Abstract
Photocatalysts have multiple applications in air purifiers, paints, and self-cleaning coatings for medical devices such as catheters, as well as in the elimination of xenobiotics. In this study, a coating of a UV-responsive photocatalyst, titanium dioxide (TiO2), was applied to an orthodontic resin. The antibacterial activity on oral bacteria as well as hydrophilic properties and mechanical properties of the TiO2-coated resin were investigated. ultraviolet A (UVA) (352 nm) light was used as the light source. Antibacterial activity was examined with or without irradiation. Measurements of early colonizers and cariogenic bacterial count, i.e., colony forming units (CFU), were performed after irradiation for different time durations. Hydrophilic properties were evaluated by water contact angle measurements. While, for the assessment of mechanical properties, flexural strength was measured by the three-point bending test. In the coat(+)light(+) samples the CFU were markedly decreased compared to the control samples. Water contact angle of the coat(+)light(+) samples was decreased after irradiation. The flexural strength of the specimen irradiated for long time showed a higher value than the required standard value, indicating that the effect of irradiation was weak. We suggest that coating with the ultraviolet responsive photocatalyst TiO2 is useful for the development of orthodontic resin with antimicrobial properties.
1. Introduction
Maintenance of good oral hygiene after an active orthodontic treatment is one of the most important procedures. In general, after an active orthodontic treatment, moved teeth and jawbone are retained by acrylic resin based retainer [1]. The retainer is typically used for at least two years. The longer the retainer is used, the more unhygienic it becomes. Gradually, micro-organisms colonize on the surfaces of retainers, just as on dentures, and they often cause stomatitis, dental caries, periodontal disease, chronic atrophic or candidiasis [2,3,4,5,6,7,8,9,10,11]. Porosities on the outer and inner surfaces of retainer also provide favorable conditions for microbial colonization [12]. Therefore, prevention of micro-organism colonization is essential for the maintenance of good oral hygiene and prevention of oral diseases.
Management of oral biofilm is important to maintain a good oral status. Common oral diseases, dental caries and periodontitis, are caused by an imbalance between biofilms and host defenses [13]. The initial process in the oral biofilm formation starts with pellicle, covering the tooth surface within a few minutes after mechanical cleaning. The pellicle plays a major role in the development and maintenance of bacterial communities. Then, the complex bacterial communities develop on the pellicle within a few days, and their components can be divided into two categories—early colonizers, and late colonizers [14]. Early colonizers that directly adhere to the pellicles are predominantly streptococci [15]. Streptococci constitute 60% to 90% of the bacteria that colonize on the teeth in the first 4 h after professional cleaning [16]. These species are mainly Gram-positive and have minor pathogenic effects on periodontal tissue. Late colonizers, such as Fusobacterium nucleatum, Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola, and Aggregatibacter actinomycetemcomitans tend to be more pathogenic than the early colonizers. The late colonizers alone cannot form a biofilm on the tooth surface, but they form a biofilm by their parasitical adherence to the early colonizers [14].
In the formation of a biofilm it is inevitable that colonizing bacteria primarily adhere to the surface of the retainer [17]. Therefore, it is critical to prevent the bacteria from adhering to the retainers. As the orthodontic acrylic resin and denture base acrylic resin have similar requirements for clinical use (ISO 20795), the results for the denture base resin are also applicable for orthodontic acrylic resin. Various approaches have been employed for the prevention of microbial biofilms. These include dental disinfection, denture cleaner, mixing resin with antibacterial agents, and coating resin with antiseptic [6,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32]. However, the biofilms are resistant to antibacterial and antifungals agents [33,34]. Moreover, a long-term use of denture cleanser corrodes metals such as clasps [35,36]. Denture cleanser affects the color stability of the denture base acrylic resin [37]. Mechanical cleaning with the adjunctive use of antimicrobial solutions is helpful in reducing biofilm growth or preventing its formation. However, such approaches rely primarily on a patient’s compliance, and may be compromised in pediatric, geriatric, and handicapped individuals. Silver nanoparticles impregnated in acrylic resin make the appliance antibacterial, but releasing silver nanoparticles from the resin is a limiting factor [24,25,27,31]. Incorporating fluorine and silver ions into resin elutes antimicrobials but it is available only for the first few weeks [27]. Moreover, elution of antimicrobial agents may result in deterioration of the mechanical properties of the retainer over time. This reduction renders the appliance more susceptible to fracture, due to its low resistance to impact, low flexural strength, or low fatigue strength [38]. Hence, novel and alternative methods to prevent the micro-organism colonization are required.
Application of a photocatalyst is one of the effective and safe approaches to remove biofilm from the dentures or retainer [39,40]. Photocatalyst reaction is defined as a photocatalyst promoted reaction on a solid surface, usually a semiconductor [41]. Titanium dioxide (TiO2) is the most studied photocatalyst [39,41,42,43,44,45,46,47,48,49,50]. TiO2 is biocompatible, nontoxic, and inexpensive. TiO2 generates reactive oxygen species (ROSs) upon ultraviolet A (UVA) irradiation, and its strong oxidative power decomposes micro-organisms and organic materials [33,42,43,44,46,50]. Further, the photocatalyst reaction can obtain superhydrophilic properties by UV irradiation, and superhydrophilicity prevents dirt accumulation on the device.
The purpose of the present study was to test the clinical applications TiO2 coated orthodontic resin based retainer. To this end, we investigated the antibacterial effects as well as mechanical, and hydrophilic properties of acrylic based orthodontic resin coated with the photocatalyst TiO2 after irradiation with UVA. Thus, we determined its clinical suitability for use as an orthodontic retainer material. First, we examined the effect of the orthodontic resin coated with TiO2 on bacteria for various irradiation durations. Further, we investigated the antimicrobial effect against early colonizers, the bacteria which are first attached to the appliances. The effects on S. mutans and S. sobrinus, which are the most well-known cariogenic bacteria, were investigated. Second, mechanical properties of the orthodontic resin are evaluated by irradiating for about 2 years, which is the recommended usage period of the orthodontic retainer. Third, the hydrophilic properties were investigated as one of the photocatalytic effects. Acquiring hydrophilic properties by decreasing the contact angle could lead to the prevention of bacterial adhesion and a self-cleaning function.
