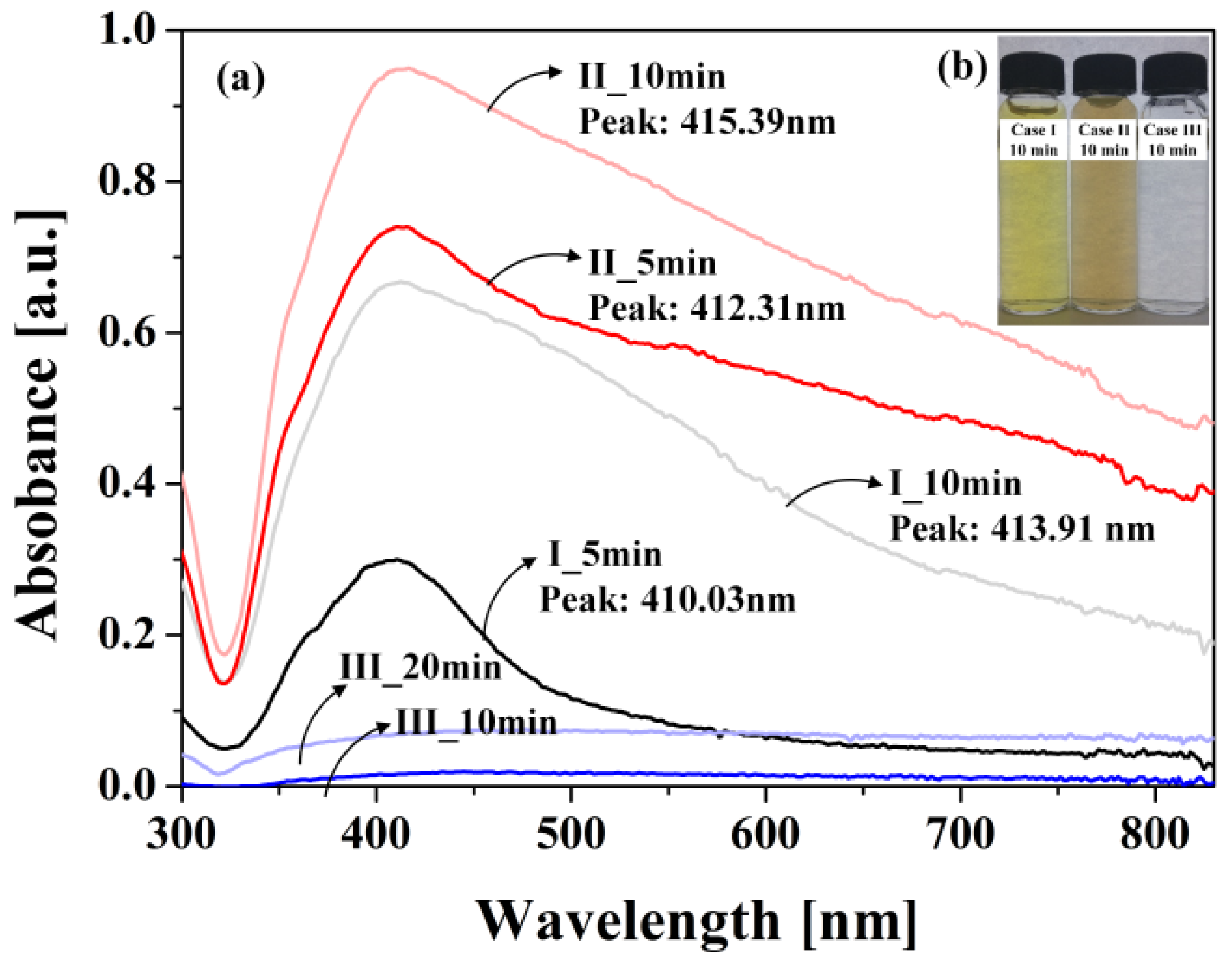
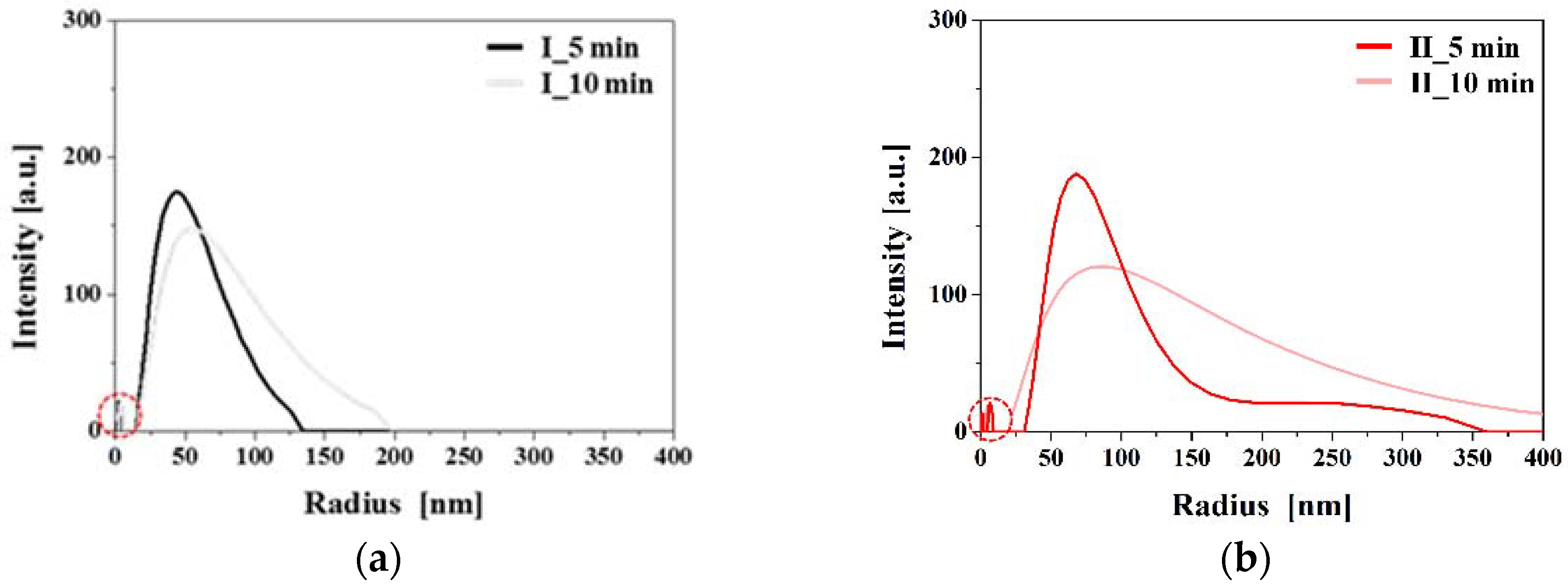
3.2. Changes in Sizes and Shapes of Ag NPs According to Bubble Formations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yoshida, T.; Yamamoto, N.; Mizutani, T.; Yamamoto, M.; Ogawa, S.; Yagi, S.; Nameki, H.; Yoshida, H. Synthesis of Ag nanoparticles prepared by a solution plasma method and application as a cocatalyst for photocatalytic reduction of carbon dioxide with water. Catal. Today 2018, 303, 320–326. [Google Scholar] [CrossRef]
- Vos, C.D.; Baneton, J.; Witzke, M.; Dille, J.; Godet, S.; Gordon, M.J.; Sankaran, R.M.; Reniers, F. A comparative study of the reduction of silver and gold salts in water by a cathodic microplasma electrode. J. Phys. D Appl. Phys. 2017, 50, 105206. [Google Scholar] [CrossRef]
- Heo, Y.K.; Kim, S.M.; Lee, S.Y. Effects of discharge duration on the size and shape of gold nanoparticles synthesized using solution plasma processing. Phys. Scr. 2010, T139, 014025. [Google Scholar] [CrossRef]
- Hieda, J.; Saito, N.; Takai, O. Exotic shapes of gold nanoparticles synthesized using plasma in aqueous solution. J. Vac. Sci. Technol. A 2008, 26, 854–856. [Google Scholar] [CrossRef]
- Bratescu, M.A.; Cho, S.P.; Takai, O.; Saito, N. Size controlled gold nanoparticles synthesized in solution plasma. J. Phys. Chem. C 2011, 115, 24569–24576. [Google Scholar] [CrossRef]
- Mun, M.K.; Lee, W.O.; Park, J.W.; Kim, D.S.; Yeom, G.Y.; Kim, D.W. Nanoparticle synthesis and modification using solution plasma process. Appl. Sci. Converg. Technol. 2016, 26, 164–173. [Google Scholar] [CrossRef]
- Saito, G.; Akiyama, T. Nanomaterial synthesis using plasma generation in liquid. J. Nanomater. 2015, 2015, 123696. [Google Scholar] [CrossRef]
- Watthanaphanit, A.; Panomsuwan, G.; Saito, N. A novel one-step synthesis of gold nanoparticles in an alginate gel matrix by solution plasma sputtering. RSC Adv. 2014, 4, 1622–1629. [Google Scholar] [CrossRef]
- Jin, S.H.; Kim, S.M.; Lee, S.Y.; Kim, J.W. Synthesis and characterization of silver nanomaterials using solution plasma process. J. Nanosci. Nanotechnol. 2014, 14, 8094–8097. [Google Scholar] [CrossRef]
