Molecular Solar Thermal Fuels with High Energy Density Based on Azobenzene Derivatives
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of bis-AZO
2.3. Characterizations
3. Results and Discussion
3.1. Chemical Composition
3.2. Photoisomerization Properties
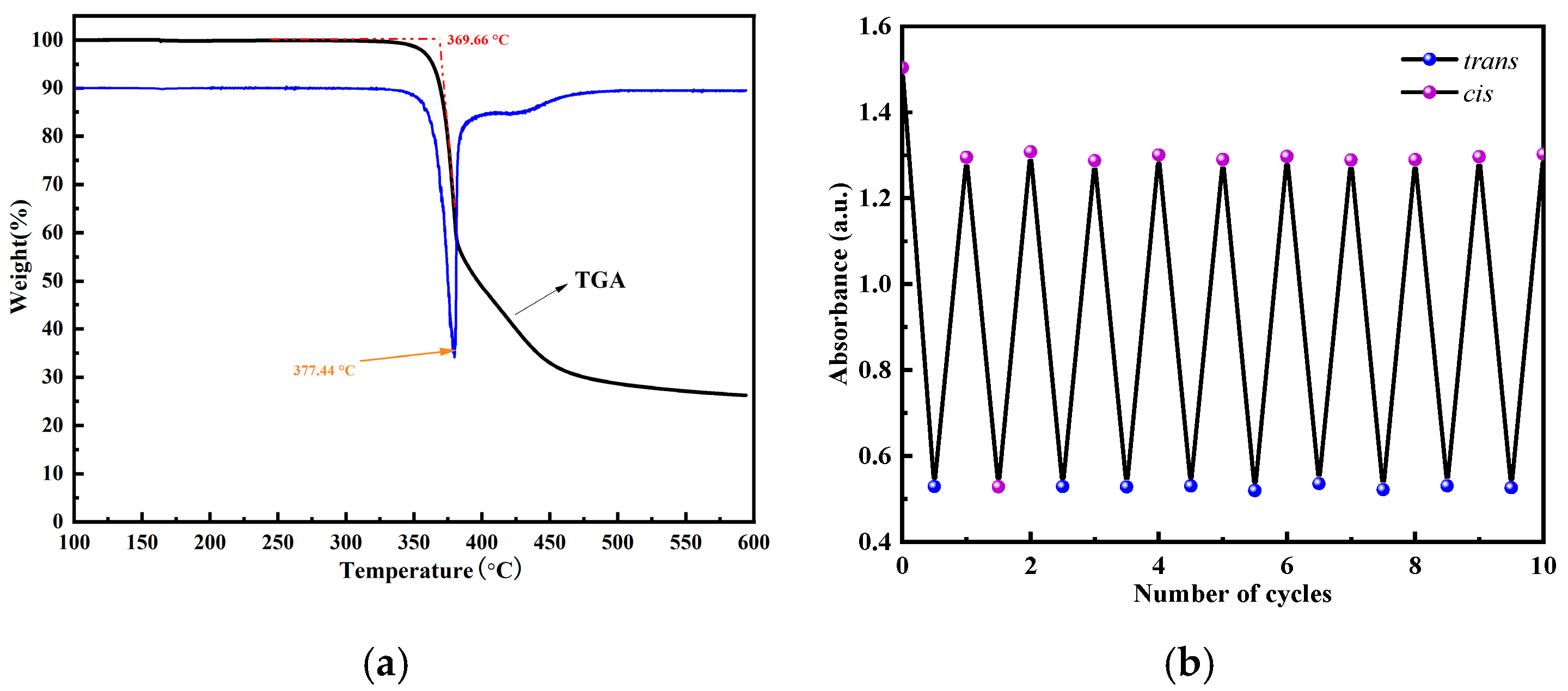
3.3. Thermal Stability and Cyclic Stability
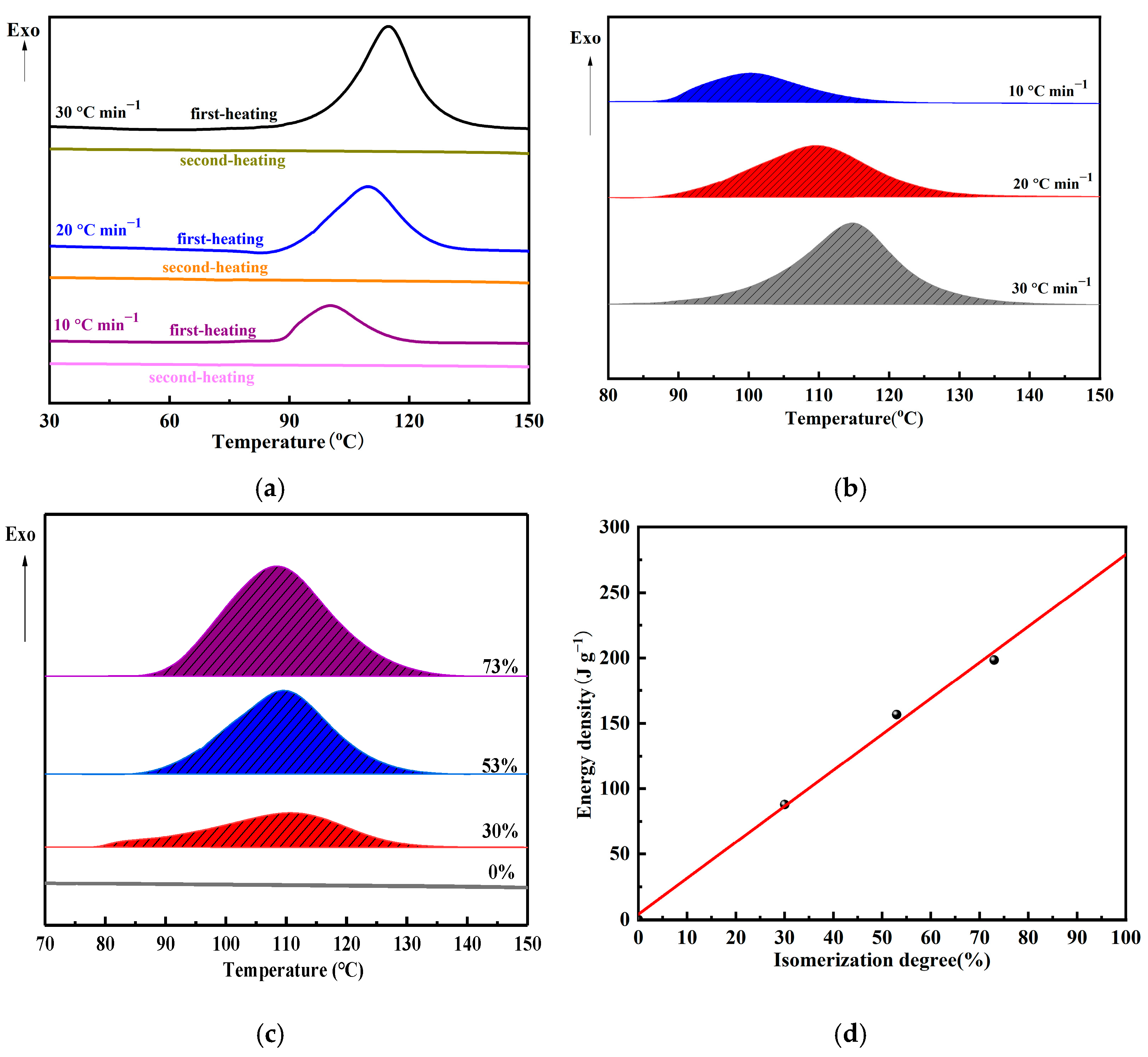
