Abstract
Current biodiesel production is costly, in part due to the catalysts added during transesterification and later washed out. We have previously shown that intact rapeseed shells can be ball-milled with an alcohol to produce biodiesel without an added catalyst. Here, we report on the activation and identity of the complexing agent within the shells of rapeseeds and sunflower seeds. Lignin, present in the cell walls of plant matter, complexes with iron and manganese within metallic media, such as in a ball mill, and acts as a catalyst support in a transesterification reaction with oil and methanol. When ball-milled with methanol, rapeseed and sunflower seeds produce up to 90% biodiesel, similar to yields produced by industrial methods. However, this new method for producing biodiesel is a greener alternative, as it requires fewer organic solvents, may reduce the time and energy required for synthesis, and may reduce the effort required for product purification.
1. Introduction
Fossil fuels are currently the most used energy source worldwide; however, as energy demands rise and the availability of fossil fuels becomes more limited, alternative energy sources are necessary. Biodiesel is one such alternative in the transportation industry. Biodiesel is synthesized using renewable oil sources such as oil-containing plants and seeds, algae, or animal fats [1,2]. In addition to being renewable, biodiesel is also biodegradable and gives off fewer emissions than diesel from fossil fuels [3]. Typically, conversion of triglycerides and alcohol to fatty acid alkyl esters (FAAEs) and glycerol is accomplished through transesterification with an alkaline catalyst such as potassium or sodium hydroxide, as shown in Figure 1 [4,5]. While using a base catalyst for transesterification leads to high yields (upwards of 95%) [6], the presence of the base can lead to saponification in the presence of free fatty acids.
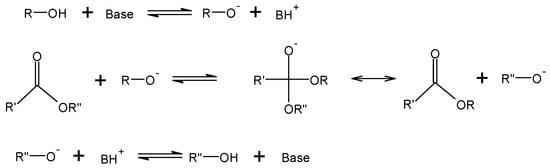
Figure 1.
Reaction mechanism of an alcohol and a triglyceride with a base catalyst.
In a previous publication we reported on a new method to produce biodiesel via ball milling. We found that the shells of rapeseeds contain a compound that can be activated to catalyze the formation of biodiesel from oil seeds [7]. This compound appears to be activated by mechanical milling, allowing us to produce biodiesel in one step. With this method, intact rapeseeds and methanol are directly transesterified, without introducing a catalyst. Additionally, the mechanically milled shells of rapeseeds lead to the conversion of other oils to biodiesel both by refluxing and by mechanical milling. In comparison to current production methods, this synthesis route is lower energy, more environmentally friendly, and does not lead to saponification, though further analysis of the products is needed. We have found that rapeseed and sunflower seed shells become catalytically active after ball milling, and we aim to find what component of the seed shells is shared between these two seeds to better understand how it becomes catalytically active. From a review of the previous literature on the chemical composition of rapeseed and sunflower seed shells, they share multiple components that could potentially become catalytic after ball milling, with cellulose and lignin being in the highest concentrations [8,9]. This is summarized in Table 1.

Table 1.
Major components of rapeseed hulls and sunflower shells in percentages based on references [8,9].
Cellulose is the most common naturally existing polymer, and it is sourced from plant fibers where it provides structure and support. Cellulose consists of D-glucopyranose rings in the lowest energy configuration, linked by β-1,4-glycosidic bonds, seen in Figure 2 below. Cellulose has been used in the production of bioethanol previously [10,11], but it has not been used for biodiesel. Hemicelluloses are a large group of polysaccharides in plants that are made up of monosaccharides such as glucuronic acid, xylose, arabinose, and rhamnose.
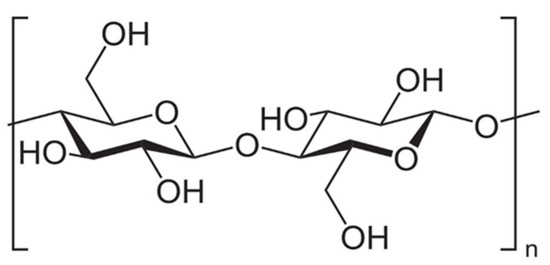
Figure 2.
Chemical structure of cellulose.
