Development and Characterization of CO2-Responsive Surfactants for Coalbed Methane Fracturing
Abstract
1. Introduction
2. Synthesis and Characterization of CO2-Responsive Surfactants
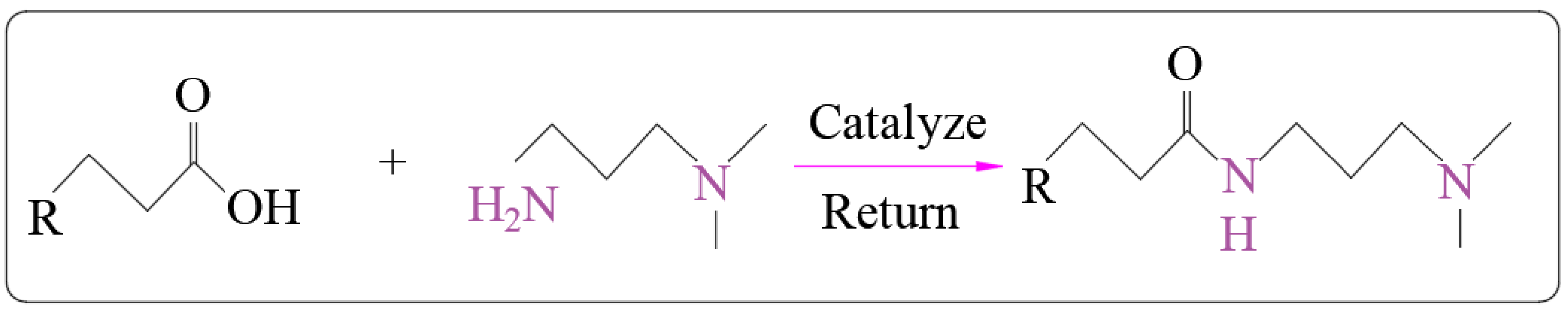
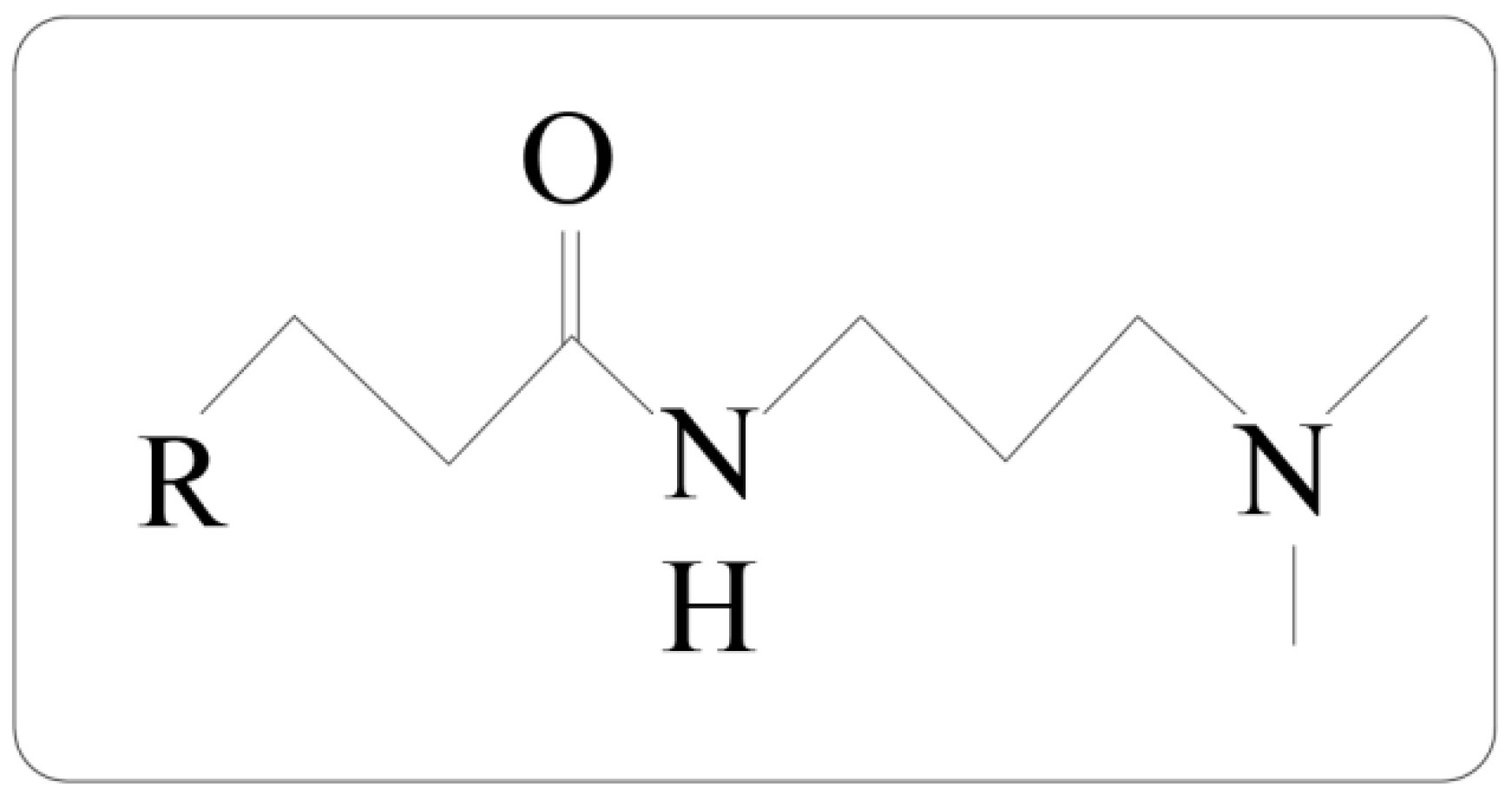
2.1. Synthesis of Surfactants
2.2. Structural Characterization of Surfactants
3. Microscopic Mechanism Research
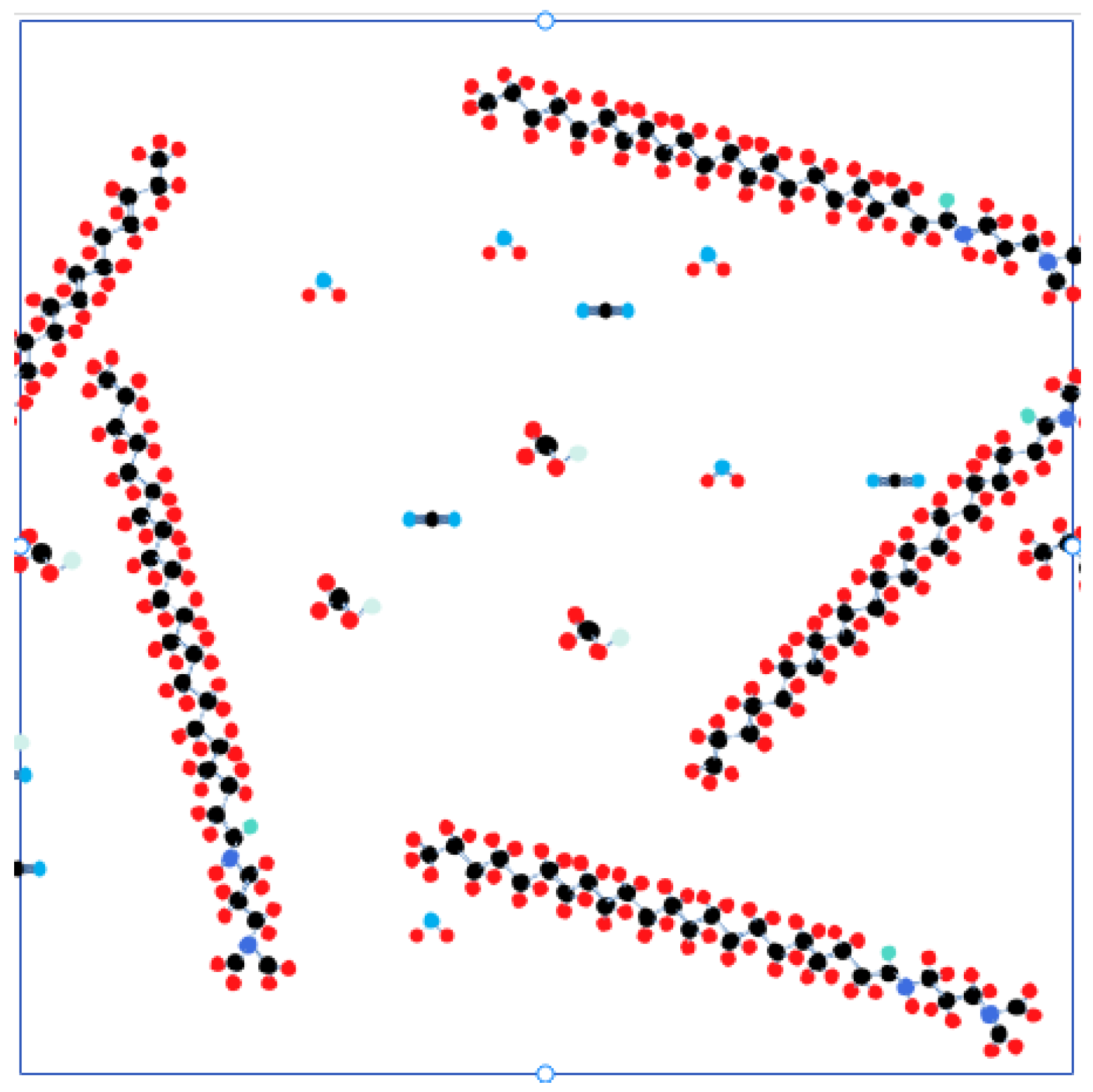
3.1. Model and Parameters
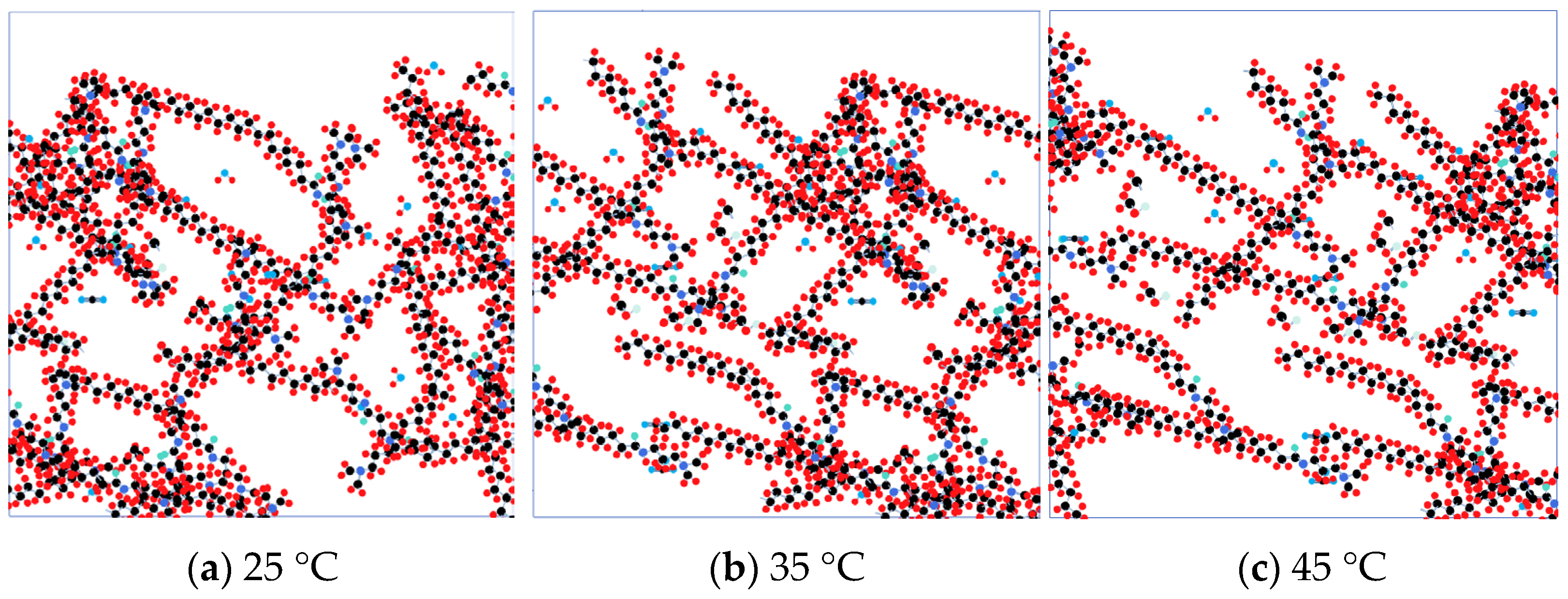
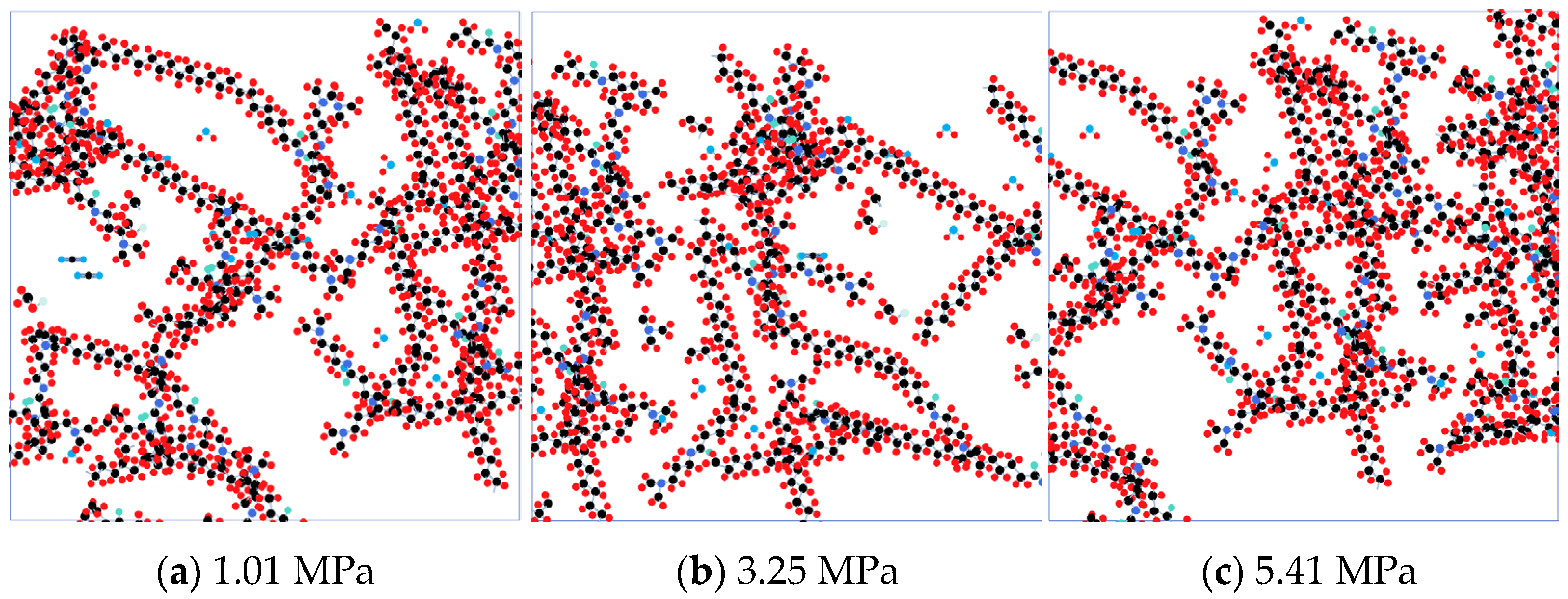
3.2. Response to Microscopic Effects
4. Performance Evaluation of CO2-Responsive Fracturing Fluid
4.1. CO2-Responsiveness
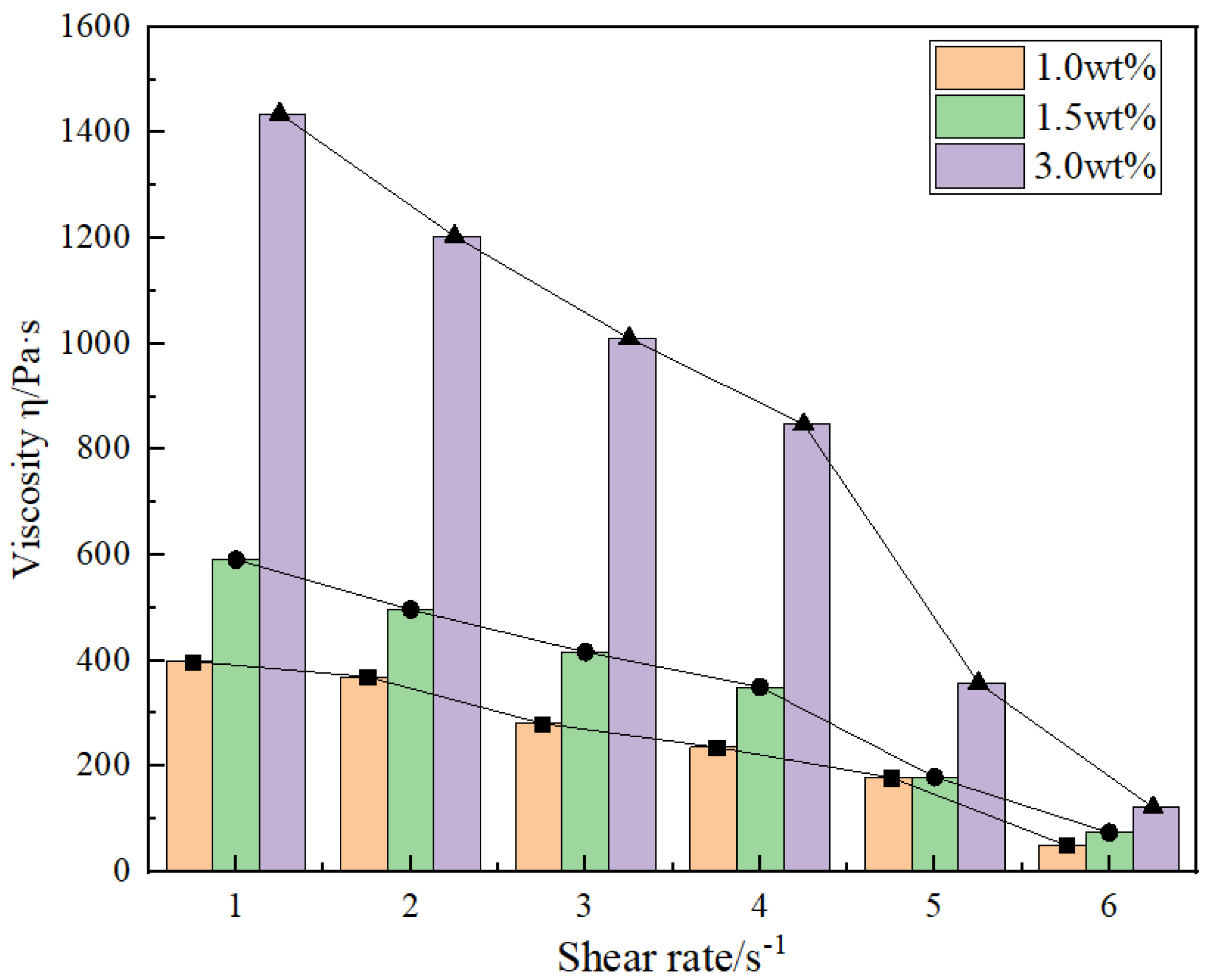
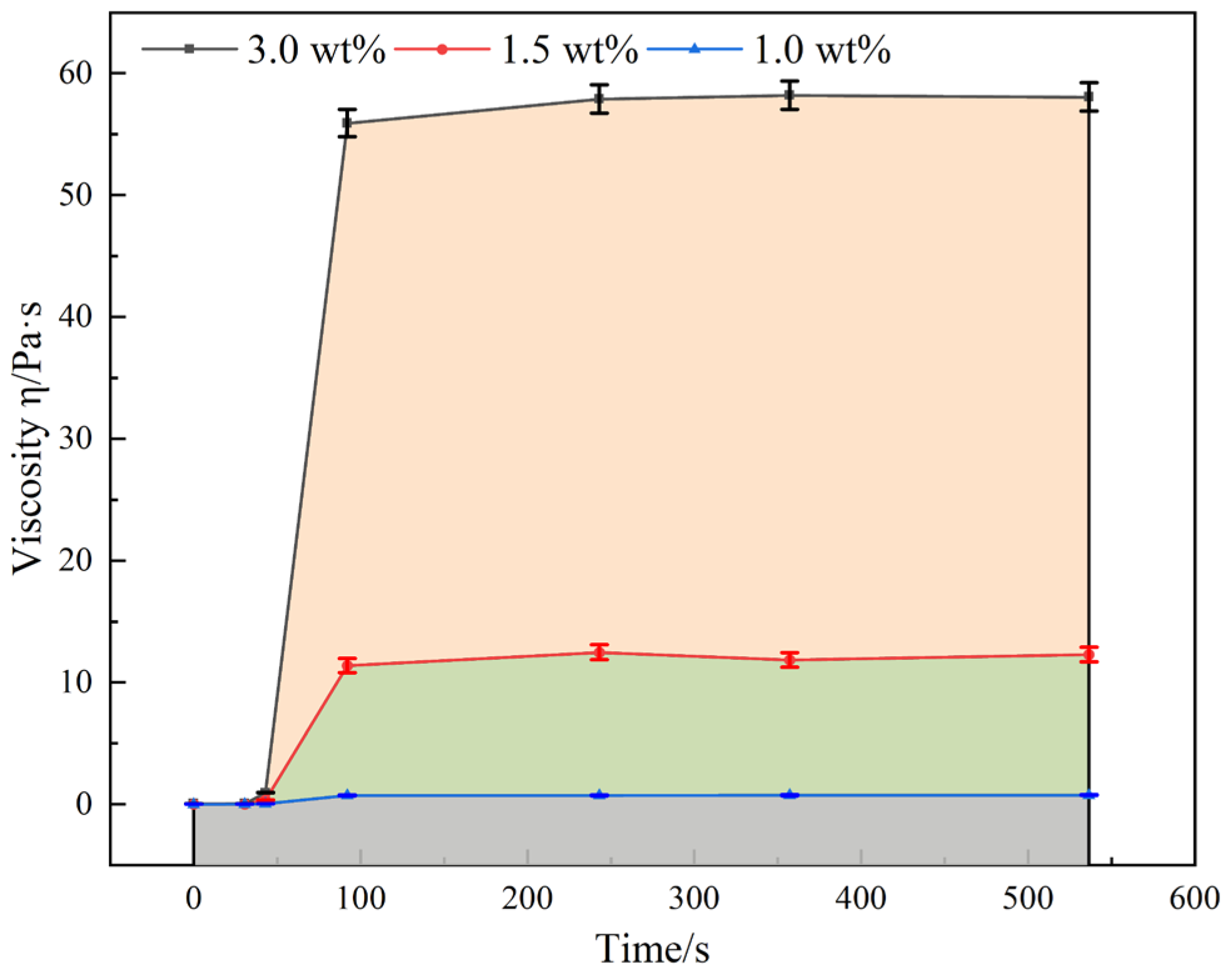
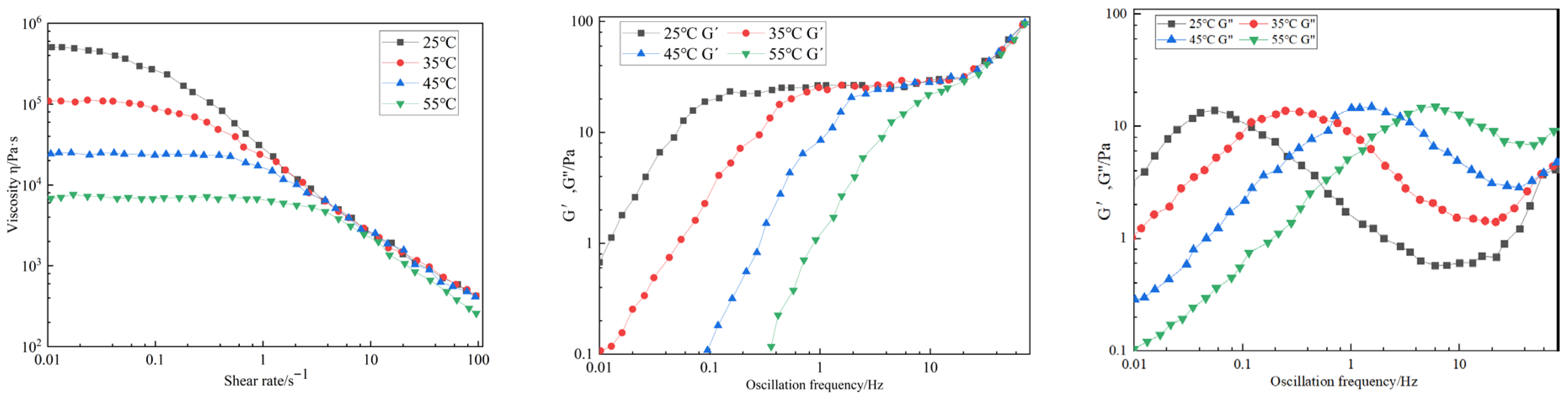
4.2. Viscoelastic Rheological Behavior
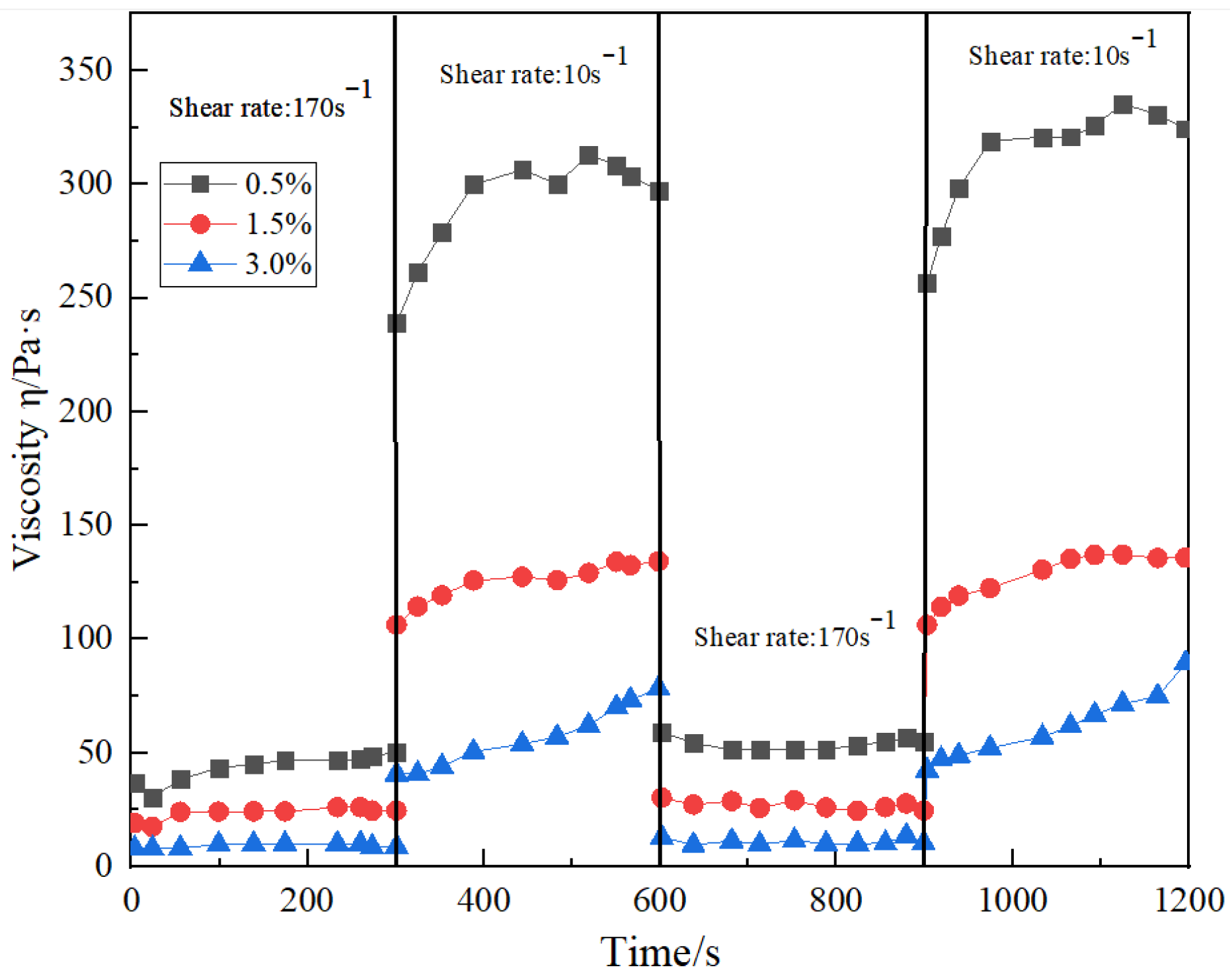
4.3. Shear Resistance
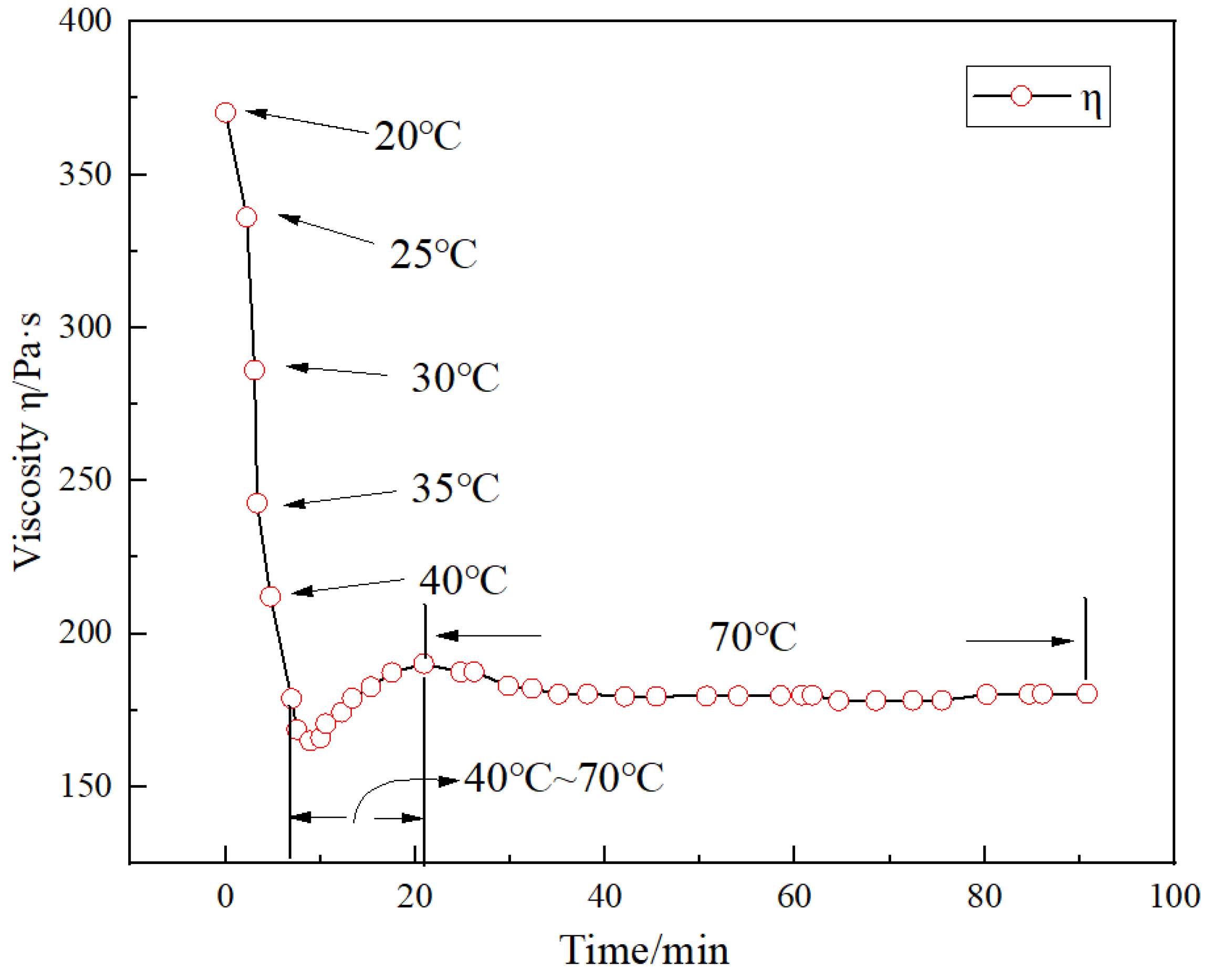
4.4. Temperature Resistance
4.5. Effects of CO2 Pressurization
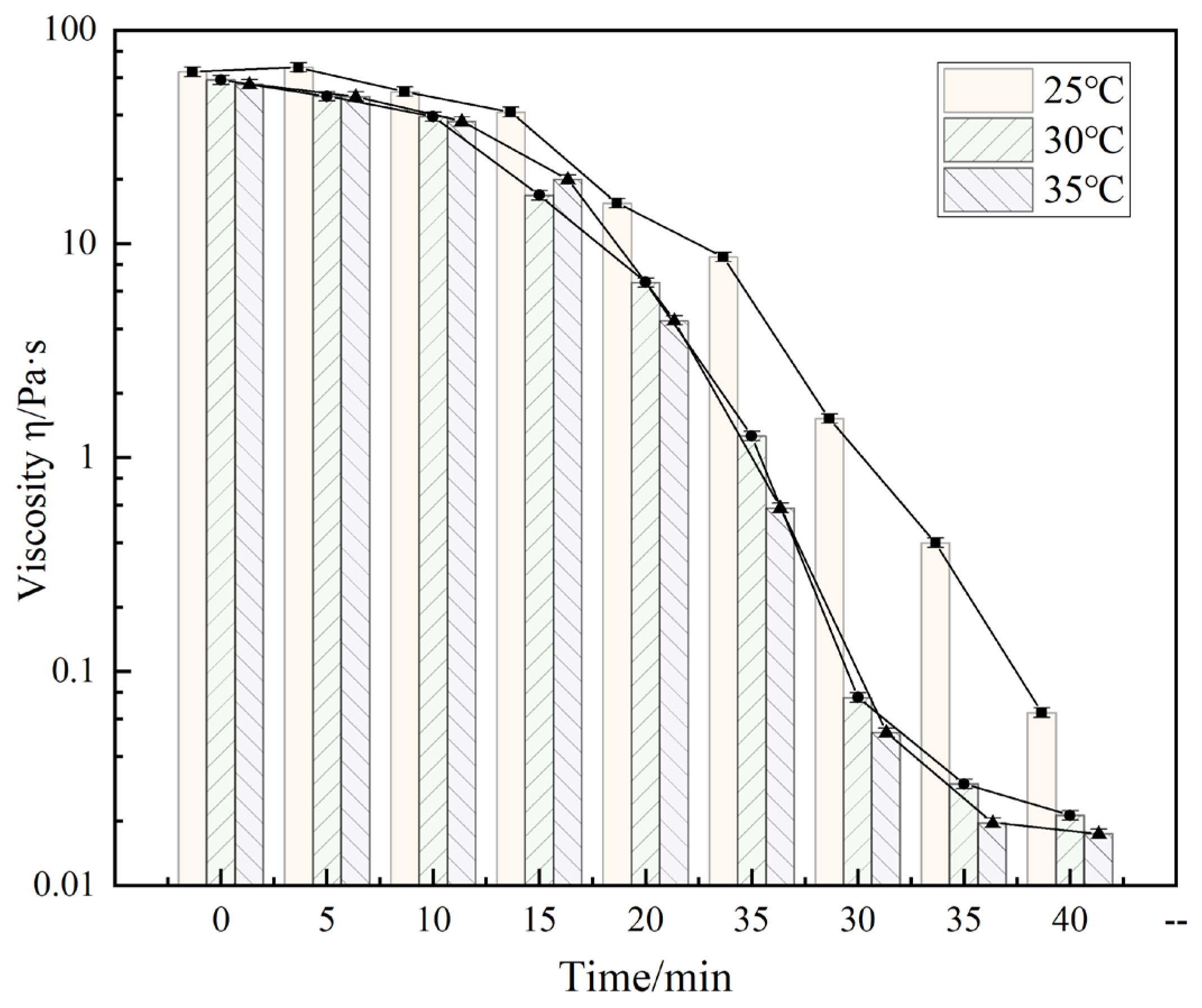
4.6. Gel Breaking Property
4.7. Biotoxicity Assessment
4.7.1. Biological Toxicity Testing
