The Effect of Hydrothermal Carbonization Temperature on Microplastic Content in Digested Sewage Sludge and Its Relation to the Fuel Properties of Hydrochars
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Reagents
2.1.2. Digested Sewage Sludge Collection
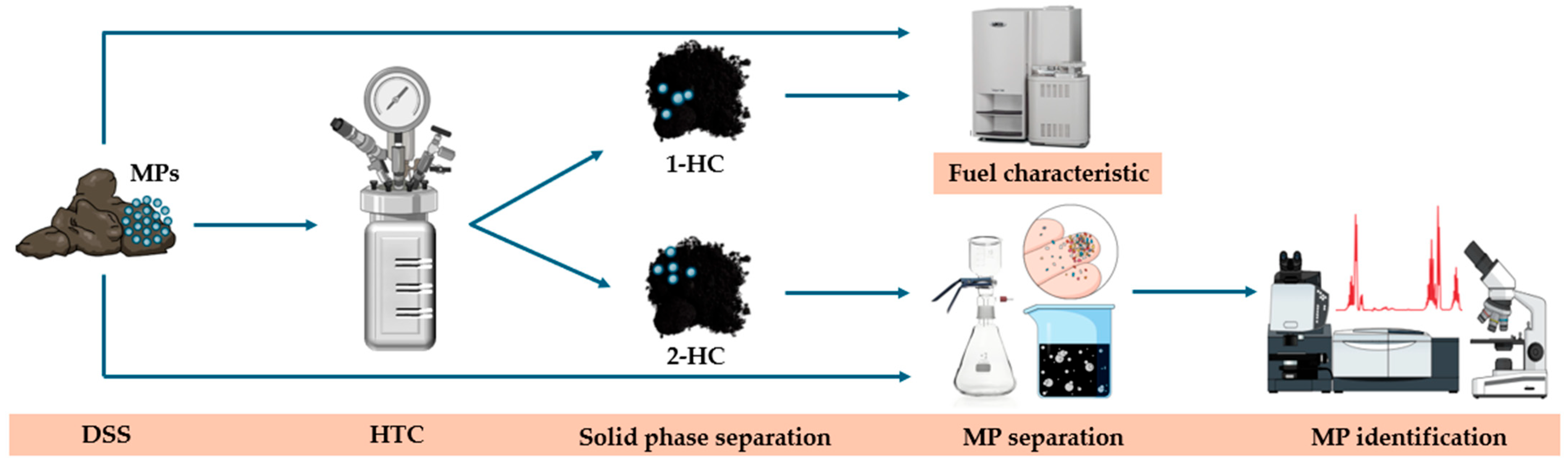
2.2. Methods
2.2.1. Hydrothermal Carbonization
2.2.2. Fuel Properties of DSS and 1-HCs
2.2.3. MP Extraction
2.2.4. MP Identification
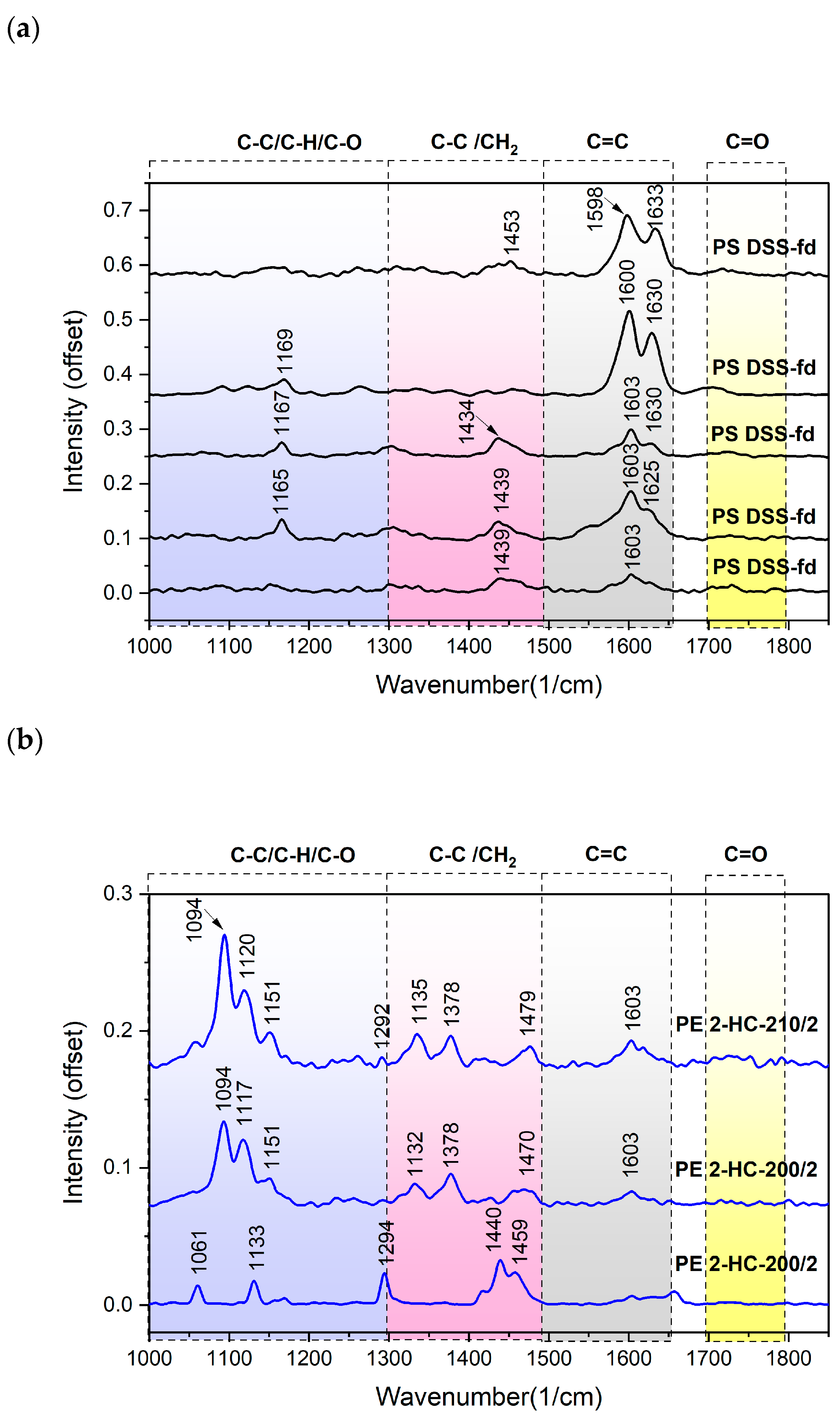
Confocal Raman Microspectroscopy (CRM)
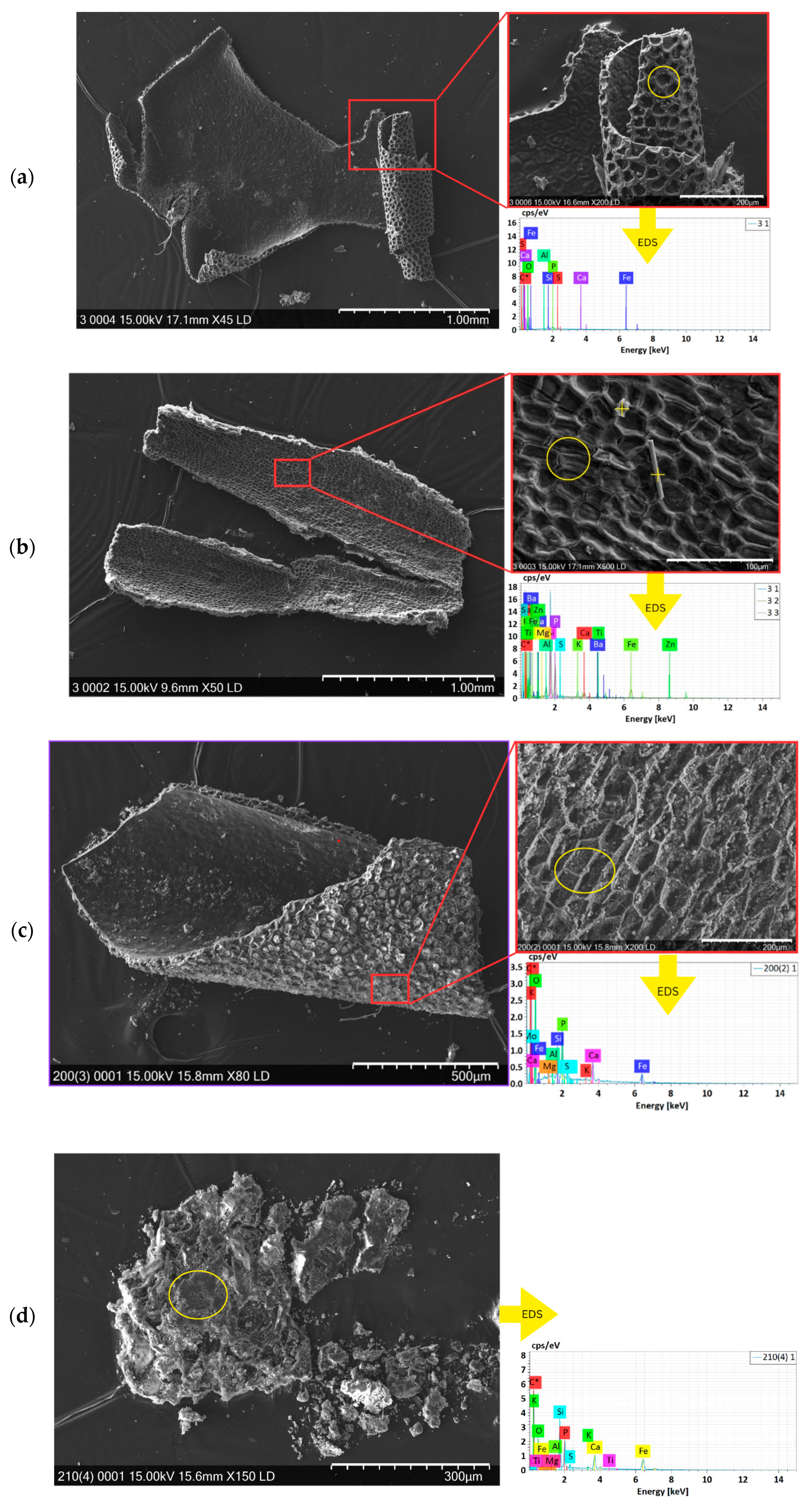
Scanning Electron Microscopy with Energy-Dispersive X-Ray Spectroscopy (SEM-EDS)
2.2.5. Quality Assurance and Quality Control
3. Results and Discussion
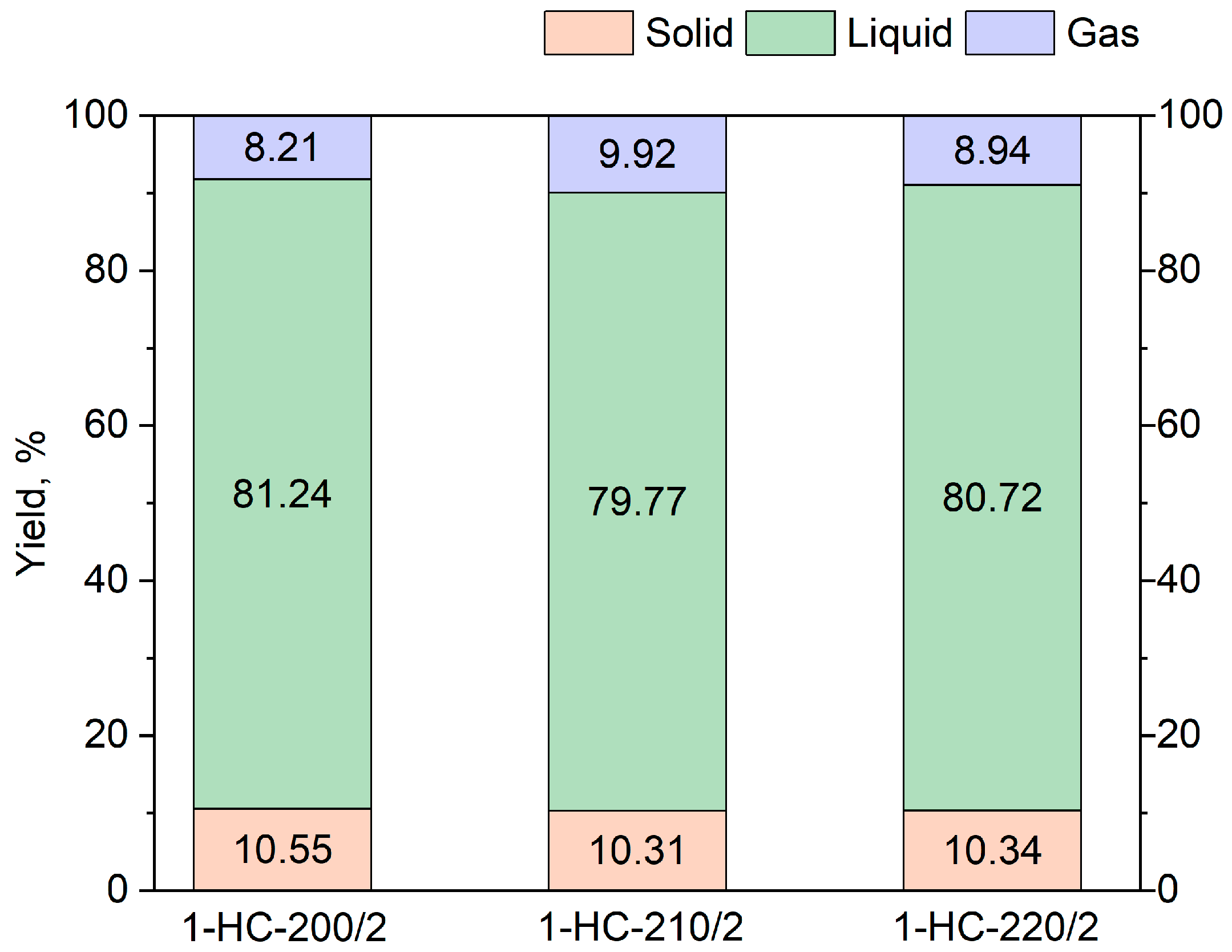
3.1. HTC Product Distribution
3.2. Fuel Properties of Sludge and Hydrochars
3.3. Microplastic Characteristics
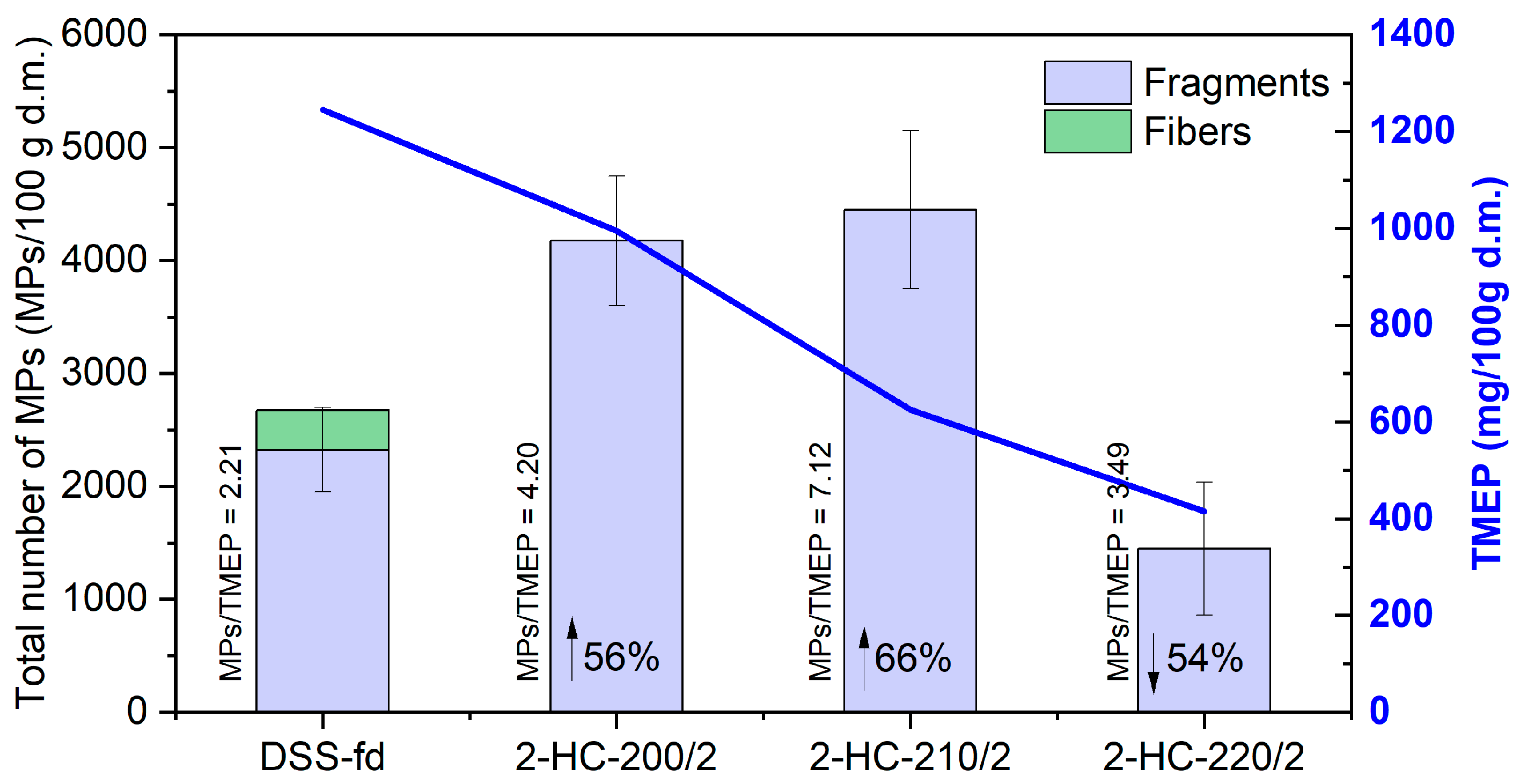
3.3.1. MP Occurrence
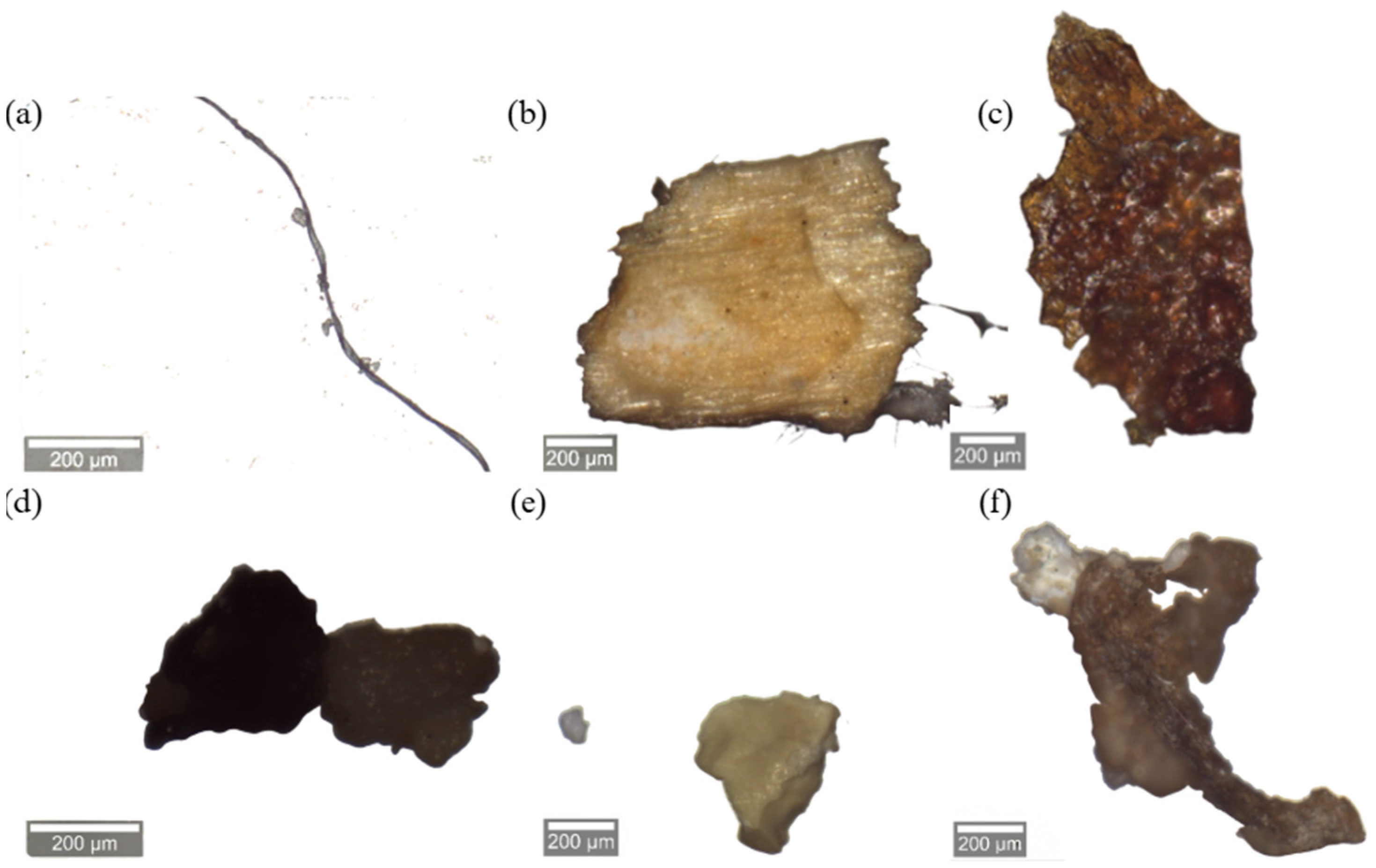
3.3.2. Polymer Identification
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, R.C.; Moore, C.J.; vom Saal, F.S.; Swan, S.H. Plastics, the Environment and Human Health: Current Consensus and Future Trends. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef]
