Impacts of the Oxygen Precursor on the Interfacial Properties of LaxAlyO Films Grown by Atomic Layer Deposition on Ge
Abstract
1. Introduction
2. Experiment
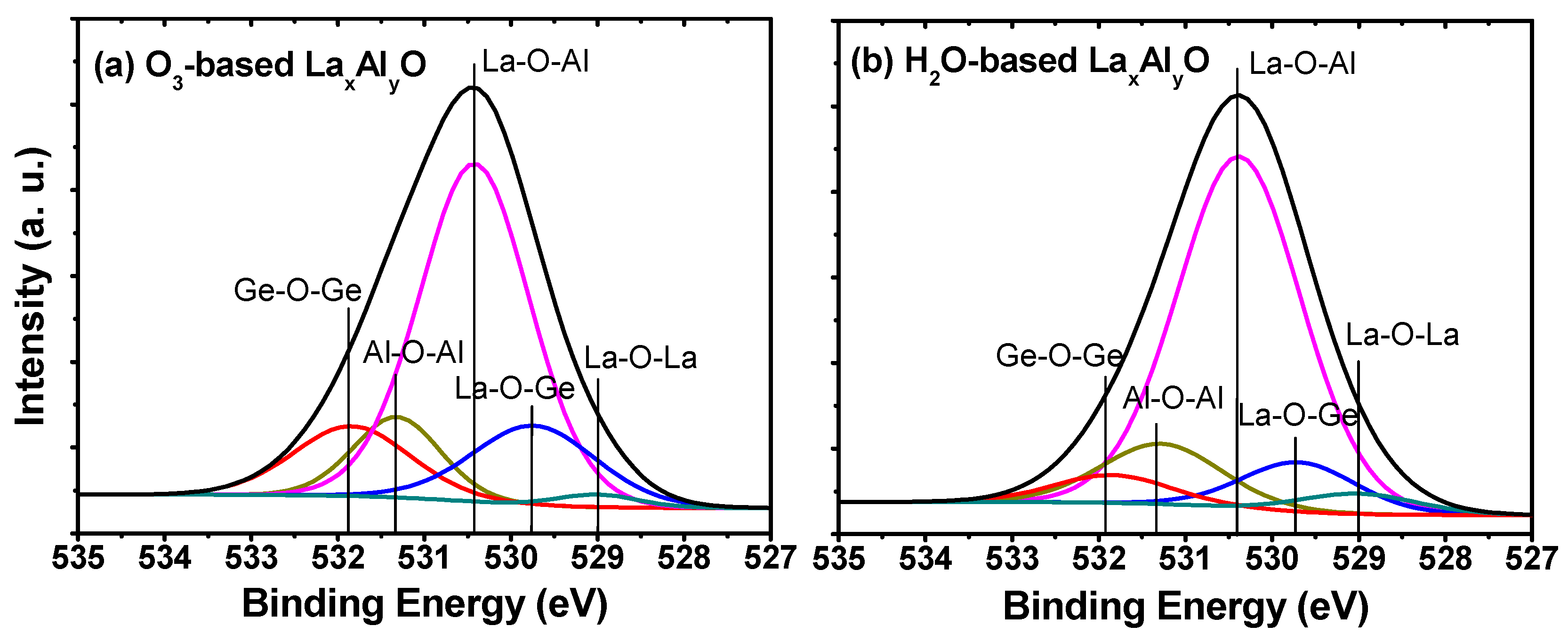
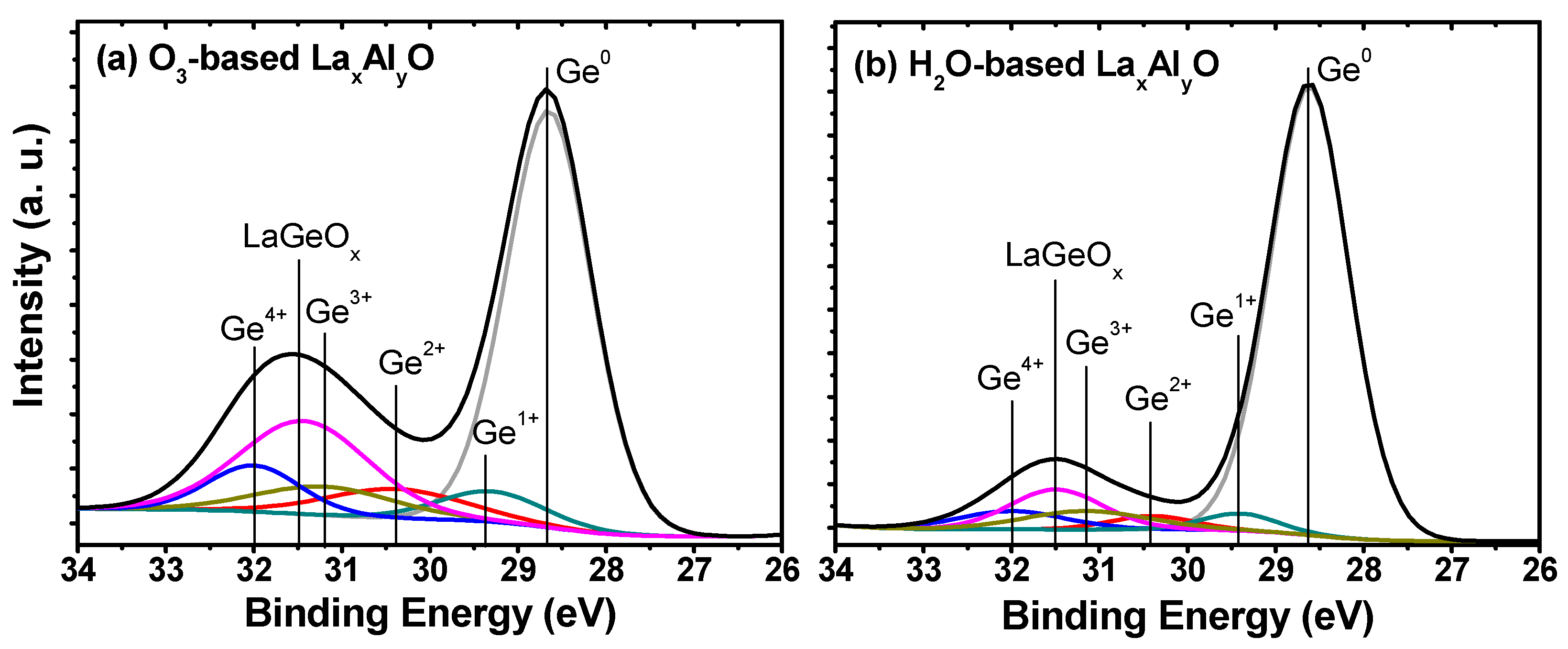
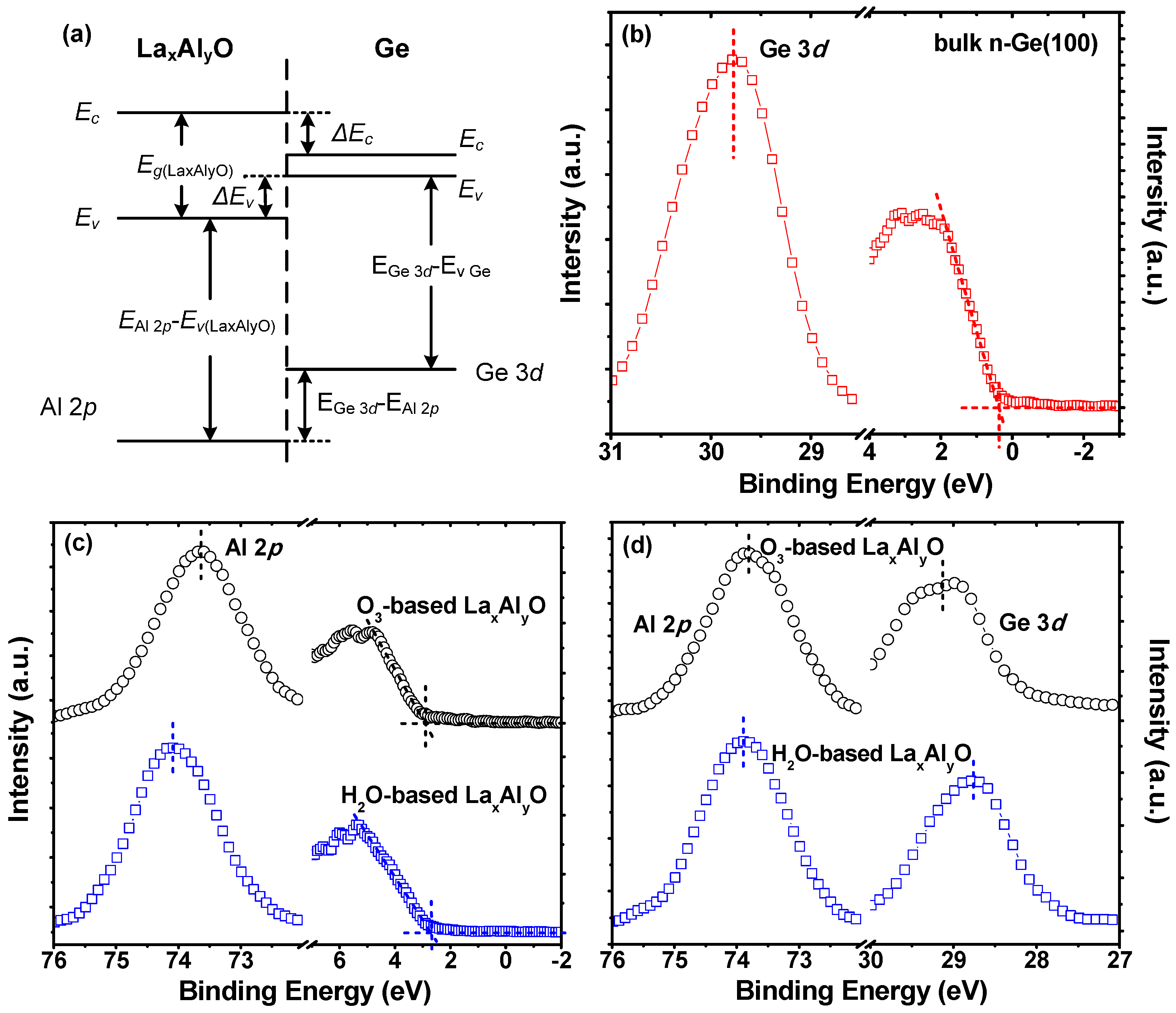
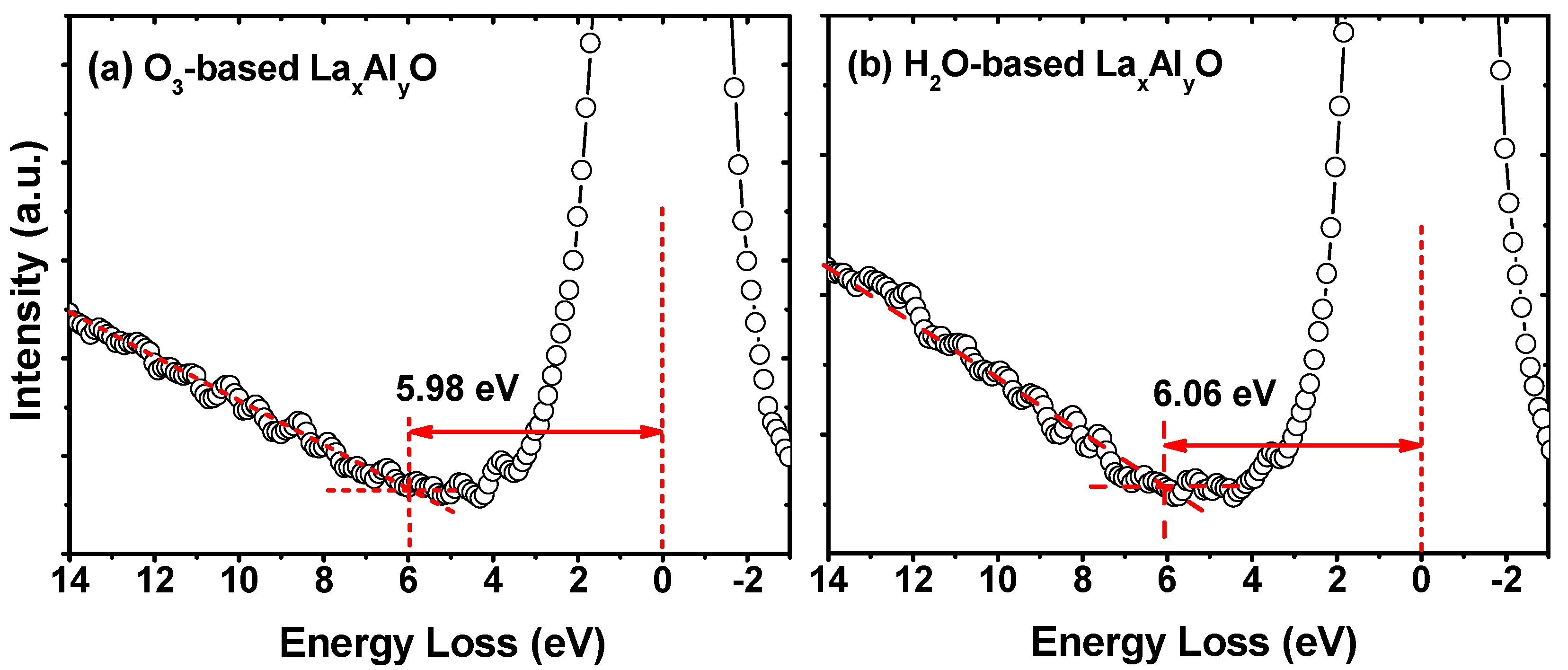
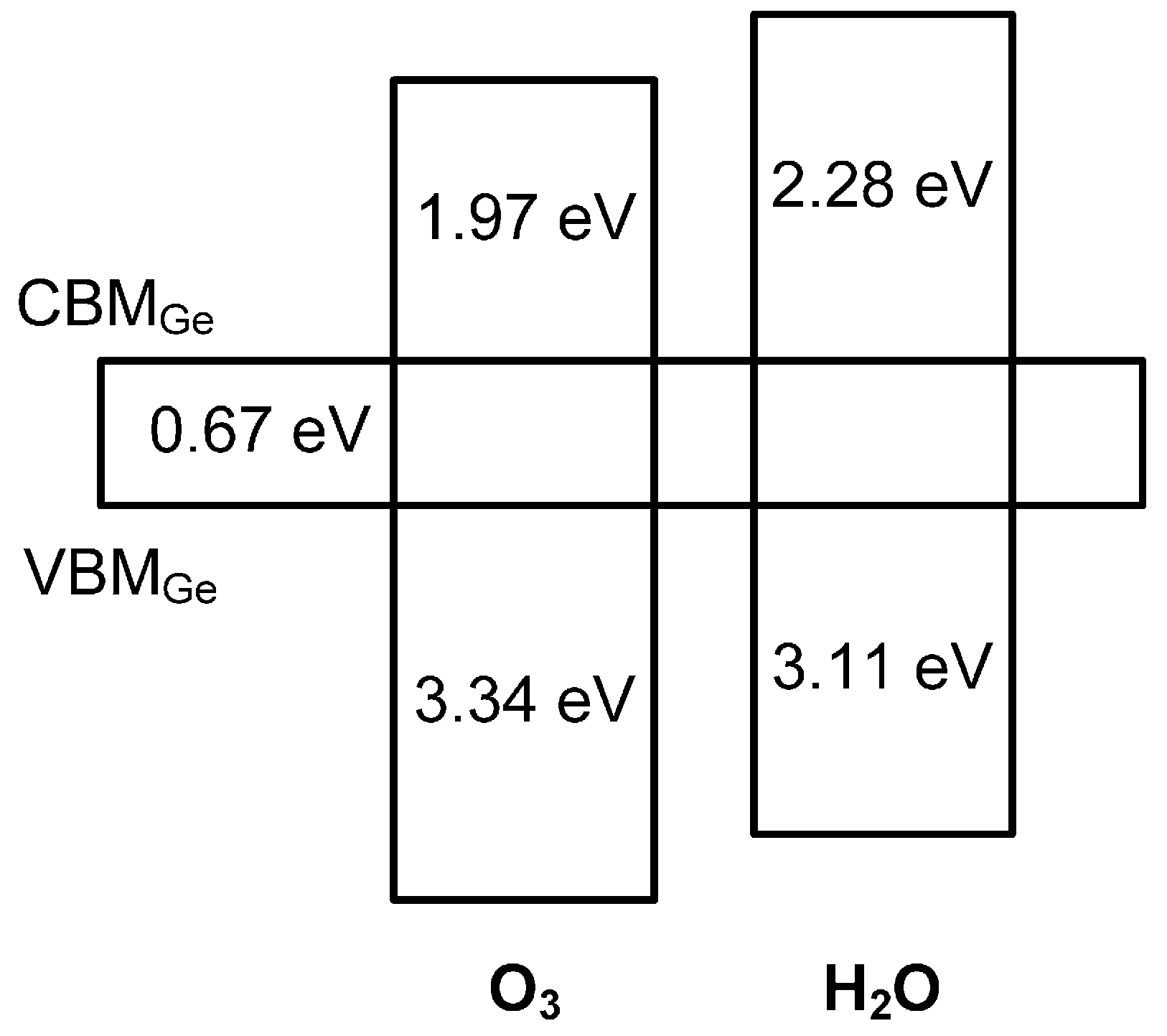
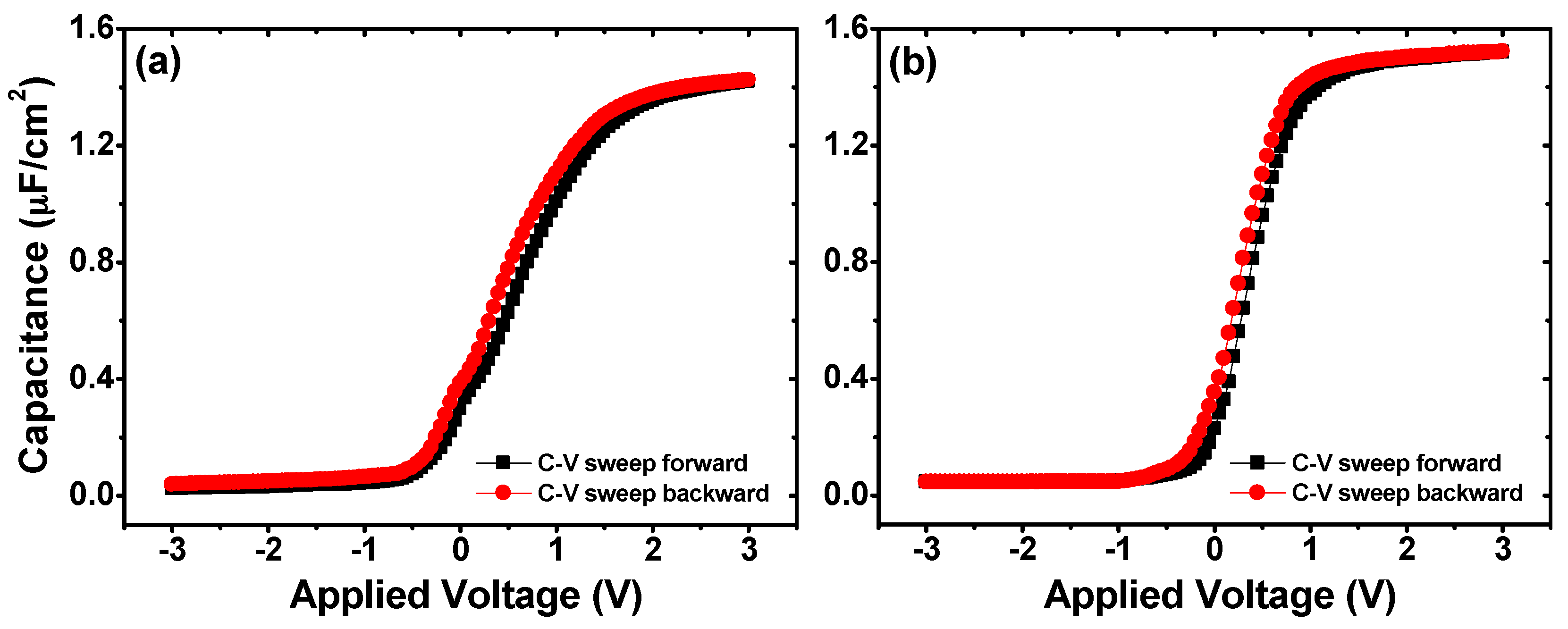
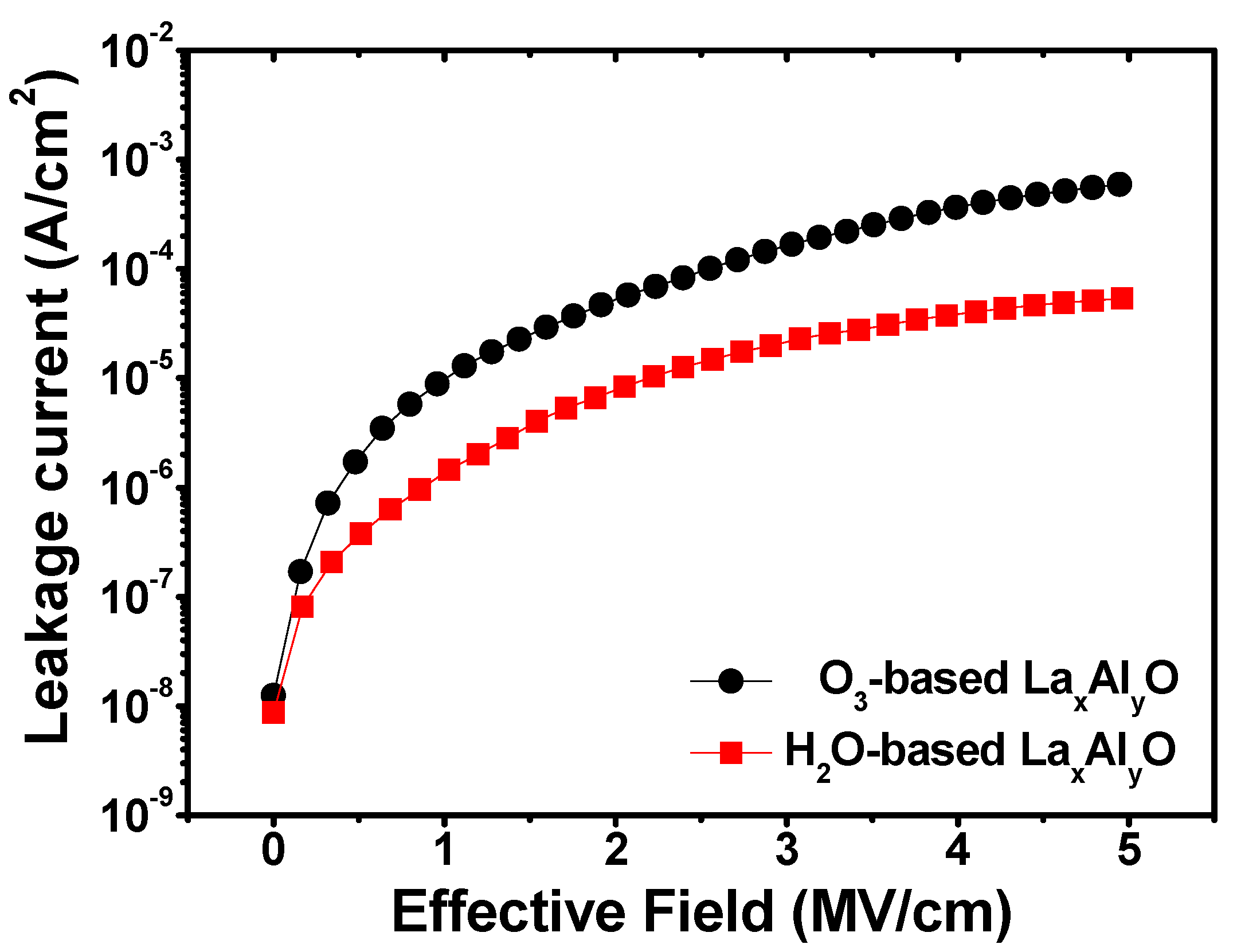
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yi, S.H.; Chang-Liao, K.S.; Wu, T.Y.; Hsu, C.W.; Huang, J.Y. High performance Ge pMOSFETs with HfO2/Hf-Cap/GeOx gate stack and suitable post metal annealing treatments. IEEE Electron Device Lett. 2017, 38, 544–547. [Google Scholar] [CrossRef]
- Xie, Q.; Deng, S.; Schaekers, M.; Lin, D.; Caymax, M.; Delabie, A.; Qu, X.P.; Jiang, Y.L.; Deduytsche, D.; Detavernier, C. Germanium surface passivation and atomic layer deposition of high-k dielectrics—A tutorial review on Ge-based MOS capacitors. Semicond. Sci. Technol. 2012, 27, 074012. [Google Scholar] [CrossRef]