4.7.2. Environmental Fate and Accumulation Effects Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fang, X.; Wu, C.; Gao, B.; Zhang, S.; Zhou, D.; Jiang, X.; Liu, N. Effects of active hydraulic fracturing fluid on the fracture propagation and structural damage of coal: Phenomena and mechanisms. Nat. Resour. Res. 2023, 32, 1761–1775. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhu, Z.; Liu, W.; Lu, H.; Fan, Z.; Nie, X.; Li, C.; Wang, J.; Ren, L. Hydraulic fracturing behaviors of shale under coupled stress and temperature conditions simulating different burial depths. Int. J. Min. Sci. Technol. 2024, 34, 783–797. [Google Scholar] [CrossRef]
- Huang, J.; Chen, J.; Lv, L.; Yu, Z.; Yao, W.; Cheng, H.; Niu, W.; Wang, J.; Zhang, J.; Qi, H. Design and Verification of a Wearable Micro-Capacitance Test System for POC Biosensing. IEEE Trans. Instrum. Meas. 2025, 74, 1–11. [Google Scholar] [CrossRef]
- Shi, Y.; Yu, W.; Zhou, D.; Ding, F.; Shu, W.; Zhang, Y.; Ju, Y.; Lei, Z. Research on the Performance of New Weighted Slippery Water Fracturing Fluid System. Open J. Appl. Sci. 2024, 14, 2101–2111. [Google Scholar] [CrossRef]
