Surface Oxidation of TiNiSn (Half-Heusler) Alloy by Oxygen and Water Vapor
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
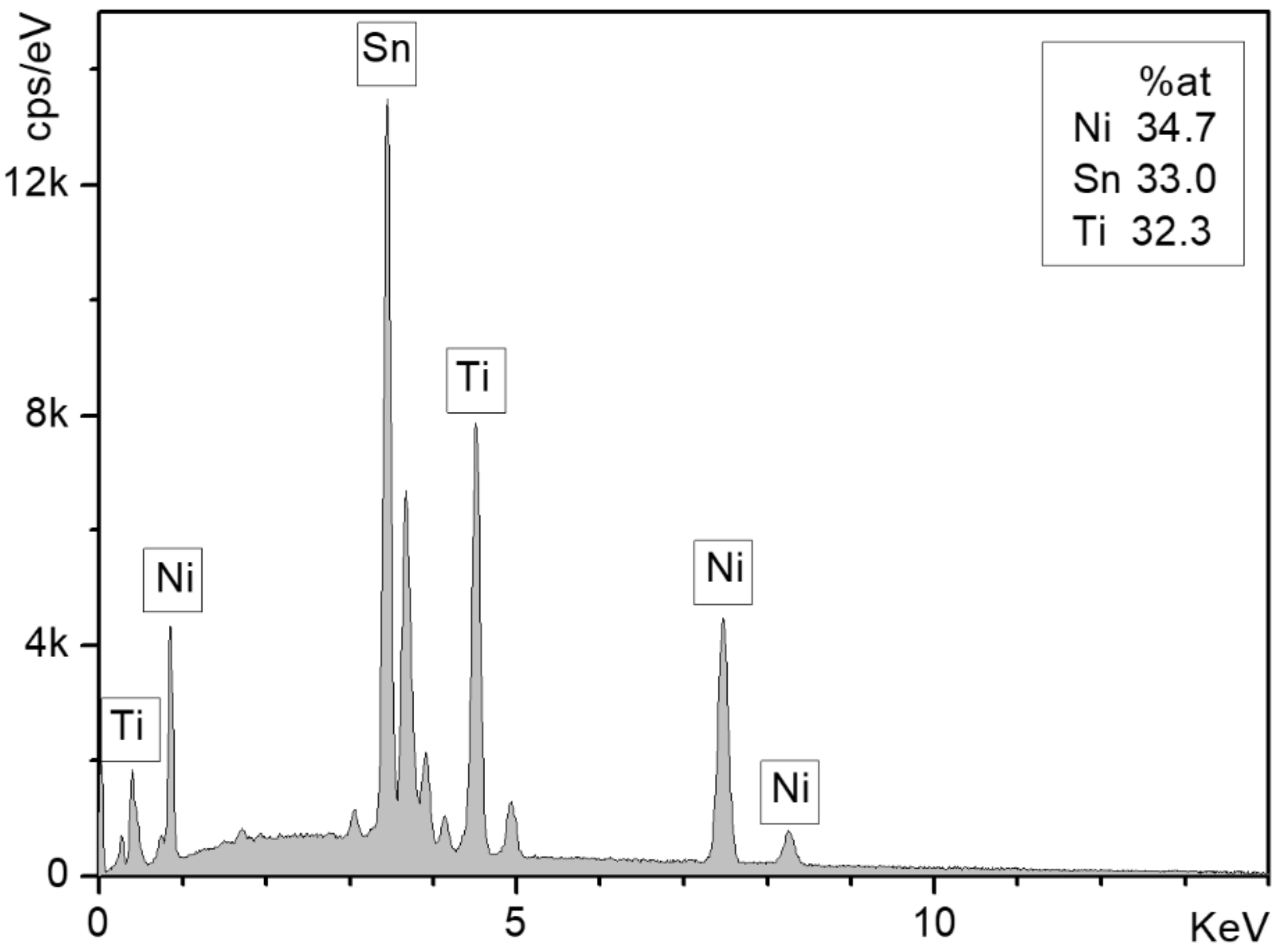
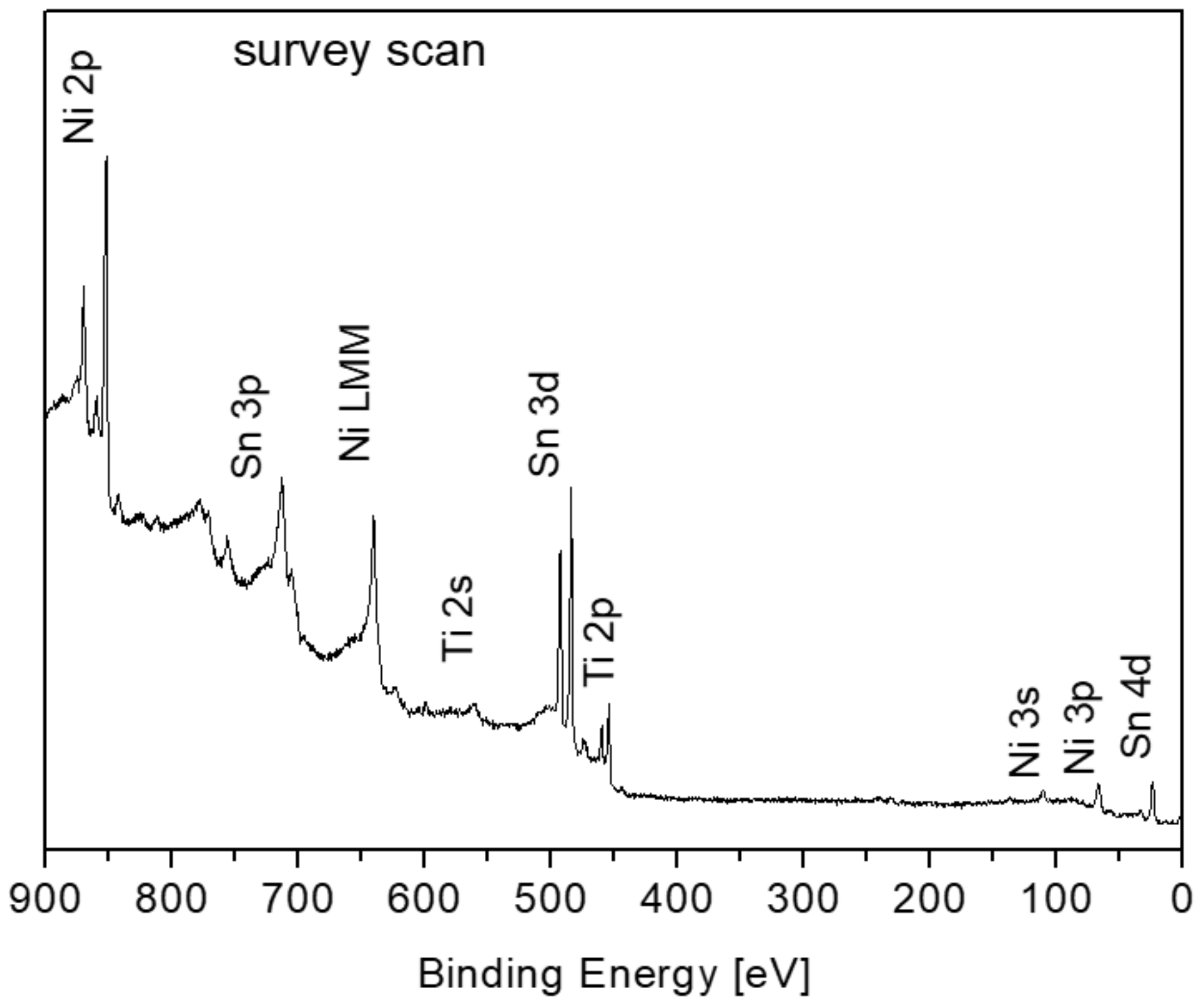
3.1. Surface Characterization and Segregation
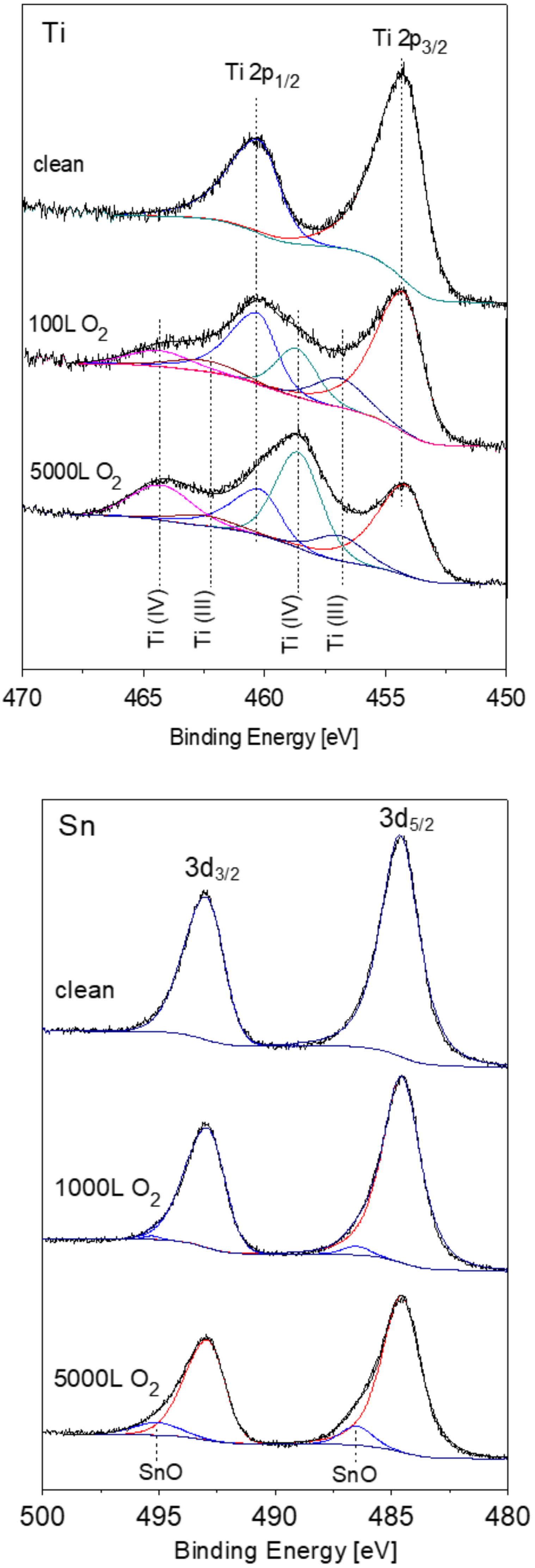
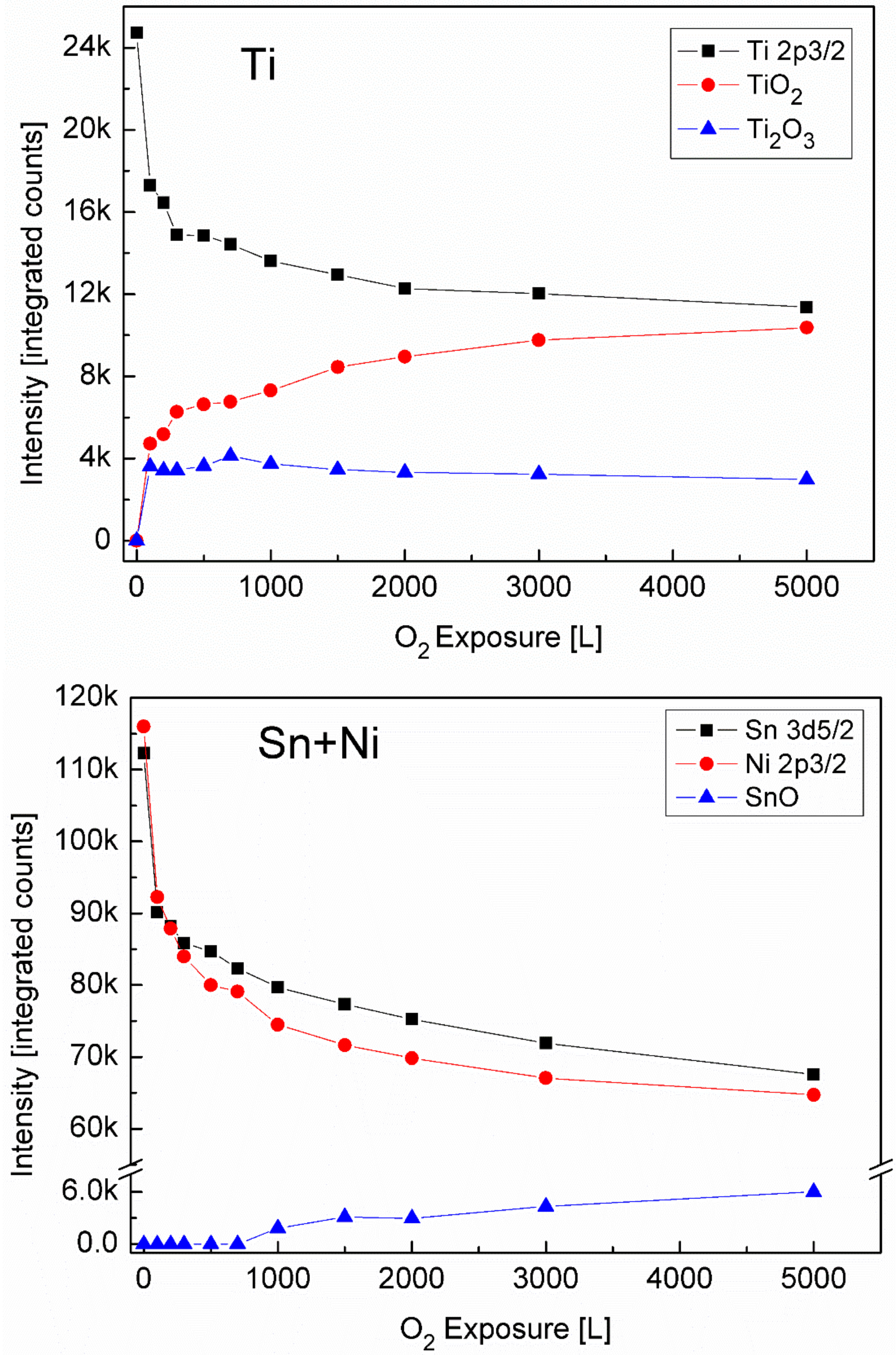
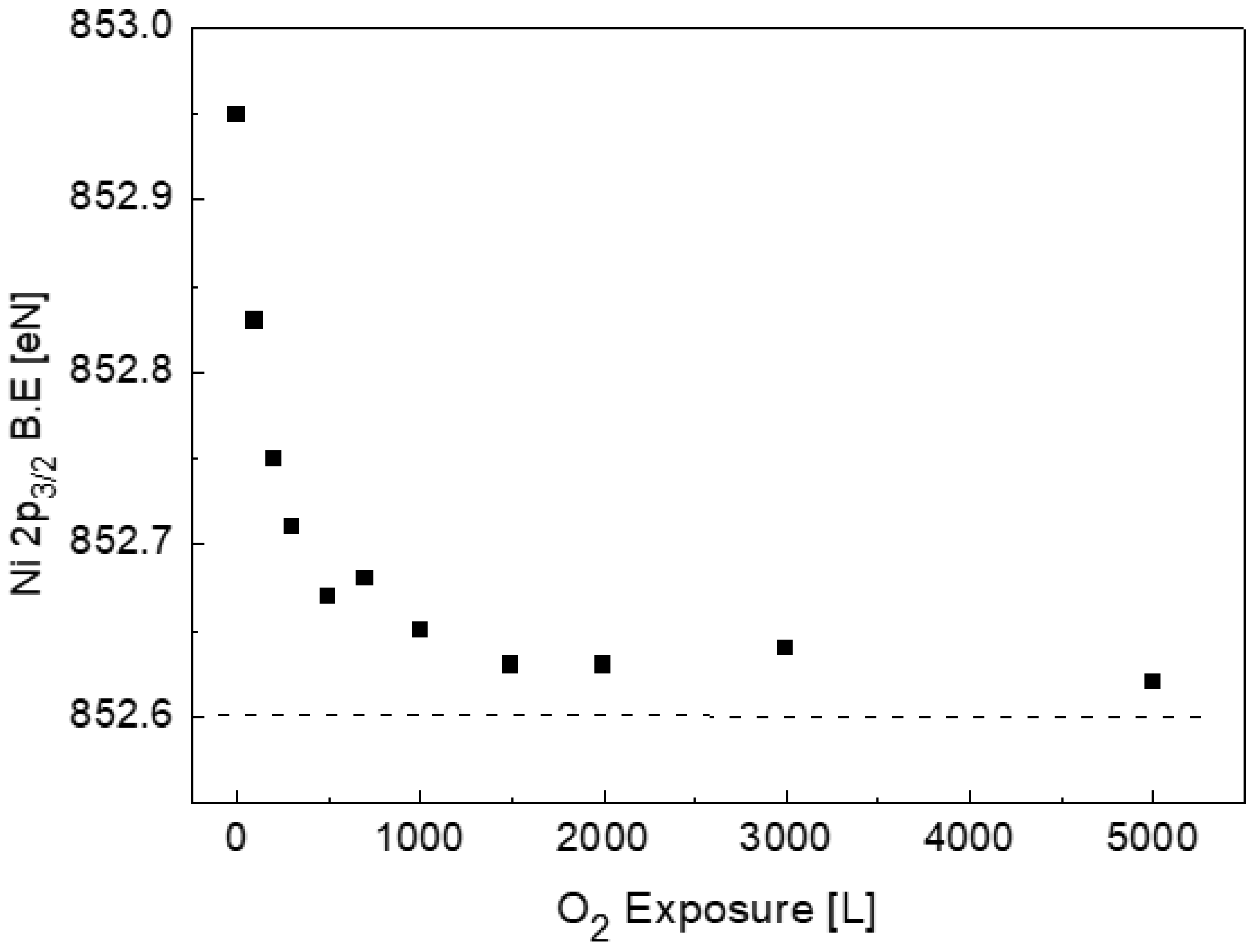
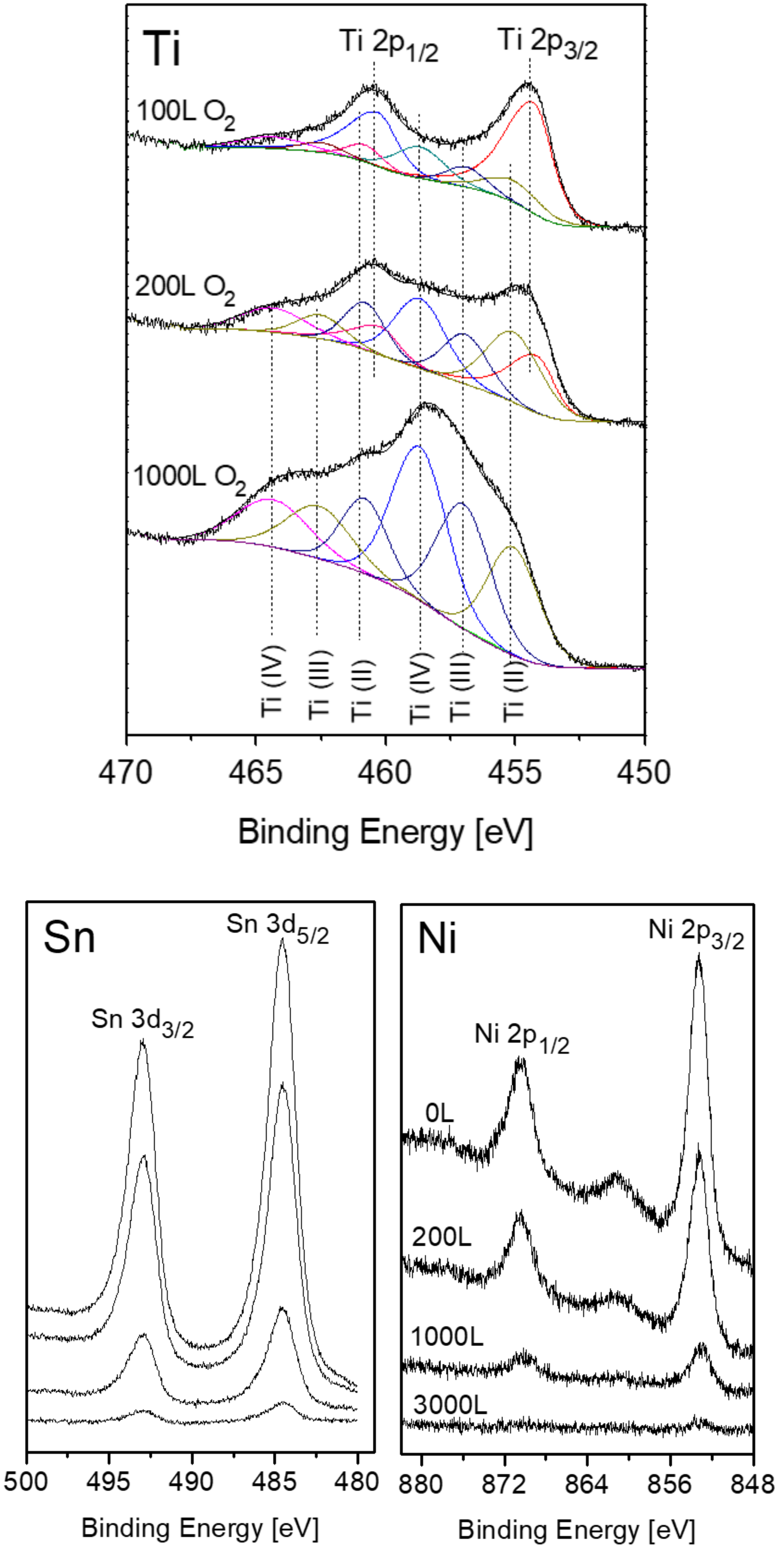
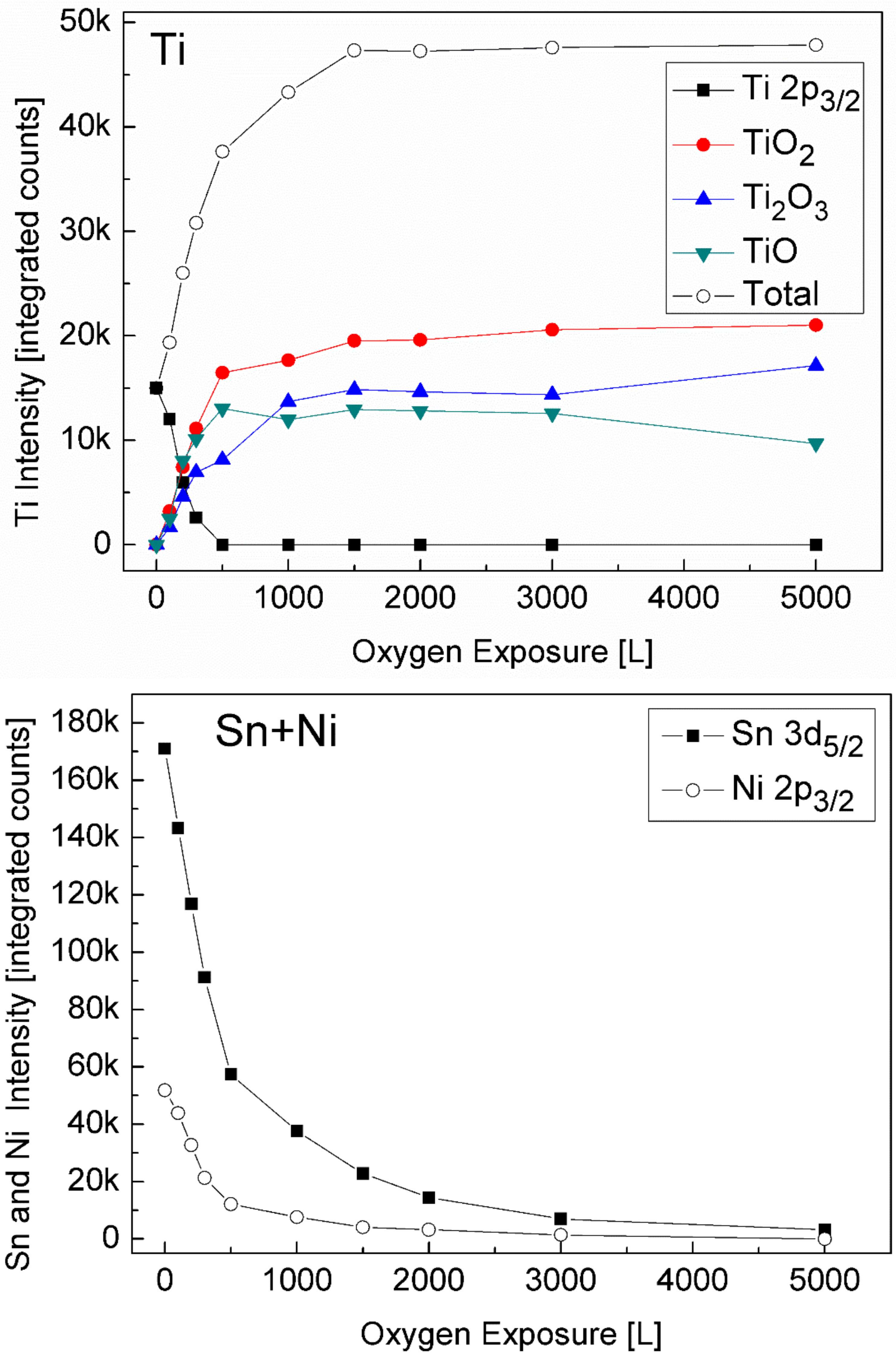
3.2. The Interaction with O2 at Room Temperature
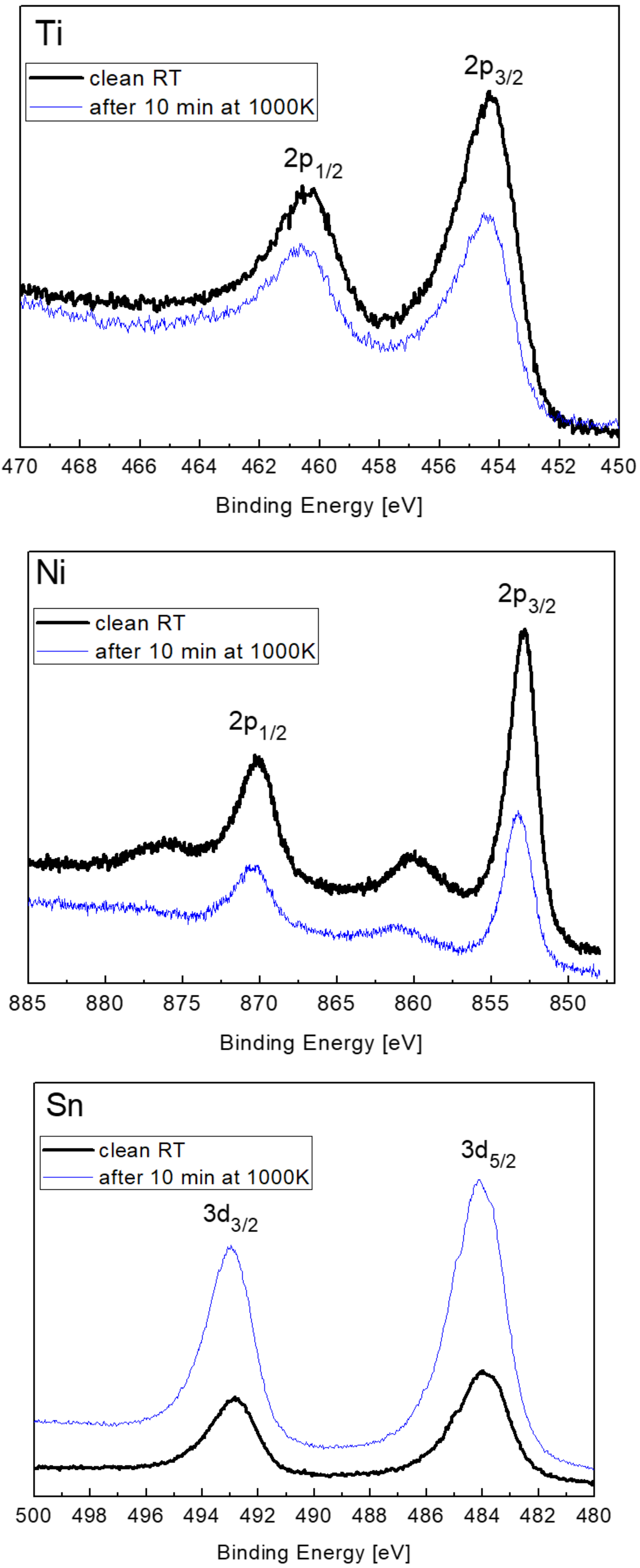
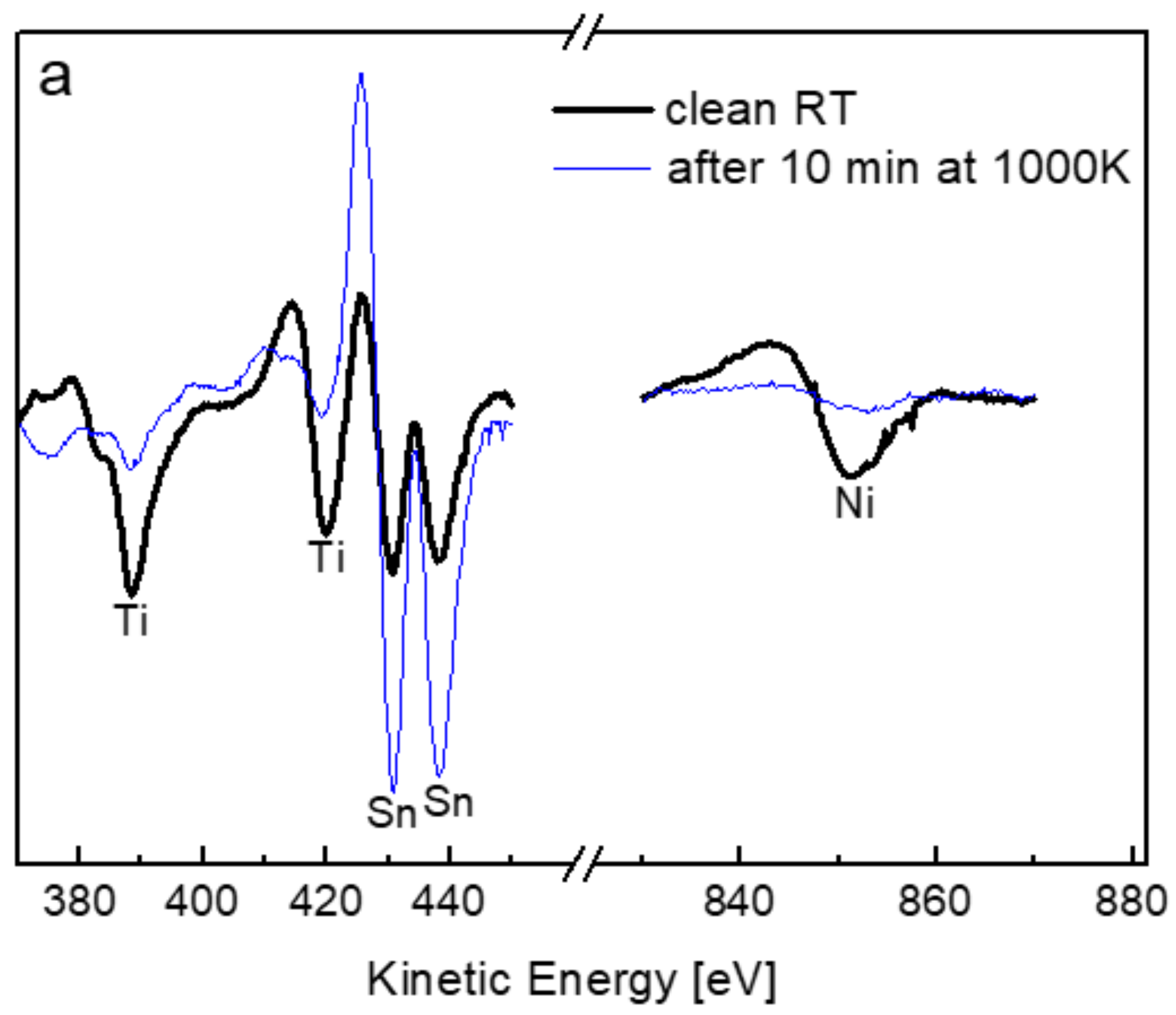
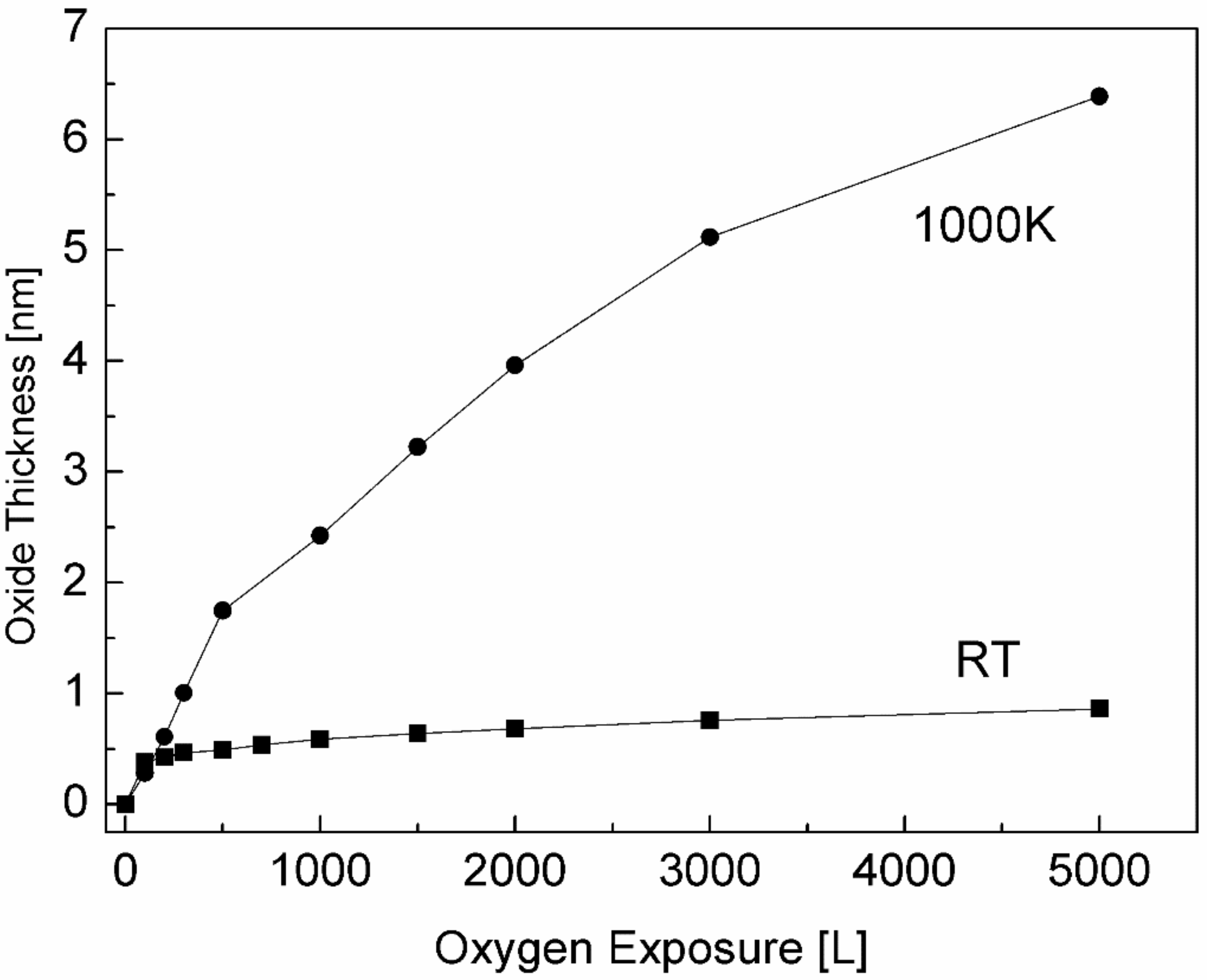
3.3. The Interaction with O2 at 1000 K
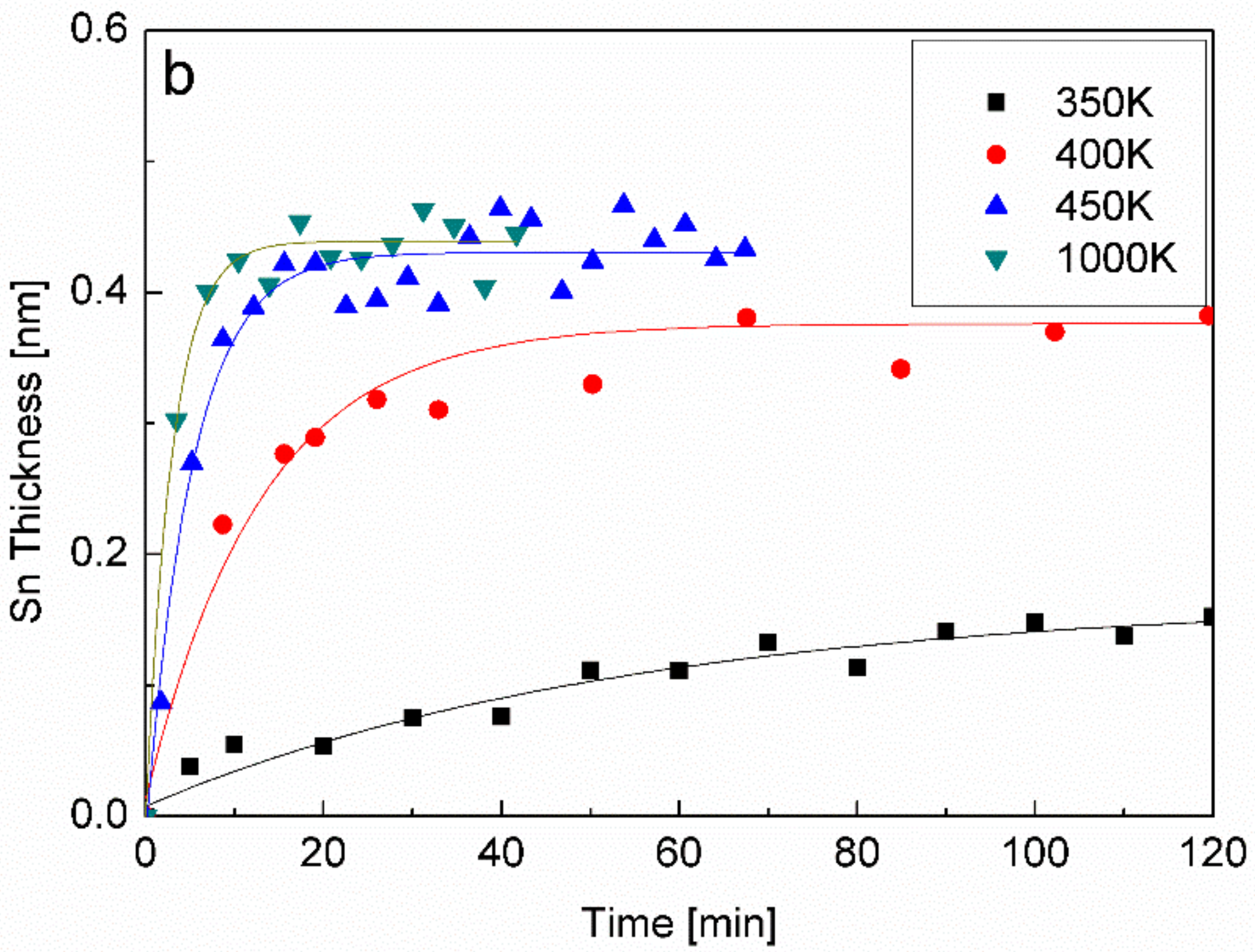
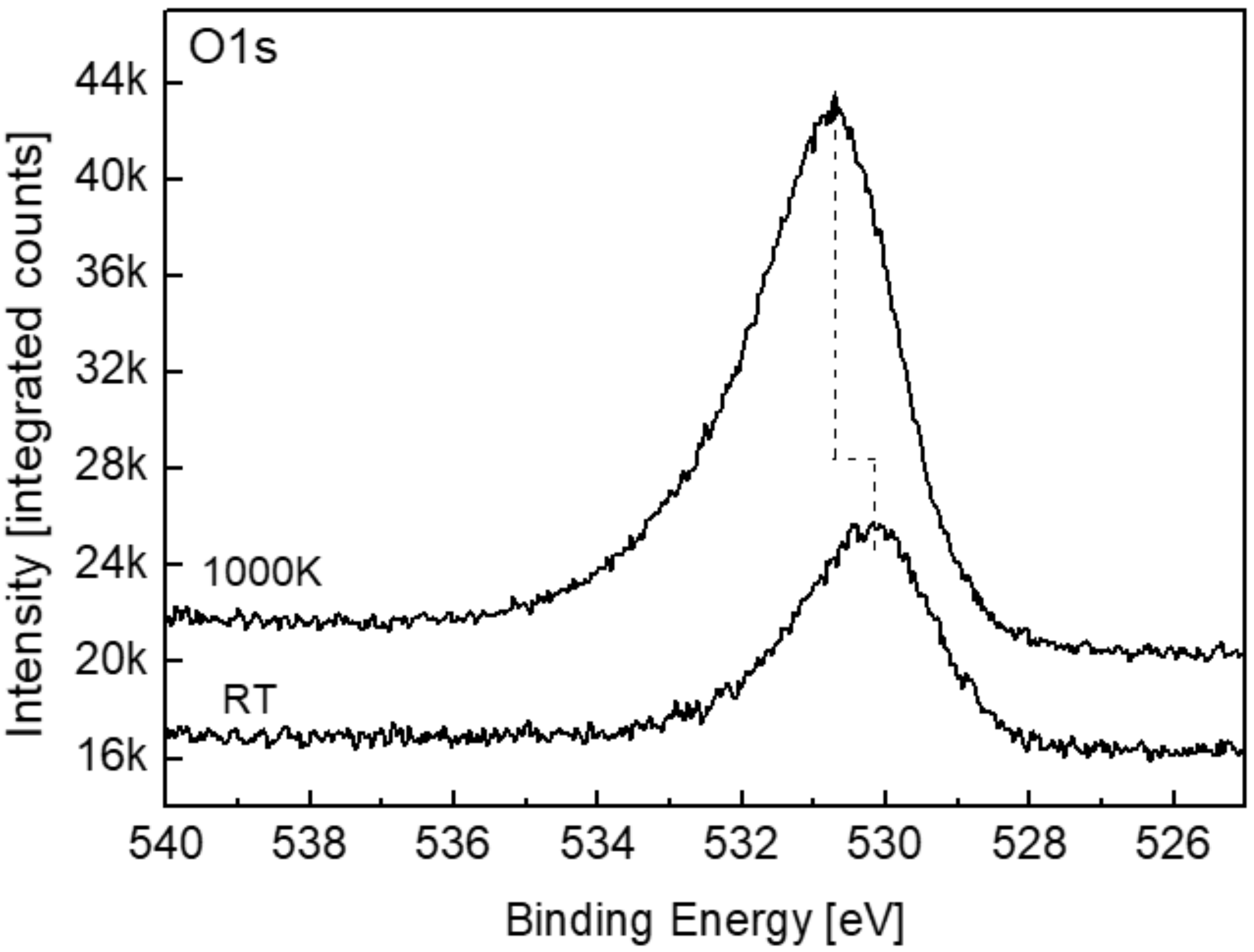
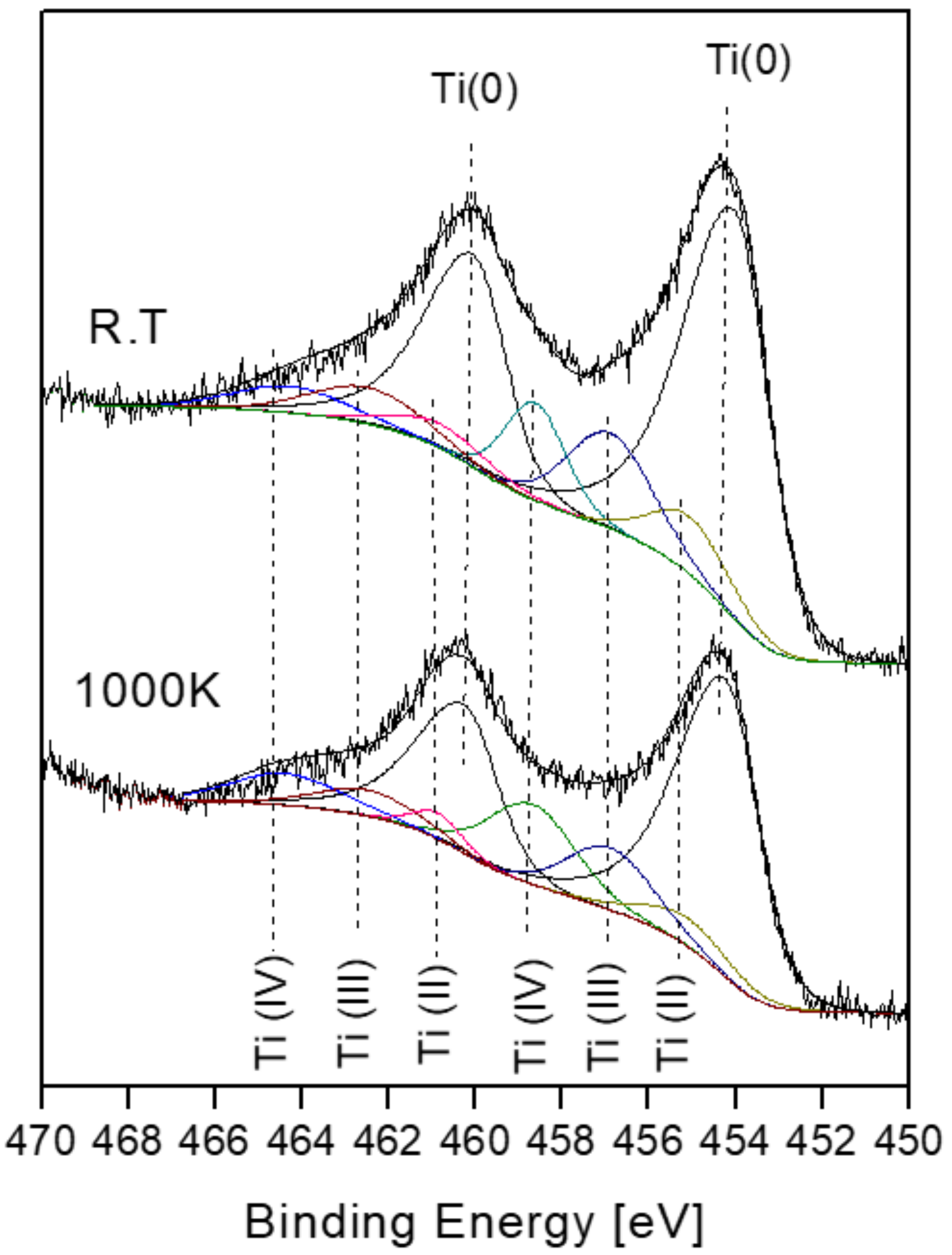
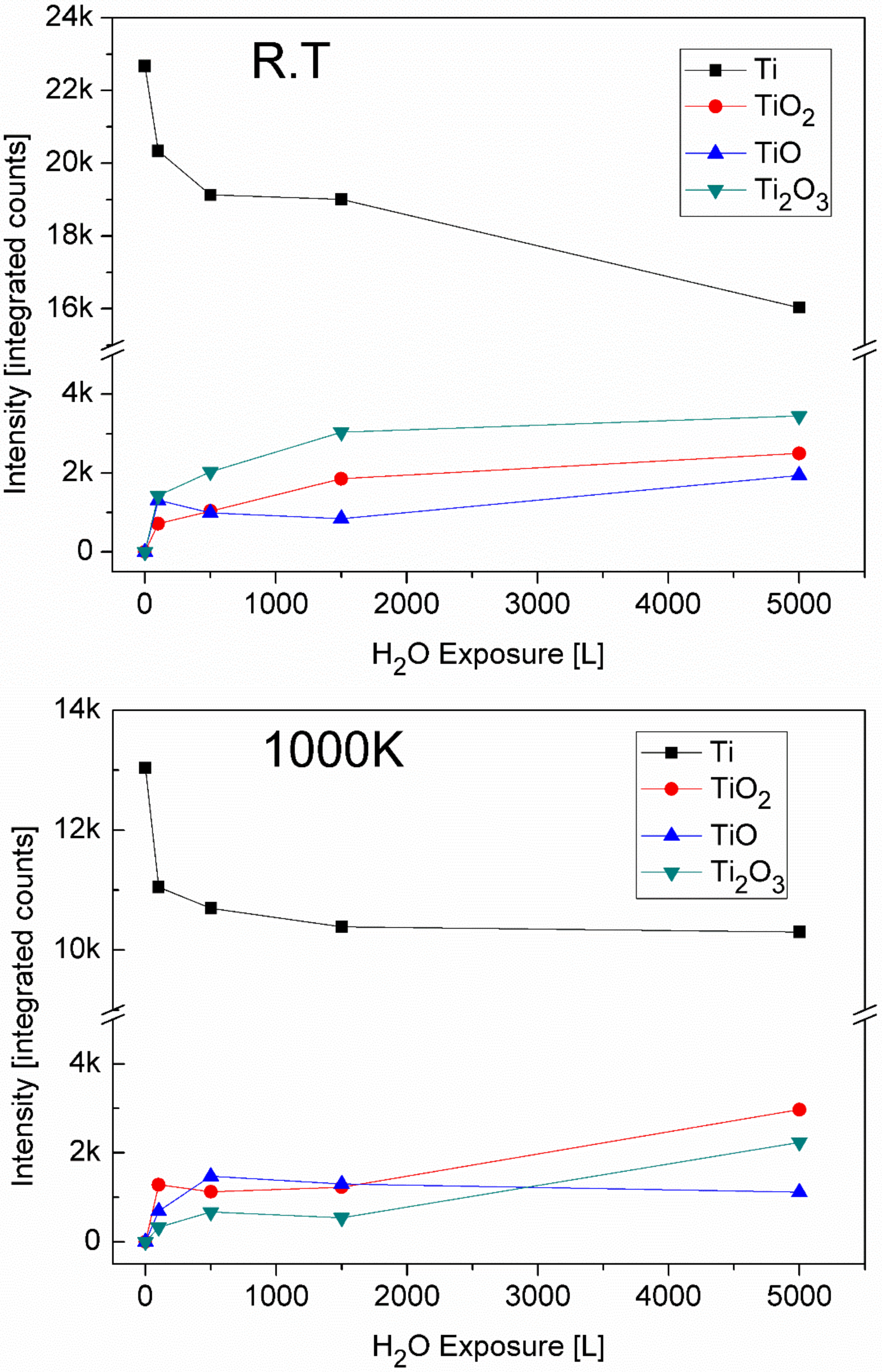
3.4. The Interaction with H2O at RT and 1000 K
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Casper, F.; Graf, T.; Chadov, S.; Balke, B.; Felser, C. Half-Heusler compounds: Novel materials for energy and spintronic applications. Semicond. Sci. Technol. 2012, 27, 063001. [Google Scholar] [CrossRef]
