Reduction and Removal of Chromium VI in Water by Powdered Activated Carbon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Powdered Activated Carbon
2.2. Determinations
2.3. Batch Tests
2.4. Parameters of SEM-EDS, Raman Spectroscopy, and XPS
3. Results and Discussion
3.1. Cr Adsorption
3.2. SEM-EDS Analysis
3.3. Raman Spectroscopy
3.4. XPS Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Richard, F.C.; Bourg, A.C.M. Aqueous geochemistry of chromium: A review. Water Res. 1991, 25, 807–816. [Google Scholar] [CrossRef]
- Sharma, D.C.; Forster, C.F. Column studies into the adsorption of chromium (VI) using sphagnum moss peat. Bioresour. Technol. 1995, 52, 261–267. [Google Scholar] [CrossRef]
- Miretzky, P.; Cirelli, A.F. Cr(VI) and Cr(III) removal from aqueous solution by raw and modified lignocellulosic materials: A review. J. Hazard. Mater. 2010, 180, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sereshti, H.; Farahani, M.V.; Baghdadi, M. Trace determination of chromium (VI) in environmental water samples using innovative thermally reduced graphene (TRG) modified SiO2 adsorbent for solid phase extraction and UV–vis spectrophotometry. Talanta 2016, 146, 662–669. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Agency, Drinking Water Contaminants-Standards and Regulations. Available online: http://www.epa.gov/safewater/contaminants/index.html (accessed on 19 May 2017).