- Seok, T.J.; Cho, Y.J.; Jin, H.S.; Kim, D.H.; Kim, D.W.; Lee, S.M.; Park, J.B.; Won, J.Y.; Kim, S.K.; Hwang, C.S.; et al. High quality interfacial sulfur passivation via H2S pre-deposition annealing for an atomic-layer-deposited HfO2 film on a Ge substrate. J. Mater. Chem. C 2016, 4, 850–856. [Google Scholar] [CrossRef]
- Simoen, E.; Mitard, J.; Hellings, G.; Eneman, G.; DeJaeger, B.; Witters, L.; Vincent, B.; Loo, R.; Delabie, A.; Sioncke, S.; et al. Challenges and opportunities in advanced Ge pMOSFETs. Mat. Sci. Semicon. Proc. 2012, 15, 588–600. [Google Scholar] [CrossRef]
- Yoshida, T.; Kato, K.; Shibayama, S.; Sakashita, M.; Taoka, N.; Takeuchi, W.; Nakatsuka, O.; Zaima, S. Interface properties of Al2O3/Ge structures with thin Ge oxide interfacial layer formed by pulsed metal organic chemical vapor deposition. Jpn. J. Appl. Phys. 2014, 53, 08LD03. [Google Scholar] [CrossRef]
- Bethge, O.; Henkel, C.; Abermann, S.; Pozzovivo, G.; Stoeger-Pollach, M.; Werner, W.S.M.; Smoliner, J.; Bertagnolli, E. Stability of La2O3 and GeO2 passivated Ge surfaces during ALD of ZrO2 high-k dielectric. Appl. Surf. Sci. 2012, 258, 3444–3449. [Google Scholar] [CrossRef]
- Sahari, S.K.; Ohta, A.; Matsui, M.; Mishima, K.; Murakami, H.; Higashi, S.; Miyazak, S. Kinetics of thermally oxidation of Ge(100) surface. J. Phys. Conf. Ser. 2013, 417, 012014. [Google Scholar] [CrossRef]