2. Materials and Methods
Autopolymerizable orthodontic acrylic resin (Ortho Crystal, Nissin Co., Tokyo, Japan), which consisted of a liquid component and a powder component, was used for this study. In the following sections, autopolymerizable orthodontic acrylic resin are referred to as ‘resin’.
2.1. Sample Preparation
According to the manufacturer’s instructions, a powder-to-liquid ratio of 10 g:4.5 mL was used. Powder and liquid components were mixed under vibration for homogenization and removal of the trapped air. The slurry was poured into an aluminum open mold and pressed using a pair of glass plates to fabricate the specimens of different dimensions: 50 mm × 50 mm × 3.0 mm (n = 60), 50 mm × 50 mm × 3.0 mm (n = 20), and 64 mm × 10 mm × 3.5 mm (n = 35) for the antibacterial properties tests, photoinduced hydrophilic tests, and mechanical properties tests, respectively. The slurry resin was immediately transferred in the polymerization equipment for a dental technique at 40 °C (manufacturer’s recommendation: 30–40 °C), and 0.25 MPa for 30 min to enhance curing (Fit Resin Multicure, Shofu Inc., Kyoto, Japan). Following preparation, each specimen was kept at room temperature in water for 12 h to eliminate the residual monomer. All test specimens were gradually grinded with waterproof polishing paper, having a grain size of approximately 30 μm (P500), 18 μm (P1000), and 15 μm (P1200). The specimens were then divided into four test groups for the assessment of the antibacterial properties and water contact angle measurement of base resin coated with thin film of photocatalytic TiO2. Uncoated resin and non-lighted resin were used as control groups for their respective experimental group.
A spin-coating methods was used to apply ultraviolet-light-responsive photocatalytic titanium dioxide (UV-TiO2) to the surface of the materials. The surface modification of the specimen with commercialized photocatalytic TiO2 (NRC 350A and 360C, Nippon Soda Co., Ltd., Tokyo, Japan) was carried out by a sol-gel thin film spin-coating method according to the manufacturer’s instructions. NRC 350A was coated to the surface of the materials. After coating, they were dried in a desiccator under 30 °C for 48 h. Then NRC 360Cwas coated, and dried in a desiccator in the same way. After the coating, the surface conditions of the materials were observed by a scanning electron microscope (SEM; JSM-5600LV, JEOL, Tokyo, Japan) at an accelerating voltage of 15 kV. Specimens were sputter-coated with Au prior to the SEM observations.
We covered samples with a glass to prevent drying, and irradiation was carried out from above. UVA from a black light source (wavelength: 352 nm, FL15BLB, Toshiba Co., Tokyo, Japan) was selected as the light source for catalytic excitation. The irradiation was performed at a distance of 10 cm (1.0 mW/cm2 under the glass).
2.2. Bacterial Strains
Streptococcus mutans ATCC 25175 (S. mutans), Streptococcus sobrinus ATCC33478 (S. sobrinus), Streptococcus gordonii ATCC 10558 (S. gordonii), Streptococcus oralis ATCC 35037 (S. oralis ATCC), Streptococcus oralis GTC 276 (S. oralis GTC), Streptococcus sanguinis ATCC 10556 (S. sanguinis), and Streptococcus mitis MRS 08-31 (S. mitis) were used in this study. S. mutans and S. sobrinus are cariogenic bacteria. S. gordonii, S. oralis ATCC, S. oralis GTC, S. sanguinis, and S. mitis are the early colonizers. These bacterial species were inoculated into 4 mL of Tryptic Soy (TS) broth (Becton, Dickinson and Company, Sparks, MD, USA) and were cultured aerobically at 37 °C for 16 h. They were harvested by centrifugation at 3000× rpm for 15 min and then suspended with phosphate buffered saline (PBS) resulting in an optical density at 540 nm (OD540 equal to 1.0).
2.3. Antibacterial Test
A micro-organism suspension (500 µL) adjusted to OD540 = 1.0 was dropped directly on the surface of the coated and non-coated specimen on ice, and UV irradiation was performed for 0, 15, 30, 60, 90, 120, 150, and 180 min. After irradiation, each bacterial cell pellet was suspended in 1 mL PBS and subjected to serial 10-fold dilutions in PBS. The dilutions of each bacteria were inoculated on MS agar (Difico Mitis Salivarius Agar (semi-selective medium for streptococci); BD Biosciences, Flanklin Lakes, NJ, USA) in petri dishes with spiral plating equipment (Eddy Jet, IUL SA, Barcelona, Spain), and petri dishes were incubated under anaerobic conditions in an AnaeroPack-Anaero box (AnaeroPack System, Mitsubishi Gas Chemical Co., Inc., Tokyo, Japan) at 37 °C for 48 h. The number of colonies was counted in accordance with the spiral plater instruction manual. Each measurement was repeated three times.
2.4. Bending Test
The rectangular plates (64 mm × 10 mm × 3.5 mm) were immersed in distilled water at 37 °C for 0, 200, 400, 600, 800, 1000, 1200 h under UVA irradiation from a distance of 10 cm. The three-point bending test was conducted using a universal testing machine (EZ Test 500 N, Shimadzu Co., Kyoto, Japan) at a crosshead speed of 5 mm/min and a span length of 50.0 mm (n = 5). Then, load-displacement curves were plotted to measure bending strength, elastic modulus, and toughness. The test was conducted according to ISO20795-2 standard.
2.5. Water Contact Angle Measurement
The rectangular plates (50 mm × 50 mm × 3.0 mm) were prepared as indicated above and spin-coated with 125 μL of the experimental coating materials on each sample. The water contact angles were measured using a contact angle device (FTA125, First Ten Ångstroms, Portsmouth, VA, USA) at 25 °C. For surface analysis of the hydrophilic characteristics, 3.5 μL of deionized water (Milli-Q Plus system, Japan Millipore, Tokyo, Japan) was dropped on the surface, and video images were taken. Video images were automatically inputted to an attached computer in which the contact angles were measured using an image analysis program (FTA32 video, First Ten Ångstroms). Water contact angles were measured every 30 min for a period of 20 s at 25 °C.
