Potential of Natural Esters as Immersion Coolant in Electric Vehicles †
Abstract
1. Introduction
2. Comparison of Performances of Different Coolants Used in Transformers and EV Engines
2.1. Cooling Systems and Coolants
2.2. Dielectric Properties
| Properties | Mineral Oils | Silicone Oils | Synthetic Esters | Vegetable Oils |
|---|---|---|---|---|
| Dielectric Breakdown (KV) | 30–35 | 36–60 | 45–70 | 82–97 |
| Kinematic Viscosity (cSt) | ||||
| at 0 °C | <76 | 81–92 | 26–50 | 77–143 |
| at 40 °C | 3–16 | 35–40 | 14–29 | 16–37 |
| at 100 °C | 2–2.5 | 15–17 | 4–6 | 4–8 |
| Pour Point (°C) | −30 to −60 | −50 to −60 | −40 to −50 | −19 to −33 |
| Flash Point (°C) | 100–170 | 300–310 | 250–270 | 315–328 |
| Fire Point (°C) | 110–185 | 340–350 | 300–310 | 350–360 |
| Thermal Conductivity (Wm−1K−1) | 0.11–0.16 | 0.15 | 0.13–0.15 | 0.16–0.19 |
| Specific heat capacity (Jkg−1K−1) | 1600–2000 | 1370–1500 | 1800–2300 | 1500–2100 |
| Dielectric Constant | 2.1 | 2.75 | 3.326 | >3 |
2.3. Comparison with Respect to Moisture Absorption, Hydrolysis Characteristics, and Oxidative Stability
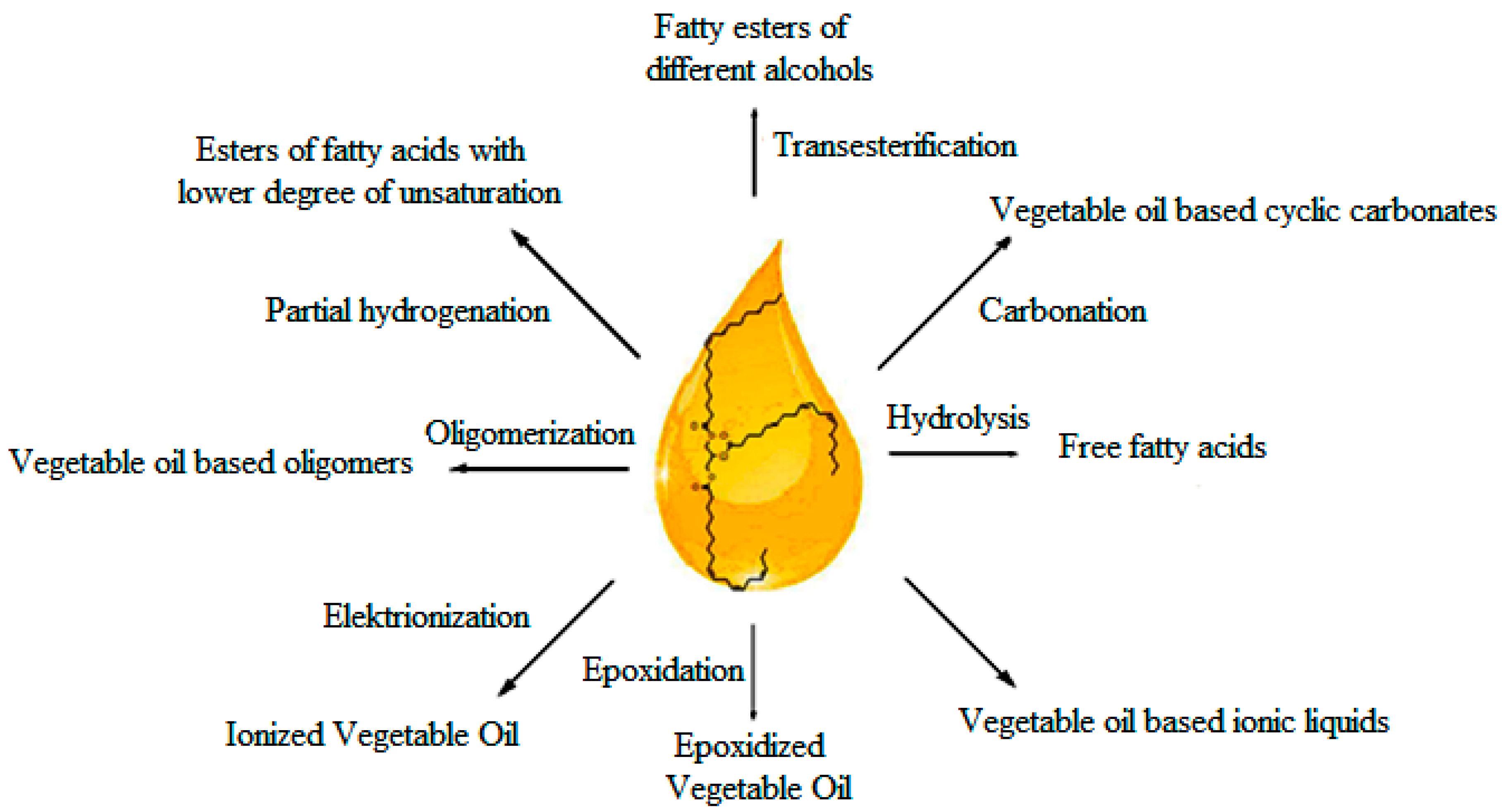
3. Modification of Natural Esters
3.1. Chemical Transformation of Fatty Esters
3.2. Improving the Performance of Esters Using Additives/Diluents
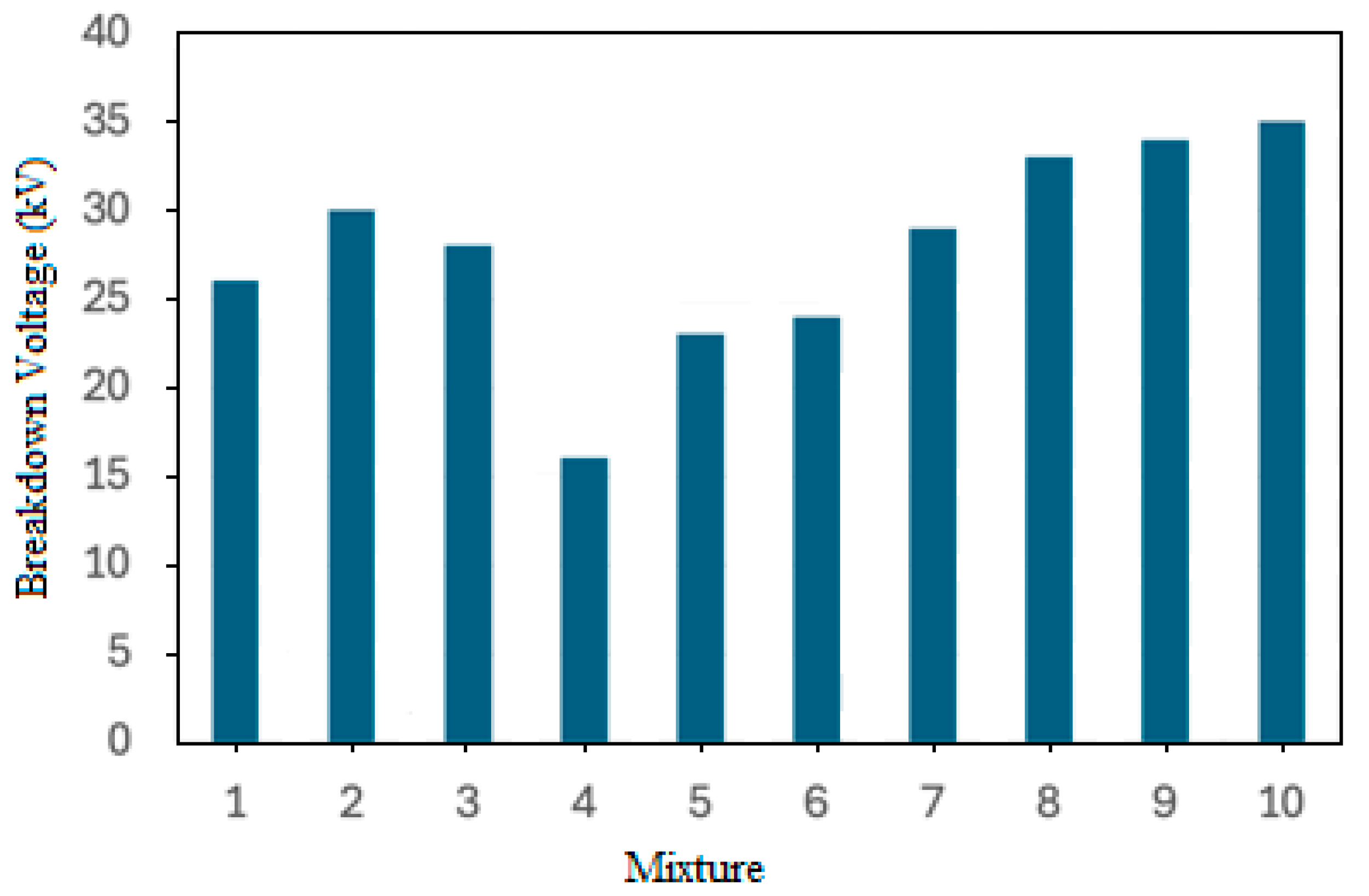
4. Promising Esters
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Faraz, A.; Ambikapathy, A.; Thangavel, S.; Logavani, K.; Arun Prasad, G. Battery electric vehicles (BEVs). In Electric Vehicles: Green Energy and Technology; Springer: Singapore, 2021; pp. 137–160. [Google Scholar]
- Sanguesa, J.A.; Torres-Sanz, V.; Garrido, P.; Martinez, F.J.; Marquez-Barja, J.M. A review on electric vehicles: Technologies and challenges. Smart Cities 2021, 4, 372–404. [Google Scholar] [CrossRef]
- Taghizad-Tavana, K.; Alizadeh, A.A.; Ghanbari-Ghalehjoughi, M.; Nojavan, S. A comprehensive review of electric vehicles in energy systems: Integration with renewable energy sources, charging levels, different types, and standards. Energies 2023, 16, 630. [Google Scholar] [CrossRef]
- Butcher, R.; Bradley, N.; Jamieson, M.; Chambers, T. Aspects of Engine Lubricant Operating Conditions and Fuel Economy Differentiation: In-Vehicle Comparisons of Standard Internal Combustion Engine with Two Types of Hybrid Electric; SAE Technical Paper 2024-01-2824; SAE International: Warrendale, PA, USA, 2024. [Google Scholar] [CrossRef]
- Arole, K.; Green, M.J.; Liang, H. Thermal and electrical properties of electric vehicle fluids. In Electric Vehicle Tribology, 1st ed.; Cabrera, L.I.F., Erdemir, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 193–206. [Google Scholar]
- Sosa, Y. Design requirements and challenges for single electric vehicle fluids. Tribol. Lubr. Technol. 2023, 79, 22–26. [Google Scholar]