- Mizutani, T.; Ogawa, S.; Murai, T.; Nameki, H.; Yoshida, T.; Yagi, S. In situ UV–Vis investigation of growth of gold nanoparticles prepared by solution plasma sputtering in NaCl solution. Appl. Surf. Sci. 2015, 354, 397–400. [Google Scholar] [CrossRef]
- Pootawang, P.; Saito, N.; Takai, O. Synthesis and characteristics of Ag, Pt bimetallic nanocomposites by arc-discharge solution plasma processing. Nanotechnology 2012, 23, 395602. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Shen, X.; Takai, O.; Saito, N. Facile fabrication of PtAu alloy clusters using solution plasma sputtering. J. Alloy. Compd. 2013, 552, 351–355. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, Y.J.; Kim, J.W.; Lee, S.Y. Facile synthesis of Pt–Pd bimetallic nanoparticles by plasma discharge in liquid and their electrocatalytic activity toward methanol oxidation in alkaline media. Thin Solid Films 2014, 572, 260–265. [Google Scholar] [CrossRef]
- Lee, S.-J.; Lee, H.; Jeon, K.-J.; Park, H.; Park, Y.-K.; Jung, S.-C. Characterization of Bimetallic Fe-Ru Oxide nanoparticles prepared by liquids-phase plasma method. Nanoscale Res. Lett. 2016, 11, 344. [Google Scholar] [CrossRef] [PubMed]
- Saito, G.; Hosokai, S.; Tsubota, M.; Akiyama, T. Influence of Solution Temperature and Surfactants on Morphologies of Tin Oxide Produced Using a Solution Plasma Technique. Cryst. Growth Des. 2012, 12, 2455–2459. [Google Scholar] [CrossRef]
- Chen, Q.; Kaneko, T.; Hatakeyama, R. Synthesis of Superfine Ethanol-Soluble CoO Nanoparticles via Discharge Plasma in Liquid. Appl. Phys. Express 2012, 5, 96201. [Google Scholar] [CrossRef]
- Nakasugi, Y.; Saito, G.; Yamashita, T.; Akiyama, T. Synthesis of nonstoichiometric titanium oxide nanoparticles using discharge in HCL solution. J. Appl. Phys. 2014, 115, 123303. [Google Scholar] [CrossRef]
- Kelgenbaeva, Z.; Omurzak, E.; Ihara, H.; Iwamoto, C.; Sulaimankulova, S.; Mashimo, T. Sn and SnO2 nanoparticles by pulsed plasma in liquid: Synthesis, characterization and applications. Phys. Status Solidi A 2015, 212, 2951–2957. [Google Scholar] [CrossRef]