- Li, J.; Wen, M.; Yang, J.; Liu, Y.; Jiang, Z.; Chen, J. Synthesis and analysis of magnetic nanoparticles within foam matrix for foam drainage gas production. Geoenergy Sci. Eng. 2024, 238, 212887. [Google Scholar] [CrossRef]
- Zheng, N.; Zhu, J.; Yang, Z.; Jiang, Z.; Yi, L.; Li, X.; Wu, C.; Long, Y.; Yi, D.; Gou, L. Study on the Synergistic Stabilization Mechanism and Performance of CO2-Responsive Viscoelastic Foam: A Leap Forward in Sustainable Fracturing Fluids. ACS Sustain. Chem. Eng. 2018, 13, 5748–5763. [Google Scholar] [CrossRef]
- He, S.; Ding, L.; Xiong, Z.; Spicer, R.; Farnsworth, A.; Valdes, P.; Wang, C.; Cai, F.; Wang, H.; Sun, Y.; et al. A distinctive Eocene Asian monsoon and modern biodiversity resulted from the rise of eastern Tibet. Sci. Bull. 2022, 21, 2245–2258. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Zhang, H.; Ma, X.; Hong, N.; Xu, J.; Liu, Y.; Chen, X.; Wang, Z.; He, L. Optimization and application of treatment technology for guanidine gum fracturing flowback fluid in deep coalbed methane wells. Acta Pet. Sin. 2023, 44, 1959–1973. [Google Scholar] [CrossRef]