Lignin is a large, amorphous, cross-linked biopolymer that is present in all plant cell walls, providing rigidity and imperviousness, Figure 2. It is one of the most common naturally existing polymers, second only to cellulose. The building blocks of lignin are hydroxyphenyl (H), guaicyl (G), and syringyl (S) groups to form the complex structure, as seen in Figure 3 below. Rapeseeds primarily consist of S and G units in lignin while sunflowers are higher in S groups than any other units [12,13]. Both the amount of lignin and the number and type of its components are dependent on the plant from which the lignin derives [14]. Typically, softwoods, such as pine and cedar, have the highest concentration of lignin at 27 to 33%. Hardwoods such as oak and walnut are 18 to 25% lignin, and herbaceous plants have the lowest concentration at 17 to 24% [15]. Hardwoods have higher concentrations of G and S units, while softwoods have more G units.
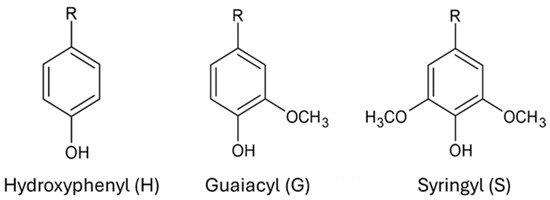
Figure 3.
Structural components of lignin.
Modified lignin has been shown to be an effective metal adsorbent with a variety of metals such as with cadmium (II), lead (II), and zinc (II) [16,17]. There is no one agreed-upon mechanism for metal uptake, but ion exchange, surface adsorption, and complexation are all plausible. While modified cellulose also adsorbs metals to an extent, sorption appears to occur primarily via added functional groups [18,19,20]. As mechanical milling of oilseeds and methanol involves natural, unmodified polymers and polysaccharides, it is not possible to compare one to one with studies of modified polymers. However, previous studies do provide valuable information on possible mechanisms for activation of the catalyst support. Adsorption isotherms and kinetics, specifically, determine adsorption efficiency based on the presence of adsorption sites in a molecule and solution pH [21].
In this paper, the results of studies into the identity of the catalyst support for ball milling intact oilseeds and methanol, methods of activation, and potential future studies on the metal binding properties of the catalytic compound are presented and discussed. This method is novel in that it presents a way to produce biodiesel through mechanochemical processes in comparison to the usage of high temperatures and/or pressures for industrial methods.
2. Materials and Methods
2.1. Materials
For milling and refluxes, ACS-grade methanol from Thermo Fisher Scientific (Waltham, MA, USA), rapeseeds (Brassica napus) from Boonetown Seed Co. (Yorktown, IN, USA), Publix (Lakeland, FL, USA), black oil sunflower seeds (Helianthus annuus), and Sussed (Wicklow, Ireland) cold-pressed extra virgin rapeseed oil were used. Microcrystalline cellulose (50 µm) and D-(+)-xylose were from Thermo Fisher Scientific; lignin (alkaline), lignin (dealkaline), D-glucuronic acid, L-(+)-arabinose, and L-(+)-rhamnose were from TCI America (Seekonk, MA, USA).
GC analyses were completed using the standards and solvent mentioned in a previous publication by Tanner et al. [7]. For grinding lignin with different metals, iron powder 99% from Alfa Aesar (Haverhill, MA, USA), manganese powder 99% from Sigma Aldrich (St. Louis, MO, USA), and chromium powder 99% from Fisher Scientific were used. Analyses were completed with a Perkin Elmer Clarus 500 Gas Chromatograph with a flame ionization detector (PerkinElmer, Waltham, MA, USA) and 4100 MIR FT-IR with ATR (Jasco, Easton, MD, USA). A Multiwave 5000 Microwave Digester (Aton Paar, Ashland, VA, USA) was used with trace metal grade nitric acid from Thermo Fisher Scientific prior to analysis via an i-Cap Q ICP-MS (Thermo Fisher Scientific, Waltham, MA, USA).
2.2. Milling of Seed Shell Components
The shell components listed in Table 1 were all tested for catalytic activation via mechanical milling. To accomplish this, 1.0 g of the individual component was added to the SPEX milling canister along with 3.0 g of rapeseed oil, 3.0 mL of methanol, and 12 3/8th in steel balls, following the previously established method by Tanner et al. [7]. Yield based on volume of product was determined according to Equation (1) below.
