Analysis of the Influence of Insulation Moisture during Long-Term Exploitation of Transformers on Their Reliability as Determined by Alternating Current Electrical Parameters
Abstract
1. Introduction
2. Materials and Methods
3. Measurements of the DC Conductivity and Dielectric Permittivity of Pressboard–Oil–Water Composite and Their Comparison with the Properties of Water
3.1. DC Properties of Moist NYTRO BIO 300X Insulating Oil-Impregnated Pressboard and Water
3.2. Dielectric Permittivity and Dielectric Relaxation of Moist Pressboard Impregnated with Insulating Oil
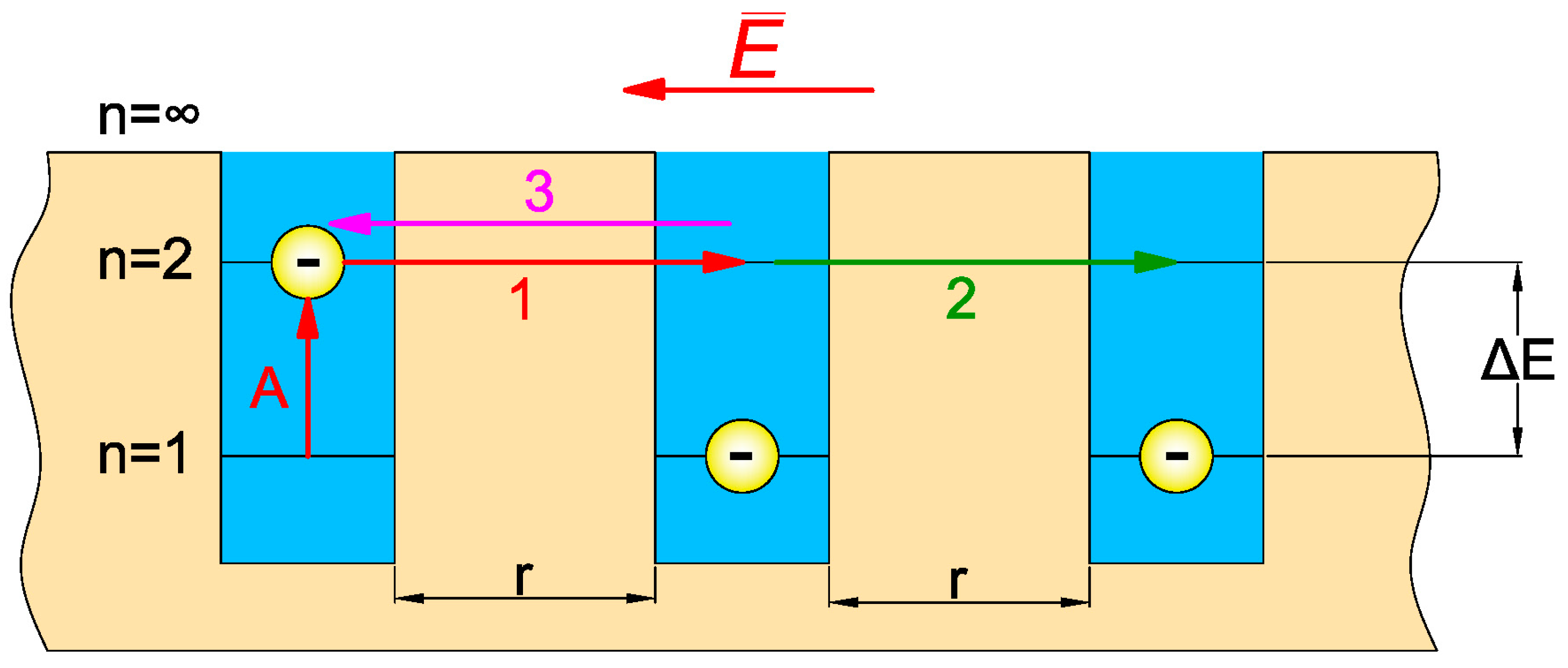
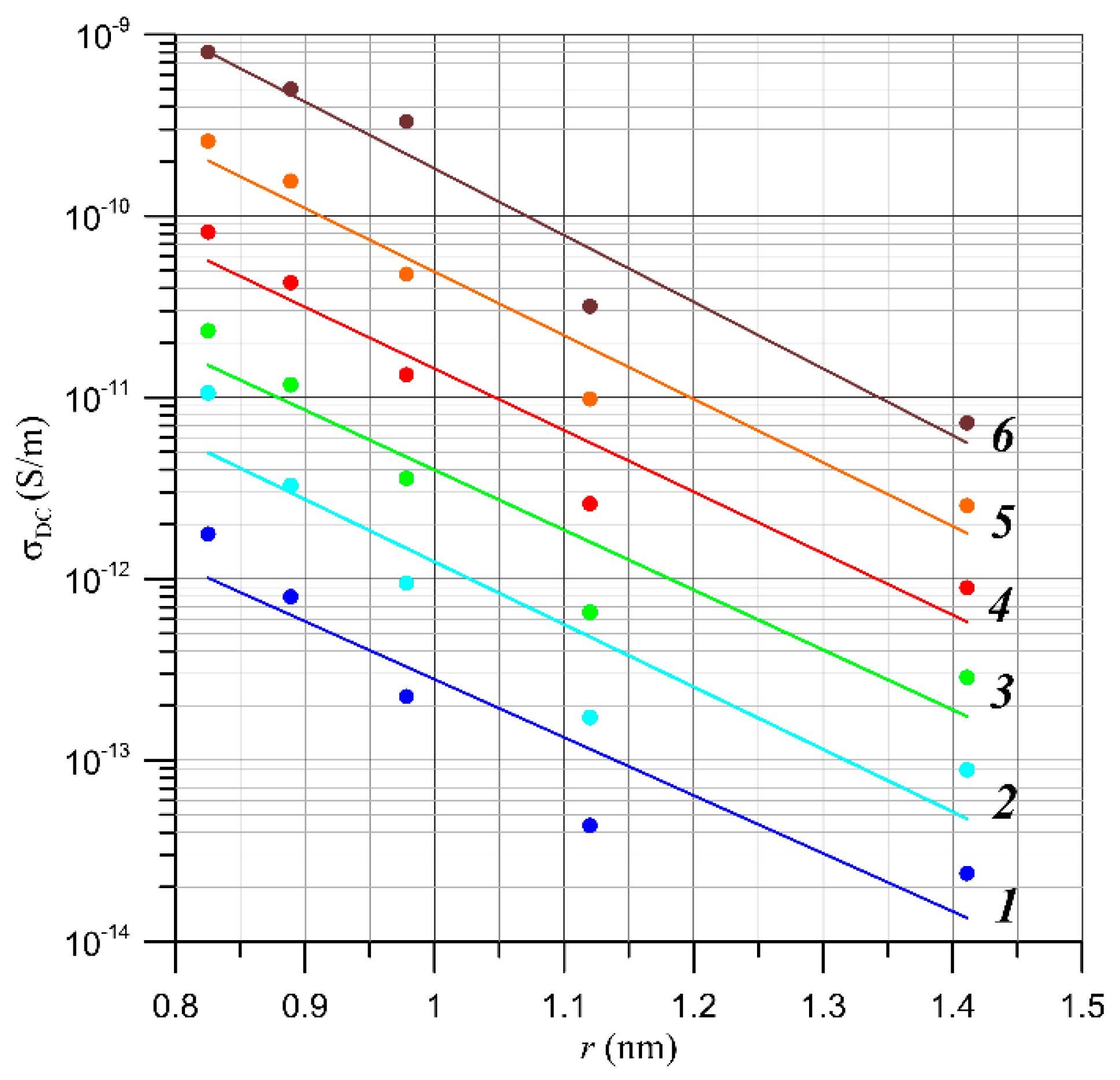
4. Analysis of AC Conductivity of Electrical Pressboard–NYTRO BIO 300X Insulating Oil–Moisture Component Using Quantum Mechanical Electron Tunnelling Phenomenon
4.1. Quantum Mechanical Electron Tunnelling Phenomenon and Associated DC Conductivity
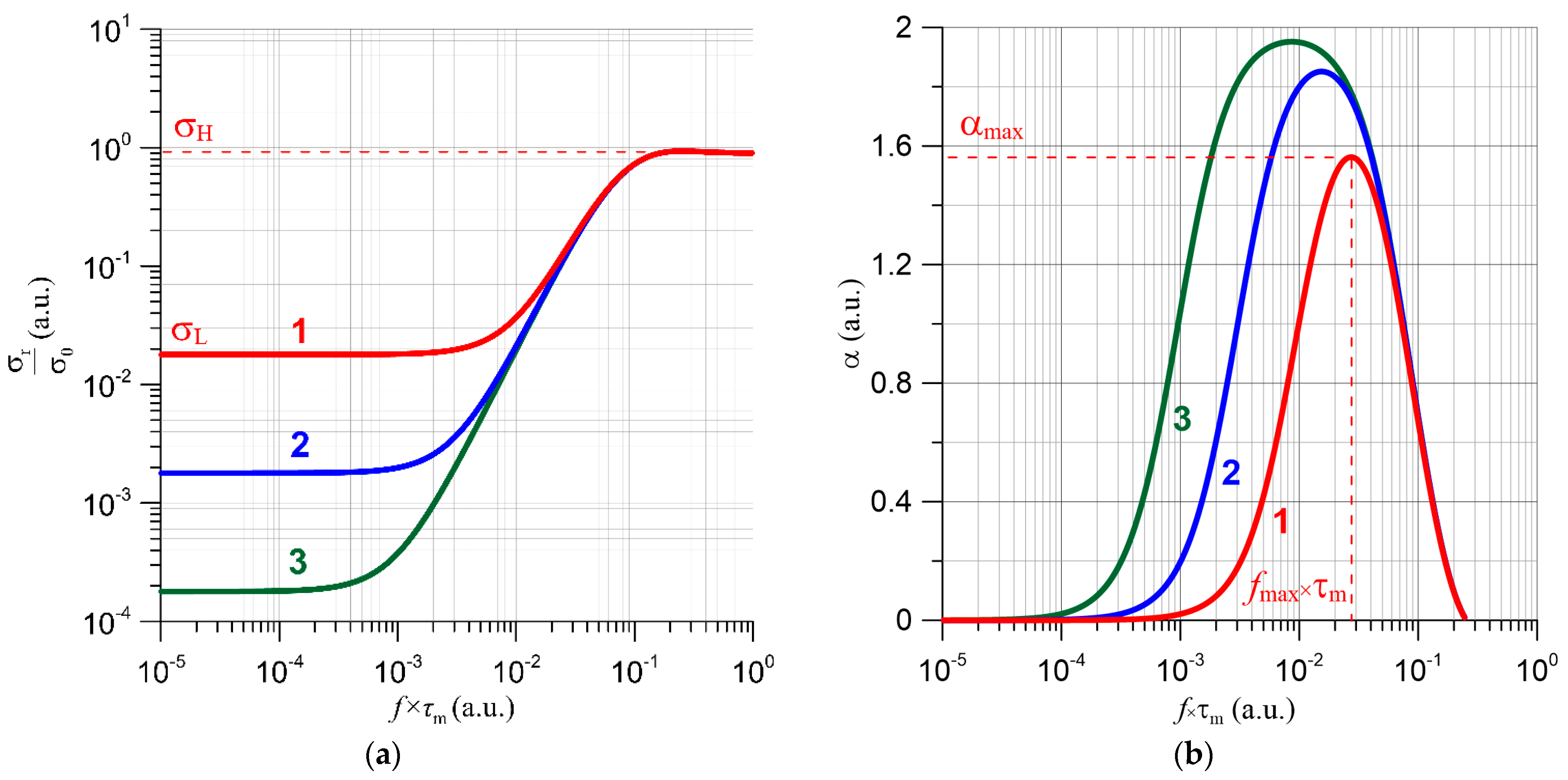
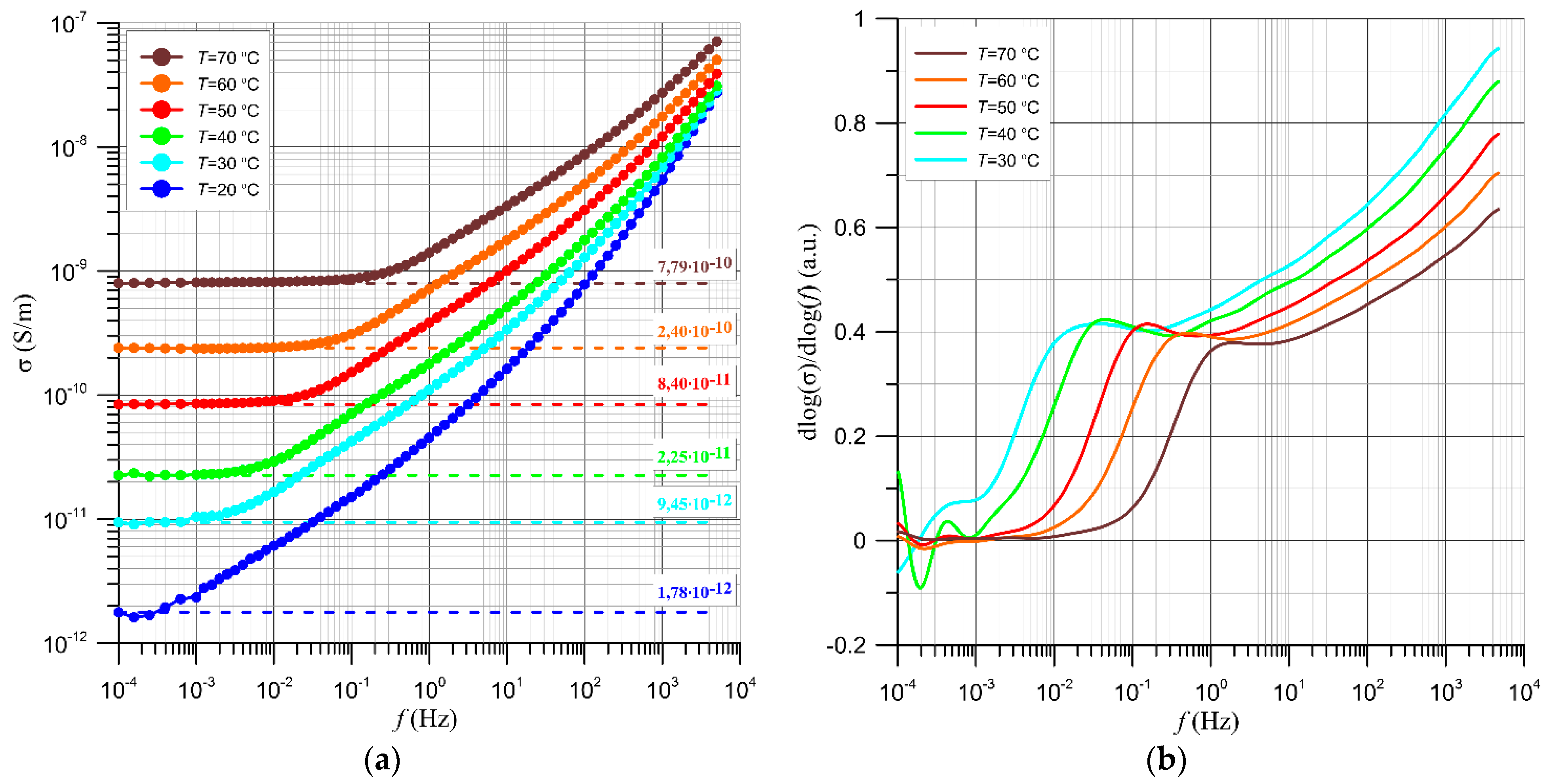
4.2. Results of Studies on AC Conductivity of Nanocomposites and Their Analysis Based on Quantum Mechanical Electron Tunnelling Phenomenon
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Graczkowski, A.; Gielniak, J. Influence of impregnating liquids on dielectric response of impregnated cellulose insulation. In Proceedings of the IEEE International Conference on Solid Dielectrics-ICSD, Potsdam, Germany, 4–9 July 2010; pp. 513–516. [Google Scholar]
- Wei, Y.; Han, W.; Li, G.; Liang, X.; Gu, Z.; Hu, K. Aging Characteristics of Transformer Oil-Impregnated Insulation Paper Based on Trap Parameters. Polymers 2021, 13, 1364. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Dai, Q.; Li, C.; Zeng, Z.; Fu, M.; Zhuo, R. The Mechanism and Diagnosis of Insulation Deterioration Caused by Moisture Ingress into Oil-Impregnated Paper Bushing. Energies 2018, 11, 1496. [Google Scholar] [CrossRef]
- Lundgaard, L.E.; Hansen, W.; Linhjell, D.; Painter, T.J. Aging of Oil-Impregnated Paper in Power Transformers. IEEE Trans. Power Deliv. 2004, 19, 230–239. [Google Scholar] [CrossRef]
- Oommen, T.V.; Prevost, T.A. Cellulose insulation in oil-filled power transformers: Part II—maintaining insulation integrity and life. IEEE Electr. Insul. Mag. 2006, 22, 5–14. [Google Scholar] [CrossRef]
- Fabre, J.; Pichon, A. Deteriorating processes and products of paper in oil application to transformers. In Proceedings of the International Conference on Large High Voltage electric Systems (CIGRE), Paris, France, 15–25 June 1960; p. 137. [Google Scholar]
- Liu, J.; Zhang, H.; Geng, C.; Fan, X.; Zhang, Y. Aging assessment model of transformer insulation based on furfural indicator under different oil/pressboard ratios and oil change. IEEE Trans. Dielectr. Electr. Insul. 2021, 28, 1061–1069. [Google Scholar] [CrossRef]
- Oommen, T.V. Moisture equilibrium in paper oil systems. In Proceedings of the 16th Electrical/Electronics Insulation Conference, Chicago, IL, USA, 3–6 October 1983; pp. 162–166. [Google Scholar]
