A Facile and Low-Cost Method to Produce Ultrapure 99.99999% Gallium
Abstract
:1. Introduction
2. Methods
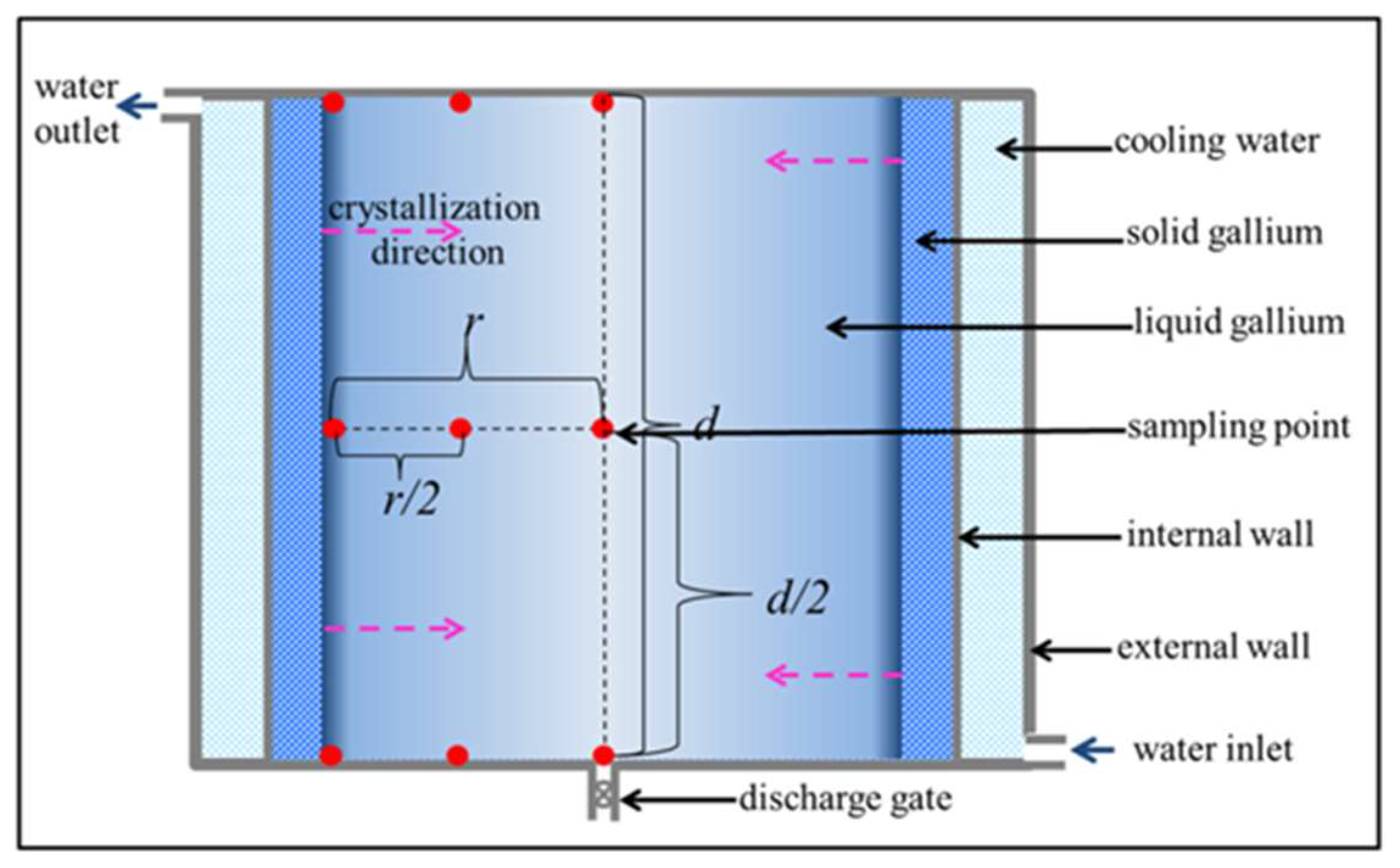
2.1. Refining Process
2.2. Quality Test
3. Results and Discussion
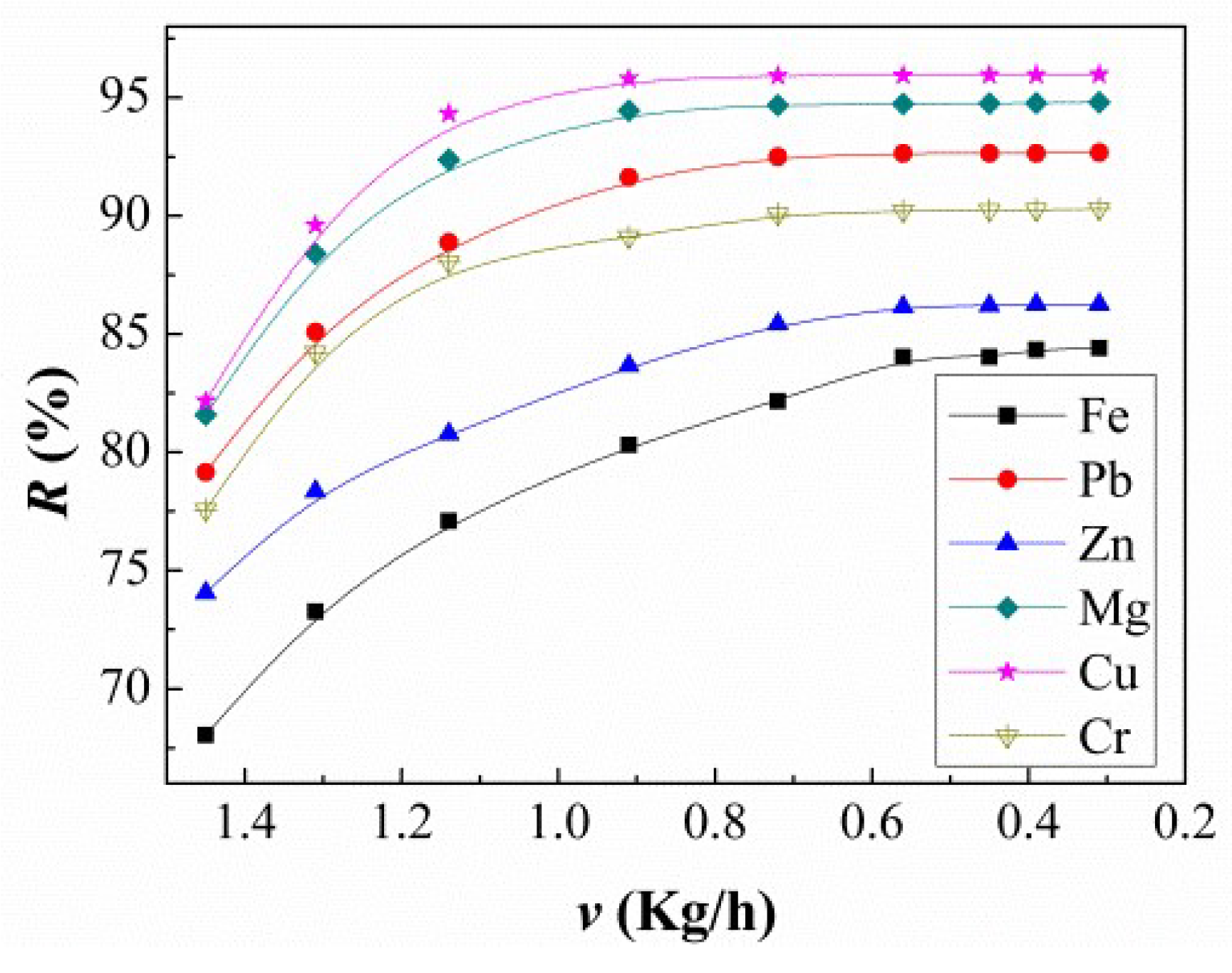
3.1. Flow Rate and Temperature of the Cooling Water Influence on the Refining Process
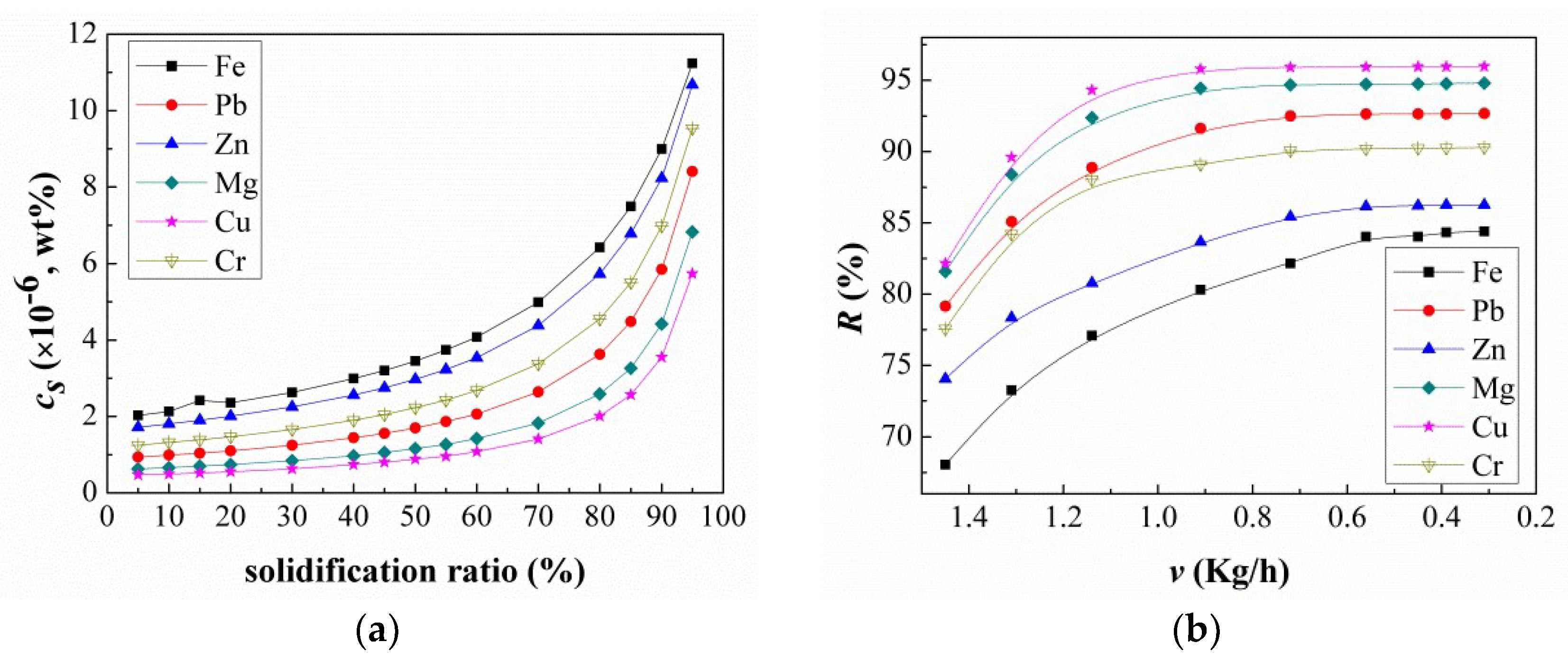
3.2. Impurity Redistribution
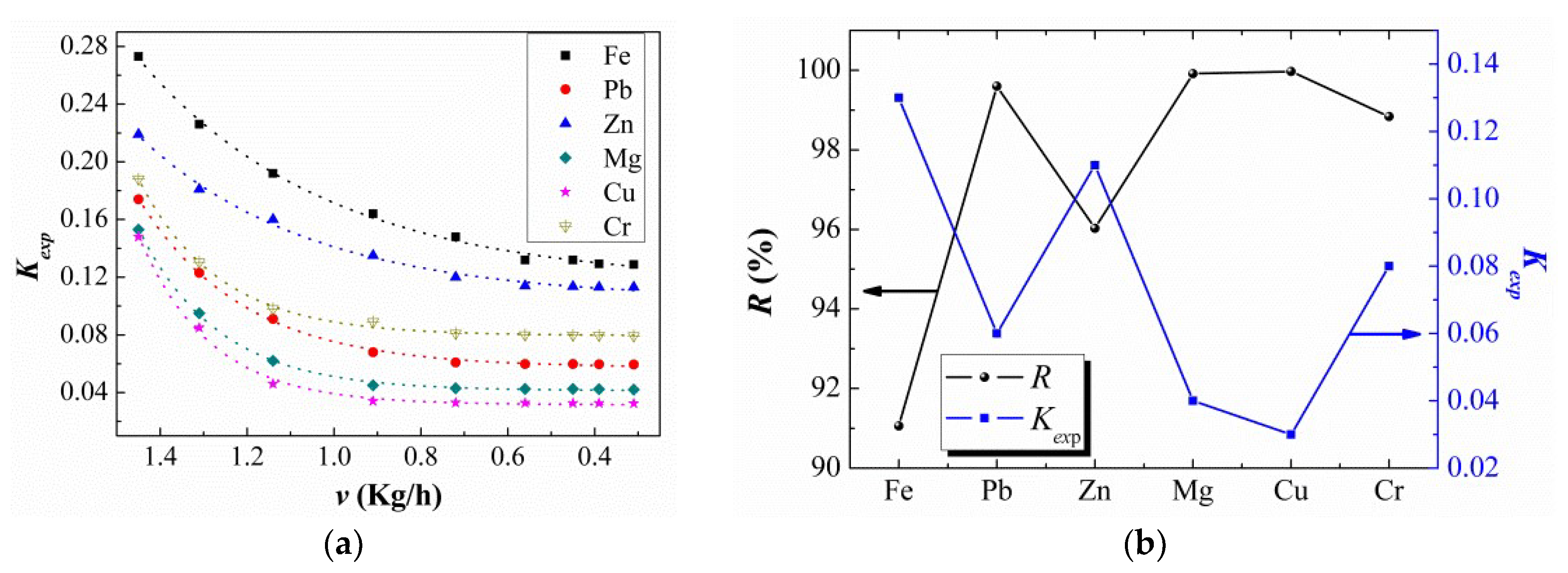
3.3. Experimental Segregation Coefficient
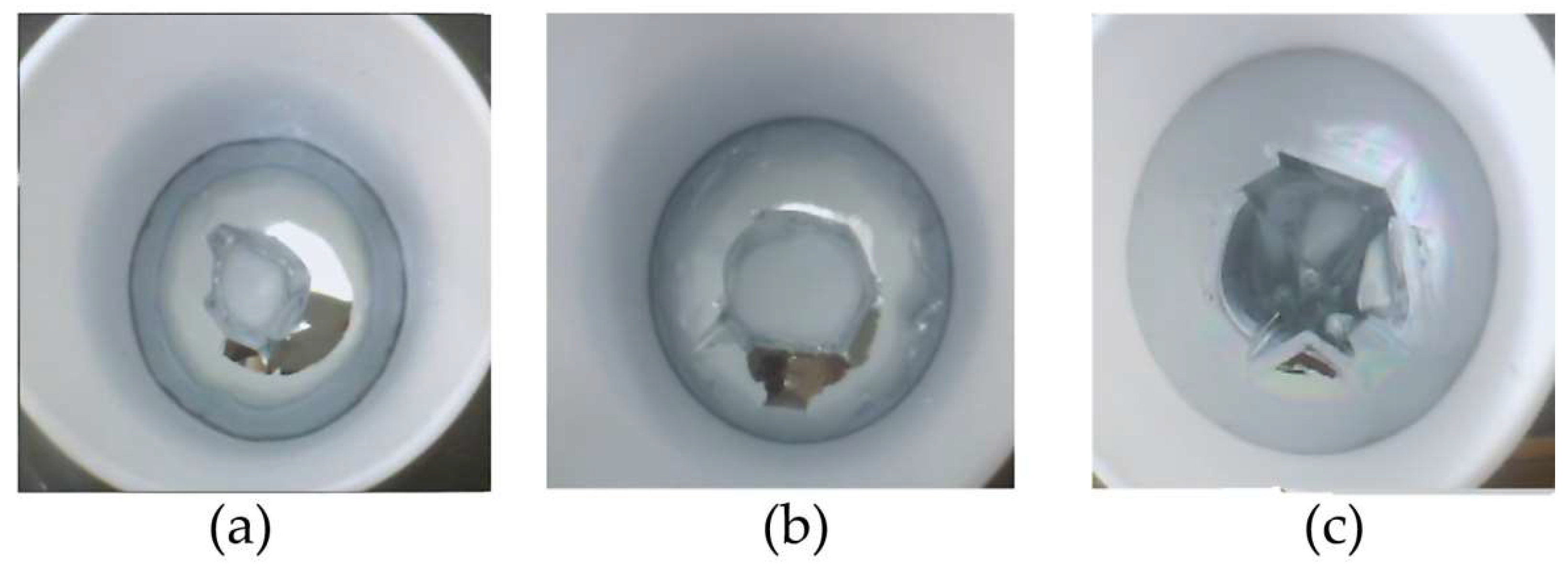
3.4. Quality of Ultrapure Gallium
4. Conclusions
5. Patent