- Hao, H.; Yao, E.; Pan, L.; Chen, R.; Wang, Y.; Xiao, H. Exploring heterogeneous drivers and barriers in MaaS bundle subscriptions based on the willingness to shift to MaaS in one-trip scenarios. Transp. Res. Part A Policy Pract. 2025, 199, 104525. [Google Scholar] [CrossRef]
- Fu, L.; Wei, M.; Liao, K.; Qianli, M.; Shao, M.; Gu, F.; Fan, Y.; Longjie, L.; Yanfeng, H. Application of environmentally stimuli-responsive materials in the development of oil and gas field. J. Pet. Sci. Eng. 2022, 219, 111088. [Google Scholar] [CrossRef]
- Dowlati, S.; Mokhtari, R.; Hohl, L.; Miller, R.; Kraume, M. Advances in CO2-switchable surfactants towards the fabrication and application of responsive colloids. Adv. Colloid Interface Sci. 2023, 315, 102907. [Google Scholar] [CrossRef]
- Lu, Y.; Li, R.; Manica, R.; Zhang, Z.; Liu, Q.; Xu, Z. CO2-responsive surfactants for greener extraction of heavy oil: A bench-scale demonstration. J. Clean. Prod. 2022, 338, 130554. [Google Scholar] [CrossRef]