2.3. Milling Times with Lignin
Alkaline lignin was milled with biodiesel reactants for varying lengths of time to determine the effect of milling time on the product. To the milling canister, 1.0 g lignin, 3.0 mL methanol, 3.0 mL rapeseed oil, and 12 steel balls were added. The canister was milled for 60, 90, 105, 120, and 135 min, and then the biodiesel was isolated and analyzed. All trials were run in triplicate.
2.4. Milling Oak Tree Shavings
As described in 2.2, Milling of Seed Shell Components, rapeseed oil, methanol, and 12 steel balls were added to the SPEX milling canister. Additionally, 0.5 g of oak tree (Quercus spp.) that had been dried and passed through a woodchipper was added to the canister for one trial and 1.0 g was added for a second trial. The ball mill was run for one hour before centrifuging and analyzing the sample via GC. All trials were run in triplicate.
2.5. Reflux of Lignin
Trials of lignin treated in different ways were tested for activity through reflux with methanol and oil. The lignin samples and their treatment methods are described in Table 2 below. For all trials, 1.0 g of lignin was used. After preparation, the samples were added to a 100 mL round-bottom flask with 8.0 mL methanol and 5.0 mL of rapeseed oil, mixing thoroughly. The round bottom was then connected to a water condenser and heated at approximately 65 °C for 4 h using a heating mantle and Variac variable transformer. The mixture was cooled to room temperature and then transferred to a centrifuge tube and centrifuged for 45 min. The middle layer was analyzed via gas chromatography, comparing to methyl ester standards. The percent composition for each methyl ester was determined based on the peak area in the chromatogram.

Table 2.
Lignin type and preparation method for samples.
2.6. Grinding of Lignin with Metals
In order to determine the effect of specific metals and of mechanical force on lignin activation as a catalyst support, an acid-washed glass mortar and pestle was used to grind 1.0 g lignin with 0.8 g iron, manganese, and chromium metals in separate trials. A fifth trial of lignin ground with a methanol-washed stainless-steel mortar and pestle was completed. Samples were ground for 1, 3, and 6 min to produce 15 different preparation methods. The lignin from each sample was isolated as necessary and subsequently added to a reflux of 8.0 mL methanol and 5.0 mL of rapeseed oil, as previously described. After centrifugation, the middle layer (biodiesel) and the bottom layer (biomass) were collected for analysis.
2.7. ICP-MS
Before inductively coupled plasma mass spectrometry (ICP-MS), samples were prepared and digested. To a 50 mL polytetrafluorethylene vessel, 0.3 g of rapeseeds and 10.0 mL of concentrated nitric acid were added. The reaction vessel was closed and loaded into the digestion rotor. The remaining samples were prepared the same way, using 0.3 g of ball-milled rapeseeds, rapeseeds ground in a stainless-steel mortar and pestle, rapeseeds ground in a glass mortar and pestle, ball-milled methanol, and biodiesel prepared from mechanical milling. The exact masses were recorded for all samples, and they were completed in triplicate. The preset digestion method used, named “plant matter,” included a 20 min ramp to 200 °C and a 10 min hold at 200 °C before entering the cooling cycle. After cooling, venting, and transferring the samples to 15 mL vials, dilutions were prepared. By volume, a 2% solution was made using 40 µL of the digested sample and 1960 µL of ultrapure deionized water. These samples were introduced to the ICP-MS, which scanned for elements including sodium, magnesium, aluminum, chromium, manganese, iron, nickel, zinc, and lead.
