Pyrogenic Carbonaceous Materials Production of Four Tropical Wood Produced by Slow Pyrolysis at Different Temperatures: Charcoal and Biochar Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Process of Carbonization and Treatment
2.3. Evaluations and Properties of Pyrogenic Carbonaceous Materials (PCMs)
2.3.1. Evaluation of Pyrogenic Carbonaceous Materials (PCMs) Yield
2.3.2. Physical and Mechanical Properties
2.3.3. Ultimate Analysis
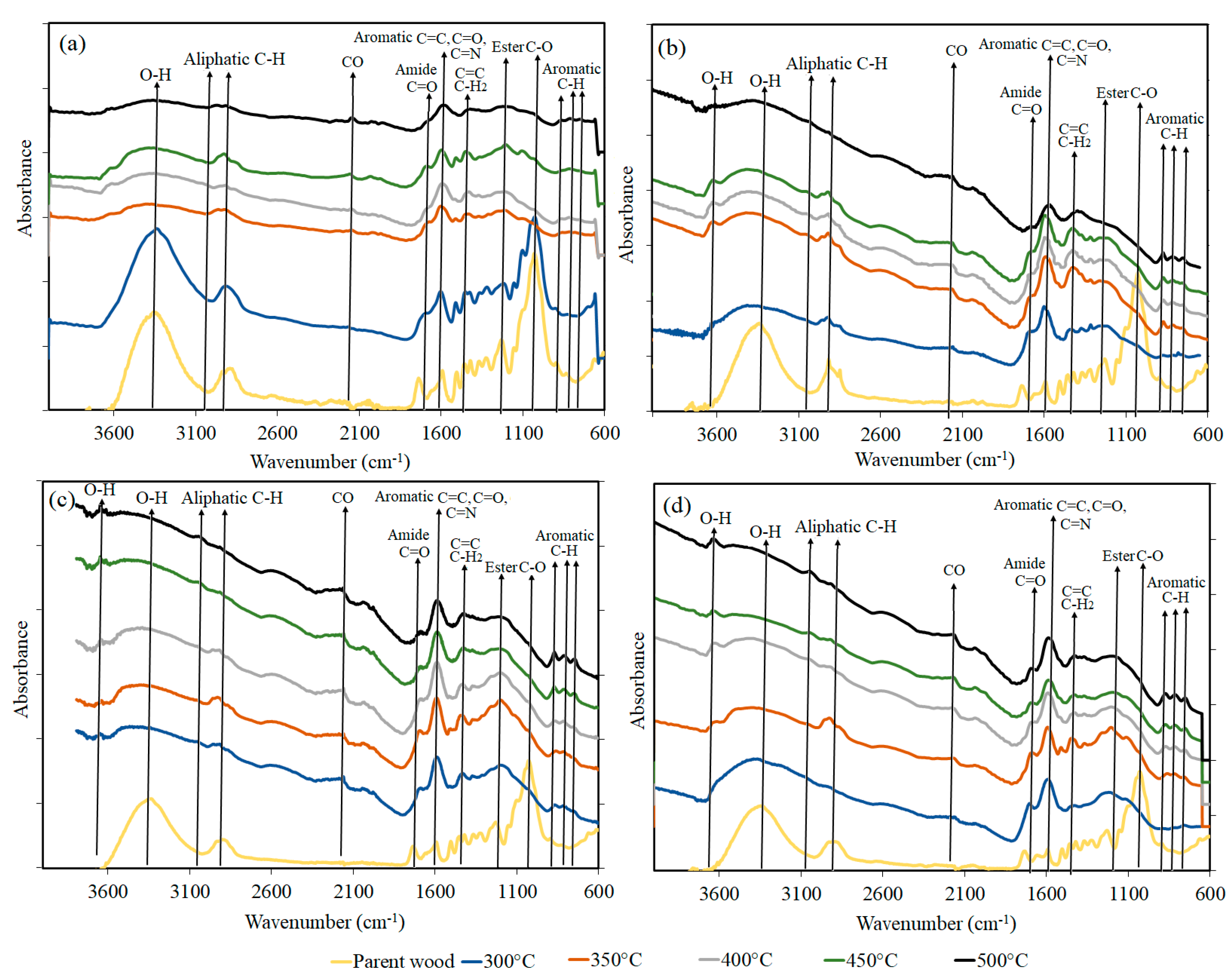
2.3.4. FTIR Analysis
- 2910 is C-H (stretching) and represents loss of hydroxyl bonds by heating;
- 2160–2170 represents CO formation due to carbonization;
- 1700 is C=O bond of hemicellulose and cellulose degradation;
- 1600 and 1630 is C=O (stretching) of the aromatic ring of lignin;
- 1434 is CH2 and CH3 (asymmetric charact) of aromatic nuclei in lignin, kept stable;
- 1206 is C-O (stretching) of C-O breakage;
- 1032 is C-O (stretching) and represents the bonds in acids, alcohols, phenols, ether, or ester groups;
- 1111 is C-O (stretching) and represents the bonds in acids, alcohols, phenols, ether, or ester groups;
- 900 is C-H of the out-of-plane of the aromatic ring;
- 878 is C-H of the out-of-plane glucose ring in cellulose and hemicellulose and for guaiacyl ring in lignin;
- 810 and 750 is C-H of the out-of-plane of the aromatic rings.