3.4. Energy Storage Performance
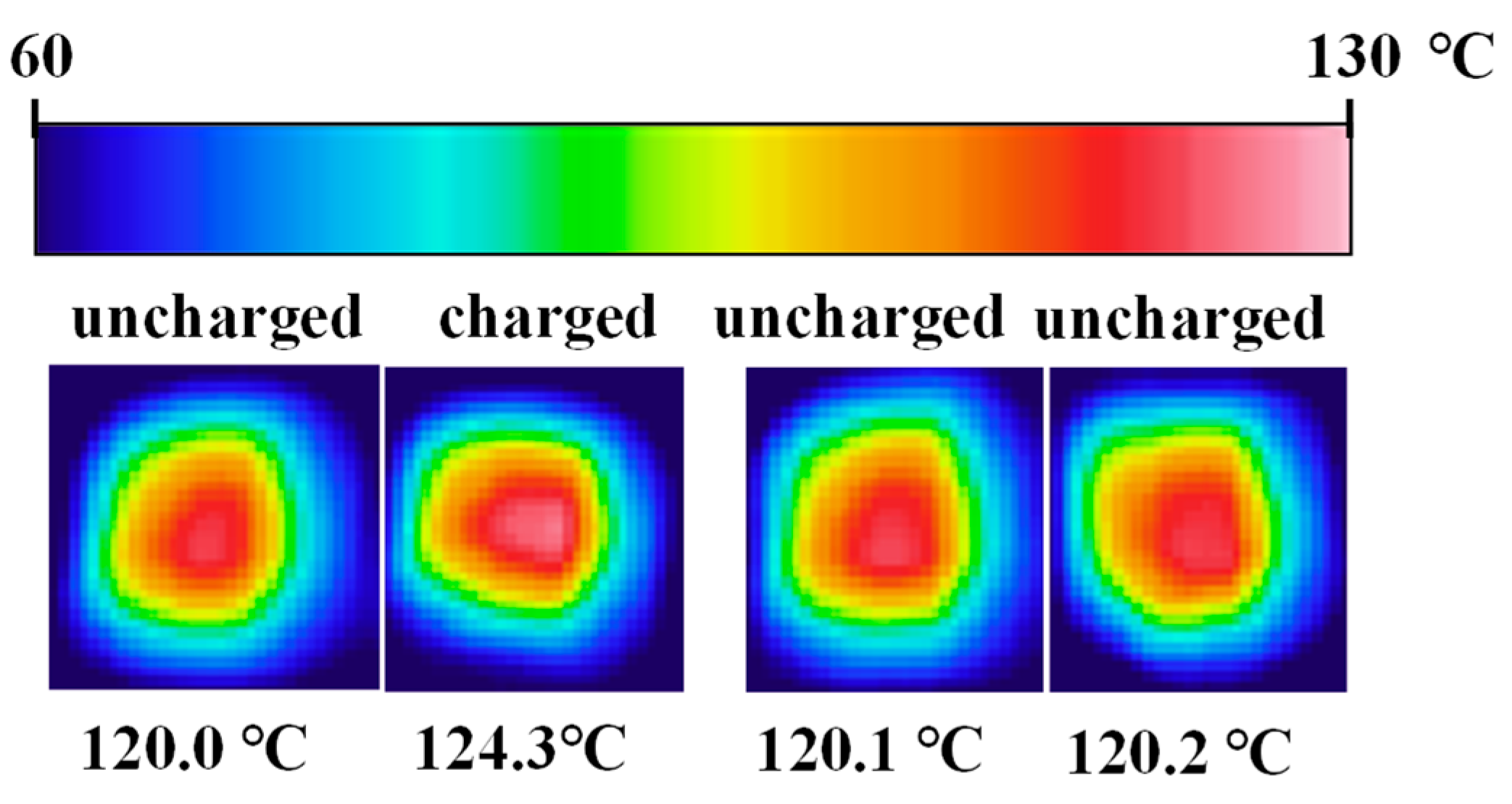
3.5. Macroscale Heat Release
4. Conclusions
- (1)
- The synthesized bis-AZO exhibited efficient and reversible photoisomerization properties in DCM, with its isomerization rate effectively regulated by irradiation intensity. Higher irradiation intensities resulted in faster isomerization, while lower intensities led to slower isomerization.
