Nanoparticle-Based Strategies to Treat Neuro-Inflammation
Abstract
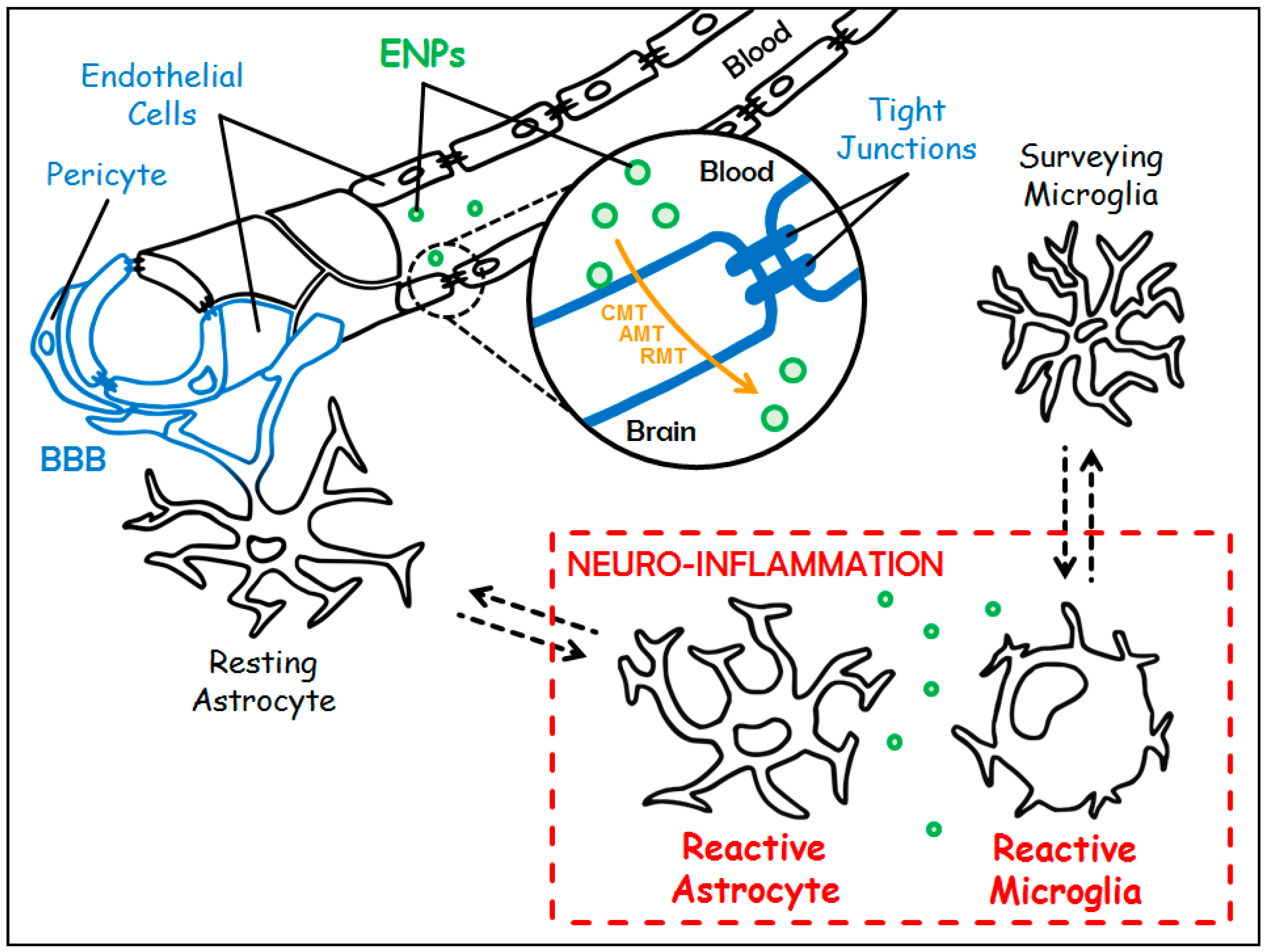
:1. Introduction
1.1. “Nanotechnology”: An Historical Perspective
1.2. “Nanotechnology” Today: A Buzzword
1.3. About Neuro-Inflammation
- (i)
- The first step is initiated upon recognition of the danger signals by microglia, its purpose is to eliminate the triggering element. It is characterized by the expression of class II antigen presenting molecules (MHC II) and costimulatory molecules (CD80, CD86), and by the secretion of pro-inflammatory cytokines (TNFα, IL-1β, IL-12…), chemokines (CCL2, CCL5…), nitric oxide (NO), and reactive oxygen species (ROS, such as superoxide anions). All are necessary to eradicate the aggressive agent;
- (ii)
- Then comes a phase of resolution of the inflammation characterized by the secretion of anti-inflammatory molecules (among others, the anti-inflammatory cytokines IL-10 and TGFβ), and tissue repair factors. This phase allows the arrest of the acute step, the healing of the injured tissue, and the return to homeostasis. A major difference with the systemic inflammatory response is that this resolution phase mediated by microglia also promotes neuroprotection and neuroreparation. On the one hand, neuroprotection is mediated through the synthesis of neurotrophic factors such as Insulin-like Growth Factor 1 (IGF1), Brain-Derived Neurotrophic Factor (BDNF), and Glial cell-Derived Neurotrophic Factor (GDNF). On the other hand, neuroreparation is mediated through the stimulation of neurogenesis by microglia, and through the plasticity of neural circuits.
