A Dimensionless Number for Evaluating the Influence of Heat Conduction in the Gas Phase on Liquid Evaporation
Abstract
1. Introduction
2. Mathematical Model
3. Results and Discussion
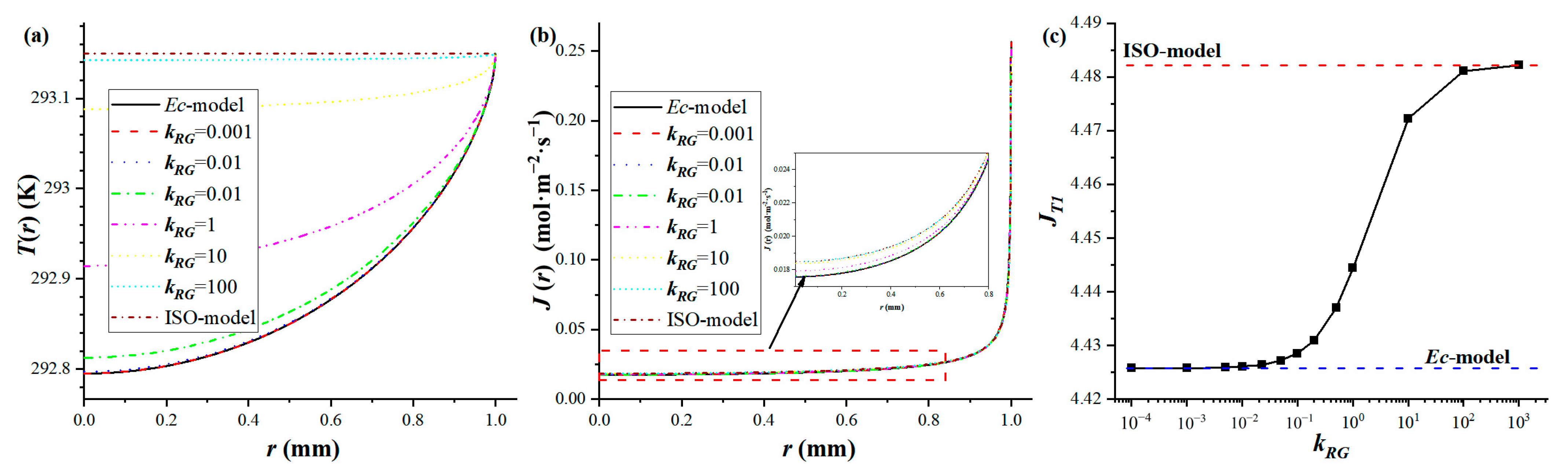
3.1. Limiting Solutions for Droplet Evaporation
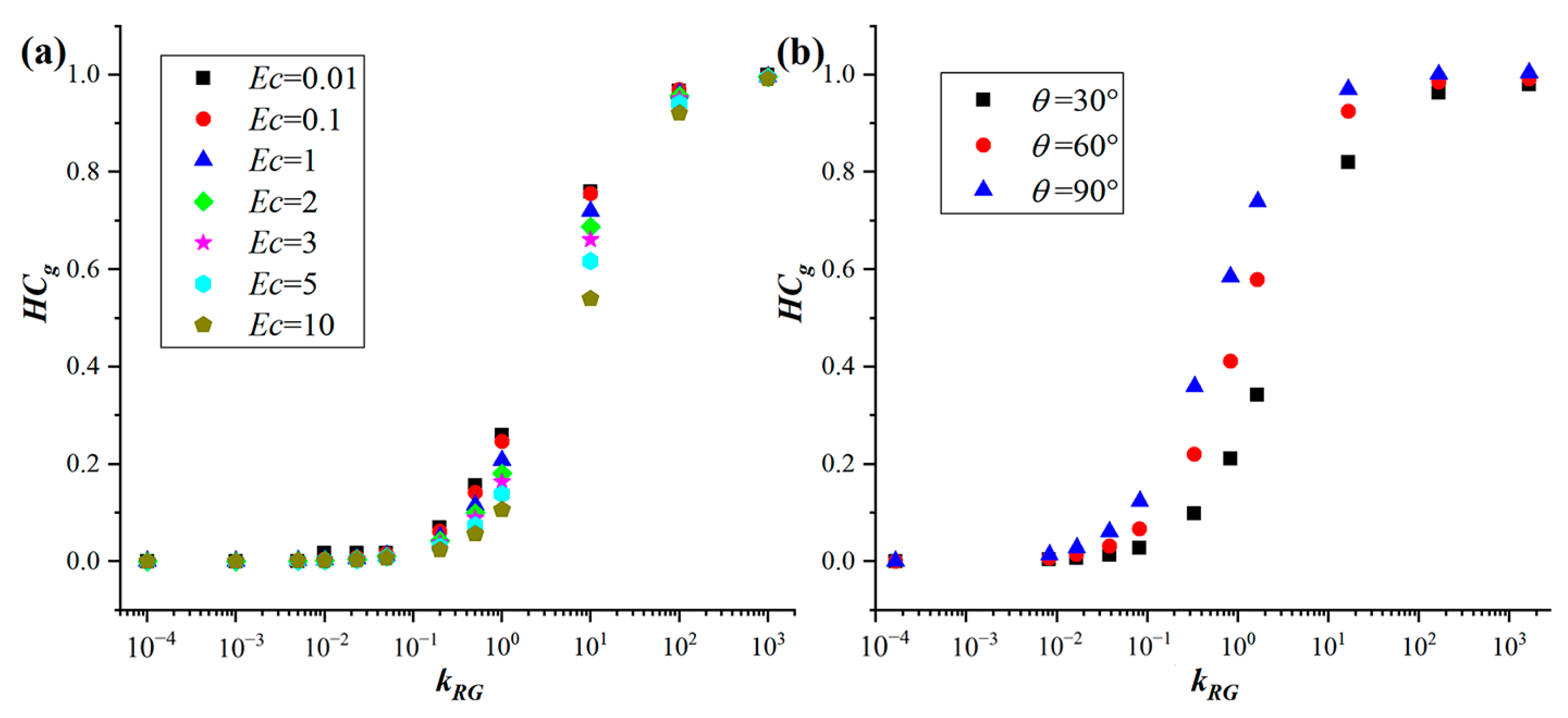
3.2. A Dimensionless Number Characterizing the Influence of Heat Conduction in Gas
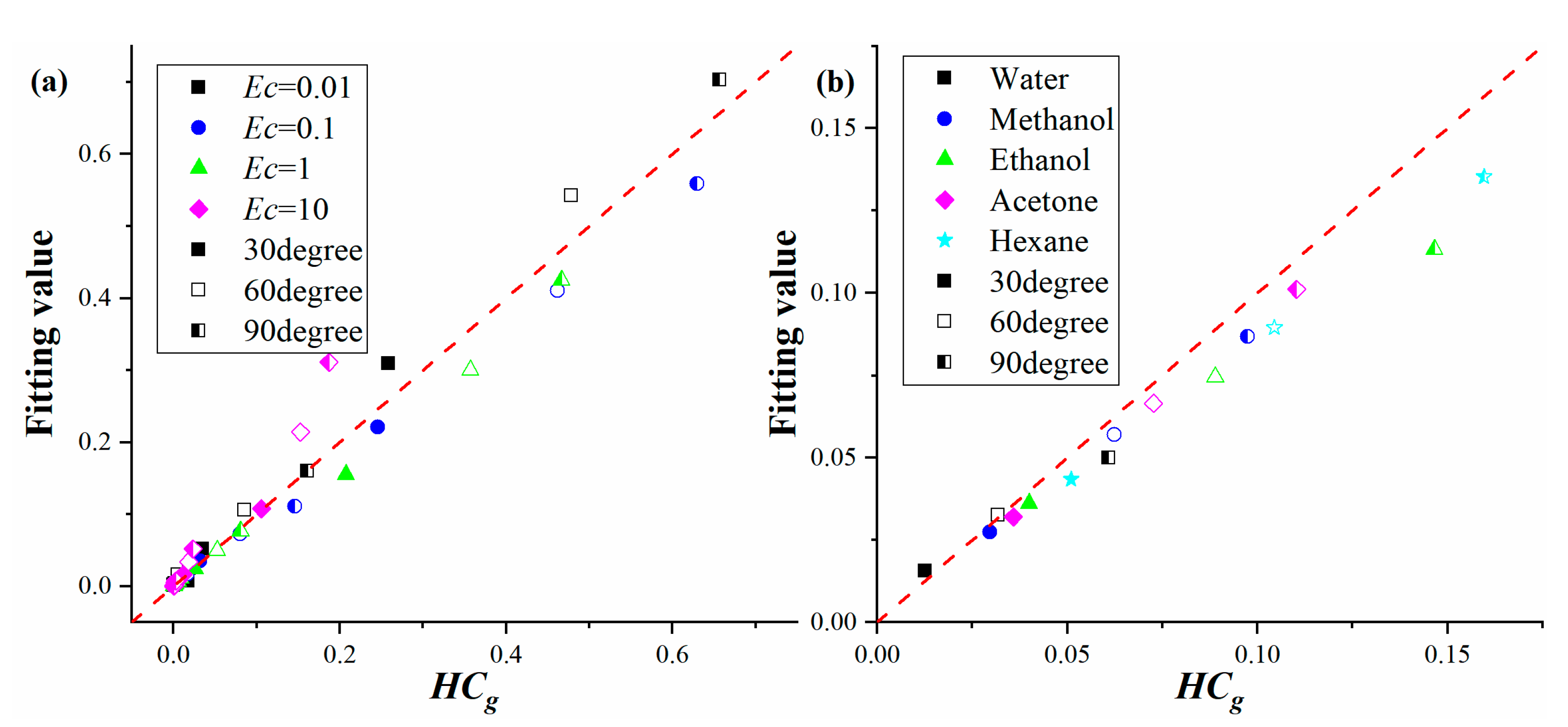
3.3. Analytic Expressions for HCg and the Droplet Evaporation Rate
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yang, Z.; Tian, H.; Wang, C.; Li, X.; Chen, X.; Chen, X.; Shao, J. Piezoelectric drop-on-demand inkjet printing with ultra-high droplet velocity. Research 2023, 6, 248. [Google Scholar] [CrossRef]
- Chen, L.; Xiao, Y.; Wu, Q.; Yan, X.; Zhao, P.; Ruan, J.; Shan, J.; Chen, D.; Weitz, D.A.; Ye, F. Emulsion designer using microfluidic three-dimensional droplet printing in droplet. Small 2021, 17, 2102579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, D.; Liu, Y.; Wittstock, G. Printing with satellite droplets. Small 2018, 14, 1802583. [Google Scholar] [CrossRef]
- Tang, Y.; Song, S.; Peng, J.; Zhang, Q.; Lin, W. An ultrasensitive lipid droplet-targeted NIR emission fluorescent probe for polarity detection and its application in liver disease diagnosis. J. Mater. Chem. B 2022, 10, 6974–6982. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Zhao, Y.; Tan, C.; Bai, L.; Huang, G.; Wang, R.; Huang, H.; Yu, X.; Guo, Y.; Chen, P. Development of a droplet digital PCR assay for the detection of BK polyomavirus. Microbiol. Spectr. 2024, 12, e01024–e01089. [Google Scholar] [CrossRef] [PubMed]