- Wang, S.K.; Kita, K.; Lee, C.H.; Tabata, T.; Nishimura, T.; Nagashio, K.; Toriumi, A. Desorption kinetics of GeO from GeO2/Ge structure. J. Appl. Phys. 2010, 105, 054104. [Google Scholar]
- Mitrovic, I.Z.; Althobaiti, M.; Weerakkody, A.D.; Dhanak, V.R.; Linhart, W.M.; Veal, T.D.; Sedghi, N.; Hall, S.; Chalker, P.R.; Tsoutsou, D.; et al. Ge interface engineering using ultra-thin La2O3 and Y2O3 films: A study into the effect of deposition temperature. J. Appl. Phys. 2014, 115, 114102. [Google Scholar] [CrossRef]
- Li, X.F.; Liu, X.J.; Cao, Y.Q.; Li, A.D.; Li, H.; Wu, D. Improved interfacial and electrical properties of atomic layer deposition HfO2 films on Ge with La2O3 passivation. Appl. Surf. Sci. 2013, 264, 783–786. [Google Scholar] [CrossRef]
- Bethge, O.; Zimmermann, C.; Lutzer, B.; Simsek, S.; Abermann, S.; Bertagnolli, E. ALD grown rare-earth high-k oxides on Ge: Lowering of the interface trap density and EOT scalability. ECS Trans. 2014, 64, 69–76. [Google Scholar] [CrossRef]
- Yang, W.; Sun, Q.Q.; Fang, R.C.; Chen, L.; Zhou, P.; Ding, S.J.; Zhang, D.W. The thermal stability of atomic layer deposited HfLaOx: Material and electrical characterization. Curr. Appl. Phys. 2012, 12, 1445–1447. [Google Scholar] [CrossRef]
