Porous Graphene Oxide Prepared on Nickel Foam by Electrophoretic Deposition and Thermal Reduction as High-Performance Supercapacitor Electrodes
Abstract
1. Introduction
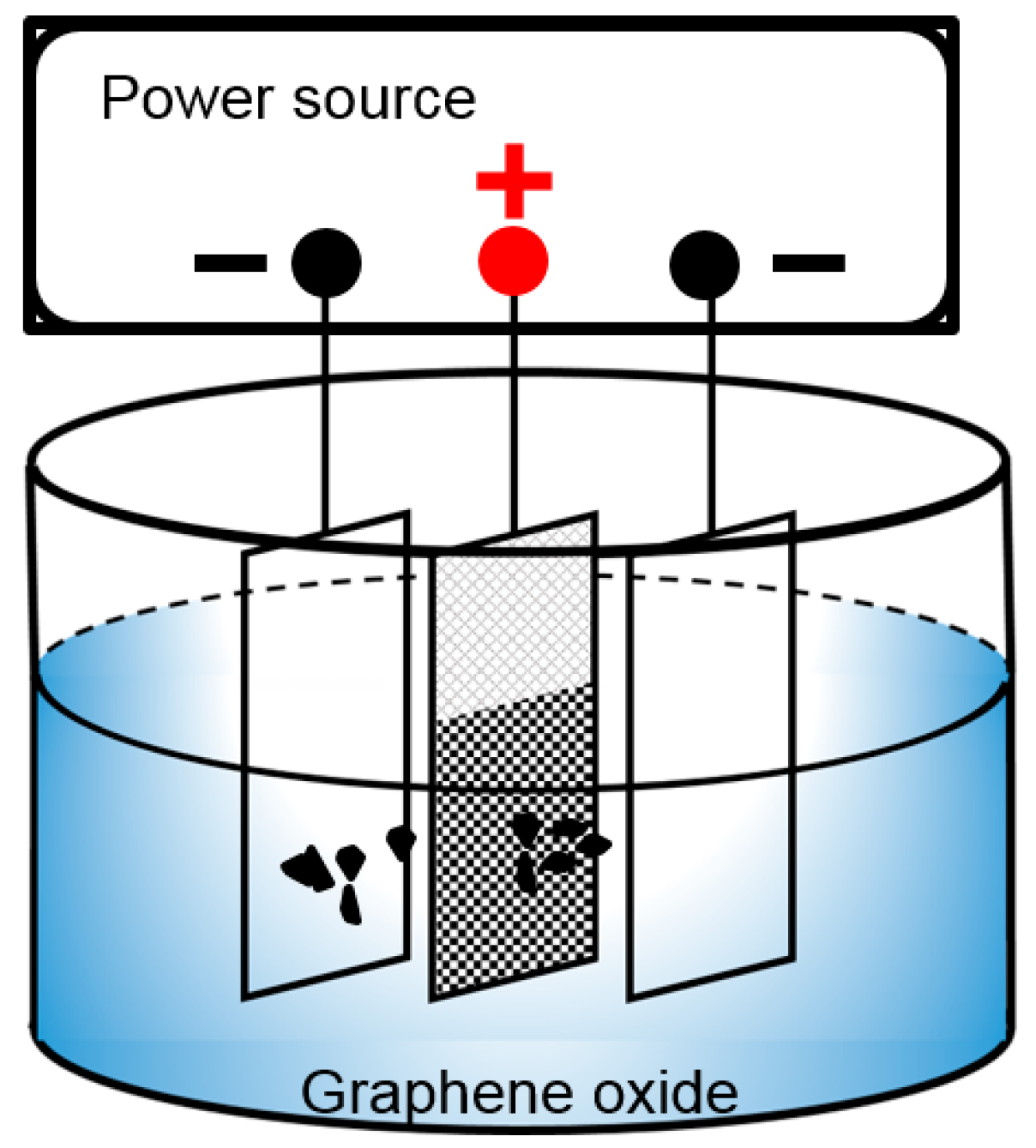
2. Experimental
3. Results and Discussion
