Selective Extraction and Hydrotreatment of Biocrude from Sewage Sludge: Toward High-Yield, Alkane-Rich, Low-Heteroatom Biofuels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
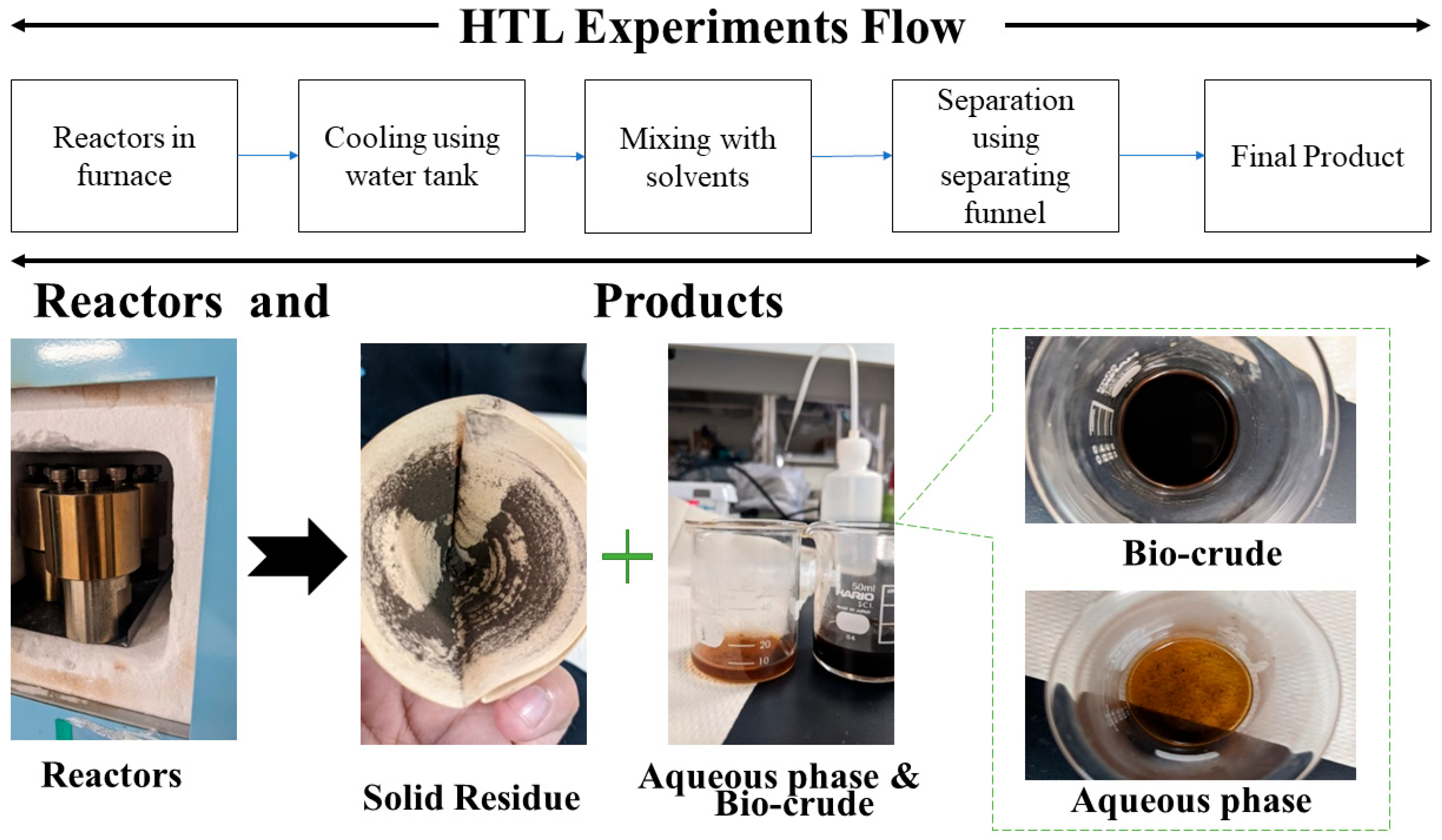
2.2. HTL Experiments
2.3. Hydrotreatment (Hydrogenation Upgrading) of HTL Biocrude
2.4. Product Analysis or Characterization
3. Results
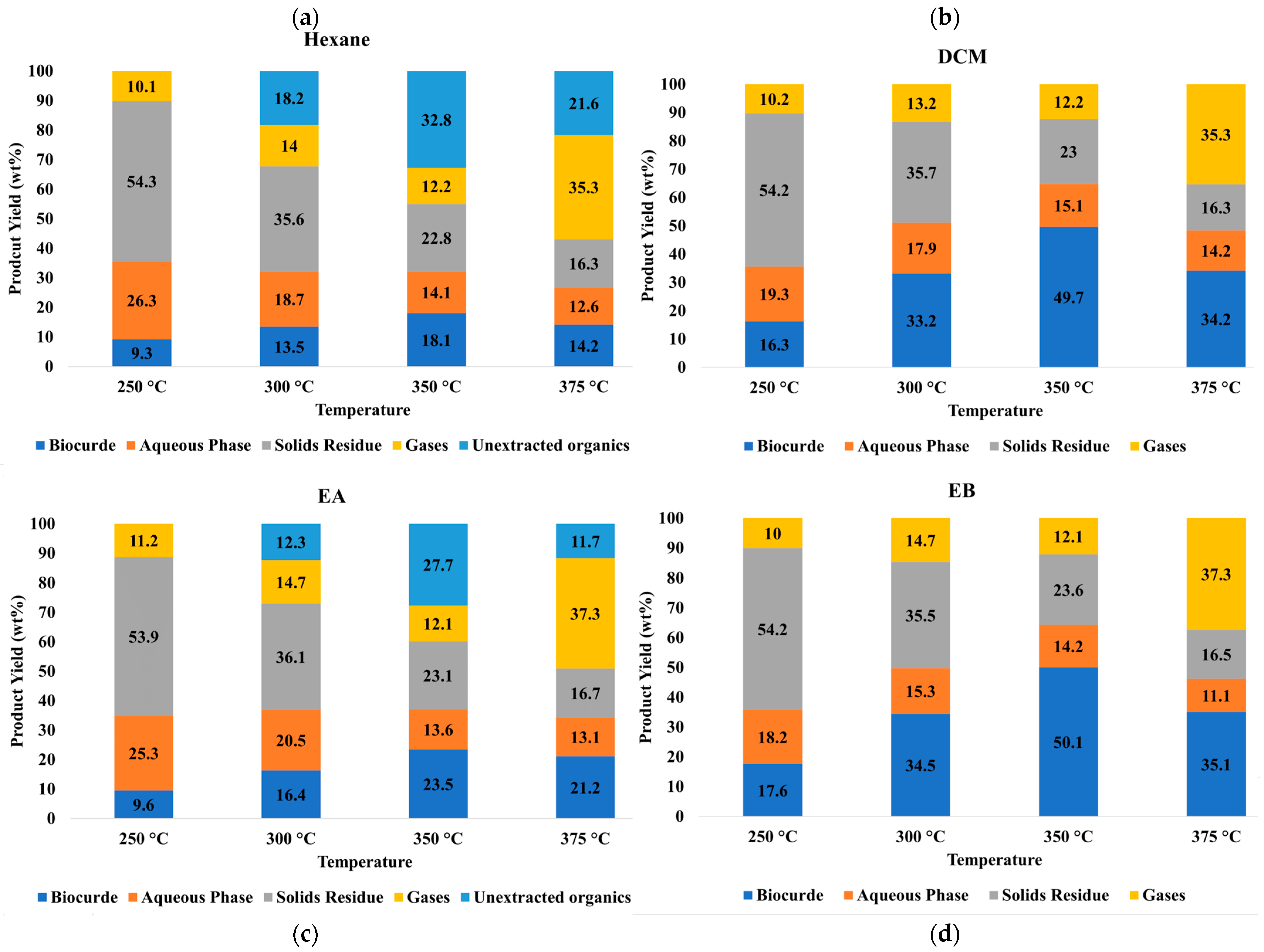
3.1. HTL Product Yields
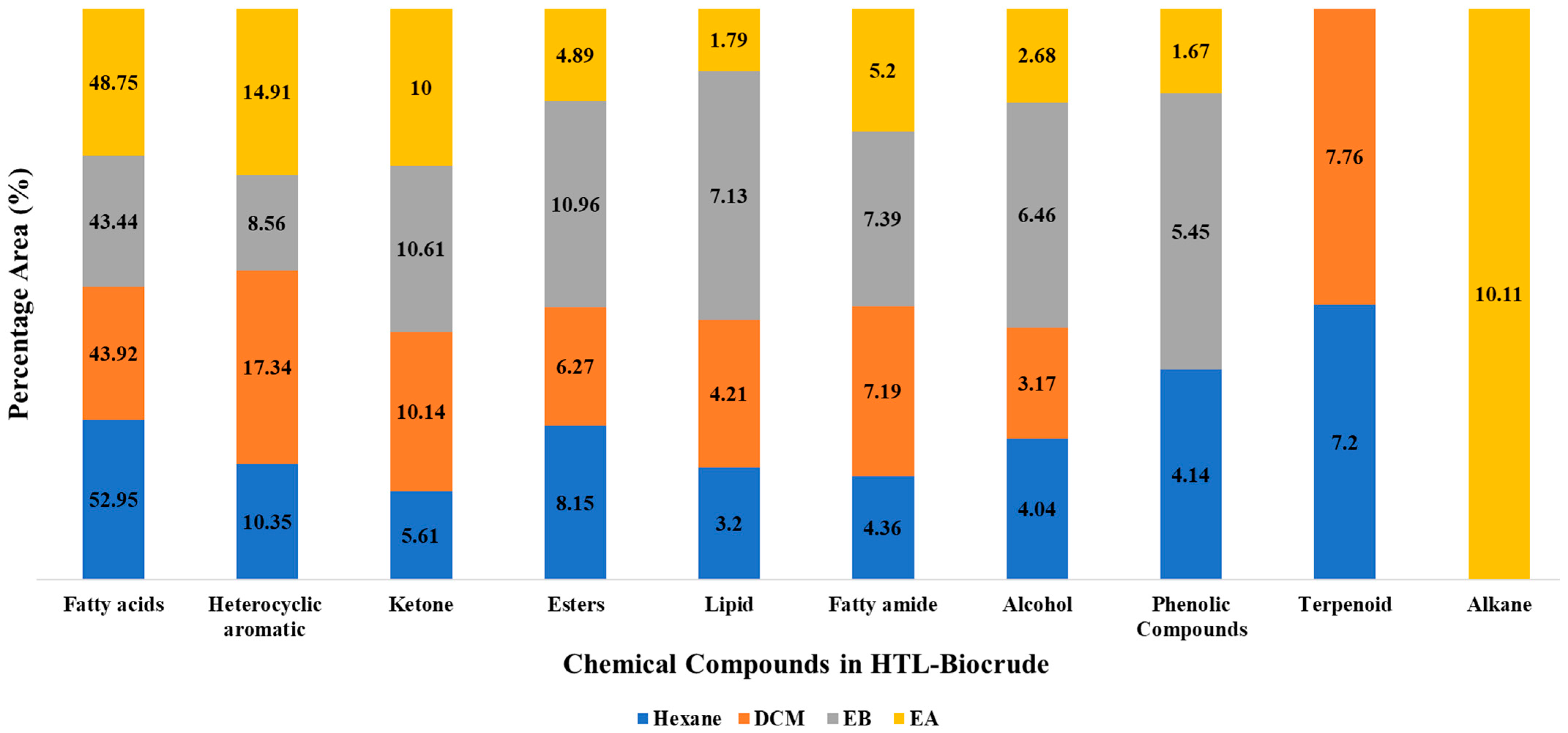
3.2. Characterization of HTL Biocrude
Chemical Compounds Composition in Biocrudes
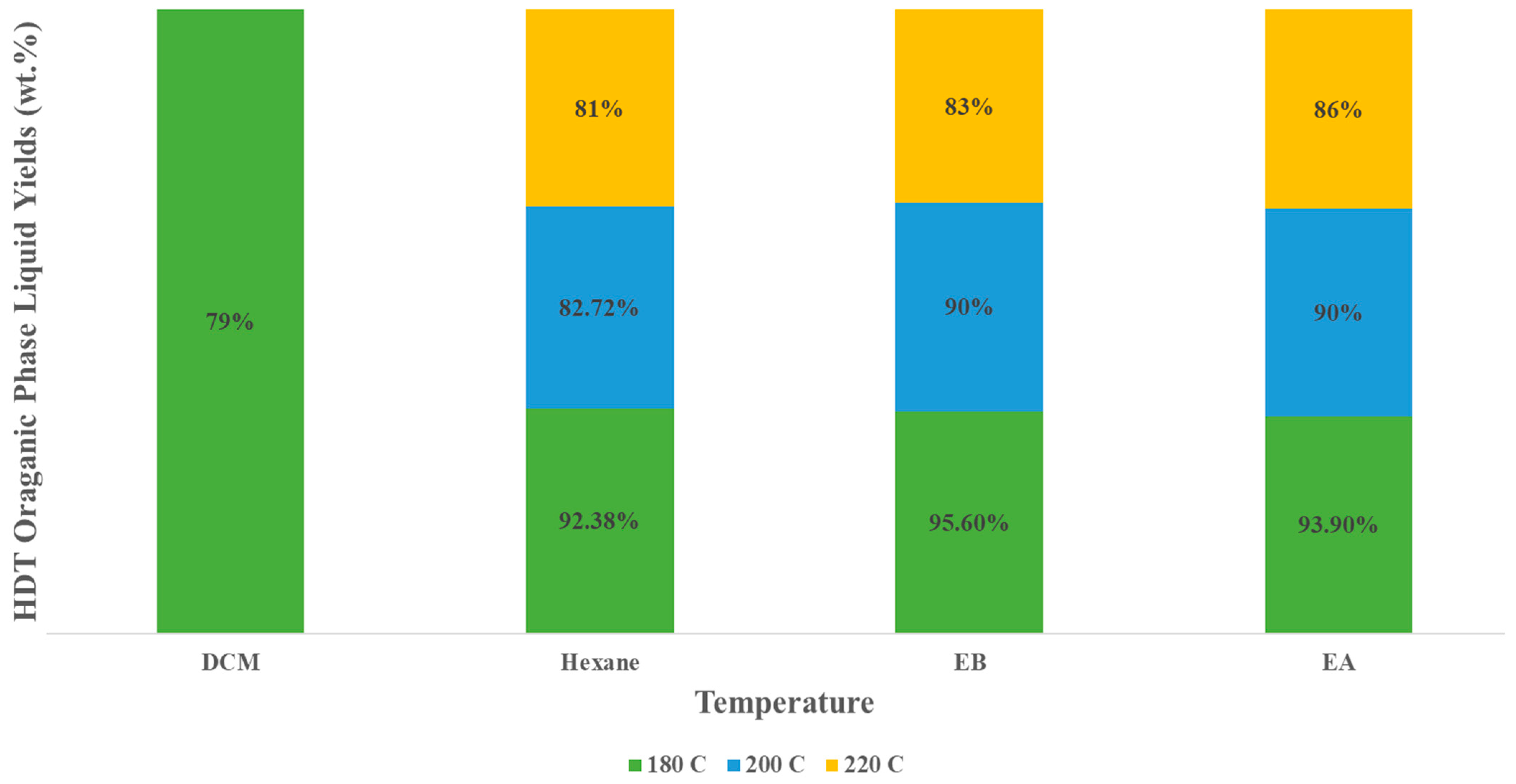
3.3. Hydrotreated Liquid Product Yield and Elemental Analysis
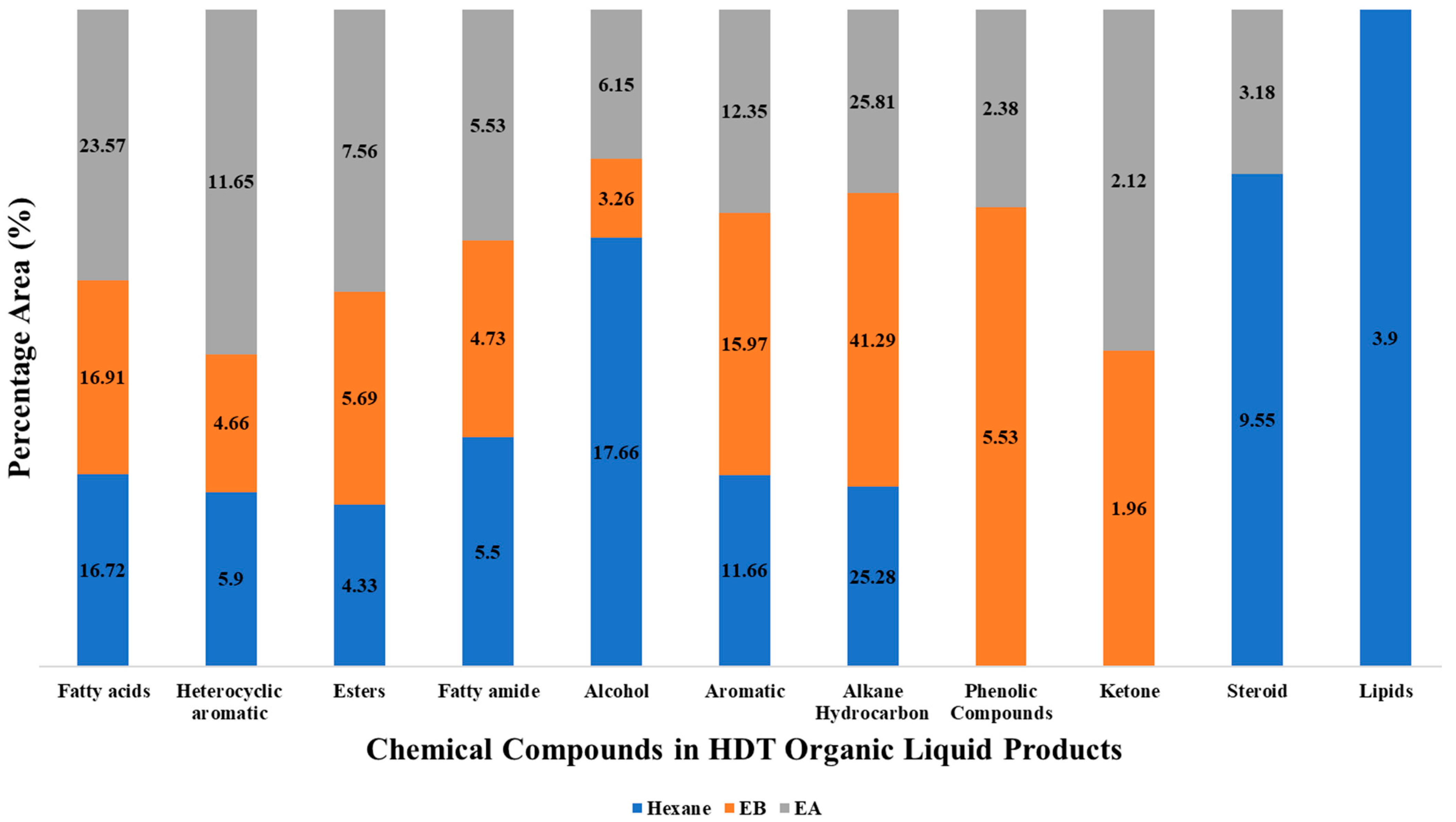
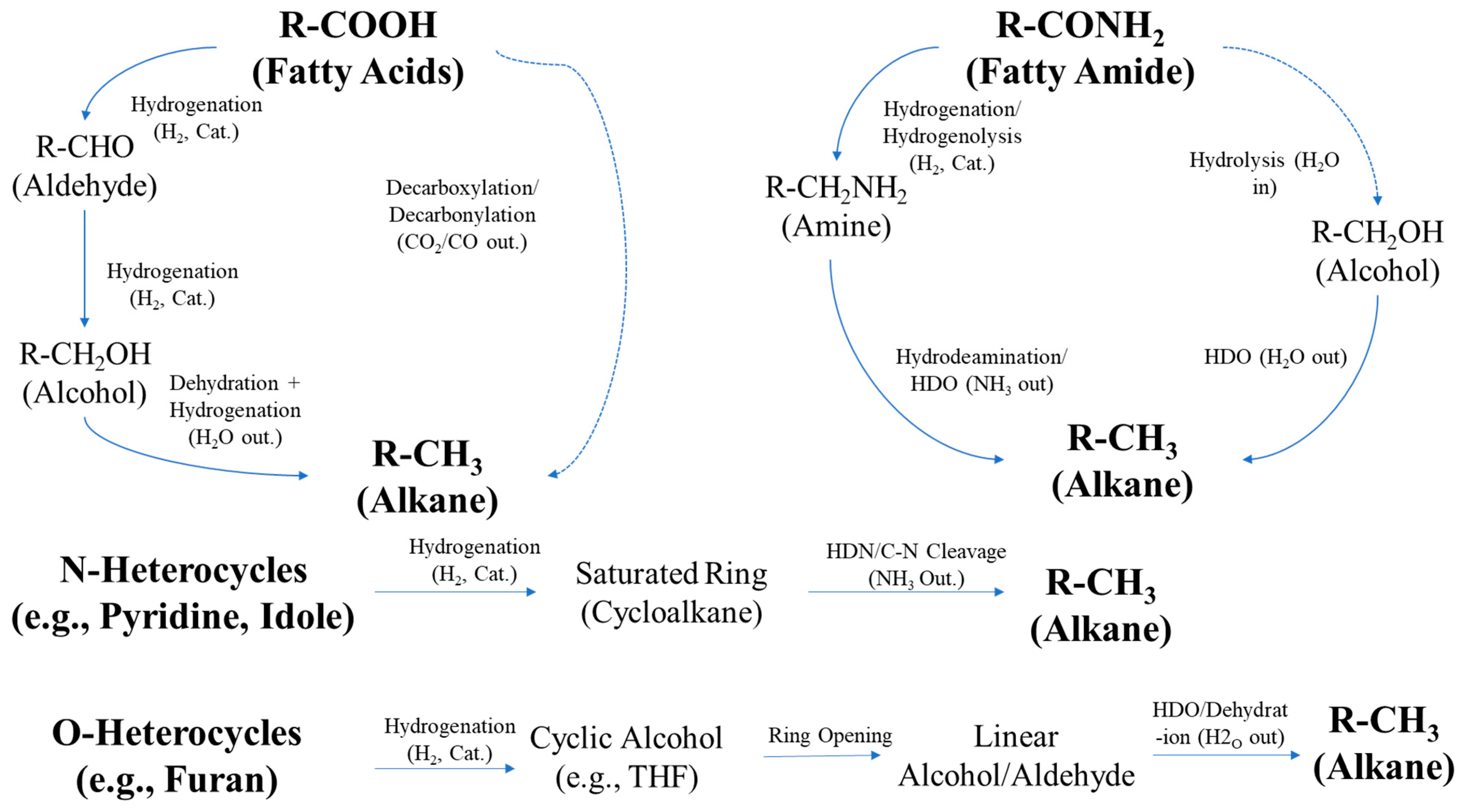
Chemical Compound Compositions in HDT Organic Liquid Product
4. Techno-Economic Comparison
5. Limitations and Future Research Direction
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quan, C.; Kamran, K.; Williams, P.T. Thermochemical conversion of sewage sludge: A critical review. Prog. Energy Combust. Sci. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Semblante, G.U.; Hai, F.I.; Bustamante, H.; Price, W.E.; Nghiem, L.D. Effects of sludge retention time on oxic-settling-anoxic process performance: Biosolids reduction and dewatering properties. Bioresour. Technol. 2016, 218, 1187–1194. [Google Scholar] [CrossRef]
- Seiple, T.E.; Coleman, A.M.; Skaggs, R.L. Municipal wastewater sludge as a sustainable bioresource in the United States. J. Environ. Manag. 2017, 197, 673–680. [Google Scholar] [CrossRef]
- Syed-Hassan, S.S.A.; Wang, Y.; Hu, S.; Su, S.; Xiang, J. Thermochemical processing of sewage sludge to energy and fuel: Fundamentals, challenges and considerations. Renew. Sustain. Energy Rev. 2017, 80, 888–913. [Google Scholar] [CrossRef]
- Mahzoun, Y. Heating Value and Energy Recovery Potential of Sewage Sludge and Suspended Solids in Municipal Wastewater Treatment Plant. Ph.D. Thesis, Kyoto University, Kyoto, Japan, 2018. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Frattarola, A.; Miino, M.C.; Padovani, S.; Katsoyiannis, I.A.; Torretta, V. Legislation for the reuse of biosolids on agricultural land in Europe: Overview. Sustainability 2019, 11, 6015. [Google Scholar] [CrossRef]