- (2)
- Due to the introduction of two azobenzene molecules, bis-AZO shows a remarkable energy density of 275.03 J g−1 with excellent thermal stability and cycling stability.
- (3)
- Bis-AZO exhibits excellent heat release behavior, achieving a significant temperature increase of approximately 4.3 °C.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| MOSTs | Molecular solar thermal fuels |
| AZO | Azobenzene |
| DMF | N, N-dimethylformamide |
| DCM | Dichloromethane |
| FT-IR | Fourier transform infrared |
| 1HNMR | Proton nuclear magnetic resonance |
| 13CNMR | Carbon nuclear magnetic resonance |
| XRD | X-ray diffraction |
| HRMS | High-resolution mass spectra |
| UV–Vis | UV–visible |
| TGA | Thermogravimetric analyzer |
| DSC | Differential scanning calorimeter |
| k | First-order kinetic constants |
| kt-c | First-order rate constants from trans to cis |
| kc-t | First-order rate constants from cis to trans |
References
- Wang, H.; Huang, J.; Song, M.J.; Hu, Y.X.; Wang, Y.F.; Lu, Z.J. Simulation and experimental study on the optical performance of a fixed-focus Fresnel lens solar concentrator using polar-axis tracking. Energies 2018, 11, 887. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, Z.; Lin, J.Q. Multi-energy complementary power systems based on solar energy: A review. Renew. Sustain. Energy Rev. 2024, 199, 114464. [Google Scholar] [CrossRef]
- Börjesson, K.; Lennartson, A.; Moth-Poulsen, K. Efficiency limit of molecular solar thermal energy collecting devices. ACS Sustain. Chem. Eng. 2013, 1, 585–590. [Google Scholar] [CrossRef]
- Dong, L.Q.; Feng, Y.Y.; Wang, L.; Feng, W. Azobenzene-based solar thermal fuels: Design, properties, and applications. Chem. Soc. Rev. 2018, 47, 7339–7368. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, T.J.; Tian, Y.C.; Akbulatov, S.; Boulatov, R. Chemical solutions for the closed-cycle storage of solar energy. Energy Environ. Sci. 2011, 4, 4449–4472. [Google Scholar] [CrossRef]
- Hu, J.; Huang, S.; Yu, M.M.; Yu, H.F. Flexible solar thermal fuel devices: Composites of fabric and a photoliquefiable azobenzene derivative. Adv. Energy Mater. 2019, 9, 1901363. [Google Scholar] [CrossRef]