- Kozlowski, C.A.; Walkowiak, W. Removal of chromium (VI) from aqueous solutions by polymer inclusion membranes. Water Res. 2002, 36, 4870–4876. [Google Scholar] [CrossRef]
- Di Nardo, A.; Di Natale, M.; Erto, A.; Musmarra, D.; Bortonea, I. Permeable reactive barrier for groundwater PCE remediation: The case study of a solid waste landfill pollution. Comput. Aided Chem. Eng. 2010, 28, 1015–1020. [Google Scholar] [CrossRef]
- Rengaraj, S.; Yeon, K.H.; Moon, S.H. Removal of chromium from water and wastewater by ion exchange resins. J. Hazard. Mater. 2001, 87, 273–287. [Google Scholar] [CrossRef]
- Wu, L.; Liao, L.; Lv, G.; Qin, F.; He, Y.; Wang, X. Micro-electrolysis of Cr(VI) in the nanoscale zero-valent iron loaded activated carbon. J. Hazard. Mater. 2013, 254, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Gholami, M.; Rahimi, M. Application and optimization in chromium-contaminated wastewater treatment of the reverse osmosis technology. Desalin. Water Treat. 2009, 9, 229–233. [Google Scholar] [CrossRef]
- Habibi, S.; Nematollahzadeh, A.; Mousavi, S.A. Nano-scale modification of polysulfone membrane matrix and the surface for the separation of chromium ions from water. Chem. Eng. J. 2015, 267, 306–316. [Google Scholar] [CrossRef]