2.4. Charcoal Characterization
2.4.1. Energetic Properties
2.4.2. TGA Analysis
2.5. Biochar Characteristics
2.6. Statistical Analysis
3. Results and Discussion
3.1. Pyrogenic Carbonaceous Materials (PCMs) Production
3.2. Physical and Mechanical Properties of PCM
3.3. FTIR Analysis
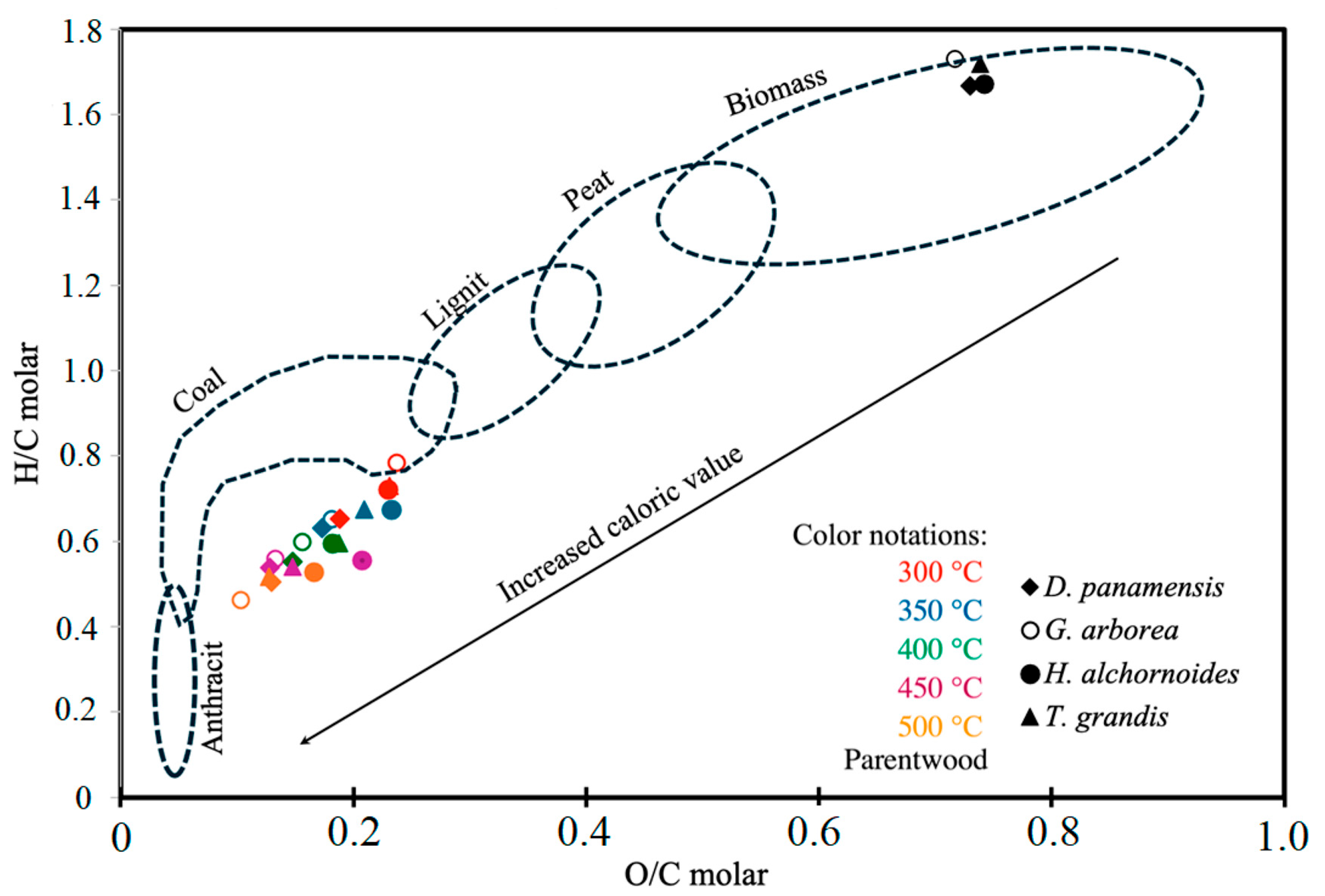
3.4. Energy and Chemical Properties
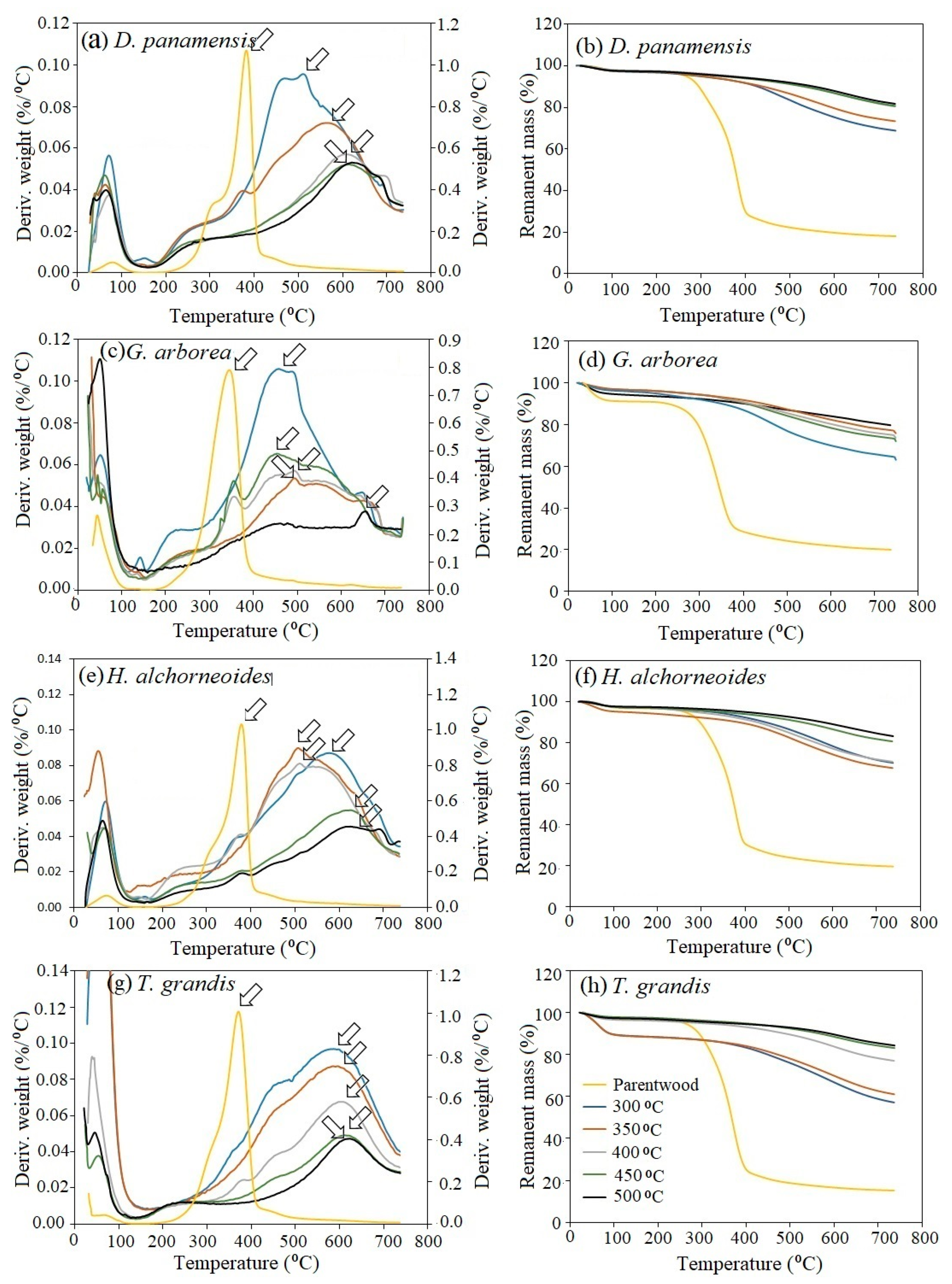
3.5. Thermogravimetric Analysis
- (i)
- Remanent mass increased with increasing pyrolysis temperatures (Figure 4b,d,f,h);
- (ii)
- Shoulders at 250 °C and 375 °C were observed in charcoal produced from D. panamensis (Figure 4a), G. arborea (Figure 4c), and H. alchorneoides (Figure 4e) obtained at 300 and 350 °C, signaling incomplete carbonization and, thus, evidencing the presence of celluloses, hemicellulose, and lignin in unchanged wood components [56];
- (iii)
- A small peak appeared near 700 °C in the DTG of D. panamensis, G. arborea, and H. alchorneoides, and was particularly visible in the charcoals obtained at 450 and 500 °C (Figure 4a,c,e).
