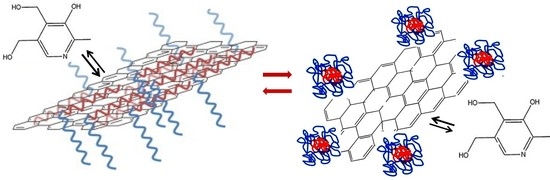
Effect of Graphene Flakes Modified by Dispersion in Surfactant Solutions on the Fluorescence Behaviour of Pyridoxine
Abstract
:1. Introduction
2. Materials and Methods
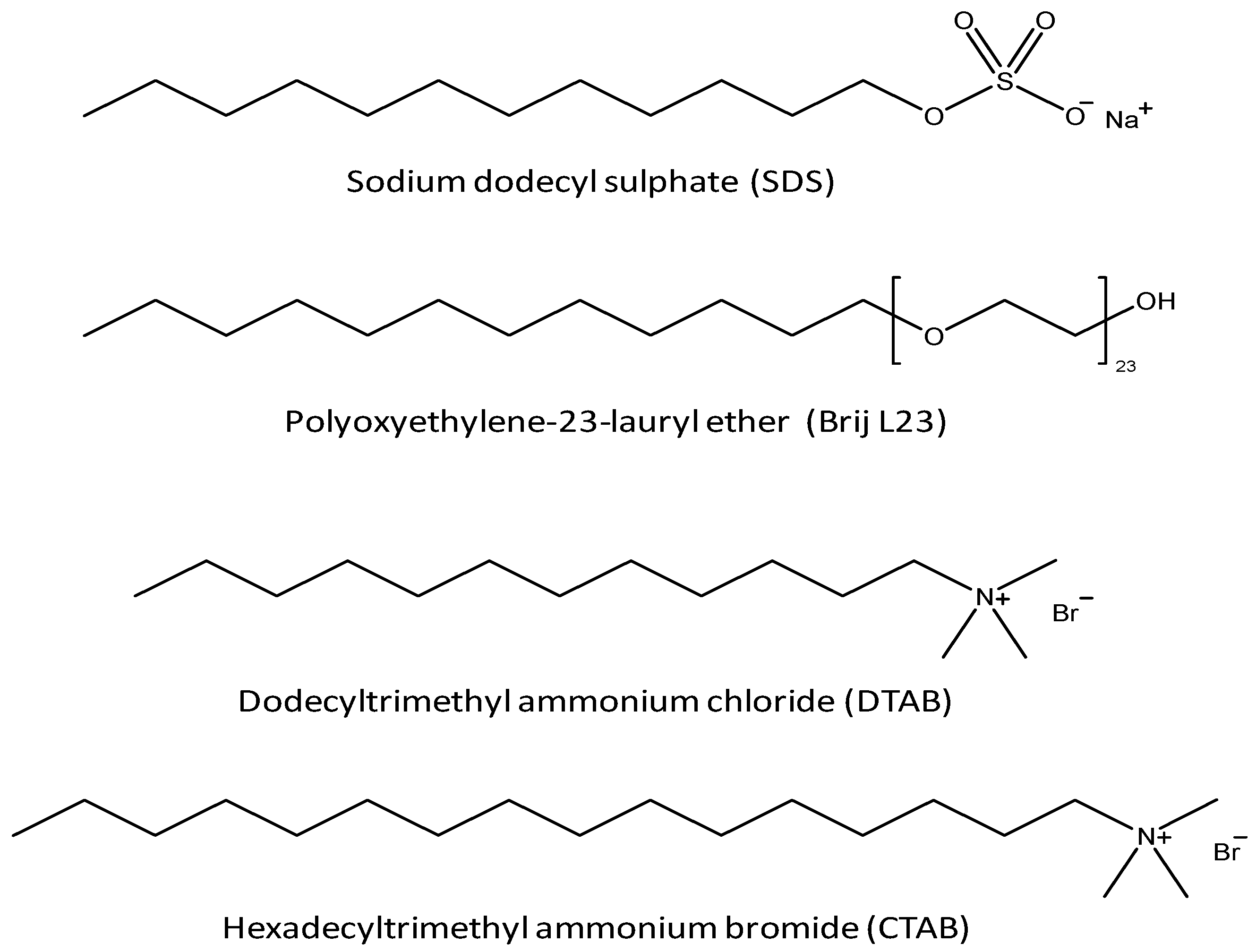
2.1. Reagents
2.2. Instrumentation
2.3. Preparation of the Vitamin Solutions and the G Dispersions in the surfactants
2.4. Fluorescence Spectra of Pyridoxine in Surfactant Solutions and G Dispersions in the Surfactants
3. Results and Discussion
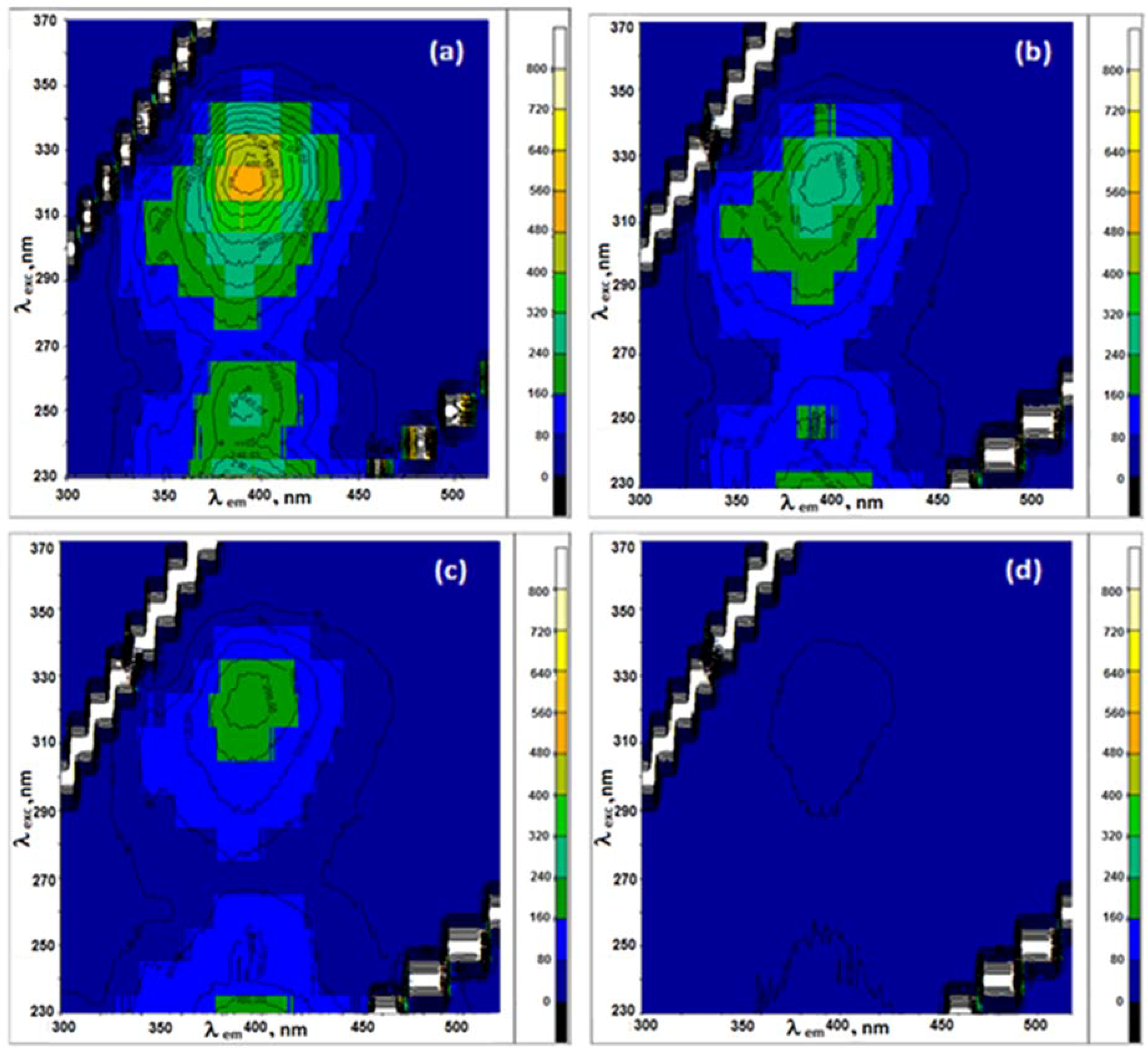
3.1. Assessment of the Quality of G Dispersions in the Different Surfactants
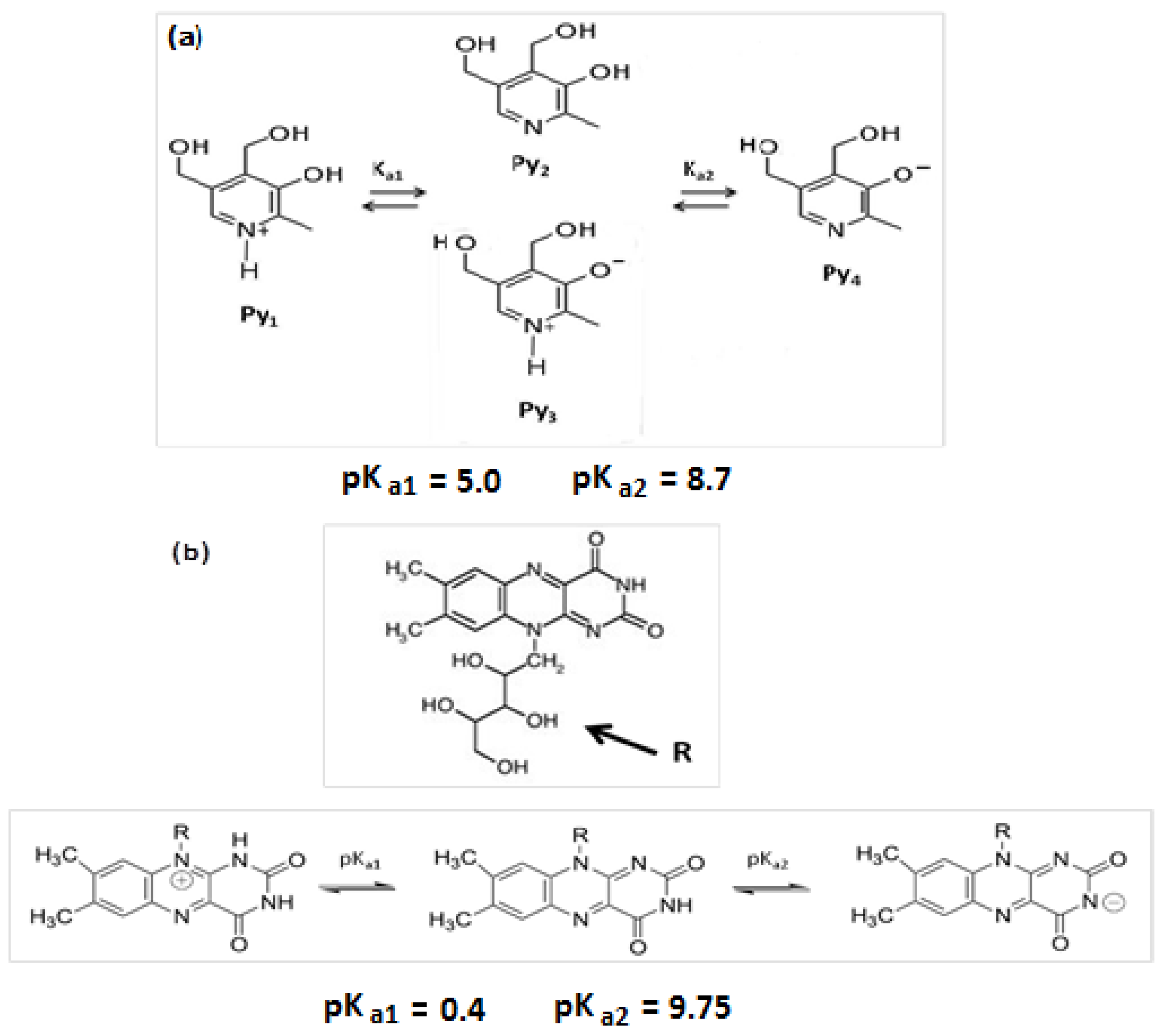
3.2. Fluorescence of Pyridoxine in Aqueous Surfactant Solutions
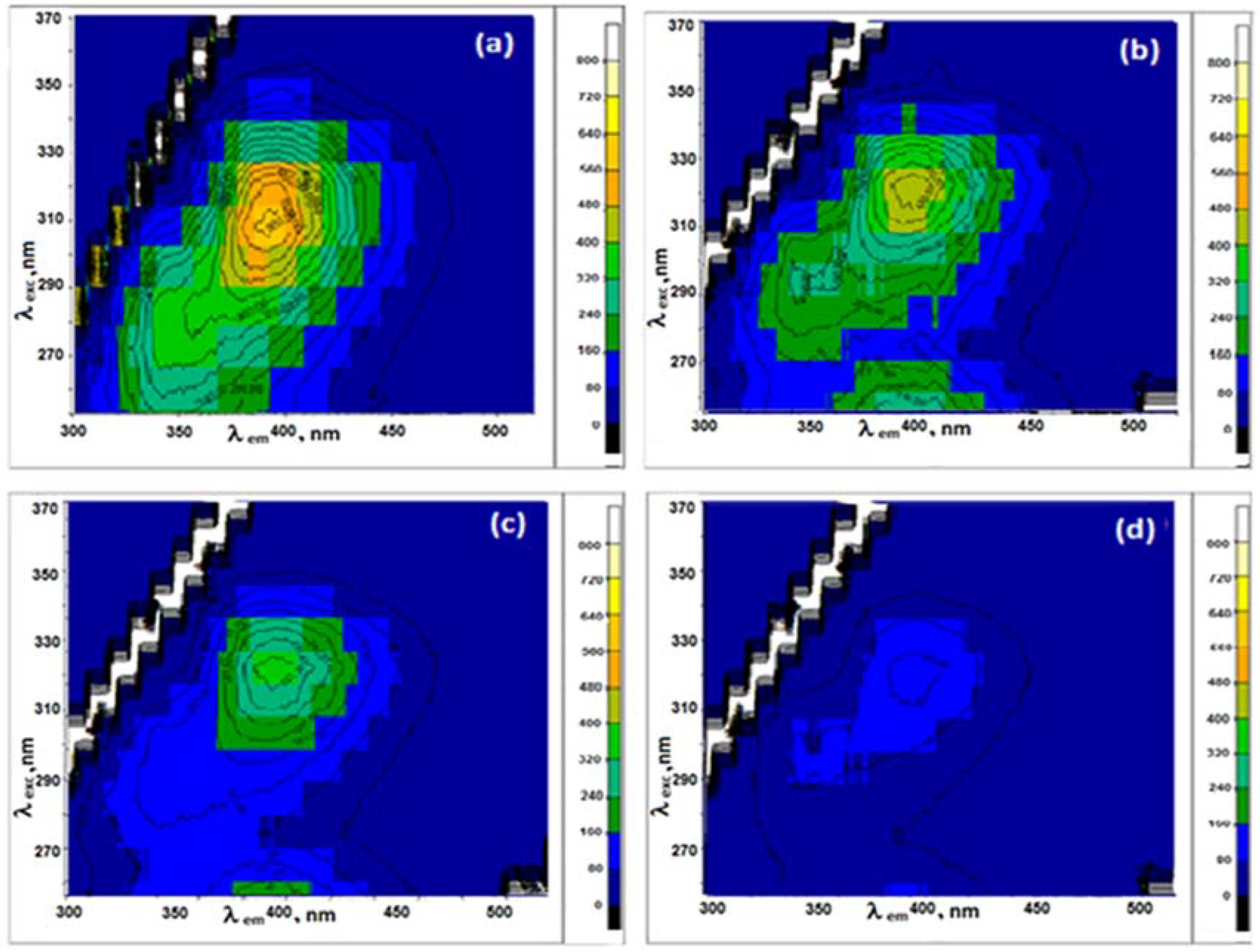
3.3. Fluorescence of Pyridoxine in the Presence of G Dispersions in the Different Surfactants
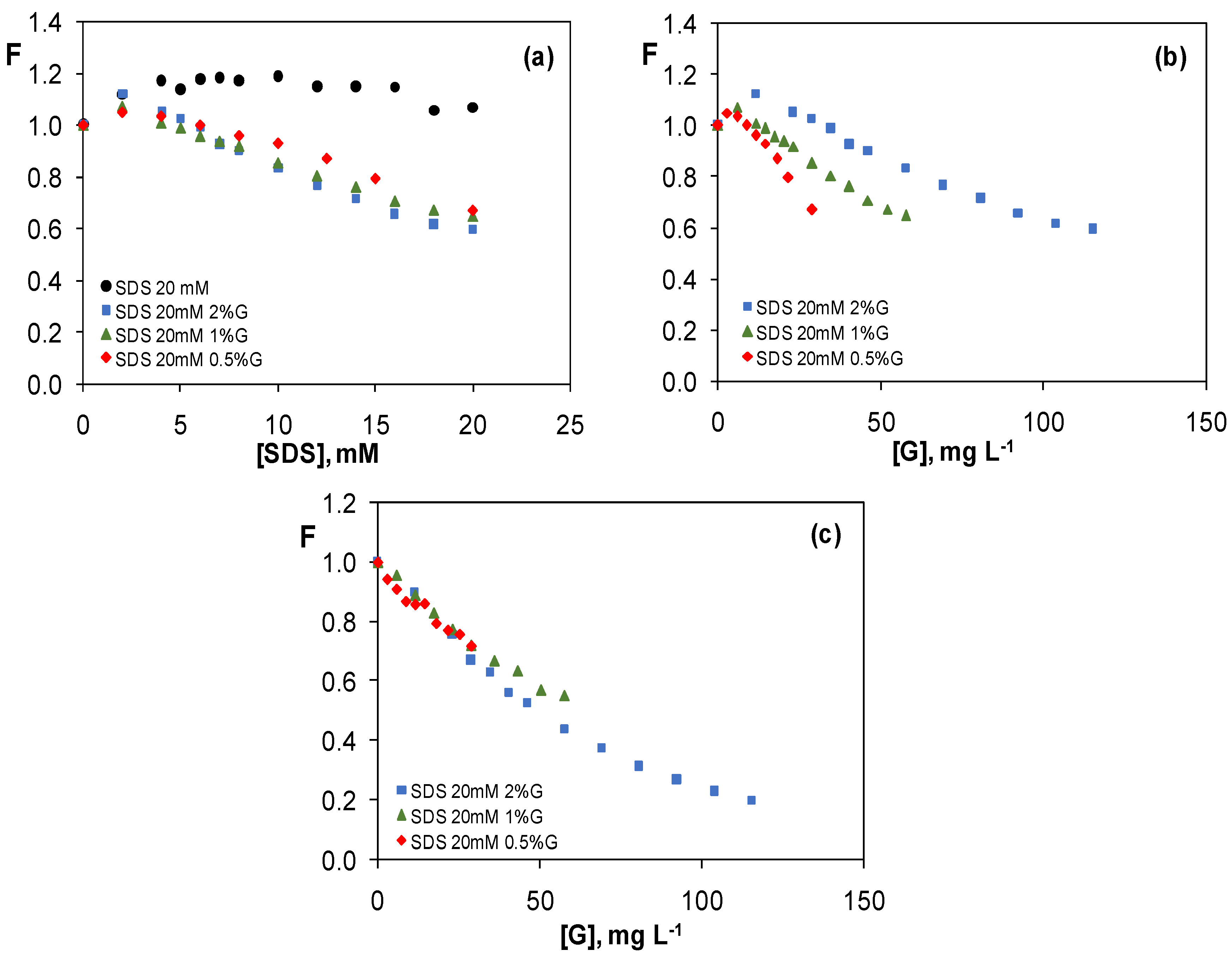
3.3.1. Graphene Dispersions in 20 mM SDS Aqueous Solutions
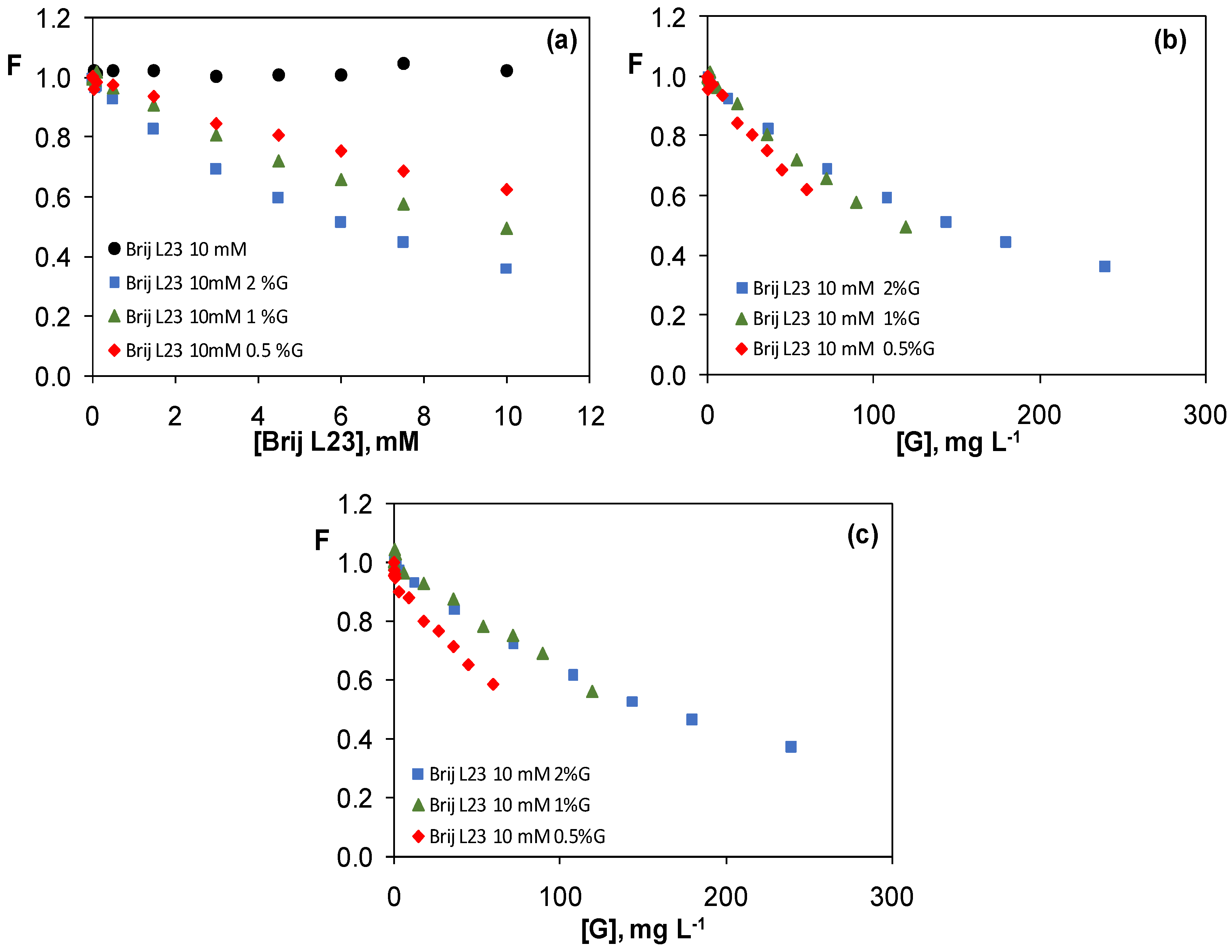
3.3.2. Graphene Dispersions in 10 mM Brij L23 Aqueous Solutions
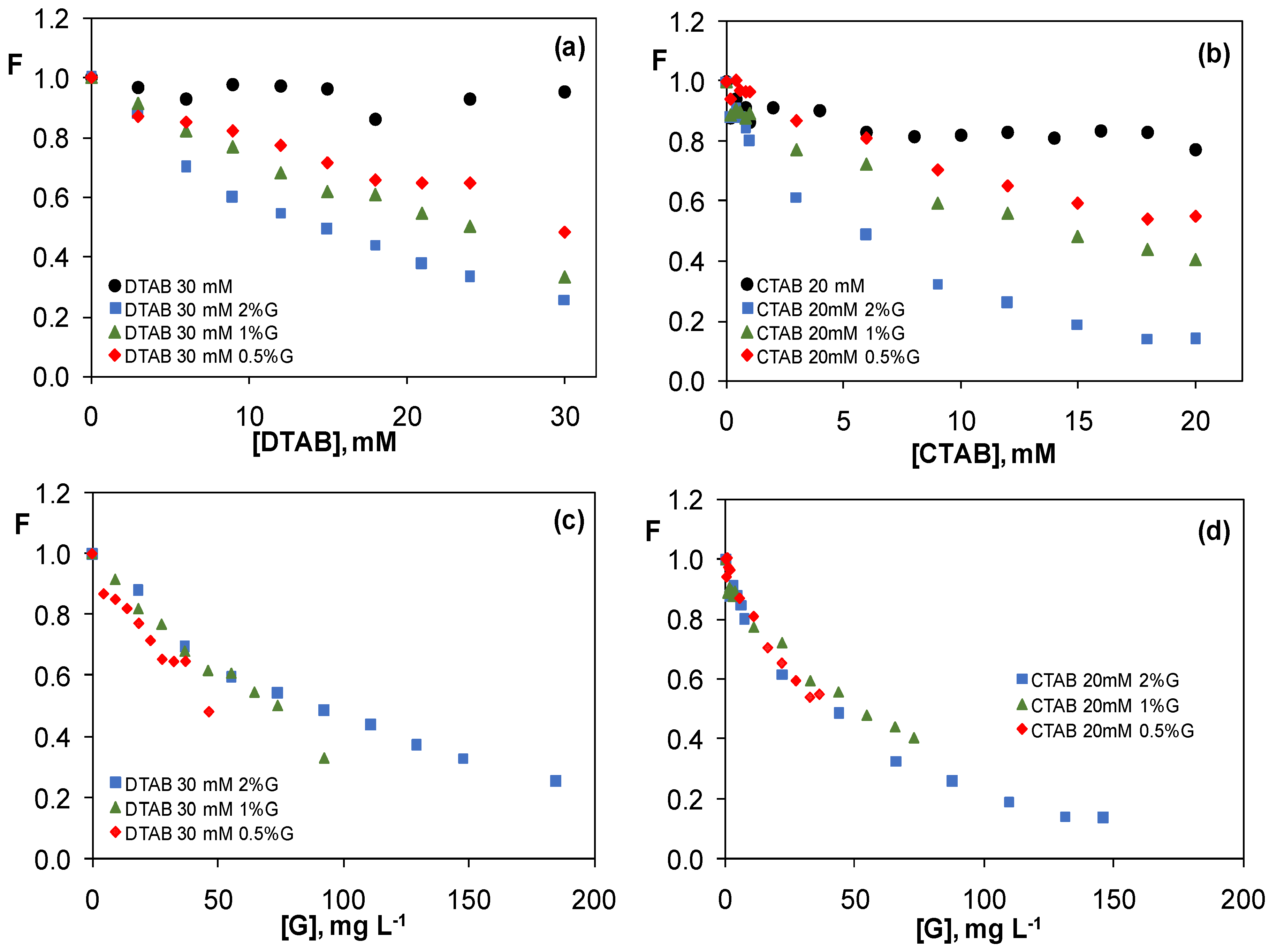
3.3.3. Graphene Dispersions in 30 mM CTAB and 20 mM DTAB Solutions
3.4. Comparison of the Quenching Effect of Graphene Dispersions in the Four Surfactants
3.5. Study of the Quenching Phenomenon
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M.; Luceño Sánchez, J.A.; Peña Capilla, R.; García Díaz, P. Recent advances in graphene/polymer nanocomposites for applications in polymer solar cells. Polymers 2018, 10, 217. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Diez-Vicente, A.L. Poly(propylene fumarate)/polyethylene glycol-modified graphene oxide nanocomposites for tissue engineering. ACS Appl. Mater. Interfaces 2016, 8, 17902–17914. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M.; Gómez-Fatou, M.A.; Ania, F.; Flores, A. Nanoindentation in Polymer Nanocomposites. Prog. Mater. Sci. 2015, 67, 1–94. [Google Scholar] [CrossRef]
- Salavagione, H.J.; Díez-Pascual, A.M.; Lázaro, E.; Vera, S.; Gomez-Fatou, M.A. Chemical sensors based on polymer composites with carbon nanotubes and graphene: The role of the polymer. J. Mater. Chem. A 2014, 2, 14289–14328. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Pang, Y.; Cui, Y.; Ma, Y.; Qian, H.; Shen, X. Fluorescence quenching of cationic organic dye by graphene: Interaction and its mechanism. Micro Nano Lett. 2012, 7, 608–612. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, C.Y.; Liu, Y. Investigation on fluorescence quenching of dyes by graphite oxide and graphene. Appl. Surf. Sci. 2011, 257, 5513–5518. [Google Scholar] [CrossRef]
- Lin, W.; Tian, B.; Zhuang, P.; Yin, J.; Zhang, C.; Li, Q.; Shih, T.; Cai, W. Graphene based fluorescence-quenching-related fermi fevel elevation and electron concentration surge. Nano Lett. 2016, 16, 5737–5741. [Google Scholar] [CrossRef] [PubMed]