- Askari, S.; Macias-Montero, M.; Velusamy, T.; Maguire, P.; Svrcek, V.; Mariotti, D. Silicon-based quantum dots: Synthesis, surface and cmposition tuning with atmospheric pressure plasma. J. Phys. D Appl. Phys. 2015, 48, 314002. [Google Scholar] [CrossRef]
- Wu, J.J.; Kondeti, V.S.S.K.; Bruggeman, P.J.; Kortshagen, U.R. Luminescent, water-soluble silicon quantum dots via micro-plasma surface treatment. J. Phys. D Appl. Phys. 2016, 49. [Google Scholar] [CrossRef]
- Omurzak, E.; Abdullaeva, Z.; Iwamoto, C.; Ihara, H.; Sulaimankulova, S.; Mashimo, T. Synthesis of Hollow Carbon Nano-Onions Using the Pulsed Plasma in Liquid. J. Nanosci. Nanotechnol. 2015, 15, 3703–3709. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Dao, V.D.; Larina, L.L.; Jung, K.D.; Choi, H.S. Solution-processable rGO–Pt nanohybrids synthesized in an aqueous fructose solution for transparent and efficient dye-sensitized solar cells. Chem. Eng. J. 2016, 283, 1285–1294. [Google Scholar] [CrossRef]
- Morishita, T.; Ueno, T.; Panomsuwan, G.; Hieda, J.; Yoshida, A.; Bratescu, M.A.; Saito, N. Fastest Formation Routes of nanocarbons in solution plasma processes. Sci. Rep. 2016, 6, 36880. [Google Scholar] [CrossRef] [PubMed]
- Abdullaeva, Z.; Omurzak, E.; Iwamoto, C.; Ihara, H.; Ganapathy, H.S.; Sulaimankulova, S.; Koinuma, M.; Mashimo, T. Pulsed Plasma Synthesis of Iron and Nickel Nanoparticles Coated by Carbon for Medical applications. Jpn. J. Appl. Phys. 2013, 52. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kim, S.M.; Kim, J.W.; Lee, S.Y. The characterization and electrocatalytic activities of carbon-supported Pt nanoparticles synthesized by the solution plasma process. Mater. Lett. 2014, 123, 184–186. [Google Scholar] [CrossRef]
- Chen, Q.; Li, J.; Li, Y. A reviews of plasma-liquid interactions for nanomaterial synthesis. J. Phys. D Appl. Phys. 2015, 48, 424005. [Google Scholar] [CrossRef]
- Bruggeman, P.; Leys, C. Non-thermal plasmas in and in contact with liquids. J. Phys. D Appl. Phys. 2009, 42, 53001. [Google Scholar] [CrossRef]
- Wang, F.; Tang, R.; Yu, H.; Gibbons, P.C.; Bruhro, W.E. Size and shape controlled synthesis of bismuth nanoparticle. Chem. Mater. 2008, 20, 3656–3662. [Google Scholar] [CrossRef]