2.6. Statistical Analysis
Antibacterial effects were analyzed by three factors: TiO2 coating, presence of UVA, and irradiated time (n = 3). The significance of differences in the antibacterial effects was examined using three-way ANOVA or two-way ANOVA. Mechanical properties were examined using one-way ANOVA, with irradiated time included as a factor (n = 5). Tukey’s HSD test was then used to determine the positions of significance. All statistical analyses were performed using IBM SPSS Statistics version 22.0 (IBM, Tokyo, Japan). A significance level of p < 0.05 was used.
3. Results
Antibacterial Test
The antimicrobial activity of the resin coated with TiO2 was examined by bacterial count of the early colonizers and cariogenic bacteria under UV irradiation. Figure 1 shows the antibacterial activity of the TiO2-coated resin surfaces against S. gordonii ATCC 10558, and S. oralis ATCC 35037 after irradiation. The coat(−)light(−) group was used as control group. The coat(+)light(−) group showed no significant differences compared with the control group. Hence, there was no effect with the coat alone. We also examined the activity with UV alone. Coat(−)light(+) induced a significant reduction in the number of colony. The number of S. gordonii was reduced from 1.6 × 107 colony-forming units/mL (CFU/mL) to 1.6 × 106 CFU/mL (after 120 min of UV irradiation), and that of S. oralis ATCC was reduced from 6.5 × 105 CFU/mL to 3.1 × 104 CFU/mL (after 90 min of UV irradiation). Consequently, about 90%, 95% colonies of S. gordonii and S. oralis ATCC were not formed on the coated plates upon irradiation (Figure 1A,B). On the other hand, coat(+)light(+) group showed a significant reduction in the CFU. The number of S. gordonii was reduced from 1.6 × 107 CFU/mL to 2.7 × 104 CFU/mL (after 120 min of UV irradiation) and that of S. oralis ATCC from 6.5 × 105 CFU/mL to 5.2 × 103 CFU/mL (after 90 min of UV irradiation). Thus, about 99.9% or more colonies of both S. gordonii and S. oralis ATCC were not formed on the coated plates upon irradiation (Figure 1A,B). Hence, coat(+)light(+) group showed a hundred times higher antibacterial effect, when compared with coat(−)light(+).
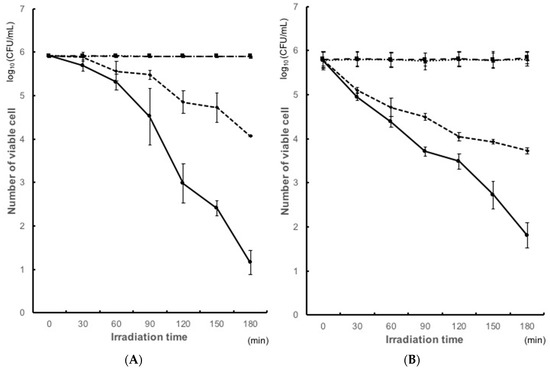
Figure 1.
Antibacterial effects of TiO2 photocatalysis against (A) Streptococcus gordonii; (B) Streptococcus oralis ATCC.  coat(+)light(+): experiment group containing powdered TiO2 with irradiation.
coat(+)light(+): experiment group containing powdered TiO2 with irradiation.  coat(−)light(−): control group without both TiO2 and irradiation.
coat(−)light(−): control group without both TiO2 and irradiation.  coat(+)light(−): experiment group in the presence of powdered TiO2 without irradiation.
coat(+)light(−): experiment group in the presence of powdered TiO2 without irradiation.  coat(−)light(−): experiment group without TiO2, but with irradiation.
coat(−)light(−): experiment group without TiO2, but with irradiation.
 coat(+)light(+): experiment group containing powdered TiO2 with irradiation.
coat(+)light(+): experiment group containing powdered TiO2 with irradiation.  coat(−)light(−): control group without both TiO2 and irradiation.
coat(−)light(−): control group without both TiO2 and irradiation.  coat(+)light(−): experiment group in the presence of powdered TiO2 without irradiation.
coat(+)light(−): experiment group in the presence of powdered TiO2 without irradiation.  coat(−)light(−): experiment group without TiO2, but with irradiation.
coat(−)light(−): experiment group without TiO2, but with irradiation.
Compared with the TiO2-noncoated samples, TiO2-coated samples showed a rapid decrease in the level of CFU of Streptococcus gordonii, particularly in the early stages (90 min and 120 min) of irradiation. Therefore, other strains were also examined at 90 and 120 min (Figure 2A,B). The cell viability on each sample before UV irradiation was set as 100%. The coat(+)light(+) clearly showed a great reduction in the cell viability. Cell viability was reduced to 0.2% for S. sobrinus, 0.9% for S. oralis GTC, 5.4% for S. mutans, and 9.9% for S. sanguinis. Clearly, the coat(+)light(+) samples exhibited the best antimicrobial performance in all microbes after 90 min UV irradiation. Similar results were observed even after 120 min of irradiation.
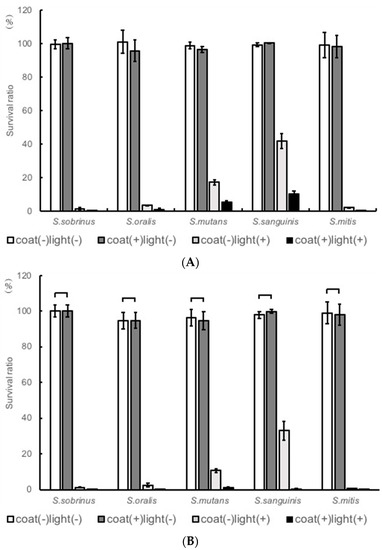
Figure 2.
Antibacterial effects on various specimens of Streptococcus sobrinus, Streptococcus oralis GTC, Streptococcus mutans, and Streptococcus sanguinis at (A) 90 min, and (B) 120 min of ultraviolet A (UVA) irradiation.