- EEA, Bio-Waste in Europe—Turning Challenges Into Opportunities, European Environment Agency. 2023. Available online: https://www.eea.europa.eu/publications/bio-waste-in-europe (accessed on 12 October 2024).
- Thomsen, L.B.S.; Carvalho, P.N.; Passos, J.S.D.; Anastasakis, K.; Bester, K.; Biller, P. Hydrothermal liquefaction of sewage sludge; energy considerations and fate of micropollutants during pilot scale processing. Water Res. 2020, 183, 116101. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, B.; Zhu, L.; Li, R.; Zhang, J.; Shi, X.; Li, X. Hydrothermal treatment and biorefinery of sewage sludge for waste reduction and production of fungal hyphae fibers and volatile fatty acids. J. Clean. Prod. 2021, 289, 125715. [Google Scholar] [CrossRef]
- Ferrentino, R.; Langone, M.; Andreottola, G. Progress toward full scale application of the anaerobic side-stream reactor (ASSR) process. Bioresour. Technol. 2019, 272, 267–274. [Google Scholar] [CrossRef]
- Elalami, D.; Carrere, H.; Monlau, F.; Abdelouahdi, K.; Oukarroum, A.; Barakat, A. Pretreatment and co-digestion of wastewater sludge for biogas production: Recent research advances and trends. Renew. Sustain. Energy Rev. 2019, 114, 109287. [Google Scholar] [CrossRef]
- Nguyễn, V.K.; Chaudhary, D.K.; Dahal, R.H.; Trinh, N.H.; Kim, J.; Chang, S.W.; Hong, Y.; La, D.D.; Nguyen, X.C.; Ngo, H.H.; et al. Review on pretreatment techniques to improve anaerobic digestion of sewage sludge. Fuel 2021, 285, 119105. [Google Scholar] [CrossRef]
- United Nations World Water Assessment Programme. Wastewater: The Untapped Resource—The United Nations World Water Development Report 2017; United Nations Educational, Scientific and Cultural Organization (UNESCO), on Behalf of the United Nations World Water Assessment Programme (WWAP). 2015. Available online: https://wedocs.unep.org/20.500.11822/20448 (accessed on 25 October 2023).
- MLIT. Ministry of Land, Infrastructure, Transport and Tourism. Systematization of Resource/Energy Recycling. MLIT. 2023. Available online: https://www.mlit.go.jp/en/mizukokudo/sewerage/index_e.html (accessed on 25 October 2023).
