Enhancing Energy Efficiency of Electric Grade Isopropyl Alcohol Production Process by Using Noble Thermally Coupled Distillation Technology
Abstract
1. Introduction
2. Methods
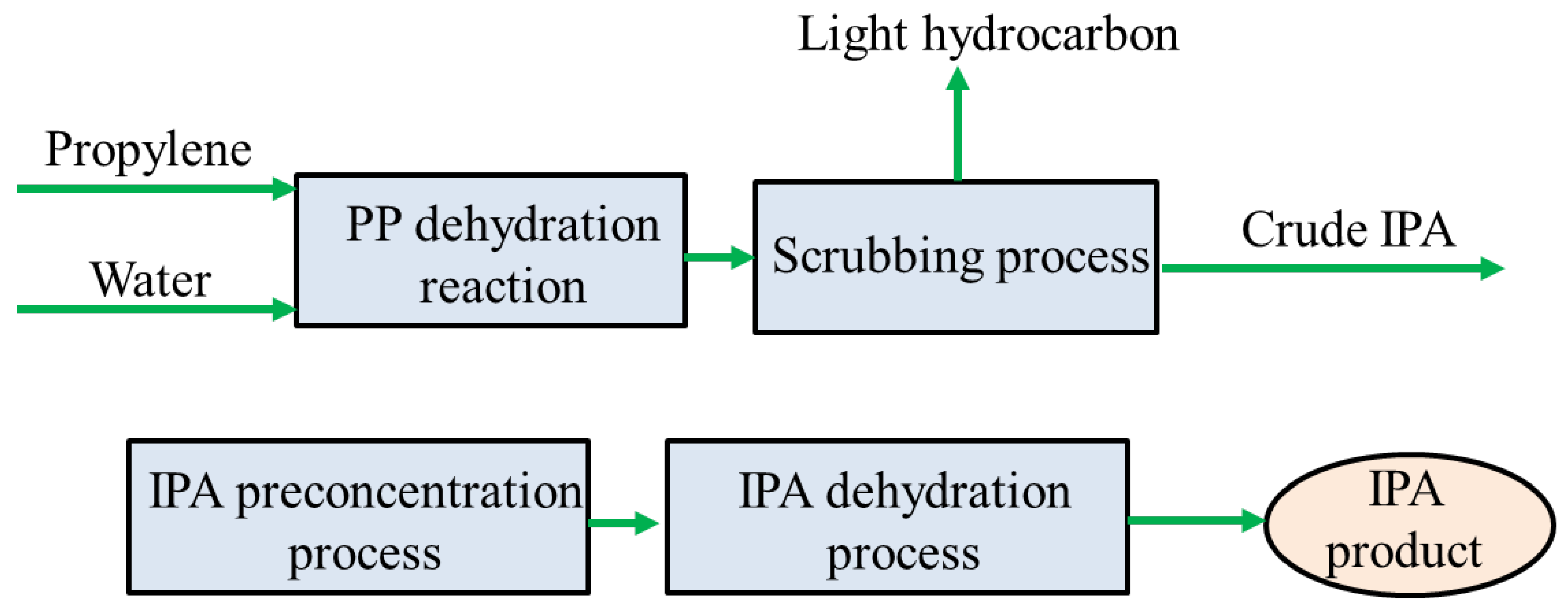
2.1. Design
2.2. Simulation
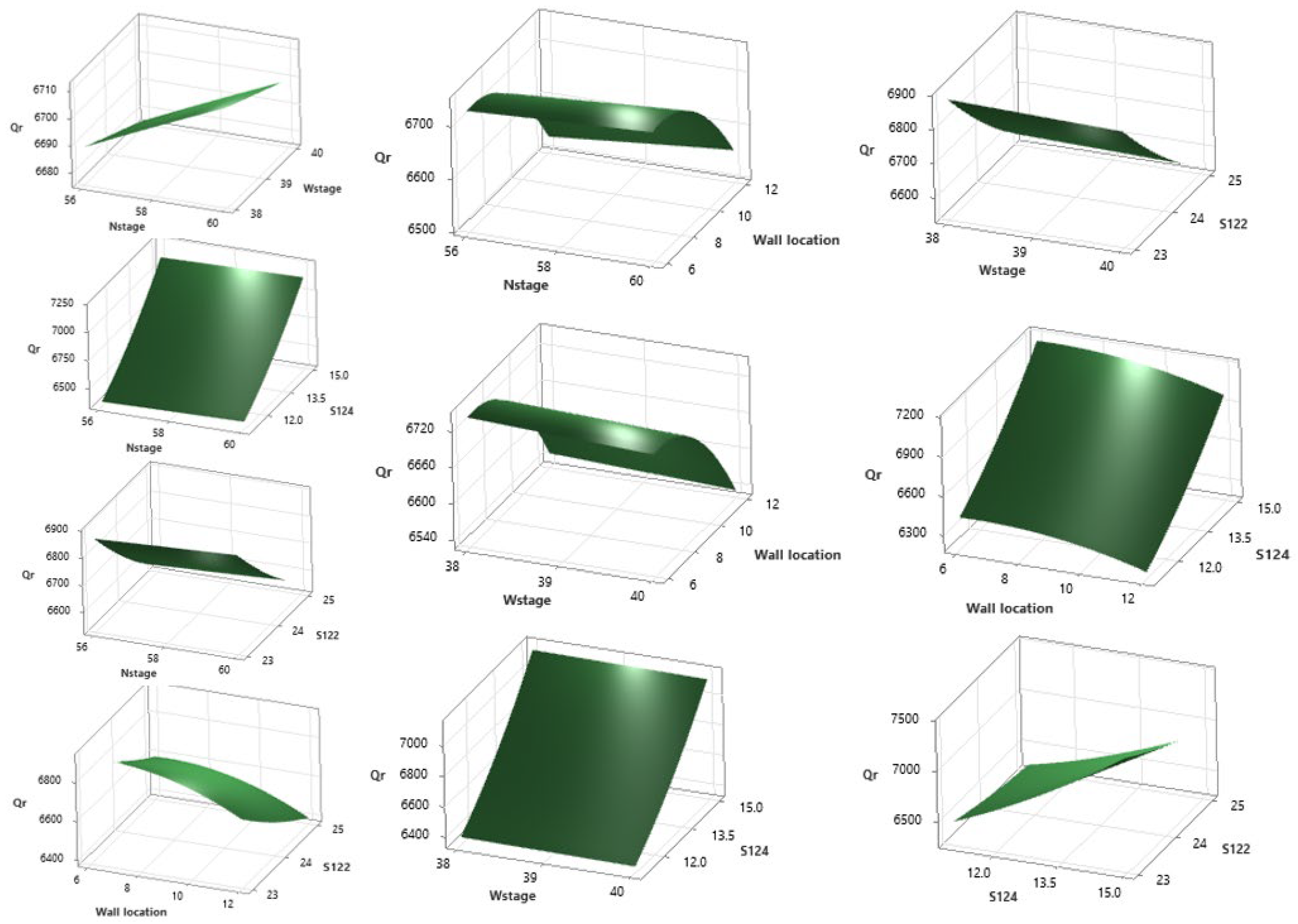
2.3. Optimization
3. Results and Discussions
3.1. Conventional Sequence: IPA Preconcentration + Extractive Distillation
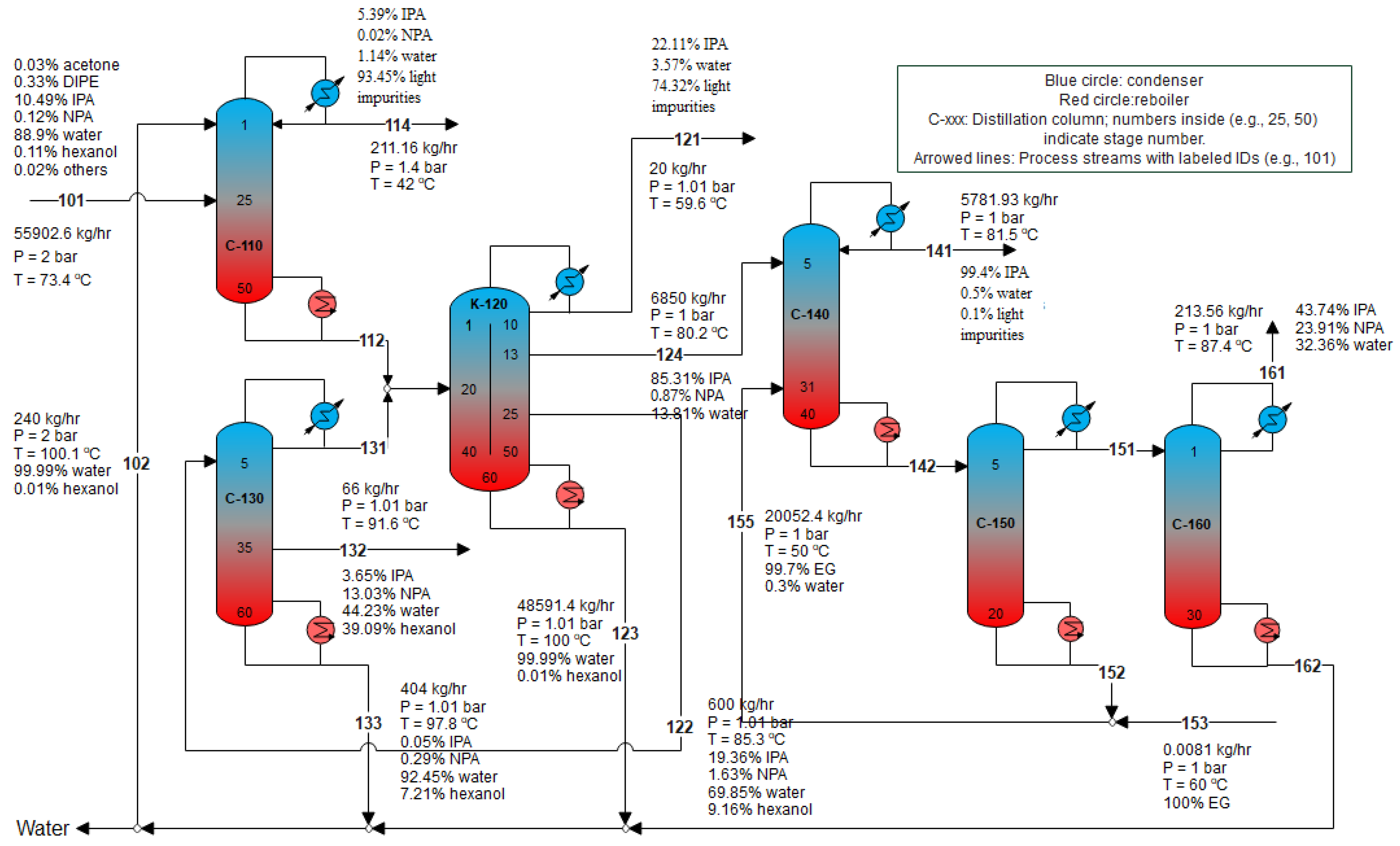
3.2. Intensifying the Side-Stream Column C-120 in the Preconcentration Process by a Kaibel Column K-120 (DWC-2 Case)
3.3. Replacing C-120 and C-130 in the Preconcentration Process by a Kaibel Column K-1230 (DWC-3 Case)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nhien, L.C.; Agarwal, N.; Lee, M. Dehydration of Isopropanol: A Comparative Review of Distillation Processes, Heat Integration, and Intensification Techniques. Energies 2023, 16, 5934. [Google Scholar] [CrossRef]
- Kittur, A.A.; Kulkarni, S.S.; Aralaguppi, M.I.; Kariduraganavar, M.Y. Preparation and Characterization of Novel Pervaporation Membranes for the Separation of Water–Isopropanol Mixtures Using Chitosan and NaY Zeolite. J. Memb. Sci. 2005, 247, 75–86. [Google Scholar] [CrossRef]
- Chua, W.J.; Rangaiah, G.P.; Hidajat, K. Design and Optimization of Isopropanol Process Based on Two Alternatives for Reactive Distillation. Chem. Eng. Process. Process Intensif. 2017, 118, 108–116. [Google Scholar] [CrossRef]