- Rogl, G.; Grytsiv, A.; Gürth, M.; Tavassoli, A.; Ebner, C.; Wünschek, A.; Puchegger, S.; Soprunyuk, V.; Schranz, W.; Bauer, E. Mechanical properties of half-Heusler alloys. Acta Mater. 2016, 107, 178–195. [Google Scholar] [CrossRef]
- Goldsmid, H. Applications of Thermoelectricity; Methuen: London, UK, 1961. [Google Scholar]
- Graf, T.; Felser, C.; Parkin, S.S.P. Simple rules for the understanding of Heusler compounds. Prog. Solid State Chem. 2011, 39, 1–50. [Google Scholar] [CrossRef]
- Kirievsky, K.; Gelbstein, Y.; Fuks, D. Phase separation and antisite defects in the thermoelectric TiNiSn half-Heusler alloys. J. Solid State Chem. 2013, 203, 247–254. [Google Scholar] [CrossRef]
- Kirievsky, K.; Shlimovich, M.; Fuks, D.; Gelbstein, Y. An ab initio study of the thermoelectric enhancement potential in nano-grained TiNiSn. Phys. Chem. Chem. Phys. 2014, 16, 20023–20029. [Google Scholar] [CrossRef] [PubMed]
- Appel, O.; Schwall, M.; Mogilyansky, D.; Köhne, M.; Balke, B.; Gelbstein, Y. Effects of microstructural evolution on the thermoelectric properties of spark-plasma-sintered Ti0.3Zr0.35Hf0.35NiSn half-Heusler compound. J. Electron. Mater. 2013, 42, 1340–1345. [Google Scholar] [CrossRef]