Table 1 shows explanatory variables related to bacterial counts by three way ANOVA. The coefficients of bacterial counts in light(+) and interaction of light(+) and coat(+) were all negative. The coefficients of light(+) for S. oralis and S. sobrinus were higher than those by interaction of light(+) and coat(+). In contrast, the coefficients of light(+) of S. gordonii, S. mutans, and S. sanguinis were higher than those by interaction of light(+) and coat(+). The coefficient of both light(+) and interaction of light(+) and coat(+) of S. mutans was the highest in all the bacteria. The coefficient for irradiation time of 120 min were significantly different in all bacterial species. In all the bacteria, the coefficient of coat(+) was almost 0. On the other hand, the coefficient of light(+) and interaction of light(+) and coat(+) were statistically significant. These results indicated that the antibacterial effect of the photocatalyst was exerted by UVA irradiation. It also showed differences in susceptibility of oral bacteria to UVA.

Table 1.
Models of three way ANOVA for changes in bacterial counts. p-values less than 0.05 were considered statistically significant.
SEM was used to observe the cross section of coating; cross sectional photographs are shown in Figure 3.
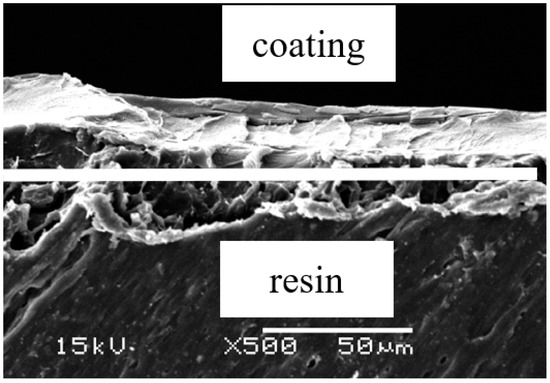
Figure 3.
Scanning electron microscopy of cross sectional photographs of the TiO2 coating.
The Flexural strength (Fs) and flexural modulus (Fm) of resin plates after UV irradiation are shown in Figure 4A,B, respectively. There was no difference between the irradiated test pieces, and all irradiated specimens fulfilled the requirements of the ISO 20795-2:2010 standard for Fs testing after 1200 h of UV irradiation (>65 MPa) (Figure 4A).
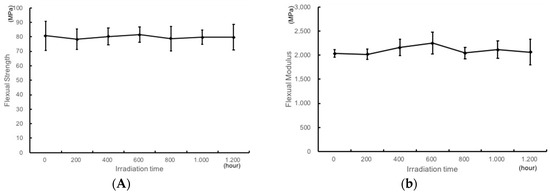
Figure 4.
Flexural strength (A) and Flexural modulus (B) of the TiO2-coated resin plates upon UV irradiation.
In the same way, all irradiated TiO2-coated specimens fulfilled the requirements of the ISO 20795-2:2010 standard for Fm testing after 1200 h of UV irradiation (>2000 MPa) (Figure 4B).
Figure 5A shows the water contact angle for TiO2-noncoated groups and TiO2-coated groups. Figure 5B shows the images illustrating the wettability of water on TiO2-coated specimens or TiO2-noncoated specimens after 120 min of UV irradiation.
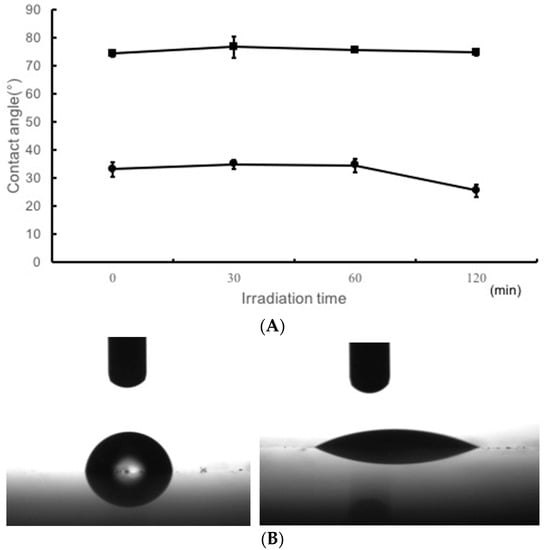
Figure 5.
(A) Water contact angle of the resin plates with TiO2-coating upon UV irradiation for 0, 30, 60, and 120 min. (B) Image shows contact angle of a water droplet on non-coated (left) and TiO2-coated resin plate (right) after 120 min of UV irradiation.
The water contact angle of TiO2-coated specimen was very small from the start of experiment, compared to that of TiO2-noncoated and it gradually decreased with time.
4. Discussion
This study demonstrates the antibacterial effects of TiO2-coating on S. mutans, S. sobrinus, and early colonizers upon UVA irradiation. When TiO2-coating and UVA were used together, a significant reduction in the microbial count was observed. In a previous study, TiO2 coated orthodontic arch wires showed the photocatalytic antibacterial effects on S. mutans, and its reduction rate was more than 99.99% by bacterial count after 1 h irradiation [51]. Also, a tissue conditioner containing a TiO2 photocatalyst decreased bacterial counts for Escherichia coli (about 90%), Staphylococcus aureus (>99.99%), and S. mutans (about 90%) after 2 h of irradiation [52]. Moreover, the photocatalytic antibacterial effects of metal specimens coated with two crystalline forms of TiO2 by thermal and anodic oxidation decreased bacterial counts for S. mutans (about 90%) after 60 min irradiation [43]. Furthermore, titanium disks coated with anatase-rich titanium dioxide (TiO2) reduced amount of viable cells of S. oralis by 40% after 24 h UVA exposure [53]. Interestingly, anodized titanium (AO) decreased survival ratio of S. sanguinis (70%) upon 2 h of UV irradiation [50]. Also, after 20 min of UV exposure to TiO2 surfaces, viabilities of S. mutans were reduced by 65% [54]. There were a few reports on S. gordonii and S. sobrinus. Finally, our results showed the bacterial count reduction rate of S. gordonii (>90%) (Figure 1), S. mutans (87%), S. sobrinus (>99%), S. oralis (98%), S. sanguinis (90%), and S. mitis (>99%) (Figure 2) after 90 min irradiation. The reduction rates of S. oralis, and S. sanguinis were better than those of past study [50,53]. The reduction rate of S. mutans was similar as previous studies [43,52,53,54]. However, a greater reduction rates of bacteria than those in our study were reported [51]. Although factors that contribute to the difference in the reduction rate are unknown, this may be due to the difference in the components of the photocatalyst used. This may also be due to the difference in the surface properties of the coating. Similar to the previous reports, the photocatalytic reaction induced a relatively mild decrease in the bacterial counts after the first 20 min of irradiation and showed a rapid decrease upon subsequent irradiation (Figure 1A) [55]. There is a difference in reaction by bacteria, but only upon irradiation for at least 90 min. Also, compared to conventional cleaning methods, cleaning is facilitated by coating TiO2. This may lead to improvement in patient’s compliance, reduced cost for equipment cleaning, and prevention of unpleasant odors.