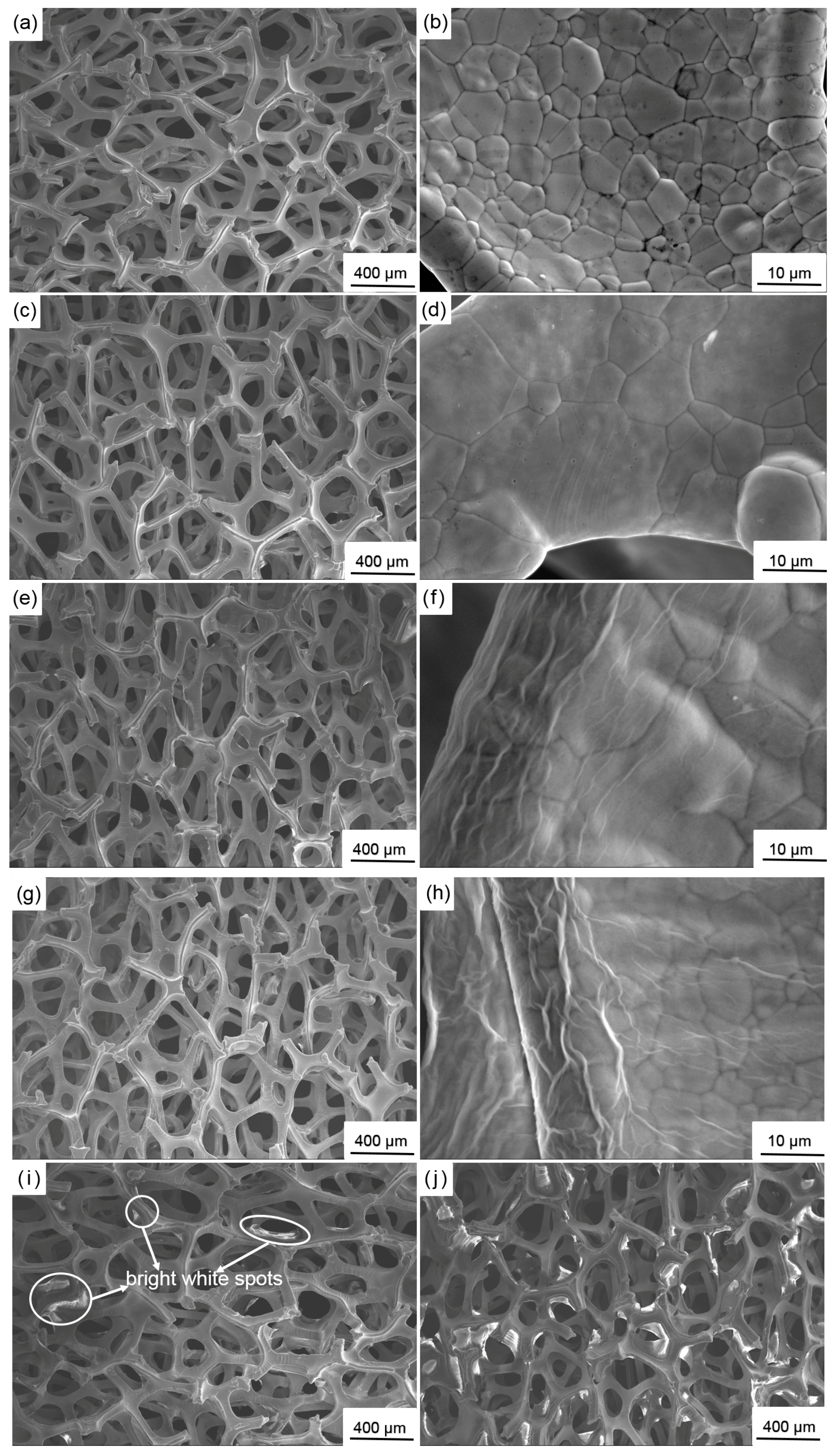
3.1. Morphological Characterization of GO
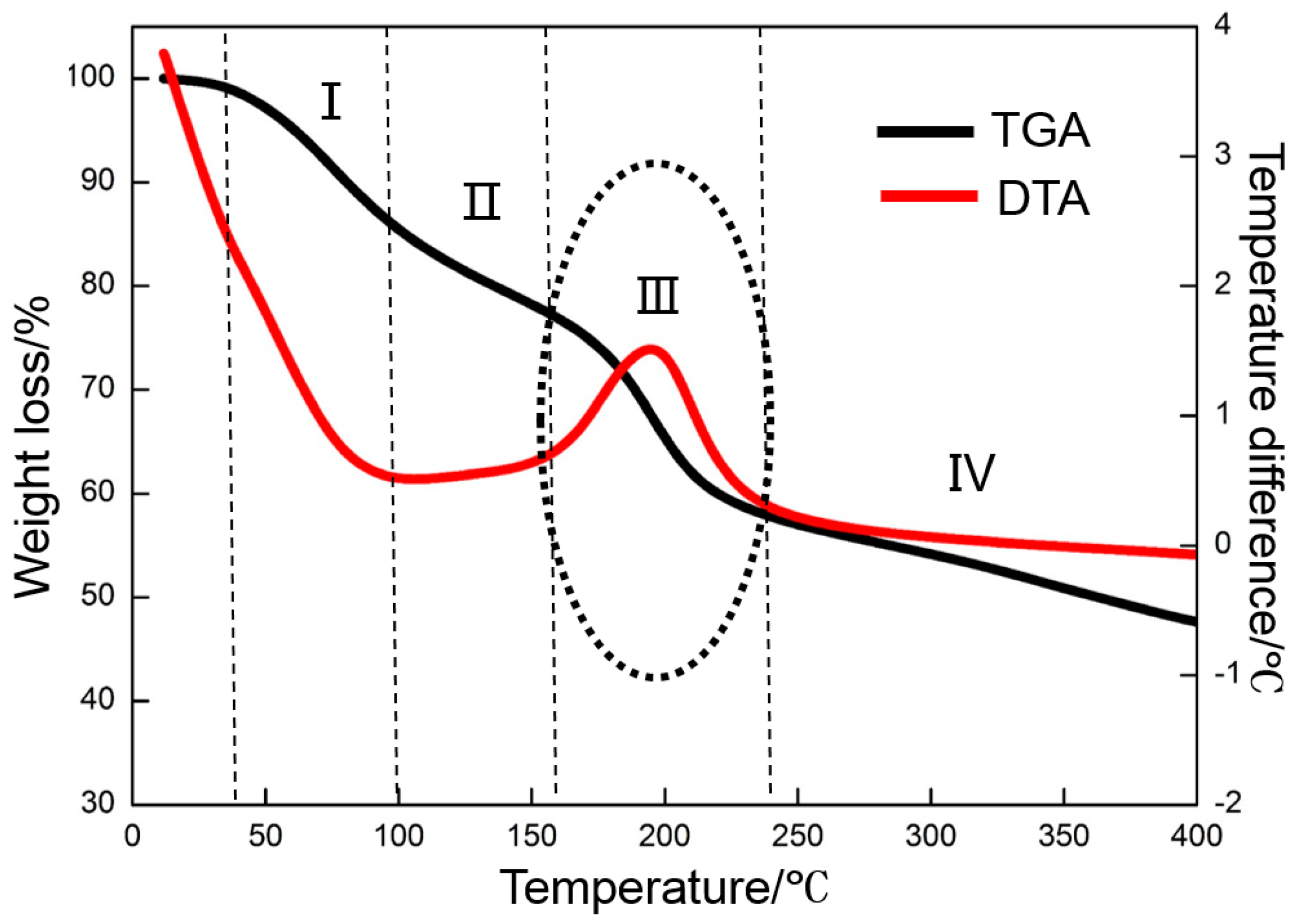
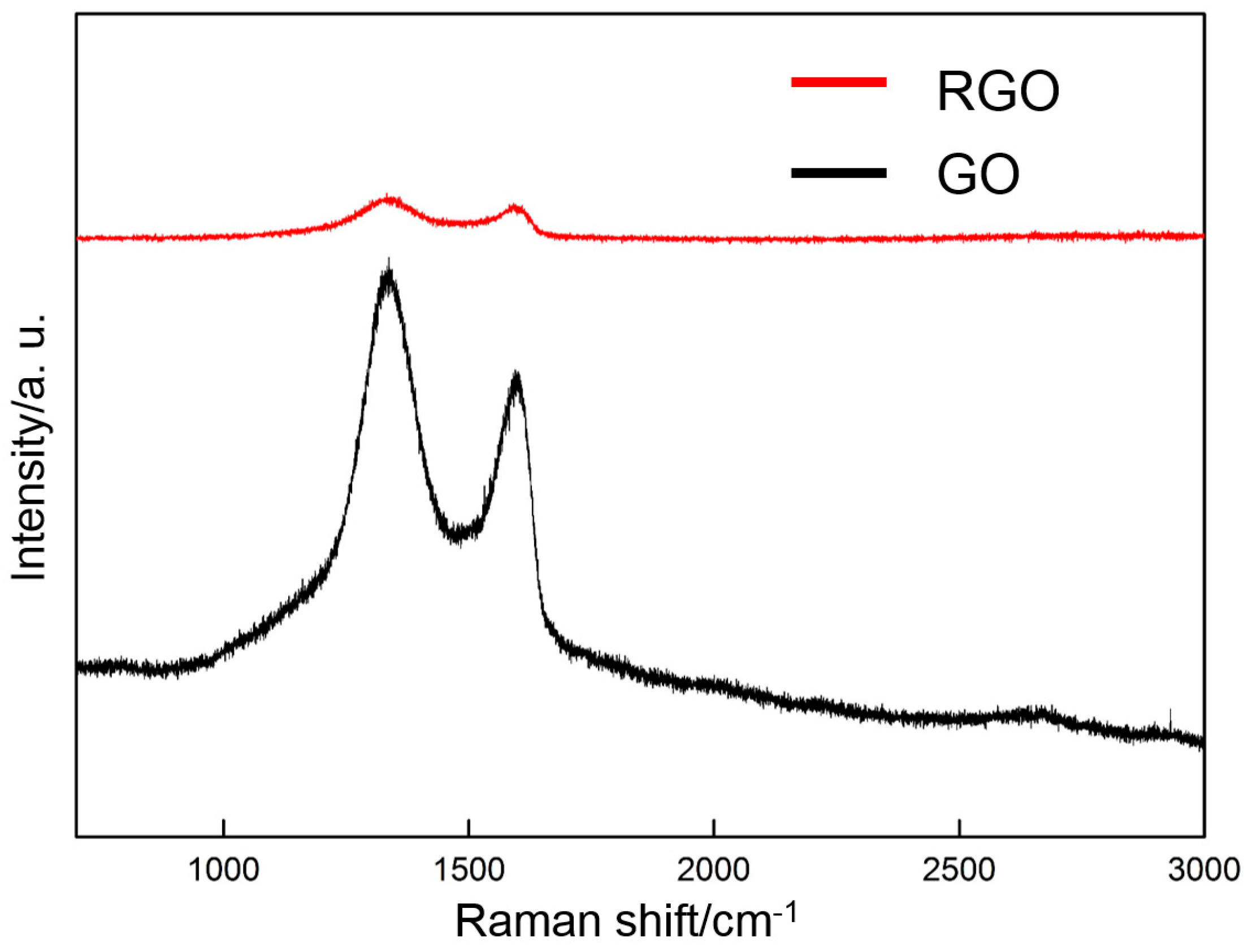
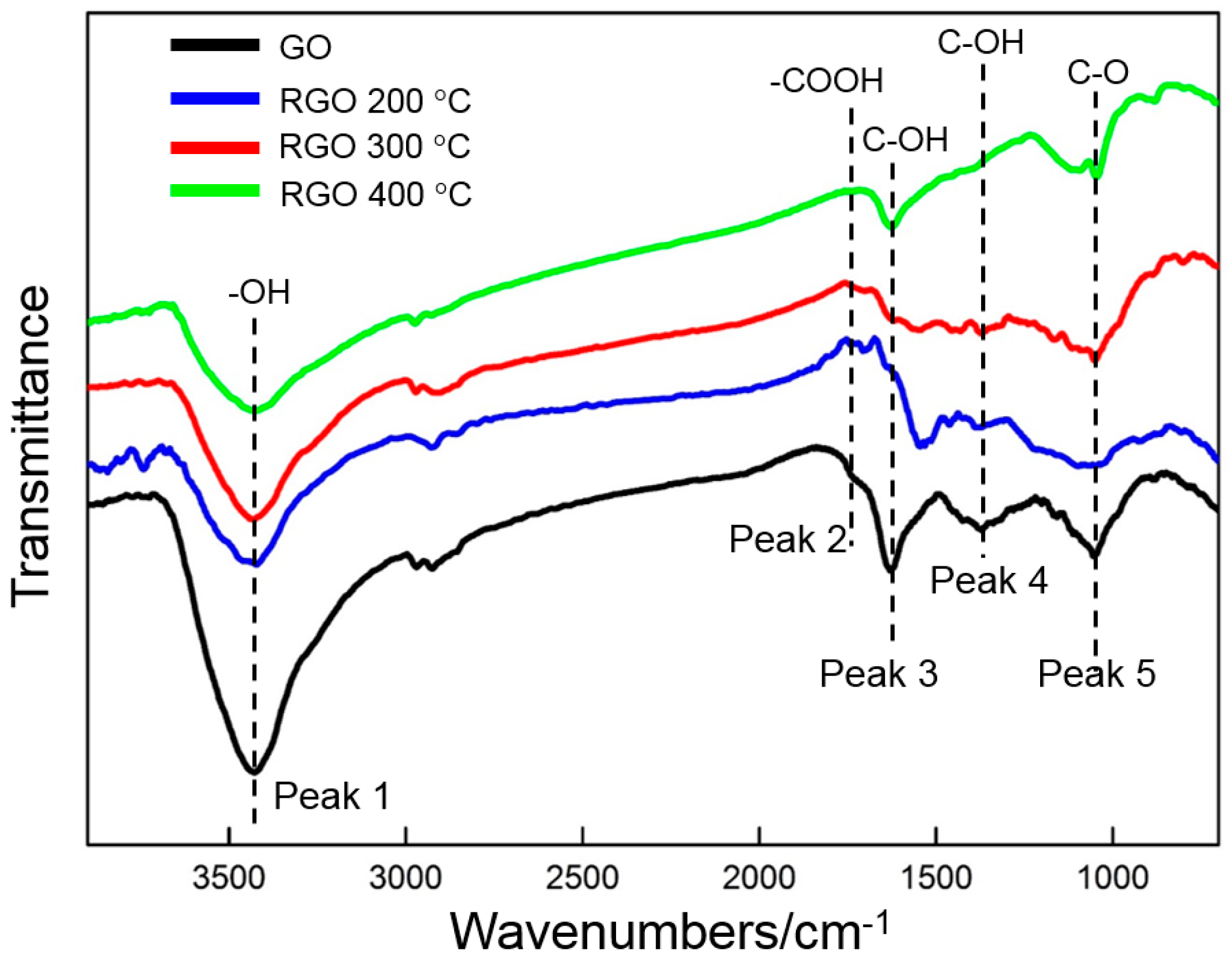
3.2. Thermal Reduction Properties of GO
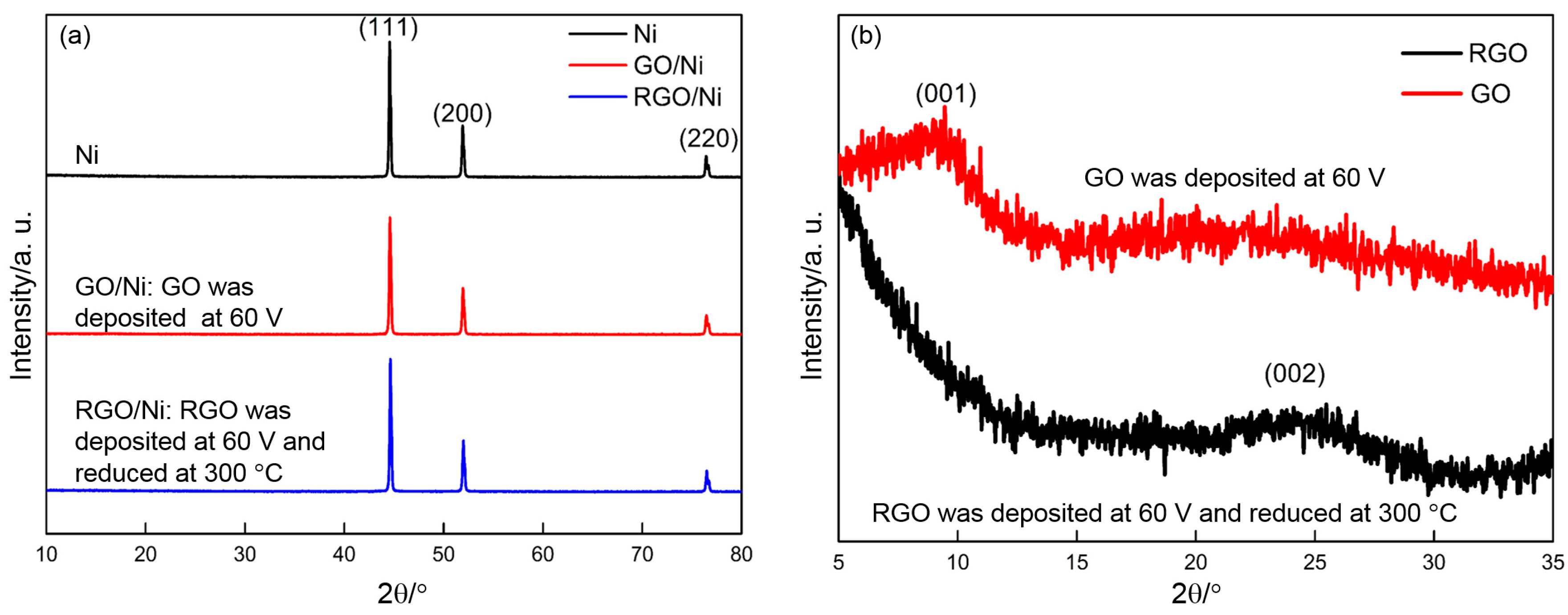