- Usman, M.; Cheng, S.; Cross, J.S. Biomass Feedstocks for Liquid Biofuels Production in Hawaii & Tropical Islands: A Review. Int. J. Renew. Energy Dev. 2021, 11, 111–132. [Google Scholar] [CrossRef]
- IEA. Transport–Topics—IEA. International Energy Agency. IEA. 2021. Available online: https://www.iea.org/topics/transport#:~:text=Transport%20has%20the%20highest%20reliance,of%20alternative%20fuels%20remains%20limited (accessed on 18 May 2024).
- Babayigit, E.; Alper, D.A.; Erdinçler, A. Direct Liquid–Liquid Lipid Extraction Method for Biodiesel Production from Sewage and Petrochemical Industry Sludges. Waste Biomass Valorization 2018, 9, 2471–2479. [Google Scholar] [CrossRef]
- Usman, M. Production of Biodiesel from Wastewater Sludge Treatment by Direct Lipids Extraction. J. Fundam. Renew. Energy Appl. 2018, 8, 1000261. [Google Scholar] [CrossRef]
- Gil, A.; Siles, J.; Martín, M.; Chica, A.; Estévez-Pastor, F.; Toro-Baptista, E. Effect of microwave pretreatment on semi-continuous anaerobic digestion of sewage sludge. Renew. Energy 2018, 115, 917–925. [Google Scholar] [CrossRef]
- Bora, A.P.; Gupta, D.P.; Durbha, K.S. Sewage sludge to bio-fuel: A review on the sustainable approach of transforming sewage waste to alternative fuel. Fuel 2020, 259, 116262. [Google Scholar] [CrossRef]
- Jahromi, H.; Adhikari, S.; Roy, P.; Hassani, E.; Pope, C.; Oh, T.-S.; Karki, Y. Production of green transportation fuels from Brassica carinata oil: A comparative study of noble and transition metal catalysts. Fuel Process. Technol. 2021, 215, 106737. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Yu, J.; Zhu, Z.; Guo, X.; Chen, G.; Pedersen, T.H.; Rosendahl, L.; Yu, X.; Wang, H. Catalytic hydrothermal liquefaction of sewage sludge over alumina-based and attapulgite-based heterogeneous catalysts. Fuel 2022, 323, 124329. [Google Scholar] [CrossRef]
- Ghodke, P.K.; Sharma, A.K.; Pandey, J.; Chen, W.; Patel, A.; Ashokkumar, V. Pyrolysis of sewage sludge for sustainable biofuels and value-added biochar production. J. Environ. Manag. 2021, 298, 113450. [Google Scholar] [CrossRef]
- Sahoo, A.K.; Saini, K.; Jindal, M.; Bhaskar, T.; Pant, K.K. Co-Hydrothermal Liquefaction of algal and lignocellulosic biomass: Status and perspectives. Bioresour. Technol. 2021, 342, 125948. [Google Scholar] [CrossRef]
- Usman, M.; Cheng, S.; Boonyubol, S.; Cross, J.S. The future of aviation soars with HTL-based SAFs: Exploring potential and overcoming challenges using organic wet feedstocks. Sustain. Energy Fuels 2023, 7, 4066–4087. [Google Scholar] [CrossRef]
- Usman, M.; Cheng, S.; Boonyubol, S.; Cross, J.S. From biomass to biocrude: Innovations in hydrothermal liquefaction and upgrading. Energy Convers. Manag. 2024, 302, 118093. [Google Scholar] [CrossRef]
- Watson, J.; Lu, J.; De Souza, R.; Si, B.; Zhang, Y.; Liu, Z. Effects of the extraction solvents in hydrothermal liquefaction processes: Biocrude oil quality and energy conversion efficiency. Energy 2018, 167, 189–197. [Google Scholar] [CrossRef]
- Jahromi, H.; Rahman, T.; Roy, P.; Adhikari, S. Hydrotreatment of solvent-extracted biocrude from hydrothermal liquefaction of municipal sewage sludge. Energy Convers. Manag. 2022, 263, 115719. [Google Scholar] [CrossRef]
- Rahman, T.; Jahromi, H.; Roy, P.; Adhikari, S.; Hassani, E.; Oh, T.-S. Hydrothermal liquefaction of municipal sewage sludge: Effect of red mud catalyst in ethylene and inert ambiences. Energy Convers. Manag. 2021, 245, 114615. [Google Scholar] [CrossRef]
- Kilgore, U.; Diaz, E.; Spry, B.; Jiang, Y.; Li, S.; Schmidt, A.; Thorson, M.R. Solvent processing for improved separation of hydrothermal liquefaction products. Sustain. Energy Fuels 2024, 8, 3279–3289. [Google Scholar] [CrossRef]
- Usman, M.; Cheng, S.; Boonyubol, S.; Cross, J.S. Evaluating Green Solvents for Bio-Oil Extraction: Advancements, challenges, and future perspectives. Energies 2023, 16, 5852. [Google Scholar] [CrossRef]
- De Jesus, S.S.; Filho, R.M. Recent advances in lipid extraction using green solvents. Renew. Sustain. Energy Rev. 2020, 133, 110289. [Google Scholar] [CrossRef]
- Janicka, P.; Płotka-Wasylka, J.; Jatkowska, N.; Chabowska, A.; Fares, M.Y.; Andruch, V.; Kaykhaii, M.; Gębicki, J. Trends in the new generation of green solvents in extraction processes. Curr. Opin. Green Sustain. Chem. 2022, 37, 100670. [Google Scholar] [CrossRef]
- Usman, M.; Cheng, S.; Boonyubol, S.; Cross, J.S. Nitrogen Minimization in Hydrothermal Liquefaction Biocrude from Sewage Sludge with Green Extraction Solvents. ACS Omega 2024, 9, 14530–14538. [Google Scholar] [CrossRef]
- Usman, M. Techno-Economic and Emissions Comparison of Waste-to-Fuel via Hydrothermal Liquefaction, Transesterification, and Incineration. J. Econ. Technol. 2024, 3, 237–250. [Google Scholar] [CrossRef]