- Rahman, M.F.; Nirgude, P. Partial discharge behaviour due to irregular-shaped copper particles in transformer oil with a different moisture content of pressboard barrier under uniform field. IET Gener. Transm. Distrib. 2019, 13, 5550–5560. [Google Scholar] [CrossRef]
- Hill, J.; Wang, Z.; Liu, Q.; Krause, C.; Wilson, G. Analysing the power transformer temperature limitation for avoidance of bubble formation. High Volt. 2019, 4, 210–216. [Google Scholar] [CrossRef]
- Garcia, B.; Villarroel, R.; Garcia, D. A Multiphysical model to study moisture dynamics in transformers. IEEE Trans. Power Deliv. 2019, 34, 1365–1373. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, Z.; Zhang, Q.; Zhang, R.; Guo, C.; Guo, W. Effect of Moisture Distribution on Surface Flashover of Oil–Paper Insulation: Fundamental Phenomena, Statistical Behavior. IEEE Trans. Dielectr. Electr. Insul. 2023, 30, 202–209. [Google Scholar] [CrossRef]
- Setayeshmehr, A.; Fofana, I.; Eichler, C.; Akbari, A.; Borsi, H.; Gockenbach, E. Dielectric spectroscopic measurements on transformer oil-paper insulation under controlled laboratory conditions. IEEE Trans. Dielectr. Electr. Insul. 2008, 15, 1100–1111. [Google Scholar] [CrossRef]
- Koch, M.; Prevost, T. Analysis of dielectric response measurements for condition assessment of oil-paper transformer insulation. IEEE Trans. Dielectr. Electr. Insul. 2012, 19, 1908–1915. [Google Scholar] [CrossRef]
- Blennow, J.; Ekanayake, C.; Walczak, K.; García, B.; Gubanski, S.M. Field experiences with measurements of dielectric response in frequency domain for power transformer diagnostics. IEEE Trans. Power Deliv. 2006, 21, 681–688. [Google Scholar] [CrossRef]
- Adekunle, A.A.; Oparanti, S.O.; Hamzat, A.; Abdelmalik, A.A. Dielectric response of vegetable oil-based nanofluid and impregnated Kraft paper for high voltage transformer insulation. J. Mol. Liq. 2023, 391, 123391. [Google Scholar] [CrossRef]
- Martínez, M.; Pleite, J. Improvement of RVM test interpretation using a Debye equivalent circuit. Energies 2020, 13, 323. [Google Scholar] [CrossRef]
- Islam, M.M.; Lee, G.; Hettiwatte, S.N. A review of condition monitoring techniques and diagnostic tests for lifetime estimation of power transformers. Electr. Eng. 2018, 100, 581–605. [Google Scholar] [CrossRef]
- Fofana, I.; Hadjadj, Y. Electrical-Based Diagnostic Techniques for Assessing Insulation Condition in Aged Transformers. Energies 2016, 9, 679. [Google Scholar] [CrossRef]
- Sarkar, S.; Sharma, T.; Baral, A.; Chatterjee, B.; Dey, D.; Chakravorti, S. An expert system approach for transformer insulation diagnosis combining conventional diagnostic tests and PDC, RVM data. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 882–891. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, J.; Zhang, Y.; Ma, Y.; Shen, Y.; Zhen, X.; Chen, Z. Effectiveness analysis and temperature effect mechanism on chemical and electrical-based transformer insulation diagnostic parameters obtained from PDC data. Energies 2018, 11, 146. [Google Scholar] [CrossRef]
