Dynamic Comparative Assessment of Long-Term Simulation Strategies for an Off-Grid PV–AEM Electrolyzer System
Abstract
1. Introduction
2. Materials and Methods
2.1. Input Data
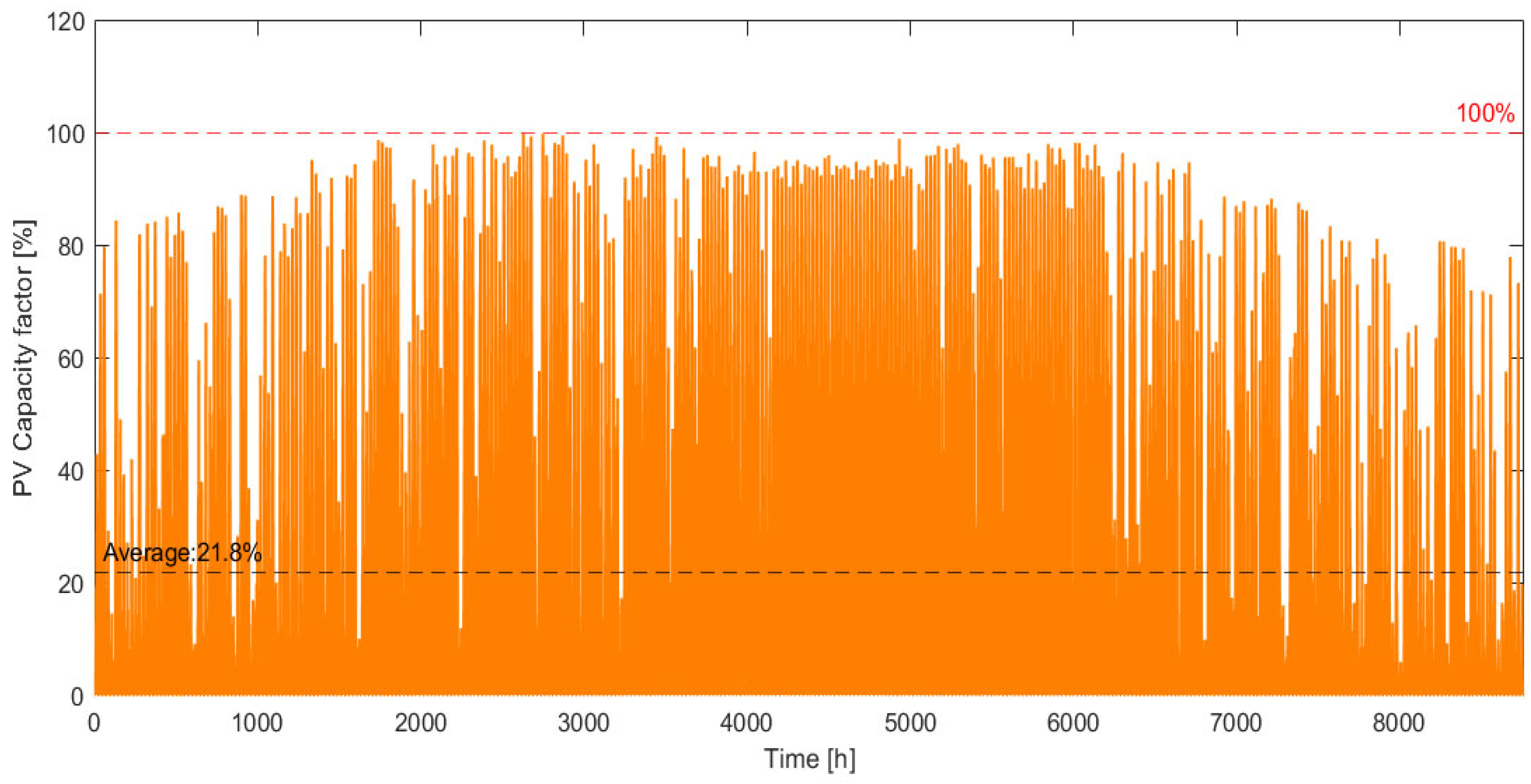
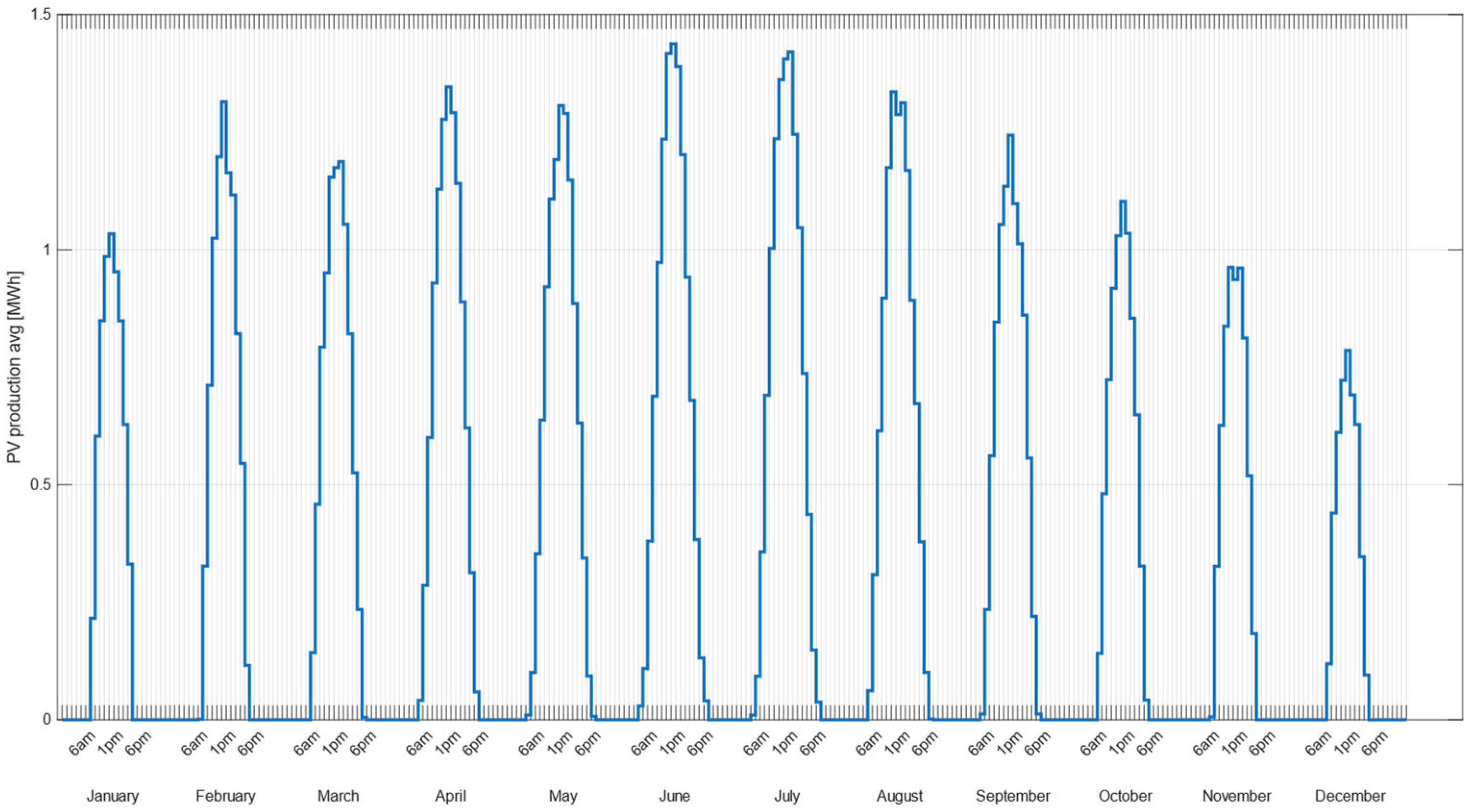
2.1.1. PV System
2.1.2. The DC/DC Converter
2.1.3. AEM Electrolyzer
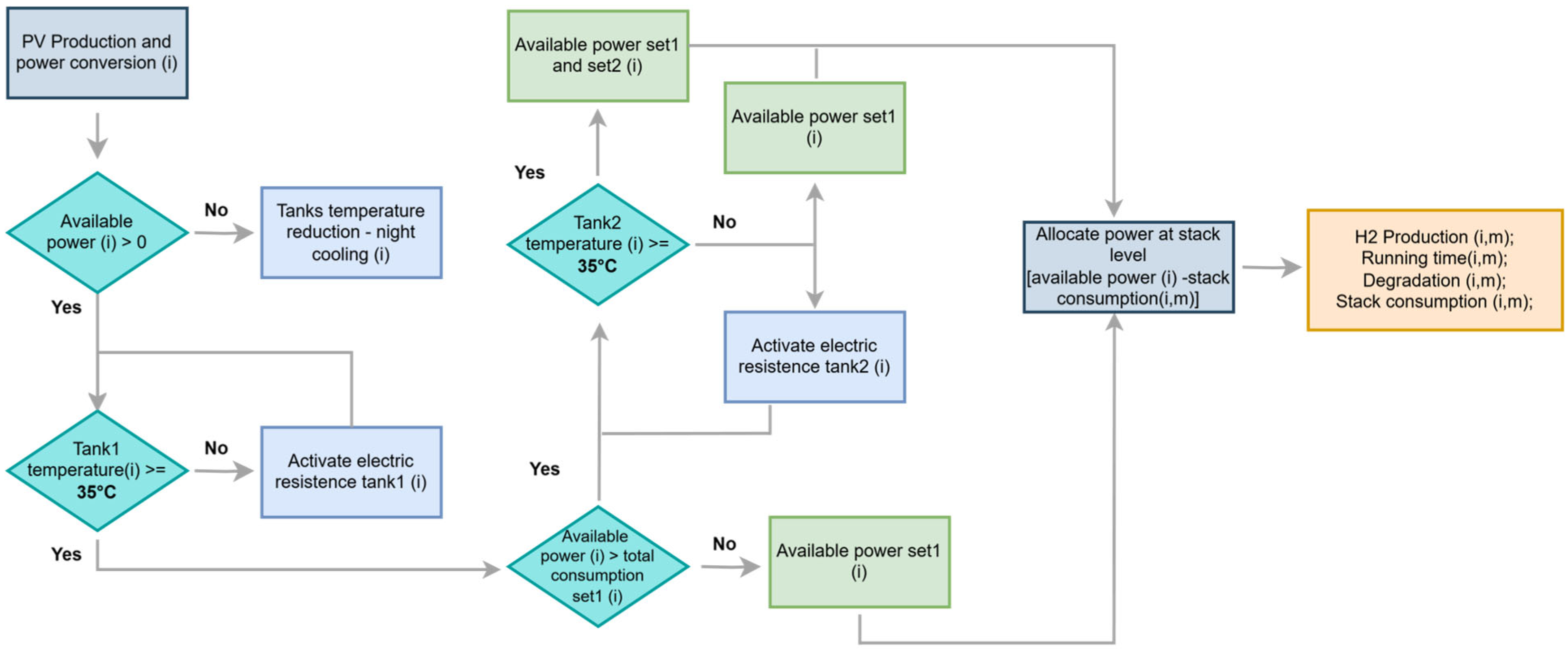
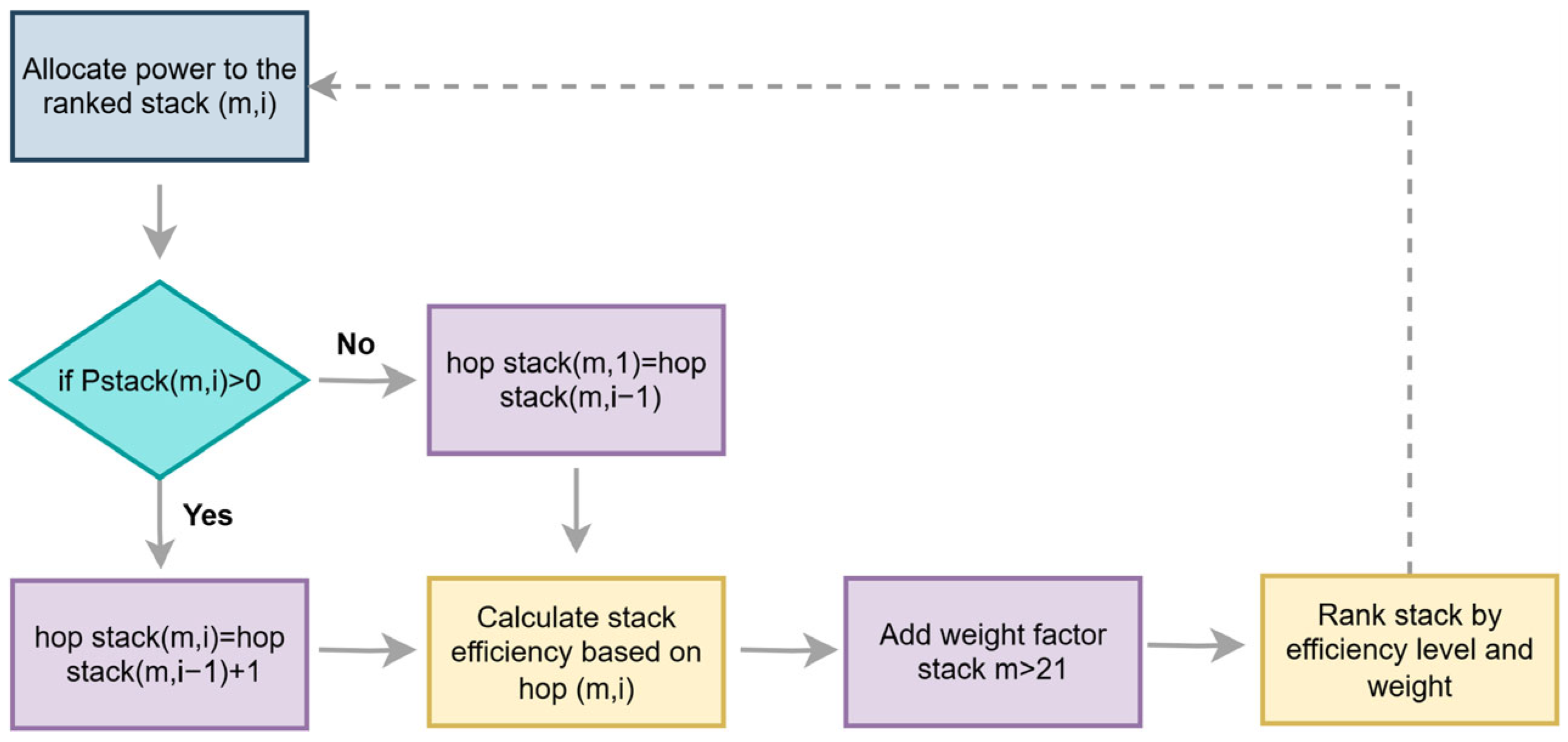
2.2. Dynamic Operation Under Variable Conditions
2.3. Simulation
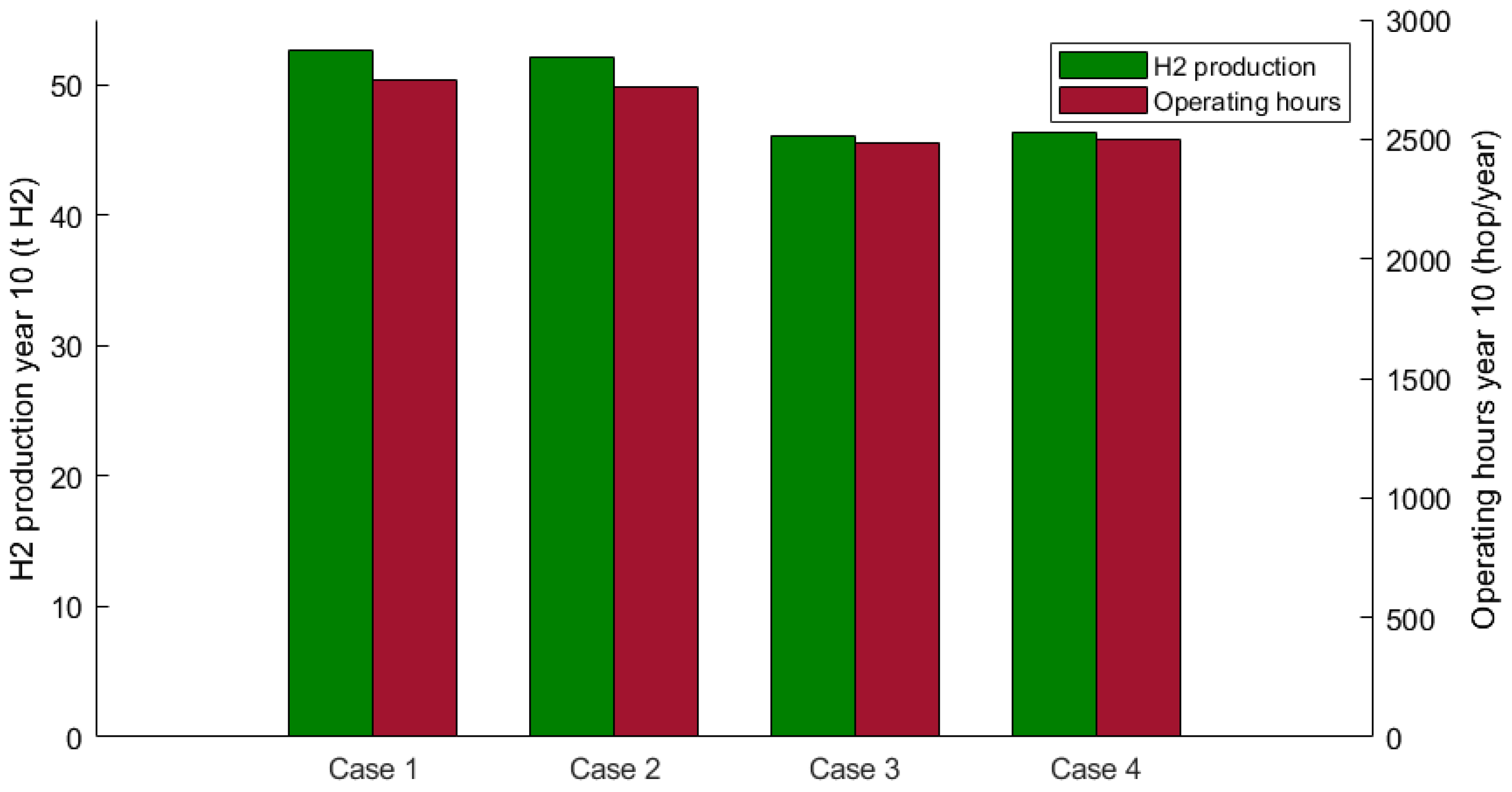
3. Results
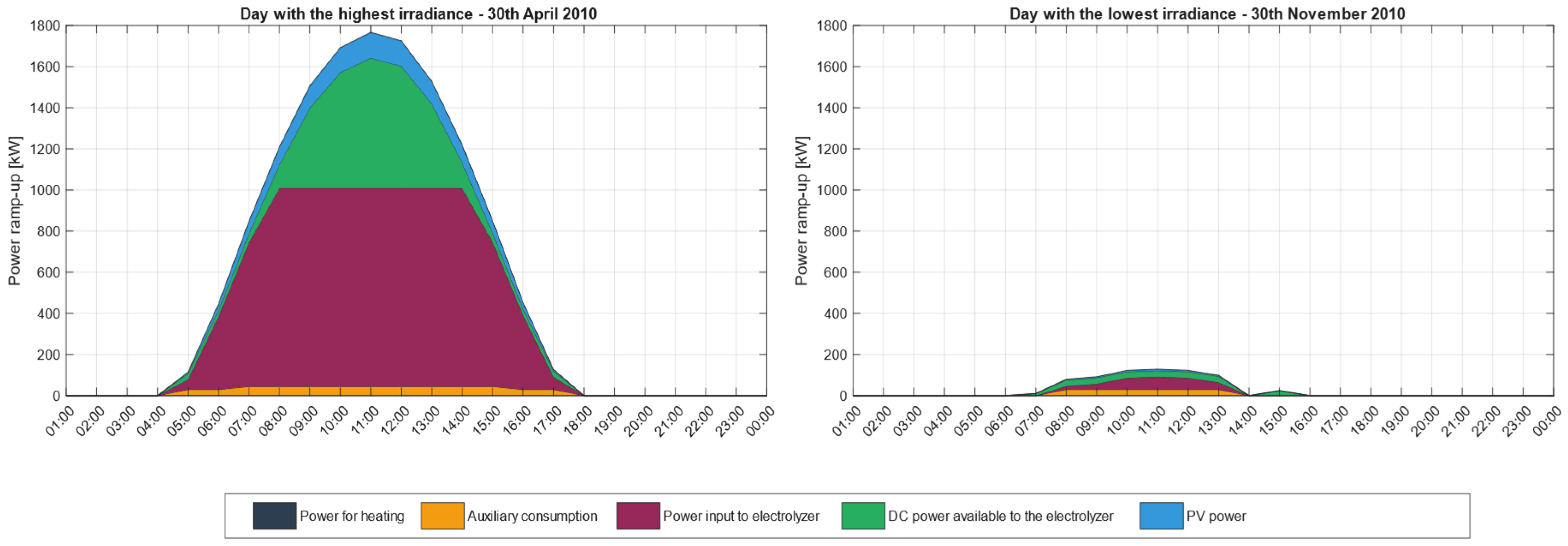
3.1. Electrical Power Distribution