1.4. About Blood-Brain Barrier
2. Engineered Nanoparticles: Promising Candidates to Tackle Neuro-Inflammation
2.1. Different Ways for Engineered Nanoparticles to Access the Central Nervous System
- (i)
- The Carrier-Mediated Transport (CMT) which is used to carry nutrients or endogenous substances into the brain. To name a few: glucose transporter-1 (GLUT-1/Slc2a1) for the uptake of glucose, and L1 and y+ for the uptake of large neutral and cationic essential amino-acids, respectively;
- (ii)
- The Adsorptive-Mediated Transcytosis (AMT) that involves electrostatic interactions between cationic compounds and negative charges of the membrane of endothelial cells prompting the formation of vesicles of endocytosis;
- (iii)
- The Receptor-Mediated Transcytosis (RMT) that relies on the expression of receptors at the luminal plasma membrane of endothelial cells (i.e., directed towards the bloodstream): transferrin receptor (TfR), LDL (Low Density Lipoprotein) Receptor-related Protein 1 and 2 (LRP-1 and -2), insulin receptor and folate receptor. This pathway warrants the entrance of endogenous macromolecules into the CNS.
2.2. Engineered Nanoparticles in Action
- (i)
- To increase blood circulating time thanks to PEG;
- (ii)
- To favor adsorptive-mediated transcytosis due to electrostatic interactions between polycationic CS and negatives charges of the membrane of the endothelial cells;
- (iii)
- To allow receptor-mediated transcytosis because of the high selectivity of OX-26 mAb for the highly expressed TfR. Two hours after intraperitoneal (IP) administration, semi-quantitative analysis revealed that ENPs were mostly located in the hippocampus and that the mean of CS-PEG-OX26 ENPs per optical field was two to three times greater than the one of CS-PEG ENPs. The authors made the proof of principle that these ENPs are able to cross the BBB and reach the brain, making them promising drug delivery systems to the CNS.
3. Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Taniguchi, N. On the basic concept of nanotechnology. In Proceedings of the International Conference on Production Engineering, Tokyo, Japan, 26–29 August 1974; Japan Society of Precision Engineering: Tokyo, Japan, 1974. [Google Scholar]
- Feynman, R.P. Plenty of room at the bottom. In Proceedings of the Meeting of the American Physical Society, Caltech, Pasadena, CA, USA, 29 December 1959. [Google Scholar]
- Drexler, K.E. Engines of Creation, the Coming Era of Nanotechnology; Anchor Books: New York, NY, USA, 1986. [Google Scholar]
- Wagner, V.; Dullaart, A.; Bock, A.K.; Zweck, A. The emerging nanomedicine landscape. Nat. Biotechnol. 2006, 24, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The ‘right’ size in nanobiotechnology. Nat. Biotechnol. 2003, 21, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Roco, M.C. Nanotechnology: Convergence with modern biology and medicine. Curr. Opin. Biotechnol. 2003, 14, 337–346. [Google Scholar] [CrossRef]
- Ball, P. Natural strategies for the molecular engineer. Nanotechnology 2002, 13, R15–R28. [Google Scholar] [CrossRef]
- Rolland, O.; Turrin, C.O.; Caminade, A.M.; Majoral, J.P. Dendrimers and nanomedicine: Multivalency in action. New J. Chem. 2009, 33, 1809–1824. [Google Scholar] [CrossRef]
- Shrikant, P.; Benveniste, E.N. The central nervous system as an immunocompetent organ: Role of glial cells in antigen presentation. J. Immunol. 1996, 157, 1819–1822. [Google Scholar] [PubMed]
- Dong, Y.; Benveniste, E.N. Immune function of astrocytes. Glia 2001, 36, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Salter, M.W.; Beggs, S. Sublime microglia: Expanding roles for the guardians of the CNS. Cell 2014, 158, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014, 14, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Chitnis, T.; Weiner, H.L. CNS inflammation and neurodegeneration. J. Clin. Investig. 2017, 127, 3577–3587. [Google Scholar] [CrossRef] [PubMed]