- Arifin, S.; Chien, I.L. Design and Control of Isopropyl Alcohol Dehydration via Homogeneous Azeotropic Distillation Using Dimethyl Sulfoxide as Extractive Agent. In Proceedings of the 2006 AIChE Annual Meeting, San Francisco, CA, USA, 12–17 November 2006. [Google Scholar]
- Zhai, J.; Chen, X.; Sun, X.; Xie, H. Economically and Thermodynamically Efficient Pressure-Swing Distillation with Heat Integration and Heat Pump Techniques. Appl. Therm. Eng. 2023, 218, 119389. [Google Scholar] [CrossRef]
- Chien, I.-L.; Chao, H.-Y.; Teng, Y.-P. Design and Control of a Complete Heterogeneous Azeotropic Distillation Column System. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2003; pp. 760–765. [Google Scholar]
- Hartanto, D.; Handayani, P.A.; Sutrisno, A.; Anugrahani, V.W.; Mustain, A.; Khoiroh, I. Isopropyl Alcohol Purification through Extractive Distillation Using Glycerol as an Entrainer: Technical Performances Simulation and Design. J. Bahan Alam Terbarukan 2019, 8, 133–143. [Google Scholar] [CrossRef]
- Wu, X.; Nho, J.; Kim, D.S.; Cho, J. Comparison of Energy Consumption of Two-Column Configuration and Three-Column Configuration in the Extractive Distillation Process for High Purity Refinement of Isopropyl Alcohol. Asian J. Chem. 2014, 26, 5223–5229. [Google Scholar] [CrossRef]
- Kalla, S.; Upadhyaya, S.; Singh, K.; Dohare, R.K.; Agarwal, M. A Case Study on Separation of IPA-Water Mixture by Extractive Distillation Using Aspen Plus. Int. J. Adv. Technol. Eng. Explor. 2016, 3, 187–193. [Google Scholar] [CrossRef]