- Appel, O.; Gelbstein, Y. A Comparison between the Effects of Sb and Bi Doping on the Thermoelectric Properties of the Ti0.3Zr0.35Hf0.35NiSn Half-Heusler Alloy. J. Electron. Mater. 2014, 43, 1976–1982. [Google Scholar] [CrossRef]
- Appel, O.; Zilber, T.; Kalabukhov, S.; Beeri, O.; Gelbstein, Y. Morphological effects on the thermoelectric properties of Ti0.3Zr0.35Hf0.35Ni1+δSn alloys following phase separation. J. Mater. Chem. C 2015, 3, 11653–11659. [Google Scholar] [CrossRef]
- Berry, T.; Fu, C.; Auffermann, G.; Fecher, G.H.; Schnelle, W.; Serrano-Sanchez, F.; Yue, Y.; Liang, H.; Felser, C. Enhancing thermoelectric performance of TiNiSn half-Heusler compounds via modulation doping. Chem. Mater. 2017, 29, 7042–7048. [Google Scholar] [CrossRef]
- Chen, S.; Ren, Z. Recent progress of half-Heusler for moderate temperature thermoelectric applications. Mater. Today 2013, 16, 387–395. [Google Scholar] [CrossRef]
- Xie, W.; Weidenkaff, A.; Tang, X.; Zhang, Q.; Poon, J.; Tritt, T.M. Recent advances in nanostructured thermoelectric half-Heusler compounds. Nanomaterials 2012, 2, 379–412. [Google Scholar] [CrossRef] [PubMed]