- McGuire, N.; Writer, S.F. Bubble trouble in electric vehicle fluids. Tribol. Lubr. Technol. 2024, 80, 62–72. [Google Scholar]
- Liu, J.; Huang, S.; Chen, H. Recent progress and prospects in oil-immersed battery thermal management system based on single-phase insulating oil: A review. J. Therm. Anal. Calorim. 2024, 149, 4263–4286. [Google Scholar] [CrossRef]
- Gangopadhyay, A.; Jost, N.; Mutyala, K.C. Lubrication regimes in battery electric vehicle power unit. In Electric Vehicle Tribology, 1st ed.; Cabrera, L.I.F., Erdemir, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 183–191. [Google Scholar]
- Fu, Z.; Zuo, W.; Li, Q.; Zhou, K.; Huang, Y.; Li, Y. Multi-objective optimization of liquid cooling plate partially filled with porous medium for thermal management of lithium-ion battery pack by RSM, NSGA-II and TOPSIS. Energy 2025, 318, 134853. [Google Scholar] [CrossRef]
- Fu, Z.; Zuo, W.; Li, Q.; Zhou, K.; Huang, Y.; Li, Y. Performance enhancement studies on the liquid cooling plate fully filled with porous medium for thermal management of lithium-ion battery pack. J. Energy Storage 2025, 116, 116072. [Google Scholar] [CrossRef]
- He, L.; Gu, Z.; Zhang, Y.; Jing, H.; Li, P. Review on thermal management of lithium-ion batteries for electric vehicles: Advances, challenges, and outlook. Energ. Fuel 2023, 37, 4835–4857. [Google Scholar] [CrossRef]
- Huang, C.; Zhu, H.; Ma, Y.; E, J. Evaluation of lithium battery immersion thermal management using a novel pentaerythritol ester coolant. Energy 2023, 284, 129250. [Google Scholar] [CrossRef]
- Roe, C.; Feng, X.; White, G.; Li, R.; Wang, H.; Rui, X.; Li, C.; Zhang, F.; Null, V.; Parkes, M.; et al. Immersion cooling for lithium-ion batteries—A review. J. Power Sources 2022, 525, 231094. [Google Scholar] [CrossRef]
- Youssef, R.; Kalogiannis, T.; Behi, H.; Pirooz, A.; Van Mierlo, J.; Berecibar, M. A comprehensive review of novel cooling techniques and heat transfer coolant mediums investigated for battery thermal management systems in electric vehicles. Energy Rep. 2023, 10, 1041–1068. [Google Scholar] [CrossRef]
- Liu, Y.H.; Aldan, G.; Huang, X.; Hao, M. Single-phase static immersion cooling for cylindrical lithium-ion battery module. Appl. Therm. Eng. 2023, 233, 121184. [Google Scholar] [CrossRef]
- Togun, H.; Aljibori, H.S.S.; Biswas, N.; Mohammed, H.I.; Sadeq, A.M.; Rashid, F.L.; Abdulrazzaq, T.; Zearah, S.A. A critical review on the efficient cooling strategy of batteries of electric vehicles: Advances, challenges, future perspectives. Renew. Sustain. Energy Rev. 2024, 203, 114732. [Google Scholar] [CrossRef]
- Claeys, S.G.; Lievens, S.S.; Van De Ven, P.; Zhou, Z.; Miller, S.J.; Schexnaydre, R.J.; Elomari, S. Ester Based Heat Transfer Fluid Useful as a Coolant for Electric Vehicles. U.S. Patent US20120164506A1, 7 November 2011. [Google Scholar]