- Vera-López, S.; Martínez, P.; San Andrés, M.P.; Díez-Pascual, A.M.; Valiente, M. Study of graphene dispersions in sodium dodecylsulfate by steady-state fluorescence of pyrene. J. Coll. Interf. Sci. 2018, 514, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M.; García-García, D.; San Andrés, M.P.; Vera, S. Determination of riboflavin based on fluorescence quenching by graphene dispersions in polyethylene glycol. RSC Adv. 2016, 6, 19686–19699. [Google Scholar] [CrossRef]
- San Andrés, M.P.; Díez-Pascual, A.M.; Palencia, S.; San Torcuato, J.; Valiente, M.; Vera, S. Fluorescence quenching of α-tocopherol by graphene dispersed in aqueous surfactant solutions. J. Lumin. 2017, 187, 169–180. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M.; Ferreira, C.H.; Andrés, M.P.S.; Valiente, M.; Vera, S. Effect of Graphene and Graphene Oxide Dispersions in Poloxamer-407 on the Fluorescence of Riboflavin: A Comparative Study. J. Phys. Chem. C 2017, 121, 830–843. [Google Scholar] [CrossRef]
- Salihoglu, O.; Kakenov, N.; Balci, O.; Balci, S.; Kocabas, C. Graphene as a reversible and spectrally selective fluorescence quencher. Sci. Rep. 2016, 6, 33911. [Google Scholar] [CrossRef] [PubMed]
- Texter, J. Graphene dispersions. Curr. Opin. Colloid Interface Sci. 2014, 19, 163–174. [Google Scholar] [CrossRef]
- Wang, S.; Yi, M.; Shen, Z. The effect of surfactants and their concentrations on the liquid-exfoliation of graphene. RSC Adv. 2016, 6, 56705–56710. [Google Scholar] [CrossRef]
- McCormick, D.B. Vitamin B6. In Present Knowledge in Nutrition (Volume 1); Bowman, B.A., Russell, R.M., Eds.; International Life Sciences Institute: Washington, DC, USA, 1996; pp. 269–277. [Google Scholar]
