Modelling Syngas Combustion from Biomass Gasification and Engine Applications: A Comprehensive Review
Abstract
1. Introduction
2. Gasification-Based Syngas Production
2.1. Gasification Process
2.2. Gasifier Type
2.3. Critical Insights on Syngas Production
3. The Oxidation and Combustion Kinetics of Syngas
3.1. Experimental Characterization of Syngas Combustion
3.2. Modelling and Kinetic Mechanisms of Syngas Combustion
3.3. Critical Insights on Kinetic Modelling
4. Application of Syngas in Internal Combustion Engines
4.1. Application of Syngas in SI Internal Combustion Engines
4.2. Application of Syngas in CI Engines
4.3. Critical Insights on Engine Applications
5. Discussion
- ⮚
- Syngas composition variability as a central challenge: The variability of syngas composition, dictated by feedstock and gasification process conditions, is consistently identified as a major factor influencing ignition delay, flame speed, and emissions. Moderate hydrogen contents (15–35 vol%) generally improve combustion stability, while CO2 and N2 act as diluents, lowering flame temperature and NOx emissions [66,90]. However, syngas produced from air-blown gasifiers is often nitrogen-rich, leading to significantly lower LHVs compared to oxygen/steam gasification pathways [33]. This compositional diversity complicates direct comparison between studies and limits the generalisation of results.
- ⮚
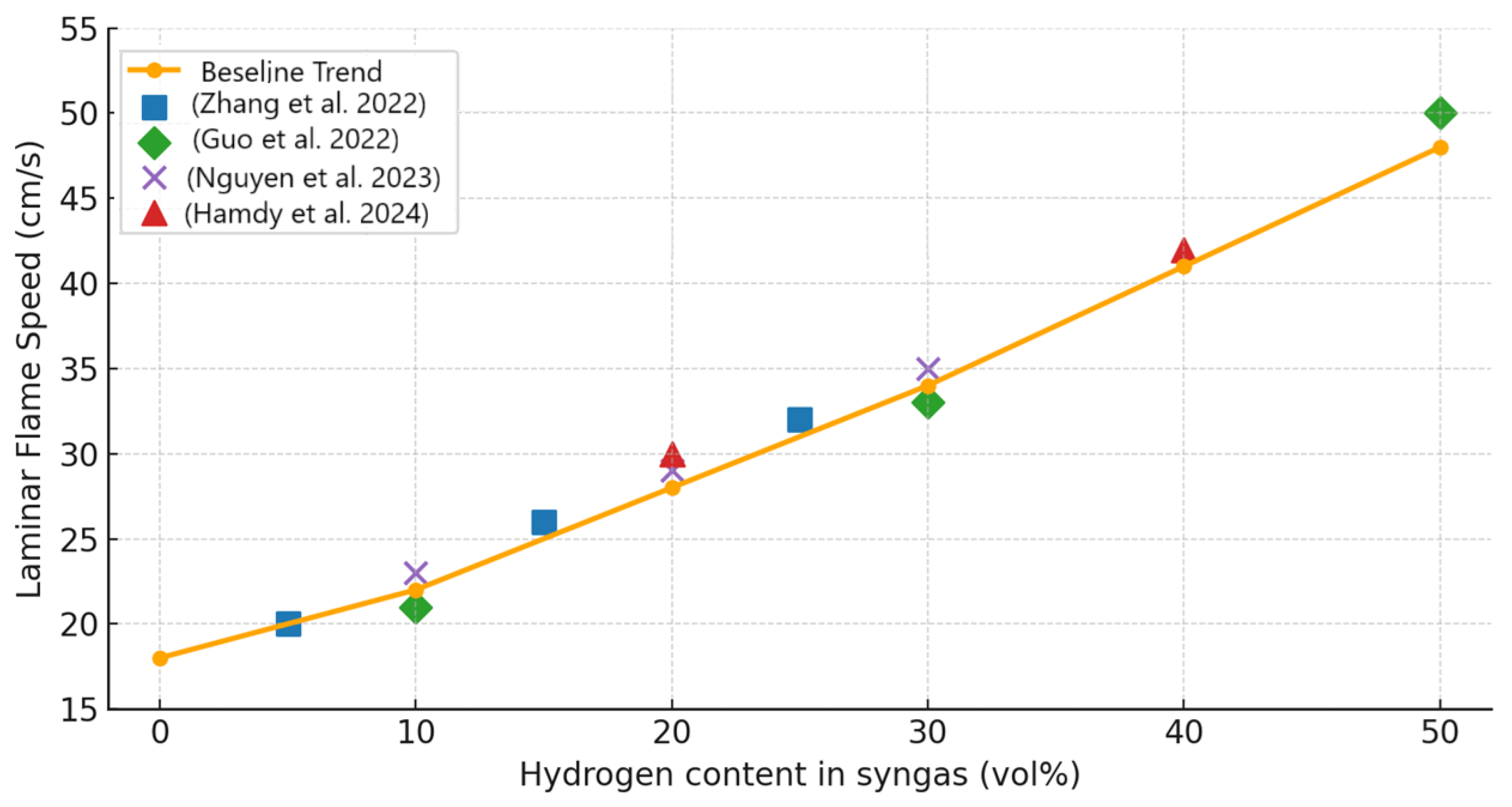
- Contradictions in the effect of hydrogen enrichment: Hydrogen is widely acknowledged as the most influential component of syngas due to its high diffusivity and reactivity. Most studies report that increasing H2 reduces ignition delay and enhances flame speed [90,120]. Yet the effect on NOx emissions remains inconsistent: some works show higher H2 fractions increase NOx due to higher flame temperatures [66], while others find the opposite under lean conditions, where faster burning reduces residence times at peak temperatures [86]. These contradictory findings highlight the strong dependence on boundary conditions, especially equivalence ratio and dilution levels.
- ⮚
- The dual role of carbon monoxide: Carbon monoxide contributes to syngas calorific value and participates in chain-branching reactions. Certain studies indicate that CO stabilises combustion and supports flame propagation [66,121], while others report inhibitory effects at high concentrations, reducing reactivity and prolonging ignition delay [78,93]. These contradictions can be attributed to the balance between CO’s role as a reactive species and its competition with H2 for oxidants. More systematic work under engine-relevant conditions is needed to clarify its role.
- ⮚
- Limitations of current kinetic and CFD models: Despite significant progress, kinetic mechanisms often fail to consistently predict laminar flame speeds and ignition delays across the full range of syngas compositions. Han et al. [121] showed that H2/CO mechanisms underpredict LFS in CO2-diluted mixtures at high pressure, necessitating refined rate constants. CFD simulations; meanwhile, frequently employ synthetic syngas mixtures with unrealistically high hydrogen contents (>50 vol%), which do not represent biomass gasification products [91]. Furthermore, turbulence–chemistry interaction models are often oversimplified, potentially compromising accuracy in transient or stratified combustion modes.
- ⮚
- Underexplored engine concepts and integration gaps: Most syngas research focuses on SI engines, with fewer studies addressing CI and dual-fuel strategies. Recent CFD studies suggest that direct-injection syngas operation in CI engines can achieve high efficiency with lower NOx emissions [91], yet this area remains underexplored compared to SI applications. Dual-fuel approaches (diesel/syngas or diesel/producer gas) also show promise for efficiency gains and NOx reduction [88], but the trade-offs in brake thermal efficiency and CO/HC emissions are not well quantified. HCCI applications of syngas are even less studied, with most models limited to zero-dimensional thermochemical approaches. Finally, there is a lack of integration between gasification studies (which define syngas variability) and engine simulations, leaving a gap on these observations, future work should focus on validating kinetic mechanisms under engine-relevant pressures, temperatures, and diluted conditions. Realistic syngas compositions reflecting biomass gasification should be employed, avoiding hydrogen-rich synthetic mixtures. Greater attention is required for CFD studies on CI, DF, and HCCI engines, which remain underrepresented compared with SI research. Systematic comparisons and meta-analyses across studies are needed to reconcile contradictory findings, particularly regarding the effects of H2 and CO on NOx. Finally, integrating gasification modelling with combustion simulations would provide end-to-end insights from feedstock to engine performance [66,119,122].
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Igini, M. Martina Igini Fossil Fuels Accounted for 82% of Global Energy Mix in 2023 Amid Record Consumption: Report. Earth.Org. 16 June 2024. Available online: https://earth.org/Fossil-Fuel-Accounted-for−82−of−Global-Energy-Mix-in−2023−Amid-Record-Consumption-Report/ (accessed on 20 August 2025).