- Mishra, D.; Haque, N.; Baral, A.; Chakravorti, S. Assessment of interfacial charge accumulation in oil-paper interface in transformer insulation from polarization-depolarization current measurements. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 1665–1673. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.; Zheng, H.; Wang, K. Feasibility of a universal approach for temperature correction in frequency domain spectroscopy of transformer insulation. IEEE Trans. Dielectr. Electr. Insul. 2018, 25, 1766–1773. [Google Scholar] [CrossRef]
- Liu, J.; Fan, X.; Zhang, Y.; Zhang, C.; Wang, Z. Aging evaluation and moisture prediction of oil-immersed cellulose insulation in field transformer using frequency domain spectroscopy and aging kinetics model. Cellulose 2020, 27, 7175–7189. [Google Scholar] [CrossRef]
- Yang, L.; Chen, J.; Gao, J.; Zheng, H.; Li, Y. Accelerating frequency domain dielectric spectroscopy measurements on insulation of transformers through system identification. IET Sci. Meas. Technol. 2018, 12, 247–254. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, G.; Zhang, N.; Wu, Q.; Zhang, P.; Liu, H.; Liu, Z. Study on temperature normalization of frequency-domain dielectric spectroscopy of oil–paper insulation. Electr. Eng. 2024. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Yu, M.H.; Zhou, W.; Liang, Y.L.; Lei, S.J.; Shi, Y.B.; Qu, L.M. Research on the variation of dielectric properties of oil-paper insulation for power equipment over a wide temperature range. High Volt. 2024. [Google Scholar] [CrossRef]
- Ekanayake, C.; Gubanski, S.M.; Graczkowski, A.; Walczak, K. Frequency Response of Oil Impregnated Pressboard and Paper Samples for Estimating Moisture in Transformer Insulation. IEEE Trans. Power Deliv. 2006, 21, 1309–1317. [Google Scholar] [CrossRef]
- Zukowski, P.; Rogalski, P.; Koltunowicz, T.N.; Kierczynski, K.; Bondariev, V. Precise measurements of the temperature-frequency dependence of the conductivity of cellulose—insulating oil—water nanoparticles composite. Energies 2020, 14, 32. [Google Scholar] [CrossRef]
- Zukowski, P.; Kierczynski, K.; Koltunowicz, T.N.; Rogalski, P.; Subocz, J.; Korenciak, D. AC conductivity measurements of liquid-solid insulation of power transformers with high water content. Measurement 2020, 165, 108194. [Google Scholar] [CrossRef]
- Kołtunowicz, T.N.; Kierczynski, K.; Okal, P.; Patryn, A.; Gutten, M. Diagnostics on the Basis of the Frequency-Temperature Dependences of the Loss Angle Tangent of Heavily Moistured Oil-Impregnated Pressboard. Energies 2022, 15, 2924. [Google Scholar] [CrossRef]
- Zukowski, P.; Rogalski, P.; Bondariev, V.; Sebok, M. Diagnostics of High Water Content Paper-Oil Transformer Insulation Based on the Temperature and Frequency Dependencies of the Loss Tangent. Energies 2022, 15, 2813. [Google Scholar] [CrossRef]
- Yuan, H.; Li, Y.; Zhou, K.; Zhou, H. Moisture Transfer Characteristics in Silicone Oil–Silicone Rubber Insulation System Considering Swelling Effect. IEEE Trans. Dielectr. Electr. Insul. 2023, 30, 2025–2032. [Google Scholar] [CrossRef]