3. Results and Discussion
3.1. Milling of Seed Shell Components
Six primary components of rapeseed and sunflower seeds were ball-milled with methanol and oil to determine a specific compound that becomes catalytic once mechanical force is applied. Only milling of lignin with methanol and oil produced biodiesel, with a percent yield of 73%, indicating that this is most likely the chemical activated by mechanical energy. Notably, this percent yield is significantly lower than that found previously by ball milling intact rapeseeds (90%) [7], suggesting that the hulls of the oilseeds impact transesterification. The hulls could provide better structural support compared to commercially available lignin, which not only originates from mixtures of plant sources, but has also been valorized through kraft or organosolv processes [22]. These methods cause significant variation in structure and functional groups [23,24], likely causing the lower yield seen here. As discussed previously, lignin has been shown to become more reactive after ball milling, so it is plausible that the same happens here and the increase in reactivity pushes the transesterification reaction forward. Cellulose and the sugars tested share overall similar structures in comparison to lignin, and with none of them leading to biodiesel formation, this further justifies that lignin, the only highly aromatic compound of the chemicals tested, would be the source of activity once mechanical force was applied. While cellulose has previously been used as a catalyst support in transesterification [25], it was determined that the addition of sulfonated groups was the primary source of catalytic activity. Contrastingly, lignin has yielded lipids used for biodiesel production upon degradation by bacteria [26,27] and has been used for biofuel production following pyrolysis [28].
3.2. Effect of Lignin Milling Time
It was found that ball milling lignin for varying lengths of time produced different methyl esters. Due to the heterogeneity of commercial lignin and this study being focused on proof of concept and the identity of the catalyst support, percent composition of each methyl ester was not considered. Rather, the presence or absence of specific FAMEs after ball milling was used as an indicator of quality. Methyl stearate was most frequently seen, appearing in at least one of the three trials run for all milling times, as seen in Table 3. The only other methyl esters produced were at the shortest milling time of 60 min, which were a shorter chain, methyl palmitate, and a longer chain, methyl eicosenate. At the longest milling time of 135 min, a shorter chain, unsaturated methyl palmitoleate, was produced. The milling times of 105 and 120 min appear to be the optimum range for lignin, as methyl stearate along was produced in all three replications of both times. When determining the best milling time, one must consider the FAMEs produced, as oxidation stability is correlated with the bis-allylic sites. The more saturated a FAME is, the more resistant it will be to oxidation and, therefore, sediment formation in the biodiesel product [29]. Based on this, the milling times of 90 to 120 min produced the highest-quality biodiesel, as methyl stearate is fully saturated, whereas methyl eicosenate in the 60 min trials and methyl palmitoleate in the 135 min trials both contain unsaturations. Milling time did not significantly affect percent yield, as the average yield by volume for all trials was 75% in comparison to the 73% yield discussed in 3.1.

Table 3.
Methyl esters produced after milling lignin, methanol, and oil for varying times. A checkmark (✓) represents the presence of the FAME.
3.3. Milling Oak Wood
Ball milling using oak wood as a possible catalyst support did not lead to biodiesel in both trials, one with 0.5 g of tree shavings and the second with 1.0 g of tree shavings. Using 0.5 g of the shavings did lead to three layers of methanol, oil, and solid shavings after centrifuging; however, the chromatogram showed no formation of methyl esters. Increasing the mass of tree shavings used was thought to increase the concentration of lignin being introduced during milling, but this caused the canister to become overfilled, as after milling, the shavings had absorbed both the oil and methanol, resulting in a compacted mass of moist sawdust instead of three discernable layers.
Both lignin concentration and the units present within the oak could play a role in the lack of activity after milling. Oak is a type of hardwood, which typically is 18 to 25% lignin with a balance of S and G units. Softwoods would be ideal to further investigate, as they have a higher concentration of lignin and more G units [30]. While it is expected that the S units would be more likely to complex with metal due to the presence of two methoxy groups compared to the one of the G units, lignin’s large and amorphous structure could create unforeseen binding sites with S or G units.
3.4. Reflux of Lignin
Refluxing lignin, both alkaline and dealkaline alike, with methanol and seed oil only yielded biodiesel when ball-milled, which led to a percent yield of 72%. Lignin, which was hand-ground using a glass mortar and pestle prior to reflux, did not lead to methyl ester production. It is possible that the mechanical forces occurring during the milling process render the lignin catalytic. However, it is more likely that the metals present within the milling media complex with the lignin. This is supported by literature in which lignin and lignin derivatives complex metals such as copper, lead, and magnesium [31,32]. This occurs through acid sites of carboxyl and phenol groups of the lignin. Therefore, applying mechanical force to the lignin likely opens acid sites, to which the metals present within the milling media then adsorb.