- IRENA. Renewable Capacity Statistics 2025; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2025. [Google Scholar]
- Toscano, G.; De Francesco, C.; Gasperini, T.; Fabrizi, S.; Duca, D.; Ilari, A. Quality Assessment and Classification of Feedstock for Bioenergy Applications Considering ISO 17225 Standard on Solid Biofuels. Resources 2023, 12, 69. [Google Scholar] [CrossRef]
- Shen, Y. Biomass Pretreatment for Steam Gasification toward H2-Rich Syngas Production—An Overview. Int. J. Hydrogen Energy 2024, 66, 90–102. [Google Scholar] [CrossRef]
- Barskov, S.; Zappi, M.; Buchireddy, P.; Dufreche, S.; Guillory, J.; Gang, D.; Hernandez, R.; Bajpai, R.; Baudier, J.; Cooper, R.; et al. Torrefaction of Biomass: A Review of Production Methods for Biocoal from Cultured and Waste Lignocellulosic Feedstocks. Renew. Energy 2019, 142, 624–642. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, W.; Fan, X. Char Derived from Sewage Sludge of Hydrothermal Carbonization and Supercritical Water Gasification: Comparison of the Properties of Two Chars. Waste Manag. 2021, 123, 88–96. [Google Scholar] [CrossRef]
- Mu, Q.; Aleem, R.D.; Liu, C.; Elendu, C.C.; Cao, C.; Duan, P.G. Oxygen Blown Steam Gasification of Different Kinds of Lignocellulosic Biomass for the Production of Hydrogen-Rich Syngas. Renew. Energy 2024, 232, 121132. [Google Scholar] [CrossRef]
- Ghodke, P.K.; Sharma, A.K.; Jayaseelan, A.; Gopinath, K.P. Hydrogen-Rich Syngas Production from the Lignocellulosic Biomass by Catalytic Gasification: A State of Art Review on Advance Technologies, Economic Challenges, and Future Prospectus. Fuel 2023, 342, 127800. [Google Scholar] [CrossRef]
- Widjaya, E.R.; Chen, G.; Bowtell, L.; Hills, C. Gasification of Non-Woody Biomass: A Literature Review. Renew. Sustain. Energy Rev. 2018, 89, 184–193. [Google Scholar] [CrossRef]
- Valizadeh, S.; Khani, Y.; Farooq, A.; Kumar, G.; Show, P.L.; Chen, W.H.; Lee, S.H.; Park, Y.K. Microalgae Gasification over Ni Loaded Perovskites for Enhanced Biohydrogen Generation. Bioresour. Technol. 2023, 372, 128638. [Google Scholar] [CrossRef]
- Akca, O.; Chen, J.; Dai, L.; Cobb, K.; Cheng, Y.; Chen, P.; Lei, H.; Ruan, R. Improving Carbon-Reduced Catalytic Gasification of Microalgae for Biohydrogen Production. Algal Res. 2024, 84, 103797. [Google Scholar] [CrossRef]
- Śpiewak, K. Gasification of Sewage Sludge—A Review. Energies 2024, 17, 4476. [Google Scholar] [CrossRef]
- Brandstätter, G.; Fürsatz, K.; Long, A.; Hannl, T.K.; Schubert, T. Exploring the Potential of Sewage Sludge for Gasification and Resource Recovery: A Review. Environ. Technol. Innov. 2025, 40, 104346. [Google Scholar] [CrossRef]
- Alves, C.T.; Onwudili, J.A.; Ghorbannezhad, P.; Kumagai, S. A Review of the Thermochemistries of Biomass Gasification and Utilisation of Gas Products. Sustain. Energy Fuels 2023, 7, 3505–3540. [Google Scholar] [CrossRef]
- Chang, Y.-J.; Chang, J.-S.; Lee, D.-J. Gasification of Biomass for Syngas Production: Research Update and Stoichiometry Diagram Presentation. Bioresour. Technol. 2023, 387, 129535. [Google Scholar] [CrossRef]
- Susastriawan, A.A.P.; Saptoadi, H. Purnomo Small-Scale Downdraft Gasifiers for Biomass Gasification: A Review. Renew. Sustain. Energy Rev. 2017, 76, 989–1003. [Google Scholar] [CrossRef]
- Rosha, P.; Dhir, A.; Mohapatra, S.K. Influence of Gaseous Fuel Induction on the Various Engine Characteristics of a Dual Fuel Compression Ignition Engine: A Review. Renew. Sustain. Energy Rev. 2018, 82, 3333–3349. [Google Scholar] [CrossRef]
- Rinaldini, C.A.; Allesina, G.; Pedrazzi, S.; Mattarelli, E.; Savioli, T.; Morselli, N.; Puglia, M.; Tartarini, P. Experimental Investigation on a Common Rail Diesel Engine Partially Fuelled by Syngas. Energy Convers. Manag. 2017, 138, 526–537. [Google Scholar] [CrossRef]
- Aslam, Z.; Li, H.; Hammerton, J.; Andrews, G.E. Combustion and Emissions Performance of Simulated Syngas/Diesel Dual Fuels in a CI Engine. In Proceedings of the SAE Powertrains, Fuels & Lubricants Conference & Exhibition, Krakow, Poland, 6–8 September 2022; SAE International: Warrendale, PA, USA, 2022. [Google Scholar]
- Arslan, A.; Dev, S.; Yousefi, A.; Stevenson, D.; Liko, B.; Butler, J.; Guo, H.; Birouk, M. Combustion and Emission Performance of a Syngas-Diesel Dual-Fuel Generator. In Proceedings of the ASME 2022 ICE Forward Conference, Indianapolis, IN, USA, 16–19 October 2022; Volume 86540, p. V001T01A006. [Google Scholar]
- Kumar, A.; Mounaïm-rousselle, C.; Brequigny, P.; Dhar, A.; Hespel, C.; Patel, C.; Kumar, D.; Duraisamy, G.; Le, L.; Sharma, N.; et al. Future of Internal Combustion Engines Using Sustainable, Scalable, and Storable E-Fuels and Biofuels for Decarbonizing Transport and Enabling Advanced Combustion Technologies. Prog. Energy Combust. Sci. 2025, 110, 101236. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, A.K.; Ağbulut, Ü. A Comprehensive Review on Biomass-Derived Producer Gas as an Alternative Fuel: A Waste Biomass-to-Energy Perspective. J. Therm. Anal. Calorim. 2025, 150, 8913–8932. [Google Scholar] [CrossRef]
- Bongomin, O.; Nzila, C.; Mwasiagi, J.I.; Maube, O. Exploring Insights in Biomass and Waste Gasification via Ensemble Machine Learning Models and Interpretability Techniques. Int. J. Energy Res. 2024, 2024, 6087208. [Google Scholar] [CrossRef]
- Kumar, S.; Palange, R.; De Blasio, C. Advancements in Gasification Technologies: Insights into Modeling Studies, Power-to-X Applications and Sustainability Assessments. Sustain. Energy Fuels 2025, 9, 4793–4831. [Google Scholar] [CrossRef]
- Jayanarasimhan, A.; Pathak, R.M.; Shivapuji, A.M.; Rao, L. Tar Formation in Gasification Systems: A Holistic Review of Remediation Approaches and Removal Methods. ACS Omega 2024, 9, 2060–2079. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Yan, X. Thermodynamic and Techno-Economic Analysis of a Biomass Gasification Tri-Generation System with Internal Combustion Engine and Stirling Engine. Int. J. Hydrogen Energy 2025, 134, 128–138. [Google Scholar] [CrossRef]
- Copa, J.R.; Tuna, C.E.; Silveira, J.L.; Boloy, R.A.M.; Brito, P.; Silva, V.; Cardoso, J.; Eusébio, D. Techno-Economic Assessment of the Use of Syngas Generated from Biomass to Feed an Internal Combustion Engine. Energies 2020, 13, 3097. [Google Scholar] [CrossRef]
- Suparmin, P.; Nurhasanah, R.; Nelwan, L.O.; Salleh, H.; Ridwan, M.; Anugerah, M. Syngas for Internal Combustion Engines, Current State, and Future Prospects: A Systematic Review. Int. J. Automot. Mech. Eng. 2024, 21, 11857–11876. [Google Scholar] [CrossRef]
- Hydrogen Council. Hydrogen Decarbonization Pathways; Hydrogen Council: Brussels, Belgium, 2021. [Google Scholar]
- Maitlo, G.; Ali, I.; Mangi, K.H.; Ali, S.; Maitlo, H.A.; Unar, I.N.; Pirzada, A.M. Thermochemical Conversion of Biomass for Syngas Production: Current Status and Future Trends. Sustainability 2022, 14, 2596. [Google Scholar] [CrossRef]
- Samad, N.A.F.A. Effects of Different Gasifying Agents on Synthesis Gas Composition from Fluidized Bed Gasification Using Raw and Torrefied Wood Sawdust. Chem. Eng. Trans. 2024, 114, 1111–1116. [Google Scholar] [CrossRef]
- Trejo, F. Review of Biomass Gasification Technologies with a Particular Focus on a Downdraft Gasifier. Processes 2025, 13, 2717. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, M.; Raheem, A.; Wang, F.; Wei, J.; Xu, D.; Song, X.; Bao, W.; Huang, A.; Zhang, S.; et al. Syngas Production from Biomass Gasification: Influences of Feedstock Properties, Reactor Type, and Reaction Parameters. ACS Omega 2023, 8, 31620–31631. [Google Scholar] [CrossRef]
- Segneri, V.; Ferrasse, J.H.; Trinca, A.; Vilardi, G. An Overview of Waste Gasification and Syngas Upgrading Processes. Energies 2022, 15, 6391. [Google Scholar] [CrossRef]
- Han, F.; Niu, Y.; Zhang, X.; Guo, Z.; Duan, S.; Liu, H.; Lu, B.; Chen, H. Performance Analysis of a Pilot Gasification System of Biomass with Stepwise Intake of Air-Steam Considering Waste Heat Utilization. Renew. Energy 2024, 236, 121498. [Google Scholar] [CrossRef]
- Tezer, Ö.; Karabağ, N.; Öngen, A.; Çolpan, C.Ö.; Ayol, A. Biomass Gasification for Sustainable Energy Production: A Review. Int. J. Hydrogen Energy 2022, 47, 15419–15433. [Google Scholar] [CrossRef]
- All Power Labs. The Five Processes of Gasification. Available online: https://www.allpowerlabs.com/gasification-explained (accessed on 20 August 2025).