- Quant, M.; Hillers-Bendtsen, A.E.; Ghasemi, S.; Erdelyi, M.; Wang, Z.H.; Muhammad, L.M.; Kann, N.; Mikkelsen, K.V.; Moth-Poulsen, K. Synthesis, characterization and computational evaluation of bicyclooctadienes towards molecular solar thermal energy storage. Chem. Sci. 2022, 13, 834–841. [Google Scholar] [CrossRef]
- Wang, Z.H.; Moïse, H.; Cacciarini, M.; Nielsen, M.B.; Morikawa, M.; Kimizuka, N.; Moth-Poulsen, K. Liquid-based multijunction molecular solar thermal energy collection device. Adv. Sci. 2021, 8, 2103060. [Google Scholar] [CrossRef]
- Orrego-Hernández, J.; Dreos, A.; Moth-Poulsen, K. Engineering of norbornadiene/quadricyclane photoswitches for molecular solar thermal energy storage applications. Acc. Chem. Res. 2020, 53, 1478–1487. [Google Scholar] [CrossRef]
- Kjeldsen, I.L.H.; Høvring, J.F.; von Buchwald, T.J.; Hillers-Bendtsen, A.E.; Mikkelsen, K.V. The effects of solvation on the back reaction and storage capabilities of solar thermal energy storage systems. Phys. Chem. Chem. Phys. 2022, 24, 5564–5577. [Google Scholar] [CrossRef]
- Schøttler, C.; Vegge, S.K.; Cacciarini, M.; Nielsen, M.B. Long-term energy storage systems based on the dihydroazulene/vinylheptafulvene photo-/thermoswitch. ChemPhotoChem 2022, 6, e202200037. [Google Scholar] [CrossRef]
- Berdnikova, D.V.; Kriesche, B.M.; Paululat, T.; Hofer, T.S. Fused bis (hemi-indigo): Broad-range wavelength-independent photoswitches. Chem. Eur. J. 2022, 28, e202202752. [Google Scholar] [CrossRef] [PubMed]
- Doronina, E.P.; Jouikov, V.; Belogolova, E.F.; Sidorkin, V.F. Isomer-selective dative bond O→M (M= Si, Ge) for designing new photochromic hemi-indigo systems. J. Organomet. Chem. 2022, 958, 122189. [Google Scholar] [CrossRef]
- Zhang, B.; Feng, Y.Y.; Feng, W. Azobenzene-based solar thermal fuels: A review. Nano-Micro Lett. 2022, 14, 138. [Google Scholar] [CrossRef]
- Duvva, N.; Chilakamarthi, U.; Giribabu, L. Recent developments in tetrathiafulvalene and dithiafulvalene based metal-free organic sensitizers for dye-sensitized solar cells: A mini-review. Sustain. Energy Fuels 2017, 1, 678–688. [Google Scholar] [CrossRef]
- Wu, S.; Li, T.X.; Zhang, Z.Y.; Li, T.; Wang, R.Z. Photoswitchable phase change materials for unconventional thermal energy storage and upgrade. Matter 2021, 4, 3385–3399. [Google Scholar] [CrossRef]