- RVM 5462—Advanced Automatic Recovery Voltage Meter for Diagnosis of Oil Paper Insulation, HAEFELY (n.d.). Available online: http://mldt.pl/files/rvm.PDF (accessed on 20 March 2024).
- Jonscher, A.K. Dielectric Relaxation in Solids; Chelsea Dielectrics Pres: London, UK, 1983. [Google Scholar]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics II. Direct Current Characteristics. J. Chem. Phys. 1942, 10, 98–105. [Google Scholar] [CrossRef]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics I. Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Davidson, D.W.; Cole, R.H. Dielectric relaxation in glycerol, propylene glycol, and n-propanol. J. Chem. Phys. 1951, 19, 1484–1490. [Google Scholar] [CrossRef]
- Davidson, D.W. Dielectric relaxation in liquids: I. The representation of relaxation behavior. Can. J. Chem. 1961, 39, 571–594. [Google Scholar] [CrossRef]
- Havriliak, S.J.; Havriliak, S.J. Dielectric and Mechanical Relaxation in Materials. Analysis, Interpretation and Application to Polymers; Hanser Publisher: Munich, Germany, 1997. [Google Scholar]
- Zukowski, P.; Kołtunowicz, T.N.; Kierczyński, K.; Subocz, J.; Szrot, M.; Gutten, M. Assessment of water content in an impregnated pressboard based on DC conductivity measurements theoretical assumptions. IEEE Trans. Dielectr. Electr. Insul. 2014, 21, 1268–1275. [Google Scholar] [CrossRef]
- Żukowski, P.; Kierczyński, K.; Kołtunowicz, T.N.; Rogalski, P.; Subocz, J. Application of elements of quantum mechanics in analysing AC conductivity and determining the dimensions of water nanodrops in the composite of cellulose and mineral oil. Cellulose 2019, 26, 2969–2985. [Google Scholar] [CrossRef]
- Zukowski, P.W.; Rodzik, A.; Shostak, Y.A.A. Dielectric constant and ac conductivity of semi-insulating Cd1-xMnxTe semiconductors. Semiconductors 1997, 31, 610–614. [Google Scholar] [CrossRef]
- Kosmulski, M. There are no nanodroplets of water in wet oil-impregnated pressboard. Cellulose 2021, 28, 5991–5992. [Google Scholar] [CrossRef]
- Fernández, I.; Ortiz, A.; Delgado, F.; Renedo, C.; Pérez, S. Comparative evaluation of alternative fluids for power transformers. Electr. Power Syst. Res. 2013, 98, 58–69. [Google Scholar] [CrossRef]
- MIDEL 7131. Increased Fire Safety; MIDEL: Manchester, UK, 2016. [Google Scholar]
- Liu, Q.; Wang, Z. Streamer characteristic and breakdown in synthetic and natural ester transformer liquids with pressboard interface under lightning impulse voltage. IEEE Trans. Dielectr. Electr. Insul. 2011, 18, 1908–1917. [Google Scholar] [CrossRef]
- Wolmarans, C.P.; Abrahams, R.; Pahlavanpour, B. Biodegradable electroinsulating fluids based on low viscosity hydrocarbons. Energetyka 2021, 9, 649–652. [Google Scholar]
- NYTRO® BIO 300X–The new bio-based alternative from Nynas. Transform. Technol. Mag. 2020, 46–51. Available online: https://www.powersystems.technology/community-hub/in-focus/nytro-bio-300x-the-new-bio-based-alternative-from-nynas.html (accessed on 22 March 2024).