- Karmakar, G.; Ghosh, P.; Sharma, B.K. Chemically modifying vegetable oils to prepare green lubricants. Lubricants 2017, 5, 44. [Google Scholar] [CrossRef]
- Mahapatra, A.; Vats, P.; Bambam, A.K.; Kumar, A.; Gajrani, K.K. Sustainable green lubricants. In Performance Characterization of Lubricants, 1st ed.; Kumar, A., Kumar, A., Kumar, A., Eds.; CRC Press: Boca Raton, FL, USA, 2024; pp. 33–47. [Google Scholar]
- Sharma, B.K.; Karmakar, G.; Shah, R.; Ghosh, P.; Sarker, M.I.; Erhan, S.Z. Sustainable Lubricant Formulations from Natural Oils: A Short Review. In Green Chemistry and Green Materials from Plant Oils and Natural Acids; Liu, Z., Kraus, G., Eds.; Royal Society of Chemistry: London, UK, 2023; Volume 83, Chapter 10; pp. 170–193. [Google Scholar]
- Rafiq, M.; Shafique, M.; Ateeq, M.; Zink, M.; Targitay, D. Natural esters as sustainable alternating dielectric liquids for transformer insulation system: Analyzing the state of the art. Clean Technol. Environ. Policy 2024, 26, 623–659. [Google Scholar] [CrossRef]
- Oparanti, S.O.; Rao, U.M.; Fofana, I. Natural esters for green transformers: Challenges and keys for improved serviceability. Energies 2022, 16, 61. [Google Scholar] [CrossRef]
- Obebe, E.O.; Hadjadj, Y.; Oparanti, S.O.; Fofana, I. Enhancing the Performance of Natural Ester Insulating Liquids in Power Transformers: A Comprehensive Review on Antioxidant Additives for Improved Oxidation Stability. Energies 2025, 18, 1690. [Google Scholar] [CrossRef]
- Nugroho, A.; Kozin, M.; Mamat, R.; Bo, Z.; Ghazali, M.F.; Kamil, M.P.; Puranto, P.; Fitriani, D.A.; Azahra, S.A.; Suwondo, K.P.; et al. Enhancing tribological performance of electric vehicle lubricants: Nanoparticle-enriched palm oil biolubricants for wear resistance. Heliyon 2024, 10, e39742. [Google Scholar] [CrossRef]
- Dekkers, M.; Ebrahimiazar, M.; Kazemi, A.; Zargartalebi, M.; Sinton, D. Screening biodegradable alternatives to mineral oil coolants. Energy Convers. Manag. 2025, 338, 119848. [Google Scholar] [CrossRef]
- Tormos, B.; Alvis-Sanchez, J. 14-Fluid characteristic and requirements for battery thermal management in battery electric vehicles. In Electric Vehicle Tribology, 1st ed.; Cabrera, L.I.F., Erdemir, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 249–264. [Google Scholar]
- Zhao, C.; Cao, W.; Dong, T.; Jiang, F. Thermal behavior study of discharging/charging cylindrical lithium-ion battery module cooled by channeled liquid flow. Int. J. Heat Mass Tran. 2018, 120, 751–762. [Google Scholar] [CrossRef]
- TotalEnergies Trials Immersion-Cooled Battery in EV Field Test. Available online: https://www.fuelsandlubes.com/totalenergies-trials-immersion-cooled-battery-in-ev-field-test/ (accessed on 19 July 2022).