The glass mortar and pestle were acid-washed prior to use as a means to remove any possible metal contamination, and lignin ground in it did not catalyze biodiesel formation when refluxed with seed oil and methanol. The same is true for untreated lignin. Additionally, obtaining the same results for both alkaline and dealkaline lignin in all pretreatment methods performed indicates that the alkalinity of the lignin itself does not determine its ability to become active, but it is instead from the ball mill environment.
3.5. Effect of Metals on Lignin
Samples of lignin were hand-ground in a glass mortar and pestle with powders of three different metals. Then, the samples were refluxed with methanol and seed oil in order to develop a sense of the time and force necessary for lignin to complex metals and thus become activated for transesterification. Of the metals tested, only iron and manganese led to biodiesel formation. Chromium did not, possibly due to it adsorbing to lignin best at a comparably lower pH (2) than iron or manganese, which adsorb best at higher pHs of 5 to 6 [33,34]. Future studies should include NMR and XPS analysis to determine the adsorption mechanisms of the metals and any differences between treatment methods such as ball milling versus using a mortar and pestle. Determination of acidic sites may also be accomplished via acid–base titration. Selective potentiometry of each metal would provide reference for results obtained from titration [31]. Lignin hand-ground with a stainless-steel mortar and pestle also produced biodiesel upon refluxing with methanol and oil. Grinding time affected the methyl esters produced; see Table 4. Lignin samples ground for 1 min, 3 min, and 6 min all led to the formation of biodiesel, indicating that only minimal mechanical activation is required for some transesterification to occur. Comparing iron and manganese, the choice of metal did not significantly impact the percent yield, as biodiesel from lignin ground with iron led to a 68% yield and biodiesel from lignin ground with manganese led to a 69% yield. These yields are lower than ball-milled samples and refluxed samples from 3.2 and 3.4, respectively, likely due to the much lower mechanical energy applied by a mortar and pestle in comparison to a ball mill.

Table 4.
Methyl esters produced after grinding lignin and iron or manganese in glass and then running reflux. A checkmark (✓) represents the presence of the FAME.
As discussed in 3.2 above, when analyzing the quality of the biodiesel produced, FAMEs with fewer unsaturations are more stable. Therefore, for lignin ground with iron, 1 min of grinding prior to reflux led to the highest-quality biodiesel with no unsaturated methyl esters. For lignin ground with manganese, six minutes of grinding was determined to be the optimal time, as methyl stearate is saturated and methyl eicosenate only contains one unsaturation.
3.6. ICP-MS Data
The calculated concentration of metals within the tested rapeseeds (RSs), biodiesel (BD), and methanol are shown in Table 5. From calculating the concentrations of metals in the samples and performing Student’s t-test on the values, ball milling had the expected effect of introducing metals into the seed shells. Specifically, sodium, chromium, manganese, iron, and lead all increased significantly by 104%, 12,000%, 63%, 32%, and 2770%, respectively. Grinding rapeseeds in stainless steel similarly caused a 19% increase in magnesium, 22,000% in chromium, 19% in iron, 181% in nickel, 16% in zinc, and 244% in lead. Comparing ball milling to grinding with a stainless-steel mortar and pestle, nickel was not introduced significantly from the milling canister and balls while it was with the mortar and pestle. The canister and balls also introduced manganese to the rapeseeds while grinding did not significantly affect the concentration of manganese in the rapeseeds. These differences are likely due to the different stainless-steel compositions of the milling media versus the mortar and pestle. The milling canister is specifically hardened steel, which has a higher carbon content than other types of steel, and it has also gone through heat treatment to make it more resistant to wear. The milling balls are 52-100 stainless steel, another wear-resistant steel that primarily consists of iron with small amounts of chromium (1.5%), manganese (0.45%), and other elements. The mortar and pestle are 18-8 stainless steel, consisting of but not limited to 18% chromium, 8% nickel, and 2% manganese, explaining why grinding introduced a significant amount of nickel while ball milling did not, as nickel was not present in the milling media. Nickel was not tested separately by grinding with lignin or seed shells in glass and then refluxing, but future studies with this metal would provide further information on the activation of lignin outside of the ball mill. Showing that nickel could cause lignin activation would provide another means of biodiesel production via a stainless-steel mortar and pestle followed by reflux with oil and methanol.