- Dakshinamurti, S.; Dakshinamurti, K. Vitamin B6. In Handbook of Vitamins, 4th ed; Zempleni, J., Rucker, R.B., Mc Cormick, D.B., Suttie, J.W., Eds.; CRC Press: New York, NY, USA, 2007; pp. 315–359. [Google Scholar]
- Vanderschuren, H.; Boycheva, S.; Li, K.T.; Szydlowski, N.; Gruissem, W.; Fitzpatrick, T.B. Strategies for vitamin B6 biofortification of plants: A dual role as a micronutrient and a stress protectant. Front. Plant Sci. 2013, 4, 143. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Mirza, T.; Qadeer, K.; Nazim, U.; Vaid, F.H. Vitamin B6: Deficiency diseases and methods of analysis. Pak. J. Pharm. Sci. 2013, 26, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.; Blazquez, S.; San Andres, M.P.; Vera, S. Determination of thiamine, riboflavin and pyridoxine in pharmaceuticals by synchronous fluorescence spectrometry in organized media. Anal. Chim. Acta 2001, 434, 193–199. [Google Scholar] [CrossRef]
- Metzler, D.E.; Snell, E.E. Spectra and ionization constants of the vitamin B6 group and related 3-hydroxypyridine derivatives. J. Am. Chem. Soc. 1955, 77, 2431–2437. [Google Scholar] [CrossRef]
- Mateos, R.; Vera, S.; Valiente, M.; Díez-Pascual, A.M.; San Andrés, M.P. Comparison of anionic, cationic and nonionic surfactants as dispersing agents for graphene based on the fluorescence of riboflavin. Nanomaterials 2017, 7, 403. [Google Scholar] [CrossRef] [PubMed]