- Baitimirova, M.; Viter, R.; Andzane, J.; Lee, A.V.D.; Voiry, D.; Iatsunskyi, I.; Coy, E.; Mikoliunaite, L.; Tumenas, S.; Załęski, K.; et al. Tuning of structural and optical properties of Graphene/ZnO nanolaminates. J. Phys. Chem. C 2016, 120, 23716–23725. [Google Scholar] [CrossRef]
- Wiemer, C.; Lamagna, L.; Fanciulli, M. Atomic layer deposition of rare-earth-based binary and ternary oxides for microelectronic applications. Semicond. Sci. Technol. 2012, 27, 251–264. [Google Scholar] [CrossRef]
- Lee, B.H.; Oh, J.; Tseng, H.H.; Jammy, R.; Huff, H. The effects of annealing ambient on the characteristics of La2O3 films deposited by RPALD. J. Electrochem. Soc. 2010, 157, H479–H482. [Google Scholar]
- Ali, K.; Choi, K.H.; Jo, J.; Yun, W.L. High rate roll-to-roll atmospheric atomic layer deposition of Al2O3 thin films towards gas diffusion barriers on polymers. Mater. Lett. 2014, 136, 90–94. [Google Scholar] [CrossRef]
- Song, J.; Kakushima, K.; Ahmet, P.; Tsutsui, K.; Sugii, N.; Hattori, T.; Iwai, H. Improvement of interfacial properties with interfacial layer in La2O3/Ge structure. Microelectron. Eng. 2007, 84, 2336–2339. [Google Scholar] [CrossRef]