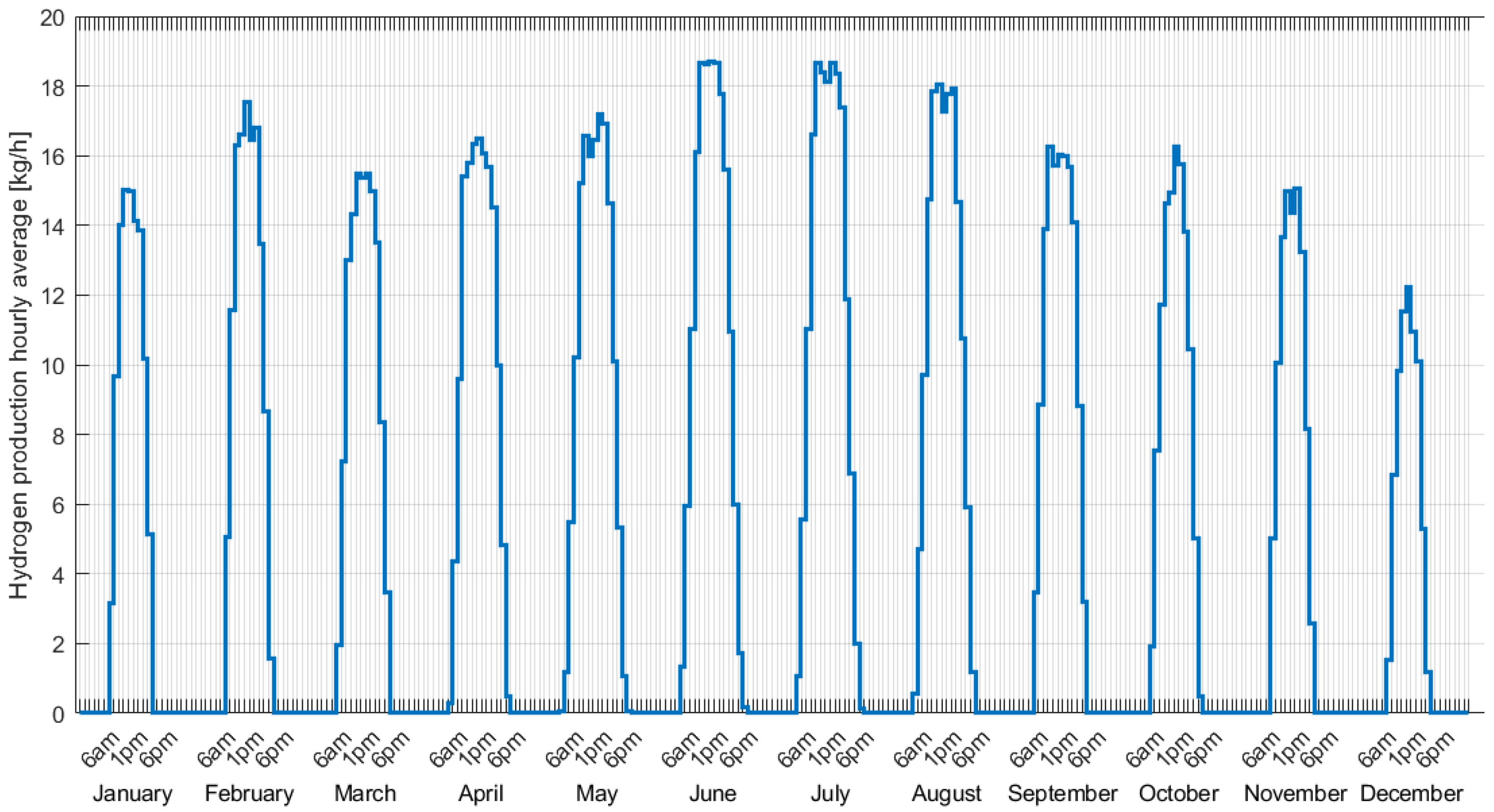
3.2. Hydrogen Production
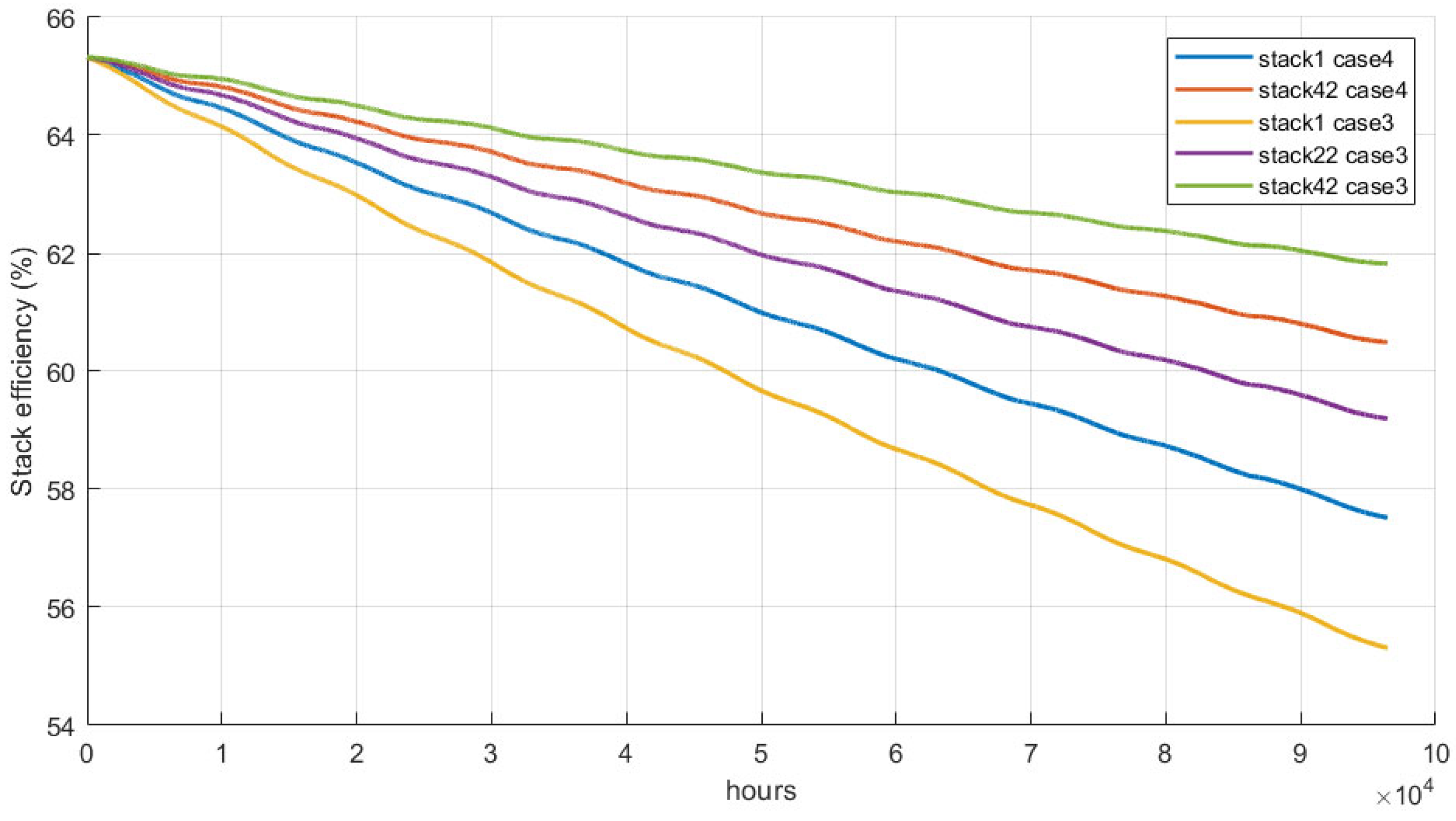
3.3. Stack Efficiency Comparison
3.4. Electrolyzer Operation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Efficiency, % | |
| A | Area, m2 |
| Global tilted irradiance, W/m2 | |
| H | Heat transfer coefficient, W/m2K |
| idx | Index |
| K | Conductivity, W/mK |
| L | Tank height, m |
| P | Power, kW |
| Q | Heat transfer, W |
| R | Thermal Resistance, Km2/W |
| T | Time, h |
| T | Temperature, K |
| U | Overall heat transfer coefficient, W/m2K |
| wt | Weight percent |
| Acronyms | |
| AEL | Alkaline Electrolysis |