- Palencia, S.; Vera, S.; Díez-Pascual, A.M.; San Andrés, M.P. Quenching of fluorene fluorescence by single-walled carbon nanotube dispersions with surfactants: Application for fluorene quantification in wastewater. Anal. Bioanal. Chem. 2015, 407, 4671–4682. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Wang, Y.; Wang, L.; Ni, Z.; Wang, Z.; Wang, R.; Koo, C.K.; Shen, Z.; Thong, J.T. Probing layer number and stacking order of few-layer graphene by Raman spectroscopy. Small 2010, 6, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Lotya, M.; King, P.J.; Khan, U.; De, S.; Coleman, J.N. High-concentration, surfactant-stabilized graphene dispersions. ACS Nano 2010, 4, 3155–3162. [Google Scholar] [CrossRef] [PubMed]
- Shannigrahi, M.; Bagchi, S. Novel fluorescent probe as aggregation predictor and micro-polarity reporter for micelles and mixed micelles. Spectrochim. Acta Part A 2005, 61, 2131–2138. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, A.G.; Korkut, S.; Punckt, C.; Aksay, I.A. Dispersion stability of functionalized graphene in aqueous sodium dodecyl sulfate solutions. Langmuir 2013, 29, 14831–14838. [Google Scholar] [CrossRef] [PubMed]
- Gou, Q.; Spada, L.; Vallejo-Lopez, M.; Lesarri, A.; Cocinero, E.J.; Caminati, W. Interactions between alkanes and aromatic molecules: A rotational study of pyridine-methane. Phys. Chem. Chem. Phys. 2014, 16, 13041–13046. [Google Scholar] [CrossRef] [PubMed]
- Kasry, A.; Ardakani, A.A.; Tulevski, G.S.; Menges, B.; Copel, M.; Vyklicky, L. Highly efficient fluorescence quenching with graphene. J. Phys. Chem. C 2012, 116, 2858–2862. [Google Scholar] [CrossRef]