- Cho, J.; Jeon, J.-K. Optimization Study on the Azeotropic Distillation Process for Isopropyl Alcohol Dehydration. Korean J. Chem. Eng. 2006, 23, 1–7. [Google Scholar] [CrossRef]
- Chien, I.-L.; Zeng, K.-L.; Chao, H.-Y. Design and Control of a Complete Heterogeneous Azeotropic Distillation Column System. Ind. Eng. Chem. Res. 2004, 43, 2160–2174. [Google Scholar] [CrossRef]
- Widagdo, S.; Seider, W.D. Journal Review. Azeotropic Distillation. AIChE J. 1996, 42, 96–130. [Google Scholar] [CrossRef]
- De Guido, G.; Monticelli, C.; Spatolisano, E.; Pellegrini, L.A. Separation of the Mixture 2-Propanol + Water by Heterogeneous Azeotropic Distillation with Isooctane as an Entrainer. Energies 2021, 14, 5471. [Google Scholar] [CrossRef]
- Gomey, A.K.; Tripathi, M.M.; Haider, M.B.; Kumar, R. Comparative Analysis of Isopropyl Alcohol Dehydration Using Ionic Liquids and Deep Eutectic Solvent. J. Ion. Liq. 2023, 3, 100069. [Google Scholar] [CrossRef]
- Spatolisano, E.; Pellegrini, L.A. Dehydration of IPA-H2O Mixture: Review of Fundamentals and Proposal of Novel Energy-Efficient Separation Schemes. Chem. Eng. Sci. 2023, 273, 118672. [Google Scholar] [CrossRef]
- Hegely, L.; Nemeth, B.; Lang, P. Isopropanol Dehydration by Batch Extractive Distillation under Variable Pressure. Sep. Purif. Technol. 2025, 369, 133178. [Google Scholar] [CrossRef]