Table 5.
Mean concentrations (µg/g sample).
Grinding the rapeseeds in glass introduced some metals to the rapeseeds, namely, sodium, chromium, zinc, and lead. This may be due to metals sticking to the glass in small quantities, despite acid washing prior to use. However, we have still shown that biodiesel is not produced after grinding rapeseed shells in glass and then refluxing. This further proves that chromium, while present, does not complex with the lignin within the rapeseed shells. The same is likely true of zinc and lead. The biodiesel itself was shown to have significantly lower metal concentrations than the untreated rapeseeds for magnesium, aluminum, iron, nickel, and zinc. Furthermore, there was no significant change in concentration for chromium, manganese, or lead. The methanol also did not hold onto metal, indicating that the shells themselves hold onto the metals, avoiding affecting the quality of the biodiesel via the mechanical milling method.
4. Conclusions
We have discovered that the catalytic support within the seed shells is lignin, which is activated by metals, manganese and iron, in the canister during ball milling. This process produces biodiesel with yields of up to 90% for lignin within rapeseeds and sunflower seeds and yields up to 75% for valorized lignin due to the increased heterogeneity of the lignin. ICP-MS showed that ball milling and grinding with stainless steel introduces metals to rapeseed shells, and the shells retain the metals after transesterification with methanol and oil. Specifically, the lignin within the rapeseed shells retains manganese and iron, complexing with them and thus providing catalytic activity during milling or refluxing. This complexation is expected to occur through acidic sites within lignin that are exposed via mechanical force. Initial scale up and energetic considerations have been investigated in [35] with the use of a planetary ball mill, which requires longer milling times but less energy consumption in comparison to the high-energy ball mill used in this study.
Author Contributions
Conceptualization, M.H. and A.T.; methodology, A.T.; software, A.T.; validation, A.T., M.H. and M.B.; formal analysis, A.T.; investigation, A.T.; resources, M.H. and M.B.; data curation, A.T.; writing—original draft preparation, A.T.; writing—review and editing, A.T. and M.H.; visualization, A.T.; supervision, M.H. and M.B.; project administration, A.T. and M.H.; funding acquisition, M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Further data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ATR | Attenuated total reflection |
| BD | Biodiesel |
| FT-IR | Fourier transform infrared spectroscopy |
| G | Guaicyl |
| GC | Gas chromatography |
| H | Hydroxyphenyl |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| IR | Infrared |
| MIR | Mid-infrared spectroscopy |
| NMR | Nuclear magnetic resonance spectroscopy |
| RS | Rapeseed |
| S | Syringyl |
| SEM | Scanning electron microscopy |
| XPS | X-ray photoelectron spectroscopy |
References
- Simsek, S.; Uslu, S. Comparative Evaluation of the Influence of Waste Vegetable Oil and Waste Animal Oil-Based Biodiesel on Diesel Engine Performance and Emissions. Fuel 2020, 280, 118613. [Google Scholar] [CrossRef]
- Labeckas, G.; Slavinskas, S. The Effect of Rapeseed Oil Methyl Ester on Direct Injection Diesel Engine Performance and Exhaust Emissions. Energy Convers. Manag. 2006, 47, 1954–1967. [Google Scholar] [CrossRef]