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moskalyk, R.R. Gallium: The backbone of the electronics industry. Miner. Eng. 2003, 16, 921–929. [Google Scholar] [CrossRef]
- Colantonio, P.; Giannini, F.; Giofrè, R.; Piazzon, L. Increasing Doherty amplifier average efficiency exploiting device knee voltage behavior. IEEE Trans. Microw. Theory Tech. 2011, 59, 2295–2305. [Google Scholar] [CrossRef]
- Yoon, J.S.; Yang, J.Y.; Lee, J.M.; Hong, S.J.; Hong, H.S. A development of the LED TMGa precursor reuse technology. Mater. Lett. 2013, 93, 153–156. [Google Scholar] [CrossRef]
- Kim, I.; Kivisaari, P.; Oksanen, J.; Suihkonen, S. Diffusion-drivens charge transport in light emitting devices. Materials 2017, 10, 1421. [Google Scholar] [CrossRef] [PubMed]
- Oon, H.S.; Cheong, K.Y. Recent development of gallium oxide thin film on GaN. Mater. Sci. Semicond. Process. 2013, 16, 1217–1231. [Google Scholar] [CrossRef]
- Liu, S.; Sweatman, K.; McDonald, S.; Nogita, K. Ga-Based Alloys in Microelectronic Interconnects: A Review. Materials 2018, 11, 1384. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.F.; Zhang, C.Y.; Zhang, Z.B.; Pun, E.Y.B.; Zhang, D.L. Low-loss Ga:Er:LiTaO3optical waveguide for integrated optics. Mater. Lett. 2018, 227, 199–201. [Google Scholar] [CrossRef]
- Li, P.W.; Zhou, W.H.; Hou, Z.L.; Wu, S.X. Synthesis of CuIn xGa 1-xSe 2 nanocrystals for potential thin film photovoltaic application under air condition. Mater. Lett. 2012, 78, 131–134. [Google Scholar] [CrossRef]