- Ransohoff, R.M. How neuroinflammation contributes to neurodegeneration. Science 2016, 353, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- Boillee, S.; Yamanaka, K.; Lobsiger, C.S.; Copeland, N.G.; Jenkins, N.A.; Kassiotis, G.; Kollias, G.; Cleveland, D.W. Onset and progression in inherited ALS determined by motor neurons and microglia. Science 2006, 312, 1389–1392. [Google Scholar] [CrossRef] [PubMed]
- Cekanaviciute, E.; Buckwalter, M.S. Astrocytes: Integrative regulators of neuroinflammation in stroke and other neurological diseases. Neurotherapeutics 2016, 13, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Faden, A.I.; Loane, D.J. Chronic neurodegeneration after traumatic brain injury: Alzheimer disease, chronic traumatic encephalopathy, or persistent neuroinflammation? Neurotherapeutics 2015, 12, 143–150. [Google Scholar] [CrossRef] [PubMed]
- The Top 10 Causes of Death. Available online: http://www.who.int/mediacentre/factsheets/fs310/en/ (accessed on 29 January 2018).
- Dementia. Available online: http://www.who.int/mediacentre/factsheets/fs362/en/ (accessed on 29 January 2018).
- Lindsley, C.W. 2014 global prescription medication statistics: Strong growth and CNS well represented. ACS Chem. Neurosci. 2015, 6, 505–506. [Google Scholar] [CrossRef] [PubMed]
- Biber, K.; Moller, T.; Boddeke, E.; Prinz, M. Central nervous system myeloid cells as drug targets: Current status and translational challenges. Nat. Rev. Drug Discov. 2016, 15, 110–124. [Google Scholar] [CrossRef] [PubMed]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Munch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Why is the global CNS pharmaceutical market so under-penetrated? Drug. Discov. Today 2002, 7, 5–7. [Google Scholar] [CrossRef]
- Reynolds, J.L.; Mahato, R.I. Nanomedicines for the treatment of CNS diseases. J. Neuroimmune Pharmacol. 2017, 12, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. The blood-brain barrier: Bottleneck in brain drug development. NeuroRx 2005, 2, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Kesselheim, A.S.; Hwang, T.J.; Franklin, J.M. Two decades of new drug development for central nervous system disorders. Nat. Rev. Drug Discov. 2015, 14, 815–816. [Google Scholar] [CrossRef] [PubMed]
- Daneman, R. The blood-brain barrier in health and disease. Ann. Neurol. 2012, 72, 648–672. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef] [PubMed]
- Cordon-Cardo, C.; O’Brien, J.P.; Casals, D.; Rittman-Grauer, L.; Biedler, J.L.; Melamed, M.R.; Bertino, J.R. Multidrug-resistance gene (P-glycoprotein) is expressed by endothelial cells at blood-brain barrier sites. Proc. Natl. Acad. Sci. USA 1989, 86, 695–698. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. From blood-brain barrier to blood-brain interface: New opportunities for CNS drug delivery. Nat. Rev. Drug Discov. 2016, 15, 275–292. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.M.; Alavijeh, M.S. Translational CNS medicines research. Drug Discov. Today 2012, 17, 1068–1078. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, M.; Kessler, J.A. Nanotechnology-novel therapeutics for CNS disorders. Nat. Rev. Neurol. 2012, 8, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Serramia, M.J.; Alvarez, S.; Fuentes-Paniagua, E.; Clemente, M.I.; Sanchez-Nieves, J.; Gomez, R.; de la Mata, J.; Munoz-Fernandez, M.A. In vivo delivery of siRNA to the brain by carbosilane dendrimer. J. Control. Release 2015, 200, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Illum, L. Transport of drugs from the nasal cavity to the central nervous system. Eur. J. Pharm. Sci. 2000, 11, 1–18. [Google Scholar] [CrossRef]