- Stylianou, S.; Simeonidis, K.; Mitrakas, M.; Zouboulis, A.; Ernst, M.; Katsoyiannis, I.A. Reductive precipitation and removal of Cr(VI) from groundwaters by pipe flocculation-microfiltration. Environ. Sci. Pollut. Res. 2017, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, J.; Liu, H.; Kang, Y. Development of a nitrogen-functionalized carbon adsorbent derived from biomass waste by diammonium hydrogen phosphate activation for Cr(VI) removal. Powder Technol. 2017, 318, 459–464. [Google Scholar] [CrossRef]
- Huang, M.; Wang, Z.; Liu, S. Reutilization of the Cr ions adsorbed on activated carbon as colorants in glass preparation. J. Environ. Chem. Eng. 2016, 4, 1555–1560. [Google Scholar] [CrossRef]
- Gallios, G.P.; Tolkou, A.K.; Katsoyiannis, I.A.; Stefusova, K.; Vaclavikova, M.; Deliyanni, E.A. Adsorption of Arsenate by Nano Scaled Activated Carbon Modified by Iron and Manganese Oxides. Sustainability 2017, 9, 1684. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Zhang, C.; Ren, L. Preparation and evaluation of activated carbon-based iron-containing adsorbents for enhanced Cr(VI) removal: Mechanism study. Chem. Eng. J. 2012, 189, 295–302. [Google Scholar] [CrossRef]
- Park, S.J.; Jang, Y.S. Pore structure and surface properties of chemically modified activated carbons for adsorption mechanism and rate of Cr(VI). J. Colloid Interface Sci. 2002, 249, 458–463. [Google Scholar] [CrossRef] [PubMed]