- Figueroa Paredes, D.A.; Sánchez, R.J.; Laoretani, D.S.; Fuentes, M.; Fernández, M.B.; Espinosa, J. Variants of the Hybrid Distillation/Pervaporation Process: Conceptual Model-Based Optimization and Environmental Analysis for IPA Dehydration. Sep. Purif. Technol. 2025, 363, 132281. [Google Scholar] [CrossRef]
- Han, D.; Chen, Y.; Shi, D. Different Extractive Distillation Processes for Isopropanol Dehydration Using Low Transition Temperature Mixtures as Entrainers. Chem. Eng. Process.-Process Intensif. 2022, 178, 109049. [Google Scholar] [CrossRef]
- Villegas-Uribe, C.A.; Alcántara-Avila, J.R.; Medina-Herrera, N.; Gómez-González, R.; Tututi-Avila, S. Temperature Control of a Kaibel, Agrawal and Sargent Dividing-Wall Distillation Columns. Chem. Eng. Process.-Process Intensif. 2021, 159, 108248. [Google Scholar] [CrossRef]
- Qian, X.; Huang, K.; Chen, H.; Yuan, Y.; Zhang, L.; Wang, S. Intensifying Kaibel Dividing-Wall Column via Vapor Recompression Heat Pump. Chem. Eng. Res. Des. 2019, 142, 195–203. [Google Scholar] [CrossRef]
- Annakou, O.; Mizsey, P. Rigorous Comparative Study of Energy-Integrated Distillation Schemes. Ind. Eng. Chem. Res. 1996, 35, 1877–1885. [Google Scholar] [CrossRef]
- Kvernland, M.; Halvorsen, I.; Skogestad, S. Model Predictive Control of a Kaibel Distillation Column. IFAC Proc. Vol. 2010, 43, 553–558. [Google Scholar] [CrossRef]