- Fofana, I.; Hemmatjou, H.; Meghnefi, F.; Farzaneh, M.; Setayeshmehr, A.; Borsi, H.; Gockenbach, E. On the frequency domain dielectric response of oil-paper insulation at low temperatures. IEEE Trans. Dielectr. Electr. Insul. 2010, 17, 799–807. [Google Scholar] [CrossRef]
- Walczak, K.; Graczkowski, A.; Gielniak, J.; Morańda, H.; Mościcka-Grzesiak, H.; Ekanayake, C.; Gubański, S. Dielectric frequency response of cellulose samples with various degree of moisture content and aging. Prz. Elektrotechniczny 2006, 1, 264–267. [Google Scholar]
- Rouabeh, J.; M’barki, L.; Hammami, A.; Jallouli, I.; Driss, A. Studies of different types of insulating oils and their mixtures as an alternative to mineral oil for cooling power transformers. Heliyon 2019, 5, e01159. [Google Scholar] [CrossRef] [PubMed]
- Zukowski, P.; Rogalski, P.; Koltunowicz, T.N.; Kierczynski, K.; Subocz, J.; Sebok, M. Influence of temperature on phase shift angle and admittance of moistened composite of cellulose and insulating oil. Measurement 2021, 185, 110041. [Google Scholar] [CrossRef]
- Zukowski, P.; Rogalski, P.; Koltunowicz, T.N.; Kierczynski, K.; Subocz, J.; Zenker, M. Cellulose Ester Insulation of Power Transformers: Researching the Influence of Moisture on the Phase Shift Angle and Admittance. Energies 2020, 13, 5511. [Google Scholar] [CrossRef]
- Landau, L.D.; Lifshitz, E.M.; Pitaevskii, L.P. Electrodynamics of Continuous Media; Butterworth-Heinemann: Oxford, UK, 1984. [Google Scholar]
- DIRANA PTM User Manual ENU; Omicron Electronics Gmbh: Wien, Austria, 2017; Available online: https://www.scribd.com/document/469939247/DIRANA-PTM-User-Manual-ENU (accessed on 20 March 2024).
- Halliday, D.; Resnick, R. Physics, Part II; John Wiley & Sons: New York, NY, USA, 1978. [Google Scholar]
- Smirnov, S.A.; Shutov, D.A.; Bobkova, E.S.; Rybkin, V.V. Chemical Composition, Physical Properties and Populating Mechanism of Some O(I) States for a DC Discharge in Oxygen with Water Cathode. Plasma Chem. Plasma Process. 2016, 36, 415–436. [Google Scholar] [CrossRef]
- ASTM D1125-95 2005; Standard Test Methods for Electrical Conductivity and Resistivity of Water. ASTM International: West Conshohocken, PA, USA, 2016.
- Light, T.S.; Licht, S.; Bevilacqua, A.C.; Morash, K.R. The fundamental conductivity and resistivity of water. Electrochem. Solid-State Lett. 2005, 8, E16. [Google Scholar] [CrossRef]
- Kendall, J. The specific conductivity of pure water in equilibrium with atmospheric carbon dioxide. J. Am. Chem. Soc. 2002, 38, 1480–1497. [Google Scholar] [CrossRef]
- Rodger, J.W. The electric conductivity of pure water. Nature 1894, 51, 42–43. [Google Scholar] [CrossRef][Green Version]
- Wang, Q.; Cha, C.S.; Lu, J.; Zhuang, L. Ionic Conductivity of Pure Water in Charged Porous Matrix. ChemPhysChem 2012, 13, 514–519. [Google Scholar] [CrossRef]
- Melnik, L.A.; Krysenko, D.A. Ultrapure Water: Properties, Production, and Use. J. Water Chem. Technol. 2019, 41, 143–150. [Google Scholar] [CrossRef]
- Stillinger, F.H. Proton Transfer Reactions and Kinetics in Water. Theor. Chem. 1978, 3, 177–234. [Google Scholar]
- Mott, N.F.; Gurney, R.W. Electronic Processes in Ionic Crystals; Clarendon Press: Oxford, UK, 1950. [Google Scholar]
- Chelkowski, A. Fizyka Dielektryków; Panstwowe Wydawnictwo Naukowe: Warszawa, Poland, 1979. (In Polish) [Google Scholar]
- Mott, N.F.; Davis, E.A. Electronic Processes in Non-Crystalline Materials, 2nd ed.; Clarendon Press: Oxford, UK; New York, NY, USA, 1979. [Google Scholar]
- Stuke, J.; Brenig, W. Amorphous and liquid semiconductors. In Proceedings of the International Conference on Amorphous and Liquid Semiconductors, Garmisch-Partenkirchen, Germany, 3–8 September 1973; Taylor & Francis: Garmisch-Partenkirchen, Germany, 1974; pp. 183–191. [Google Scholar]
- Ravich, Y.I.; Nemov, S.A. Hopping conduction via strongly localized impurity states of indium in PbTe and its solid solutions. Semiconductors 2002, 36, 1–20. [Google Scholar] [CrossRef]
- Żukowski, P.; Kołtunowicz, T.; Partyka, J.; Węgierek, P.; Komarov, F.F.; Mironov, A.M.; Butkievith, N.; Freik, D. Dielectric properties and model of hopping conductivity of GaAs irradiated by H+ ions. Vacuum 2007, 81, 1137–1140. [Google Scholar] [CrossRef]
- Koltunowicz, T.N.; Zukowski, P.; Bondariev, V.; Czarnacka, K.; Boiko, O.; Fedotova, J.A.; Kasiuk, J.V. Study of dielectric function of (FeCoZr)x(CaF2)(100−x) nanocomposites produced with a beam of argon ions. J. Alloys Compd. 2015, 650, 262–267. [Google Scholar] [CrossRef]
- Żukowski, P.; Gałaszkiewicz, P.; Bondariev, V.; Okal, P.; Pogrebnjak, A.; Kupchishin, A.; Ruban, A.; Pogorielov, M.; Kołtunowicz, T.N. Comparative measurements and analysis of the electrical properties of nanocomposites TixZr1-xC+α-Cy (0.0 ≤ x ≤ 1.0). Materials 2022, 15, 7908. [Google Scholar] [CrossRef] [PubMed]
- Moyal, J.E. Theory of ionization fluctuations. Philos. Mag. 1955, 46, 263–280. [Google Scholar] [CrossRef]
- Landau, L.D.; Lifshitz, E.M. Quantum Mechanics (Third Edition, Revised and Enlarged); Pergamon Press: Oxford, UK, 1977; Available online: http://triton.itep.ru/ions/Physics/ALICE/Landau/LANDAULIFSHITZVOL.3QUANTUMMECHANICSNON-RELATIVISTICTHEORY_English.pdf (accessed on 20 March 2024).