- Kobya, M. Adsorption, kinetic and equilibrium studies of Cr(VI) by hazelnut shell activated carbon. Adsorpt. Sci. Technol. 2004, 22, 51–64. [Google Scholar] [CrossRef]
- Yang, J.; Yu, M.; Chen, W. Adsorption of hexavalent chromium from aqueous solution by activated carbon prepared from longan seed: Kinetics, equilibrium and thermodynamics. J. Ind. Eng. Chem. 2015, 21, 414–422. [Google Scholar] [CrossRef]
- Gong, X.; Li, W.; Wang, K.; Hu, J. Study of the adsorption of Cr(VI) by tannic acid immobilised powdered activated carbon from micro-polluted water in the presence of dissolved humic acid. Bioresour. Technol. 2013, 141, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Ihsanullah; Al-Khaldi, F.A.; Abu-Sharkh, B.; Abulkibash, A.M.; Qureshi, M.I.; Laoui, T.; Atieh, M.A. Effect of acid modification on adsorption of hexavalent chromium (Cr(VI)) from aqueous solution by activated carbon and carbon nanotubes. Desalin. Water Treat. 2016, 57, 7232–7244. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.S.; Park, J.M. XAS and XPS studies on chromium-binding groups of biomaterial during Cr(VI) biosorption. J. Colloid Interface Sci. 2008, 317, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, I.; Şahin, M.; Orhan, R.; Erdem, M. Preparation and characterization of activated carbon from grape stalk by zinc chloride activation. Fuel Process. Technol. 2014, 125, 200–206. [Google Scholar] [CrossRef]