- Yang, Z.; He, S.; Fang, Y.; Zhang, Y. Viscoelastic Fluid Formed by Ultralong-Chain Erucic Acid–Base Ionic Liquid Surfactant Responds to Acid/Alkaline, CO2, and Light. J. Agric. Food Chem. 2021, 69, 3094–3102. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.; Wang, Y.; Li, X. CO2-switchable viscoelastic fluids based on a pseudogemini surfactant. Langmuir 2013, 29, 4187–4192. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, Z.; Dreiss, C.A.; Wang, Y.; Fei, C.; Feng, Y. Smart wormlike micelles switched by CO2 and air. Soft. Matter. 2013, 9, 6217–6221. [Google Scholar] [CrossRef]
- Lu, W.; Wang, J.; Wang, T.; Zhang, K.; Jiang, X.; Zhao, H. Visual style prompt learning using diffusion models for blind face restoration. Pattern Recognit. 2025, 161, 111312. [Google Scholar] [CrossRef]
- Zhang, C.; Yu, C.; Gu, Z.-H.; Liu, K.; Wu, P.-K.; Li, Z.-M. Characterization and optimization of oil–gas interfacial tension during CO2/N2 injection in heavy oil reservoirs: Experimental study and regression model. Pet. Sci. 2025, 22, 2516–2534. [Google Scholar] [CrossRef]