- Hsieh, A.; Chang, H.; Hsu, C. The bubble to jetting transition mechanism of plasmas in NaNO3 solutions sustained by pulsed power. J. Phys. D Appl. Phys. 2012, 45, 415202. [Google Scholar] [CrossRef]
- Chang, H.W.; Hsu, C.C. Plasmas in Saline Solution Sustained Using Bipolar Pulsed Power Source: Tailoring the Discharge Behavior Using the Negative Pulses. Plasma Chem. Plasma Process. 2013, 33, 581–591. [Google Scholar] [CrossRef]
- Sobel, A. Gas-discharge displays: The state of the art. IEEE Trans. Electron Devices 1977, 24, 835–847. [Google Scholar] [CrossRef]
- Uehara, S.; Ishihara, K.; Nishiyama, H. Development of a capillary plasma pump with vapor bubble for water purification: Experimental and theoretical investigation. J. Phys. D Appl. Phys. 2016, 49, 405202. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, X.; Yan, H.; Huang, Y.; Liu, Z.; Yan, K. Synthesis of SnO2 nanoparticles using a solution plasma and their gas-sensing properties. Appl. Phys. Lett. 2017, 110. [Google Scholar] [CrossRef]
- Qian, J.; Joshi, R.P.; Kolb, J.; Schoenbach, K.H.; Dickens, J.; Neuber, A.; Butcher, M.; Cevallos, M.; Krompholz, H.; Schamiloglu, E.; et al. Microbubble-based model analysis of liquid breakdown initiation by a submicrosecond pulse. J. Appl. Phys. 2005, 97, 113304. [Google Scholar] [CrossRef]
- Sommers, B.S.; Foster, J.E. Nonlinear oscillations of gas bubbles submerged in water: Implications for plasma breakdown. J. Phys. D Appl. Phys. 2012, 45, 415203. [Google Scholar] [CrossRef]
- Foster, J.E. Plasma-based water purification: Challenges and prospects for the future. Phys. Plasmas 2017, 24, 055501. [Google Scholar] [CrossRef]
- Vinod, M.; Gopchandran, K.G. Au, Ag and Au:Ag colloida nanoparticles synthesized by pulsed laser ablations as SERS substrates. Prog. Nat. Sci. Mater. 2014, 24, 569–578. [Google Scholar] [CrossRef]
- He, X.; Zhao, X.; Li, Y.; Sui, X. shape-controlled synthesis for silver: Trangular/hexagonal nanoplates, chain-like nanoplate assemblies, and nanobelts. J. Mater. Res. 2009, 24, 2200–2209. [Google Scholar] [CrossRef]
- Xin, J.; Yin, X.; Chen, S.; Wu, A. Synthesis of uniform and stable silver nanoparticles by a gold seed-mediated growth approach in a buffer system. J. Exp. Nanosci. 2012, 9, 382–390. [Google Scholar] [CrossRef]