- Dubey, R.; Bajpai, J.; Bajpai, A.K. Green synthesis of graphene sand composite (GSC) as novel adsorbent for efficient removal of Cr(VI) ions from aqueous solution. J. Water Process Eng. 2015, 5, 83–94. [Google Scholar] [CrossRef]
- Shimodaira, N.; Masui, A. Raman spectroscopic investigations of activated carbon materials. J. Appl. Phys. 2002, 92, 902–909. [Google Scholar] [CrossRef]
- Xu, C.; Qiu, B.; Gu, H.; Yang, X.; Wei, H.; Huang, X.; Guo, Z. Synergistic interactions between activated carbon fabrics and toxic hexavalent chromium. ECS J. Solid State Sci. Technol. 2014, 3, M1–M9. [Google Scholar] [CrossRef]
- ASTM International. Standard Test Method for Carbon Black-Total and External Surface Area by Nitrogen Adsorption; ASTM D6556-10; ASTM International: West Conshohocken, PA, USA, 2010. [Google Scholar]
- ASTM International. Standard Test Method for Determination of Iodine Number of Activated Carbon; ASTM D4607-94; ASTM International: West Conshohocken, PA, USA, 2011. [Google Scholar]
- Shi, B. Analysis of existing forms of Cr6+ in waste water. Electroplat. Pollut. Control 1986, 6, 30–33. [Google Scholar]
- Babel, S.; Kurniawan, T.A. Cr(VI) removal from synthetic wastewater using coconut shell charcoal and commercial activated carbon modified with oxidizing agents and/or chitosan. Chemosphere 2004, 54, 951–967. [Google Scholar] [CrossRef] [PubMed]