- Schena, M.; Shalon, D.; Davis, R.W.; Brown, P.O. Quantitative monitoring of gene expression patterns with a complementary DNA microarray. Science 1995, 270, 467–470. [Google Scholar] [CrossRef]
- Jing, J.; Reed, J.; Huang, J.; Hu, X.; Clarke, V.; Edington, J.; Housman, D.; Anantharaman, T.S.; Huff, E.J.; Mishra, B. Automated high resolution optical mapping using arrayed, fluid-fixed DNA molecules. Proc. Natl. Acad. Sci. USA 1998, 95, 8046–8051. [Google Scholar] [CrossRef]
- Kolegov, K.S.; Barash, L.Y. Applying droplets and films in evaporative lithography. Adv. Colloid. Interface. Sci. 2020, 285, 102271. [Google Scholar] [CrossRef]
- Vasiliev, L.L. Heat pipes in modern heat exchangers. Appl. Therm. Eng. 2005, 25, 1–19. [Google Scholar] [CrossRef]
- Shailesh, K.; Naresh, Y.; Banerjee, J. Heat transfer performance of a novel PCM based heat sink coupled with heat pipe: An experimental study. Appl. Therm. Eng. 2023, 229, 120552. [Google Scholar] [CrossRef]
- Malla, L.K.; Dhanalakota, P.; Mahapatra, P.S.; Pattamatta, A. Thermal and flow characteristics in a flat plate pulsating heat pipe with ethanol-water mixtures: From slug-plug to droplet oscillations. Int. J. Heat Mass Transf. 2022, 194, 123066. [Google Scholar] [CrossRef]
- Picknett, R.G.; Bexon, R. The evaporation of sessile or pendant drops in still air. J. Colloid Interface Sci. 1977, 61, 336–350. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Hu, H.; Larson, R.G. Evaporation of a sessile droplet on a substrate. J. Phys. Chem. B 2002, 106, 1334–1344. [Google Scholar] [CrossRef]
- Larson, R.G. Transport and deposition patterns in drying sessile droplets. AIChE J. 2014, 60, 1538–1571. [Google Scholar] [CrossRef]