- Qian, X.; Huang, K.; Jia, S.; Chen, H.; Yuan, Y.; Zhang, L.; Wang, S. Composition/Temperature Cascade Control for a Kaibel Dividing-Wall Distillation Column by Combining PI Controllers and Model Predictive Control Integrated with Soft Sensor. Comput. Chem. Eng. 2019, 126, 292–303. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, K.; Chen, H.; Qian, X.; Zang, L.; Zhang, L.; Wang, S. Comparing Composition Control Structures for Kaibel Distillation Columns. Processes 2020, 8, 218. [Google Scholar] [CrossRef]
- Kaibel, G. Distillation Columns with Vertical Partitions. Chem. Eng. Technol. 1987, 10, 92–98. [Google Scholar] [CrossRef]
- Wang, X.; Yu, X.; Xie, L.; Li, M.; Zhang, Y. Energy-Saving Columns: Design and Control of a Kaibel and a Multi-Sidestream Column for Separating Hydrocarbon Mixture. Chem. Eng. Process.-Process Intensif. 2018, 133, 66–82. [Google Scholar] [CrossRef]
- Ghadrdan, M.; Halvorsen, I.J.; Skogestad, S. Optimal Operation of Kaibel Distillation Columns. Chem. Eng. Res. Des. 2011, 89, 1382–1391. [Google Scholar] [CrossRef]
- Soraya Lopez-Saucedo, E.; Chen, Q.; Grossmann, I.E.; Caballero, J.A. Kaibel Column: Modeling and Optimization. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1183–1188. [Google Scholar]
- Wu, Y.C.; Hsu, P.H.-C.; Chien, I.-L. Critical Assessment of the Energy-Saving Potential of an Extractive Dividing-Wall Column. Ind. Eng. Chem. Res. 2013, 52, 5384–5399. [Google Scholar] [CrossRef]