- Selvi, K.; Pattabhi, S.; Kadirvelu, K. Removal of Cr(VI) from aqueous solution by adsorption onto activated carbon. Bioresour. Technol. 2001, 80, 87–89. [Google Scholar] [CrossRef]
- McIntosh, D.; Khabashesku, V.N.; Barrera, E.V. Benzoyl peroxide initiated in situ functionalization, processing, and mechanical properties of single-walled carbon nanotube—Polypropylene composite fibers. J. Phys. Chem. C 2007, 111, 1592–1600. [Google Scholar] [CrossRef]
- Zhi, C.Y.; Bai, X.D.; Wang, E.G. Raman characterization of boron carbonitride nanotubes. Appl. Phys. Lett. 2002, 80, 3590–3592. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef]
- Iyyamperumal, E.; Wang, S.; Dai, L. Vertically aligned BCN nanotubes with high capacitance. ACS Nano 2012, 6, 5259–5265. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Yap, P.S.; Lim, T.M.; Lim, T.T. Adsorption-photocatalytic degradation of Acid Red 88 by supported TiO2: Effect of activated carbon support and aqueous anions. Chem. Eng. J. 2011, 171, 1098–1107. [Google Scholar] [CrossRef]
- Liu, H.; Liang, S.; Gao, J.; Ngo, H.H.; Guo, W.; Guo, Z.; Li, Y. Enhancement of Cr(VI) removal by modifying activated carbon developed from Zizania caduciflora with tartaric acid during phosphoric acid activation. Chem. Eng. J. 2014, 246, 168–174. [Google Scholar] [CrossRef]