3.3. XRD Results
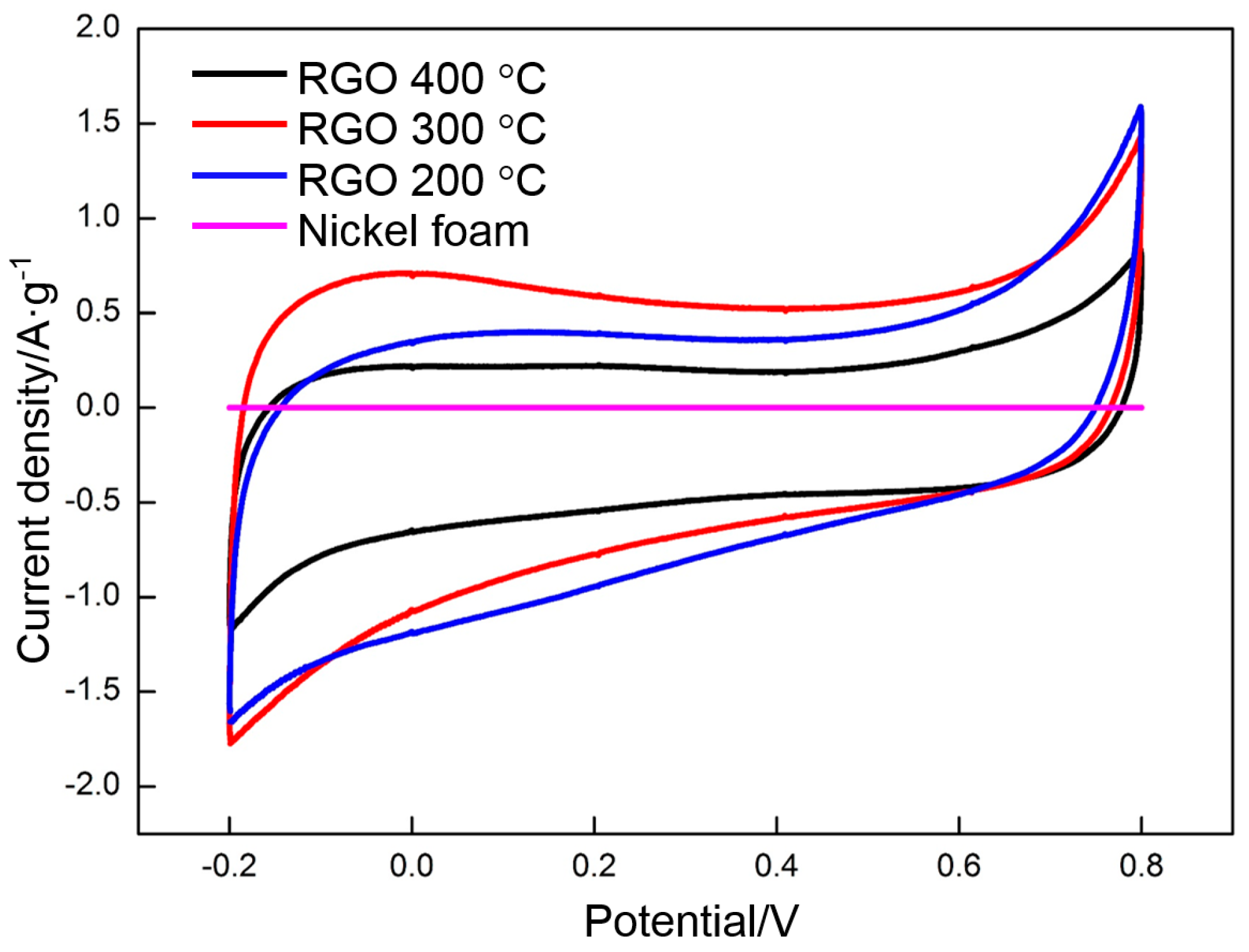
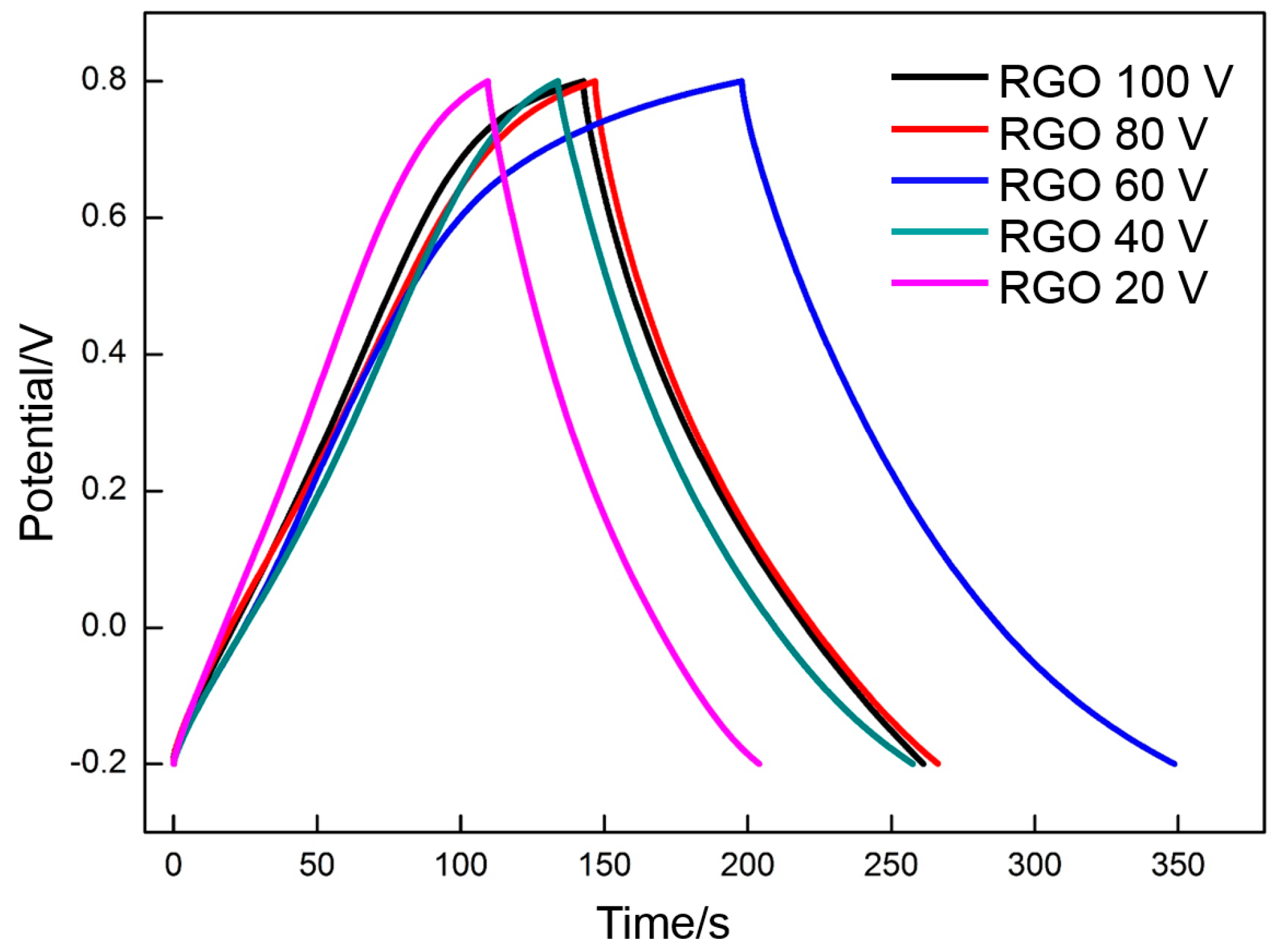
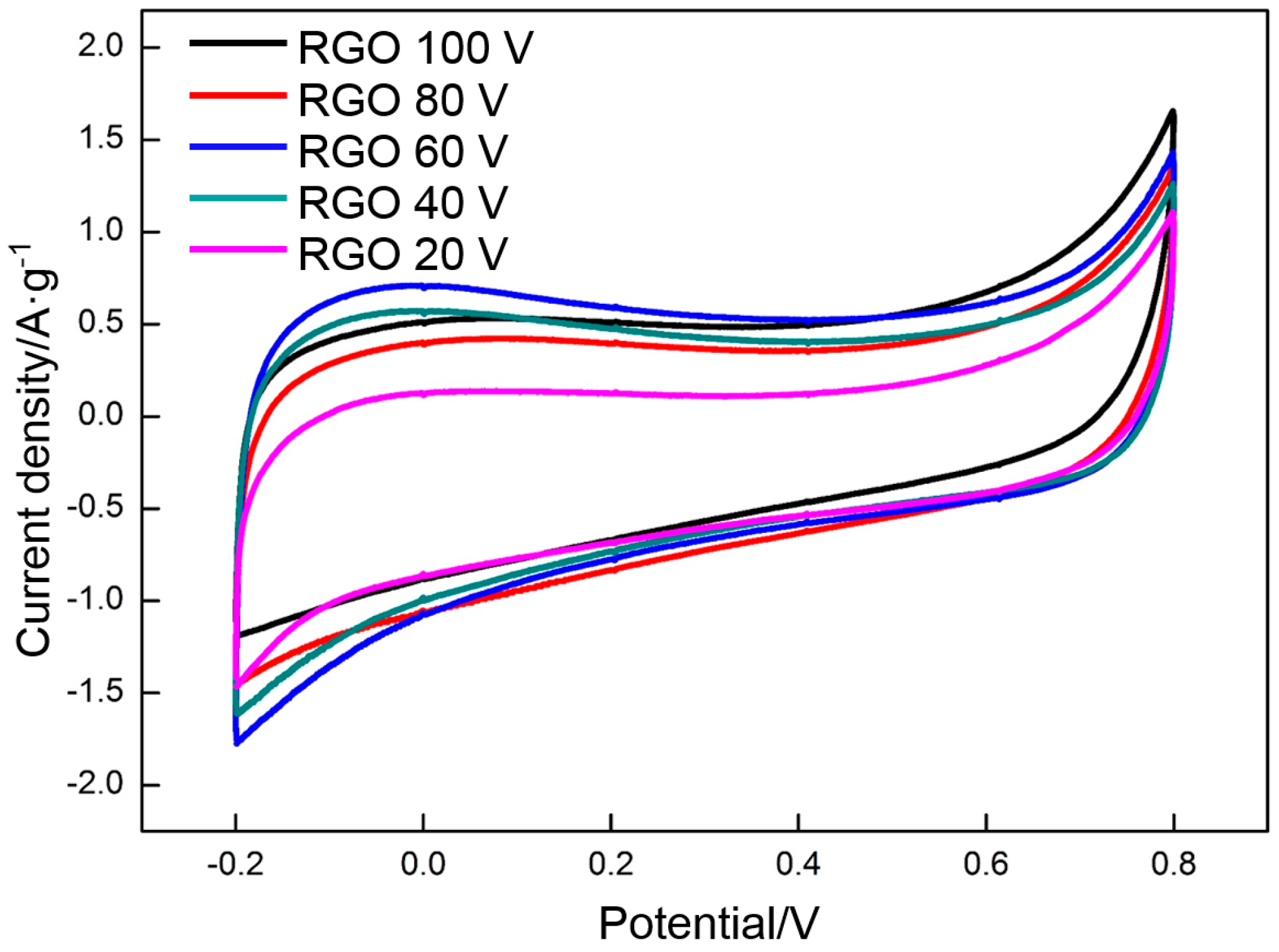
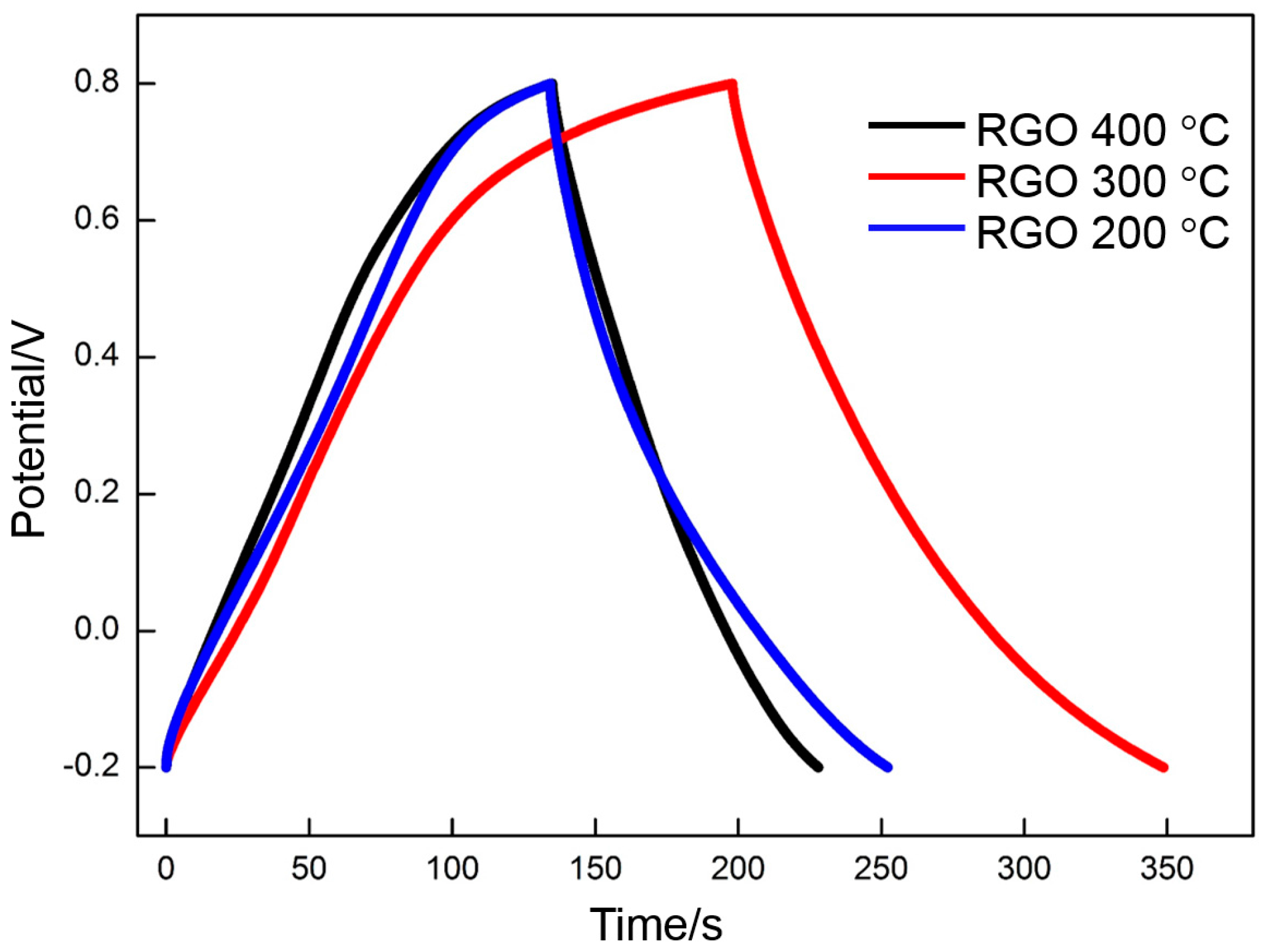
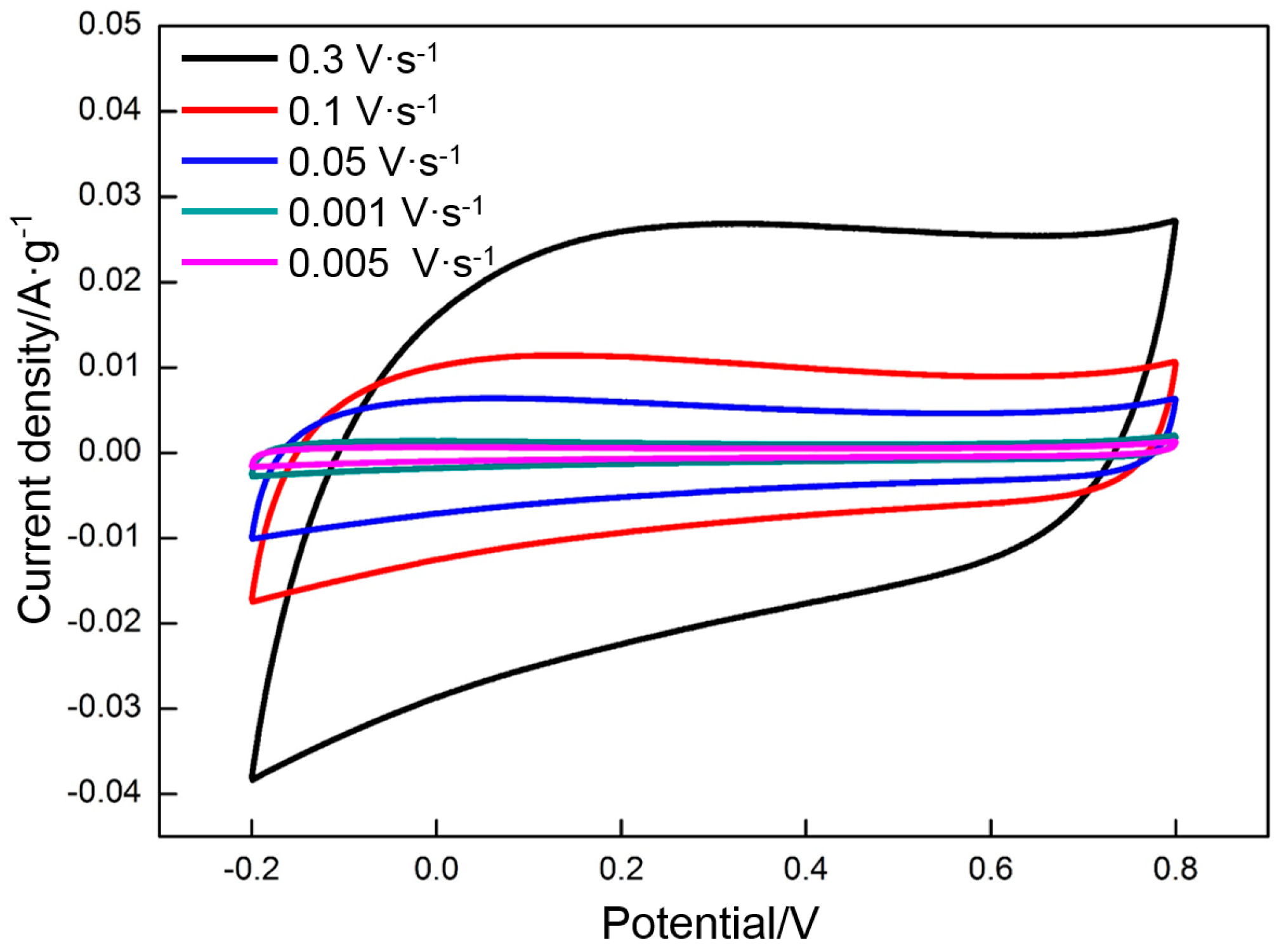
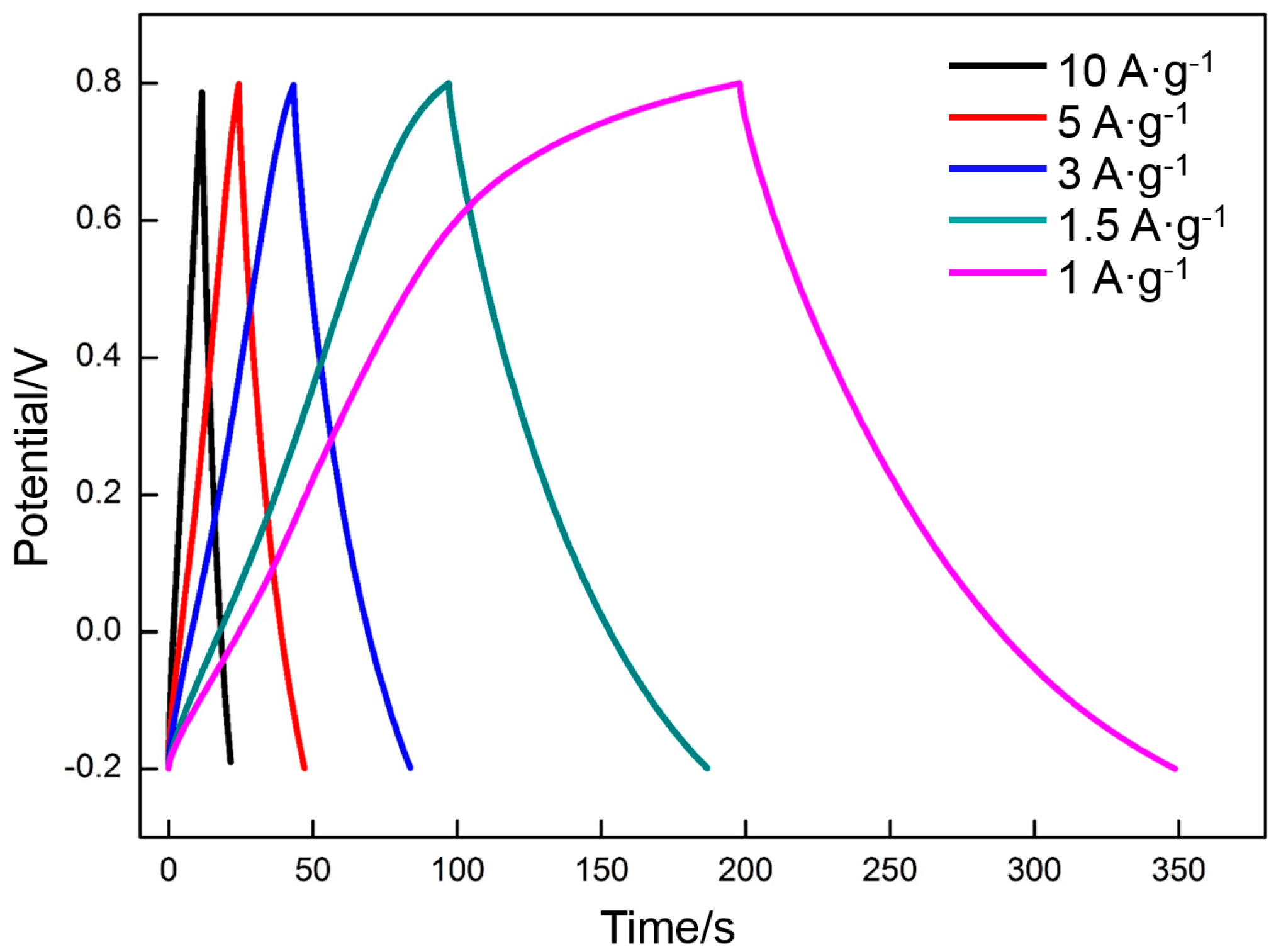