- Allen, S.; Allen, D.; Phoenix, V.R.; Le Roux, G.; Jimenez, P.D.; Simonneau, A.; Binet, S.; Galop, D. Atmospheric Transport and Deposition of Microplastics in a Remote Mountain Catchment. Nat. Geosci. 2019, 12, 339–344. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.L.; Kelly, F.J. Plastic and Human Health: A Micro Issue? Environ. Sci. Technol. 2017, 51, 6634–6647. [Google Scholar] [CrossRef] [PubMed]
- Kurniawan, S.B.; Mohd Said, N.S.; Imron, M.F.; Abdullah, S.R.S. Microplastic Pollution in the Environment: Insights into Emerging Sources and Potential Threats. Environ. Technol. Innov. 2021, 23, 101790. [Google Scholar] [CrossRef]
- Carnevale Miino, M.; Galafassi, S.; Zullo, R.; Torretta, V.; Rada, E.C. Microplastics Removal in Wastewater Treatment Plants: A Review of the Different Approaches to Limit Their Release in the Environment. Sci. Total Environ. 2024, 930, 172675. [Google Scholar] [CrossRef]
- Habib, R.Z.; Thiemann, T.; Al Kendi, R. Microplastics and Wastewater Treatment Plants—A Review. J. Water Resour. Prot. 2020, 12, 1–35. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chiueh, P.-T.; Lo, S.-L. Energy Recovery from Sewage Sludge: Product Characteristics, Heating Value Prediction and Reaction Kinetics. Chemosphere 2021, 268, 128783. [Google Scholar] [CrossRef]
- Khanh Nguyen, V.; Kumar Chaudhary, D.; Hari Dahal, R.; Hoang Trinh, N.; Kim, J.; Chang, S.W.; Hong, Y.; Duc La, D.; Nguyen, X.C.; Hao Ngo, H.; et al. Review on Pretreatment Techniques to Improve Anaerobic Digestion of Sewage Sludge. Fuel 2021, 285, 119105. [Google Scholar] [CrossRef]
- Alper, K.; Tekin, K.; Karagöz, S.; Ragauskas, A.J. Sustainable Energy and Fuels from Biomass: A Review Focusing on Hydrothermal Biomass Processing. Sustain. Energy Fuels 2020, 4, 4390–4414. [Google Scholar] [CrossRef]
- European Environment Agency. Greenhouse Gas Intensities of Transport Fuels in the EU in 2021 Monitoring; European Environment Agency: Copenhagen, Denmark, 2023. [Google Scholar]
- Wilk, M.; Gajek, M.; Śliz, M.; Czerwińska, K.; Lombardi, L. Hydrothermal Carbonization Process of Digestate from Sewage Sludge: Chemical and Physical Properties of Hydrochar in Terms of Energy Application. Energies 2022, 15, 6499. [Google Scholar] [CrossRef]