- Delabie, A.; Alian, A.; Bellenger, F.; Caymax, M.; Conard, T.; Franquet, A.; Sioncke, S.; Elshocht, V.S.; Heyns, M.M.; Meuris, M. H2O- and O3-based atomic layer deposition of high-κ dielectric films on GeO2 passivation layers. J. Electrochem. Soc. 2009, 156, G163–G167. [Google Scholar] [CrossRef]
- Spiga, S.; Wiemer, C.; Tallarida, G.; Scarel, G.; Ferrari, S.; Seguini, G.; Fanciulli, M. Effects of the oxygen precursor on the electrical and structural properties of HfO2 films grown by atomic layer deposition on Ge. Appl. Phys. Lett. 2005, 87, 112904. [Google Scholar] [CrossRef]
- Oh, I.K.; Kim, M.K.; Lee, J.S.; Lee, C.W.; Lansalot-Matras, C.; Noh, W.; Park, J.; Noorid, A.; Thompson, D.; Chu, S.; et al. The effect of La2O3-incorporation in HfO2 dielectrics on Ge substrate by atomic layer deposition. Appl. Surf. Sci. 2013, 287, 349–354. [Google Scholar] [CrossRef]
- Kraut, E.A.; Grant, R.W.; Waldrop, J.R.; Kowalczyk, S.P. Precise determination of the valence-band edge in X-Ray photoemission spectra: Application to measurement of semiconductor interface potentials. Phys. Rev. Lett. 1980, 44, 1620. [Google Scholar] [CrossRef]