- Wei, Y.; Zhuang, D.; Zhao, M.; Zhang, N.; Yu, X.; Sun, R.; Zhang, L.; Lyu, X.; Peng, X.; Wei, J. Fabrication of wide band-gap CuGaSe2solar cells for tandem device applications by sputtering from a ternary target and post selenization treatment. Mater. Lett. 2018, 230, 128–131. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, Y.; Xiao, Y.; Fan, Y. Recovery of gallium from Bayer liquor: A review. Hydrometallurgy 2012, 125, 115–124. [Google Scholar] [CrossRef]
- Swain, B.; Mishra, C.; Kang, L.; Park, K.S.; Lee, C.G.; Hong, H.S. Recycling process for recovery of gallium from GaN an e-waste of LED industry through ball milling, annealing and leaching. Environ. Res. 2015, 138, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Liu, Z.; Li, Y.; Wilson, B.P.; Lundström, M. Recovery and separation of gallium(III) and germanium(IV) from zinc refinery residues: Part I: Leaching and iron(III) removal. Hydrometallurgy 2017, 169, 564–570. [Google Scholar] [CrossRef]
- Lu, F.; Xiao, T.; Lin, J.; Ning, Z.; Long, Q.; Xiao, L.; Huang, F.; Wang, W.; Xiao, Q.; Lan, X.; et al. Resources and extraction of gallium: A review. Hydrometallurgy 2017, 174, 105–115. [Google Scholar] [CrossRef]
- Ober, J.A. Mineral Commodity Summaries 2018; U.S. Geological Survey: Reston, VA, USA, 2018.