- Wilk, M.; Czerwińska, K.; Śliz, M.; Imbierowicz, M. Hydrothermal Carbonization of Sewage Sludge: Hydrochar Properties and Processing Water Treatment by Distillation and Wet Oxidation. Energy Rep. 2023, 9, 39–58. [Google Scholar] [CrossRef]
- Czerwińska, K.; Śliz, M.; Wilk, M. Thermal Disposal of Post-Processing Water Derived from the Hydrothermal Carbonization Process of Sewage Sludge. Waste Biomass Valorization 2024, 15, 1671–1680. [Google Scholar] [CrossRef]
- Czerwińska, K.; Mikusińska, J.; Błoniarz, A.; Śliz, M.; Wilk, M. The Effect of Residence Time during the Hydrothermal Carbonization Process of Sewage Sludge on the Properties of Hydrochar. Energies 2024, 17, 3380. [Google Scholar] [CrossRef]
- Cao, Z.; Hülsemann, B.; Wüst, D.; Oechsner, H.; Lautenbach, A.; Kruse, A. Effect of Residence Time during Hydrothermal Carbonization of Biogas Digestate on the Combustion Characteristics of Hydrochar and the Biogas Production of Process Water. Bioresour. Technol. 2021, 333, 125110. [Google Scholar] [CrossRef]