- Lennartson, A.; Roffey, A.; Moth-Poulsen, K. Designing photoswitches for molecular solar thermal energy storage. Tetrahedron Lett. 2015, 56, 1457–1465. [Google Scholar] [CrossRef]
- Masutani, K.; Morikawa, M.A.; Kimizuka, N. A liquid azobenzene derivative as a solvent-free solar thermal fuel. Chem. Commun. 2014, 50, 15803–15806. [Google Scholar] [CrossRef]
- Ishiba, K.; Morikawa, M.A.; Chikara, C.; Yamada, T.; Iwase, K.; Kawakita, M.; Kimizuka, N. Photoliquefiable ionic crystals: A phase crossover approach for photon energy storage materials with functional multiplicity. Angew. Chem. Int. Ed. 2015, 54, 1532–1536. [Google Scholar] [CrossRef]
- Cho, E.N.; Zhitomirsky, D.; Han, G.G.D.; Liu, Y.; Grossman, J.C. Molecularly engineered azobenzene derivatives for high energy density solid-state solar thermal fuels. ACS Appl. Mater. Interfaces 2017, 9, 8679–8687. [Google Scholar] [CrossRef]
- Han, G.G.D.; Li, H.S.; Grossman, J.C. Optically-controlled long-term storage and release of thermal energy in phase-change materials. Nat. Commun. 2017, 8, 1446. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, M.A.; Yamanaka, Y.; Hui, J.K.H.; Kimizuka, N. Photoliquefaction and phase transition of m-bisazobenzenes give molecular solar thermal fuels with a high energy density. RSC Adv. 2023, 13, 24031–24037. [Google Scholar] [CrossRef]
- Durgun, E.; Grossman, J.C. Photoswitchable molecular rings for solar-thermal energy storage. J. Phys. Chem. Lett. 2013, 4, 854–860. [Google Scholar] [CrossRef] [PubMed]
- Piwowarczyk, M.; Deptuch, A.; Drzewicz, A.; Juszyńska-Gałązka, E.; Gałązka, M.; Galewski, Z. Mesomorphism of (E)-4-[(4-propyloxyphenyl) diazenyl] alkanoates (3OABOOCm). Thermochim. Acta 2024, 731, 179649. [Google Scholar] [CrossRef]
- Yu, X.J.; Wang, Z.B.; Buchholz, M.; Füllgrabe, N.; Grosjean, S.; Bebensee, F.; Bräse, S.; Wöll, C.; Heinke, L. Cis-to-trans isomerization of azobenzene investigated by using thin films of metal-organic frameworks. Phys. Chem. Chem. Phys. 2015, 17, 22721–22725. [Google Scholar] [CrossRef]
- Wu, S.; Butt, H.J. Solar-thermal energy conversion and storage using photoresponsive azobenzene-containing polymers. Macromol. Rapid Commun. 2020, 41, 1900413. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.Q.; Chen, Y.H.; Zhai, F.; Tang, L.; Gao, W.C.; Tang, J.W.; Feng, Y.Y.; Feng, W. Azobenzene-based solar thermal energy storage enhanced by gold nanoparticles for rapid, optically-triggered heat release at room temperature. J. Mater. Chem. A 2020, 8, 18668–18676. [Google Scholar] [CrossRef]