- Smith, R.J.; Lotya, M.; Coleman, J.N. The importance of repulsive potential barriers for the dispersion of G using surfactants. New J. Phys. 2010, 12, 125008. [Google Scholar] [CrossRef]
- Meng, W.; Gall, E.; Ke, F.; Zeng, Z.; Kopchick, B.; Timsina, R.; Qiu, X. Structure and interaction of graphene oxide-cetyltrimethylammonium bromide complexation. J. Phys. Chem. C 2015, 119, 21135–21140. [Google Scholar] [CrossRef]
- Zhang, X.F.; Shao, X. π–π binding ability of different carbon nano-materials with aromatic phthalocyanine molecules: Comparison between graphene, graphene oxide and carbon nanotubes. J. Photochem. Photobiol. A 2014, 278, 69–74. [Google Scholar] [CrossRef]
- Bavali, A.; Parvin, P.; Mortazavi, S.Z.; Nourazar, S.S. Laser induced fluorescence spectroscopy of various carbon nanostructures (GO, G and nanodiamond) in Rd6G solution. Biomed. Opt. Express 2015, 6, 1679–1693. [Google Scholar] [CrossRef] [PubMed]
- Wanless, E.J.; Ducker, W.A. Organization of sodium dodecyl sulfate at the graphite-solution interface. J. Phys. Chem. 1996, 100, 3207–3214. [Google Scholar] [CrossRef]
- Tummala, N.R.; Striolo, A. Role of counterion condensation in the self-assembly of SDS surfactants at the water-graphite interface. J. Phys. Chem. B 2008, 112, 1987–2000. [Google Scholar] [CrossRef] [PubMed]
- Guardia, L.; Fernández-Merino, M.J.; Paredes, J.I.; Solís-Fernández, P.; Villar-Rodil, S.; Martínez-Alonso, A.; Tascón, J.M.D. High-throughput production of pristine G in an aqueous dispersion assisted by non-ionic surfactants. Carbon 2011, 49, 1653–1662. [Google Scholar] [CrossRef]