- Golsanami, N.; Sun, J.; Zhang, Z. A review on the applications of the nuclear magnetic resonance (NMR) technology for investigating fractures. J. Appl. Geophys. 2016, 133, 30–38. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, L.; Wan, X.; Tang, Y.; Liu, Q.; Li, W.; Liao, J. Synthesis and Characterization of a Temperature-Sensitive Microcapsule Gelling Agent for High-Temperature Acid Release. ACS Omega 2024, 9, 20849–20858. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xia, H.; Yu, D.; Cheng, J.; Li, J. Outlier detection method based on high-density iteration. Inf. Sci. 2024, 662, 120286. [Google Scholar] [CrossRef]
- Mark, P.; Nilsson, L. Structure and dynamics of liquid water with different long-range interaction truncation and temperature control methods in molecular dynamics simulations. J. Comput. Chem. 2003, 23, 1211. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Ranjith, P.G. Insights into interfacial behaviours of surfactant and polymer: A molecular dynamics simulation. J. Mol. Liq. 2022, 346, 117865. [Google Scholar] [CrossRef]
- Bai, S.; Kubelka, J.; Piri, M. Atomistic molecular dynamics simulations of surfactant-induced wettability alteration in crevices of calcite nanopores. Energy Fuels 2020, 34, 3135–3143. [Google Scholar] [CrossRef]
- Qi, H.; Hu, Z.; Yang, Z.; Zhang, J.; Wu, J.J.; Cheng, C.; Wang, C.; Zheng, L. Capacitive aptasensor coupled with microfluidic enrichment for real-time detection of trace SARS-CoV-2 nucleocapsid protein. Anal. Chem. 2022, 6, 2812–2819. [Google Scholar] [CrossRef]
- Michalet, X. Mean square displacement analysis of single-particle trajectories with localization error: Brownian motion in an isotropic medium. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2010, 82, 041914. [Google Scholar] [CrossRef]
- Mahmood, A.; Irfan, A.; Wang, J.L. Molecular level understanding of the chalcogen atom effect on chalcogen-based polymers through electrostatic potential, non-covalent interactions, excited state behaviour, and radial distribution function. Polym. Chem. 2022, 13, 5993–6001. [Google Scholar] [CrossRef]
- SY/T 5107-2016; The Evaluation Measurement for Properties of Water-Based Fracturing Fluid. Petroleum Industry Press: Beijing, China, 2016.