| AEM | Anion Exchange Membrane Electrolysis |
| BoP | Balance of Plant |
| CF | Capacity Factor |
| DC | Direct Current |
| DF | Degradation Rate |
| EL | Electrolyzer |
| EU | European Union |
| GTI | Global Tilted Irradiance |
| H+ | Hydrogen Ions |
| HER | Hydrogen Evolution Reaction |
| HRS | Hydrogen Refuelling Station |
| I-V | Current-Voltage |
| KOH | Potassium Hydroxide |
| LHV | Lower Heating Value |
| MEA | Membrane Electrode Assembly |
| MPTT | Maximum Power Point Tracking |
| OER | Oxygen Evolution Reaction |
| OH− | Hydroxide Ion |
| PEMEL | Proton Exchange Membrane Electrolysis |
| PV | Photovoltaic |
| SMR | Steam Methane Reforming |
| TRL | Technology Readiness Level |
| Subscripts | |
| cond | Conduction |
| conv | Convection |
| el | Electrical |
| ext | External |
| int | Internal |
| nom | Nominal |
| sys | System |
| tot | Total |
References
- Wolf, E.; Fung, I.; Hoskins, B.; Mitchell, J.F.B.; Palmer, T.; Santer, B. Climate Change: Evidence & Causes. 2020. The Royal Society. Available online: https://www.nap.edu/catalog/18373 (accessed on 1 February 2024).
- Sarsar, L.; Echaoui, A. Empirical analysis of the economic complexity boost on the impact of energy transition on economic growth: A panel data study of 124 countries. Energy 2024, 294, 130712. [Google Scholar] [CrossRef]
- European Council. Fit for 55. European Green Deal. Available online: https://www.consilium.europa.eu/en/policies/green-deal/fit-for-55-the-eu-plan-for-a-green-transition/ (accessed on 1 February 2024).
- Farrell, N. Policy design for green hydrogen. Renew. Sustain. Energy Rev. 2023, 178, 113216. [Google Scholar] [CrossRef]
- European Hydrogen Observatory. Hydrogen Production. Available online: https://observatory.clean-hydrogen.europa.eu/hydrogen-landscape/production-trade-and-cost/hydrogen-production (accessed on 27 November 2024).