- Kumaran, A.T.; Hemavathi, S. Optimization of Lithium-ion battery thermal performance using dielectric fluid immersion cooling technique. Process Saf. Environ. Prot. 2024, 189, 768–781. [Google Scholar] [CrossRef]
- Sundin, D.W.; Sponholtz, S. Thermal Management of Li-Ion Batteries with Single-Phase Liquid Immersion Cooling. IEEE Open J. Veh. Technol. 2020, 1, 82–92. [Google Scholar] [CrossRef]
- Chen, D.; Jiang, J.; Kim, G.; Yang, C.; Pesaran, A. Comparison of different cooling methods for lithium ion battery cells. Appl. Therm. Eng. 2016, 94, 846–854. [Google Scholar] [CrossRef]
- Liu, J.; Chen, H.; Yang, M.; Huang, S.; Wang, K. Comparative study of natural ester oil and mineral oil on the applicability of the immersion cooling for a battery module. Renew. Energy 2024, 224, 120187. [Google Scholar] [CrossRef]
- Nogueira, T.; Carvalho, J.; Magano, J. Eco-friendly ester fluid for power transformers versus mineral oil: Design considerations. Energies 2022, 15, 5418. [Google Scholar] [CrossRef]
- Jacob, J.; Preetha, P.; Thiruthi Krishnan, S. Review on natural ester and nanofluids as an environmental friendly alternative to transformer mineral oil. IET Nanodielectr. 2020, 3, 33–43. [Google Scholar] [CrossRef]
- Deshpande, Y.V.; Raut, S.; Madankar, T.A.; Andhare, A. Influence of biodegradable coolants on machinability improvement—A review. Adv. Mater. Process. Technol. 2024, 10, 3229–3264. [Google Scholar] [CrossRef]
- Rallabandi, S.; Issac Selvaraj, R.V. Advancements in battery cooling techniques for enhanced performance and safety in electric vehicles: A comprehensive review. Energy Technol. 2024, 12, 2301404. [Google Scholar] [CrossRef]
- Rodríguez, E.; Rivera, N.; Fernández-González, A.; Pérez, T.; González, R.; Hernández Battez, A. Electrical compatibility of transmission fluids in electric vehicles. Tribol. Int. 2022, 171, 107544. [Google Scholar] [CrossRef]
- Zhan, J.; Deng, Y.; Ren, J.; Gao, Y.; Liu, Y.; Rao, S.; Li, W.; Gao, Z. Cell design for improving low-temperature performance of lithium-ion batteries for electric vehicles. Batteries 2023, 9, 373. [Google Scholar] [CrossRef]
- Daccord, R.; Kientz, T.; Bouillot, A. Aging of a dielectric fluid used for direct contact immersion cooling of batteries. Front. Mech. Eng. 2023, 9, 1212730. [Google Scholar] [CrossRef]
- Chavan, S.; Venkateswarlu, B.; Prabakaran, R.; Salman, M.; Joo, S.W.; Choi, G.S.; Kim, S.C. Thermal runaway and mitigation strategies for electric vehicle lithium-ion batteries using battery cooling approach: A review of the current status and challenges. J. Energy Storage 2023, 72, 108569. [Google Scholar] [CrossRef]
- Trimbake, A.; Singh, C.P.; Krishnan, S. Mineral Oil Immersion Cooling of Lithium-Ion Batteries: An Experimental Investigation. J. Electrochem. Energy Convers. Storage 2021, 19, 021007. [Google Scholar] [CrossRef]