- Katayama, T.; Kim, S.W.; Kimura, Y.; Mishima, Y. The effects of quaternary additions on thermoelectric properties of TiNiSn-based half-Heusler alloys. J. Electron. Mater. 2003, 32, 1160–1165. [Google Scholar] [CrossRef]
- Schierning, G.; Chavez, R.; Schmechel, R.; Balke, B.; Rogl, G.; Rogl, P. Concepts for medium-high to high temperature thermoelectric heat-to-electricity conversion: A review of selected materials and basic considerations of module design. Transl. Mater. Res. 2015, 2, 025001. [Google Scholar] [CrossRef]
- Gürth, M.; Rogl, G.; Romaka, V.; Grytsiv, A.; Bauer, E.; Rogl, P. Thermoelectric high ZT half-Heusler alloys Ti1−x−yZrxHfyNiSn (0 ≤ x ≤ 1; 0 ≤ y ≤ 1). Acta Mater. 2016, 104, 210–222. [Google Scholar] [CrossRef]
- Bankina, V.; Fedorova, O.; Leytus, G. X-ray study of TiNiSn and ZrNiSn intermetallics oxidation. Mater. Sci. Forum 1993, 133–136, 575–580. [Google Scholar] [CrossRef]
- Gałązka, K.; Populoh, S.; Sagarna, L.; Karvonen, L.; Xie, W.; Beni, A.; Schmutz, P.; Hulliger, J.; Weidenkaff, A. Phase formation, stability, and oxidation in (Ti, Zr, Hf) NiSn half-Heusler compounds. Phys. Status Solidi A 2014, 211, 1259–1266. [Google Scholar] [CrossRef]