- IEA. World Energy Outlook; IEA: Paris, France, 2024. [Google Scholar]
- Aminov, R.Z.; Bairamov, A.N.; Filippov, S.P. Comprehensive assessment of the effectiveness of the hydrogen production and transportation system. Int. J. Hydrogen Energy 2024, 86, 1358–1375. [Google Scholar] [CrossRef]
- Hydrogen Europe. Clean Energy Monitor; Hydrogen Europe: Brussels, Belgium, 2024. [Google Scholar]
- Jia, J.; Seitz, L.C.; Benck, J.D.; Huo, Y.; Chen, Y.; Ng, J.W.D.; Bilir, T.; Harris, J.S.; Jaramillo, T.F. Solar water splitting by photovoltaic-electrolysis with a solar-to-hydrogen efficiency over 30%. Nat. Commun. 2016, 7, 13237. [Google Scholar] [CrossRef]
- Van, L.P.; Hoang, L.H.; Duc, T.N. A comprehensive review of direct coupled photovoltaic-electrolyser system: Sizing techniques, operating strategies, research progress, current challenges, and future recommendations. Int. J. Hydrogen Energy 2023, 48, 25231–25249. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, S.; Zhong, Y.; Sun, Z.; Peng, Y. A predictive control method for multi-electrolyzer off-grid hybrid hydrogen production systems with photovoltaic power prediction. Int. J. Hydrogen Energy 2024, 84, 383–393. [Google Scholar] [CrossRef]
- Khalilnejad, A.; Abbaspour, A.; Sarwat, A.I. Multi-level optimization approach for directly coupled photovoltaic-electrolyser system. Int. J. Hydrogen Energy 2016, 41, 11884–11894. [Google Scholar] [CrossRef]
- Laoun, B.; Khellaf, A.; Naceur, M.W.; Kannan, A.M. Modeling of solar photovoltaic-polymer electrolyte membrane electrolyzer direct coupling for hydrogen generation. Int. J. Hydrogen Energy 2016, 41, 10120–10135. [Google Scholar] [CrossRef]
- Gallardo, F.; García, J.; Ferrario, A.M.; Comodi, G.; Chiu, J.N. Assessing sizing optimality of OFF-GRID AC-linked solar PV-PEM systems for hydrogen production. Int. J. Hydrogen Energy 2022, 47, 27303–27325. [Google Scholar] [CrossRef]
- Şahin, M.E. A photovoltaic powered electrolysis converter system with maximum power point tracking control. Int. J. Hydrogen Energy 2020, 45, 9293–9304. [Google Scholar] [CrossRef]
- Benghanem, M.; Chettibi, N.; Mellit, A.; Almohamadi, H. Type-2 fuzzy-logic based control of photovoltaic-hydrogen production systems. Int. J. Hydrogen Energy 2023, 48, 35477–35492. [Google Scholar] [CrossRef]
- Mohammadi, A.; Mehrpooya, M. A comprehensive review on coupling different types of electrolyzer to renewable energy sources. Energy 2018, 158, 632–655. [Google Scholar] [CrossRef]
- Mallapragada, D.S.; Gençer, E.; Insinger, P.; Keith, D.W.; O’Sullivan, F.M. Can Industrial-Scale Solar Hydrogen Supplied from Commodity Technologies Be Cost Competitive by 2030? Cell Rep. Phys. Sci. 2020, 1, 100174. [Google Scholar] [CrossRef]
- Vostakola, M.F.; Ozcan, H.; El-Emam, R.S.; Horri, B.A. Recent Advances in High-Temperature Steam Electrolysis with Solid Oxide Electrolysers for Green Hydrogen Production. Energies 2023, 16, 3327. [Google Scholar] [CrossRef]
- IRENA. Green Hydrogen Cost Reduction; Scaling up Electrolysers to Meet the 1.5 C Climate Goal; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2020; Available online: www.irena.org/publications (accessed on 27 November 2024).
- Lopez, V.A.M.; Ziar, H.; Haverkort, J.W.; Zeman, M.; Isabella, O. Dynamic operation of water electrolyzers: A review for applications in photovoltaic systems integration. Renew. Sustain. Energy Rev. 2023, 182, 113407. [Google Scholar] [CrossRef]
- Lange, H.; Klose, A.; Lippmann, W.; Urbas, L. Technical evaluation of the flexibility of water electrolysis systems to increase energy flexibility: A review. Int. J. Hydrogen Energy 2023, 48, 15771–15783. [Google Scholar] [CrossRef]
- Abdelsalam, R.A.; Mohamed, M.; Farag, H.E.Z.; El-Saadany, E.F. Green hydrogen production plants: A techno-economic review. Energy Convers. Manag. 2024, 319, 118907. [Google Scholar] [CrossRef]
- Hu, S.; Guo, B.; Ding, S.; Yang, F.; Dang, J.; Liu, B.; Gu, J.; Ma, J.; Ouyang, M. A comprehensive review of alkaline water electrolysis mathematical modeling. Appl. Energy 2022, 327, 120099. [Google Scholar] [CrossRef]
- Aili, D.; Kraglund, M.R.; Rajappan, S.C.; Serhiichuk, D.; Xia, Y.; Deimede, V.; Kallitsis, J.; Bae, C.; Jannasch, P.; Henkensmeier, D.; et al. Electrode Separators for the Next-Generation Alkaline Water Electrolyzers. Am. Chem. Soc. 2023, 8, 1900–1910. [Google Scholar] [CrossRef]
- Xu, G.; Wu, Y.; Tang, S.; Wang, Y.; Yu, X.; Ma, M. Optimal design of hydrogen production processing coupling alkaline and proton exchange membrane electrolyzers. Energy 2024, 302, 131827. [Google Scholar] [CrossRef]
- Han, B.; Steen, S.M.; Mo, J.; Zhang, F.Y. Electrochemical performance modeling of a proton exchange membrane electrolyzer cell for hydrogen energy. Int. J. Hydrogen Energy 2015, 40, 7006–7016. [Google Scholar] [CrossRef]
- Hernández-Gómez, Á.; Ramirez, V.; Guilbert, D. Investigation of PEM electrolyzer modeling: Electrical domain, efficiency and specific energy consumption. Int. J. Hydrogen Energy 2020, 45, 14625–14639. [Google Scholar] [CrossRef]
- Patonia, A.; Poudineh, R. Cost-Competitive Green Hydrogen: How to Lower the Cost of Electrolysers? Oxford Institute for Energy Studies: Oxford, UK, 2022. [Google Scholar]
- Park, Y.S.; Liu, F.; Diercks, D.; Braaten, D.; Liu, B.; Duan, C. High-performance anion exchange membrane water electrolyzer enabled by highly active oxygen evolution reaction electrocatalysts: Synergistic effect of doping and heterostructure. Appl. Catal. B Environ. 2022, 318, 121824. [Google Scholar] [CrossRef]
- Vincent, I.; Bessarabov, D. Low cost hydrogen production by anion exchange membrane electrolysis: A review. Renew. Sustain. Energy Rev. 2018, 81, 1690–1704. [Google Scholar] [CrossRef]