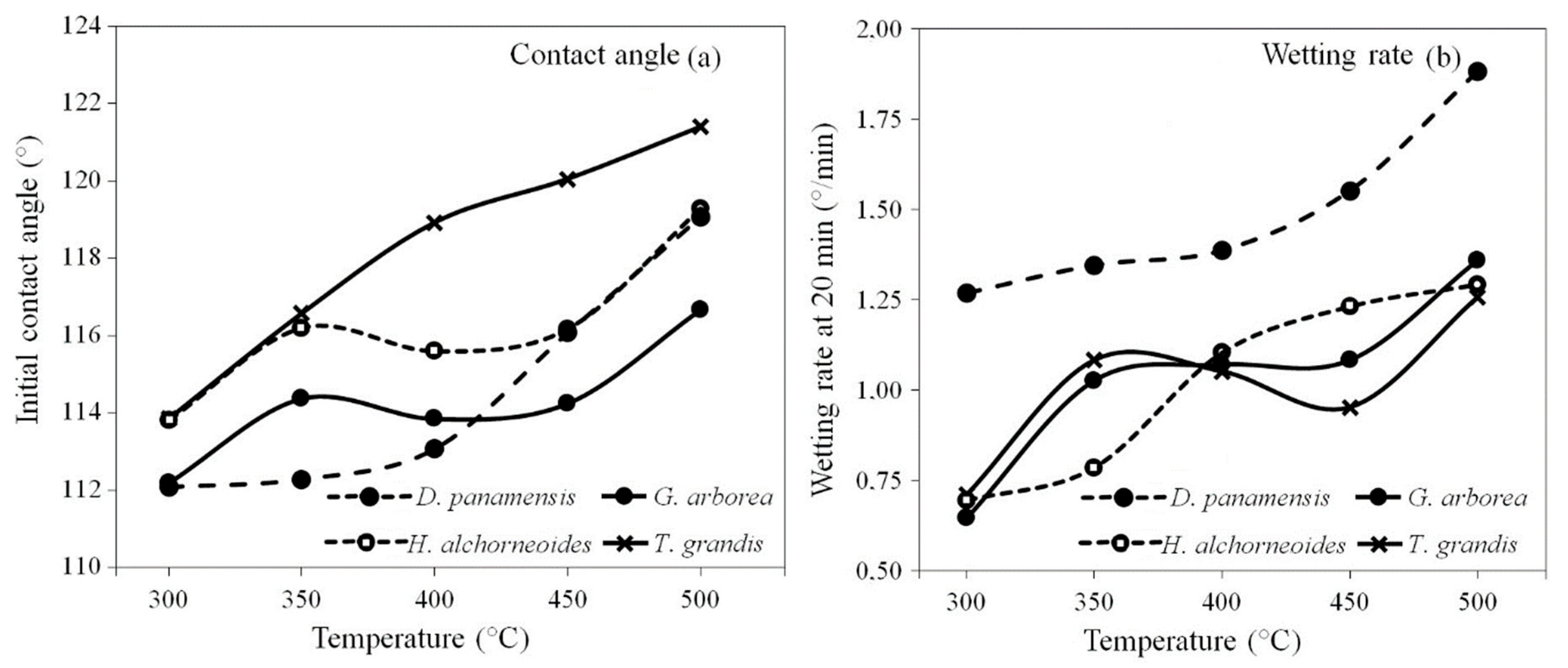
3.6. Biochar Properties
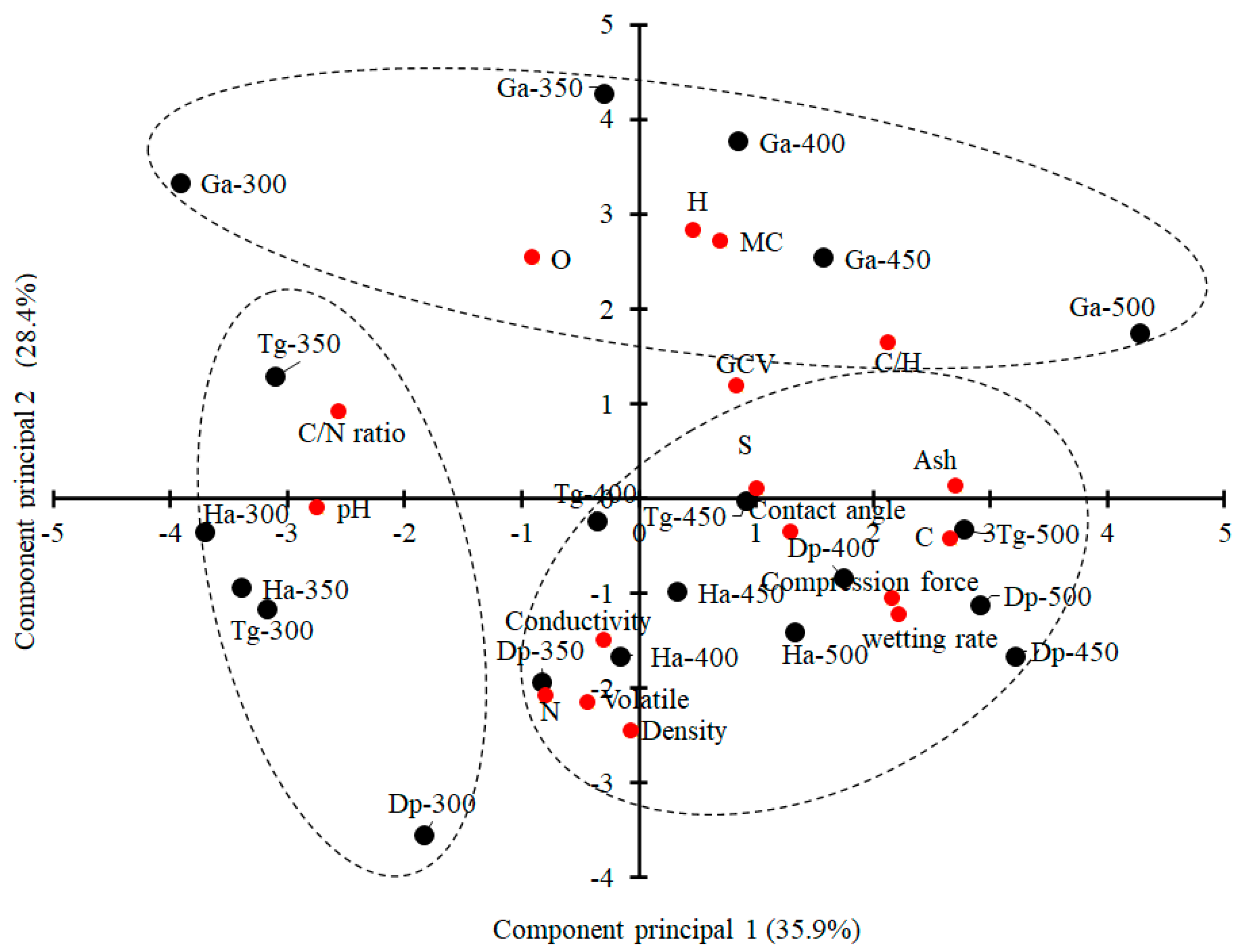
3.7. Multivariate Analysis
- The first group groups charcoal of H. alchorneoides and T. grandis pyrolyzed at 300 °C and 350 °C (low temperatures) and that of D. panamensis of pyrolysis temperature 300 °C. This group is associated with pH and C/N ratio;
- The second group is formed by all the charcoals of G. arborea, and is associated with O and H content, C/H ratio, MC, and GCV;
- The third group includes the charcoals of H. alchorneoides, T. grandis, and D. panamensis pyrolyzed at 350 °C to 500 °C; it is associated with contact angle, wetting rate, ash, N and C content, compression force, conductivity, volatility, and density.
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kan, T.; Strezov, V.; Evans, T.J. Lignocellulosic biomass pyrolysis: A review of product properties and effects of pyrolysis parameters. Renew. Sustain. Energy Rev. 2016, 57, 1126–1140. [Google Scholar] [CrossRef]
- Patel, A.; Agrawal, B.; Rawal, B.R. Pyrolysis of biomass for efficient extraction of biofuel. Energy Sources Part A 2020, 42, 1649–1661. [Google Scholar] [CrossRef]
- Hagemann, N.; Spokas, K.; Schmidt, H.P.; Kägi, R.; Böhler, M.A.; Bucheli, T.D. Activated carbon, biochar and charcoal: Linkages and synergies across pyrogenic carbon’s ABCs. Water 2018, 10, 182. [Google Scholar] [CrossRef]
- Velmurugan, V. Review of research and development on pyrolysis process. Mater. Today Proc. 2022, 49, 3679–3686. [Google Scholar] [CrossRef]
- Dhyani, V.; Bhaskar, T. A comprehensive review on the pyrolysis of lignocellulosic biomass. Renew. Energy 2018, 129, 695–716. [Google Scholar] [CrossRef]
- Zadeh, Z.E.; Abdulkhani, A.; Aboelazayem, O.; Saha, B. Recent insights into lignocellulosic biomass pyrolysis: A critical review on pretreatment, characterization, and products upgrading. Processes 2020, 8, 799. [Google Scholar] [CrossRef]
- Wang, S.; Dai, G.; Yang, H.; Luo, Z. Lignocellulosic biomass pyrolysis mechanism: A state-of-the-art review. Prog. Energy Combust. Sci. 2017, 62, 33–86. [Google Scholar] [CrossRef]
- Zhou, N.; Thilakarathna, W.W.; He, Q.S.; Rupasinghe, H.V. A review: Depolymerization of lignin to generate high-value bio-products: Opportunities, challenges, and prospects. Front. Energy Res. 2022, 9, 758744. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.; Sivashanmugam, P.; Kavitha, S.; Kannah, Y.; Varjani, S.; AdishKumar, S.; Kumar, G. Lignocellulosic biomass-based pyrolysis: A comprehensive review. Chemosphere 2022, 286, 131824. [Google Scholar] [CrossRef]
- Urrutia, R.I.; Gutierrez, V.S.; Stefanazzi, N.; Volpe, M.A.; González, J.O.W. Pyrolysis liquids from lignocellulosic biomass as a potential tool for insect pest management: A comprehensive review. Ind. Crop. Prod. 2022, 177, 114533. [Google Scholar] [CrossRef]
- Rangabhashiyam, S.; Balasubramanian, P. The potential of lignocellulosic biomass precursors for biochar production: Performance, mechanism and wastewater application-a review. Ind. Crop. Prod. 2019, 128, 405–423. [Google Scholar] [CrossRef]