- Xu, X.; Feng, J.; Li, W.Y.; Wang, G.; Feng, W.; Yu, H.F. Azobenzene-containing polymer for solar thermal energy storage and release: Advances, challenges, and opportunities. Prog. Polym. Sci. 2024, 149, 101782. [Google Scholar] [CrossRef]
- Cai, F.; Song, T.; Yang, B.; Lv, X.; Zhang, L.; Yu, H.F. Enhancement of solar thermal fuel by microphase separation and nanoconfinement of a block copolymer. Chem. Mater. 2021, 33, 9750–9759. [Google Scholar] [CrossRef]
- Kucharski, T.J.; Ferralis, N.; Kolpak, A.M.; Zheng, J.O.; Nocera, D.G.; Grossman, J.C. Templated assembly of photoswitches significantly increases the energy-storage capacity of solar thermal fuels. Nat. Chem. 2014, 6, 441–447. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, J.; Feng, W.; Zhao, X.; Wang, T.; Li, C.H.; Luo, W. Molecular regulation of nano-structured solid-state AZO-SWCNTs assembly film for the high-energy and short-term solar thermal storage. Sol. Energy Mater. Sol. Cells 2019, 193, 198–205. [Google Scholar] [CrossRef]
- Yang, W.X.; Feng, Y.Y.; Si, Q.Y.; Yan, Q.H.; Long, P.; Dong, L.Q.; Fu, L.X.; Feng, W. Efficient cycling utilization of solar-thermal energy for thermochromic displays with controllable heat output. J. Mater. Chem. A 2019, 7, 97–106. [Google Scholar] [CrossRef]
- Han, G.D.; Park, S.S.; Liu, Y.; Zhitomirsky, D.; Cho, E.; Dincă, M.; Grossman, J.C. Photon energy storage materials with high energy densities based on diacetylene-azobenzene derivatives. J. Mater. Chem. A 2016, 4, 16157–16165. [Google Scholar] [CrossRef]
- Xu, X.T.; Zhang, P.; Wu, B.; Xing, Y.M.; Shi, K.; Fang, W.H.; Yu, H.F.; Wang, G.J. Photochromic dendrimers for photoswitched solid-to-liquid transitions and solar thermal fuels. ACS Appl. Mater. Interfaces 2020, 12, 50135–50142. [Google Scholar] [CrossRef]
- Morikawa, M.; Yamanaka, Y.; Kimizuka, N. Liquid bisazobenzenes as molecular solar thermal fuel with enhanced energy density. Chem. Lett. 2022, 51, 402–406. [Google Scholar] [CrossRef]