- Chen, M.; Coleman, B.; Gaburici, L.; Prezgot, D.; Jakubek, Z.J.; Sivarajah, B.; Vermaire, J.C.; Lapen, D.R.; Velicogna, J.R.; Princz, J.I.; et al. Identification of Microplastics Extracted from Field Soils Amended with Municipal Biosolids. Sci. Total Environ. 2024, 907, 168007. [Google Scholar] [CrossRef]
- Prus, Z.; Wilk, M. Microplastics in Sewage Sludge: Worldwide Presence in Biosolids, Environmental Impact, Identification Methods and Possible Routes of Degradation, Including the Hydrothermal Carbonization Process. Energies 2024, 17, 4219. [Google Scholar] [CrossRef]
- Jiang, C.; Chen, Z.; Lu, B.; Li, Z.; Zhang, S.; Liu, Y.; Luo, G. Hydrothermal Pretreatment Reduced Microplastics in Sewage Sludge as Revealed by the Combined Micro-Fourier Transform Infrared (FTIR) and Raman Imaging Analysis. Chem. Eng. J. 2022, 450, 138163. [Google Scholar] [CrossRef]
- Xu, Z.; Bai, X. Microplastic Degradation in Sewage Sludge by Hydrothermal Carbonization: Efficiency and Mechanisms. Chemosphere 2022, 297, 134203. [Google Scholar] [CrossRef]
- Jose, S.; Lonappan, L.; Cabana, H. Hydrothermal Carbonization as a Promising Approach towards the Removal of Polyethylene Microplastics and Trace Organic Contaminants from Wastewater Sludge. J. Environ. Chem. Eng. 2025, 13, 118262. [Google Scholar] [CrossRef]