- Wang, X.; He, Y.; Song, G. A Graphene oxide-rhodamine 6G. Nanocomposite as turn on fluorescence probe for selective detection of DNA. Phys. Procedia 2012, 25, 394–400. [Google Scholar] [CrossRef]
- Dong, H.; Gao, W.; Yan, F.; Ji, H.; Ju, H. Fluorescence resonance energy transfer between quantum dots and graphene oxide for sensing biomolecules. Anal. Chem. 2010, 82, 5511–5517. [Google Scholar] [CrossRef] [PubMed]
- Li, S. Analysis of the Interactions between Graphene Oxide and Biomolecules and Protein Fibrillation Using Surface Chemistry and Spectroscopy. Ph.D. Thesis, University of Miami, Coral Gables, FL, USA, August 2014. [Google Scholar]
- Ranjit, S.; Levitus, M. Probing the interaction between fluorophores and DNA nucleotides by fluorescence correlation spectroscopy and fluorescence quenching. Photochem. Photobiol. 2012, 88, 782–791. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Pramanik, S.; Banerjee, P.; Bhattacharya, S.C. Interaction of 1-anthracene sulphonate with cationic micelles of alkyl trimethyl ammonium bromides (CnTAB): A spectroscopic study. Colloids Surf. A 2008, 317, 585–591. [Google Scholar] [CrossRef]


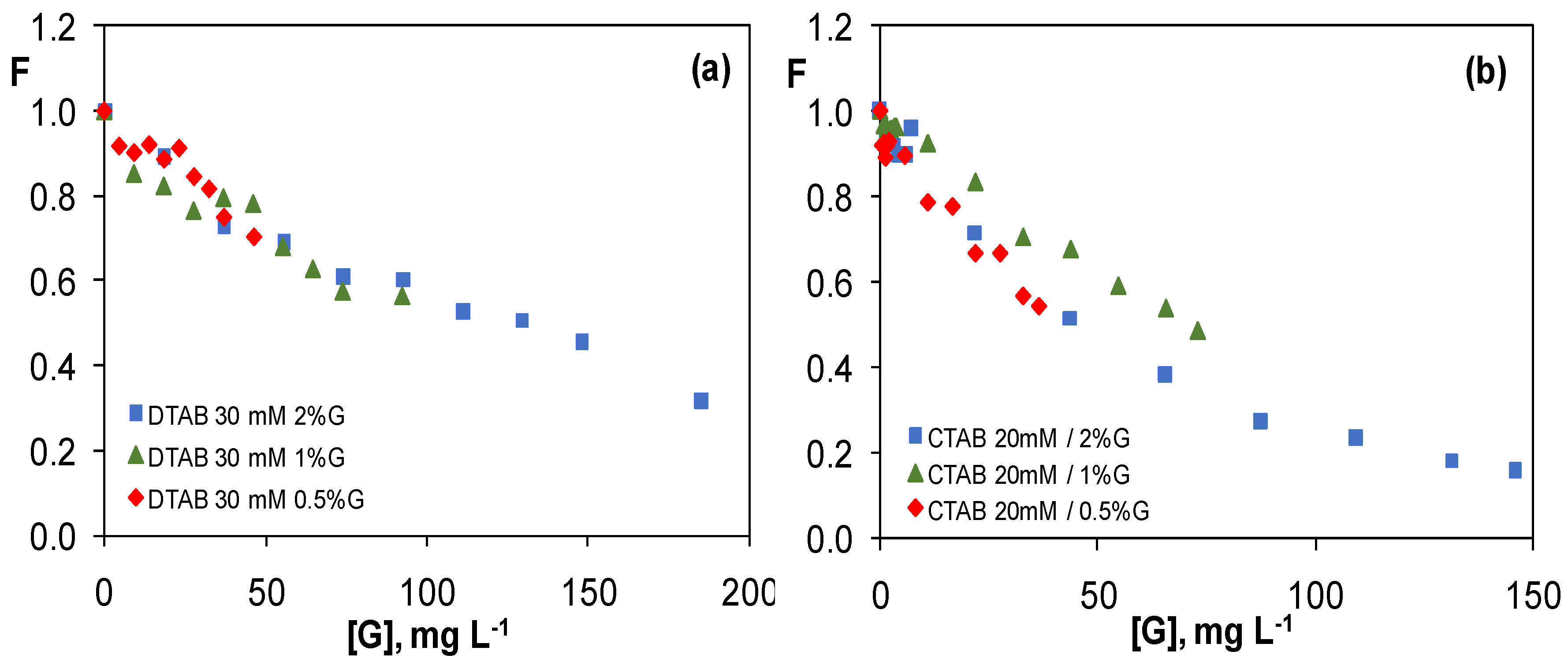
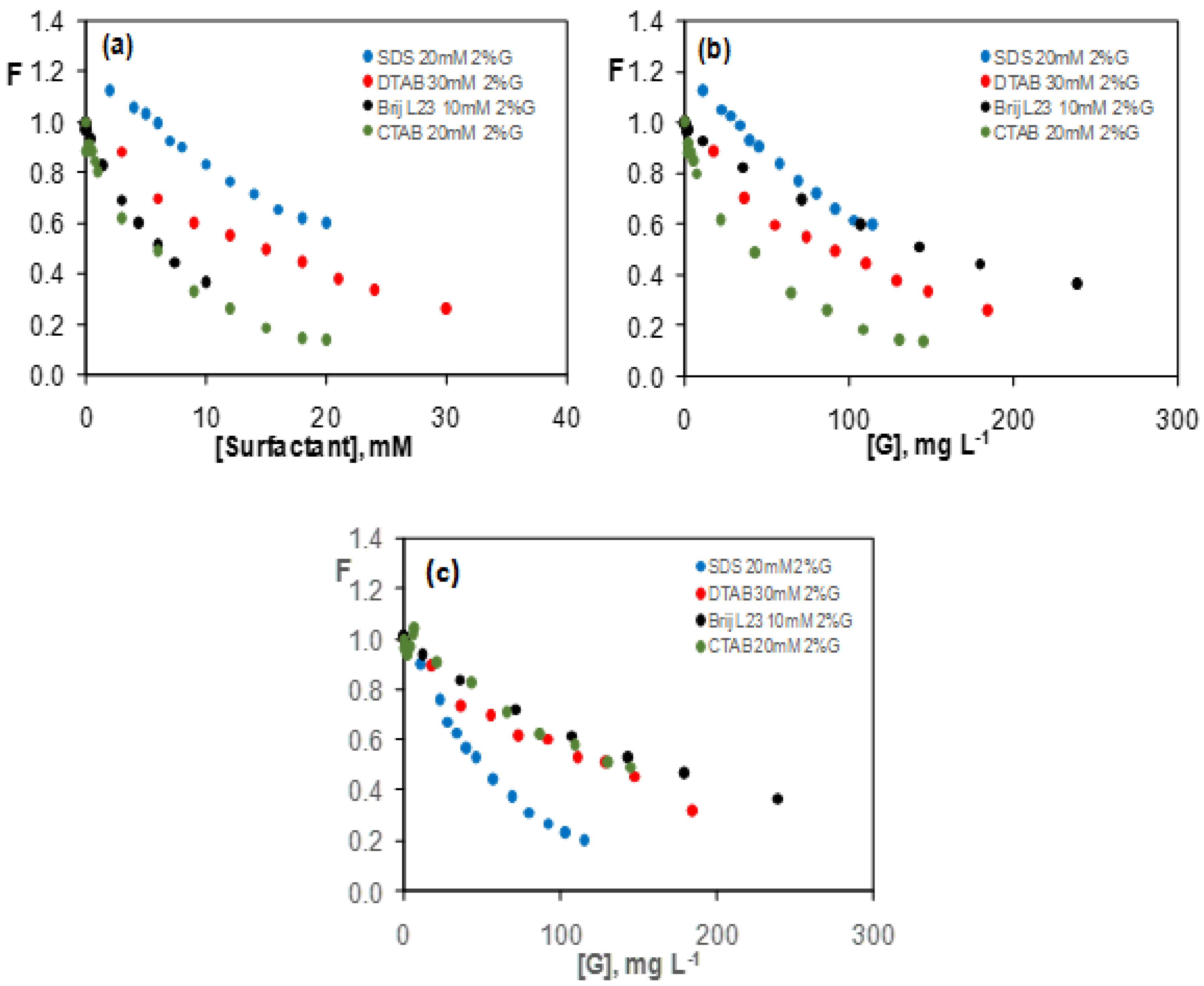
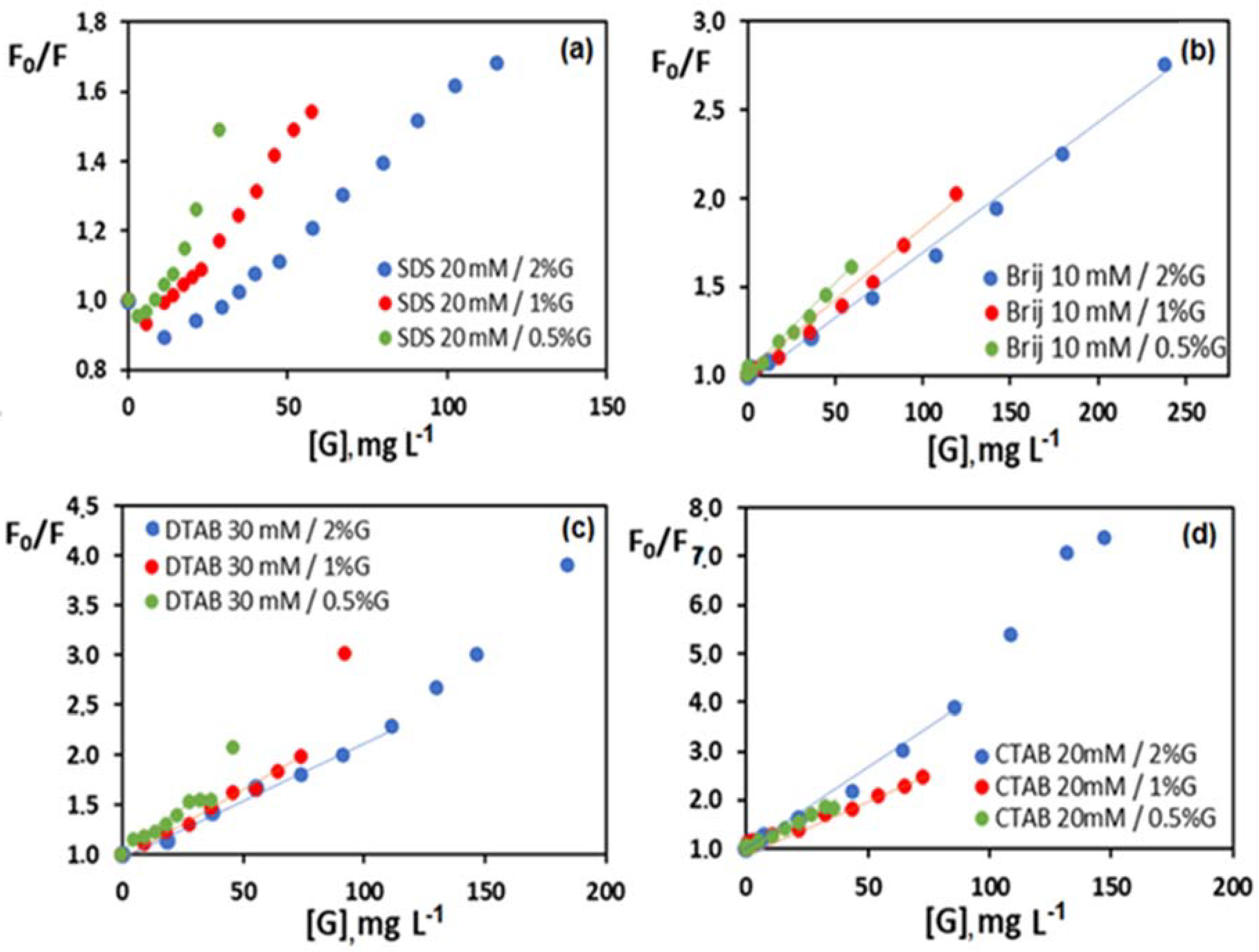
| Dispersion | [Surfactant] (mM) | [G] (mg L−1) | K (L mg−1) |
|---|---|---|---|
| 30 mM DTAB/0.5% G | 0–18 | 0–29 | 0.017 ± 0.008 |
| 30 mM DTAB/1% G | 0–18 | 0–58 | 0.012 ± 0.004 |