- Zhang, K.; Ma, L.; Xu, X.; Luo, J.; Guo, D. Temperature distribution along the surface of evaporating droplets. Phys. Rev. E 2014, 89, 32404. [Google Scholar] [CrossRef]
- Hu, H.; Larson, R.G. Analysis of the effects of Marangoni stresses on the microflow in an evaporating sessile droplet. Langmuir 2005, 21, 3972–3980. [Google Scholar] [CrossRef] [PubMed]
- Ristenpart, W.D.; Kim, P.G.; Domingues, C.; Wan, J.; Stone, H.A. Influence of substrate conductivity on circulation reversal in evaporating drops. Phys. Rev. Lett. 2007, 99, 234502. [Google Scholar] [CrossRef]
- Zang, D.; Tarafdar, S.; Tarasevich, Y.Y.; Choudhury, M.D.; Dutta, T. Evaporation of a Droplet: From physics to applications. Phys. Rep. 2019, 804, 1–56. [Google Scholar] [CrossRef]
- Yang, X.; Jiang, Z.; Lyu, P.; Ding, Z.; Man, X. Deposition pattern of drying droplets. Commun. Theor. Phys. 2021, 73, 47601. [Google Scholar] [CrossRef]
- Lohse, D.; Zhang, X. Physicochemical hydrodynamics of droplets out of equilibrium. Nat. Rev. Phys. 2020, 2, 426–443. [Google Scholar] [CrossRef]
- Sefiane, K.; Wilson, S.K.; David, S.; Dunn, G.J.; Duffy, B.R. On the effect of the atmosphere on the evaporation of sessile droplets of water. Phys. Fluids 2009, 21, 062101. [Google Scholar] [CrossRef]
- Dunn, G.J.; Wilson, S.K.; Duffy, B.R.; David, S.; Sefiane, K. The strong influence of substrate conductivity on droplet evaporation. J. Fluid Mech. 2009, 623, 329–351. [Google Scholar] [CrossRef]
- Ait Saada, M.; Chikh, S.; Tadrist, L. Evaporation of a sessile drop with pinned or receding contact line on a substrate with different thermophysical properties. Int. J. Heat Mass Transf. 2013, 58, 197–208. [Google Scholar] [CrossRef]