- Sun, S.D.; Liang, S.F.; Xu, W.C.; Wang, M.H.; Gao, J.G.; Zhang, Q.J.; Wu, S. Photoswitches with different numbers of azo chromophores for molecular solar thermal storage. Soft Matt. 2022, 18, 8840–8849. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Qiu, Q.F.; Usuba, J.; Wan, J.; Han, G.G.D. Photoinduced solid-liquid phase transition and energy storage enabled by the design of linked double photoswitches. ACS Mater. Au 2023, 4, 30–34. [Google Scholar] [CrossRef]
- Slavov, C.; Yang, C.; Schweighauser, L.; Boumrifak, C.; Dreuw, A.; Wegner, H.A.; Wachtveitl, J. Connectivity matters-ultrafast isomerization dynamics of bisazobenzene photoswitches. Phys. Chem. Chem. Phys. 2016, 18, 14795–14804. [Google Scholar] [CrossRef]
- Dong, D.F.; Li, T. Multi-azo photoswitches for improved molecular solar thermal energy storage. ChemPhotoChem 2024, 8, e202400007. [Google Scholar] [CrossRef]
- Sharma, A.; Bekir, M.; Lomadze, N.; Santer, S. Photo-isomerization kinetics of azobenzene containing surfactant conjugated with polyelectrolyte. Molecules 2020, 26, 19. [Google Scholar] [CrossRef]
- Arya, P.; Jelken, J.; Lomadze, N.; Santer, S.; Bekir, M. Kinetics of photo-isomerization of azobenzene containing surfactants. J. Chem. Phys. 2020, 152, 024904. [Google Scholar] [CrossRef] [PubMed]
- Tamaoki, N.; Yamaoka, T. Light-intensity dependence in the photochromism of dibenzo [2.2](4, 4′)-azobenzenophane. J. Chem. Soc. Perkin Trans. 2 1991, 6, 873–878. [Google Scholar] [CrossRef]
- Bleger, D.; Dokic, J.; Peters, M.V.; Grubert, L.; Saalfrank, P.; Hecht, S. Electronic decoupling approach to quantitative photoswitching in linear multiazobenzene architectures. J. Phys. Chem. B 2011, 115, 9930–9940. [Google Scholar] [CrossRef]
- Wang, G.J.; Yuan, D.; Yuan, T.T.; Dong, J.; Feng, N.; Han, G.X. A visible light responsive azobenzene-functionalized polymer: Synthesis, self-assembly, and photoresponsive properties. J. Polym. Sci. Pol. Chem. 2015, 53, 2768–2775. [Google Scholar] [CrossRef]