- Wilk, M.; Śliz, M.; Czerwińska, K.; Gajek, M.; Kalemba-Rec, I. Improvements in Dewaterability and Fuel Properties of Hydrochars Derived from Hydrothermal Co-Carbonization of Sewage Sludge and Organic Waste. Renew. Energy 2024, 227, 120547. [Google Scholar] [CrossRef]
- Akaniro, I.R.; Zhang, R.; Tsang, C.H.M.; Wang, P.; Yang, Z.; Zhao, J. Exploring the Potential of Hydrothermal Treatment for Microplastics Removal in Digestate. ACS Sustain. Chem. Eng. 2024, 12, 14187–14199. [Google Scholar] [CrossRef]
- PKN-ISO/TS 12902:2007; Solid Mineral Fuels—Determination of Total Carbon, Hydrogen and Nitrogen—Instrumental Method. Polish Committee for Standardization: Warsaw, Poland, 2007.
- Channiwala, S.A.; Parikh, P.P. A Unified Correlation for Estimating HHV of Solid, Liquid and Gaseous Fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- ISO 17246:2010; Coal—Proximate Analysis. International Organization for Standardization: Geneva, Switzerland, 2010.
- ASTM D7582-15; Standard Test Methods for Proximate Analysis of Coal and Coke by Macro Thermogravimetric Analysis. ASTM International: West Conshohocken, PA, USA, 2015.