- Oh, S.; Hwang, H.; Choi, H.S.; Choi, J.W. The effects of noble metal catalysts on the bio-oil quality during the hydrodeoxygenative upgrading process. Fuel 2015, 153, 535–543. [Google Scholar] [CrossRef]
- Lee, E.H.; Park, R.-S.; Kim, H.; Park, S.; Jung, S.; Jeon, J.; Kim, S.C.; Park, Y.-K. Hydrodeoxygenation of guaiacol over Pt loaded zeolitic materials. J. Ind. Eng. Chem. 2016, 37, 18–21. [Google Scholar] [CrossRef]
- Jahromi, H.; Agblevor, F.A. Upgrading of pinyon-juniper catalytic pyrolysis oil via hydrodeoxygenation. Energy 2017, 141, 2186–2195. [Google Scholar] [CrossRef]
- Niaounakis, M. Treatments and Uses. In Management of Marine Plastic Debris; Elsevier: Amsterdam, The Netherlands, 2017; pp. 215–315. [Google Scholar] [CrossRef]
- Fan, Y.; Hornung, U.; Dahmen, N. Hydrothermal liquefaction of sewage sludge for biofuel application: A review on fundamentals, current challenges and strategies. Biomass Bioenergy 2022, 165, 106570. [Google Scholar] [CrossRef]
- Li, B.; Song, H.; Yang, T.; Liu, E.; Li, R. Hydrothermal liquefaction of sewage sludge and model compound: Heavy metals distribution and behaviors. J. Anal. Appl. Pyrolysis 2023, 169, 105800. [Google Scholar] [CrossRef]
- Xu, D.; Lin, G.; Liu, L.; Wang, Y.; Jing, Z.; Wang, S. Comprehensive evaluation on product characteristics of fast hydrothermal liquefaction of sewage sludge at different temperatures. Energy 2018, 159, 686–695. [Google Scholar] [CrossRef]
- Villaver, W.S.; Carpio, R.B.; Yap, K.J.R.; De Leon, R.L. Effects of temperature and reaction time on yield and properties of biocrude oil produced by hydrothermal liquefaction of Spirulina platensis. Int. J. Smart Grid Clean Energy 2018, 7, 32–41. [Google Scholar] [CrossRef]
- Shah, A.A.; Toor, S.; Conti, F.; Nielsen, A.H.; Rosendahl, L. Hydrothermal liquefaction of high ash containing sewage sludge at sub and supercritical conditions. Biomass Bioenergy 2020, 135, 105504. [Google Scholar] [CrossRef]
- Seehar, T.H.; Toor, S.; Sharma, K.; Nielsen, A.H.; Pedersen, T.H.; Rosendahl, L. Influence of process conditions on hydrothermal liquefaction of eucalyptus biomass for biocrude production and investigation of the inorganics distribution. Sustain. Energy Fuels 2021, 5, 1477–1487. [Google Scholar] [CrossRef]
- De Souza Costa, P.; Mata, R.A.; Pinto, M.F.; Paradela, F.; Dutra, F.R. Hydrothermal liquefaction of microalgae for the production of biocrude and value-added chemicals. Chem. Eng. Trans. 2022, 94, 865–870. [Google Scholar] [CrossRef]
- Xue, Y.; Chen, H.; Zhao, W.; Yang, C.; Ma, P.; Han, S. A review on the operating conditions of producing bio-oil from hydrothermal liquefaction of biomass. Int. J. Energy Res. 2016, 40, 865–877. [Google Scholar] [CrossRef]
- Bayat, H.; Dehghanizadeh, M.; Jarvis, J.M.; Brewer, C.E.; Jena, U. Hydrothermal liquefaction of food waste: Effect of process parameters on product yields and chemistry. Front. Sustain. Food Syst. 2021, 5, 658592. [Google Scholar] [CrossRef]
- Cravotto, C.; Fabiano-Tixier, A.-S.; Claux, O.; Abert-Vian, M.; Tabasso, S.; Cravotto, G.; Chemat, F. Towards Substitution of Hexane as Extraction Solvent of Food Products and Ingredients with No Regrets. Foods 2022, 11, 3412. [Google Scholar] [CrossRef]
- Comandella, D.; Bignami, M.; Fürst, P.; Grob, K.; Mengelers, M.; Cascio, C.; Xiftou, K.; Croera, C.; Lambré, C. Technical Report on the Need for Re-Evaluation of the Safety of Hexane Used as an Extraction Solvent in the Production of Foodstuffs and Food Ingredients. EFSA Support. Publ. 2024, 21, 9001E. [Google Scholar] [CrossRef]
- Groß, P.; Ihmels, H. Studies about the Effect of Halogenated Solvents on the Fluorescence Properties of 9-Aryl-Substituted Isoquinolinium Derivatives—A Case Study. J. Fluoresc. 2024, 35, 2407–2414. [Google Scholar] [CrossRef]
- Carré, P.; Berthold, S.; Piofczyk, T.; Bothe, S.; Hadjiali, S. Solvent Solutions: Comparing Extraction Methods for Edible Oils and Proteins in a Changing Regulatory Landscape. Part 1: Physical-Properties. OCL 2024, 31, 12. [Google Scholar] [CrossRef]
- Badawy, T.; Williamson, J.; Xu, H. Laminar Burning Characteristics of Ethyl Propionate, Ethyl Butyrate, Ethyl Acetate, Gasoline and Ethanol Fuels. Fuel 2016, 183, 627–640. [Google Scholar] [CrossRef]
- Byrne, F.P.; Forier, B.; Bossaert, G.; Hoebers, C.; Farmer, T.J.; Hunt, A.J. A Methodical Selection Process for the Development of Ketones and Esters as Bio-Based Replacements for Traditional Hydrocarbon Solvents. Green Chem. 2018, 20, 4003–4011. [Google Scholar] [CrossRef]
- Usman, M.; Cheng, S.; Cross, J.S. Biodiesel production from urban and suburban municipal sewage sludges in Tokyo, Japan. In Proceedings of the International Conference and Utility Exhibition on Energy, Environment and Climate Change (ICUE 2022), Pattaya, Thailand, 26–28 October 2022. [Google Scholar] [CrossRef]