- Shklovskii, B.I.; Efros, A.L. Electronic Properties of Doped Semiconductors; Springer: Berlin/Heidelberg, Germany, 1984. [Google Scholar]
- Van Vlack, L.H. Materials Science for Engineers; Addison-Wesley Publishing Company: Boston, MA, USA, 1970. [Google Scholar]
- Milnes, A.G.; Arthur, G. Deep Impurities in Semiconductors; Wiley: New York, NY, USA, 1973. [Google Scholar]
- Granovsky, V.V.L.; Musin, A.K.; Golubev, V.S.; Solnsev, G.S. Electric Current in Gas. Steady-State Current; Science: Moscow, Russia, 1971. [Google Scholar]
- Linstrom, P.J.; Mallard, W.G. NIST Chemistry Webbook, NIST Standard Reference Database Number 69, Volume 20899; National Institute of Standards and Technology: Gaithersburg, MD, USA, 1998. [Google Scholar]
- Zavilopulo, A.N.; Chipev, F.F.; Shpenik, O.B. Ionization of Nitrogen, Oxygen, Water, and Carbon Dioxide Molecules by Near-Threshold Electron Impact. Tech. Phys. 2005, 50, 402. [Google Scholar] [CrossRef]
- Kovtun, Y.V. Mean energy of water molecule ionization by electron impact. Tech. Phys. 2015, 60, 1110–1118. [Google Scholar] [CrossRef]
- Wolmarans, C.P.; Fairholm, R. A New Sustainable, Readily Biodegradable and High-Performance Insulating Liquid for Power Transformers. In Proceedings of the 26th International Conference and Exhibition on Electricity Distribution—CIRED 2021, Online Conference, 20–23 September 2021. [Google Scholar] [CrossRef]