- Worek, J.; Styszko, K. Comparative Study of Matrix Etching Methods for the Separation of Microplastics from Environmental Samples. Desalination Water Treat. 2025, 322, 101140. [Google Scholar] [CrossRef]
- Casella, C.; Sol, D.; Laca, A.; Díaz, M. Microplastics in Sewage Sludge: A Review. Environ. Sci. Pollut. Res. 2023, 30, 63382–63415. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, J.; Zhao, X.; Gu, X.; Ji, R. Separation and Identification of Microplastics from Soil and Sewage Sludge. Environ. Pollut. 2019, 254, 113076. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, Y.; Gao, Y.; Li, X.; Gong, Y. Study on the Extraction Method of Microplastic System in Textile Wastewater. Polymers 2023, 15, 1394. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Park, K.Y. Hydrothermal Carbonization of Anaerobically Digested Sludge for Solid Fuel Production and Energy Recovery. Fuel 2014, 130, 120–125. [Google Scholar] [CrossRef]
- Roslan, S.Z.; Zainudin, S.F.; Mohd Aris, A.; Chin, K.B.; Musa, M.; Mohamad Daud, A.R.; Syed Hassan, S.S.A. Hydrothermal Carbonization of Sewage Sludge into Solid Biofuel: Influences of Process Conditions on the Energetic Properties of Hydrochar. Energies 2023, 16, 2483. [Google Scholar] [CrossRef]
- Xie, L.; Gou, L.; Wang, Y.; Dai, L. Co-Hydrothermal Carbonization of Sewage Sludge and Polyvinyl Chloride for the Production of High-Quality Solid Fuel with Low Nitrogen Content. Sci. Total Environ. 2022, 804, 150094. [Google Scholar] [CrossRef]
- Wang, L.; Li, A.; Chang, Y. Hydrothermal Treatment Coupled with Mechanical Expression at Increased Temperature for Excess Sludge Dewatering: Heavy Metals, Volatile Organic Compounds and Combustion Characteristics of Hydrochar. Chem. Eng. J. 2016, 297, 1–10. [Google Scholar] [CrossRef]
- Musivand, S.; Bracciale, M.P.; Damizia, M.; De Filippis, P.; de Caprariis, B. Viable Recycling of Polystyrene via Hydrothermal Liquefaction and Pyrolysis. Energies 2023, 16, 4917. [Google Scholar] [CrossRef]
- Saad, M.; Bucki, M.; Bujok, S.; Pawcenis, D.; Rijavec, T.; Górecki, K.; Bratasz, Ł.; Kralj Cigić, I.; Strlič, M.; Kruczała, K. The Impact of Heat and Humidity on Unplasticized Poly(Vinyl Chloride). Polym. Degrad. Stab. 2025, 238, 111334. [Google Scholar] [CrossRef]
- Kimukai, H.; Kodera, Y.; Koizumi, K.; Okada, M.; Yamada, K.; Hiaki, T.; Saido, K. Low Temperature Decomposition of Polystyrene. Appl. Sci. 2020, 10, 5100. [Google Scholar] [CrossRef]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef]
- Wu, J.; Gao, J.; Pei, Y.; Luo, K.; Yang, W.; Wu, J.; Yue, X.; Wen, J.; Luo, Y. Microplastics in Agricultural Soils: A Comprehensive Perspective on Occurrence, Environmental Behaviors and Effects. Chem. Eng. J. 2024, 489, 151328. [Google Scholar] [CrossRef]
- Lares, M.; Ncibi, M.C.; Sillanpää, M.; Sillanpää, M. Occurrence, Identification and Removal of Microplastic Particles and Fibers in Conventional Activated Sludge Process and Advanced MBR Technology. Water Res. 2018, 133, 236–246. [Google Scholar] [CrossRef]
- Letwin, N.V.; Gillespie, A.W.; Ijzerman, M.M.; Kudla, Y.M.; Csajaghy, J.D.; Prosser, R.S. Characterizing the Microplastic Content of Biosolids in Southern Ontario, Canada. Environ. Toxicol. Chem. 2023, 43, 793–806. [Google Scholar] [CrossRef]
- Styszko, K.; Bolesta, W.; Worek, J.; Prus, Z.; Cwynar, K.; Pyssa, J.; Uchmanowicz, D.; Frydel, L.; Daso, A.P.; Kasprzyk-Hordern, B. Tracking Nonregulated Micropollutants in Sewage Sludge: Antimicrobials, OH-PAHs, and Microplastics—Environmental Risks, Fertilizer Implications and Energy Considerations. Energy Rep. 2025, 13, 4756–4768. [Google Scholar] [CrossRef]
- Worek, J.; Kawoń, K.; Chwiej, J.; Berent, K.; Rego, R.; Styszko, K. Assessment of the Presence of Microplastics in Stabilized Sewage Sludge: Analysis Methods and Environmental Impact. Appl. Sci. 2024, 15, 1. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Dement’ev, K.I.; Palankoev, T.A.; Alekseeva, O.A.; Babkin, I.A.; Maksimov, A.L. Thermal Depolymerization of Polystyrene in Highly Aromatic Hydrocarbon Medium. J. Anal. Appl. Pyrolysis 2019, 142, 104612. [Google Scholar] [CrossRef]