- Kraut, E.A.; Grant, R.W.; Waldrop, J.R.; Kowalczyk, S.P. Semiconductor core-level to valence-band maximum binding-energy differences: Precise determination by X-ray photoelectron spectroscopy. Phys. Rev. B 1983, 28, 1965. [Google Scholar] [CrossRef]
- Zhu, Y.; Jain, N.; Mohata, D.K.; Datta, S.; Lubyshev, D.; Fastenau, J.M.; Liu, A.K.; Hudait, M.K. Band offset determination of mixed As/Sb type-II staggered gap heterostructure for n-channel tunnel field effect transistor application. J. Appl. Phys. 2013, 113, 024319. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Fang, Z.B.; Liu, S.Y.; Tan, Y.S.; Chen, J.J. Band offsets of La2O3 films on Ge substrates grown by radio frequency magnetron sputtering. Mater. Lett. 2014, 116, 43–45. [Google Scholar] [CrossRef]
- Suzuki, M. Comprehensive study of lanthanum aluminate high-dielectric-constant gate oxides for advanced CMOS devices. Materials 2012, 5, 443–477. [Google Scholar] [CrossRef]
- Viter, R.; Iatsunskyi, I.; Fedorenko, V.; Tumenas, S.; Balevicius, Z.; Ramanavicius, A.; Balme, S.; Kempiński, M.; Nowaczyk, G.; Jurga, S.; et al. Enhancement of electronic and optical properties of ZnO/Al2O3 nanolaminate coated electrospun nanofibers. J. Phys. Chem. C 2016, 120, 5124–5132. [Google Scholar] [CrossRef]