- Havilah, P.R.; Sharma, A.K.; Govindasamy, G.; Matsakas, L.; Patel, A. Biomass Gasification in Downdraft Gasifiers: A Technical Review on Production, Up-Gradation and Application of Synthesis Gas. Energies 2022, 15, 3938. [Google Scholar] [CrossRef]
- Carmo-Calado, L.; Hermoso-Orzáez, M.J.; La Cal-Herrera, J.; Brito, P.; Terrados-Cepeda, J. Techno-Economic Evaluation of Downdraft Fixed Bed Gasification of Almond Shell and Husk as a Process Step in Energy Production for Decentralized Solutions Applied in Biorefinery Systems. Agronomy 2023, 13, 2278. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, R.; Sun, Z.; Zhang, B.; Wang, Z.; Liu, J.; Yang, B.; Wu, Z. Carbon-Negative Syngas Production: A Comprehensive Assessment of Biomass Pyrolysis Coupling Chemical Looping Reforming. AIChE J. 2023, 69, e18254. [Google Scholar] [CrossRef]
- Miccio, F.; Polchri, L.; Murri, A.N.; Landi, E.; Medri, V. Chemical Looping Gasification of Biomass Char in Fluidized Bed and CO2-Enriched Atmosphere. Biomass Convers. Biorefinery 2025, 15, 11561–11571. [Google Scholar] [CrossRef]
- Fang, S.; Zheng, X.; Lin, Y.; Ding, L.; Yan, S.; Li, J.; Huang, Z.; Huang, H. Evaluation of the Chemical Looping Gasification Characteristics of Kitchen Waste Using CuFe2O4 and NiFe2O4 as Oxygen Carriers. Energy 2024, 312, 133617. [Google Scholar] [CrossRef]
- Mishra, S.; Upadhyay, R.K. Review on Biomass Gasification: Gasifiers, Gasifying Mediums, and Operational Parameters. Mater. Sci. Energy Technol. 2021, 4, 329–340. [Google Scholar] [CrossRef]
- Kurkela, E. Pilot-Scale Development of Pressurized Fixed-Bed Gasification for Synthesis Gas Production from Biomass Residues. Biomass Convers. Biorefinery 2023, 13, 6553–6574. [Google Scholar] [CrossRef]
- Meng, F.; Meng, J.; Zhang, D. Influence of Higher Equivalence Ratio on the Biomass Oxygen Gasification in a Pilot Scale Fixed Bed Gasifier. J. Renew. Sustain. Energy 2018, 10, 53101. [Google Scholar] [CrossRef]
- Yao, X.; Zhao, Z.; Li, J.; Zhang, B.; Zhou, H.; Xu, K. Experimental Investigation of Physicochemical and Slagging Characteristics of Inorganic Constituents in Ash Residues from Gasification of Different Herbaceous Biomass. Energy 2020, 198, 117367. [Google Scholar] [CrossRef]
- Hongrapipat, J.; Rauch, R.; Pang, S.; Liplap, P.; Arjharn, W.; Messner, M.; Henrich, C.; Koch, M.; Hofbauer, H. Co-Gasification of Refuse Derived Fuel and Wood Chips in the Nong Bua Dual Fluidised Bed Gasification Power Plant in Thailand. Energies 2022, 15, 7363. [Google Scholar] [CrossRef]
- Jayah, T.H.; Aye, L.; Fuller, R.J.; Stewart, D.F. Computer Simulation of a Downdraft Wood Gasifier for Tea Drying. Biomass Bioenergy 2003, 25, 459–469. [Google Scholar] [CrossRef]
- de Sales, C.A.V.B.; Maya, D.M.Y.; Lora, E.E.S.; Jaén, R.L.; Reyes, A.M.M.; González, A.M.; Andrade, R.V.; Martínez, J.D. Experimental Study on Biomass (Eucalyptus Spp.) Gasification in a Two-Stage Downdraft Reactor by Using Mixtures of Air, Saturated Steam and Oxygen as Gasifying Agents. Energy Convers. Manag. 2017, 145, 314–323. [Google Scholar] [CrossRef]
- Couto, N.; Silva, V.; Cardoso, J.; Rouboa, A. 2nd Law Analysis of Portuguese Municipal Solid Waste Gasification Using CO2/Air Mixtures. J. CO2 Util. 2017, 20, 347–356. [Google Scholar] [CrossRef]
- Luz, F.C.; Rocha, M.H.; Lora, E.E.S.; Venturini, O.J.; Andrade, R.V.; Leme, M.M.V.; Del Olmo, O.A. Techno-Economic Analysis of Municipal Solid Waste Gasification for Electricity Generation in Brazil. Energy Convers. Manag. 2015, 103, 321–337. [Google Scholar] [CrossRef]
- Bhoi, P.R.; Huhnke, R.L.; Kumar, A.; Indrawan, N.; Thapa, S. Co-Gasification of Municipal Solid Waste and Biomass in a Commercial Scale Downdraft Gasifier. Energy 2018, 163, 513–518. [Google Scholar] [CrossRef]
- Olgun, H.; Ozdogan, S.; Yinesor, G. Results with a Bench Scale Downdraft Biomass Gasifier for Agricultural and Forestry Residues. Biomass Bioenergy 2011, 35, 572–580. [Google Scholar] [CrossRef]
- Onokwai, A.O.; Ajisegiri, E.S.A.; Okokpujie, I.P.; Ibikunle, R.A.; Oki, M.; Dirisu, J.O. Characterization of Lignocellulose Biomass Based on Proximate, Ultimate, Structural Composition, and Thermal Analysis. Mater. Today Proc. 2022, 65, 2156–2162. [Google Scholar] [CrossRef]
- Lee, J.; Sohn, D.; Lee, K.; Park, K.Y. Solid Fuel Production through Hydrothermal Carbonization of Sewage Sludge and Microalgae Chlorella Sp. from Wastewater Treatment Plant. Chemosphere 2019, 230, 157–163. [Google Scholar] [CrossRef]
- Alfarra, F.; Ozcan, H.K.; Cihan, P.; Ongen, A.; Guvenc, S.Y.; Ciner, M.N. Artificial Intelligence Methods for Modeling Gasification of Waste Biomass: A Review; Springer: Berlin/Heidelberg, Germany, 2024; Volume 196, ISBN 0123456789. [Google Scholar]
- Alptekin, F.M.; Celiktas, M.S. Review on Catalytic Biomass Gasification for Hydrogen Production as a Sustainable Energy Form and Social, Technological, Economic, Environmental, and Political Analysis of Catalysts. ACS Omega 2022, 7, 24918–24941. [Google Scholar] [CrossRef]