- Lochhead, J.J.; Thorne, R.G. Intranasal delivery of biologics to the central nervous system. Adv. Drug Deliv. Rev. 2012, 64, 614–628. [Google Scholar] [CrossRef] [PubMed]
- Illum, L. Nasal drug delivery—Possibilities, problems and solutions. J. Control. Release 2003, 87, 187–198. [Google Scholar] [CrossRef]
- Hu, K.; Shi, Y.; Jiang, W.; Han, J.; Huang, S.; Jiang, X. Lactoferrin conjugated PEG-PLGA nanoparticles for brain delivery: Preparation, characterization and efficacy in Parkinson’s disease. Int. J. Pharm. 2011, 415, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Tong, Y.; Bai, L.; Ye, L.; Zhong, L.; Duan, X.; Zhu, Y. Lactoferrin functionalized PEG-PLGA nanoparticles of shikonin for brain targeting therapy of glioma. Int. J. Biol. Macromol. 2018, 107, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.K.; Agarwal, S.; Seth, B.; Yadav, A.; Nair, S.; Bhatnagar, P.; Karmakar, M.; Kumari, M.; Chauhan, L.K.; Patel, D.K.; et al. Curcumin-loaded nanoparticles potently induce adult neurogenesis and reverse cognitive deficits in Alzheimer’s disease model via canonical Wnt/beta-catenin pathway. ACS Nano 2014, 8, 76–103. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, Y.S.R.; Etman, S.M.; Abdelmonsif, D.A.; Abdallah, O.Y. Intranasal piperine-loaded chitosan nanoparticles as brain-targeted therapy in Alzheimer’s disease: Optimization, biological efficacy, and potential toxicity. J. Pharm. Sci. 2015, 104, 3544–3556. [Google Scholar] [CrossRef] [PubMed]
- Hernando, S.; Herran, E.; Figueiro-Silva, J.; Pedraz, J.L.; Igartua, M.; Carro, E.; Hernandez, R.M. Intranasal administration of TAT-conjugated lipid nanocarriers loading GDNF for Parkinson’s disease. Mol. Neurobiol. 2017, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Rait, A.; Garrido-Sanabria, E.R.; Pirollo, K.F.; Harford, J.B.; Chang, E.H. Nanotherapeutics for gene modulation that prevents apoptosis in the brain and fatal neuroinflammation. Mol. Ther. 2018, 26, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ye, M.; An, C.; Pan, L.; Ji, L. The effect of cationic albumin-conjugated PEGylated tanshinone IIA nanoparticles on neuronal signal pathways and neuroprotection in cerebral ischemia. Biomaterials 2013, 34, 6893–6905. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Yan, Z.; Hu, K.; Pang, Z.; Cheng, X.; Guo, L.; Zhang, Q.; Jiang, X.; Fang, L.; Lai, R. Odorranalectin-conjugated nanoparticles: Preparation, brain delivery and pharmacodynamic study on Parkinson’s disease following intranasal administration. J. Control. Release 2011, 151, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.; Gandham, S.K.; Panicucci, R.; Amiji, M.M. Intranasal brain delivery of cationic nanoemulsion-encapsulated TNFalpha siRNA in prevention of experimental neuroinflammation. Nanomedicine 2016, 12, 987–1002. [Google Scholar] [CrossRef] [PubMed]
- Voigt, N.; Henrich-Noack, P.; Kockentiedt, S.; Hintz, W.; Tomas, J.; Sabel, B.A. Surfactants, not size or zeta-potential influence blood-brain barrier passage of polymeric nanoparticles. Eur. J. Pharm. Biopharm. 2014, 87, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, B.; Xie, S.; Yang, B.; Xu, Q.; Tan, J. Superparamagnetic iron oxide nanoparticles modified with Tween 80 pass through the intact blood-brain barrier in rats under magnetic field. ACS Appl. Mater. Interfaces 2016, 8, 11336–11341. [Google Scholar] [CrossRef] [PubMed]