- Smith, J.D.; Cappa, C.D.; Drisdell, W.S.; Cohen, R.C.; Saykally, R.J. Raman thermometry measurements of free evaporation from liquid water droplets. J. Am. Chem. Soc. 2006, 128, 12892–12898. [Google Scholar] [CrossRef]
- Sefiane, K.; Bennacer, R. An expression for droplet evaporation incorporating thermal effects. J. Fluid Mech. 2011, 667, 260–271. [Google Scholar] [CrossRef]
- Wilson, S.K.; D’Ambrosio, H. Evaporation of sessile droplets. Annu. Rev. Fluid Mech. 2023, 55, 481–509. [Google Scholar] [CrossRef]
- Xu, X.; Ma, L. Analysis of the effects of evaporative cooling on the evaporation of liquid droplets using a combined field approach. Sci. Rep. 2015, 5, 8614. [Google Scholar] [CrossRef] [PubMed]
- Girard, F.; Antoni, M.; Sefiane, K. On the effect of Marangoni flow on evaporation rates of heated water drops. Langmuir 2008, 24, 9207–9210. [Google Scholar] [CrossRef]
- Girard, F.; Antoni, M. Influence of substrate heating on the evaporation dynamics of pinned water droplets. Langmuir 2008, 24, 11342–11345. [Google Scholar] [CrossRef]
- Pan, Z.; Dash, S.; Weibel, J.A.; Garimella, S.V. Assessment of water droplet evaporation mechanisms on hydrophobic and superhydrophobic substrates. Langmuir 2013, 29, 15831–15841. [Google Scholar] [CrossRef] [PubMed]
- Ait Saada, M.; Chikh, S.; Tadrist, L. Numerical investigation of heat and mass transfer of an evaporating sessile drop on a horizontal surface. Phys. Fluids 2010, 22, 112115. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, L.; Xu, X.; Luo, J. Combined effects of underlying substrate and evaporative cooling on the evaporation of sessile liquid droplets. Soft Matter 2015, 11, 5632–5640. [Google Scholar] [CrossRef] [PubMed]