- Carraretto, C.; Macor, A.; Mirandola, A.; Stoppato, A.; Tonon, S. Biodiesel as alternative fuel: Experimental analysis and energetic evaluations. Energy Convers. Manag. 2004, 29, 2195–2211. [Google Scholar] [CrossRef]
- Thirumarimurugan, M.; Sivakumar, V.; Merly-Xavier, A.; Prabhakaran, D.; Kannadasan, T. Preparation of Biodiesel from Sunflower Oil by Transesterification. Int. J. Biosci. Biochem. Bioinform. 2012, 2, 441–444. [Google Scholar] [CrossRef]
- Ashouri, R.; Jafari, D.; Esfandyari, M.; Vatankhah, G.; Mahdavi, M. Valorization of Slaughterhouse Wastes Through Transesterification for Sustainable Biodiesel Production Using Potassium Hydroxide as a Heterogeneous Catalyst. J. Clean. Prod. 2024, 447, 141596. [Google Scholar] [CrossRef]
- Lee, D.W.; Park, Y.M.; Lee, K.Y. Heterogeneous Base Catalysts for Transesterification in Biodiesel Synthesis. Catal. Surv. Asia 2009, 13, 63–77. [Google Scholar] [CrossRef]
- Tanner, A.; Baranek, M.; Eastlack, T.; Butts, B.; Beazley, M.; Hampton, M. Biodiesel Production Directly from Rapeseeds. Water 2023, 15, 2595. [Google Scholar] [CrossRef]
- Carré, P.; Citeau, M.; Robin, G.; Estorges, M. Hull Content and Chemical Composition of Whole Seeds, Hulls and Germs in Cultivars of Rapeseed (Brassica Napus). OCL 2016, 23, A302. [Google Scholar] [CrossRef]
- Asad, M.; Brahim, M.; Ziegler-Devin, I.; Boussetta, N.; Brosse, N. Chemical Characterization of Non-Saccharidic and Saccharidic Components of Rapeseed Hulls and Sunflower Shells. BioRes 2017, 12, 3143–3153. [Google Scholar] [CrossRef]
- Nagarajan, S.; Skillen, N.C.; Irvine, J.T.S.; Lawton, L.A.; Robertson, P.K.J. Cellulose II as Bioethanol Feedstock and Its Advantages over Native Cellulose. Renew. Sustain. Energy Rev. 2017, 77, 182–192. [Google Scholar] [CrossRef]
- Carroll, A.; Somerville, C. Cellulosic Biofuels. Annu. Rev. Plant Biol. 2009, 60, 165–182. [Google Scholar] [CrossRef] [PubMed]
- Brahim, M.; Boussetta, N.; Grimi, N.; Vorobiev, E.; Zieger-Devin, I.; Brosse, N. Pretreatment Optimization from Rapeseed Straw and Lignin Characterization. Ind. Crops Prod. 2017, 95, 643–650. [Google Scholar] [CrossRef]
- Ziebell, A.L.; Barb, J.G.; Sandhu, S.; Moyers, B.T.; Sykes, R.W.; Doeppke, C.; Gracom, K.L.; Carlile, M.; Marek, L.F.; Davis, M.F.; et al. Sunflower as a Biofuels Crop: An Analysis of Lignocellulosic Chemical Properties. Biomass Bioenerg. 2013, 59, 208–217. [Google Scholar] [CrossRef]
- Vanholme, R.; Demedts, B.; Morreel, K.; Ralph, J.; Boerjan, W. Lignin Biosynthesis and Structure. Plant Physiol. 2010, 153, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Shah, T.W.; Zhihe, L.; Zhiyu, L.; Andong, Z. Composition and Role of Lignin in Biochemicals. In Lignin—Chemistry, Structure, and Application, 1st ed.; Sand, A., Tuteja, J., Eds.; Intech Open: London, UK, 2022; pp. 149–200. [Google Scholar] [CrossRef]
- Srivastava, S.K.; Singh, A.K.; Sharma, A. Studies on the Uptake of Lead and Zinc by Lignin Obtained from Black Liquor—A Paper Industry Waste Material. Environ. Technol. 1994, 15, 353–361. [Google Scholar] [CrossRef]
- Demirbas, A. Adsorption of Lead and Cadmium Ions in Aqueous Solutions onto Modified Lignin from Alkali Glycerol Delignication. J. Hazard. Mater. 2004, 109, 221–226. [Google Scholar] [CrossRef]
- Hokkanen, S.; Bhatnagar, A.; Sillanpää, M. A Review on Modification Methods to Cellulose-Based Adsorbents to Improve Adsorption Capacity. Water Res. 2016, 91, 156–173. [Google Scholar] [CrossRef] [PubMed]