3.4. Electrochemical Properties of RGO
4. Conclusions
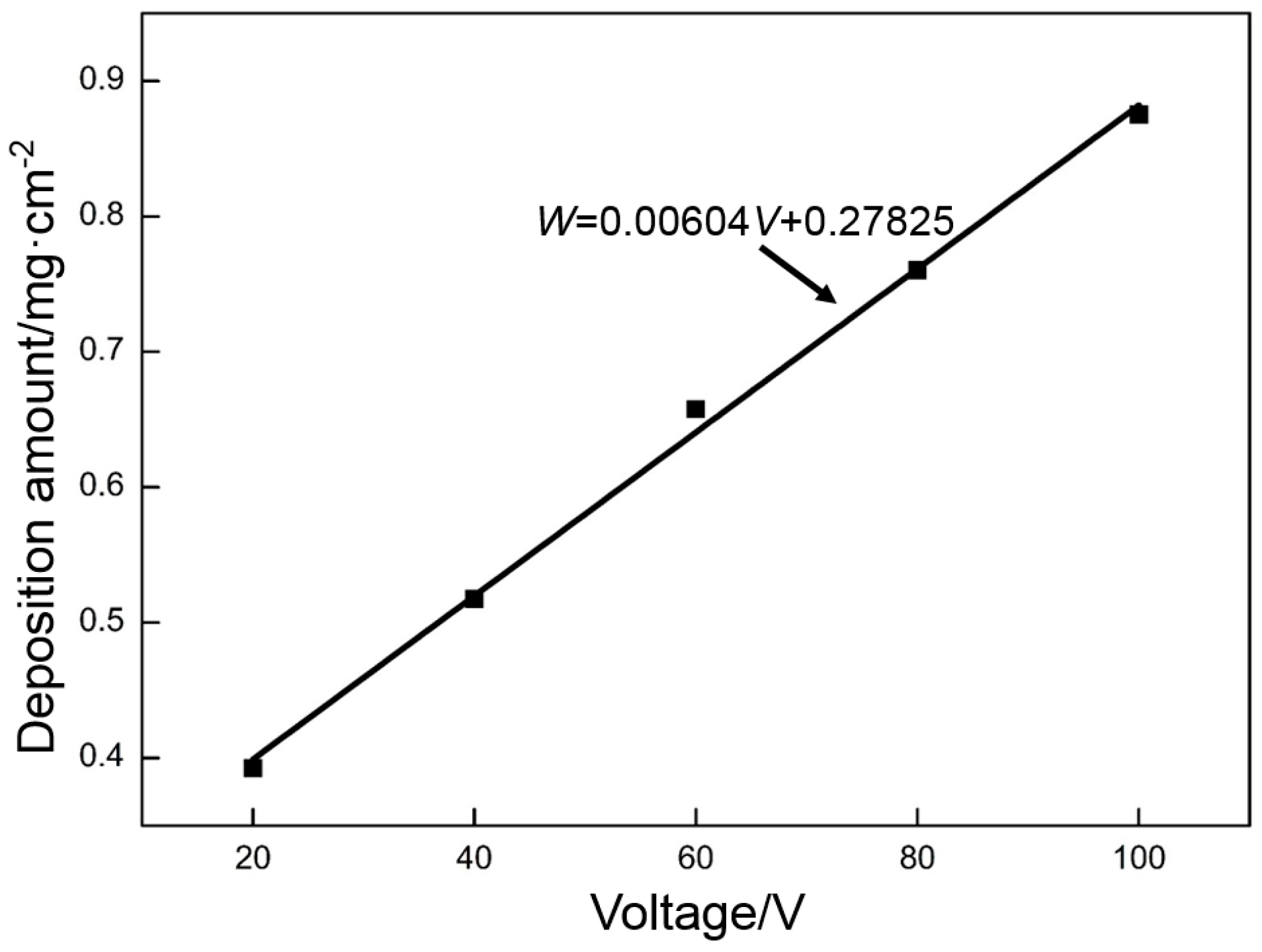
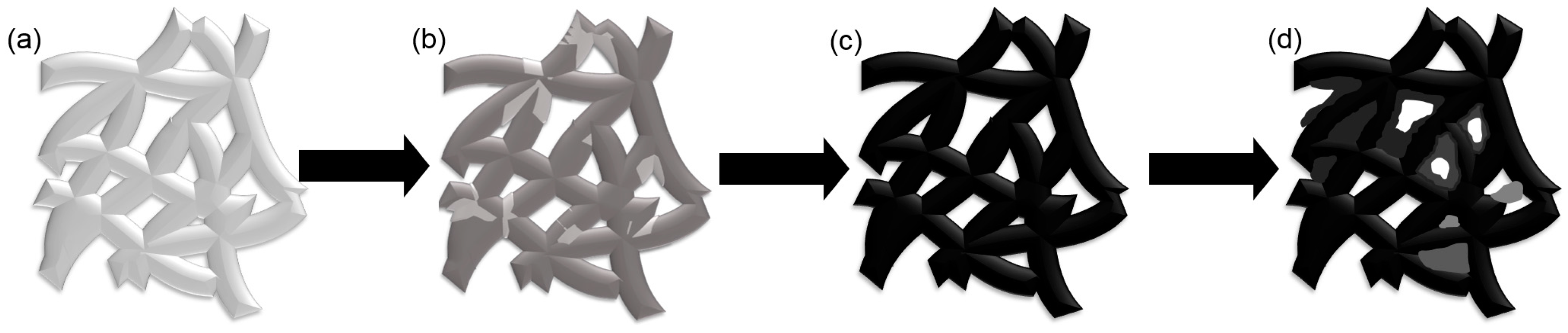
- Deposition voltage has a significant effect on the morphologies of deposited GO. At a low deposition voltage (less than 60 V), the frameworks in the nickel foam are not completely covered with GO. Some holes around the frameworks are covered at a high deposition voltage (higher than 60 V). The frameworks in the nickel foam are only covered with a uniform dense deposition layer at the deposition voltage of 60 V.