- Koffie, R.M.; Farrar, C.T.; Saidi, L.J.; William, C.M.; Hyman, B.T.; Spires-Jones, T.L. Nanoparticles enhance brain delivery of blood-brain barrier-impermeable probes for in vivo optical and magnetic resonance imaging. Proc. Natl. Acad. Sci. USA 2011, 108, 18837–18842. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cai, P.; Shalviri, A.; Henderson, J.T.; He, C.; Foltz, W.D.; Prasad, P.; Brodersen, P.M.; Chen, Y.; DaCosta, R.; et al. A multifunctional polymeric nanotheranostic system delivers doxorubicin and imaging agents across the blood-brain barrier targeting brain metastases of breast cancer. ACS Nano 2014, 8, 9925–9940. [Google Scholar] [CrossRef] [PubMed]
- Betzer, O.; Shilo, M.; Opochinsky, R.; Barnoy, E.; Motiei, M.; Okun, E.; Yadid, G.; Popovtzer, R. The effect of nanoparticle size on the ability to cross the blood-brain barrier: An in vivo study. Nanomedicine 2017, 12, 1533–1546. [Google Scholar] [CrossRef] [PubMed]
- Monsalve, Y.; Tosi, G.; Ruozi, B.; Belletti, D.; Vilella, A.; Zoli, M.; Vandelli, M.A.; Forni, F.; Lopez, B.L.; Sierra, L. PEG-g-chitosan nanoparticles functionalized with the monoclonal antibody OX26 for brain drug targeting. Nanomedicine 2015, 10, 1735–1750. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Lu, Y.M.; Wang, H.; Liu, J.; Liao, M.H.; Hong, L.J.; Tao, R.R.; Ahmed, M.M.; Liu, P.; Liu, S.S.; et al. The effect of lipid nanoparticle PEGylation on neuroinflammatory response in mouse brain. Biomaterials 2013, 34, 7960–7970. [Google Scholar] [CrossRef] [PubMed]
- Colvin, V.L. The potential environmental impact of engineered nanomaterials. Nat. Biotechnol. 2003, 21, 1166–1170. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. A review of clinical translation of inorganic nanoparticles. AAPS J. 2015, 17, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Ehmann, F.; Sakai-Kato, K.; Duncan, R.; Hernan Perez de la Ossa, D.; Pita, R.; Vidal, J.M.; Kohli, A.; Tothfalusi, L.; Sanh, A.; Tinton, S.; et al. Next-generation nanomedicines and nanosimilars: EU regulators’ initiatives relating to the development and evaluation of nanomedicines. Nanomedicine 2013, 8, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Hamburg, M.A. Science and regulation. FDA’s approach to regulation of products of nanotechnology. Science 2012, 336, 299–300. [Google Scholar] [CrossRef] [PubMed]
- Hoet, P.; Legiest, B.; Geys, J.; Nemery, B. Do nanomedicines require novel safety assessments to ensure their safety for long-term human use? Drug Saf. 2009, 32, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Pita, R.; Ehmann, F.; Papaluca, M. Nanomedicines in the EU—regulatory overview. AAPS J. 2016, 18, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, M.; Moshtaghian, J.; Ghaedi, K.; Jafari Dinani, N.; Naderi, G. Effects of silver nanoparticle on the developing liver of rat pups after maternal exposure. Iran. J. Pharm. Res. 2017, 16, 685–693. [Google Scholar] [PubMed]
- Brohi, R.D.; Wang, L.; Talpur, H.S.; Wu, D.; Khan, F.A.; Bhattarai, D.; Rehman, Z.U.; Farmanullah, F.; Huo, L.J. Toxicity of nanoparticles on the reproductive system in animal models: A review. Front. Pharmacol. 2017, 8, 606. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, T.G.; Stueckle, T.A.; Antonini, J.A.; Rojanasakul, Y.; Castranova, V.; Yang, Y.; Wang, L. Potential toxicity and underlying mechanisms associated with pulmonary exposure to iron oxide nanoparticles: Conflicting literature and unclear risk. Nanomaterials 2017, 7, 307. [Google Scholar] [CrossRef] [PubMed]
- Iversen, T.G.; Skotland, T.; Sandvig, K. Endocytosis and intracellular transport of nanoparticles: Present knowledge and need for future studies. Nano Today 2011, 6, 176–185. [Google Scholar] [CrossRef]
- Cao, Y.; Long, J.; Liu, L.; He, T.; Jiang, L.; Zhao, C.; Li, Z. A review of endoplasmic reticulum (ER) stress and nanoparticle (NP) exposure. Life Sci. 2017, 186, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; Germolec, D.R.; Weaver, J.L. Evaluation of nanoparticle immunotoxicity. Nat. Nanotechnol. 2009, 4, 411–414. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D.; Italiani, P.; Palomba, R.; Decuzzi, P.; Duschl, A.; Fadeel, B.; Moghimi, S.M. Nanoparticles and innate immunity: New perspectives on host defence. Semin. Immunol. 2017, 34, 33–51. [Google Scholar] [CrossRef] [PubMed]