- Sakheta, A.; Raj, T.; Nayak, R.; O’Hara, I.; Ramirez, J. Improved Prediction of Biomass Gasification Models through Machine Learning. Comput. Chem. Eng. 2024, 191, 108834. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Tong, Y.; Wang, X. Understanding and Optimizing the Gasification of Biomass Waste with Machine Learning. Green Chem. Eng. 2022, 4, 123–133. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Z.; Zhong, H.; Cheng, K. Essential Aspects of the CFD Software Modelling of Biomass Gasification Processes in Downdraft Reactors. RSC Adv. 2024, 14, 28724–28739. [Google Scholar] [CrossRef] [PubMed]
- Efremov, C.; Le, T.T.; Paramasivam, P.; Rudzki, K.; Osman, S.; Chau, T.H. Improving Syngas Yield and Quality from Biomass/Coal Co-Gasification Using Cooperative Game Theory and Local Interpretable Model-Agnostic Explanations. Int. J. Hydrogen Energy 2024, 96, 892–907. [Google Scholar] [CrossRef]
- Li, J.; Suvarna, M.; Pan, L.-J.; Zhao, Y.; Wang, X. A Hybrid Data-Driven and Mechanistic Modelling Approach for Hydrothermal Gasification. Appl. Energy 2021, 304, 117674. [Google Scholar] [CrossRef]
- Awais, M.; Omar, M.M.; Munir, A.; Li, W.; Ajmal, M.; Hussain, S.; Ahmad, S.A.; Ali, A. Co-Gasification of Different Biomass Feedstock in a Pilot-Scale (24 KWe) Downdraft Gasifier: An Experimental Approach. Energy 2022, 238, 121821. [Google Scholar] [CrossRef]
- Xu, H.; Liu, F.; Sun, S.; Meng, S.; Zhao, Y. A Systematic Numerical Study of the Laminar Burning Velocity of Iso-Octane/Syngas/Air Mixtures. Chem. Eng. Sci. 2019, 195, 598–608. [Google Scholar] [CrossRef]
- Nagar, V.; Kaushal, R. A Review of Recent Advancement in Plasma Gasification: A Promising Solution for Waste Management and Energy Production. Int. J. Hydrogen Energy 2024, 77, 405–419. [Google Scholar] [CrossRef]
- Costa, M.; Piazzullo, D. The Effects of Syngas Composition on Engine Thermal Balance in a Biomass Powered CHP Unit: A 3D CFD Study. Energies 2024, 17, 738. [Google Scholar] [CrossRef]
- Yin, G.; Wang, C.; Zhou, M.; Zhou, Y.; Hu, E.; Huang, Z. Experimental and Kinetic Study on Laminar Flame Speeds of Ammonia/Syngas/Air at a High Temperature and Elevated Pressure. Front. Energy 2022, 16, 263–276. [Google Scholar] [CrossRef]
- Jithin, E.V.; Raghuram, G.K.S.; Keshavamurthy, T.V.; Velamati, R.K.; Prathap, C.; Varghese, R.J. A Review on Fundamental Combustion Characteristics of Syngas Mixtures and Feasibility in Combustion Devices. Renew. Sustain. Energy Rev. 2021, 146, 111178. [Google Scholar] [CrossRef]
- Shang, R.; Zhuang, Z.; Yang, Y.; Li, G. Laminar Flame Speed of H2/CH4/Air Mixtures with CO2 and N2 Dilution. Int. J. Hydrogen Energy 2022, 47, 32315–32329. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Elbaz, A.M.; He, Y.; Chen, C.; Zhu, Y.; Roberts, W.L. Effects of CO2 Dilution and CH4 Addition on Laminar Burning Velocities of Syngas at Elevated Pressures: An Experimental and Modeling Study. Energy Fuels 2021, 35, 18733–18745. [Google Scholar] [CrossRef]
- Kousheshi, N.; Yari, M.; Paykani, A.; Saberi Mehr, A.; de la Fuente, G.F. Effect of Syngas Composition on the Combustion and Emissions Characteristics of a Syngas/Diesel RCCI Engine. Energies 2020, 13, 212. [Google Scholar] [CrossRef]
- Qian, Y.; Sun, S.; Ju, D.; Shan, X.; Lu, X. Review of the State-of-the-Art of Biogas Combustion Mechanisms and Applications in Internal Combustion Engines. Renew. Sustain. Energy Rev. 2017, 69, 50–58. [Google Scholar] [CrossRef]
- Bhaduri, S.; Jeanmart, H.; Contino, F. EGR Control on Operation of a Tar Tolerant HCCI Engine with Simulated Syngas from Biomass. Appl. Energy 2018, 227, 159–167. [Google Scholar] [CrossRef]
- Jothiprakash, G.; Balasubramaniam, P.; Sundaram, S.; Ramesh, D. Catalytic Biomass Gasification for Syngas Production: Recent Progress in Tar Reduction and Future Perspectives. Biomass 2025, 5, 37. [Google Scholar] [CrossRef]
- Guo, P.; Liu, S.; Chang, X.; Wang, Z.; Xu, C.; Hu, L.; Lu, J. ScienceDirect The Synergistic Effect of Equivalence Ratio and Initial Temperature on Laminar Flame Speed of Syngas. Int. J. Hydrogen Energy 2022, 47, 23106–23117. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, S.; Su, Y.; Luo, Z.; Wang, T. Experimental Study of the Influence of H2/CO on the CH4 Explosion Pressure and Thermal Behaviors. ACS Omega 2022, 47, 23106–23117. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Bui, V.G.; Nguyen, X.B.; Nguyen, L.C.T.; Pham, M.M.; Truong, T.H.; Phung, M.T.; Bui, V.H. Experimental Study of Explosion Characteristics and General Correlations of Lean H2/CO/CH4/Air Mixtures in Quiescence. Int. J. Energy Res. 2023, 2023, 8080573. [Google Scholar] [CrossRef]
- Hamdy, M.; Abdelhalim, A.; Haque, M.A.; Abdelhafez, A.; Nemitallah, M.A. Flow, Combustion and Species Fields of Premixed CH4/H2/CO Syngas Oxy-Flames on a Swirl Burner: Effects of Syngas Composition. Int. J. Hydrogen Energy 2024, 68, 1398–1411. [Google Scholar] [CrossRef]
- Wang, S.; Elbaz, A.M.; Wang, G.; Wang, Z.; Roberts, W.L. Turbulent Flame Speed of NH3/CH4/H2/H2O/Air-Mixtures: Effects of Elevated Pressure and Lewis Number. Combust. Flame 2023, 247, 112488. [Google Scholar] [CrossRef]
- Zhao, H.; Yuan, C.; Li, G.; Tian, F. The Propagation Characteristics of Turbulent Expanding Flames of Methane/Hydrogen Blending Gas. Energies 2024, 17, 5997. [Google Scholar] [CrossRef]
- Uka, D.; Blagojević, B.; Alioui, O.; Boublia, A.; Elboughdiri, N.; Benguerba, Y.; Jurić, T.; Popović, B.M. An Innovative and Environmentally Friendly Approach for Resveratrol Solubilization and Bioaccessibility Enhancement by Using Natural Deep Eutectic Solvents. J. Mol. Liq. 2023, 391, 123411. [Google Scholar] [CrossRef]