- GO is reduced in a very narrow temperature range of 160 to 240 °C. The thermal reduction of GO at 300 °C contributes to the enhancement of the EDLC. At the same time, several of the oxygen-containing functional groups in GO are maintained, which is beneficial to the increase in pseudocapacitance.
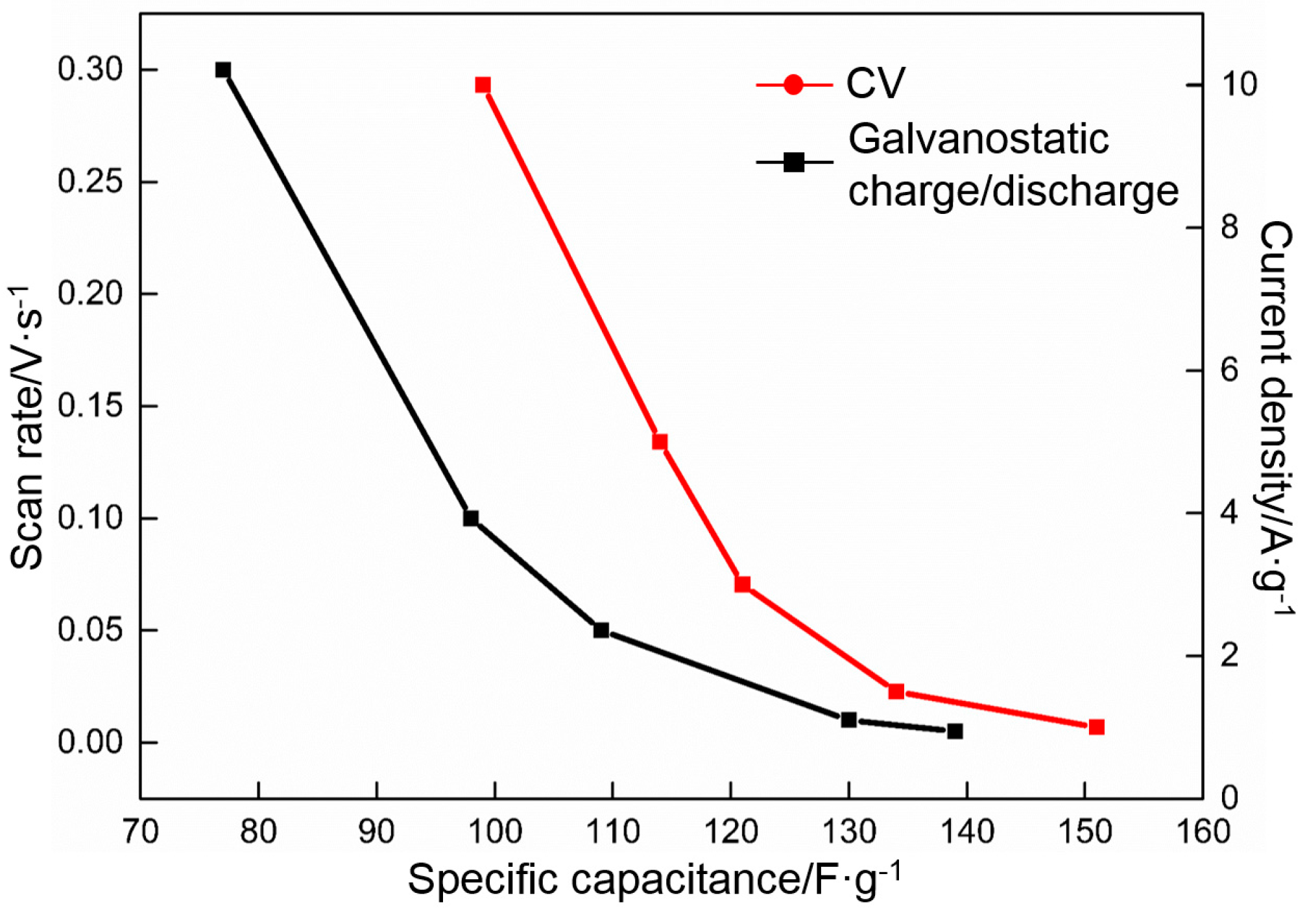
- The specific capacitance of RGO is closely related to the deposition voltage and the thermal reduction temperature. The RGO deposited at 60 V and thermally reduced at 300 °C exhibits the highest specific capacitance of all. The specific capacitances calculated by using CV and galvanostatic charge/discharge are 139 and 151 F·g−1, respectively.
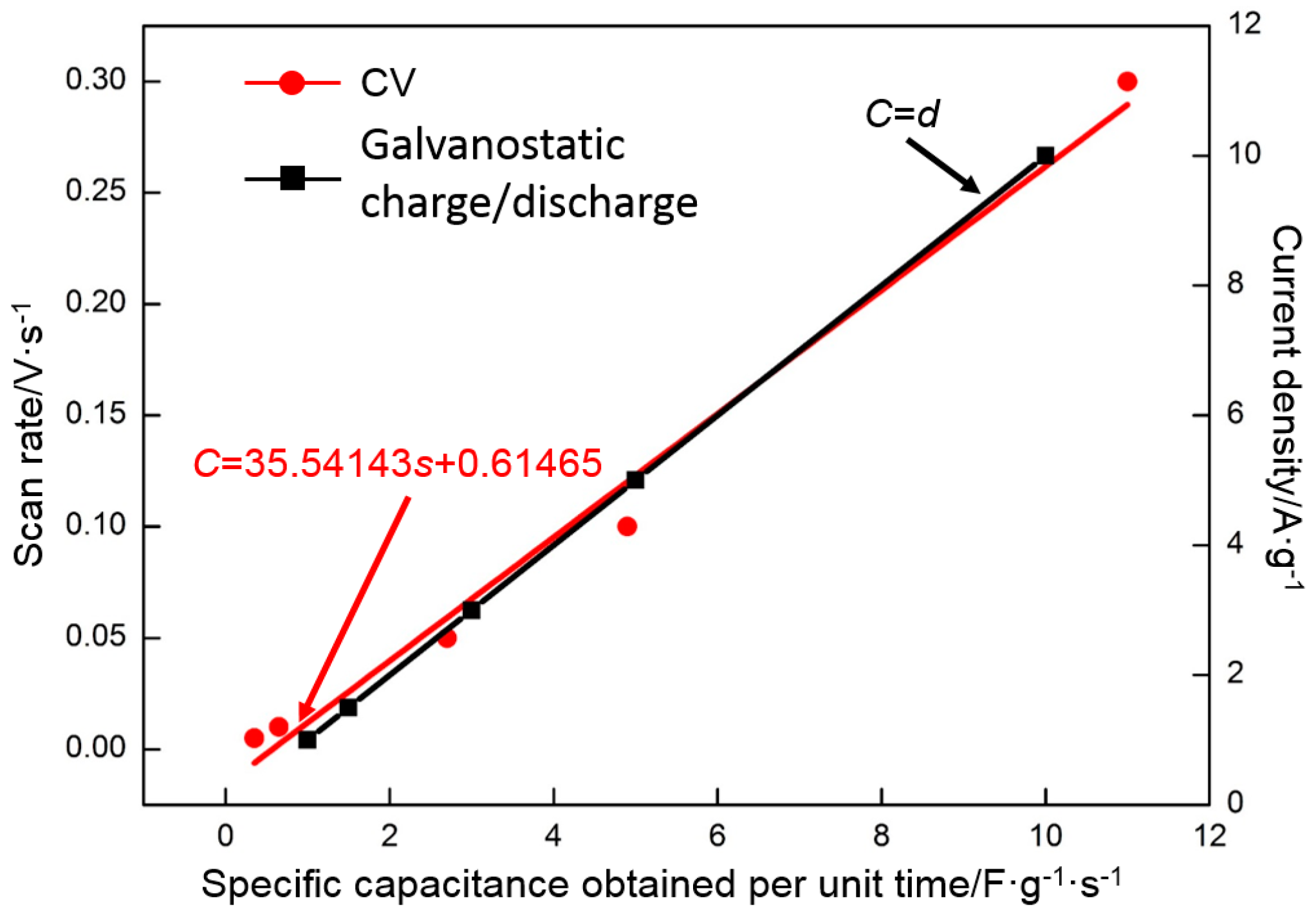
- The specific capacitance of RGO is also connected with the charge/discharge rate. Along with the increase in charge/discharge rate, the specific capacitance presents the decreasing trend, accompanied with the increase in charge/discharge time (CV: 0.005 V·s−1, 139 F·g−1/400 s; 0.01 V·s−1, 130 F·g−1/200 s; 0.05 V·s−1, 109 F·g−1/40 s; 0.1 V·s−1, 98 F·g−1/20 s; 0.3 V·s−1, 77 F·g−1/6.7 s. Galvanostatic charge/discharge: 1 A·g−1, 151 F·g−1/151 s; 1.5 A·g−1, 135 F·g−1/90 s; 3 A·g−1, 121 F·g−1/40.4 s; 5 A·g−1, 114 F·g−1/20.8 s; 10 A·g−1 100 F·g−1/10 s). The specific capacitance obtained per unit time (F·g−1·s−1) is used to characterize the charge/discharge efficiency. The index is increased linearly with the charge/discharge rate (CV: 0.005 V·s−1, 0.35 F·g−1·s−1; 0.01 V·s−1, 0.65 F·g−1·s−1; 0.05 V·s−1, 2.7 F·g−1·s−1; 0.1 V·s−1, 4.9 F·g−1·s−1; 0.3 V·s−1, 11 F·g−1·s−1. Galvanostatic charge/discharge: 1 A·g−1, 1 F·g−1·s−1; 1.5 A·g−1, 1.5 F·g−1·s−1; 3 A·g−1, 3 F·g−1·s−1; 5 A·g−1, 5 F·g−1·s−1; 10 A·g−1, 10 F·g−1·s−1).