- Berche, A.; Jund, P. Oxidation of half-Heusler NiTiSn materials: Implications for thermoelectric applications. Intermetallics 2018, 92, 62–71. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Lau, L.W.; Gerson, A.; Smart, R.S.C. X-ray photoelectron spectroscopic chemical state quantification of mixed nickel metal, oxide and hydroxide systems. Surf. Interface Anal. 2009, 41, 324–332. [Google Scholar] [CrossRef] [Green Version]
- Biesinger, M.C.; Lau, L.W.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- X-Ray Photoelectron Spectroscopy (XPS) Reference Pages. Available online: http://www.xpsfitting.com (accessed on 25 May 2018).
- Lu, G.; Bernasek, S.L.; Schwartz, J. Oxidation of a polycrystalline titanium surface by oxygen and water. Surf. Sci. 2000, 458, 80–90. [Google Scholar] [CrossRef]
- Moulder, J.; Stickle, W.; Sobol, P.; Bomben, K. Handbook of X-Ray Photoelectron Spectroscopy; Perkin-Elmer: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Polak, M.; Rubinovich, L. The interplay of surface segregation and atomic order in alloys. Surf. Sci. Rep. 2000, 38, 127–194. [Google Scholar] [CrossRef]
- Vitos, L.; Ruban, A.; Skriver, H.L.; Kollar, J. The surface energy of metals. Surf. Sci. 1998, 411, 186–202. [Google Scholar] [CrossRef]
- Watts, J.F.; Wolstenholme, J. An Introduction to Surface Analysis by XPS and AES; John Wiley & Sons Ltd.: Chichester, UK, 2003. [Google Scholar]
- Powell, C.J.; Jablonski, A. NIST Electron Inelastic-Mean-Free-Path Database-Version 1.2; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2010. [Google Scholar]
- Seah, M.; Lea, C. Surface segregation and its relation to grain boundary segregation. Philos. Mag. 1975, 31, 627–645. [Google Scholar] [CrossRef]
- Viefhaus, H.; Rüsenberg, M. Electron spectroscopic studies of tin surface segregation on iron single crystal surfaces. Surf. Sci. 1985, 159, 1–23. [Google Scholar] [CrossRef]
- Wallace, W.; McMahon, C., Jr. Is the multilayer segregation of tin to the iron (100) surface an equilibrium phenomenon? Surf. Sci. 1992, 260, L1–L6. [Google Scholar] [CrossRef]
- Tan, J.; Walmsley, J.C.; Holme, B.; Nordmark, H.; Nisancioglu, K. Surface segregation of tin by heat treatment of dilute aluminium–tin alloys. Corros. Sci. 2013, 68, 204–213. [Google Scholar] [CrossRef]