- Hu, X.; Bai, F.; Yu, C.; Yan, F. Experimental Study of the Laminar Flame Speeds of the CH4/H2/CO/CO2/N2 Mixture and Kinetic Simulation in Oxygen-Enriched Air Condition. ACS Omega 2020, 5, 33372–33379. [Google Scholar] [CrossRef] [PubMed]
- Burke, M.P.; Dryer, F.L.; Ju, Y. Assessment of Kinetic Modeling for Lean H2/CH4/O2/Diluent Flames at High Pressures. Proc. Combust. Inst. 2011, 33, 905–912. [Google Scholar] [CrossRef]
- Kéromnès, A.; Metcalfe, W.K.; Heufer, K.A.; Donohoe, N.; Das, A.K.; Sung, C.J.; Herzler, J.; Naumann, C.; Griebel, P.; Mathieu, O.; et al. An Experimental and Detailed Chemical Kinetic Modeling Study of Hydrogen and Syngas Mixture Oxidation at Elevated Pressures. Combust. Flame 2013, 160, 995–1011. [Google Scholar] [CrossRef]
- Li, X.; You, X.; Wu, F.; Law, C.K. Uncertainty Analysis of the Kinetic Model Prediction for High-Pressure H2/CO Combustion. Proc. Combust. Inst. 2015, 35, 617–624. [Google Scholar] [CrossRef]
- Olm, C.; Zsély, I.G.; Varga, T.; Curran, H.J.; Turányi, T. Comparison of the Performance of Several Recent Syngas Combustion Mechanisms. Combust. Flame 2015, 162, 1793–1812. [Google Scholar] [CrossRef]
- Costa, M.; La Villetta, M.; Massarotti, N.; Piazzullo, D.; Rocco, V. Numerical Analysis of a Compression Ignition Engine Powered in the Dual-Fuel Mode with Syngas and Biodiesel. Energy 2017, 137, 969–979. [Google Scholar] [CrossRef]
- Wiemann, S.; Hegner, R.; Atakan, B.; Schulz, C.; Kaiser, S.A. Combined Production of Power and Syngas in an Internal Combustion Engine—Experiments and Simulations in SI and HCCI Mode. Fuel 2018, 215, 40–45. [Google Scholar] [CrossRef]
- Costa, M.; Prati, M.V.; De Simio, L.; Iannaccone, S.; Piazzullo, D. CFD Study of a CI Engine Powered in the Dual-Fuel Mode with Syngas and Waste Vegetable Oil. J. Phys. Conf. Ser. 2021, 1868, 012014. [Google Scholar] [CrossRef]
- Pérez Gordillo, D.S.; Mantilla González, J.M. Computational Study of the Effects of Ignition Parameters Changes on a Spark Ignition Engine Fueled With Syngas. J. Energy Resour. Technol. 2022, 144, 112306. [Google Scholar] [CrossRef]
- Perrone, D.; Castiglione, T.; Morrone, P.; Pantano, F.; Bova, S. Energetic, Economic and Environmental Performance Analysis of a Micro-Combined Cooling, Heating and Power (CCHP) System Based on Biomass Gasification. Energies 2023, 16, 6911. [Google Scholar] [CrossRef]
- Kan, X.; Zhou, D.; Yang, W.; Zhai, X.; Wang, C.-H. An Investigation on Utilization of Biogas and Syngas Produced from Biomass Waste in Premixed Spark Ignition Engine. Appl. Energy 2018, 212, 210–222. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, Z.; Zhu, Z. Effects of Syngas Addition on Combustion Characteristics of Gasoline Surrogate Fuel. ACS Omega 2023, 8, 3929–3944. [Google Scholar] [CrossRef]
- Shivapuji, A.M.; Dasappa, S. Influence of Fuel Hydrogen Fraction on Syngas Fueled SI Engine: Fuel Thermo-Physical Property Analysis and in-Cylinder Experimental Investigations. Int. J. Hydrogen Energy 2015, 40, 10308–10328. [Google Scholar] [CrossRef]
- Gamiño, B.; Aguillón, J. Numerical Simulation of Syngas Combustion with a Multi-Spark Ignition System in a Diesel Engine Adapted to Work at the Otto Cycle. Fuel 2010, 89, 581–591. [Google Scholar] [CrossRef]
- Shivapuji, A.M.; Dasappa, S. Selection and Thermodynamic Analysis of a Turbocharger for a Producer Gas-Fuelled Multi-Cylinder Engine. Proc. Inst. Mech. Eng. Part A J. Power Energy 2014, 228, 340–356. [Google Scholar] [CrossRef]
- Kim, S.; Kim, J. Feasibility Assessment of Hydrogen-Rich Syngas Spark-Ignition Engine for Heavy-Duty Long-Distance Vehicle Application. Energy Convers. Manag. 2022, 252, 115048. [Google Scholar] [CrossRef]
- Hariharan, D.; Rahimi Boldaji, M.; Yan, Z.; Mamalis, S.; Lawler, B. Single-Fuel Reactivity Controlled Compression Ignition through Catalytic Partial Oxidation Reformation of Diesel Fuel. Fuel 2020, 264, 116815. [Google Scholar] [CrossRef]
- Olanrewaju, F.O.; Li, H.; Aslam, Z.; Hammerton, J.; Lovett, J.C. Analysis of the Effect of Syngas Substitution of Diesel on the Heat Release Rate and Combustion Behaviour of Diesel-Syngas Dual Fuel Engine. Fuel 2022, 312, 122842. [Google Scholar] [CrossRef]
- Starik, A.M.; Korobov, A.N.; Titova, N.S. Combustion Improvement in HCCI Engine Operating on Synthesis Gas via Addition of Ozone or Excited Oxygen Molecules to the Charge: Modeling Study. Int. J. Hydrogen Energy 2017, 42, 10475–10484. [Google Scholar] [CrossRef]
- Neshat, E.; Saray, R.K.; Hosseini, V. Effect of Reformer Gas Blending on Homogeneous Charge Compression Ignition Combustion of Primary Reference Fuels Using Multi Zone Model and Semi Detailed Chemical-Kinetic Mechanism. Appl. Energy 2016, 179, 463–478. [Google Scholar] [CrossRef]
- Li, W.; Zou, C.; Yao, H.; Lin, Q.; Fu, R.; Luo, J. An Optimized Kinetic Model for H2/CO Combustion in CO2 Diluent at Elevated Pressures. Combust. Flame 2022, 241, 112093. [Google Scholar] [CrossRef]
- Xia, W.; Huang, C.; Yang, J.; Zou, C.; Song, Y. Experimental and Modeling Study of Ignition Delay Times of Natural Gas with CO 2 Dilution. Fuel 2024, 358, 130148. [Google Scholar] [CrossRef]