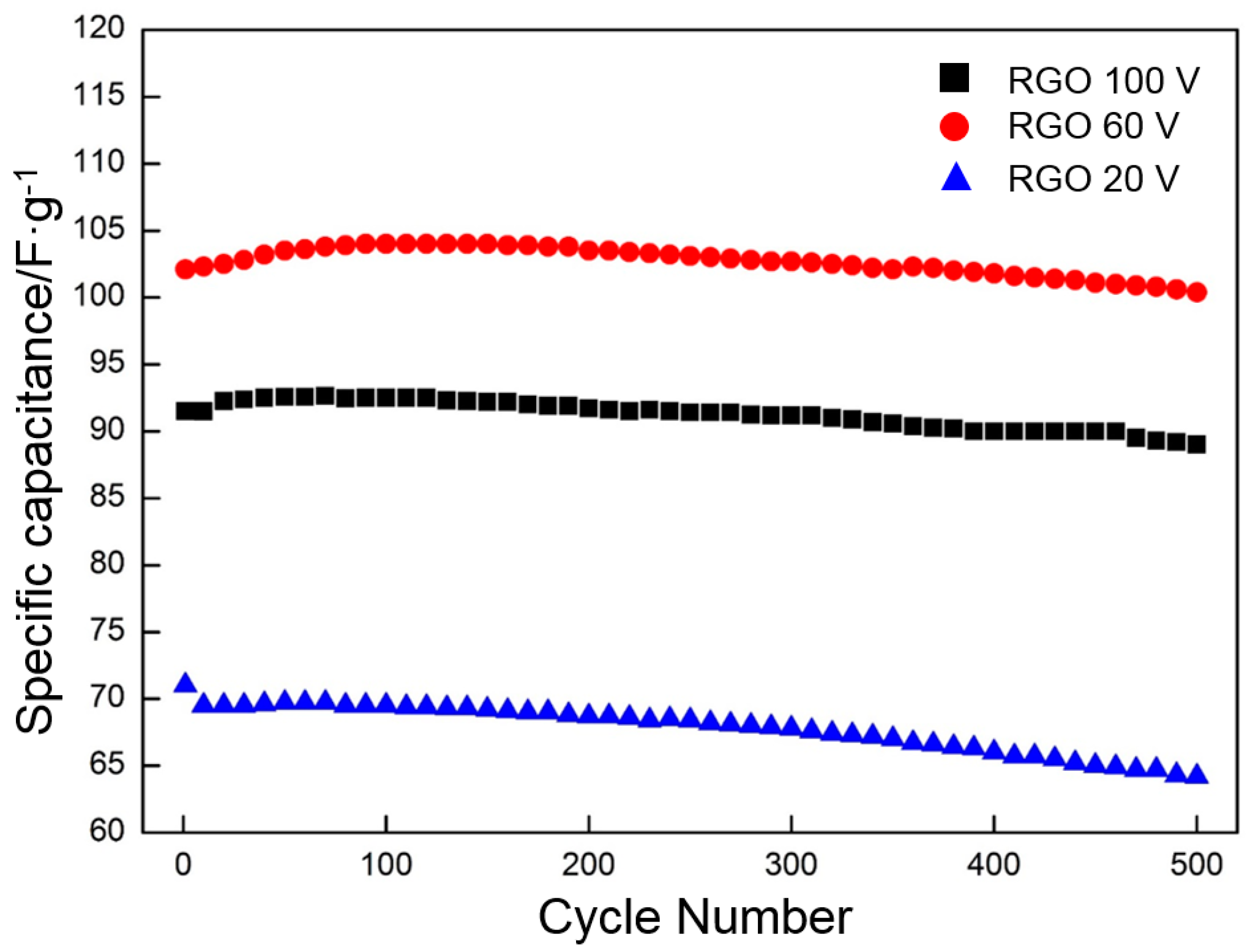
- The RGO deposited at 60 V and thermally reduced at 300 °C exhibits an excellent cycle stability and maintains approximately 98% of the initial specific capacitance after 500 cycles.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hao, J.; Liao, Y.; Zhong, Y.; Shu, D.; He, C.; Guo, S.; Huang, Y.; Zhong, J.; Hu, L. Three-dimensional graphene layers prepared by a gas-foaming method for supercapacitor applications. Carbon 2015, 94, 879–887. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, J.; Zhang, Q.; You, B.; Chen, G. Three dimensional Ni foam-supported graphene oxide for binder-free pseudocapacitor. Electrochim. Acta 2015, 152, 216–221. [Google Scholar] [CrossRef]
- Miller, J.R.; Simon, P. Electrochemical Capacitors for Energy Management. Science 2008, 321, 651–652. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Qin, X.; Wang, X.; Wang, Y.; Tao, H.; Wu, Q.; Yang, L.; Hu, Z. Carbon Nanocages as Supercapacitor Electrode Materials. Adv. Mater. 2012, 24, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Zhai, D.; Li, B.; Kang, F.; Du, H.; Xu, C. Preparation of mesophase-pitch-based activated carbons for electric double layer capacitors with high energy density. Microporous Mesoporous Mater. 2010, 130, 224–228. [Google Scholar] [CrossRef]
- Xu, B.; Wu, F.; Mu, D.; Dai, L.; Cao, G.; Zhang, H.; Chen, S.; Yang, Y. Activated carbon prepared from PVDC by NaOH activation as electrode materials for high performance EDLCs with non-aqueous electrolyte. Int. J. Hydrogen Energy 2010, 35, 632–637. [Google Scholar] [CrossRef]
- Lu, W.; Yushin, G. Electrical double layer capacitors with activated sucrose-derived carbon electrodes. Carbon 2011, 49, 4830–4838. [Google Scholar]
- Liu, H.J.; Wang, J.; Wang, C.X.; Xia, Y.Y. Ordered Hierarchical Mesoporous/Microporous Carbon Derived from Mesoporous Titanium-Carbide/Carbon Composites and its Electrochemical Performance in Supercapacitor. Adv. Energy Mater. 2011, 1, 1101–1108. [Google Scholar] [CrossRef]
- Liu, H.J.; Wang, X.M.; Cui, W.J.; Dou, Y.Q.; Zhao, D.Y.; Xia, Y.Y. Highly ordered mesoporous carbon nanofiber arrays from a crab shell biological template and its application in supercapacitors and fuel cells. J. Mater. Chem. 2010, 20, 4223–4230. [Google Scholar] [CrossRef]
- Huang, C.H.; Zhang, Q.; Chou, T.C.; Chen, C.M.; Su, D.S.; Doong, R.A. Three-Dimensional Hierarchically Ordered Porous Carbons with Partially Graphitic Nanostructures for Electrochemical Capacitive Energy Storage. ChemSusChem 2012, 5, 563–571. [Google Scholar] [CrossRef] [PubMed]
- An, K.H.; Kim, W.S.; Park, Y.S.; Choi, Y.C.; Lee, S.M.; Chung, D.C.; Bae, D.J.; Lim, S.C.; Lee, Y.H. Supercapacitors Using Single-Walled Carbon Nanotube Electrodes. Adv. Mater. 2001, 13, 497–500. [Google Scholar] [CrossRef]
- Hiraoka, T.; Izadi-Najafabadi, A.; Yamada, T.; Futaba, D.N.; Yasuda, S.; Tanaike, O.; Hatori, H.; Yumura, M.; Iijima, S.; Hata, K. Compact and Light Supercapacitor Electrodes from a Surface-Only Solid by Opened Carbon Nanotubes with 2200 m2·g−1 Surface Area. Adv. Funct. Mater. 2010, 20, 422–428. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.; Ganesh, K.J.; Cai, W.P.; Ferreira, J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Wang, J. Graphene-based materials for supercapacitor electrodes—A review. J. Mater. 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Alazmi, A.; Rasul, S.; Patole, S.P.; Costa, P.M.F.J. Comparative study of synthesis and reduction methods for graphene oxide. Polyhedron 2016, 116, 153–161. [Google Scholar] [CrossRef]
- Sun, L.; Wang, H.; Eid, K.; Alshehri, S.M.; Malgras, V.; Yamauchi, Y.; Wang, L. One-Step Synthesis of Dendritic Bimetallic PtPd Nanoparticles on Reduced Graphene Oxide and Its Electrocatalytic Properties. Electrochim. Acta 2016, 188, 845–851. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, G.; Gao, P.; Bi, S.; Tang, X.; Wang, D. High-performance supercapacitor of macroscopic graphene hydrogels by partial reduction and nitrogen doping of graphene oxide. Electrochim. Acta 2016, 221, 167–176. [Google Scholar] [CrossRef]
- Yang, D.; Bock, C. Laser reduced graphene for supercapacitor applications. J. Power Sources 2017, 337, 73–81. [Google Scholar] [CrossRef]
- Hsieh, C.-T.; Hsu, S.-M.; Lin, J.-Y. Fabrication of Graphene-Based Electrochemical Capacitors. Jpn. J. Appl. Phys. 2012, 51, 01AH06. [Google Scholar] [CrossRef]
- Tegou, E.; Pseiropoulos, G.; Filippidou, M.K.; Chatzandroulis, S. Low-temperature thermal reduction of graphene oxide films in ambient atmosphere: Infra-red spectroscopic studies and gas sensing applications. Microelectron. Eng. 2016, 159, 146–150. [Google Scholar] [CrossRef]
- Tayel, M.B.; Soliman, M.M.; Ebrahim, S.; Harba, M.E. Sprayed polyaniline layer onto chemically reduced graphene oxide as electrode for high performance supercapacitor. Synth. Met. 2016, 217, 237–243. [Google Scholar] [CrossRef]
- Ma, C.; Chen, Z.; Fang, M.; Lu, H. Controlled synthesis of graphene sheets with tunable sizes by hydrothermal cutting. J. Nanopart. Res. 2012, 14, 2–9. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, G.; Jiang, Z.; Yao, Z.; Yue, M. Solvothermal synthesis of graphene nanosheets as the electrode materials for supercapacitors. Ionics 2015, 21, 801–808. [Google Scholar] [CrossRef]
- Tao, H.-C.; Zhua, S.-C.; Yang, X.-L.; Zhang, L.-L.; Nia, S.-B. Systematic investigation of reduced graphene oxide foams for high-performance supercapacitors. Electrochim. Acta 2016, 190, 168–177. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Q.; Wei, T.; Jiang, L.; Zhang, M.; Jing, X.; Fan, Z. Template-Assisted Low Temperature Synthesis of Functionalized Graphene for Ultrahigh Volumetric Performance Supercapacitors. ACS Nano 2014, 8, 4720–4729. [Google Scholar] [CrossRef] [PubMed]
- Xia, K.; Li, Q.; Zheng, L.; You, K.; Tian, X.; Han, B.; Gao, Q.; Huan, Z.; Chen, G.; Zhou, C. Controllable fabrication of 2D and 3D porous graphene architectures using identical thermally exfoliated graphene oxides as precursors and their application as supercapacitor electrodes. Microporous Mesoporous Mater. 2017, 237, 228–236. [Google Scholar] [CrossRef]
- Li, Z.; Chang, T.; Yun, G.; Guo, J.; Yang, B. 2D tin dioxide nanoplatelets decorated graphene with enhanced performance supercapacitor. J. Alloys Compd. 2014, 586, 353–359. [Google Scholar] [CrossRef]
- Xiao, P.; Wang, D.; Lang, J. Comparison in Factors Affecting Electrochemical Properties of Thermal-Reduced Graphene Oxide for Supercapacitors. J. Electrochem. 2014, 20, 553–562. [Google Scholar]
- Wang, Y.; Shi, Z.; Huang, Y.; Ma, Y.; Wang, C.; Chen, M.; Chen, Y. Supercapacitor Devices Based on Graphene Materials. J. Phys. Chem. C 2009, 113, 13103–13107. [Google Scholar] [CrossRef]