- Yang, W.; Xu, M.; Bai, H.; Meng, Y.; Wang, L.; Shi, L.; Pei, Y.; Zhang, J.; Zheng, L. Concentration depth distribution of grain boundary segregation measured by wavelength dispersive X-ray spectroscopy. Ultramicroscopy 2015, 159, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Senkovskiy, B.; Usachov, D.Y.; Fedorov, A.; Vilkov, O.Y.; Shelyakov, A.; Adamchuk, V. Electronic structure of Ti–Ni alloys: An XPS and NEXAFS study. J. Alloys Compd. 2012, 537, 190–196. [Google Scholar] [CrossRef]
- Weast, R.C. Handbook of Chemistry and Physics, 51st ed.; Chemical Rubber Company: Cleveland, OH, USA, 1970. [Google Scholar]
- Fuentes, G.; Elizalde, E.; Yubero, F.; Sanz, J. Electron inelastic mean free path for Ti, TiC, TiN and TiO2 as determined by quantitative reflection electron energy-loss spectroscopy. Surf. Interface Anal. 2002, 33, 230–237. [Google Scholar] [CrossRef]
- Azoulay, A.; Shamir, N.; Volterra, V.; Mintz, M. Water vapor interactions with polycrystalline titanium surfaces. Surf. Sci. 1999, 422, 141–153. [Google Scholar] [CrossRef]