- Jamsran, N.; Park, H.; Lee, J.; Oh, S.; Kim, C.; Lee, Y.; Kang, K. Influence of Syngas Composition on Combustion and Emissions in a Homogeneous Charge Compression Ignition Engine. Fuel 2021, 306, 121774. [Google Scholar] [CrossRef]
- Park, H.; Lee, J.; Jamsran, N.; Oh, S.; Kim, C.; Lee, Y.; Kang, K. Comparative Assessment of Stoichiometric and Lean Combustion Modes in Boosted Spark-Ignition Engine Fueled with Syngas. Energy Convers. Manag. 2021, 239, 114224. [Google Scholar] [CrossRef]
- Fan, G.; Zheng, Z.; Zhu, Z. Combustion and Emission Characteristics of Gasoline Engine Blended Combustion Syngas. ACS Omega 2022, 7, 26375–26395. [Google Scholar] [CrossRef]
- Caligiuri, C.; Baškovič, U.Ž.; Renzi, M.; Seljak, T.; Opresnik, S.R.; Baratieri, M.; Katrašnik, T. Complementing Syngas with Natural Gas in Spark Ignition Engines for Power Production: Effects on Emissions and Combustion. Energies 2021, 14, 3688. [Google Scholar] [CrossRef]
- Bui, V.; Bui, T.M.T.; Tran, V.; Huang, Z.; Hoang, A.T.; Tarelko, W.; Bui, V.; Pham, X.M.; Nguyen, P.Q.P. Flexible Syngas-Biogas-Hydrogen Fueling Spark-Ignition Engine Behaviors with Optimized Fuel Compositions and Control Parameters. Int. J. Hydrogen Energy 2022, 48, 6722–6737. [Google Scholar] [CrossRef]
- Bates, R.; Dölle, K. Syngas Use in Internal Combustion Engines—A Review. Adv. Res. 2017, 10, 1–8. [Google Scholar] [CrossRef]
- Li, B.; Zhong, F.; Wang, R.; Jiang, Y.; Han, R. A Comparative Analysis of the Performances of a Syngas Port Injection plus Gasoline Direct Injection Combined-Injection Spark-Ignition Engine under Lean-Homogeneous Charge and Lean-Stratified Charge Modes. Energy 2024, 308, 132883. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.; Yu, B.; Li, X.; Zhang, L.; Zhao, Y.; Sun, S. Experimental and Kinetic Modeling Study on Laminar Flame Speeds and Emission Characteristics of Oxy-Ammonia Premixed Flames. Int. J. Hydrogen Energy 2024, 63, 857–870. [Google Scholar] [CrossRef]
- de Campos, V.; Carmo-Calado, L.; Mota-Panizio, R.; Matos, V.; Silva, V.B.; Brito, P.S.; Eusébio, D.F.L.; Tuna, C.E.; Silveira, J.L. A Waste-to-Energy Technical Approach: Syngas–Biodiesel Blend for Power Generation. Energies 2023, 16, 7384. [Google Scholar] [CrossRef]
- Krishnamoorthi, M.; Sreedhara, S.; Duvvuri, P.P. Experimental, Numerical and Exergy Analyses of a Dual Fuel Combustion Engine Fuelled with Syngas and Biodiesel/Diesel Blends. Appl. Energy 2020, 263, 114643. [Google Scholar] [CrossRef]
- Talibi, M.; Hellier, P.; Ladommatos, N. Combustion and Exhaust Emission Characteristics, and in-Cylinder Gas Composition, of Hydrogen Enriched Biogas Mixtures in a Diesel Engine. Energy 2017, 124, 397–412. [Google Scholar] [CrossRef]
- Lei, Y.; Wu, Y.; Qiu, T.; Zhou, D.; Lian, X.; Jin, W. Experimental Study of Dual-Fuel Diesel/Natural Gas High-Pressure Injection. ACS Omega 2023, 8, 519–528. [Google Scholar] [CrossRef]
- Yaliwal, V.S.; Banapurmath, N.R.; Gaddigoudar, P.; Patil, N.D.; Harari, P.A. Performance and Emission Characteristics of a Diesel Engine Operated on Diverse Modes Using Renewable and Sustainable Fuels Derived from Dairy Scum and Municipal Solid Waste. Mater. Today Proc. 2021, 47, 2592–2597. [Google Scholar] [CrossRef]
- Nguyen, V.N.; Nayak, B.; Singh, T.J.; Nayak, S.K.; Cao, D.N.; Le, H.C.; Nguyen, X.P. Investigations on the Performance, Emission and Combustion Characteristics of a Dual-Fuel Diesel Engine Fueled with Induced Bamboo Leaf Gaseous Fuel and Injected Mixed Biodiesel-Diesel Blends. Int. J. Hydrogen Energy 2024, 54, 397–417. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Tsolakis, A.; Alagumalai, A.; Mahian, O.; Lam, S.S.; Pan, J.; Peng, W.; Tabatabaei, M.; Aghbashlo, M. Use of Hydrogen in Dual-Fuel Diesel Engines. Prog. Energy Combust. Sci. 2023, 98, 101100. [Google Scholar] [CrossRef]
- Saxena, M.; Ranjane, V.; Maurya, R. Crank Angle Based Exergy Analysis of Syngas Fuelled Homogeneous Charge Compression Ignition Engine. SAE Tech. Pap. 2022, 01, 1037. [Google Scholar] [CrossRef]
- Martinez-boggio, S.; Lacava, P.T.; Carvalho, F.S. De Combustion Diagnosis in a Spark-Ignition Engine Fueled with Syngas at Different CO/H2 and Diluent Ratios. Gases 2024, 4, 97–116. [Google Scholar] [CrossRef]
- Han, W.; Dai, P.; Gou, X.; Chen, Z. A Review of Laminar Flame Speeds of Hydrogen and Syngas Measured from Propagating Spherical Flames. Appl. Energy Combust. Sci. 2020, 1–4, 100008. [Google Scholar] [CrossRef]
- Ali, K.; Kim, C.; Lee, Y.; Oh, S.; Kim, K. A Numerical Study to Investigate the Effect of Syngas Composition and Compression Ratio on the Combustion and Emission Characteristics of a Syngas-Fueled HCCI Engine. J. Energy Resour. Technol. 2020, 142, 092301. [Google Scholar] [CrossRef]
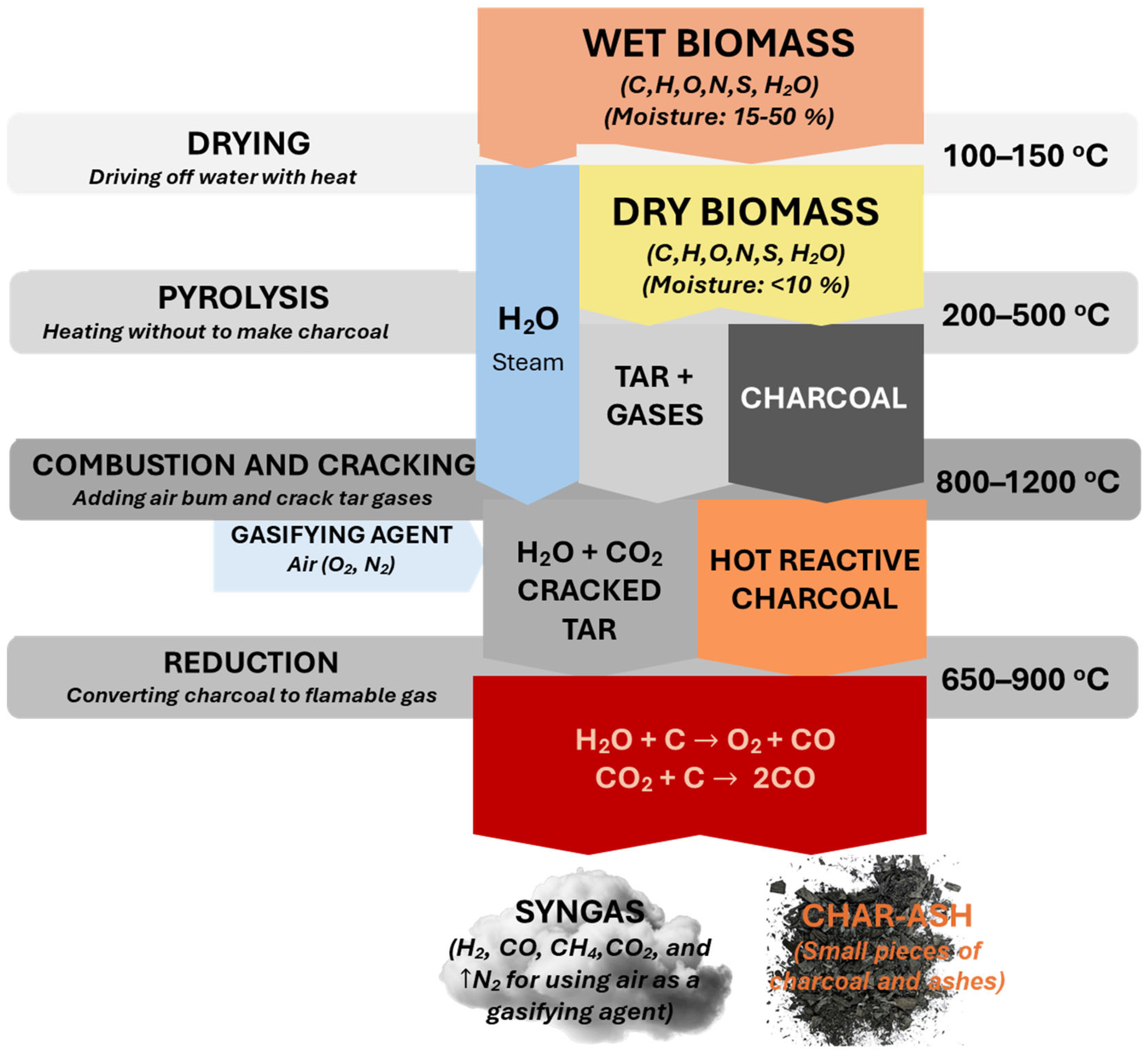
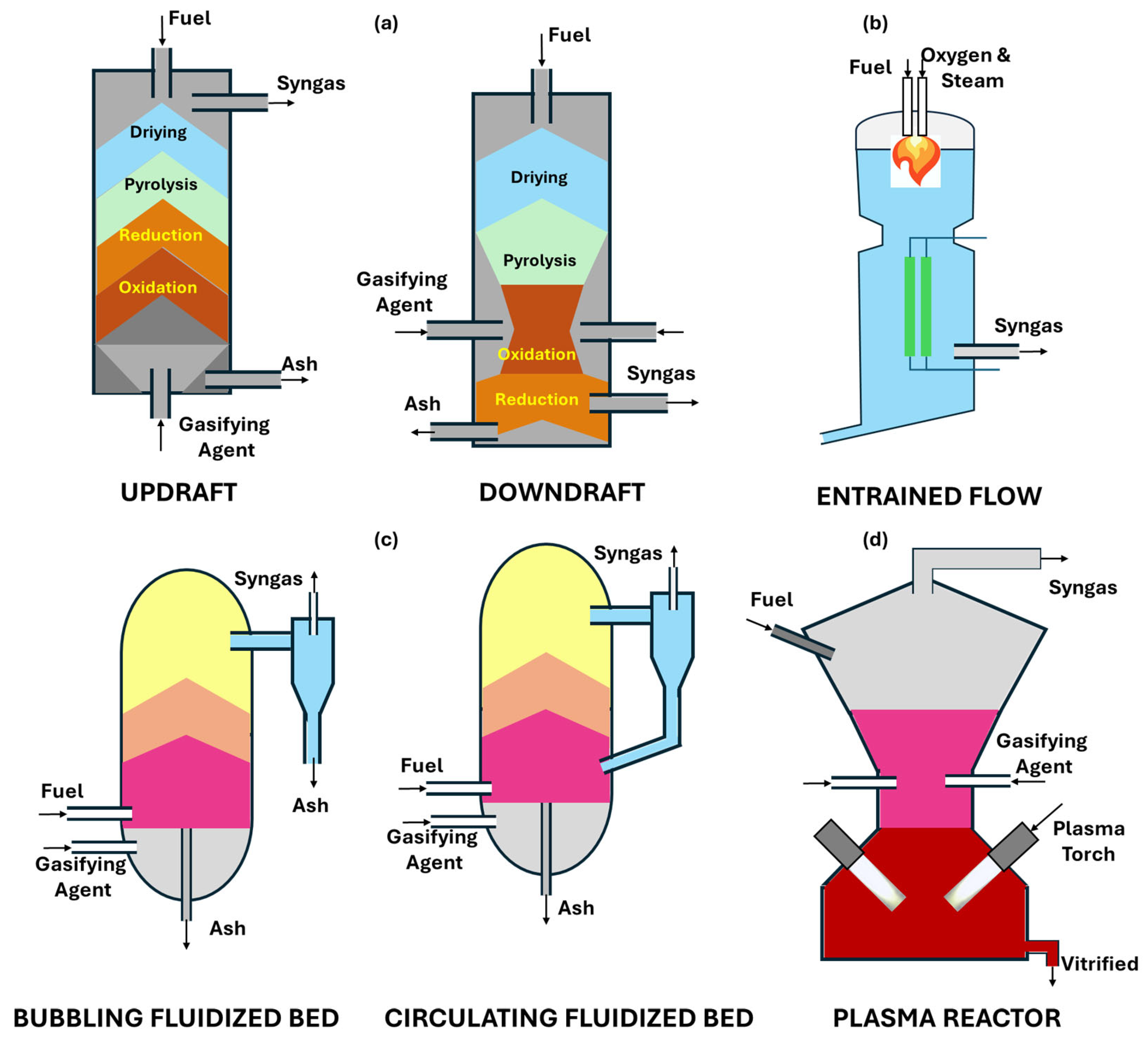
| Gasifying Agent | H2 (vol%) | CO (vol%) | CO2 (vol%) | CH4 (vol%) | N2 (vol%) | LHV (MJ·Nm−3) | Ref. |
|---|---|---|---|---|---|---|---|
| Air | 8–18 | 15–25 | 5–15 | 0–3 | 45–55 | 4–7 | [32,34] |
| Oxygen | 20–30 | 45–55 | 5–15 | 0–5 | 0–3 | 10–12 | [31,32] |
| Steam | 40–55 | 15–25 | 5–12 | 2–6 | 0–3 | 12–14 | [32,33] |
| Oxygen–steam | 30–45 | 35–50 | 5–12 | 1–5 | 0–3 | 10–14 | [31,35] |
| Reaction Name | Mechanism | kJ.mol−1 |
|---|---|---|
| Reactions of combustion | −111 −283 −393 −242 | |