- Jiosseu, J.L.; Mengournou, G.M.; Nkoutcha, E.T.; Imano, A.M. Effect of ageing of monoesters and mineral oil on the propagation of creeping discharges. J. Electrostat. 2023, 123, 103810. [Google Scholar] [CrossRef]
- Tiwari, R.; Agrawal, P.S.; Belkhode, P.N.; Ruatpuia, J.V.L.; Rokhum, S.L. Hazardous effects of waste transformer oil and its prevention: A review. Next Sustain. 2024, 3, 100026. [Google Scholar] [CrossRef]
- Amin, D.; Walvekar, R.; Khalid, M.; Vaka, M.; Mubarak, N.M.; Gupta, T.C.S.M. Recent Progress and Challenges in Transformer Oil Nanofluid Development: A Review on Thermal and Electrical Properties. IEEE Access. 2019, 7, 151422–151438. [Google Scholar] [CrossRef]
- McShane, C.P. Vegetable-oil-based dielectric coolants. IEEE Ind. Appl. Mag. 2002, 8, 34–41. [Google Scholar] [CrossRef]
- Rozga, P.; Beroual, A.; Przybylek, P.; Jaroszewski, M.; Strzelecki, K. A review on synthetic ester liquids for transformer applications. Energies 2020, 13, 6429. [Google Scholar] [CrossRef]
- Lyutikova, M.N.; Ridel, A.V.; Konovalov, A.A. Dielectric Liquids: Past, Present, Future. Power Technol. Eng. 2023, 57, 615–622. [Google Scholar] [CrossRef]
- Park, T.W.; Han, S.H. Numerical analysis of local hot-spot temperatures in transformer windings by using alternative dielectric fluids. Electr Eng. 2015, 97, 261–268. [Google Scholar] [CrossRef]
- Li, S.; Zhao, X.; Liao, R.; Yang, L.; Guo, P. Study on ageing characteristics of insulating pressboard impregnated by mineral-vegetable oil. In Proceedings of the IEEE Conference on Electrical Insulation and Dielectric Phenomena, Toronto, ON, Canada, 16–19 October 2016; IEEE: New York, NY, USA, 2016. [Google Scholar]
- Fofana, I.; Wasserberg, V.; Borsi, H.; Gockenbach, E. Challenge of mixed insulating liquids for use in high-voltage transformers. II. Investigations of mixed liquid impregnated paper insulation. IEEE Electr. Insul. Mag. 2002, 18, 5–16. [Google Scholar] [CrossRef]
- Qin, J.; Peng, X.; Qiu, Q.; Tang, C. A new type of nano APTES-hBN modified palm oil as natural ester insulating oil with upgraded thermal aging characteristics. Renew. Energy 2022, 200, 743–750. [Google Scholar] [CrossRef]
- Gutiérrez, C.M.; Alonso, C.O.; Diego, C.F.; Diego, I.F.; Salas, C.O.; Köseoğlu, A.K.; Altay, R.; Fernández, A.O. Compatibility of Esters with Cellulosic Insulation Materials. In Alternative Liquid Dielectrics for High Voltage Transformer Insulation Systems: Performance Analysis and Applications, 1st ed.; Rao, U.M., Fofana, I., Sarathi, R., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 43–84. [Google Scholar]