- Lubwama, M.; Yiga, V.A.; Ssempijja, I.; Lubwama, H.N. Thermal and mechanical characteristics of local firewood species and resulting charcoal produced by slow pyrolysis. Biomass Convers. Biorefin. 2021, 13, 6689–6704. [Google Scholar] [CrossRef]
- Hernández-Chaverri, R.A.; Buenrostro-Figueroa, J.J.; Prado-Barragán, L.A. Biomass: Biorefinery as a model to boost the bioeconomy in Costa Rica, a review. Agron. Mesoamerican 2021, 32, 1047–1070. [Google Scholar] [CrossRef]
- Castro-Vargas, M.S.; Werner, M. Regulation by impasse: Pesticide registration, capital and the state in Costa Rica. Environ. Plan. E Nat. Space 2023, 6, 901–922. [Google Scholar] [CrossRef]
- Valenciano-Salazar, J.A.; André, F.J.; Martín-de Castro, G. Sustainability and firms’ mission in a developing country: The case of voluntary certifications and programs in Costa Rica. J. Environ. Plan. Manag. 2022, 65, 2029–2053. [Google Scholar] [CrossRef]
- Rubio-Jovel, K.; Sellare, J.; Damm, Y.; Dietz, T. SDGs trade-offs associated with voluntary sustainability standards: A case study from the coffee sector in Costa Rica. Sustain. Dev. 2023, 32, 917–939. [Google Scholar] [CrossRef]
- Sahoo, K.; Kumar, A.; Chakraborty, J.P. A comparative study on valuable products: Bio-oil, biochar, non-condensable gases from pyrolysis of agricultural residues. J. Mater. Cycles Waste Manag. 2021, 23, 186–204. [Google Scholar] [CrossRef]
- Dhar, S.A.; Sakib, T.U.; Hilary, L.N. Effects of pyrolysis temperature on production and physicochemical characterization of biochar derived from coconut fiber biomass through slow pyrolysis process. Biomass Conv. Bioref. 2022, 12, 2631–2647. [Google Scholar] [CrossRef]
- Balaguer-Benlliure, V.; Moya, R.; Gaitán-Álvarez, J. Physical and energy characteristics, compression strength and Chemical modification of charcoal produced from sixteen tropical woods in Costa Rica. J. Sustain. For. 2023, 42, 151–169. [Google Scholar] [CrossRef]
- Valverde, J.C.; Arias, D.; Campos, R.; Jiménez, M.F.; Brenes, L. Forest and agro-industrial residues and bioeconomy: Perception of use in the energy market in Costa Rica. Energy Ecol. Environ. 2021, 6, 232–243. [Google Scholar] [CrossRef]
- Chaves, M.; Torres, C.; Tenorio, C.; Moya, R.; Arias-Aguilar, D. Syngas characterization and electric performance evaluation of gasification process using forest plantation biomass. Waste Biomass Valor. 2024, 15, 1291–1308. [Google Scholar] [CrossRef]
- Kumar, R.; Strezov, V.; Weldekidan, H.; He, J.; Singh, S.; Kan, T.; Dastjerdi, B. Lignocellulose biomass pyrolysis for bio-oil production: A review of biomass pre-treatment methods for production of drop-in fuels. Renew. Sustain. Energy Rev. 2020, 123, 109763. [Google Scholar] [CrossRef]
- Song, B.; Almatrafi, E.; Tan, X.; Luo, S.; Xiong, W.; Zhou, C.; Qin, M.; Liu, Y.; Cheng, M.; Zend, G.; et al. Biochar-based agricultural soil management: An application-dependent strategy for contributing to carbon neutrality. Renew. Sustain. Energy Rev. 2022, 164, 112529. [Google Scholar] [CrossRef]