- Park, C.-S.; Kim, D.H.; Shin, B.J.; Kim, D.Y.; Lee, H.-K.; Tae, H.-S. Conductive polymer synthesis with single-crystallinity via a novel plasma polymerization technique for gas sensor applications. Materials 2016, 9, 812. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Park, C.-S.; Kim, W.H.; Shin, B.J.; Hong, J.G.; Park, T.S.; Seo, J.H.; Tae, H.-S. Influence of guide-tube and bluff-body on advanced atmospheric pressure plasma source for single-crystlline polymer nanoparticle synthesis at low temperature. Phys. Plasmas 2017, 24, 023505. [Google Scholar] [CrossRef]
- Cobley, C.M.; Skrabalak, S.E.; Campbell, D.J.; Xia, Y. Shape-controlled synthesis of silver nanoparticles for plasmonic and sensing applications. Plasmonics 2009, 4, 171–179. [Google Scholar] [CrossRef]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-J.; Shin, J.-G.; Park, C.-S.; Kum, D.S.; Shin, B.J.; Kim, J.Y.; Park, H.-D.; Choi, M.; Tae, H.-S. In-Liquid Plasma Process for Size- and Shape-Controlled Synthesis of Silver Nanoparticles by Controlling Gas Bubbles in Water. Materials 2018, 11, 891. https://doi.org/10.3390/ma11060891
Kim H-J, Shin J-G, Park C-S, Kum DS, Shin BJ, Kim JY, Park H-D, Choi M, Tae H-S. In-Liquid Plasma Process for Size- and Shape-Controlled Synthesis of Silver Nanoparticles by Controlling Gas Bubbles in Water. Materials. 2018; 11(6):891. https://doi.org/10.3390/ma11060891
Chicago/Turabian StyleKim, Hyun-Jin, Jun-Goo Shin, Choon-Sang Park, Dae Sub Kum, Bhum Jae Shin, Jae Young Kim, Hyung-Dal Park, Muhan Choi, and Heung-Sik Tae. 2018. "In-Liquid Plasma Process for Size- and Shape-Controlled Synthesis of Silver Nanoparticles by Controlling Gas Bubbles in Water" Materials 11, no. 6: 891. https://doi.org/10.3390/ma11060891
APA StyleKim, H.-J., Shin, J.-G., Park, C.-S., Kum, D. S., Shin, B. J., Kim, J. Y., Park, H.-D., Choi, M., & Tae, H.-S. (2018). In-Liquid Plasma Process for Size- and Shape-Controlled Synthesis of Silver Nanoparticles by Controlling Gas Bubbles in Water. Materials, 11(6), 891. https://doi.org/10.3390/ma11060891