- Wang, S.-J.; Chen, W.-Y.; Chang, W.-T.; Hu, C.-C.; Cheng, S.-H. Optimal Design of Mixed Acid Esterification and Isopropanol Dehydration Systems via Incorporation of Dividing-Wall Columns. Chem. Eng. Process. Process Intensif. 2014, 85, 108–124. [Google Scholar] [CrossRef]
- Sommer, A.; Heitmann, W.; Bricker, R. Method of Preparing Alcohols Having Two to Four Carbon Atoms by Catalytic Hydration of Olefins. U.S. Patent 4,351,970, 28 September 1982. [Google Scholar]
- Cignitti, S.; Rodriguez-Donis, I.; Abildskov, J.; You, X.; Shcherbakova, N.; Gerbaud, V. CAMD for Entrainer Screening of Extractive Distillation Process Based on New Thermodynamic Criteria. Chem. Eng. Res. Des. 2019, 147, 721–733. [Google Scholar] [CrossRef]
- Noshadi, I.; Amin, N.A.S.; Parnas, R.S. Continuous Production of Biodiesel from Waste Cooking Oil in a Reactive Distillation Column Catalyzed by Solid Heteropolyacid: Optimization Using Response Surface Methodology (RSM). Fuel 2012, 94, 156–164. [Google Scholar] [CrossRef]
- Wang, H.; Li, C.; Li, C. RSM Optimization of the Operating Parameters for a Butanol Distillation Column. Asia-Pac. J. Chem. Eng. 2012, 7, 117–123. [Google Scholar] [CrossRef]
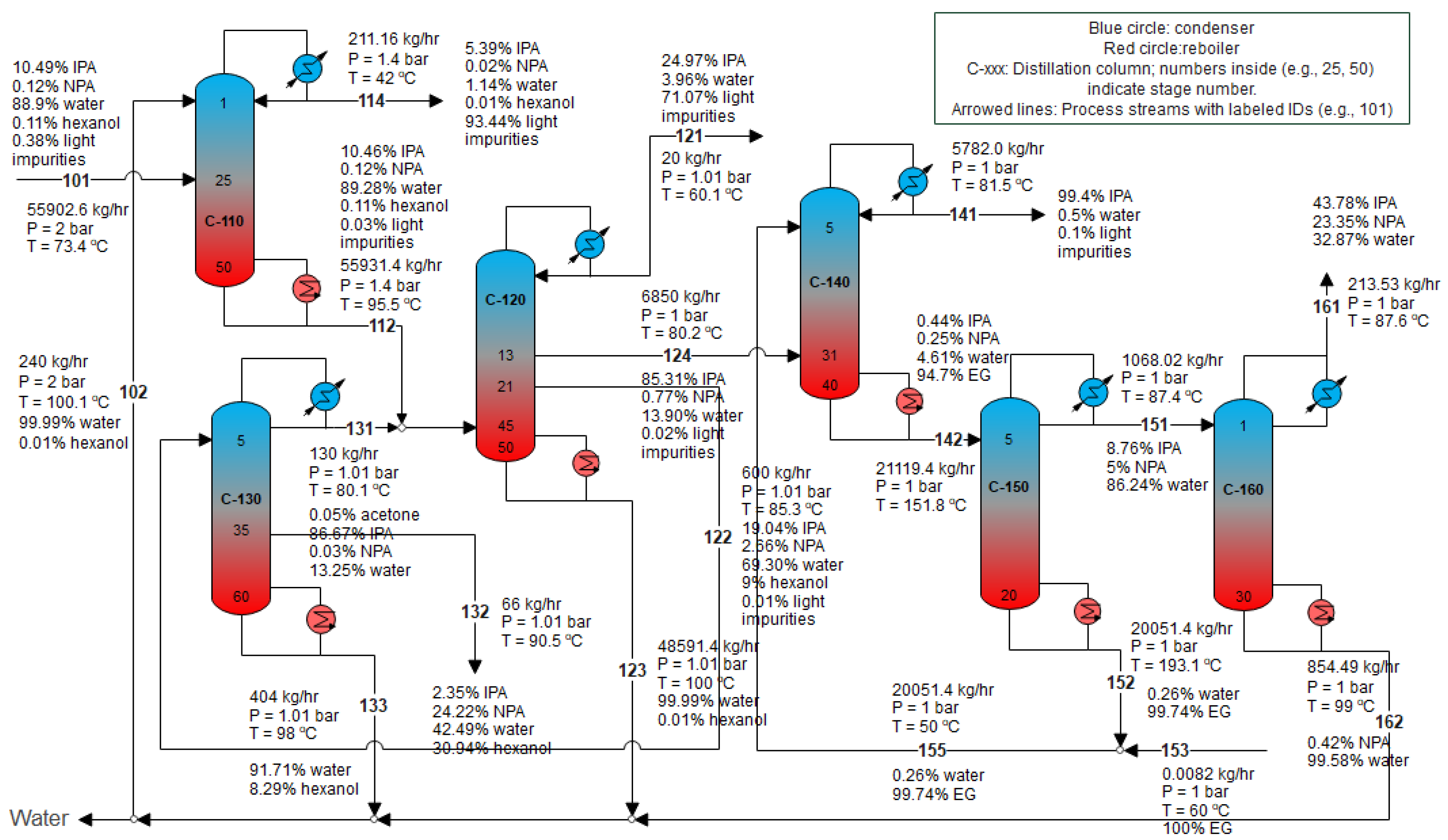


| Component | Mass Fraction |
|---|---|
| IPA | 0.10449 |
| NPA | 0.0012 |
| Hexanol | 0.0011 |
| Water | 0.889 |
| Light impurities | 0.0038 |
| Column | Reboiler Duty, kW | ||||
|---|---|---|---|---|---|
| Base Case | DWC-2 | DWC-3 | Δ (DWC-2 vs. Base) | Δ (DWC-3 vs. Base) | |
| C-110 | 1498 | 1498 | 1498 | 0 | 0 |