| Boudouard reaction | +172 | |
| Water gas reaction | +131 | |
| Water gas shift reaction | −41 | |
| Methanation reactions | −75 −206 −165 | |
| Reforming of methane with steam | CH4 + H2O = CO + 3H2 | +206 |
| Partial oxidation of methane | CH4 + 0.5O2 = CO + 2H2 | −36 |
| Reforming of methane with CO2 | CH4 + CO2 = 2CO + 2H2 | +247 |
| Biomass | Ultimate Analysis (wt.%) | Proximate Analysis (wt.%) | LHV (MJ/kg) | Ref. | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | O | N | S | FC | VM | Ash | W | |||
| Wood chips | 49.6 | 6.0 | 43.8 | 0.5 | 0.1 | 10.7 | 49.1 | 1.4 | 38.7 | 9.9 | [47] |
| Rubber wood | 50.0 | 6.5 | 42.0 | 0.2 | − | 19.2 | 80.1 | 0.7 | − | − | [48] |
| Wood | 50.4 | 5.9 | 43.3 | <0.1 | <0.01 | – | – | 0.3 | − | 19.0 | [27] |
| Eucalyptus | 49.0 | 6.3 | 44.4 | 0.2 | 0.1 | 15.2 | 72.7 | 0.8 | 11.3 | 18.4 | [49] |
| MSW Portugal | 49.7 | 7.2 | 41.6 | 0.8 | 0.7 | 5.1 | 74.1 | 14.3 | 6.5 | 19.6 | [50] |
| MSW Brazil | 48.0 | 6.3 | 43.6 | 1.4 | 0.7 | 5.5 | 75.2 | 8.6 | 10.7 | 14.4 | [51] |
| Switchgrass | 49.6 | 5.7 | 40.4 | 0.3 | 0.05 | 17.5 | 78.6 | 3.9 | 7.7 | 16.5 | [52] |
| Hazelnut shells | 45.9 | 5.7 | 48.2 | 0.1 | 0.01 | 18.2 | 68.2 | 1.1 | 12.4 | 17.4 | [53] |
| Sugarcane straw | 44.8 | 5.9 | 48.9 | 0.1 | 0.3 | 13.3 | 77.3 | 9.6 | 0.9 | 15.7 | [54] |
| Rice husk | 40.8 | 5.3 | 53.4 | 0.4 | 0.2 | 16.4 | 61.8 | 20.9 | 0.9 | 14.9 | |
| RDF pellets | 71.5 | 10.7 | 17.0 | 0.7 | 0.1 | 4.4 | 74.9 | 16.5 | 4.3 | 25.7 | [47] |
| Microalgae | 50.9 | 7.0 | 31.0 | 10.2 | 0.8 | 12.6 | 80.7 | 6.7 | − | 20.8 | [55] |
| Sewage sludge | 52.3 | 7.9 | 32.6 | 6.4 | 0.8 | 10.6 | 72.3 | 17.1 | − | 18.6 | [55] |
| Focus | Modeling or Control Approach | Insights | Ref. |
|---|---|---|---|
| Hybrid modeling | Aspen Plus + Machine learning (ML) | Enhanced prediction accuracy | [58] |
| ML optimization | Gradient boosting | Impact of temperature and feedstock | [59] |
| CFD modeling | ANSYS Fluent | Reactor design strategies | [60] |
| ML combined with Explainable Artificial Intelligence (XAI) | XGBoost, SHAP/LIME | Improving syngas yield and feedstock quality | [61] |
| Hybrid ML and mechanistic modeling | GBR + Aspen Plus | Optimization of H2−enriched syngas production | [62] |
| Engine Type | Simulation Method | Engine/Experimental Info | Fuel Composition (% vol.) | Objective(s) | Finding(s) | Ref. |
|---|---|---|---|---|---|---|
| SI | KIVA4–CHEMKIN | 4–stroke, 4–cyl SI engine, CR = 12.9:1, rpm = 1500, ϕ = 0.8, IT variable | SG: H2 17; CO 15; CH4 4; CO2 15; O 0.14; N2 53Biogas: CH4 65; CO2 35 | Effects of IT, H2, and CH4 contents on SI engine fueled with SG and biogas | Under WOT and MBT: ITE: SG 39% > Biogas 37.5%; NOx: SG 3.3 g/kWh < Biogas 7.2 g/kWh. At advanced ITs: ↑ H2 (11–20%) less sensitive to NOx than ↑CH4 (55–88%) | [92] |
| SI | 3D CFD (engine cycle, detailed kinetics) | Biomass-fueled micro-CHP SI engine | H2 20–30; CO 25–40; CO2/N2 balance | Assess syngas composition effects on engine thermal balance | Inert dilution (CO2/N2) ↓ LHV & cylinder temps; moderate H2 supports efficient operation | [66] |
| SI | 3D CFD (KIVA–4 + GRI−Mech 3.0) | SI engine, full-cycle CFD | H2 15–35; CO 20–40; CO2 10–20; CH4 2–5 | Optimize SI engine performance with biomass syngas | ↑ H2 → faster ignition; CO2 dilution → ↓ flame temp & NOx | [90] |
| SI | Data synthesis + CFD benchmarking | Typical gasifier syngas ranges; validation for CFD | H2 20–30; CO 25–45; CO2 5–15; CH4 0–5 | Provide realistic syngas input data for kinetic/CFD models | Steam/oxy-steam gasification ↑ H2 fraction & LHV; air-blown yields low H2, high N2 | [33] |
| SI | Multidimensional CFD + kinetic analysis | SI engine conditions, syngas blends with variable CO/H2 | H2 15–30; CO 20–40; CH4 0–5; balance inerts | Quantify impact of CO/H2 ratio and diluents | ↑ H2 → shorter ignition delay & ↑ reactivity; CO ensures flame stability | [93] |
| SI | CHEMKIN | 4–stroke, 2–cyl SI research engine, CR = 11:1, 1500 rpm | SG: H2 12.8–37.2; CO 11.5–16.4; CH4 2.3–3.6; CO2 10.8–24.7; N2 18.1–62.6 | Model impact of H2 fraction on energy balance & efficiency | ↑H2 12.8→19.4% → ↑ brake thermal efficiency; ↑ H2 up to 37.2% → ↓ brake thermal efficiency | [94] |
| CI (retrofitted to SI) | 2−D thermodynamic code + PHOENICS | 4–stroke, 6–cyl CI engine, CR = 16.3 (CI mode), 1100 rpm | SG: H2 6; CO 25; CH4 5; CO2 11; N2 53 | Simulate SG combustion in CI engines used in SI mode | Predicts primary parameters of SG combustion in adapted CI engines | [95] |
| CI (retrofitted to SI) | Zero-dimensional model | 4–stroke, 6–cyl CI engine, CR = 16.5 (CI), 10.5 (SI), rpm = 1500 | SG: H2 19; CO 19; CH4 2; CO2 12; H2O 2; N2 46 | Assess SG impact on engine performance with turbocharger | Imbalance in turbocharger → ↓ engine power; optimization can mitigate losses | [96] |
| CI | 1–D multicylinder engine model (GT-SUITE) | 4–stroke, 1–cyl CI engine, CR = 15:1, rpm = 1800 | SG: H2 70; CO 15; CO2 15 | Evaluate SI engine feasibility with H2–rich SG | 15% fuel consumption reduction vs. NG bus; ↓ thermal efficiency under high load | [97] |
| CI | 3D CFD (engine simulation, DI syngas) | Micro-CHP CI engine | H2 22–30; CO 28–38; CO2 8–12; CH4 2–4; N2 balance | Evaluate direct injection of biomass syngas in CI engines | High efficiency & reduced NOx under DI syngas | [89] |