- Moriceau, P.; Grzybowska, B.; Gengembre, L.; Barbaux, Y. Oxidative dehydrogenation of isobutane on Cr–Ce–O oxide: II. Physical characterizations and determination of the chromium active species. Appl. Catal. A Gen. 2000, 199, 73–82. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Ou, J.L.; Duan, Z.K.; Xing, Z.J.; Wang, Y. Adsorption of Cr(VI) on bamboo bark-based activated carbon in the absence and presence of humic acid. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 108–116. [Google Scholar] [CrossRef]
- Di Natale, F.; Erto, A.; Lancia, A.; Musmarra, D. Equilibrium and dynamic study on hexavalent chromium adsorption onto activated carbon. J. Hazard. Mater. 2015, 281, 47–55. [Google Scholar] [CrossRef] [PubMed]
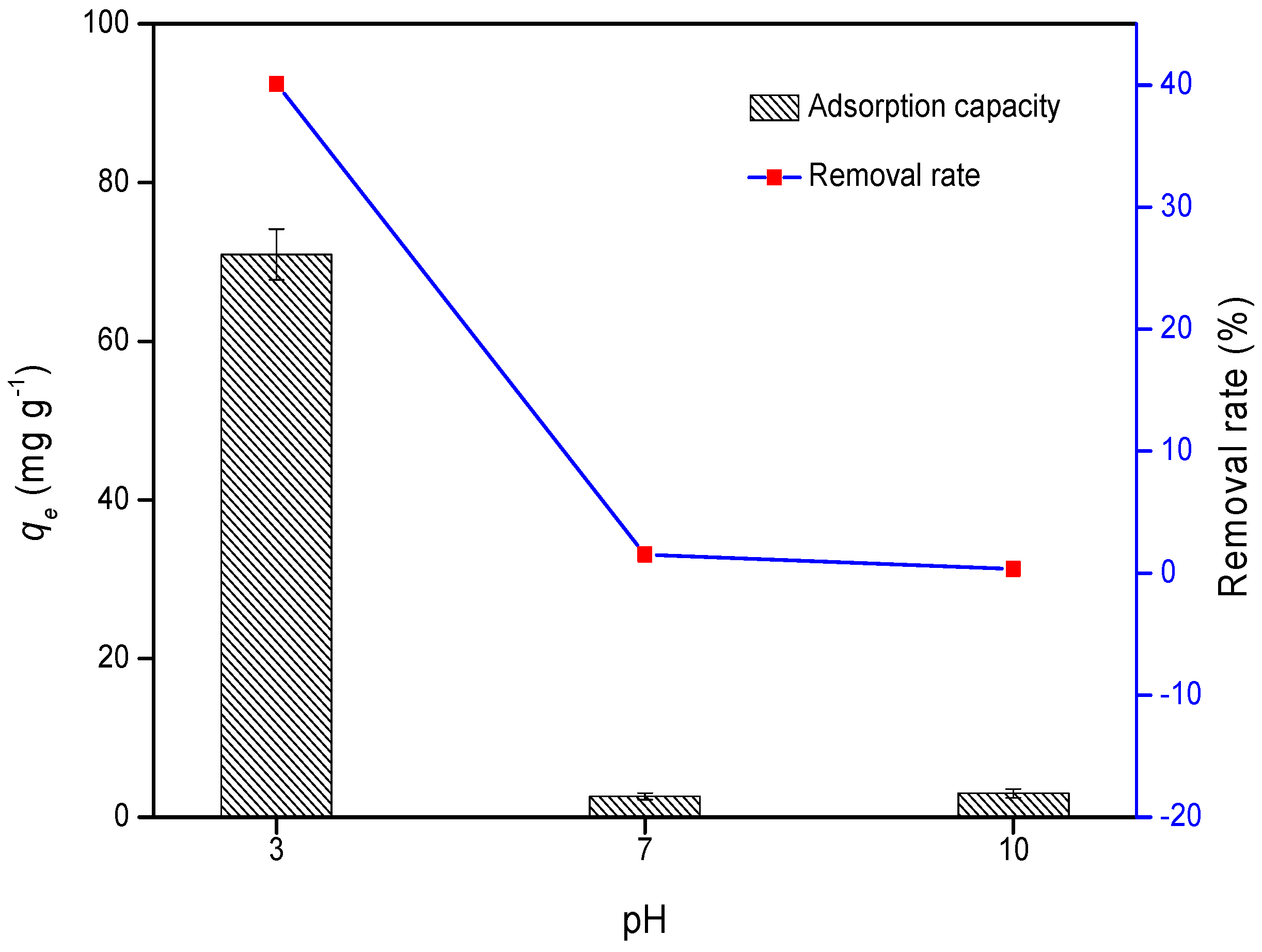
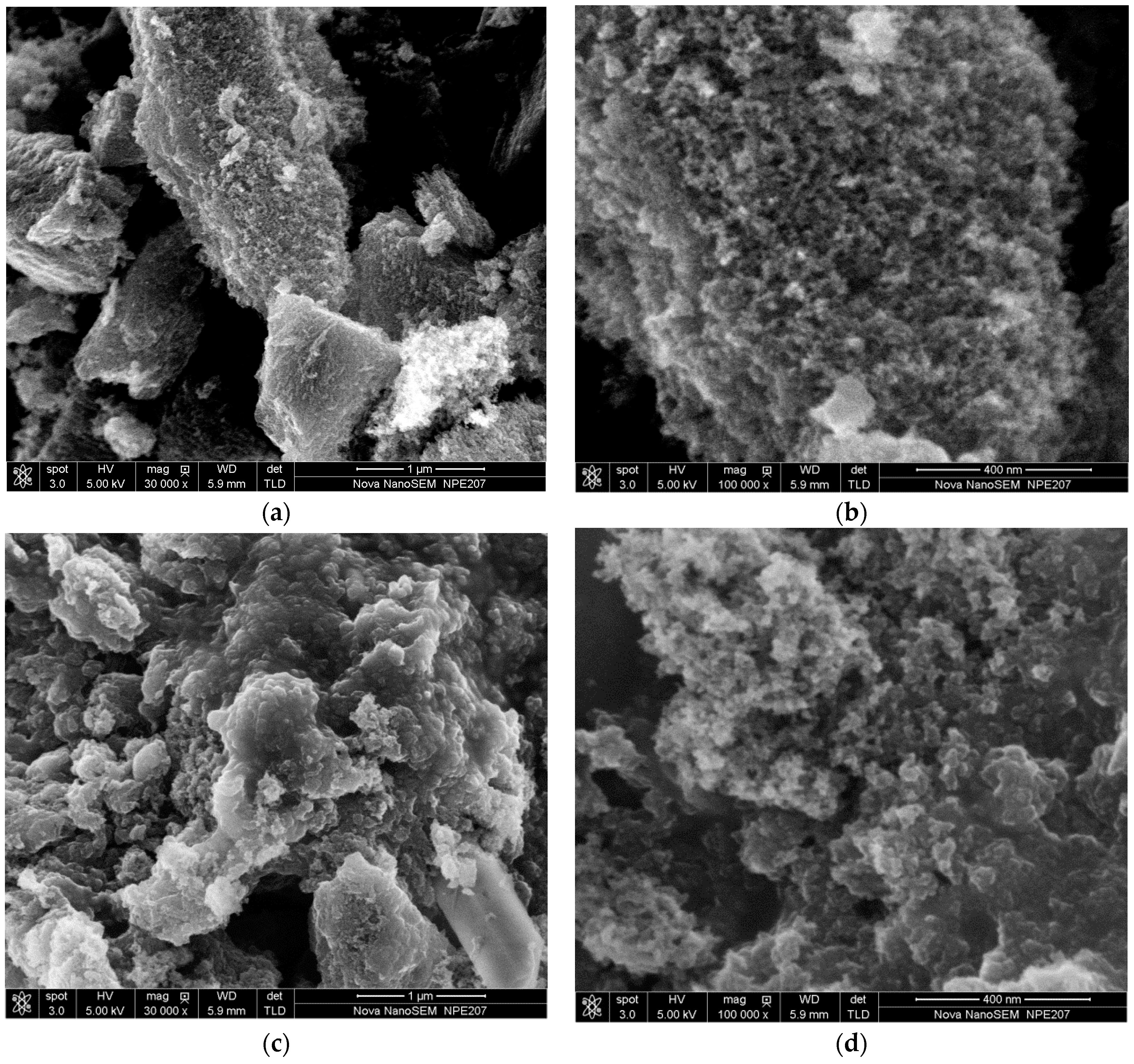
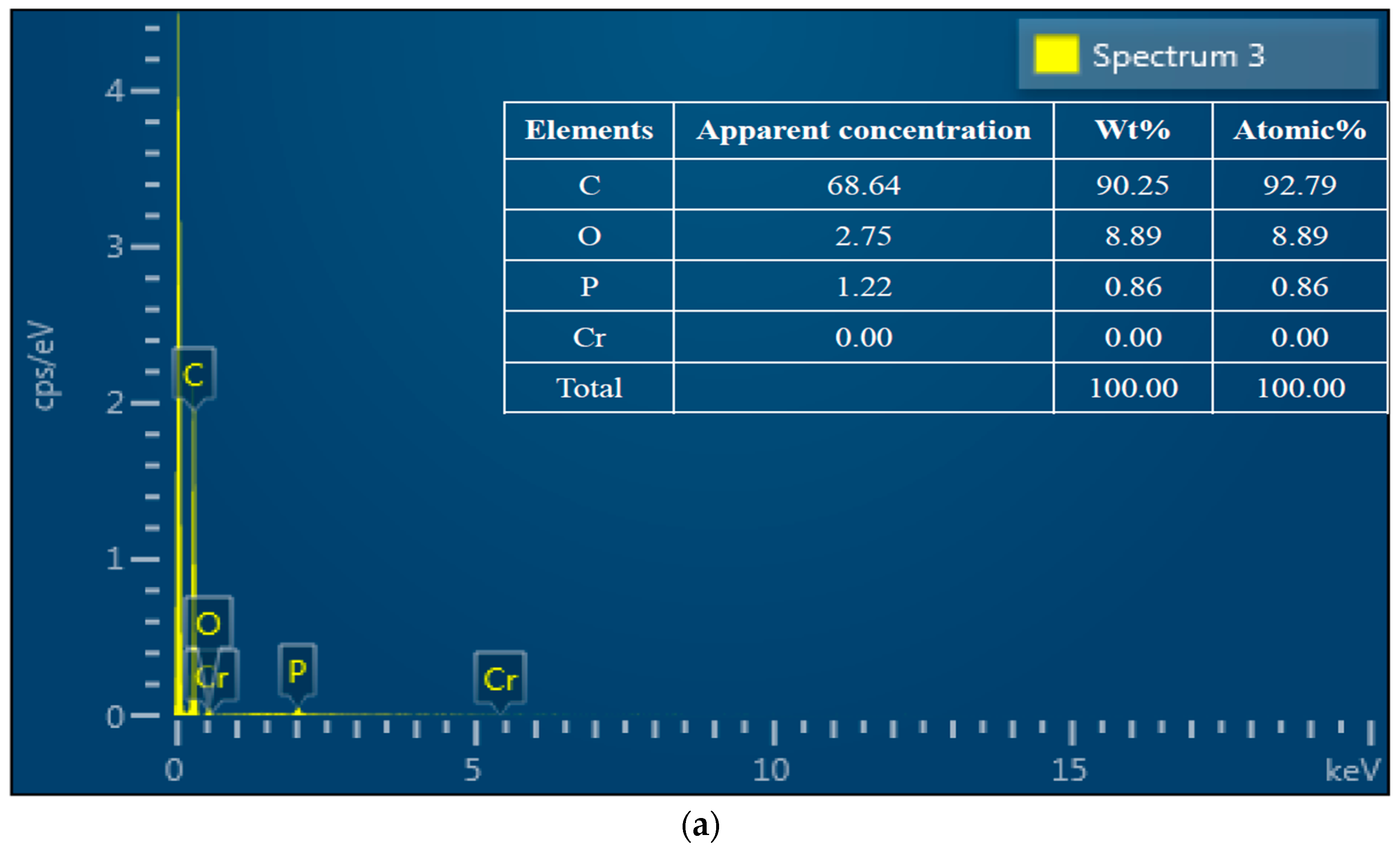
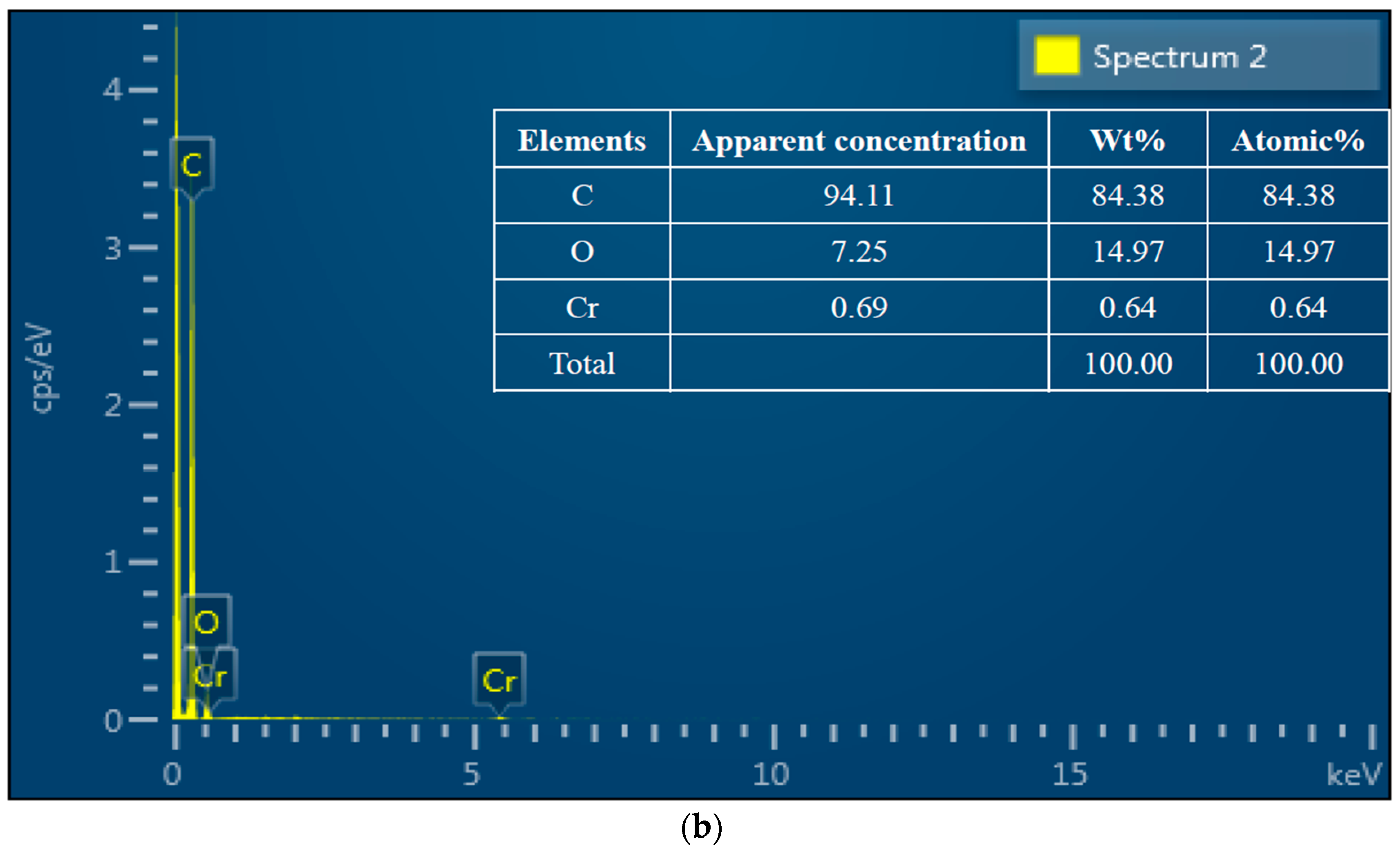
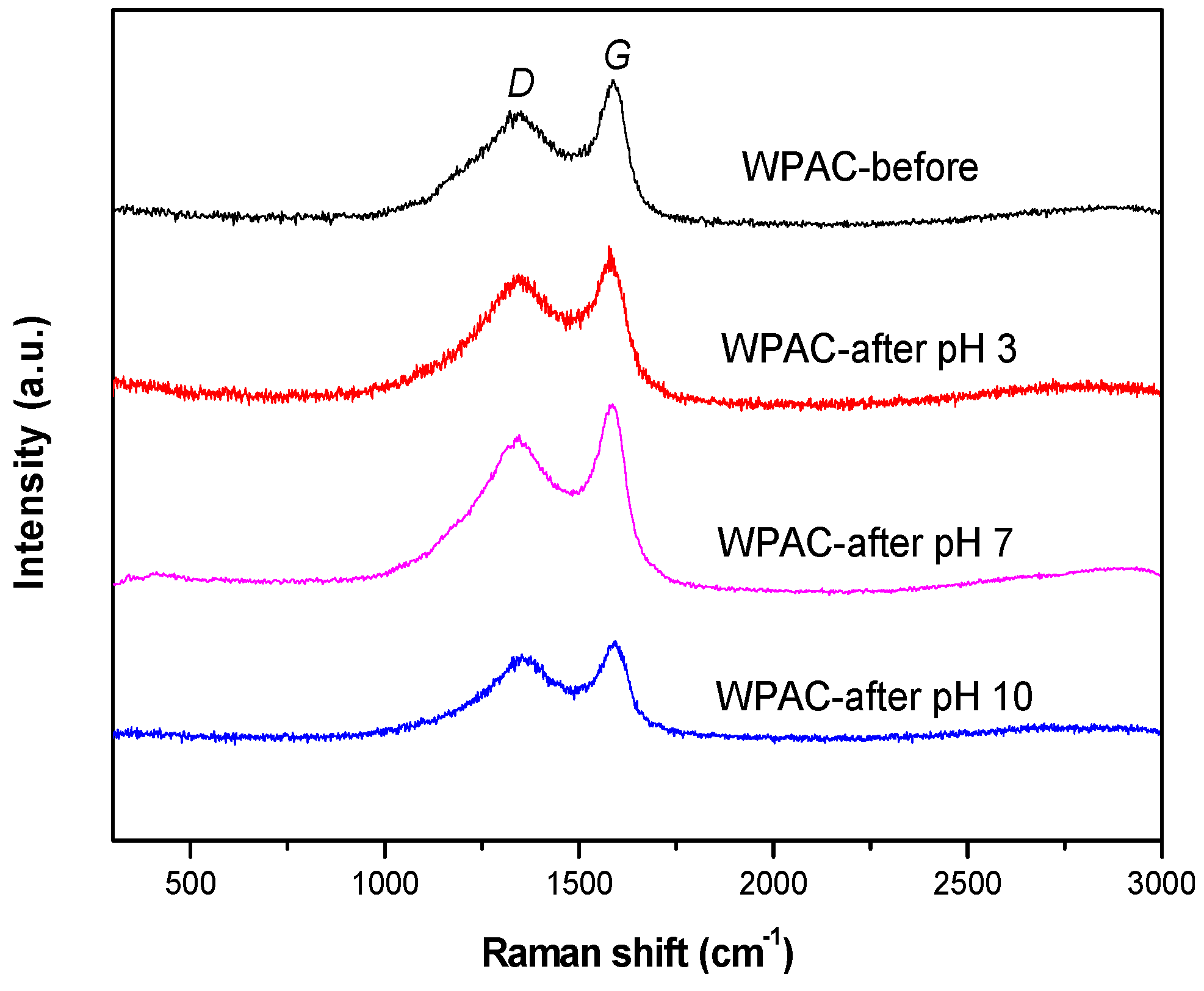
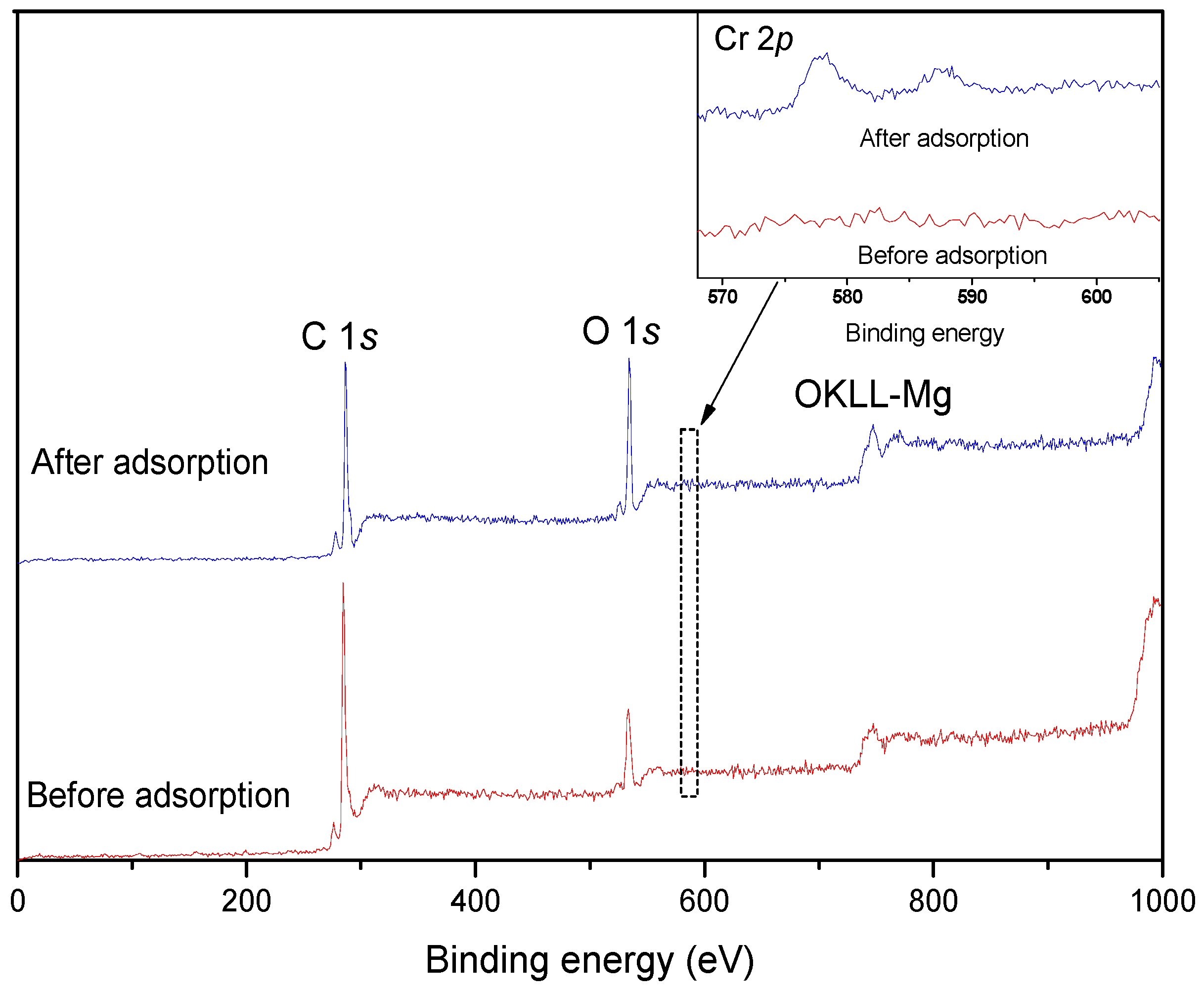
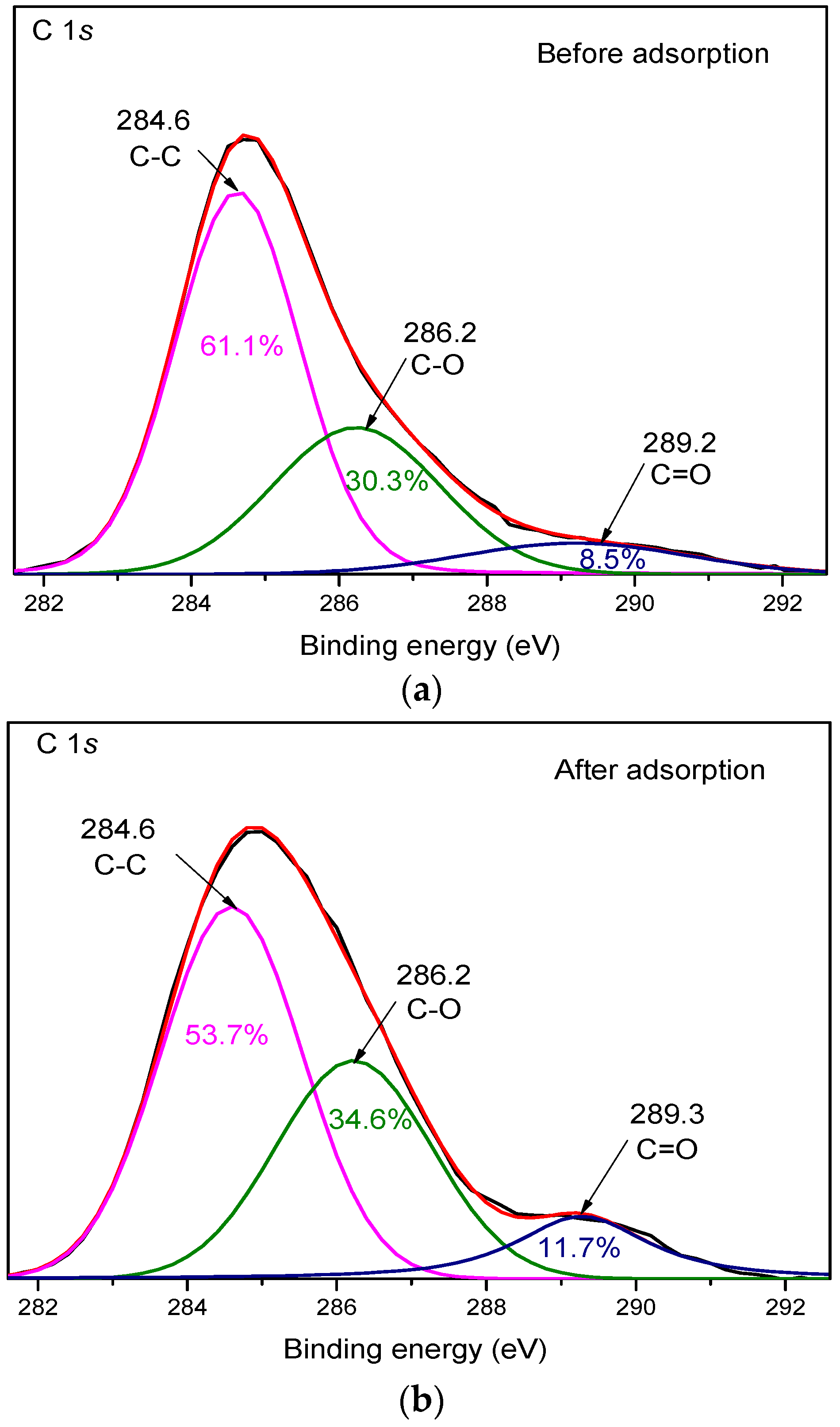
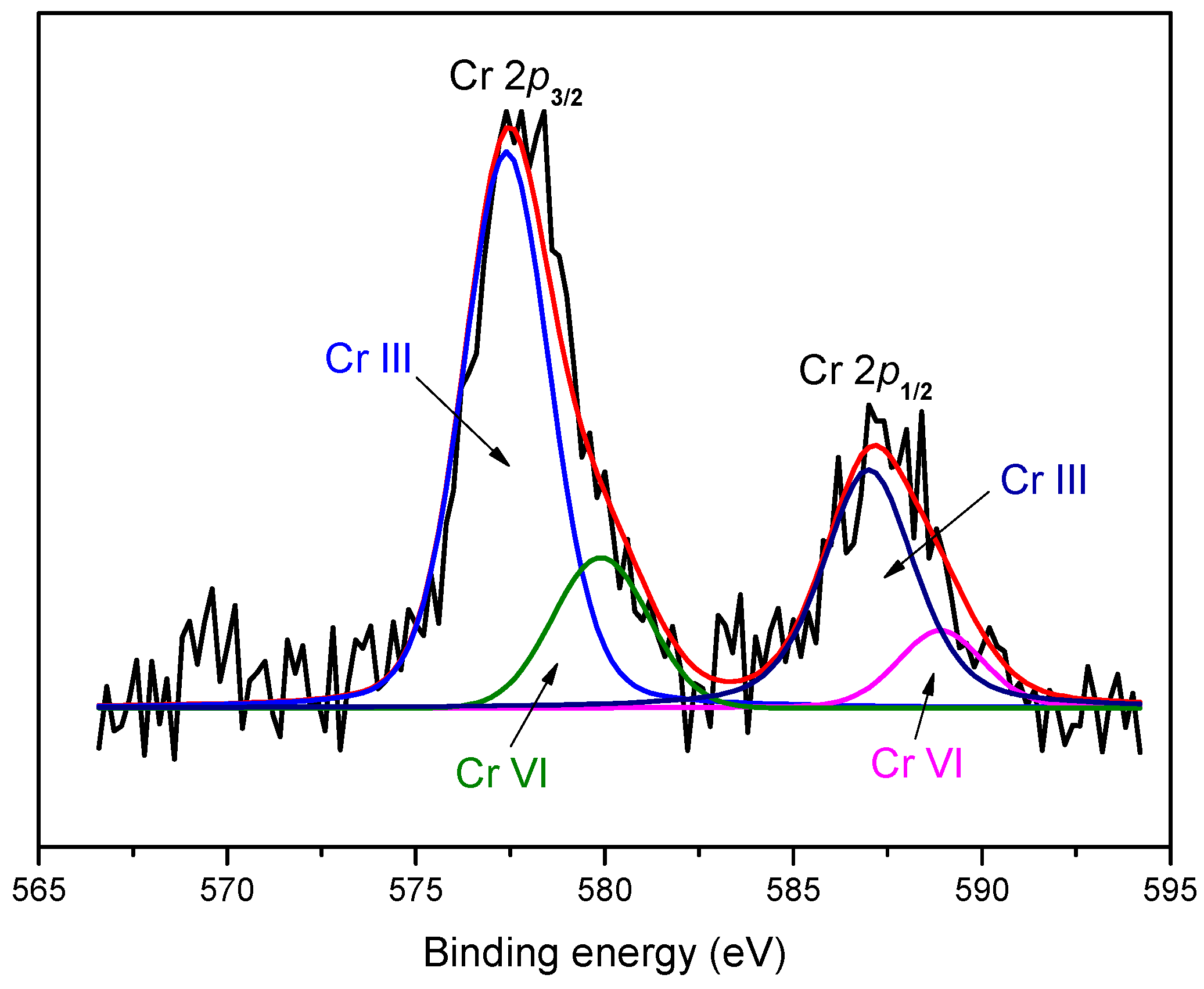
| Sample | ID/IG | D-Band Width (cm−1) | G-Band Width (cm−1) |
|---|---|---|---|
| WPAC-before | 3.22 | 288.52 | 340.30 |
| WPAC-after pH 10 | 3.17 | 209.63 | 224.80 |
| WPAC-after pH 7 | 3.22 | 328.08 | 359.45 |
| WPAC-after pH 3 | 3.84 | 327.44 | 310.65 |
| Sample | C | O | Cr | O/C |
|---|---|---|---|---|
| Before adsorption | 80.58% | 18.43% | 0 | 0.24 |
| After adsorption | 78.38% | 20.54% | 1.08% | 0.32 |