| C-120 | 6970 | 6525 (K-120) | 7314 (K-1230) | −445 | +344 |
| C-130 | 2508 | 2088 | 0 | −420 | −2508 |
| C-140 | 4922 | 4933 | 4934 | +11 | +12 |
| C-150 | 2623 | 2547 | 2533 | −76 | −90 |
| C-160 | 96 | 96 | 92 | 0 | −4 |
| Total | 18,617 | 17,688 | 16,371 | −929 | −2246 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agarwal, N.; Nga, N.N.; Nhien, L.C.; Hanifah, R.A.; Kim, M.; Lee, M. Enhancing Energy Efficiency of Electric Grade Isopropyl Alcohol Production Process by Using Noble Thermally Coupled Distillation Technology. Energies 2025, 18, 4159. https://doi.org/10.3390/en18154159
Agarwal N, Nga NN, Nhien LC, Hanifah RA, Kim M, Lee M. Enhancing Energy Efficiency of Electric Grade Isopropyl Alcohol Production Process by Using Noble Thermally Coupled Distillation Technology. Energies. 2025; 18(15):4159. https://doi.org/10.3390/en18154159
Chicago/Turabian StyleAgarwal, Neha, Nguyen Nhu Nga, Le Cao Nhien, Raisa Aulia Hanifah, Minkyu Kim, and Moonyong Lee. 2025. "Enhancing Energy Efficiency of Electric Grade Isopropyl Alcohol Production Process by Using Noble Thermally Coupled Distillation Technology" Energies 18, no. 15: 4159. https://doi.org/10.3390/en18154159
APA StyleAgarwal, N., Nga, N. N., Nhien, L. C., Hanifah, R. A., Kim, M., & Lee, M. (2025). Enhancing Energy Efficiency of Electric Grade Isopropyl Alcohol Production Process by Using Noble Thermally Coupled Distillation Technology. Energies, 18(15), 4159. https://doi.org/10.3390/en18154159