- Yan, J.; Fan, Z.; Wei, T.; Qian, W.; Zhang, M.; Wei, F. Fast and reversible surface redox reaction of graphene-MnO2 composites as supercapacitor electrodes. Carbon 2010, 48, 3825–3833. [Google Scholar] [CrossRef]
- Ezeigwea, E.R.; Tana, M.T.T.; Khiewa, P.; Siong, C.W. One-step green synthesis of graphene/ZnO nanocomposites for electrochemical capacitors. Ceram. Int. 2015, 41, 715–724. [Google Scholar] [CrossRef]
- Liu, X.; Qi, X.; Zhang, Z.; Ren, L.; Hao, G.; Liu, Y.; Wang, Y.; Huang, K.; Wei, X.; Li, J.; et al. Electrochemically reduced graphene oxide with porous structure as a binder-free electrode for high-rate supercapacitors. RSC Adv. 2014, 4, 13673–13679. [Google Scholar] [CrossRef]
- Jeong, H.T.; Kim, B.C.; Higgins, M.J.; Wallace, G.G. Higgins Highly stretchable reduced graphene oxide (rGO)/single-walled carbon nanotubes (SWNTs) electrodes for energy storage devices. Electrochim. Acta 2015, 163, 149–160. [Google Scholar] [CrossRef]
- Zabihi, F.; Xie, Y.; Gao, S.; Eslamian, M. Morphology, conductivity, and wetting characteristics of PEDOT: PSS thin films deposited by spin and spray coating. Appl. Surf. Sci. 2015, 338, 163–177. [Google Scholar] [CrossRef]
- Deng, C.; Jiang, J.; Liu, F.; Fang, L.; Wang, J.; Li, D.; Wu, J. Influence of graphene oxide coatings on carbon fiber by ultrasonically assisted electrophoretic deposition on its composite interfacial property. Surf. Coat. Technol. 2015, 272, 176–181. [Google Scholar] [CrossRef]
- Huang, S.; Wu, G.; Chen, C.; Yang, Y.; Zhang, S.; Lu, C. Electrophoretic deposition and thermal annealing of a graphene oxide thin film on carbon fiber surfaces. Carbon 2013, 52, 605–620. [Google Scholar] [CrossRef]
- Wang, C.; Li, J.; Sun, S.; Li, X.; Zhao, F.; Jiang, B.; Huang, Y. Electrophoretic deposition of graphene oxide on continuous carbon fibers for reinforcement of both tensile and interfacial strength. Compos. Sci. Technol. 2016, 135, 46–53. [Google Scholar] [CrossRef]
- Ghasemi, S.; Hosseinzadeh, R.; Jafari, M. MnO2 nanoparticles decorated on electrophoretically deposited graphene nanosheets for high performance supercapacitor. Int. J. Hydrogen Energy 2015, 40, 1037–1046. [Google Scholar] [CrossRef]
- Xiong, C.; Li, T.; Zhu, Y.; Zhao, T.; Dang, A.; Li, H.; Ji, X.; Shang, Y.; Khan, M. Two-step approach of fabrication of interconnected nanoporous 3D reduced graphene oxide-carbon nanotube-polyaniline hybrid as a binder-free supercapacitor electrode. J. Alloys Compd. 2017, 695, 1248–1259. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Yu, P.; Ma, Y. Electrophoretic deposition of graphene nanosheets on nickel foams for electrochemical capacitors. J. Power Sources 2010, 195, 3031–3035. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, D.; Tang, P.; Wang, Y.; Xu, C.; Li, H. MnO2/graphene/nickel foam composite as high performance supercapacitor electrode via a facile electrochemical deposition strategy. Mater. Lett. 2012, 76, 127–130. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Dong, Q.; Yang, C.; Lu, Y. Electrophoretic deposition of graphene oxide on graphite fiber cloth and the electrochemical performance as electrode of supercapacitor. J. Mater. Sci. 2017, 28, 6308–6319. [Google Scholar] [CrossRef]
- Ito, J.; Nakamura, J.; Natori, A. Semiconducting nature of the oxygen-adsorbed graphene sheet. J. Appl. Phys. 2008, 103, 1137121. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Han, F.; Cai, X. The effect of heat treatment on the electrical conductivity of highly conducting graphene films. New Carbon Mater. 2012, 27, 266–270. [Google Scholar] [CrossRef]
- Tong, H.; Zhu, J.; Chen, J.; Han, Y.; Yang, S.; Ding, B.; Zhang, X. Electrochemical reduction of GO and its electrochemical capacitive performance. J. Solid State. Electrochem. 2013, 17, 2857–2863. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, Y.; Jin, M.; Kim, E.; Bae, J.; Lee, Y. Thermal stability of graphite oxide. Chem. Phys. Lett. 2009, 470, 255–258. [Google Scholar] [CrossRef]
- An, S.; Zhu, Y.; Lee, S.; Stoller, M.D.; Emilsson, T.; Park, S.; Velamakanni, A.; An, J.; Ruoff, R.S. Thin Film Fabrication and Simultaneous Anodic Reduction of Deposited GO Platelets by Electrophoretic Deposition. J. Phys. Chem. Lett. 2010, 1, 1259–1263. [Google Scholar] [CrossRef]
- Casiraghi, C.; Hartschuh, A.; Qian, H.; Piscanec, S.; Georgi, C.; Fasoli, A.; Novoselov, K.S.; Basko, D.M.; Ferrari, A.C. Raman Spectroscopy of Graphene Edges. Nano Lett. 2009, 9, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.J.; Zhao, Q.K.; Li, T.Y.; Yan, J.; Ren, Y.M.; Feng, J.; Wei, T. Easy synthesis of porous graphene nanosheets and their use in supercapacitors. Carbon 2012, 50, 1699–1712. [Google Scholar] [CrossRef]
- Wu, X.; Yang, D.; Wang, C.; Jiang, Y.; Wei, T.; Fan, Z. Functionalized three-dimensional graphene networks for high performance supercapacitors. Carbon 2015, 92, 26–30. [Google Scholar] [CrossRef]
- An, K.H.; Kim, W.S.; Park, Y.S.; Moon, J.M.; Bae, D.J.; Lim, S.C.; Lee, Y.S.; Lee, Y.H. Electrochemical Properties of High-Power Supercapacitors Using Single-Walled Carbon Nanotube Electrodes. Adv. Funct. Mater. 2011, 5, 387–392. [Google Scholar]
| Electrode | Specific Capacitance (F·g−1) | Current Density or Scan Rate | References |
|---|---|---|---|
| RGO | 151 | 1 A·g−1 | This work |
| ACs | 145 | 20 A·g−1 | [5] |
| ACs | 155 | 0.05 A·g−1 | [6] |
| ACs | 160 | 0.1 A·g−1 | [7] |
| OHMMC | 146 | 0.1 A·g−1 | [8] |
| MCNAs | 152 | 0.1 A·g−1 | [9] |
| 3D HOPC | 73.4 | 3 mV·s−1 | [10] |
| CNTs | 180 | 100 mV·s−1 | [11] |
| SWNT | 114 | 1 mV·s−1 | [12] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Li, J.; Huang, W. Porous Graphene Oxide Prepared on Nickel Foam by Electrophoretic Deposition and Thermal Reduction as High-Performance Supercapacitor Electrodes. Materials 2017, 10, 936. https://doi.org/10.3390/ma10080936
Xu Y, Li J, Huang W. Porous Graphene Oxide Prepared on Nickel Foam by Electrophoretic Deposition and Thermal Reduction as High-Performance Supercapacitor Electrodes. Materials. 2017; 10(8):936. https://doi.org/10.3390/ma10080936
Chicago/Turabian StyleXu, Yunhe, Jun Li, and Wenxin Huang. 2017. "Porous Graphene Oxide Prepared on Nickel Foam by Electrophoretic Deposition and Thermal Reduction as High-Performance Supercapacitor Electrodes" Materials 10, no. 8: 936. https://doi.org/10.3390/ma10080936
APA StyleXu, Y., Li, J., & Huang, W. (2017). Porous Graphene Oxide Prepared on Nickel Foam by Electrophoretic Deposition and Thermal Reduction as High-Performance Supercapacitor Electrodes. Materials, 10(8), 936. https://doi.org/10.3390/ma10080936