UVA irradiation alone showed a decrease in bacterial counts. Furthermore, the photocatalytic activities of TiO2 coating decreased significantly in bacterial counts of S. gordonii, S. oralis ATCC 35037 (Figure 1), S. sobrinus, S. mutans, S. oralis GTC, S. sanguinis, and S. mitis (Figure 2). When uncoated resins were irradiated with UVA light, all the bacteria reduced in counts. These reductions in the light(+)coat(−) groups were expected outcomes and followed a similar trend as a previous study, which reported that the viability of S. mutans decreased significantly after 60 min of UVA irradiation as compared to the control which was not irradiated [43]. The decrease may be associated with the cell-damaging effect of UVA. Coat(+)light(+) group showed a higher antibacterial effect, as compared to the coat(−)light(+) group. The difference of antibacterial effect between these group was explained in the Table 1.
The primary step in photocatalytic decomposition consists of hydroxyl radical attack on the bacterial cell wall [48]. This leads to increased permeability which allows radicals to reach and damage the cytoplasmic membrane causing lipid peroxidation and thereby causing membrane disorder [48]. The antibacterial effect of TiO2 is associated to this disorder of cytoplasmic membrane [48,56]. We propose that the observed bacterial type-dependent variation in the antimicrobial effects may be due to differential effects of hydroxyl radicals on distinct bacterium species [57,58]. The antibacterial effect of the TiO2 coating for various organisms is determined primarily by the complexity and density of the cell walls, as well as by the types of micro-organisms [59].
In this study, we observed that UVA irradiation has antibacterial effect. In cariogenic bacteria, S. mutans was more resistant to UVA than S. sobrinus. This may be because of the higher GC content of S. sobrinus than S. mutans. It has been proposed that species with genomes exhibiting a high GC content are more susceptible to UV-induced mutagenesis [60]. Also, our results showed that the coefficients for S. sanguinis and S. gordonii in light(+) treatment were low. Consistently, it has been demonstrated that S. sanguinis and S. gordonii have higher resistance to H2O2 [61].
In all bacteria, S. mutans was the most resistant to UVA. Conversely, S. sobrinus and S. oralis were highly susceptible to UVA. Several factors may contribute to the cause of these variable responses to UVA among species. For instance, the production of various ROSs involved in inducing UV damage may vary among species. In addition, the method of defending and repairing DNA damage differs among bacteria [61,62,63]. Also, the oxidative damage to biomolecules and counteracting protective mechanisms underlie the variability in UVA sensitivity among different bacterial species [64]. However, the reason of higher sensitivity of S. oralis and S. sobrinus to UV irradiation is unknown (Table 1). Further experiments are needed to understand the mechanistic basis for the variable susceptibility of oral bacteria to UVA.
In general, orthodontic patients are young, and oral care is a major problem for these patients. S. mutans and S. sobrinus are the most harmful cariogenic bacteria and TiO2 coating have shown to be effective against them.
Bacteria form biofilms on the surface of the device. During biofilm formation, adherent bacteria produce a polymer matrix in which the community becomes embedded and biofilm bacteria are notoriously resistant to antimicrobial substances [65]. Once they are established on the exposed surface of a dental device, removal of biofilms can be extremely difficult. Effective methodology for cleaning of dental device is not well established. Application of photocatalyst is considered one of the potential strategy to overcome these problems. Therefore, further study is necessary for understanding the effects of TiO2 on various organisms not only in vitro conditions but also in vivo and intraoral conditions.
With regard to flexural strength, there was no significant difference in bending strength even after irradiation for a long time. UV irradiated specimens fulfilled the requirements of the ISO 20795-2 standard for Fs testing (Figure 4A). Similarly, regarding strength modulus, there was no difference in bending strength even after irradiation for a long time (Figure 4B). These data imply that the resin can withstand irradiation for a long time to ensure a long-term clinical use of orthodontics. Even when irradiating for about 2 years, which is the recommended use period of the retainer, the durability was satisfactory. It was shown that clinical application is achievable.
Moreover, TiO2-coating improved the hydrophilic properties of the surface of the denture base acrylic resin (Figure 5A,B). A previous study reported that the water contact angle of surfaces in TiO2-coated resin was 68.1 ± 3.4 degrees [66]. However, TiO2-coating makes the resin surface more hydrophilic, with a water contact angle of 25.4 ± 2.1 degrees (Figure 5). It has been reported that TiO2 coating applied to acrylic resin inhibits the adhesion of S. sanguinis and C. albicans organisms [67,68]. In this study, enhancement in the hydrophilic properties of acrylic resin based orthodontic resin surface suppresses the adhesion of early colonizer, the subsequent adhesion of other microbes, which could reduce the total number of microbes adhering to orthodontic resin. Suppression of early colonizer could reduce further bacterial adhesion thereby reducing the risk of systemic disease. Moreover, improvement in the hydrophilic properties of orthodontic resin surface can suppress adhesion of other dirt such as food debris. Even without irradiation, the coat(+) group showed higher hydrophilicity making it easier to remove dirt.
We would like to establish the novel home care method for orthodontic retainer, with use of TiO2-coating and UV irradiation. As one of the clinical applications, patients place the retainer under the UV lamp and they can also easily clean it at home. This cleaning methods is very simple. In addition, cleaning up the device can be carried out by a professional at the time of visit to the clinic. Our method can be applied not only to a retainer but also to other orthodontic appliances (expansion plate, functional orthodontic appliance), as well as to denture base and occlusal splint.