- Shwartz, A.; Shamir, N.; Froumin, N.; Zalkind, S.; Edry, I.; Haim, A.; Mintz, M. Initial oxidation of TiFe1−xMnx (x = 0–0.3) by low dose exposures to H2O and O2. J. Alloys Compd. 2014, 610, 6–10. [Google Scholar]
- Matmor, M.; Cohen, S.; Rafailov, G.; Vaknin, M.; Shamir, N.; Gouder, T.; Zalkind, S. Surface characterization of U(AlxSi1−x)3 alloy and its interaction with O2 and H2O, at room temperature. J. Nucl. Mater. 2018, 499, 29–37. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Appel, O.; Cohen, S.; Beeri, O.; Shamir, N.; Gelbstein, Y.; Zalkind, S. Surface Oxidation of TiNiSn (Half-Heusler) Alloy by Oxygen and Water Vapor. Materials 2018, 11, 2296. https://doi.org/10.3390/ma11112296
Appel O, Cohen S, Beeri O, Shamir N, Gelbstein Y, Zalkind S. Surface Oxidation of TiNiSn (Half-Heusler) Alloy by Oxygen and Water Vapor. Materials. 2018; 11(11):2296. https://doi.org/10.3390/ma11112296
Chicago/Turabian StyleAppel, Oshrat, Shai Cohen, Ofer Beeri, Noah Shamir, Yaniv Gelbstein, and Shimon Zalkind. 2018. "Surface Oxidation of TiNiSn (Half-Heusler) Alloy by Oxygen and Water Vapor" Materials 11, no. 11: 2296. https://doi.org/10.3390/ma11112296