- Edge, L.F.; Schloma, D.G. Measurement of the band offsets between amorphous LaAlO3 and silicon. Appl. Phys. Lett. 2004, 84, 726. [Google Scholar] [CrossRef]
- Perego, M.; Scarel, G.; Fanciulli, M.; Fedushkin, I.L.; Skatova, A.A. Fabrication of GeO2 layers using a divalent Ge precursor. Appl. Phys. Lett. 2007, 90, 162115. [Google Scholar] [CrossRef]
- Mi, Y.Y.; Wang, S.J.; Chai, J.W.; Pan, J.S.; Huan, A.C.H.; Ning, M.; Ong, C.K. Energy-band alignments at LaAlO3 and Ge interfaces. Appl. Phys. Lett. 2006, 89, 202107. [Google Scholar] [CrossRef]
- Hauser, J.R.; Ahmed, K. Characterization of ultra-thin oxides using electrical C-V and I-V measurements. AIP Conf. Proc. 1998, 449, 235. [Google Scholar]
- SZE, S.M.; NG, K.K. Physics of Semiconductor Devices, 3rd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2006; pp. 223–236. [Google Scholar]
- Lamagna, L.; Wiemer, C.; Perego, M.; Volkos, S.N.; Baldovino, S.; Tsoutsou, D.; Schamm-Chardon, S.; Coulon, P.E.; Fanciulli, M. O3-based atomic layer deposition of hexagonal La2O3 films on Si(100) and Ge(100) substrates. J. Appl. Phys. 2010, 108, 084108. [Google Scholar] [CrossRef]
- Swaminathan, S.; Sun, Y.; Pianetta, P.; McIntyre, P.C. Ultrathin ALD-Al2O3 layers for Ge(001) gate stacks: Local composition evolution and dielectric properties. J. Appl. Phys. 2011, 110, 094105. [Google Scholar] [CrossRef]