One of the important aspect of our method is biocompatibility. It has been reported that in animals the TiO2-coated resin has no irritation to the oral mucosa, nor does it cause skin sensitization. Any elution of components from the coating has no deleterious effects on the tissues [69].
Overall, we demonstrate that the TiO2-coated resin exposed to UVA irradiation shows great reduction of microbial counts when compared with uncoated and coated without UVA-exposed samples. In addition, the durability of the specimen showed a higher value than the required standard value, indicating that the effect of irradiation was small. In conclusion, the results of this preliminary study suggest that the antibacterial effect of TiO2-coated resin can be beneficial in long-lasting orthodontic treatments.
5. Conclusions
The antimicrobial activity of the resin coated with TiO2 was examined by bacterial count of the early colonizers and cariogenic bacteria under UV irradiation. The results of present study suggest that coating with the ultraviolet responsive photocatalyst TiO2 is useful for antimicrobial properties of removable orthodontic resin based retainer.
Author Contributions
A.K., Y.N., T.O., T.S., R.N., T.H., H.K., Y.N., and N.H. conceived of and designed the experiments; A.K. and T.S. performed the experiments; A.K. and Y.N. analyzed the data; Y.N., T.O., R.N., and T.H. contributed materials/analysis tools; A.K., Y.N., T.O., and Y.N. wrote the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors are grateful to Nihon Sotatu Co., for supplying the TiO2 coating.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Littlewood, S.J.; Millett, D.T.; Doubleday, B.; Bearn, D.R.; Worthington, H.V. Retention procedures for stabilising tooth position after treatment with orthodontic braces. Cochrane Database Syst. Rev. 2006, 51, 94–95. [Google Scholar]
- Batoni, G.; Pardini, M.; Giannotti, A.; Ota, F.; Giuca, M.R.; Gabriele, M.; Campa, M.; Senesi, S. Effect of removable orthodontic appliances on oral colonisation by mutans streptococci in children. Eur. J. Oral Sci. 2001, 109, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Bjerklin, K.; Gärskog, B.; Rönnerman, A. Proximal caries increment in connection with orthodontic treatment with removable appliances. Br. J. Orthod. 1983, 10, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Hibino, K.; Wong, R.W.; Hagg, U.; Samaranayake, L.P. The effects of orthodontic appliances on candida in the human mouth. Int. J. Paediatr. Dent. 2009, 19, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Kiyoko, T.; Kazuhiko, N.; Sonoko, M.; Atsuko, T.; Takashi, O. Clinical and microbiological evaluations of acute periodontitis in areas of teeth applied with orthodontic bands. Pediatr. Dent. J. 2005, 15, 212–218. [Google Scholar]
- Kuroki, K.; Hayashi, T.; Sato, K.; Asai, T.; Okano, M.; Kominami, Y.; Takahashi, Y.; Kawai, T. Effect of self-cured acrylic resin added with an inorganic antibacterial agent on Streptococcus mutans. Dent. Mater. J. 2010, 29, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Madurantakam, P.; Kumar, S. Fixed and removable orthodontic retainers and periodontal health. Evid. Based Dent. 2017, 18, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Tomsett, K.; Wickes, B.L.; López-Ribot, J.L.; Redding, S.W. Denture stomatitis: A role for candida biofilms. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 98, 53–59. [Google Scholar] [CrossRef]
- Turkoz, C.; Canigur Bavbek, N.; Kale Varlik, S.; Akca, G. Influence of thermoplastic retainers on Streptococcus mutans and Lactobacillus adhesion. Am. J. Orthod. Dentofac. Orthop. 2012, 141, 598–603. [Google Scholar] [CrossRef] [PubMed]
- Zharmagambetova, A.; Tuleutayeva, S.; Akhmetova, S. Microbiological aspects of the orthodontic treatment. Georgian Med. News 2017, 264, 39–43. [Google Scholar]
- Zingler, S.; Pritsch, M. Association between clinical and salivary microbial parameters during treatment with removable orthodontic appliances with or without use of fluoride mouth rinse. Eur. J. Paediatr. Dent. 2016, 17, 181–187. [Google Scholar] [PubMed]
- Shay, K. Denture hygiene: A review and update. J. Contemp. Dent. Pract. 2000, 1, 28–41. [Google Scholar] [PubMed]
- Song, W.S.; Lee, J.K.; Park, S.H.; Um, H.S.; Lee, S.Y.; Chang, B.S. Comparison of periodontitis-associated oral biofilm formation under dynamic and static conditions. J. Periodontal Implant Sci. 2017, 47, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Andersen, R.N.; Blehert, D.S.; Egland, P.G.; Foster, J.S.; Palmer, R.J. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 486–505. [Google Scholar] [CrossRef] [PubMed]
- Teles, F.R.; Teles, R.P.; Sachdeo, A.; Uzel, N.G.; Song, X.Q.; Torresyap, G.; Singh, M.; Papas, A.; Haffajee, A.D.; Socransky, S.S. Comparison of microbial changes in early redeveloping biofilms on natural teeth and dentures. J. Periodontol. 2012, 83, 1139–1148. [Google Scholar] [CrossRef] [PubMed]
- Nyvad, B.; Kilian, M. Microbiology of the early colonization of human enamel and root surfaces in vivo. Scand. J. Dent. Res. 1987, 95, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Verran, J.; Motteram, K.L. The effect of adherent oral streptococci on the subsequent adherence of candida albicans to acrylic in vitro. J. Dent. 1987, 15, 73–76. [Google Scholar] [CrossRef]
- Da Silva, P.M.; Acosta, E.J.; Pinto Lde, R.; Graeff, M.; Spolidorio, D.M.; Almeida, R.S.; Porto, V.C. Microscopical analysis of candida albicans biofilms on heat-polymerised acrylic resin after chlorhexidine gluconate and sodium hypochlorite treatments. Mycoses 2011, 54, e712–e717. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, I.M.; Cruz, P.C.; da Silva, C.H.; de Souza, R.F.; Paranhos Hde, F.; Candido, R.C.; Marin, J.M.; de Souza-Gugelmin, M.C. Effervescent tablets and ultrasonic devices against candida and mutans streptococci in denture biofilm. Gerodontology 2011, 28, 264–270. [Google Scholar] [CrossRef] [PubMed]