- Kirkwood, J.G.; Boggs, E.M. The radial distribution function in liquids. J. Chem. Phys. 1942, 10, 394–402. [Google Scholar] [CrossRef]
- SY/T 6376-2022; The Environmental Technical Requirements for Oilfield Chemical Agents. Petroleum Industry Press: Beijing, China, 2022.
- Lv, W.; Dong, M.; Sarma, H.; Li, Y.; Li, Z.; Sun, J.; Gong, H. Effects of CO2-philic nonionic polyether surfactants on miscibility behaviors of CO2–hydrocarbon systems: Experimental and simulation approach. Chem. Eng. J. 2024, 464, 142701. [Google Scholar] [CrossRef]
- Wang, X.; Song, Y.; Wu, P.; Zhang, G.; Guo, J.; Li, Y. Rheological properties of hydroxypropyl guar gum fracturing fluids: Effects of cross-linking agents. Carbohydr. Polym. 2025, 366, 123837. [Google Scholar] [CrossRef]
- SY/T 6787-2010; Technical Requirements of Environmental Protection for Water-Soluble Oilfield Chemicals. Petroleum Industry Press: Beijing, China, 2010.
- SY/T 6788-2010; Evaluation Procedures of Environmental Protection for Water-Soluble Oilfield Chemicals. Petroleum Industry Press: Beijing, China, 2010.
- Sun, L.; Shi, W.; Tian, X.; Li, J.; Zhao, B.; Wang, S.; Tan, J. A plane stress measurement method for CFRP material based on array LCR waves. NDT E Int. 2025, 151, 103318. [Google Scholar] [CrossRef]
- Allen, D.; Wiencek, M.; Kelly, M.; Solomons, K.; Jeffries, M. Exploring Alternatives for Marine Toxicity Testing: Initial Evaluation of Fish Embryo and Mysid Tests. Environ. Toxicol. Chem. 2024, 43, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Nazar, M.; Ahmad, A.; Hussain, S.M.S.; Moniruzzaman, M. Binary mixture of ionic liquid and span 80 for oil spill remediation: Synthesis and performance evaluation. Mar. Pollut. Bull. 2024, 202, 116311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, X. Understanding the relationship between coopetition and startups’ resilience: The role of entrepreneurial ecosystem and dynamic exchange capability. J Bus Ind Mark 2025, 2, 527–542. [Google Scholar] [CrossRef]









| Experimental Instruments | Purity | Molecular Weight | Manufacturer |
|---|---|---|---|
| Erucic | 90% | 338.57 g/mol | Bailingwei Technology Co., Ltd., Shenzhen, China |
| N.N-Dimethylaminopropylamine | 99% | 102.18 g/mol | Bailingwei Technology Co., Ltd. |
| Catalyst (sodium fluoride) | AR | 41.99 g/mol | China National Pharmaceutical Group Chemical Reagent Co., Ltd. Shanghai, China |
| Dehydrating agent (calcium chloride) | analytical pure | 110.98 g/mol | Xilong Chemical Co., Ltd. Shanghai, China |
| acetone | analytical pure | 58.08 g/mol | Yantai Shuangshuang Chemical Co., Ltd. Yantai, China |
| EC50/(mg·L−1) | <1 | 1~100 | 101~1000 | 1000~10,000 | >10,000 |
|---|---|---|---|---|---|
| Toxicity level | Highly toxic | Moderately toxic | Toxic | Slightly toxic | Non-toxic |
| 96 h LC50/(μg·L−1) | <1 | 1~100 | 101~1000 | 1000~10,000 | >10,000 |
|---|---|---|---|---|---|
| Toxicity level | Highly toxic | Moderately toxic | Toxic | Slightly toxic | Non-toxic |
| Evaluation Sample | EC50/(mg·L−1) | 96 h LC50/(μg·L−1) | Biological Toxicity Classification |
|---|---|---|---|
| 1.0 wt% C22ZEA aqueous solutions | 1.94 × 106 | 8 × 105 | non-toxic |
| 1.5 wt% C22ZEA aqueous solutions | 1.83 × 106 | 6 × 105 | non-toxic |
| 3.0 wt% C22ZEA aqueous solutions | 8.24 × 105 | 3 × 105 | non-toxic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.-H.; Xu, T.-F.; Zhang, Q.-H.; Lin, F.-J. Development and Characterization of CO2-Responsive Surfactants for Coalbed Methane Fracturing. Energies 2025, 18, 5084. https://doi.org/10.3390/en18195084
Li Z-H, Xu T-F, Zhang Q-H, Lin F-J. Development and Characterization of CO2-Responsive Surfactants for Coalbed Methane Fracturing. Energies. 2025; 18(19):5084. https://doi.org/10.3390/en18195084
Chicago/Turabian StyleLi, Zhi-Heng, Teng-Fei Xu, Qing-Hua Zhang, and Fu-Jin Lin. 2025. "Development and Characterization of CO2-Responsive Surfactants for Coalbed Methane Fracturing" Energies 18, no. 19: 5084. https://doi.org/10.3390/en18195084
APA StyleLi, Z.-H., Xu, T.-F., Zhang, Q.-H., & Lin, F.-J. (2025). Development and Characterization of CO2-Responsive Surfactants for Coalbed Methane Fracturing. Energies, 18(19), 5084. https://doi.org/10.3390/en18195084