- Meyer, S.; Wahl, S.; Molchanov, A.; Neckermann, K.; Möller, C.; Lauer, K.; Hagendorf, C. Influence of the feedstock purity on the solar cell efficiency. Sol. Energy Mater. Sol. Cells 2014, 130, 668–672. [Google Scholar] [CrossRef]
- Greber, J.F. Gallium and Gallium Compounds. Kirk-Othmer Encycl. Ind. Chem. 2000. [Google Scholar] [CrossRef]
- Huang, F.; Chen, R.; Guo, J.; Ding, H.; Su, Y. Removal of metal impurities in metallurgical grade silicon by cold crucible continuous melting and directional solidification. Sep. Purif. Technol. 2017, 188, 67–72. [Google Scholar] [CrossRef]
- Wen, S.; Jiang, D.; Shi, S.; Tan, Y.; Li, P.; Gu, Z.; Zhang, X. Determination and controlling of crystal growth rate during silicon purification by directional solidification. Vacuum 2016, 125, 75–80. [Google Scholar] [CrossRef]
- Wen, S.; Jiang, D.; Li, P.; Tan, Y. Back diffusion of iron impurity during silicon purification by vacuum directional solidification. Vacuum 2015, 119, 270–275. [Google Scholar] [CrossRef]
- Martorano, M.A.; Neto, J.B.B.F.; Oliveira, T.S.; Tsubaki, T.O. Refining of metallurgical silicon by directional solidification. Mater. Sci. Eng. B Solid-State Mater. Adv. Technol. 2011, 176, 217–226. [Google Scholar] [CrossRef]
- Huang, S.; Ma, W.; Wei, K.; Li, S.; Morita, K. A model for distribution of aluminum in silicon refined by vacuum directional solidification. Vacuum 2013, 96, 12–17. [Google Scholar] [CrossRef]

| Impurity | Fe | Pb | Zn | Mg | Cu | Cr | Purity % |
|---|---|---|---|---|---|---|---|
| Before refining | 15 | 56 | 24 | 76 | 107 | 40 | 99.9938 |
| After refining | 0.93 | 0.68 | 1.05 | 0.34 | 0.22 | 0.97 | 99.9999958 |
| Removal ratio (%) | 93.79 | 98.78 | 95.64 | 99.55 | 99.79 | 97.58 | – |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, K.; Li, Y.; Zhang, J.; Zhao, Q. A Facile and Low-Cost Method to Produce Ultrapure 99.99999% Gallium. Materials 2018, 11, 2308. https://doi.org/10.3390/ma11112308
Pan K, Li Y, Zhang J, Zhao Q. A Facile and Low-Cost Method to Produce Ultrapure 99.99999% Gallium. Materials. 2018; 11(11):2308. https://doi.org/10.3390/ma11112308
Chicago/Turabian StylePan, Kefeng, Ying Li, Jiawei Zhang, and Qing Zhao. 2018. "A Facile and Low-Cost Method to Produce Ultrapure 99.99999% Gallium" Materials 11, no. 11: 2308. https://doi.org/10.3390/ma11112308
APA StylePan, K., Li, Y., Zhang, J., & Zhao, Q. (2018). A Facile and Low-Cost Method to Produce Ultrapure 99.99999% Gallium. Materials, 11(11), 2308. https://doi.org/10.3390/ma11112308