| 30 mM DTAB/2% G | 0–18 | 0–116 | 0.012 ± 0.002 |
| 20 mM CTAB/0.5% G | 0–20 | 0–37 | 0.026 ± 0.002 |
| 20 mM CTAB/1% G | 0–20 | 0–74 | 0.019 ± 0.002 |
| 20 mM CTAB/2% G | 0–12 | 0–87 | 0.032 ± 0.006 |
| 10 mM Brij L23/0.5% G | 0–10 | 0–60 | 0.010 ± 0.001 |
| 10 mM Brij L23/1% G | 0–10 | 0–120 | 0.008 ± 0.001 |
| 10 mM Brij L23/2% G | 0–10 | 0–240 | 0.0071 ± 0.0007 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateos, R.; García-Zafra, A.; Vera-López, S.; San Andrés, M.P.; Díez-Pascual, A.M. Effect of Graphene Flakes Modified by Dispersion in Surfactant Solutions on the Fluorescence Behaviour of Pyridoxine. Materials 2018, 11, 888. https://doi.org/10.3390/ma11060888
Mateos R, García-Zafra A, Vera-López S, San Andrés MP, Díez-Pascual AM. Effect of Graphene Flakes Modified by Dispersion in Surfactant Solutions on the Fluorescence Behaviour of Pyridoxine. Materials. 2018; 11(6):888. https://doi.org/10.3390/ma11060888
Chicago/Turabian StyleMateos, Rocío, Alba García-Zafra, Soledad Vera-López, María Paz San Andrés, and Ana María Díez-Pascual. 2018. "Effect of Graphene Flakes Modified by Dispersion in Surfactant Solutions on the Fluorescence Behaviour of Pyridoxine" Materials 11, no. 6: 888. https://doi.org/10.3390/ma11060888
APA StyleMateos, R., García-Zafra, A., Vera-López, S., San Andrés, M. P., & Díez-Pascual, A. M. (2018). Effect of Graphene Flakes Modified by Dispersion in Surfactant Solutions on the Fluorescence Behaviour of Pyridoxine. Materials, 11(6), 888. https://doi.org/10.3390/ma11060888