- Usman, M.; Cheng, S.; Cross, J.S. Biodiesel production from wet sewage sludge and reduced CO2 emissions compared to incineration in Tokyo, Japan. Fuel 2023, 341, 127614. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, R.; Yang, M.; Fang, L.; Wu, Y.; Wu, K.; Ya, L.; Gong, J. Catalytic hydrothermal liquefaction for bio-oil production over CNTs supported metal catalysts. Chem. Eng. Sci. 2017, 161, 299–307. [Google Scholar] [CrossRef]
- Wang, F.; Shen, H.; Liu, T.; Yang, X.; Yang, Y.; Guo, Y. Formation of pyrazines in Maillard model systems: Effects of structures of Lysine-Containing Dipeptides/Tripeptides. Foods 2021, 10, 273. [Google Scholar] [CrossRef]
- Kłosowski, G.; Mikulski, D.; Pielech-Przybylska, K. Pyrazines Biosynthesis by Bacillus Strains Isolated from Natto Fermented Soybean. Biomolecules 2021, 11, 1736. [Google Scholar] [CrossRef]
- Zhu, Z.; Toor, S.; Rosendahl, L.; Yu, D.; Chen, G. Influence of alkali catalyst on product yield and properties via hydrothermal liquefaction of barley straw. Energy 2015, 80, 284–292. [Google Scholar] [CrossRef]
- Luo, L.; Dai, L.; Savage, P.E. Catalytic hydrothermal liquefaction of soy protein concentrate. Energy Fuels 2015, 29, 3208–3214. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, Y.; Zhang, J.; Xia, S.; Hermanowicz, S.W. Effects of short-time aerobic digestion on extracellular polymeric substances and sludge features of waste activated sludge. Chem. Eng. J. 2016, 299, 177–183. [Google Scholar] [CrossRef]
- Nie, E.; Zheng, G.; Gao, D.; Chen, T.; Yang, J.; Wang, Y.; Wang, X. Emission characteristics of VOCs and potential ozone formation from a full-scale sewage sludge composting plant. Sci. Total Environ. 2019, 659, 664–672. [Google Scholar] [CrossRef]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gándara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef]
- Golubev, O.V.; Egazar’yants, S.V.; Matevosyan, D.V.; Naranov, E.R.; Maksimov, A.L.; Karakhanov, E.A. Development of Protective-Layer Catalysts for Removal of Chlorine Compounds from Diesel Fractions. Russ. J. Appl. Chem. 2018, 91, 2040–2045. [Google Scholar] [CrossRef]
- Mitra, S.; Sulakhe, S.; Shown, B.; Mandal, S.; Das, A. Organic chlorides in petroleum crude oil: Challenges for refinery and mitigations. ChemBioEng Rev. 2022, 9, 319–332. [Google Scholar] [CrossRef]
- Gandarias, I.; Arias, P.L. Hydrotreating catalytic processes for oxygen removal in the upgrading of Bio-Oils and Bio-Chemicals. In Liquid, Gaseous and Solid Biofuels—Conversion Techniques; InTech eBooks: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef]
- Rogers, K.; Zheng, Y. Selective Deoxygenation of Biomass-Derived Bio-oils within Hydrogen-Modest Environments: A Review and New Insights. ChemSusChem 2016, 9, 1750–1772. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Xu, Y.; Tang, Y. Temperature-Dependent hydrogenation, hydrodeoxygenation, and hydrogenolysis of anisole on nickel catalysts. Catalysts 2023, 13, 1418. [Google Scholar] [CrossRef]
- Mussa, N.-S.; Toshtay, K.; Capron, M. Catalytic Applications in the production of hydrotreated Vegetable Oil (HVO) as a renewable fuel: A review. Catalysts 2024, 14, 452. [Google Scholar] [CrossRef]
- CHEM, Libretexts, 1.7: 1.7-Chemical properties III- Catalytic Hydrogenation. Chemistry LibreTexts, 2017. Available online: https://chem.libretexts.org/Courses/Brevard_College/CHE_202%3A_Organic_Chemistry_II/01%3A_Aldehydes_and_Ketones/1.07%3A_1.7-Chemical_properties_III-_Catalytic_Hydrogenation#:~:text=The%20simplest%20large%2Dscale%20procedure,The%20product%20is%20an%20alcohol (accessed on 23 September 2024).
- Witsuthammakul, A.; Sooknoi, T. Selective hydrodeoxygenation of bio-oil derived products: Ketones to olefins. Catal. Sci. Technol. 2015, 5, 3639–3648. [Google Scholar] [CrossRef]
- He, Z.; Xu, H.; Zhou, C.; Xin, Z.; Liu, J.; Shen, B. A kinetic model for in situ coking denitrification of heavy oil with high nitrogen content based on starch using a structure-oriented lumping method. RSC Adv. 2018, 8, 32707–32718. [Google Scholar] [CrossRef]
- Hu, Y.; Li, J.; Wang, S.; Xu, L.; Barati, B.; Cao, B.; Wang, H.; Xie, K.; Wang, Q. Catalytic fast hydropyrolysis of seaweed biomass with different zeolite catalysts to produce high-grade bio-oil. Process Saf. Environ. Prot. 2021, 146, 69–76. [Google Scholar] [CrossRef]
| Proximate Analysis | |||||||
| SS Dewatered | Moisture | Volatiles | Ash | Fixed Carbon | |||
| (%) | wt% (dry basis) | ||||||
| 75 | 76 | 11.3 | 12.7 | ||||
| Ultimate Analysis | |||||||
| SS Dewatered | Ash | C | H | N | S | O a | HHV (MJ/kg) |
| wt% (dry basis) | |||||||
| 11.3 | 44.34 | 6.37 | 5.89 | 0.62 | 31.48 | 18.52 | |
| Solvent | Polarity Index | Hansen Solubility Parameters (MPa0.5) | Boiling Point (°C) | Density (g/mL) | Reference |
|---|---|---|---|---|---|
| Hexane | ~0.1–0.2 | δD: 14.9, δP: 0, δH: 0 | 68.7 | 0.654 | [49,50] |
| DCM | 3.1 | δD: 17.0, δP: 7.3, δH: 7.1 | 39.8 | 1.33 | [51,52] |