- Ando, T. Ultimate scaling of high-κ gate dielectrics: Higher-κ or interfacial layer scavenging? Materials 2012, 5, 478–500. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Liu, H.; Wang, X.; Wang, Y.; Wang, S. Impacts of the Oxygen Precursor on the Interfacial Properties of LaxAlyO Films Grown by Atomic Layer Deposition on Ge. Materials 2017, 10, 856. https://doi.org/10.3390/ma10080856
Zhao L, Liu H, Wang X, Wang Y, Wang S. Impacts of the Oxygen Precursor on the Interfacial Properties of LaxAlyO Films Grown by Atomic Layer Deposition on Ge. Materials. 2017; 10(8):856. https://doi.org/10.3390/ma10080856
Chicago/Turabian StyleZhao, Lu, Hongxia Liu, Xing Wang, Yongte Wang, and Shulong Wang. 2017. "Impacts of the Oxygen Precursor on the Interfacial Properties of LaxAlyO Films Grown by Atomic Layer Deposition on Ge" Materials 10, no. 8: 856. https://doi.org/10.3390/ma10080856
APA StyleZhao, L., Liu, H., Wang, X., Wang, Y., & Wang, S. (2017). Impacts of the Oxygen Precursor on the Interfacial Properties of LaxAlyO Films Grown by Atomic Layer Deposition on Ge. Materials, 10(8), 856. https://doi.org/10.3390/ma10080856