- Oparanti, S.O.; Fofana, I.; Jafari, R.; Zarrougui, R.; Abdelmalik, A.A. Canola oil: A renewable and sustainable green dielectric liquid for transformer insulation. Ind. Crops Prod. 2024, 215, 118674. [Google Scholar] [CrossRef]
- Martin, D.; Saha, T.; Mcpherson, L. Condition monitoring of vegetable oil insulation in in-service power transformers: Some data spanning 10 years. IEEE Electr. Insul. Mag. 2017, 33, 44–51. [Google Scholar] [CrossRef]
- Rao, U.M.; Fofana, I.; Rozga, P.; Picher, P.; Sarkar, D.K.; Karthikeyan, R. Influence of Gelling in Natural Esters Under Open Beaker Accelerated Thermal Aging. IEEE Trans. Dielectr. Electr. Insul. 2023, 30, 413–420. [Google Scholar] [CrossRef]
- Lu, W.; Liu, Q.; Wang, Z.D. Gelling behaviour of natural ester transformer liquid under thermal ageing. In Proceedings of the 2012 International Conference on High Voltage Engineering and Application, Shanghai, China, 17–20 September 2012; IEEE: New York, NY, USA, 2012. [Google Scholar]
- Tenbohlen, S.; Koch, M. Aging Performance and Moisture Solubility of Vegetable Oils for Power Transformers. IEEE Trans. Power Syst. 2010, 25, 825–830. [Google Scholar] [CrossRef]
- McShane, C.P.; Corkran, J.L.; Rapp, K.J.; Luksich, J. Natural Ester Dielectric Fluid Development. In Proceedings of the 2005/2006 IEEE/PES Transmission and Distribution Conference and Exhibition, Dallas, TX, USA, 21–24 May 2006; IEEE: New York, NY, USA, 2006. [Google Scholar]
- Shinde, R. Global Footprint of Free Breathing Transformer with Natural Ester. In Proceedings of the 2019 IEEE 20th International Conference on Dielectric Liquids (ICDL), Rome, Italy, 23–27 June 2019; IEEE: New York, NY, USA, 2019. [Google Scholar]
- Ortiz, A.; Delgado, F.; Ortiz, F.; Fernandez, I.; Santisteban, A. The aging impact on the cooling capacity of a natural ester used in power transformers. Appl. Therm. Eng. 2018, 144, 797–803. [Google Scholar] [CrossRef]
- Abdelmalik, A.A. Chemically modified palm kernel oil ester: A possible sustainable alternative insulating fluid. Sustain. Mater. Technol. 2014, 1–2, 42–51. [Google Scholar] [CrossRef]
- Goud, V.V.; Patwardhan, A.V.; Pradhan, N.C. Kinetics of in situ epoxidation of natural unsaturated triglycerides catalysed by acidic ion exchange resin. Ind. Eng. Chem. Res. 2007, 46, 3078–3085. [Google Scholar] [CrossRef]
- Hwang, H.S.; Erhan, S.Z. Modification of epoxidized soybean oil for lubricant formulation with improved oxidative stability and low pour point. J. Am. Oil Chem. Soc. 2001, 78, 1179–1184. [Google Scholar] [CrossRef]
- Sharma, B.K.; Adhvaryu, A.; Liu, Z.; Erhan, S. Chemical modification of vegetable oils for lubricant applications. J. Am. Oil Chem. Soc. 2006, 83, 129–136. [Google Scholar] [CrossRef]