- Zignani, S.C.; Faro, M.L.; Carbone, A.; Italiano, C.; Trocino, S.; Monforte, G.; Aricò, A. Performance and stability of a critical raw materials-free anion exchange membrane electrolysis cell. Electrochim. Acta 2022, 413, 140078. [Google Scholar] [CrossRef]
- Li, S.; Xin, Z.; Luo, Y.; Pan, J.; Liao, G.; Li, Q.; Sun, Y.; Feng, Z.; Tan, R. Recent advances in the development of single atom catalysts for oxygen evolution reaction. Int. J. Hydrogen Energy 2024, 82, 1081–1100. [Google Scholar] [CrossRef]
- Kim, J.H.; Jo, H.J.; Han, S.M.; Kim, Y.J.; Kim, S.Y. Recent advances in electrocatalysts for anion exchange membrane water electrolysis: Design strategies and characterization approaches. Energy Mater. 2025, 5, 500099. [Google Scholar] [CrossRef]
- Du, N.; Roy, C.; Peach, R.; Turnbull, M.; Thiele, S.; Bock, C. Anion-Exchange Membrane Water Electrolyzers. Am. Chem. Soc. 2022, 122, 11830–11895. [Google Scholar] [CrossRef]
- Titheridge, L.J.; Marshall, A.T. Techno-economic modelling of AEM electrolysis systems to identify ideal current density and aspects requiring further research. Int. J. Hydrogen Energy 2024, 49, 518–532. [Google Scholar] [CrossRef]
- Li, S.; Liu, T.; Zhang, W.; Wang, M.; Zhang, H.; Qin, C.; Zhang, L.; Chen, Y.; Jiang, S.; Liu, D.; et al. Highly efficient anion exchange membrane water electrolyzers via chromium-doped amorphous electrocatalysts. Nat. Commun. 2024, 15, 1–11. [Google Scholar] [CrossRef]
- Guo, D.; Chi, J.; Yu, H.; Jiang, G.; Shao, Z. Self-Supporting NiFe Layered Double Hydroxide ‘Nanoflower’ Cluster Anode Electrode for an Efficient Alkaline Anion Exchange Membrane Water Electrolyzer. Energies 2022, 15, 4645. [Google Scholar] [CrossRef]
- Silva, A.S.-D.; Hartert, A.; Oestreicher, V.; Romero, J.; Jaramillo-Hernández, C.; Muris, L.J.J.; Thorez, G.; Vieira, B.J.C.; Ducourthial, G.; Fiocco, A.; et al. Scalable synthesis of NiFe-layered double hydroxide for efficient anion exchange membrane electrolysis. Nat. Commun. 2025, 16, 6138. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Xu, H.M.; Huang, C.J.; Shuai, T.Y.; Zhan, Q.N.; Li, G.R. Recent advances in the synthesis of transition metal hydroxyl oxide catalysts and their application in electrocatalytic oxygen evolution reactions. Nanoscale 2024, 16, 19970–19997. [Google Scholar] [CrossRef] [PubMed]
- Falcão, D.S.; Pinto, A.M.F.R. A review on PEM electrolyzer modelling: Guidelines for beginners. J. Clean. Prod. 2020, 261, 121184. [Google Scholar] [CrossRef]
- Brezak, D.; Kovač, A.; Firak, M. MATLAB/Simulink simulation of low-pressure PEM electrolyzer stack. Int. J. Hydrogen Energy 2023, 48, 6158–6173. [Google Scholar] [CrossRef]
- Möller, M.C.; Krauter, S. Hybrid Energy System Model in Matlab/Simulink Based on Solar Energy, Lithium-Ion Battery and Hydrogen. Energies 2022, 15, 2201. [Google Scholar] [CrossRef]
- Tjarks, G.; Gibelhaus, A.; Lanzerath, F.; Müller, M.; Bardow, A.; Stolten, D. Energetically-optimal PEM electrolyzer pressure in power-to-gas plants. Appl. Energy 2018, 218, 192–198. [Google Scholar] [CrossRef]
- Toghyani, S.; Fakhradini, S.; Afshari, E.; Baniasadi, E.; Jamalabadi, M.Y.A.; Shadloo, M.S. Optimization of operating parameters of a polymer exchange membrane electrolyzer. Int. J. Hydrogen Energy 2019, 44, 6403–6414. [Google Scholar] [CrossRef]
- Ruuskanen, V.; Koponen, J.; Kosonen, A.; Hehemann, M.; Keller, R.; Niemelä, M.; Ahola, J. Power quality estimation of water electrolyzers based on current and voltage measurements. J. Power Sources 2020, 450, 227603. [Google Scholar] [CrossRef]
- IRENA. Geopolitics of the Energy Transformation: The Hydrogen Factor; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2022. [Google Scholar]
- Tuinema, B.W.; Adabi, E.; Ayivor, P.K.; Suárez, V.G.; Liu, L.; Perilla, A.; Ahmad, Z.; Torres, J.L.R.; van der Meijden, M.A.; Palensky, P. Modelling of large-sized electrolysers for realtime simulation and study of the possibility of frequency support by electrolysers. IET Gener. Transm. Distrib. 2020, 14, 1985–1992. [Google Scholar] [CrossRef]
- Xu, X.; Hu, W.; Liu, W.; Wang, D.; Huang, Q.; Huang, R.; Chen, Z. Risk-based scheduling of an off-grid hybrid electricity/hydrogen/gas/refueling station powered by renewable energy. J. Clean. Prod. 2021, 315, 128155. [Google Scholar] [CrossRef]
- Huang, C.; Zong, Y.; You, S.; Traholt, C. Analytical Modeling and Control of Grid-Scale Alkaline Electrolyzer Plant for Frequency Support in Wind-Dominated Electricity-Hydrogen Systems. IEEE Trans. Sustain. Energy 2023, 14, 217–232. [Google Scholar] [CrossRef]
- Matute, G.; Yusta, J.M.; Correas, L.C. Techno-economic modelling of water electrolysers in the range of several MW to provide grid services while generating hydrogen for different applications: A case study in Spain applied to mobility with FCEVs. Int. J. Hydrogen Energy 2019, 44, 17431–17442. [Google Scholar] [CrossRef]
- Stansberry, J.M.; Brouwer, J. Experimental dynamic dispatch of a 60 kW proton exchange membrane electrolyzer in power-to-gas application. Int. J. Hydrogen Energy 2020, 45, 9305–9316. [Google Scholar] [CrossRef]
- Wallnöfer-Ogris, E.; Brouwer, J. A review on understanding and identifying degradation mechanisms in PEM water electrolysis cells: Insights for stack application, development and research. Int. J. Hydrogen Energy 2024, 65, 381–397. [Google Scholar] [CrossRef]
- Batalla, B.S.; Laube, A.; Hofer, A.; Struckmann, T.; Bachmann, J.; Weidlich, C. Degradation studies of proton exchange membrane water electrolysis cells with low platinum group metals—Catalyst coating achieved by atomic layer deposition. Int. J. Hydrogen Energy 2022, 47, 39719–39730. [Google Scholar] [CrossRef]
- Höglinger, M.; Kartusch, S.; Eder, J.; Grabner, B.; Macherhammer, M.; Trattner, A. Advanced testing methods for proton exchange membrane electrolysis stacks. Int. J. Hydrogen Energy 2024, 77, 598–611. [Google Scholar] [CrossRef]
- MATLAB, Version 9.13 (R2022b); MathWorks, Inc.: Natick, MA, USA, 2022.