| Sample | Parameters | C-C | C-O | C=O |
|---|---|---|---|---|
| Before adsorption | Peak position (eV) | 284.6 | 286.2 | 289.2 |
| Peak area | 55,087.02 | 27,323.53 | 7691.37 | |
| After adsorption | Peak position (eV) | 284.6 | 286.2 | 289.3 |
| Peak area | 35,748.62 | 23,006.97 | 7755.30 |
| Species | Parameters | Cr 2p3/2 | Cr 2p1/2 |
|---|---|---|---|
| Cr(III) | Peak position (eV) | 577.4 | 586.9 |
| Peak area | 5759.27 | 2879.63 | |
| Cr(VI) | Peak position (eV) | 579.9 | 588.9 |
| Peak area | 1549.87 | 774.93 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; An, D.; Sun, S.; Gao, J.; Qian, L. Reduction and Removal of Chromium VI in Water by Powdered Activated Carbon. Materials 2018, 11, 269. https://doi.org/10.3390/ma11020269
Chen Y, An D, Sun S, Gao J, Qian L. Reduction and Removal of Chromium VI in Water by Powdered Activated Carbon. Materials. 2018; 11(2):269. https://doi.org/10.3390/ma11020269
Chicago/Turabian StyleChen, Yanan, Dong An, Sainan Sun, Jiayi Gao, and Linping Qian. 2018. "Reduction and Removal of Chromium VI in Water by Powdered Activated Carbon" Materials 11, no. 2: 269. https://doi.org/10.3390/ma11020269
APA StyleChen, Y., An, D., Sun, S., Gao, J., & Qian, L. (2018). Reduction and Removal of Chromium VI in Water by Powdered Activated Carbon. Materials, 11(2), 269. https://doi.org/10.3390/ma11020269