- Prata, J.C.; Paço, A.; Reis, V.; da Costa, J.P.; Fernandes, A.J.S.; da Costa, F.M.; Duarte, A.C.; Rocha-Santos, T. Identification of Microplastics in White Wines Capped with Polyethylene Stoppers Using Micro-Raman Spectroscopy. Food Chem. 2020, 331, 127323. [Google Scholar] [CrossRef]
- Luo, H.; Zhao, Y.; Li, Y.; Xiang, Y.; He, D.; Pan, X. Aging of Microplastics Affects Their Surface Properties, Thermal Decomposition, Additives Leaching and Interactions in Simulated Fluids. Sci. Total Environ. 2020, 714, 136862. [Google Scholar] [CrossRef] [PubMed]
- Mais, L.; Melis, N.; Vacca, A.; Mascia, M. Electrochemical Removal of PET and PE Microplastics for Wastewater Treatment. Environ. Sci. 2024, 10, 399–407. [Google Scholar] [CrossRef]
- Hao, L.; Ma, H.; Xing, B. Surface Characteristics and Adsorption Properties of Polypropylene Microplastics by Ultraviolet Irradiation and Natural Aging. Sci. Total Environ. 2024, 944, 173962. [Google Scholar] [CrossRef]
- Liu, X.; Sun, P.; Qu, G.; Jing, J.; Zhang, T.; Shi, H.; Zhao, Y. Insight into the Characteristics and Sorption Behaviors of Aged Polystyrene Microplastics through Three Type of Accelerated Oxidation Processes. J. Hazard. Mater. 2021, 407, 124836. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Skrzypek, G.; Hernández-Sánchez, C.; Ortega-Zamora, C.; González-Sálamo, J.; González-Curbelo, M.Á.; Hernández-Borges, J. Microplastic-Adsorbed Organic Contaminants: Analytical Methods and Occurrence. Trends Anal. Chem. 2021, 136, 116186. [Google Scholar] [CrossRef]

| Sample/ Parameter | Ultimate Analysis | Proximate Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O | HHV | M | A | VM | FC | |
| % | % | % | % | % | MJ/kg | % | % | % | % | |
| DSS-d | 28.0660 24.0880 28.1400 | 5.3334 4.5771 5.5261 | 4.3146 2.6743 4.4014 | 1.2975 1.3006 1.3015 | 15.6660 22.0606 15.9425 | 13.8136 10.8987 14.0475 | 12.76 12.8 12.63 | 33.86 33.80 33.36 | 50.35 46.47 46.97 | 3.03 6.93 7.04 |
| 1-HC-200/2 | 30.4500 30.2470 30.3740 | 4.2101 4.1820 3.7538 | 3.4318 3.4231 3.4114 | 1.1900 1.1800 | 11.1481 11.1279 11.8708 | 13.4612 13.3519 12.7057 | 1.23 1.18 1.19 | 49.53 49.84 49.40 | 41.15 41.19 41.25 | 8.09 7.79 8.16 |
| 1-HC-210/2 | 30.5910 30.5120 | 4.1887 4.0978 | 3.2281 3.2558 | 1.1500 1.1800 | 10.2922 10.3544 48.4100 | 13.5670 13.4256 | 1.9 1.84 1.82 | 49.80 49.94 49.77 | 40.3 40.24 40.18 | 8.00 7.98 8.23 |
| 1-HC-220/2 | 28.341 28.3920 29.8050 | 3.8217 3.8213 3.9093 | 2.9405 2.9505 2.9235 | 1.1800 1.1500 | 12.6668 12.6562 11.3822 | 12.0997 12.1114 12.7340 | 1.90 1.68 1.91 | 50.33 50.50 50.07 | 42.72 42.7 42.52 | 5.05 5.12 5.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prus, Z.; Szkadłubowicz, K.; Mikusińska, J.; Dróżdż, A.; Brunarska, I.; Chwiej, J.; Styszko, K.; Wilk, M. The Effect of Hydrothermal Carbonization Temperature on Microplastic Content in Digested Sewage Sludge and Its Relation to the Fuel Properties of Hydrochars. Energies 2025, 18, 5105. https://doi.org/10.3390/en18195105
Prus Z, Szkadłubowicz K, Mikusińska J, Dróżdż A, Brunarska I, Chwiej J, Styszko K, Wilk M. The Effect of Hydrothermal Carbonization Temperature on Microplastic Content in Digested Sewage Sludge and Its Relation to the Fuel Properties of Hydrochars. Energies. 2025; 18(19):5105. https://doi.org/10.3390/en18195105
Chicago/Turabian StylePrus, Zuzanna, Klaudia Szkadłubowicz, Joanna Mikusińska, Agnieszka Dróżdż, Irena Brunarska, Joanna Chwiej, Katarzyna Styszko, and Małgorzata Wilk. 2025. "The Effect of Hydrothermal Carbonization Temperature on Microplastic Content in Digested Sewage Sludge and Its Relation to the Fuel Properties of Hydrochars" Energies 18, no. 19: 5105. https://doi.org/10.3390/en18195105
APA StylePrus, Z., Szkadłubowicz, K., Mikusińska, J., Dróżdż, A., Brunarska, I., Chwiej, J., Styszko, K., & Wilk, M. (2025). The Effect of Hydrothermal Carbonization Temperature on Microplastic Content in Digested Sewage Sludge and Its Relation to the Fuel Properties of Hydrochars. Energies, 18(19), 5105. https://doi.org/10.3390/en18195105