- Wolmarans, C.; Gamil, A.; Al-Abadi, A.; Milone, M.; Jornaan, J.; Hellberg, R. Type Testing of 80 MVA Power Transformer with a new Bio-based, Biodegradable and Low Viscosity Insulating liquid. In Proceedings of the Conference: CIGRE Session, Paris, France, 28 August–2 September 2022; Volume A2–PS2, p. 11125. [Google Scholar]
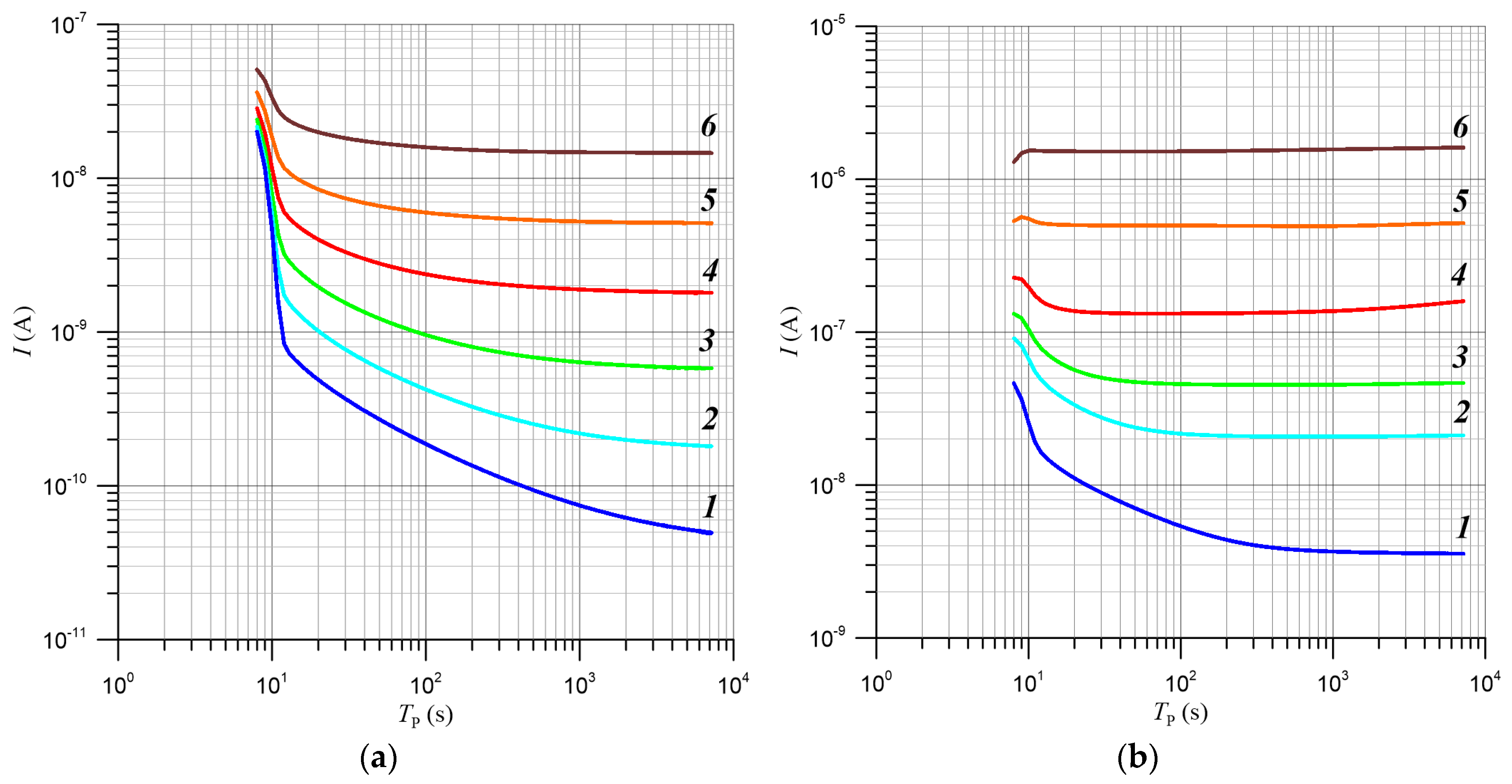
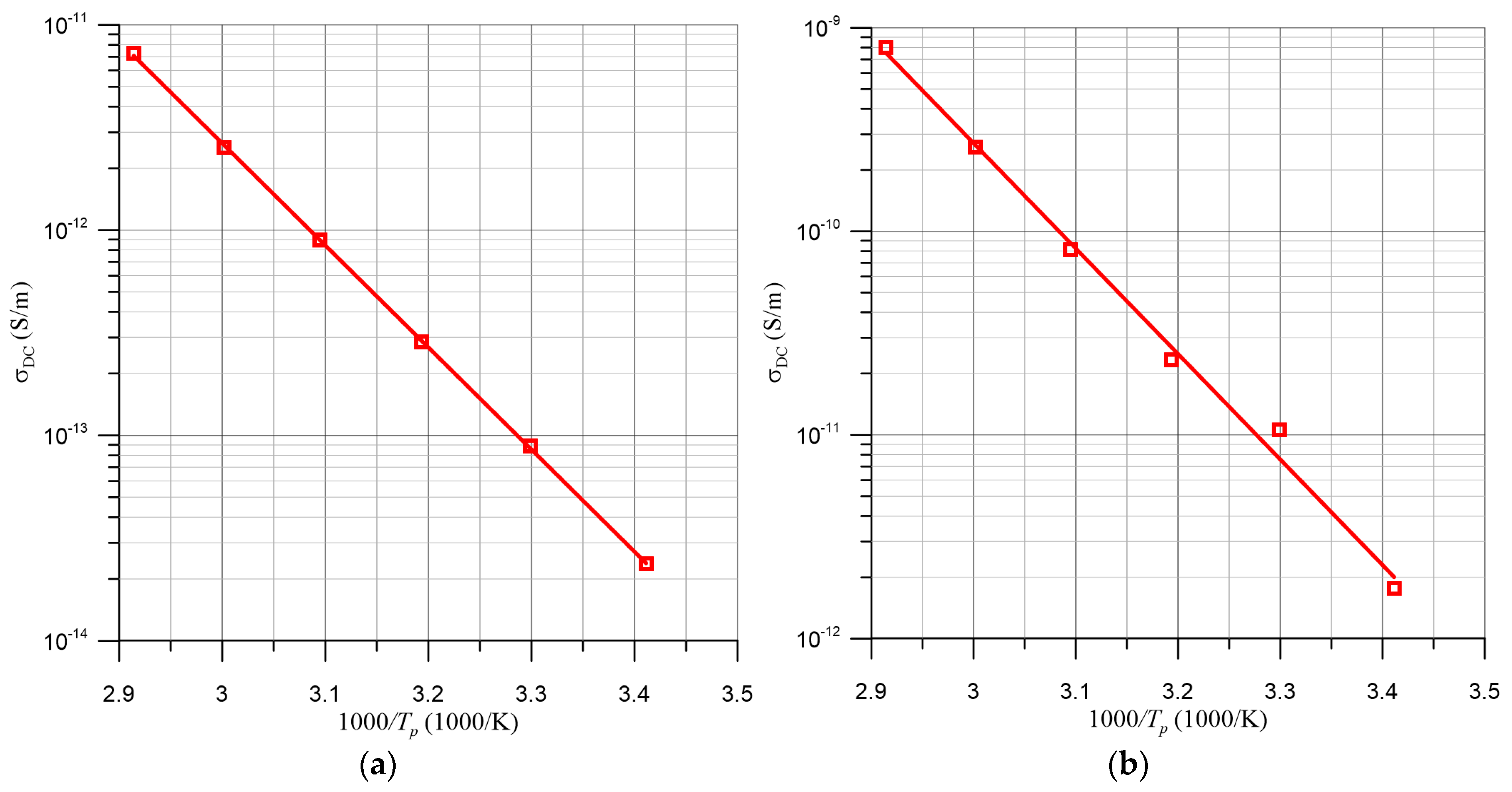
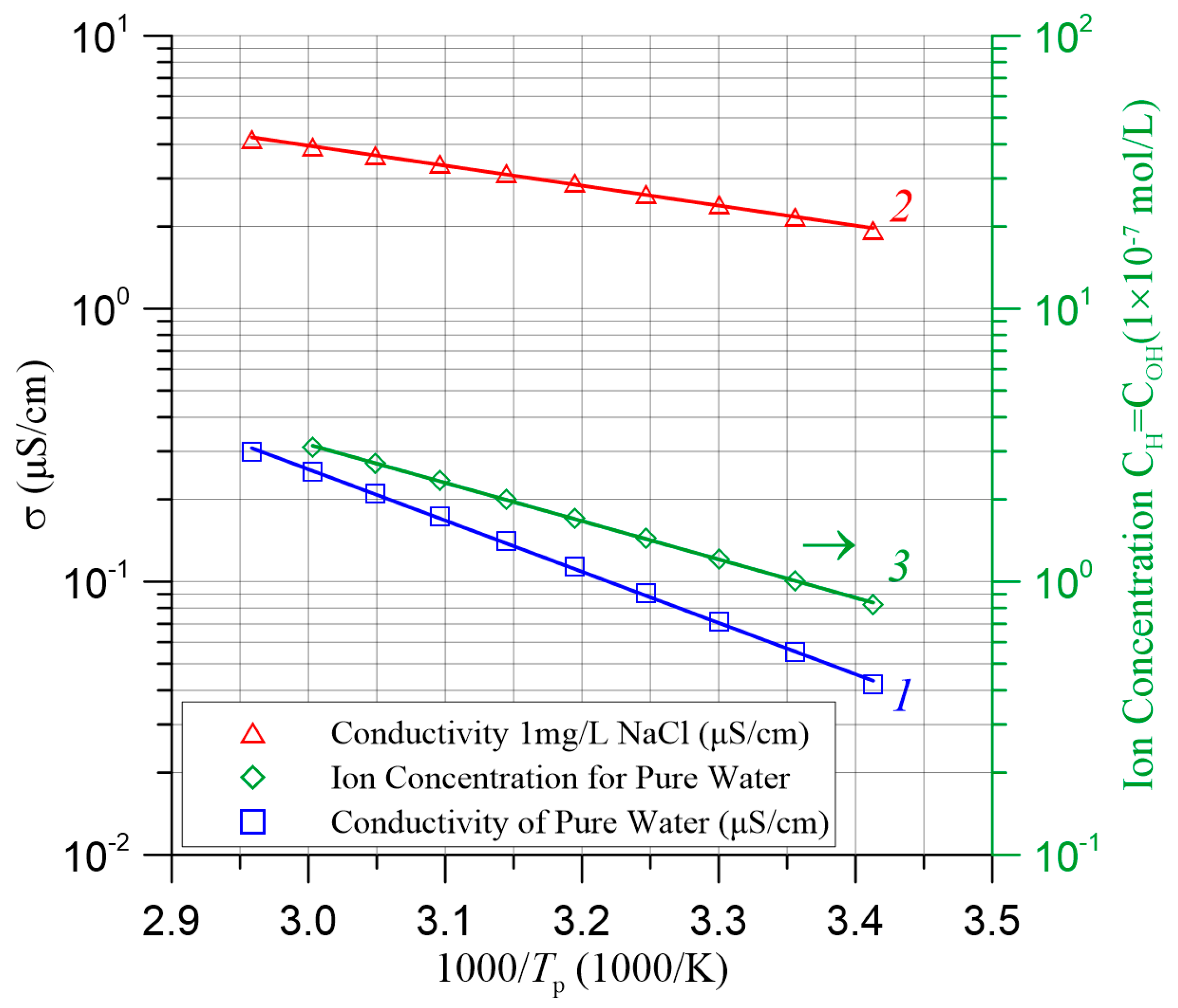
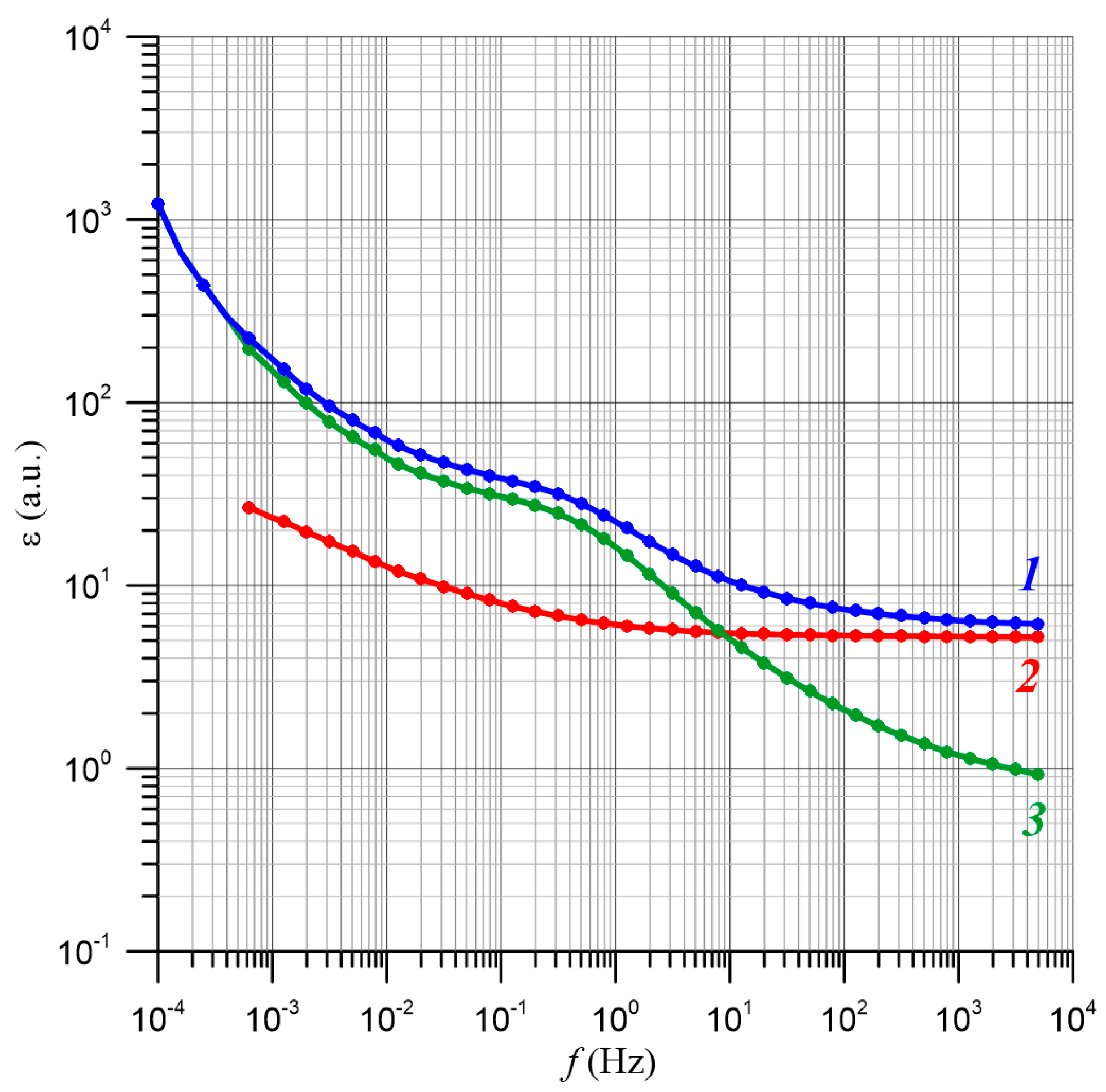
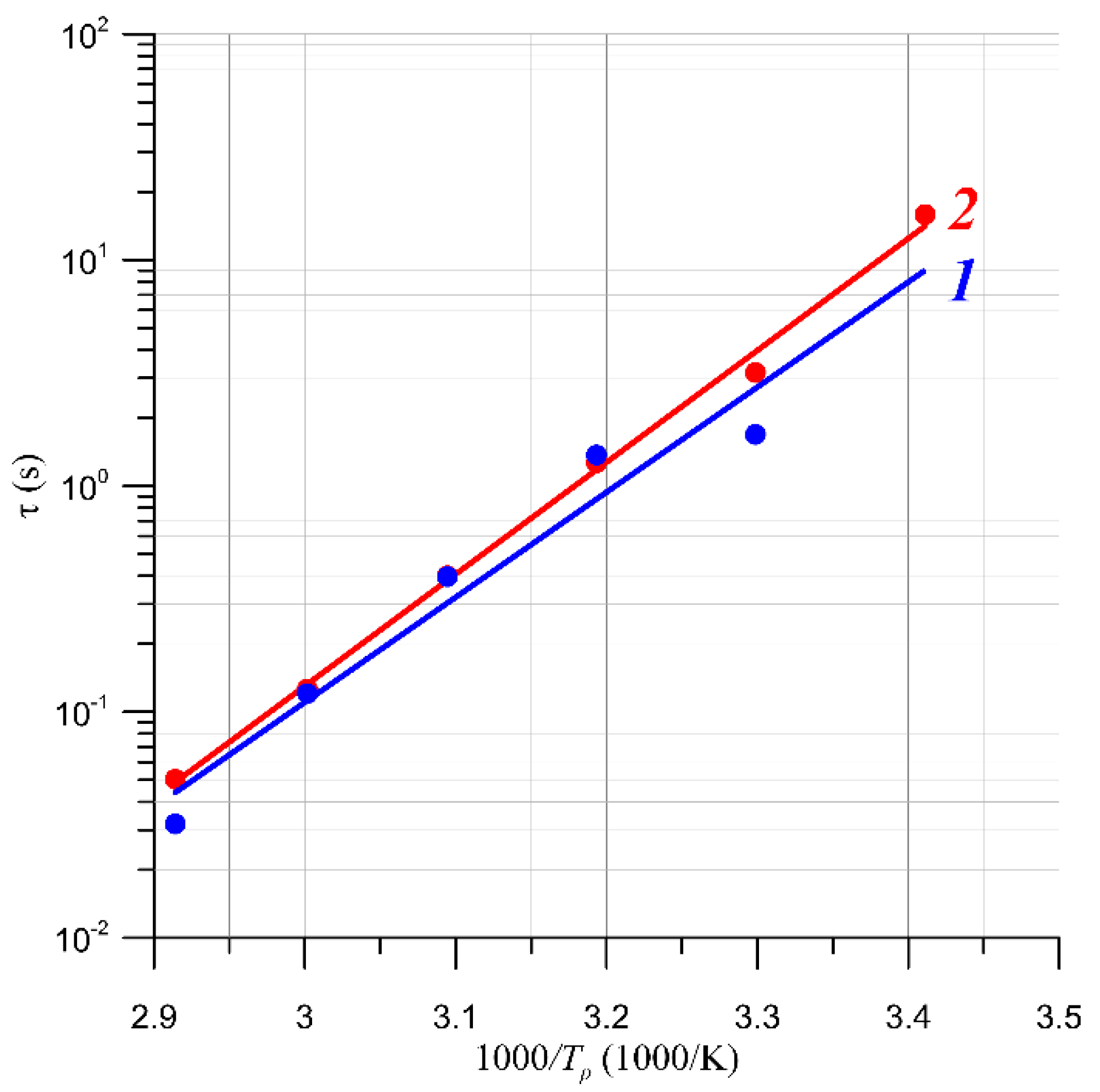
| TK, K | 2/RBD, nm−1 | R2 |
|---|---|---|
| 293.15 | 7.3641 | 0.869 |
| 303.15 | 7.9324 | 0.861 |
| 313.15 | 7.6094 | 0.902 |
| 323.15 | 7.8158 | 0.928 |
| 333.15 | 8.0708 | 0.952 |
| 343.15 | 8.4717 | 0.953 |
| Average | 7.88 | 0.911 |
| Deviation | 0.349 | 0.0366 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zukowski, P.; Kierczynski, K.; Rogalski, P.; Bondariev, V.; Zenker, M.; Pajak, R.; Szrot, M.; Molenda, P.; Koltunowicz, T.N. Analysis of the Influence of Insulation Moisture during Long-Term Exploitation of Transformers on Their Reliability as Determined by Alternating Current Electrical Parameters. Energies 2024, 17, 1952. https://doi.org/10.3390/en17081952