| DF (Diesel/Syngas) | 3D CONVERGE CFD | 4–stroke, 1–cyl Ricardo Hydra CI engine, CR = 15.5:1, rpm = 1200 | Main fuel: Diesel; SG: H2 3; CO 11; CO2 3.4; CH4 1.4; C2H4 2.9; N2 78.2 | Study RCCI with diesel & reformed diesel | ↑ ITE, ↓ NOx, ↓ THC, ↑ controllability, ↑ CO & CO2 | [98] |
| DF (Diesel/Producer Gas) | Heat Release Rate model (CONVERGE) | 4−stroke, 1–cyl Yanmar CI engine, CR = 20.9:1 | Main fuel: Diesel; PG: H2 15; CO 20; CH4 4; CO2 12; O2 1; N2 48 | Effect of PG substitution of diesel on HRR & combustion | PG/diesel: delayed combustion start, ↓ max BTE, ↓ max pressure, ↓ NOx | [99] |
| HCCI | Zero-dimensional single-zone thermochemical model | HCCI engine | SG: H2 50; CO 50 | Improve HCCI combustion with ozone or excited oxygen | Ignition accelerated before TDC → ↓ intake temperature; 1% singlet delta oxygen ↑ power 7–14% | [100] |
| HCCI | Multi-zone model + semi-detailed kinetics | 4–stroke, 1–cyl Waukesha CFR engine, CR = 11.5–19:1, rpm = 800 | Main fuel: PRFs; SG: 0–30% replacement, H2/CO = 1/1 | Effect of syngas addition on HCCI performance | ↓ H2 abstraction rate, ↑ fuel decomposition time, ↓ combustion start, ↓ peak pressure/temp, ↓ NOx, ↑ HC & CO | [101] |
| Objective | Fuel Composition (% vol.) | Experimental Setup | Key Findings | Ref. |
|---|---|---|---|---|
| 100% syngas in SI engine vs. gasoline | H2: 13–19; CO: 16–24; CH4: 1–6; CO2: 9–14; N2: balance | 4−stroke, 1−cyl, 5.5 kW SI engine | ↓ Power (~20%); ↑ CO2; ↓ NOx & CO vs. gasoline | [110] |
| Syngas/CH4/gasoline blends | H2: 23–40; CO: 23–39; CH4: 11–26; CO2: 10–28 | 2−cyl Lombardini SI engine, 2000–4500 rpm | H2−rich syngas improves efficiency; ↑ NOx; ↓ CO | [105] |
| Ignition timing & supercharging | H2/CO ≈ 40/39; CH4 traces | SI engine under boost & IT variation | ↑ H2, ↑ CO, ↓ CO2 improve efficiency; supercharging ↑ NOx | [87] |
| CFD with hydrogen enrichment | H2: 17; CO: 15; CH4: 4; CO2: 15; N2: 53 | 4−cyl SI engine, 1500 rpm | ↑ H2 improves ITE; ↓ NOx vs. biogas | [92] |
| DI−SI syngas vs. CNG | H2: 50; CO: 50 (+CH4 20%) | 1−cyl, DISI, CR = 14:1, 1500–2400 rpm | ↑ Heat release & efficiency; ↓ torque vs. CNG | [104] |
| Equivalence ratio & CR effects | H2: 60; CO: 40; LHV = 20.4 MJ/kg | 1−cyl SI engine, CR = 9–11, 1200 rpm | ↑ CR improves lean burn; syngas achieves lowest misfire limit | [111] |
| Producer gas in retrofitted CI → SI | H2: 8.5; CO:30.5; CH4: 0.3; CO2: 4.8; N2: 49.6 | 1−cyl CI engine, CR = 9–17, 1000–2000 rpm | ↑ CR improves BTE (11→24%); ↓ smoke; ↑ CO | [17] |
| Stoichiometric vs. lean SG | H2: 30; CO: 25; CO2: 45 | 1−cyl CI engine, CR = 13–17, 1800 rpm | ↑ CR increases ITE & HRR; ↓ combustion duration; ↓ gross power | [19] |
| Objective | Fuel Composition (% vol.) | Experimental Setup | Key Findings | Ref. |
|---|---|---|---|---|
| Efficiency & emissions vs. H2/CO ratio | H2: 100–0; CO:0–100 | Yanmar L100V CI, CR = 21.2:1 | ↑ H2 → ↑ efficiency & NOx; ↑ CO → ↓ performance & NOx | [19] |
| Effect of load & H2/CO ratio | H2/CO = 100/0—0/100 | Kirloskar CI, 1500 rpm, CR = 17.5:1 | Max diesel replacement ~70%; H2−rich → ↑ efficiency, ↑ NOx | [113] |
| CFD & exergy analysis of DF mode | H2/CO ≈ 50/50 | DF CI simulations | Syngas/diesel ↓ soot & CO; ↑ NOx at high load | [114] |
| PG/diesel dual mode | PG—H2: 15–19; CO: 18–22; N2: 45–55 | Kirloskar CI, CR = 17:1 | PG ↓ NOx & soot, but ↓ BTE | [17] |
| PG/diesel vs. PG/biodiesel DF | PG—H2: 12; CO: 10; CH4: 1.5; CO2: 15; N2: 59 | 1−cyl Kirloskar AV1 CI, CR = 5–20, 1450–1600 rpm | DF feasible without mods; BTE order: BD > D > D/PG > BD/PG; BD/PG ↓ smoke by 16% | [22] |
| Pilot injection optimization | PG—H2: 20; CO: 20; N2: 60 | AVL 5402 CI, 1500 rpm | Split injection ↑ efficiency; ↓ emissions | [115] |
| PG + biodiesel (DiSOME) DF | PG—H2: 15–19; CO: 18–22; N2: 45–55 | Apex CI engine, CR = 17.5:1, 1500 rpm | DF ↑ BTE by ~6%; ↓ HC & CO; ↑ NOx vs. HCCI | [116] |
| Modified DF diesel with PG | PG—H2: 15–19; CO: 18–22; N2: 45–55 | Kirloskar TAF−1 CI, CR = 17:1, 1500 rpm | PG ↓ BTE, ↑ fuel consumption, ↓ NOx & HC | [117] |
| H2 vs. PG DF | H2: 100 PG—H2: 15–19; CO: 18–22; N2: 45–55 | DF CI | H2 → ↑ BTE, ↓ fuel use, ↑ HRR; PG → ↓ BTE, ↑ CO & HC | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rey, J.R.C.; Longo, A.; Rijo, B.; Mateos-Pedrero, C.; Brito, P.; Nobre, C. Modelling Syngas Combustion from Biomass Gasification and Engine Applications: A Comprehensive Review. Energies 2025, 18, 5112. https://doi.org/10.3390/en18195112
Rey JRC, Longo A, Rijo B, Mateos-Pedrero C, Brito P, Nobre C. Modelling Syngas Combustion from Biomass Gasification and Engine Applications: A Comprehensive Review. Energies. 2025; 18(19):5112. https://doi.org/10.3390/en18195112
Chicago/Turabian StyleRey, José Ramón Copa, Andrei Longo, Bruna Rijo, Cecilia Mateos-Pedrero, Paulo Brito, and Catarina Nobre. 2025. "Modelling Syngas Combustion from Biomass Gasification and Engine Applications: A Comprehensive Review" Energies 18, no. 19: 5112. https://doi.org/10.3390/en18195112
APA StyleRey, J. R. C., Longo, A., Rijo, B., Mateos-Pedrero, C., Brito, P., & Nobre, C. (2025). Modelling Syngas Combustion from Biomass Gasification and Engine Applications: A Comprehensive Review. Energies, 18(19), 5112. https://doi.org/10.3390/en18195112