- Moya, R.; Tenorio, C.; Quesada-Kimzey, J. Charcoal production of four tropical wood produced with slow pyrolysis in different temperatures: Yield of different products and condition of pyrolysis into the reactor. Biomass Conv. Bioref. 2024, 10, 1–8. [Google Scholar] [CrossRef]
- Rodriguez-Solis, A.; Badilla-Valverde, Y.; Moya, R. Agronomic effects of Tectona grandis biochar from wood residues on the growth of young Cedrela odorata plants in a nursery. Agronomy 2021, 11, 2079. [Google Scholar] [CrossRef]
- Villagra-Mendoza, K.; Masís-Meléndez, F.; Quesada-Kimsey, J.; García-González, C.A.; Horn, R. Physicochemical changes in loam soils amended with bamboo biochar and their influence in tomato production yield. Agronomy 2021, 11, 2052. [Google Scholar] [CrossRef]
- Berrocal-Mendéz, N.; Moya, R. Production, cost and properties of charcoal produced after logging and sawing, by the earth pit method from Tectona grandis wood residues. J. Indian Acad. Wood Sci. 2022, 19, 121–132. [Google Scholar] [CrossRef]
- Haryanto, A.; Hidayat, W.; Hasanudin, U.; Iryani, D.A.; Kim, S.; Lee, S.; Yoo, J. Valorization of Indonesian wood wastes through pyrolysis: A review. Energies 2021, 14, 1407. [Google Scholar] [CrossRef]
- Okekunle, P.O.; Ogunshola, A.D.; Babayemi, O.A.; Abodunrin, E.D.; Daramola, O.M. Fuel characterization of bio-oil from fast pyrolysis of Tectona grandis in a fixed bed reactor at different temperatures (400–700 °C). Int. J. Energy Clean Environ. 2021, 22, 1–14. [Google Scholar] [CrossRef]
- Aswin, S.; Ranjithkumar, S.P.; Sivamani, S. Modelling and simulation of pyrolysis of teak (Tectona grandis) sawdust. In Biofuel Production Technologies: Critical Analysis for Sustainability, Clean Energy Production Technologies; Springer: Berlin/Heidelberg, Germany, 2020; pp. 325–342. [Google Scholar] [CrossRef]
- Gupta, G.K.; Gupta, P.K.; Mondal, M.K. Experimental process parameters optimization and in-depth product characterizations for teak sawdust pyrolysis. Waste Manag. 2019, 87, 499–511. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Sheeba, K.N. Generation of fuel char through biomass slow pyrolysis. Energy Source Part A 2017, 39, 599–605. [Google Scholar] [CrossRef]
- Adegoke, I.A.; Ogunsanwo, O.Y.; Ige, A.R. Bio-fuel properties and elemental analysis of bio-oil produced from pyrolysis of Gmelina arborea. Acta Chem. Malays. 2021, 5, 38–41. [Google Scholar] [CrossRef]
- Moya, R.; Tenorio, C. Application of the steaming step during kiln drying of lumber of two tropical species with high growth stress presence. Dry Technol. 2022, 42, 3231–3240. [Google Scholar] [CrossRef]
- Gaitán-Alvarez, J.; Berrocal, A.; Mantanis, G.I.; Moya, R.; Araya, F. Acetylation of tropical hardwood species from forest plantations in Costa Rica: An FTIR spectroscopic analysis. J. Wood Sci. 2020, 66, 49. [Google Scholar] [CrossRef]