- Lei, C.; Sun, Y.; Tsang, D.C.W.; Lin, D. Environmental transformations and ecological effects of iron-based nanoparticles. Environ. Pollut. 2017, 232, 10–30. [Google Scholar] [CrossRef] [PubMed]
- Schwab, F.; Zhai, G.; Kern, M.; Turner, A.; Schnoor, J.L.; Wiesner, M.R. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants—Critical review. Nanotoxicology 2016, 10, 257–278. [Google Scholar] [CrossRef] [PubMed]
- Libralato, G.; Galdiero, E.; Falanga, A.; Carotenuto, R.; de Alteriis, E.; Guida, M. Toxicity effects of functionalized quantum dots, gold and polystyrene nanoparticles on target aquatic biological models: A review. Molecules 2017, 22, 1439. [Google Scholar] [CrossRef] [PubMed]
- Mahaye, N.; Thwala, M.; Cowan, D.A.; Musee, N. Genotoxicity of metal based engineered nanoparticles in aquatic organisms: A review. Mutat. Res. 2017, 773, 134–160. [Google Scholar] [CrossRef] [PubMed]
| ENP Type | Customization (Targeting Ligand) | Therapeutics | Route of Administration and Animal Model | Reference |
|---|---|---|---|---|
| polyethylene gycol–polylactide–polyglycolide (PEG-PLGA) | Lactoferrin (Lf) targeting the Transferrin Receptor (TfR) on endothelial cells | Urocortin (URO) | Intravenous (IV) Rat model of Parkinson’s disease (PD) | [40] |
| PEG-PLGA | Lactoferrin (Lf) targeting the TfR on endothelial cells | Shikonin (SHK) | IV Healthy rats only | [41] |
| PLGA | Non applicable (NA) | Curcumin (Cur) | Intraperitoneal (IP) Rat model of Alzheimer’s disease (AD) | [42] |
| Tripolyphosphate cross-linked cationic chitosan (CS) | NA | Piperine (PIP) | Intranasal (IN) Rat model of sporadic dementia of AD type | [43] |
| Nanostructured Lipid Carrier (NLC) coated with cationic CS | TransActivator of Transcription (TAT) | Glial cell-Derived Neurotrophic Factor (GDNF) | IN Mouse model of PD | [44] |
| cationic nanoliposomes (scL) | Single-chain fragment from the variable region of anti-TfR monoclonal antibody (TfRscFv) | siRNA against TNFα | IV Lipopolysaccharide (LPS)-induced neuro-inflammation in mice | [45] |
| PEG polymeric poly lactic acid (PLA) | Cationic bovine serum albumin (CBSA) | Tanshinone IIA (TIIA) | Rat model of cerebral ischemic stroke | [46] |
| PEG-PLGA | Odorranalectin (OL), targeting l-fucose expressed on the olfactory epithelium | URO | IN Rat model of PD | [47] |
| Cationic lipids nanoemulsions (SNE) | NA | siRNA against TNFα | IN LPS-induced neuro-inflammation in rats | [48] |
| Poly-Butyl-CyanoAcrylate (PBCA) | Non-ionic surfactants (in particular Tween 80) with or without cationic resin DEAE (both interacting with circulating apoliproprotein E) | NA | IV Healthy rats only | [49] |
| Gold nanoparticles (GNP) | Insulin (INS) | NA | IV Healthy mice only | [53] |
| CS-PEG | Anti-TfR monoclonal antibody OX26 | NA | IP Healthy mice only | [54] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poupot, R.; Bergozza, D.; Fruchon, S. Nanoparticle-Based Strategies to Treat Neuro-Inflammation. Materials 2018, 11, 270. https://doi.org/10.3390/ma11020270
Poupot R, Bergozza D, Fruchon S. Nanoparticle-Based Strategies to Treat Neuro-Inflammation. Materials. 2018; 11(2):270. https://doi.org/10.3390/ma11020270
Chicago/Turabian StylePoupot, Rémy, Dylan Bergozza, and Séverine Fruchon. 2018. "Nanoparticle-Based Strategies to Treat Neuro-Inflammation" Materials 11, no. 2: 270. https://doi.org/10.3390/ma11020270
APA StylePoupot, R., Bergozza, D., & Fruchon, S. (2018). Nanoparticle-Based Strategies to Treat Neuro-Inflammation. Materials, 11(2), 270. https://doi.org/10.3390/ma11020270