- Moser, B.R.; Sharma, B.K.; Doll, K.M.; Erhan, S.Z. Diester from oleic acid: Synthesis, low temperature properties, and oxidation stability. J. Am. Oil Chem. Soc. 2007, 84, 675–680. [Google Scholar] [CrossRef]
- Yang, T.; Wang, F.; Yao, D.; Li, J.; Zheng, H.; Yao, W.; Lv, Z.; Huang, Z. Low-Temperature Property Improvement on Green and Low-Carbon Natural Ester Insulating Oil. IEEE Trans. Dielectr. Electr. Insul. 2022, 29, 1459–1464. [Google Scholar] [CrossRef]
- Madavan, R.; Saroja, S.; Karthick, A.; Murugesan, S.; Mohanavel, V.; Velmurugan, P.; Al Obaid, S.; Alfarraj, S.; Sivakumar, S. Performance analysis of mixed vegetable oil as an alternative for transformer insulation oil. Biomass Convers. Biorefin. 2022, 15, 1939–1944. [Google Scholar] [CrossRef]
- Radha, R.; Iruthayarajan, M.W.; Bakrutheen, M. Performance of natural high oleic ester based blended oil insulation for transformer. In Proceedings of the 10th IEEE International Conference on Intelligent Systems and Control (ISCO), Coimbatore, India, 7–8 January 2016; pp. 1–5. [Google Scholar] [CrossRef]
- Beldjilali, A.; Idir, O.; Saidi-Amroun, N.; Saidi, M.; Moulai, H. Electrical and physicochemical properties and transient charging currents in mineral and vegetable oils mixture. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1739–1748. [Google Scholar] [CrossRef]
- Abeysundara, D.C.; Weerakoon, C.; Lucas, J.R.; Gunatunga, K.A.I.; Obadage, K.C. Coconut oil as an alternative to transformer oil. In Proceedings of the ERU Symposium, Moratuwa, Sri Lanka; 2001. [Google Scholar]
- Pagger, E.P.; Pattanadech, N.; Uhlig, F.; Muhr, M. Dielectric Insulating Liquids. In Biological Insulating Liquids; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Deepa, S.N.; Srinivasan, A.D.; Anusha, M.; Veeramanju, K.T.; Chaitra, M.R. Review on the Addition of Antioxidants and Nanoparticles to Natural Ester as an Alternative to Transformer Oil. In Proceedings of the International Conference on Advances in Renewable Energy and Electric Vehicles, Nitte, India, 22–23 December 2022; Springer Nature: Singapore, 2022; pp. 217–258. [Google Scholar]
- Hamid, M.H.A.; Ishak, M.T.; Din, M.F.M.; Suhaimi, N.S.; Katim, N.I.A. Dielectric properties of natural ester oils used for transformer application under temperature variation. In Proceedings of the IEEE International Conference on Power and Energy (PECon), Melaka, Malysia, 28–29 November 2016; IEEE: New York, NY, USA, 2017. [Google Scholar]
- Boss, P.; Oommen, T.V. New insulating fluids for transformers based on biodegradable high oleic vegetable oil and ester fluid. In Proceedings of the IEEE Colloquium on Insulating Liquids, Leatherhead, UK, 27 May 1999; IET: London, UK, 2002. [Google Scholar]