- Moya, R.; Tenorio, C.; Muñoz, F.; Salas, J.; Berrocal, A. Tecnología de Madera de Plantaciones Forestales: Fichas Técnicas; Editorial Tecnológica de Costa Rica; Editorial de la Universidad de Costa Rica: San Pedro, Costa Rica, 2019. [Google Scholar]
- ASTM D1762-84; Standard Test Method for Chemical Analysis of Wood Charcoal. ASTM International: West Conshohocken, PA, USA, 2021. [CrossRef]
- Ahmed, A.; Abu Bakar, M.S.; Azad, A.K.; Sukri, R.S.; Phusunti, N. Intermediate pyrolysis of Acacia cincinnata and Acacia holosericea species for bio-oil and biochar production. Energy Convers. Manag. 2018, 176, 393–408. [Google Scholar] [CrossRef]
- Kaur, V.; Kaur, B.; Kaur, K.; Kaur, M.; Kaur, S. Preparation andharacterizationn of charcoal material derived from bamboo for the adsorption of sulphur contaminated water. Lond. J. Res. Sci. Nat. Form. 2018, 18, 824557. [Google Scholar]
- Liu, Z.; Huang, Y.; Zhao, G. Preparation and characterization of activated carbon fibers from liquefied wood by ZnCl2 activation. BioResources 2016, 11, 3178–3190. [Google Scholar] [CrossRef]
- Ozdemir, I.; Şahin, M.; Orhan, R.; Erdem, M. Preparation and characterization of activated carbon from grape stalk by zinc chloride activation. Fuel Process. Technol. 2014, 125, 200–206. [Google Scholar] [CrossRef]
- Rousset, P.; Figueiredo, C.; De Souza, M.; Quirino, W. Pressure effect on the quality of eucalyptus wood charcoal for the steel industry: A statistical analysis approach. Fuel Process. Technol. 2011, 92, 1890–1897. [Google Scholar] [CrossRef]
- Ren, X.; Cai, H.; Chang, J.; YongMing, F. TG-FTIR study on the pyrolysis properties of lignin from different kinds of woody biomass. Pap. Biomater. 2018, 3, 1–7. [Google Scholar] [CrossRef]
- ASTM D5865M-19; Standard Test Method for Gross Calorific Value of Coal and Coke No Title. ASTM International: West Conshohocken, PA, USA, 2019. [CrossRef]
- ASTM D3173M-17a; Standard Test Method for Proximate Analysis of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2017. [CrossRef]
- Masís-Meléndez, F.; Segura-Chavarría, D.; García-González, C.A.; Quesada-Kimsey, J.; Villagra-Mendoza, K. Variability of physical and chemical properties of TLUD stove derived biochars. Appl. Sci. 2020, 10, 507. [Google Scholar] [CrossRef]
- Bachmann, J.; Horton, R.; Van Der Ploeg, R.R.; Woche, S. Modified sessile drop method for assessing initial soil–water contact angle of sandy soil. Soil Sci. Soc. Am. J. 2000, 64, 564–567. [Google Scholar] [CrossRef]
- Diboma, B.S.; Atiotsia, V.H.; Che, L.C.; Essomba, P.B.; Bot, B.V.; Tamba, J.G. Gasification of charcoal derived fr m tropical wood residues in an updraft fixed bed reactor. Bioresour. Techn. Rep. 2023, 21, 101308. [Google Scholar] [CrossRef]
- Dufourny, A.; Van De Steene, L.; Humbert, G.; Guibal, D.; Martin, L.; Blin, J. Influence of pyrolysis conditions and the nature of the wood on the quality of charcoal as a reducing agent. J. Anal. Appl. Pyrolysis 2019, 137, 1–13. [Google Scholar] [CrossRef]