- Sin, S.L.; Gan, L.H.; Hu, X.; Tam, K.C.; Gan, Y.Y. Photochemical and thermal isomerizations of azobenzene-containing amphiphilic diblock copolymers in aqueous micellar aggregates and in film. Macromolecules 2005, 38, 3943–3948. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, J.X.; Luo, W.; Quan, X.Q.; Li, H.J.; Huang, J.; Feng, W. High-energy and light-actuated phase change composite for solar energy storage and heat release. Surf. Interfaces 2021, 24, 101071. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, R.; Luo, W.; Hu, Y.; Wang, H.; Wang, C.H.; Li, X.X.; Huang, J. Photoguided AZO-phase change composite for high-energy solar storage and heat release at near ambient temperature. J. Energy Storage 2024, 101, 113974. [Google Scholar] [CrossRef]
- Taoda, H.; Hayakawa, K.; Kawase, K.; Yamakita, H. Photochemical conversion and storage of solar energy by azobenzene. J. Chem. Eng. Japan 1987, 20, 265–270. [Google Scholar] [CrossRef]
- Kunz, A.; Heindl, A.H.; Dreos, A.; Wang, Z.H.; Moth-Poulsen, K.; Becker, J.; Wegner, H.A. Intermolecular London dispersion interactions of azobenzene switches for tuning molecular solar thermal energy storage systems. ChemPlusChem 2019, 84, 1145–1148. [Google Scholar] [CrossRef]

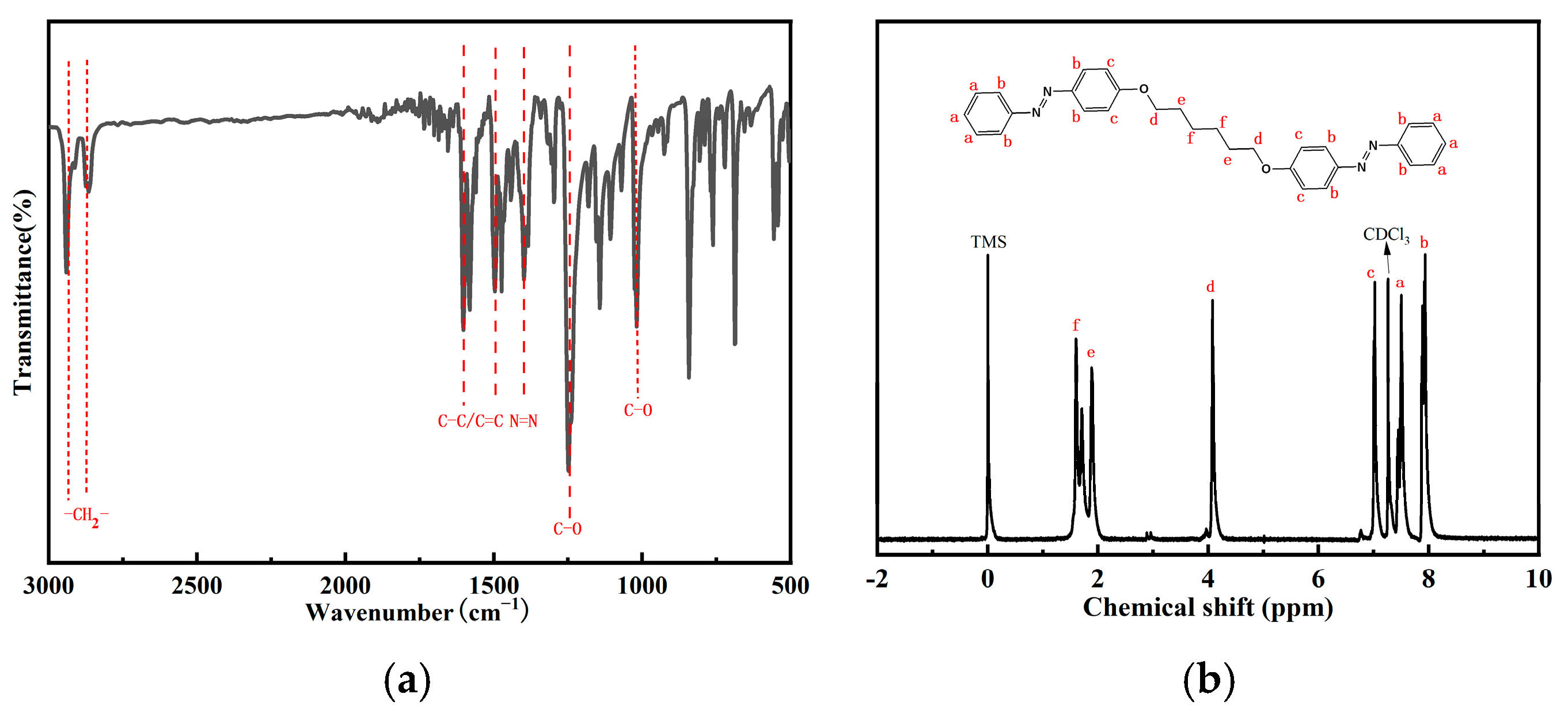
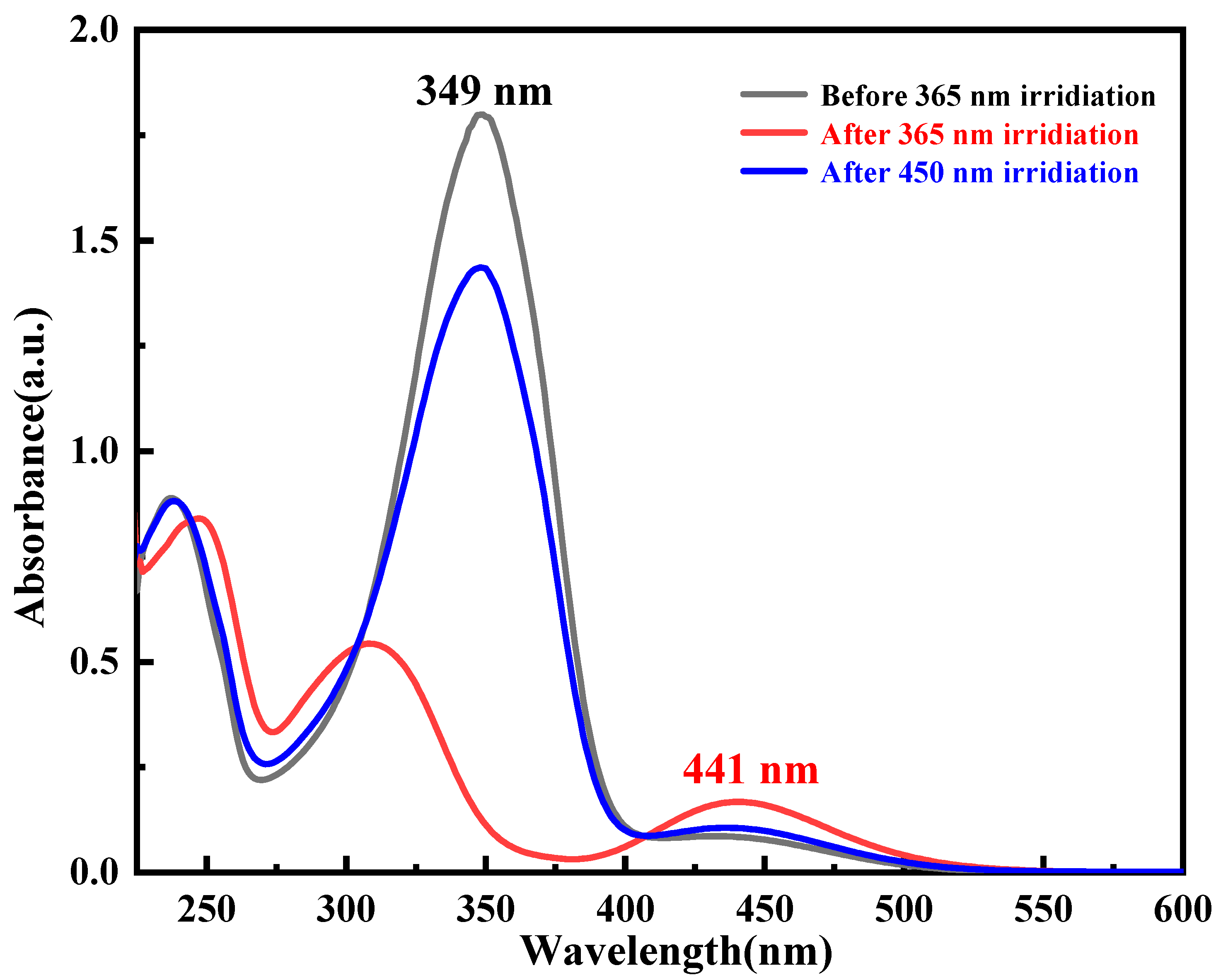

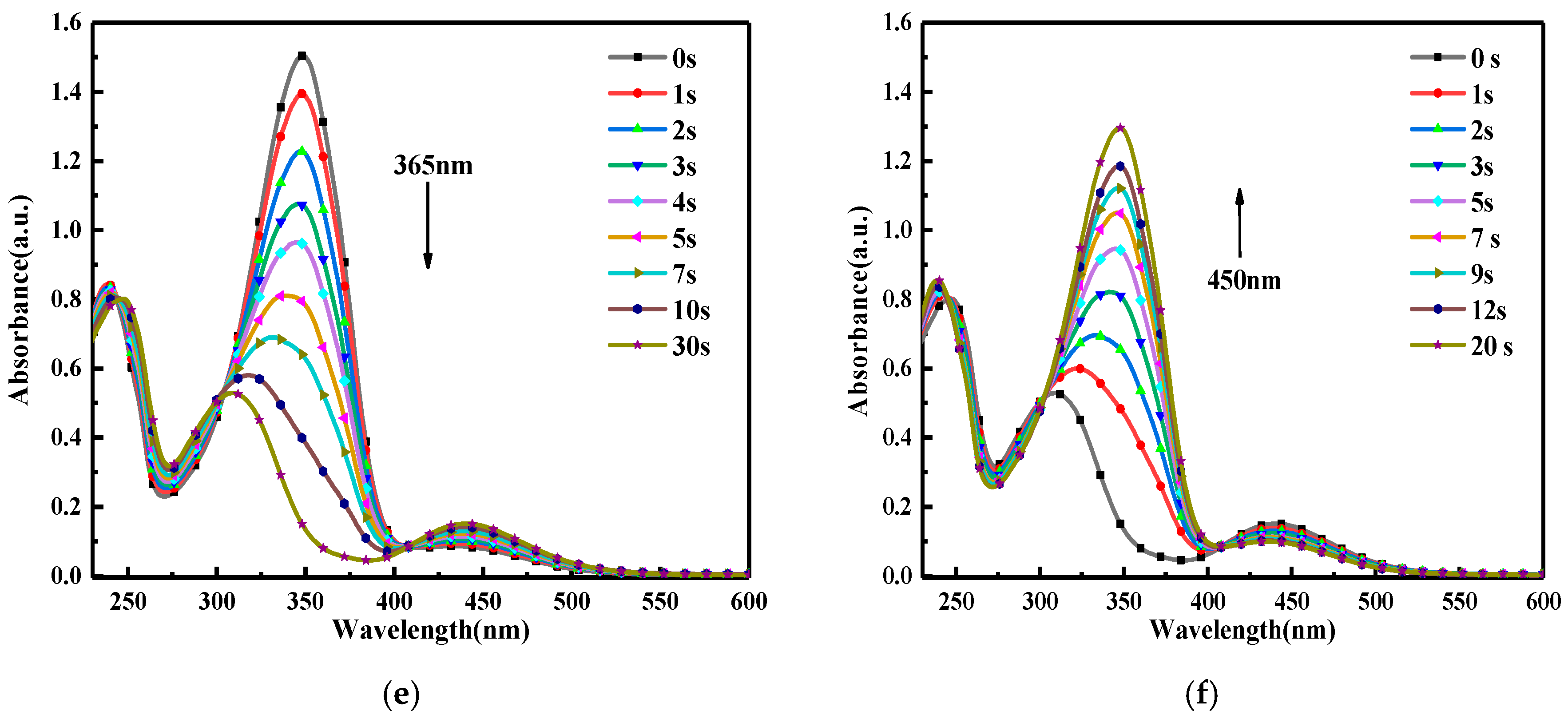
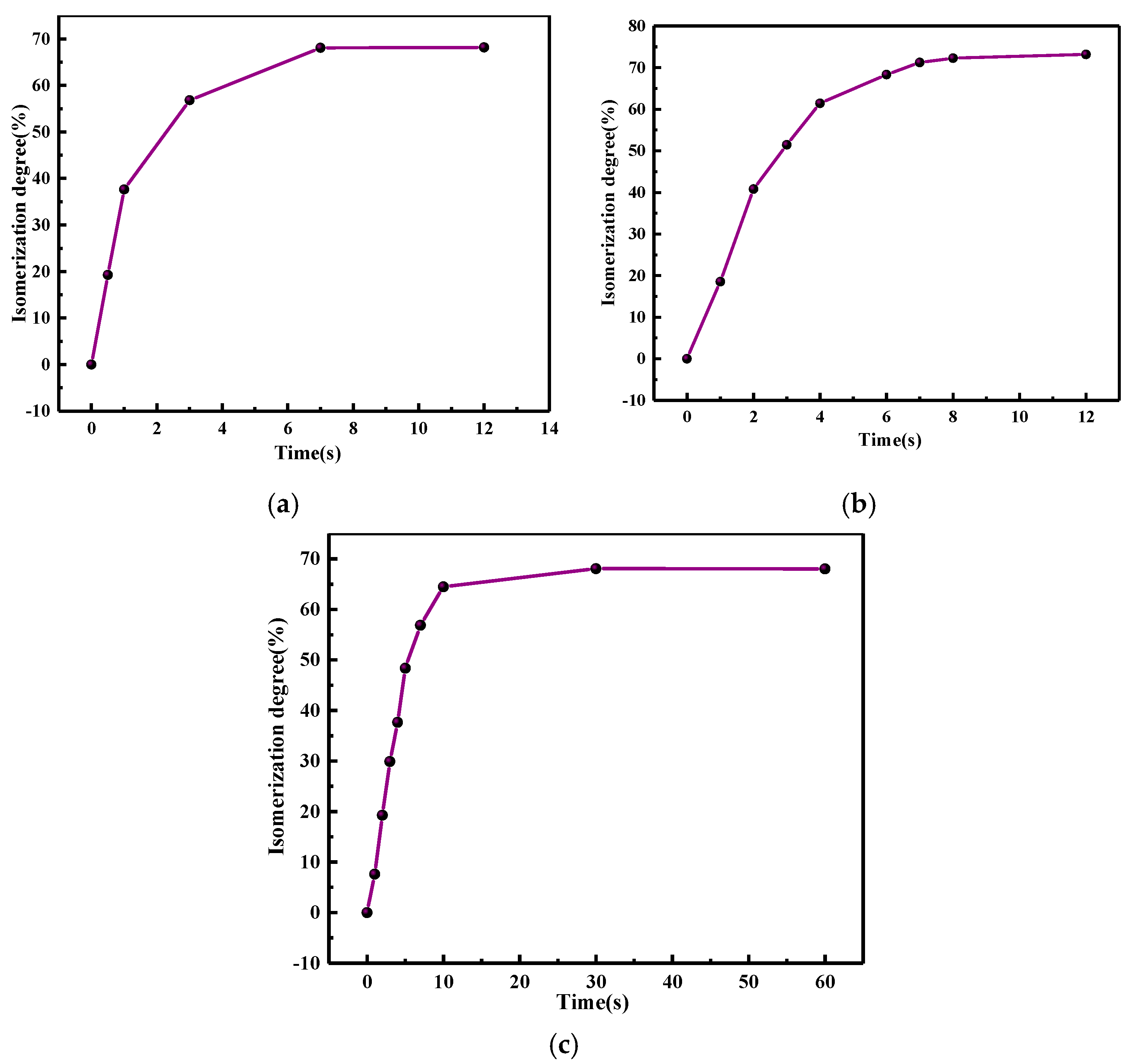
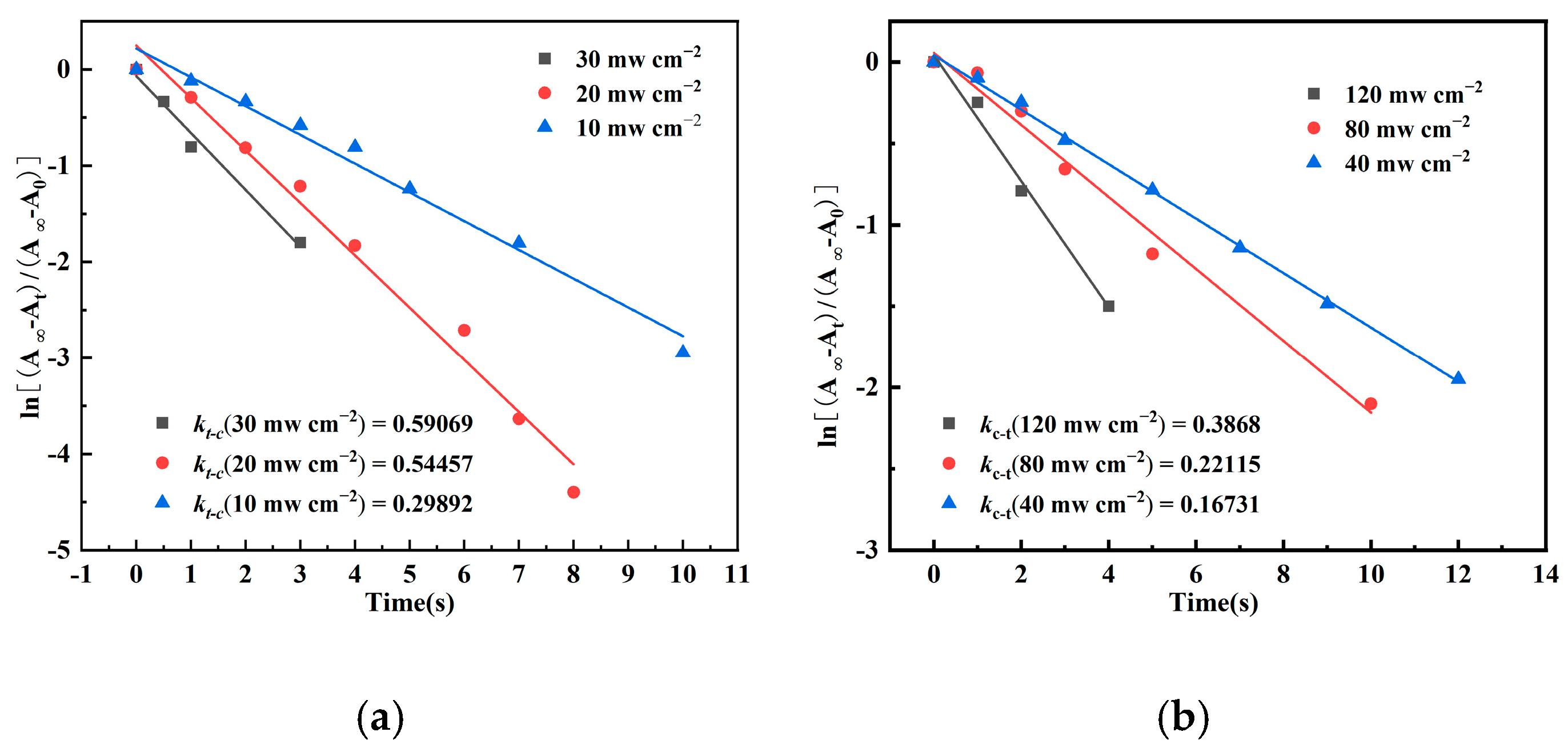
| Light Wavelength (nm) | Irradiation Intensity (mW cm−2) | k(t-c) (s−1) | k(c-t) (s−1) |
|---|---|---|---|
| 365 | 30 | 0.59069 | |
| 20 | 0.54457 | ||
| 10 | 0.29892 | ||
| 450 | 120 | 0.3868 | |
| 80 | 0.22115 | ||
| 40 | 0.16731 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Liu, R.; Guo, Y.; Wang, H.; Luo, W.; Huang, J. Molecular Solar Thermal Fuels with High Energy Density Based on Azobenzene Derivatives. Energies 2025, 18, 2672. https://doi.org/10.3390/en18112672
Jiang Y, Liu R, Guo Y, Wang H, Luo W, Huang J. Molecular Solar Thermal Fuels with High Energy Density Based on Azobenzene Derivatives. Energies. 2025; 18(11):2672. https://doi.org/10.3390/en18112672
Chicago/Turabian StyleJiang, Yan, Rui Liu, Yupeng Guo, Hai Wang, Wen Luo, and Jin Huang. 2025. "Molecular Solar Thermal Fuels with High Energy Density Based on Azobenzene Derivatives" Energies 18, no. 11: 2672. https://doi.org/10.3390/en18112672
APA StyleJiang, Y., Liu, R., Guo, Y., Wang, H., Luo, W., & Huang, J. (2025). Molecular Solar Thermal Fuels with High Energy Density Based on Azobenzene Derivatives. Energies, 18(11), 2672. https://doi.org/10.3390/en18112672