- De Freitas Fernandes, F.S.; Pereira-Cenci, T.; da Silva, W.J.; Ricomini Filho, A.P.; Straioto, F.G.; Cury, A.A.D.B. Efficacy of denture cleansers on Candida spp. Biofilm formed on polyamide and polymethyl methacrylate resins. J. Prosthet. Dent. 2011, 105, 51–58. [Google Scholar] [CrossRef]
- De Souza, R.F.; de Freitas Oliveira Paranhos, H.; Lovato da Silva, C.H.; Abu-Naba’a, L.; Fedorowicz, Z.; Gurgan, C.A. Interventions for cleaning dentures in adults. Cochrane Database Syst. Rev. 2009, CD007395. [Google Scholar] [CrossRef] [PubMed]
- Dhamande, M.M.; Pakhan, A.J.; Thombare, R.U.; Ghodpage, S.L. Evaluation of efficacy of commercial denture cleansing agents to reduce the fungal biofilm activity from heat polymerized denture acrylic resin: An in vitro study. Contemp. Clin. Dent. 2012, 3, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Farhadian, N.; Usefi Mashoof, R.; Khanizadeh, S.; Ghaderi, E.; Farhadian, M.; Miresmaeili, A. Streptococcus mutans counts in patients wearing removable retainers with silver nanoparticles vs. those wearing conventional retainers: A randomized clinical trial. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, D.R.; Gorup, L.F.; Takamiya, A.S.; de Camargo, E.R.; Filho, A.C.; Barbosa, D.B. Silver distribution and release from an antimicrobial denture base resin containing silver colloidal nanoparticles. J. Prosthodont. 2012, 21, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Oei, J.D.; Zhao, W.W.; Chu, L.; DeSilva, M.N.; Ghimire, A.; Rawls, H.R.; Whang, K. Antimicrobial acrylic materials with in situ generated silver nanoparticles. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Regis, R.R.; Zanini, A.P.; Della Vecchia, M.P.; Silva-Lovato, C.H.; Oliveira Paranhos, H.F.; de Souza, R.F. Physical properties of an acrylic resin after incorporation of an antimicrobial monomer. J. Prosthodont. 2011, 20, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Shinonaga, Y.; Arita, K. Antibacterial effect of acrylic dental devices after surface modification by fluorine and silver dual-ion implantation. Acta Biomater. 2012, 8, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Sodagar, A.; Kassaee, M.Z.; Akhavan, A.; Javadi, N.; Arab, S.; Kharazifard, M.J. Effect of silver nano particles on flexural strength of acrylic resins. J. Prosthodont. Res. 2012, 56, 120–124. [Google Scholar] [CrossRef] [PubMed]
- Sousa, F.A.; Paradella, T.C.; Koga-Ito, C.Y.; Jorge, A.O. Effect of sodium bicarbonate on candida albicans adherence to thermally activated acrylic resin. Braz. Oral Res. 2009, 23, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.P.; Senna, P.M.; Silva, W.J.; Del Bel Cury, A.A. Long-term efficacy of denture cleansers in preventing Candida spp. Biofilm recolonization on liner surface. Braz. Oral Res. 2010, 24, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Wady, A.F.; Machado, A.L.; Zucolotto, V.; Zamperini, C.A.; Berni, E.; Vergani, C.E. Evaluation of candida albicans adhesion and biofilm formation on a denture base acrylic resin containing silver nanoparticles. J. Appl. Microbiol. 2012, 112, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Wolff, M.S.; Larson, C. The cariogenic dental biofilm—Good, bad or just something to control? Braz. Oral. Res. 2009, 23, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Chandra, J.; Mukherjee, P.K.; Leidich, S.D.; Faddoul, F.F.; Hoyer, L.L.; Douglas, L.J.; Ghannoum, M.A. Antifungal resistance of candidal biofilms formed on denture acrylic in vitro. J. Dent. Res. 2001, 80, 903–908. [Google Scholar] [CrossRef] [PubMed]
- Jagger, D.C.; Harrison, A. Denture cleaning the best approach. Br. Dent. J. 1995, 178, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Budtz-Jørgensen, E. Materials and methods for cleaning dentures. J. Prosthet. Dent. 1979, 42, 619–623. [Google Scholar] [CrossRef]
- Fitjer, L.C.; Jonas, I.E.; Kappert, H.F. Corrosion susceptibility of lingual wire extensions in removable appliances. An in vitro study. J. Orofac. Orthop. 2002, 63, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Murata, H.; Li, Y.; Sadamori, S.; Hamada, T. Influence of denture cleansers on the color stability of three types of denture base acrylic resin. J. Prosthet. Dent. 2009, 10, 205–213. [Google Scholar] [CrossRef]
- Rantala, L.I.; Lastumäki, T.M.; Peltomäki, T.; Vallittu, P.K. Fatigue resistance of removable orthodontic appliancereinforced with glass fibre weave. J. Oral Rehabil. 2003, 30, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Yamada, M.; Ueda, T.; Sakurai, K. Reduction of biofilm formation on titanium surface with ultraviolet-c pre-irradiation. J. Biomater. Appl. 2014, 29, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Tomomi, S.; Susumu, I.; Tsuyoshi, O.; Hiroyuki, K.; Yusuke, M.; Nobuhiro, H.; Yoshiki, N. Evaluation of antibacterial activity of visible light-responsive TiO2-based photocatalyst coating on orthodontic materials against cariogenic bacteria. Asian Pacfic. J. Dent. 2016, 16, 15–22. [Google Scholar]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Chung, H.; Choi, W.; Yoon, J. Linear correlation between inactivation of E. coli and oh radical concentration in TiO2 photocatalytic disinfection. Water Res. 2004, 38, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Chung, C.J.; Oh, K.T.; Choi, Y.J.; Kim, K.H. Photocatalytic antibacterial effect of TiO2 film of tiag on streptococcus mutans. Angle Orthod. 2009, 79, 528–532. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, K.H.; Choy, K.C.; Oh, K.T.; Kim, K.N. Photocatalytic antibacterial effect of TiO2 film formed on ti and tiag exposed to lactobacillus acidophilus. J. Biomed. Mater. Res. B Appl. Biomater. 2007, 80, 353–359. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, T.; Fujishima, A. Photoelectrochemical properties of TiO2 photocatalyst and its applications for environmental purification. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 247–262. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Hosseinpour, S.; Tang, F.; Wang, F.; Livingstone, R.A.; Schlegel, S.J.; Ohto, T.; Bonn, M.; Nagata, Y.; Backus, E. Chemisorbed and physisorbed water at the TiO2/water interface. J. Phys. Chem. Lett. 2017, 8, 2195–2199. [Google Scholar] [CrossRef] [PubMed]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Studies on photokilling of bacteria on TiO2 thin film. J. Photochem. Photobiol. A Chem. 2003, 156, 227–233. [Google Scholar] [CrossRef]
- Takeuchi, M.; Sakamoto, K.; Martra, G.; Coluccia, S.; Anpo, M. Mechanism of photoinduced superhydrophilicity on the TiO2 photocatalyst surface. J. Phys. Chem. B 2005, 109, 15422–15428. [Google Scholar] [CrossRef] [PubMed]
- Unosson, E.; Tsekoura, E.K.; Engqvist, H.; Welch, K. Synergetic inactivation of staphylococcus epidermidis and streptococcus mutansin a TiO2/H2O2/uv system. Biomatter 2013, 3, e26727. [Google Scholar] [CrossRef] [PubMed]
- ÖZyildiz, F.; Uzel, A.; Hazar, A.S.; GÜDen, M.; ÖLmez, S.; Aras, I.; Karaboz, İ. Photocatalytic antimicrobial effect of TiO2 anatase thin-film–coated orthodontic arch wires on 3 oral pathogens. Turkish J. Biol. 2014, 38, 289–295. [Google Scholar] [CrossRef]
- Uchimaru, M.; Sakai, T.; Moroi, R.; Shiota, S.; Shibata, Y.; Deguchi, M.; Sakai, H.; Yamashita, Y.; Terada, Y. Antimicrobial and antifungal effects of tissue conditioners containing a photocatalyst. Dent. Mater. J. 2011, 30, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Westas, E.; Hayashi, M.; Cecchinato, F.; Wennerberg, A.; Andersson, M.; Jimbo, R.; Davies, J.R. Bactericidal effect of photocatalytically-active nanostructured TiO2 surfaces on biofilms of the early oral colonizer, streptococcus oralis. J. Biomed. Mater. Res. A 2017, 105, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Han, J.; Lim, B.; Lim, Y. Comparison of ultraviolet light-induced photocatalytic bactericidal effect on modified titanium implant surfaces. Int. J. Oral Maxillofac. Implants 2011, 26, 39–44. [Google Scholar] [PubMed]
- Kayano, S.; Yoshihiko, K.; Kazuhito, H.; Akira, F. Batericidal and detoxification effects of TiO2 film photocatalysts. Environ. Sci. Technol. 1998, 32, 726–728. [Google Scholar]
- Saito, T.; Iwase, T.; Horie, J.; Morioka, T. Mode of photocatalytic bactericidal action of powdered semiconductor TiO2 on mutans streptococci. J. Photochem. Photobiol. B 1992, 14, 369–379. [Google Scholar] [CrossRef]
- Dongari, A.I.; Miyasaki, K.T. Sensitivity of actinobaciiius actinomycetemcomitans and haemophiius apiiropiiiius to oxicjative killing. Oral Microbiol. Immunol. 1991, 6, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Jagger, J. Near-uv radiation effects on microorganisms. Photochem. Photobiol. 1981, 34, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Maness, P.; Smolinski, S.; Blake, D.; Huang, Z.; Wolfrum, E.; Jacoby, W. Bactericidal activity of photocatalytic TiO2 reaction: Toward an understanding of its killing mechanism. Appl. Environ. Microbiol. 1999, 65, 4094–4098. [Google Scholar] [PubMed]
- Matallana-Surget, S.; Meador, J.; Joux, F.; Douki, T. Effect of the gc content of DNA on the distribution of uvb-induced bipyrimidinephotoproducts. Photochem. Photobiol. Sci. 2008, 7, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, J.M.; Weinbauer, M.G.; Herndl, G.J. Interspecific variability in sensitivity to uv radiation and subsequent recovery in selected isolates of marine bacteria. Appl. Environ. Microbiol. 2000, 66, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Matallana-Surget, S.; Douki, T.; Cavicchioli, R.; Joux, F. Remarkable resistance to uvb of the marine bacterium photobacterium angustum explained by an unexpected role of photolyase. Photoch. Photobio. Sci. 2009, 8, 1313–1320. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Lopes, S.; Baptista, I.; Henriques, I.; Gomes, N.C.; Almeida, A.; Correia, A.; Cunha, A. Diversity in uv sensitivity and recovery potential among bacterioneuston and bacterioplankton isolates. Lett. Appl. Microbiol. 2011, 52, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Oliveira, V.; Baptista, I.; Henriques, I.; Gomes, N.C.; Almeida, A.; Correia, A.; Cunha, A. Wavelength dependence of biological damage induced by uv radiation on bacteria. Arch. Microbiol. 2013, 195, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, P.; Das, J.; Foley, I. Biofilm susceptibility to antimicrobials. Adv. Dent. Res. 1997, 11, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Kado, D.; Sakurai, K.; Sugiyama, T.; Ueda, T. Evaluation of cleanability of a titanium dioxide (TiO2)-coated acrylic resin denture base. Prosthodont. Res. Pract. 2005, 4, 69–76. [Google Scholar] [CrossRef]
- Arai, T.; Ueda, T.; Sugiyama, T.; Sakurai, K. Inhibiting microbial adhesion to denture base acrylic resin by titanium dioxide coating. J. Oral Rehabil. 2009, 36, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Obata, T.; Ueda, T.; Sakurai, K. Inhibition of denture plaque by TiO2 coating on denture base resins in the mouth. J. Prosthet. Dent. 2017, 118, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, M.; Ueda, T.; Sawaki, K.; Kawaguchi, M.; Sakurai, K. Biocompatibility of a titanium dioxide-coating method for denture base acrylic resin. Gerodontology 2016, 33, 539–544. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).