- Photovoltaic Geographical Information System (PVGIS). Version 5.2; 2017. Available online: https://re.jrc.ec.europa.eu/pvg_tools/en/#MR (accessed on 3 July 2024).
- Heterojunction Hyper-Ion Series Bifacial Module. 2024. Available online: https://en.risenenergy.com/ (accessed on 3 July 2024).
- Marocco, P.; Gandiglio, M.; Santarelli, M. Optimal design of PV-based grid-connected hydrogen production systems. J. Clean. Prod. 2024, 434, 140007. [Google Scholar] [CrossRef]
- Vizza, D.; Caponi, R.; Bocci, E.; Del Zotto, L.; Bassano, C. Cost effective hydrogen production of coupled photovoltaic and electrolyzer systems considering plant lifetime and geographical location. Energy Convers. Manag. X 2025, 27, 101136. [Google Scholar] [CrossRef]
- Lopez, V.A.M.; Ziar, H.; Zeman, M.; Isabella, O. Optimization of a stand-alone PV system for efficient hydrogen production using an alkaline water electrolyzer. In Proceedings of the 23rd World Hydrogen Energy Conference: Bridging Continents by H2, WHEC, Istanbul, Turkey, 26–30 June 2022; International Association for Hydrogen Energy, IAHE: Miami, FL, USA, 2022; pp. 1020–1022. [Google Scholar]
- EPC Bidirectional DC/DC Converters. 2024. Available online: https://epicpowerconverters.com/products/dc-dc-converters/ (accessed on 3 July 2024).
- Rakousky, C.; Reimer, U.; Wippermann, K.; Kuhri, S.; Carmo, M.; Lueke, W.; Stolten, D. Polymer electrolyte membrane water electrolysis: Restraining degradation in the presence of fluctuating power. J. Power Sources 2017, 342, 38–47. [Google Scholar] [CrossRef]
- Siracusano, S.; Trocino, S.; Briguglio, N.; Pantò, F.; Aricò, A.S. Analysis of performance degradation during steady-state and load-thermal cycles of proton exchange membrane water electrolysis cells. J. Power Sources 2020, 468, 228390. [Google Scholar] [CrossRef]
- Mulk, W.U.; Aziz, A.R.A.; Ismael, M.A.; Ghoto, A.A.; Ali, S.A.; Younas, M.; Gallucci, F. Electrochemical hydrogen production through anion exchange membrane water electrolysis (AEMWE): Recent progress and associated challenges in hydrogen production. Int. J. Hydrogen Energy 2024, 94, 1174–1211. [Google Scholar] [CrossRef]
- Azam, A.M.I.N.; Ragunathan, T.; Zulkefli, N.N.; Masdar, M.S.; Majlan, E.H.; Yunus, R.M.; Shamsul, N.S.; Husaini, T.; Shaffee, S.N.A. Investigation of Performance of Anion Exchange Membrane (AEM) Electrolysis with Different Operating Conditions. Polymers 2023, 15, 1301. [Google Scholar] [CrossRef]
- Bender, G.; Dinh, H.N. HydroGEN: Low-Temperature Electrolysis (LTE) and LTE/Hybrid Supernode; National Renewable Energy Laboratory (NREL): Golden, CO, USA, 2020. [Google Scholar]
- Kuhnert, E.; Mayer, K.; Heidinger, M.; Rienessel, C.; Hacker, V.; Bodner, M. Impact of intermittent operation on photovoltaic-PEM electrolyzer systems: A degradation study based on accelerated stress testing. Int. J. Hydrogen Energy 2024, 55, 683–695. [Google Scholar] [CrossRef]
- Campbell-Stanway, C.; Becerra, V.; Prabhu, S. Techno-economic analysis with electrolyser degradation modelling in green hydrogen production scenarios. Int. J. Hydrogen Energy 2025, 106, 80–95. [Google Scholar] [CrossRef]
- Gladik, A.; Riedel, M.; Eichel, R.A. Anion exchange membrane electrolysis at work—Investigating impact of starting parameters and start–stop operation on cold start behavior and degradation. J. Power Sources 2025, 628, 235878. [Google Scholar] [CrossRef]
- Bergman, T.L.; Incropera, F.P.; DeWitt, D.P.; Lavine, A.S. Fundamentals of Heat and Mass Transfer; John Wiley & Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Peet, M.J.; Hasan, H.S.; Bhadeshia, H.K.D.H. Prediction of thermal conductivity of steel. Int. J. Heat Mass Transf. 2011, 54, 2602–2608. [Google Scholar] [CrossRef]
- Yener, T.; Yener, Ş.Ç.; Mutlu, R. Convection Coefficient Estimation of Still Air Using an Infrared Thermometer and Curve-Fitting. J. Eng. Technol. Appl. Sci. 2019, 4, 95–103. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caponi, R.; Vizza, D.; Bassano, C.; Del Zotto, L.; Bocci, E. Dynamic Comparative Assessment of Long-Term Simulation Strategies for an Off-Grid PV–AEM Electrolyzer System. Energies 2025, 18, 4209. https://doi.org/10.3390/en18154209
Caponi R, Vizza D, Bassano C, Del Zotto L, Bocci E. Dynamic Comparative Assessment of Long-Term Simulation Strategies for an Off-Grid PV–AEM Electrolyzer System. Energies. 2025; 18(15):4209. https://doi.org/10.3390/en18154209
Chicago/Turabian StyleCaponi, Roberta, Domenico Vizza, Claudia Bassano, Luca Del Zotto, and Enrico Bocci. 2025. "Dynamic Comparative Assessment of Long-Term Simulation Strategies for an Off-Grid PV–AEM Electrolyzer System" Energies 18, no. 15: 4209. https://doi.org/10.3390/en18154209
APA StyleCaponi, R., Vizza, D., Bassano, C., Del Zotto, L., & Bocci, E. (2025). Dynamic Comparative Assessment of Long-Term Simulation Strategies for an Off-Grid PV–AEM Electrolyzer System. Energies, 18(15), 4209. https://doi.org/10.3390/en18154209