- Gurgel, L.V.A.; Júnior, O.K.; Gil, R.P.d.F.; Gil, L.F. Adsorption of Cu(II), Cd(II), and Pb(II) from Aqueous Single Metal Solutions by Cellulose and Mercerized Cellulose Chemically Modified with Succinic Anhydride. Bioresour. Technol. 2008, 99, 3077–3083. [Google Scholar] [CrossRef]
- Kenawy, I.M.; Hafez, M.A.H.; Ismail, M.A.; Hashem, M.A. Adsorption of Cu(II), Cd(II), Hg(II), Pb(II) and Zn(II) from Aqueous Single Metal Solutions by Guanyl-Modified Cellulose. Int. J. Biol. Macromol. 2018, 107, 1538–1549. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Z. Application of lignin and its derivatives in adsorption of heavy metal ions in water: A review. ACS Sustain. Chem. Eng. 2018, 6, 7181–7192. [Google Scholar] [CrossRef]
- Kropat, M.; Liao, M.; Park, H.; Salem, K.; Johnson, S.; Argyropoulos, D. A perspective of lignin processing and utilization technologies for composites and plastics with emphasis on technical and market trends. Bioresources 2021, 16, 2084–2115. [Google Scholar] [CrossRef]
- Argyropoulos, D. The emerging bio-refinery industry needs to refine lignin prior to use. J. Biotechnol. Biomater. 2014, 6, e001. [Google Scholar] [CrossRef]
- Pan, H.; Sun, G.; Zhao, T. Synthesis and characterization of aminated lignin. Int. J. Biol. Macromol. 2013, 59, 221–226. [Google Scholar] [CrossRef]
- Saikia, K.; Ngaosuwan, K.; Assabumrungrat, S.; Singh, B.; Okoye, P.; Rashid, U.; Rokhum, S. Sulphonated cellulose-based carbon as a green heterogeneous catalyst for biodiesel production: Process optimization and kinetic studies. Biomass Bioenerg. 2023, 173, 106799. [Google Scholar] [CrossRef]
- Zhang, K.; Rong, X.; Abomohra, A.; Xie, S.; Yu, Z.; Guo, Q.; Liu, P.; Peng, L.; Li, X.A. sustainable approach for efficient conversion of lignin into biodiesel accompanied by biological pretreatment of corn straw. Energy Convers. Manag. 2019, 199, 111928. [Google Scholar] [CrossRef]
- Bhatia, S.; Gurav, R.; Choi, T.-R.; Han, Y.; Park, Y.-L.; Park, J.; Jung, H.-R.; Yang, S.-Y.; Song, H.-S.; Kim, S.-H.; et al. Bioconversion of barley straw lignin into biodiesel using Rhodococcus sp. YHY01. Bioresour. Technol. 2019, 289, 121704. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.; Dasan, Y.; Khan, I. Extraction of Lignin from Biomass for Biodiesel Production, 1st ed.; Springer Nature: Cham, Switzerland, 2015; pp. 169–176. [Google Scholar] [CrossRef]
- Cosgrove, J.; Church, D.; Pryor, W. The kinetics of the autoxidation of polyunsaturated fatty acids. Lipids 1987, 22, 299–304. [Google Scholar] [CrossRef]
- Sakakibara, A. A structural model of softwood lignin. Wood Sci. Technol. 1980, 14, 89–100. [Google Scholar] [CrossRef]
- Garcia-Valls, R.; Hatton, T. Metal ion complexation with lignin derivatives. Chem. Eng. J. 2003, 94, 99–105. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, S.; Shan, X. Adsorption of metal ion on lignin. J. Hazard. Mater. 2008, 151, 134–142. [Google Scholar] [CrossRef]
- Liang, F.-B.; Song, Y.-L.; Huang, C.-P.; Zhang, J.; Chen, B.-H. Adsorption of Hexavalent Chromium on a Lignin-Based Resin: Equilibrium, Thermodynamics, and Kinetics. J. Environ. Chem. Eng. 2013, 1, 1301–1308. [Google Scholar] [CrossRef]
- Lalvani, S.; Hubner, A.; Wiltowski, T. Chromium Adsorption by Lignin. Energy Sources 2000, 22, 45–56. [Google Scholar] [CrossRef]
- Tanner, S. A Greener Way to Synthesize Biodiesel: Mechanochemistry with no Added Catalyst. Graduate Thesis, University of Central Florida, Orlando, FL, USA, 2025. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).