| Parameters | Unit | Water | Methanol | Acetone | Ethanol | Hexane |
|---|---|---|---|---|---|---|
| kL | kgms−3K−1 | 0.604 | 0.203 | 0.161 | 0.167 | 0.1167 |
| ρL | kgm−3 | 998 | 790 | 788 | 785 | 655 |
| CL | m2s−2K−1 | 4180 | 2530 | 2170 | 2438 | 2270 |
| HL | m2s2 | 2.45 × 106 | 1.2 × 106 | 5.49 × 105 | 8.455 × 105 | 3.843 × 105 |
| b | kgm−3K−1 | 1.11 × 103 | 9.47 × 10−3 | 2.84 × 10−2 | 7.08 × 10−3 | 3.41 × 10−2 |
| c0 | kgm−3 | 0.0194 | 0.186 | 0.637 | 0.1417 | 0.7022 |
| D | m2s−1 | 2.44 × 10−5 | 1.5 × 10−5 | 1.06 × 10−5 | 1.19 × 10−5 | 7.32 × 10−6 |
| kG | kgms−3K−1 | 0.023 | 0.023 | 0.023 | 0.023 | 0.023 |
| Ec | 1 | 0.10986 | 0.83970 | 1.02652 | 0.42656 | 0.82199 |
| kRG | 1 | 0.03808 | 0.11330 | 0.12772 | 0.14286 | 0.19709 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Xu, X. A Dimensionless Number for Evaluating the Influence of Heat Conduction in the Gas Phase on Liquid Evaporation. Energies 2025, 18, 3233. https://doi.org/10.3390/en18133233
Xu L, Xu X. A Dimensionless Number for Evaluating the Influence of Heat Conduction in the Gas Phase on Liquid Evaporation. Energies. 2025; 18(13):3233. https://doi.org/10.3390/en18133233
Chicago/Turabian StyleXu, Longfei, and Xuefeng Xu. 2025. "A Dimensionless Number for Evaluating the Influence of Heat Conduction in the Gas Phase on Liquid Evaporation" Energies 18, no. 13: 3233. https://doi.org/10.3390/en18133233
APA StyleXu, L., & Xu, X. (2025). A Dimensionless Number for Evaluating the Influence of Heat Conduction in the Gas Phase on Liquid Evaporation. Energies, 18(13), 3233. https://doi.org/10.3390/en18133233