| EA | 4.4 | δD: 15.8, δP: 5.3, δH: 7.2 | 77 | 0.902 | [52,53] |
| EB | 3.1–3.7 | δD: 16.1, δP: 3.7, δH: 5.0–6.4 | 121 | 0.879 | [52,54] |
| Solvents | Temperature | Biocrude Yields | C | H | N | S | O a | HHV |
|---|---|---|---|---|---|---|---|---|
| (°C) | wt.% on Dry SS Basis | MJ/kg | ||||||
| DCM | 250 | 16.3 ± 0.82 | 18.01 ± 0.9 | 2.26 ± 0.11 | 1.3 ± 0.06 | 0.20 ± 0.01 | 11.03 ± 0.55 | 7.36 ± 0.37 |
| 300 | 33.2 ± 1.66 | 36.67 ± 1.83 | 4.61 ± 0.23 | 2.65 ± 0.13 | 0.39 ± 0.02 | 22.46 ± 1.12 | 14.99 ± 0.75 | |
| 350 | 49.7 ± 2.49 | 54.9 ± 2.74 | 6.9 ± 0.34 | 3.96 ± 0.2 | 0.53 ± 0.03 | 33.62 ± 1.68 | 22.44 ± 1.12 | |
| 375 | 34.2 ± 1.71 | 37.78 ± 1.89 | 4.75 ± 0.24 | 2.72 ± 0.14 | 0.35 ± 0.02 | 23.13 ± 1.16 | 15.44 ± 0.77 | |
| Hexane | 250 | 9.3 ± 0.47 | 31.66 ± 1.58 | 6.32 ± 0.32 | 0.12 ± 0.11 | NA | 13.28 ± 0.66 | 17.39 ± 0.87 |
| 300 | 13.5 ± 0.68 | 45.96 ± 2.3 | 9.17 ± 0.46 | 0.17 ± 0.01 | NA | 19.28 ± 0.96 | 25.24 ± 1.26 | |
| 350 | 18.1 ± 0.91 | 61.62 ± 3.08 | 12.3 ± 0.62 | 0.23 ± 0.01 | NA | 25.85 ± 1.29 | 33.84 ± 1.69 | |
| 375 | 14.2 ± 0.71 | 48.34 ± 2.42 | 9.65 ± 0.48 | 0.18 ± 0.01 | NA | 20.28 ± 1.01 | 26.55 ± 1.33 | |
| EB | 250 | 17.6 ± 0.88 | 20.68 ± 1.03 | 3.22 ± 0.16 | 0.11 ± 0.01 | NA | 11.1 ± 0.56 | 9.62 ± 0.48 |
| 300 | 34.5 ± 1.72 | 40.54 ± 2.03 | 6.32 ± 0.32 | 0.22 ± 0.01 | NA | 21.76 ± 1.09 | 18.85 ± 0.94 | |
| 350 | 50.1 ± 2.51 | 58.87 ± 2.94 | 9.18 ± 0.46 | 0.32 ± 0.02 | 0.03 ± 0.00 | 31.6 ± 1.58 | 27.38 ± 1.37 | |
| 375 | 35.1 ± 1.76 | 41.24 ± 2.06 | 6.43 ± 0.32 | 0.22 ± 0.01 | 0.02 ± 0.00 | 22.14 ± 1.11 | 19.18 ± 0.96 | |
| EA | 250 | 9.6 ± 0.48 | 19.33 ± 0.97 | 3.46 ± 0.17 | 0.09 ± 0.0 | NA | 17.96 ± 0.9 | 8.26 ± 0.41 |
| 300 | 16.4 ± 0.82 | 33.02 ± 1.65 | 5.91 ± 0.3 | 0.16 ± 0.01 | NA | 30.68 ± 1.53 | 14.12 ± 0.71 | |
| 350 | 23.5 ± 1.18 | 47.31 ± 2.37 | 8.47 ± 0.42 | 0.23 ± 0.01 | NA | 43.96 ± 2.2 | 20.23 ± 1.01 | |
| 375 | 21.2 ± 1.06 | 42.68 ± 2.13 | 7.64 ± 0.38 | 0.21 ± 0.01 | NA | 39.66 ± 1.98 | 18.25 ± 0.91 | |
| HDT of HTL Biocrude | DCM | Hexane | EB | EA | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 180 | 200 | 220 | 180 | 200 | 220 | 180 | 200 | 220 | 180 | 200 | 220 |
| Organic liquid yield (wt%) | 79 ± 0.35 | 0 | 0 | 90.38 ± 0.27 | 80.02 ± 0.17 | 79.6 ± 0.15 | 93.5 ± 0.07 | 90.2 ± 0.10 | 85.7 ± 0.10 | 91.2 ± 0.05 | 88.6 ± 0.10 | 85.2 ± 0.10 |
| Aqueous phase (wt%) | 0 | 0 | 0 | 2.0 ± 0.05 | 2.7 ± 0.05 | 3.1 ± 0.05 | 2.1 ± 0.05 | 2.9 ± 0.05 | 3.3 ± 0.05 | 2.3 ± 0.05 | 2.7 ± 0.05 | 3 ± 0.05 |
| Elemental Compositions (wt%) | ||||||||||||
| C | 56.1 ± 0.10 | - | - | 68.7 ± 0.10 | 70.87 ± 0.10 | 74.33 ± 0.10 | 65.3 ± 0.10 | 69.8 ± 0.10 | 72.18 ± 0.10 | 54.8 ± 0.10 | 57.75 ± 0.05 | 60.35 ± 0.05 |
| H | 7.3 ± 0.10 | - | - | 11.79 ± 0.05 | 13.92 ± 0.05 | 14.71 ± 0.10 | 10.94 ± 0.05 | 12.5 ± 0.05 | 13.91 ± 0.05 | 9.71 ± 0.01 | 10.2 ± 0.05 | 11.81 ± 0.05 |
| N | 3.93 ± 0.01 | - | - | 0.20 ± 0.01 | 0.17 ± 0.01 | 0.15 ± 0.01 | 0.28 ± 0.01 | 0.23 ± 0.01 | 0.21 ± 0.01 | 0.20 ± 0.01 | 0.19 ± 0.01 | 0.19 ± 0.01 |
| S | 0.50 ± 0.01 | - | - | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| O a | 32.17 ± 0.101 | - | - | 19.31 ± 0.05 | 15.04 ± 0.10 | 10.81 ± 0.05 | 23.48 ± 0.08 | 17.47 ± 0.05 | 13.7 ± 0.05 | 35.29 ± 0.08 | 31.86 ± 0.05 | 27.65 ± 0.05 |
| H/C (molar ratio) | 1.56 ± 0.01 | 2.05 ± 0.01 | 2.35 ± 0.01 | 2.37 ± 0.01 | 2.01 ± 0.05 | 2.14 ± 0.02 | 2.31 ± 0.01 | 2.12 ± 0.01 | 2.11 ± 0.01 | 2.34 ± 0.01 | ||
| N/C (molar ratio) | 0.06 ± 0.00 | 0.002 ± 0.0001 | 0.002 ± 0.0001 | 0.001 ± 0.0001 | 0.002 ± 0.0001 | 0.002 ± 0.0001 | 0.002 ± 0.0001 | 0.003 ± 0.0005 | 0.002 ± 0.0000 | 0.002 ± 0.0000 | ||
| HHV (MJ/kg) | 23.68 ± 0.03 | 36.66 ± 0.10 | 41.24 ± 0.05 | 44.31 ± 0.05 | 33.54 ± 0.05 | 38.39 ± 0.05 | 41.91 ± 0.05 | 26.1 ± 0.05 | 28.42 ± 0.05 | 32.38 ± 0.10 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usman, M.; Cheng, S.; Boonyubol, S.; Aziz, M.; Cross, J.S. Selective Extraction and Hydrotreatment of Biocrude from Sewage Sludge: Toward High-Yield, Alkane-Rich, Low-Heteroatom Biofuels. Energies 2025, 18, 4568. https://doi.org/10.3390/en18174568
Usman M, Cheng S, Boonyubol S, Aziz M, Cross JS. Selective Extraction and Hydrotreatment of Biocrude from Sewage Sludge: Toward High-Yield, Alkane-Rich, Low-Heteroatom Biofuels. Energies. 2025; 18(17):4568. https://doi.org/10.3390/en18174568
Chicago/Turabian StyleUsman, Muhammad, Shuo Cheng, Sasipa Boonyubol, Muhammad Aziz, and Jeffrey S. Cross. 2025. "Selective Extraction and Hydrotreatment of Biocrude from Sewage Sludge: Toward High-Yield, Alkane-Rich, Low-Heteroatom Biofuels" Energies 18, no. 17: 4568. https://doi.org/10.3390/en18174568
APA StyleUsman, M., Cheng, S., Boonyubol, S., Aziz, M., & Cross, J. S. (2025). Selective Extraction and Hydrotreatment of Biocrude from Sewage Sludge: Toward High-Yield, Alkane-Rich, Low-Heteroatom Biofuels. Energies, 18(17), 4568. https://doi.org/10.3390/en18174568