Zukowski P, Kierczynski K, Rogalski P, Bondariev V, Zenker M, Pajak R, Szrot M, Molenda P, Koltunowicz TN. Analysis of the Influence of Insulation Moisture during Long-Term Exploitation of Transformers on Their Reliability as Determined by Alternating Current Electrical Parameters. Energies. 2024; 17(8):1952. https://doi.org/10.3390/en17081952
Chicago/Turabian StyleZukowski, Pawel, Konrad Kierczynski, Przemyslaw Rogalski, Vitalii Bondariev, Marek Zenker, Rafal Pajak, Marek Szrot, Pawel Molenda, and Tomasz N. Koltunowicz. 2024. "Analysis of the Influence of Insulation Moisture during Long-Term Exploitation of Transformers on Their Reliability as Determined by Alternating Current Electrical Parameters" Energies 17, no. 8: 1952. https://doi.org/10.3390/en17081952
APA StyleZukowski, P., Kierczynski, K., Rogalski, P., Bondariev, V., Zenker, M., Pajak, R., Szrot, M., Molenda, P., & Koltunowicz, T. N. (2024). Analysis of the Influence of Insulation Moisture during Long-Term Exploitation of Transformers on Their Reliability as Determined by Alternating Current Electrical Parameters. Energies, 17(8), 1952. https://doi.org/10.3390/en17081952