- Hosier, I.L.; Guushaa, A.; Westenbrink, E.W.; Rogers, C.; Vaughan, A.S.; Swingler, S.G. Aging of biodegradable oils and assessment of their suitability for high voltage applications. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 728–738. [Google Scholar] [CrossRef]
- Rafiq, M.; Lv, Y.Z.; Zhou, Y.; Ma, K.B. Use of vegetable oils as transformer oils—A review. Renew. Sustain. Energy Rev. 2015, 52, 308–324. [Google Scholar] [CrossRef]
- Oommen, T.V.; Claiborne, C. Biodegradable Insulating Fluid from High Oleic Vegetable Oils. In Proceedings of the CIGRE Symposium, Paris, France, 15–17 September 1998; CIGRE: Paris, France, 1998. [Google Scholar]
- Brentin, R. Soybean Oil: Lubricating Performance. In Proceedings of the 2019 STLE Annual Meeting, Nashville, TN, USA, 19–23 May 2019. [Google Scholar]
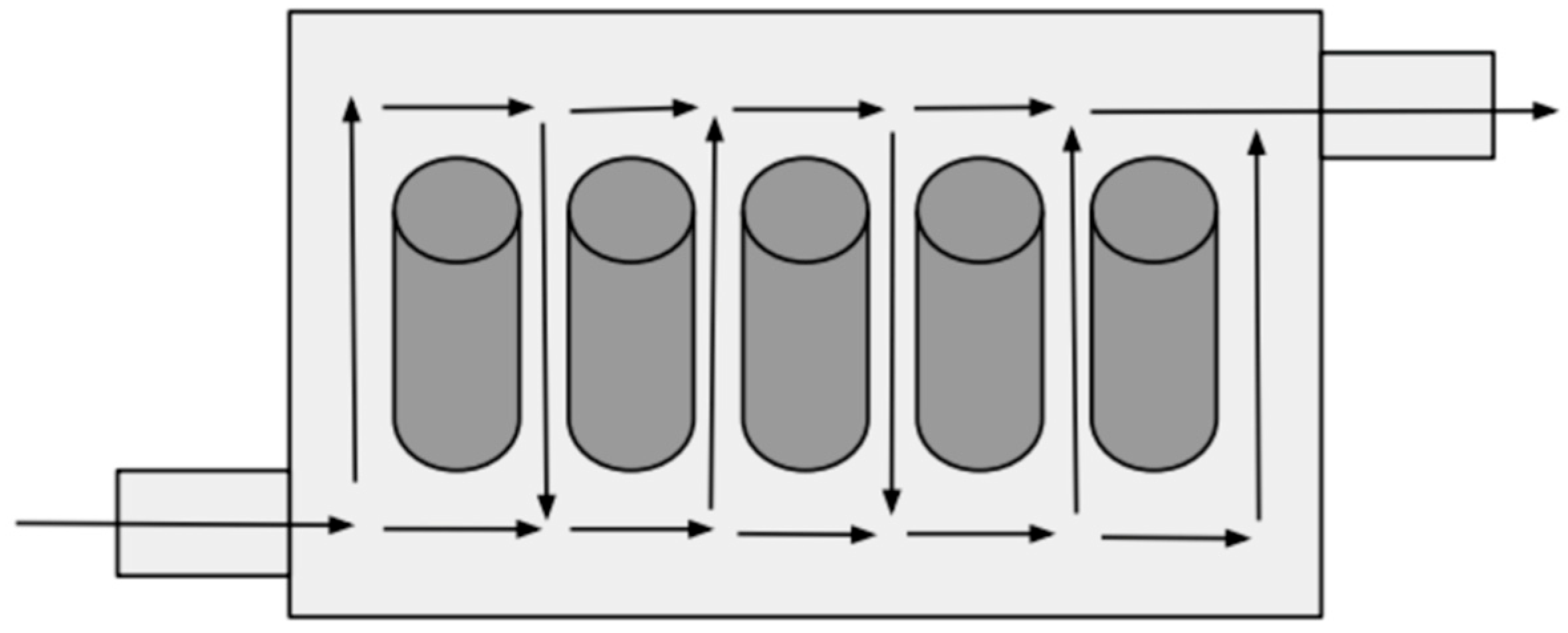
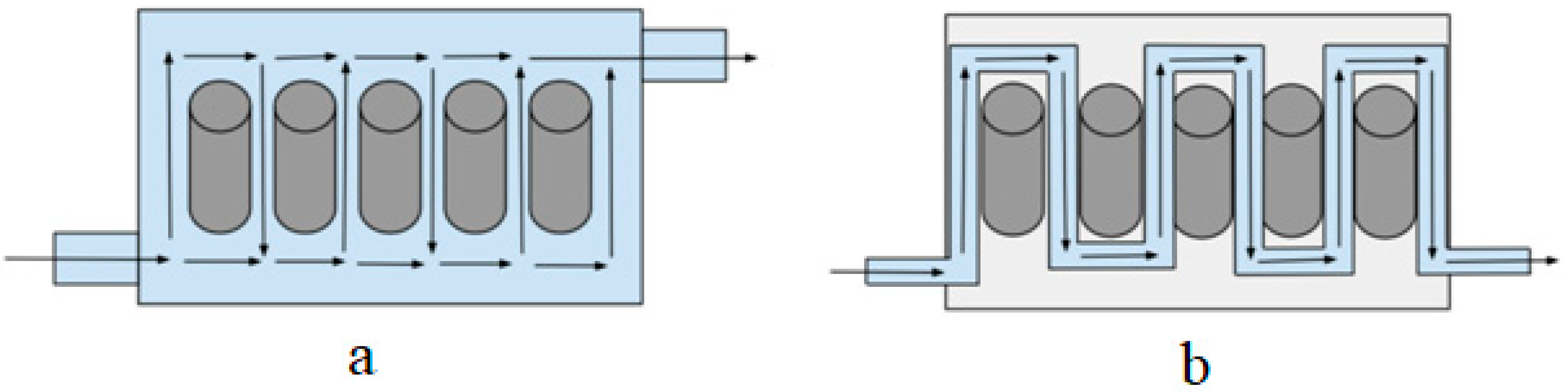
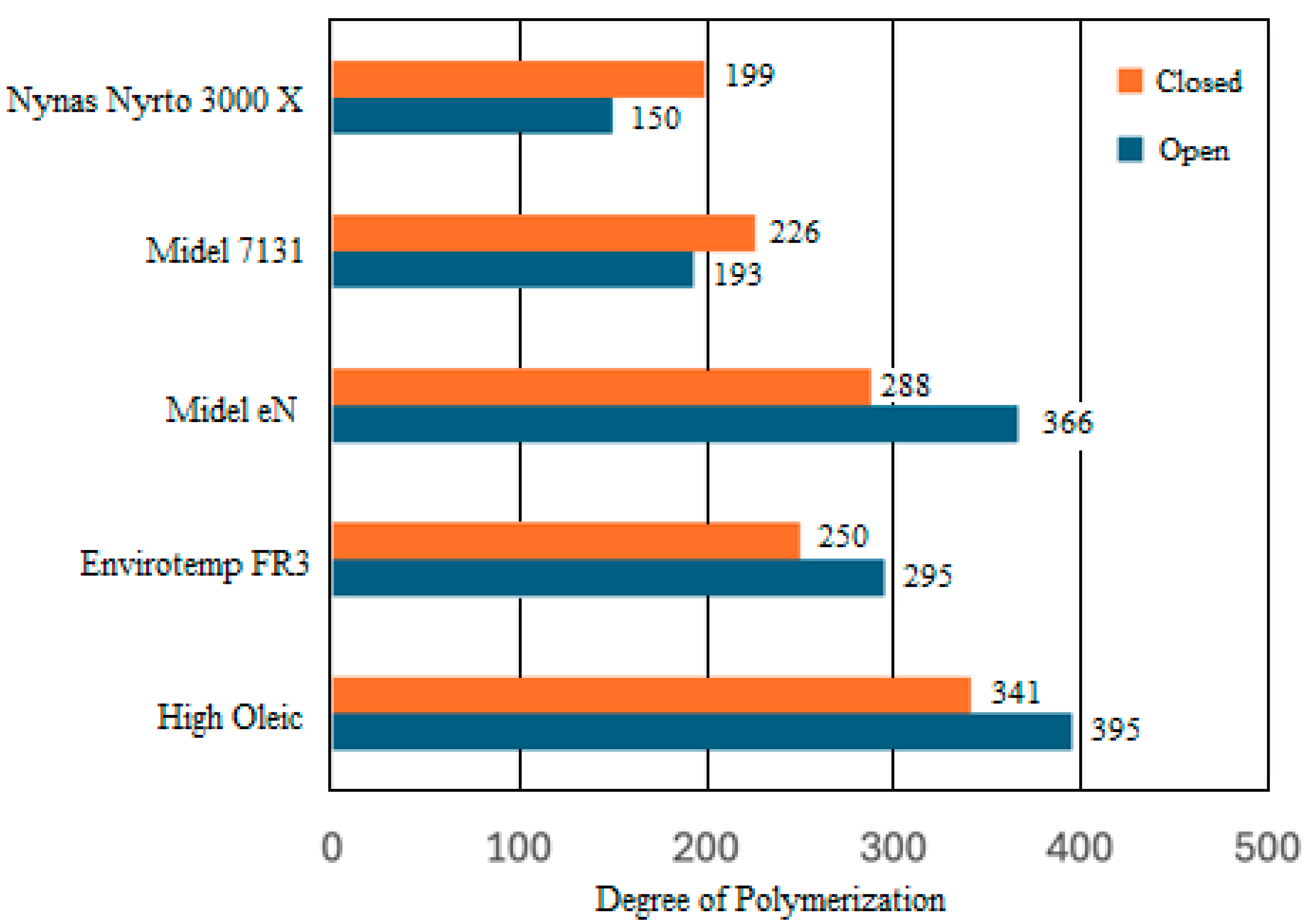
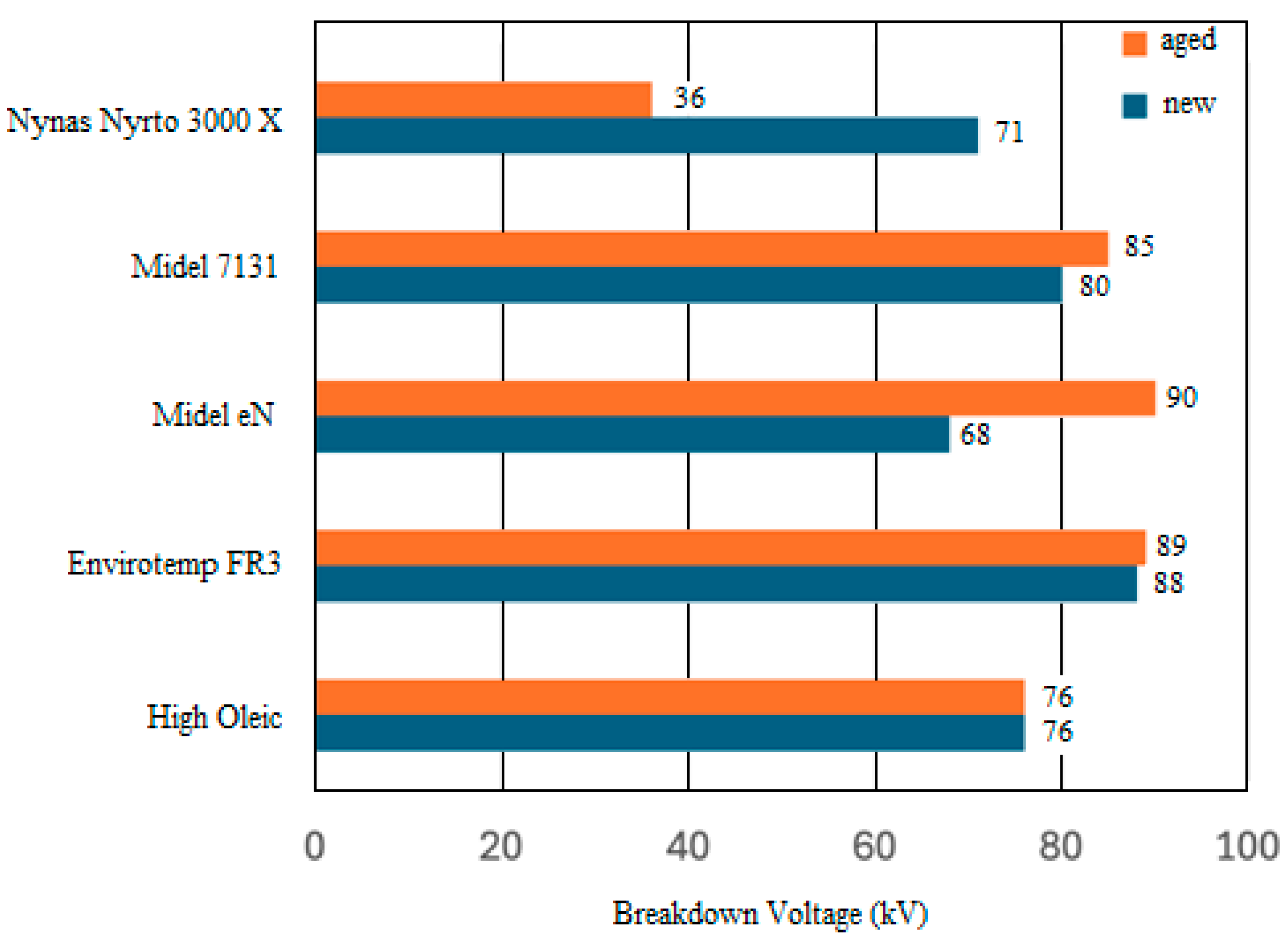
| BTMS | Thermal Conductivity (Wm−1K−1) | Specific Heat Capacity (Jkg−1K−1) | Convective Heat-Transfer Coefficient (Wm−2K−1) |
|---|---|---|---|
| Air cooling | 0.0242 | 1006 | 10–100 |
| Indirect Cooling (water–glycol) | 0.3892 | 3323 | 10,000 |
| Immersion cooling single-phase | 0.129–0.15 | 1370–2241 | 2000 |
| Immersion cooling double-phase | 0.075 | 1300 | 20,800 |
| Vegetable Oils (Normal/ Transesterified) | Kinematic Viscosity at 40 °C (cSt) | Pour Point (°C) | Flash Point (°C) |
|---|---|---|---|
| Rapeseed oil | 36.14 | −8 | 297 |
| Transesterified Rapeseed oil | 4.615 | −18 | 175 |
| Jatropha oil | 32.44 | 3 | ≥240 |
| Transesterified Jatropha oil | 10.45 | 0 | 191 |
| Palm Kernel oil | 44.49 | 26 | 239 |
| Transesterified Palm Kernel oil | 4.57 | −6 | 148 |
| Neem oil | 43.75 | 7 | 209 |
| Transestrified Neem oil | 5.53 | 3 | 175 |
| Canola oil | 33.4 | −24 | 280 |
| Transestrified Canola oil | 3.9 | −25 | 167 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, R.; Huang, C.; Karmakar, G.; Erhan, S.Z.; Sarker, M.I.; Sharma, B.K. Potential of Natural Esters as Immersion Coolant in Electric Vehicles. Energies 2025, 18, 4145. https://doi.org/10.3390/en18154145
Shah R, Huang C, Karmakar G, Erhan SZ, Sarker MI, Sharma BK. Potential of Natural Esters as Immersion Coolant in Electric Vehicles. Energies. 2025; 18(15):4145. https://doi.org/10.3390/en18154145
Chicago/Turabian StyleShah, Raj, Cindy Huang, Gobinda Karmakar, Sevim Z. Erhan, Majher I. Sarker, and Brajendra K. Sharma. 2025. "Potential of Natural Esters as Immersion Coolant in Electric Vehicles" Energies 18, no. 15: 4145. https://doi.org/10.3390/en18154145
APA StyleShah, R., Huang, C., Karmakar, G., Erhan, S. Z., Sarker, M. I., & Sharma, B. K. (2025). Potential of Natural Esters as Immersion Coolant in Electric Vehicles. Energies, 18(15), 4145. https://doi.org/10.3390/en18154145