- Yuan, T.; He, W.; Yin, G.; Xu, S. Comparison of bio-chars formation derived from fast and slow pyrolysis of walnut shell. Fuel 2020, 261, 116450. [Google Scholar] [CrossRef]
- Lima, M.D.R.; Massuque, J.; Bufalino, L.; Trugilho, P.F.; Ramalho, F.M.G.; Protásio, T.; Hein, P.R.G. Clarifying the carbonization temperature effects on the production and apparent density of charcoal derived from Amazonia wood wastes. J. Anal. Appl. Pyrolysis 2022, 166, 105636. [Google Scholar] [CrossRef]
- Weber, K.; Quicker, P. Properties of biochar. Fuel 2018, 217, 240–261. [Google Scholar] [CrossRef]
- Zhang, J.; You, C. Water holding capacity and absorption properties of wood chars. Energy Fuels 2013, 27, 2643–2648. [Google Scholar] [CrossRef]
- Mothé, C.G.; Miranda, I.C. Characterization of sugarcane and coconut fibers by thermal analysis and FTIR. J. Therm. Anal. Calorim. 2009, 97, 661–665. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Andersen, L.K.; Vassileva, C.G. An overview of the composition and application of biomass ash. Part 1. Phase–mineral and chemical composition and classification. Fuel 2013, 105, 40–76. [Google Scholar] [CrossRef]
- Várhegyi, G.; Szabó, P.; Antal, M.J. Kinetics of charcoal devolatilization. Energy Fuels 2022, 16, 724–731. [Google Scholar] [CrossRef]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Physicochemical and sorptive properties of biochars derived from woody and herbaceous biomass. Chemosphere 2015, 134, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Elleuch, A.; Halouani, K.; Li, Y. Investigation of chemical and electrochemical reactions mechanisms in a direct carbon fuel cell using olive wood charcoal as sustainable fuel. J. Power Sources 2015, 281, 350–361. [Google Scholar] [CrossRef]
- Singh, B.; Dolk, M.M.; Shen, Q.; Camps-Arbestain, M. Biochar pH, electrical conductivity and liming potential. In Biochar: A Guide to Analytical Methods; CRC Press: Boca Raton, FL, USA, 2017; p. 23. [Google Scholar]
- Joseph, S.; Peacocke, C.; Lehmann, J.; Monroe, P. Developing a biochar classifi cation and test methods. In Biochar for Environmental Management: Science and Technology, 1st ed.; Lehmann, J., Joseph, S., Eds.; Routledge: London, UK, 2009. [Google Scholar]
- Paz-Ferreiro, J.; Nieto, A.; Méndez, A.; Askeland, M.P.J.; Gascó, G. Biochar from Biosolids Pyrolysis: A Review. Int. J. Environ. Res. Public Health 2018, 15, 956. [Google Scholar] [CrossRef]
- Cantrell, K.B.; Hunt, P.G.; Uchimiya, M.; Novak, J.M.; Ro, K.S. Impact of pyrolysis temperature and manure source on physicochemical characteristics of biochar. Bioresour. Technol. 2012, 107, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Alkharabsheh, H.M.; Seleiman, M.F.; Battaglia, M.L.; Shami, A.; Jalal, R.S.; Alhammad, B.A.; Almutairi, K.F.; Al-Saif, A.M. Biochar and its broad impacts in soil quality and fertility, nutrient leaching, and crop productivity: A review. Agronomy 2021, 11, 993. [Google Scholar] [CrossRef]
- Xu, H.; Cai, A.; Wu, D.; Liang, G.; Xiao, J.; Xu, M.; Colinet, G.; Zhang, W. Effects of biochar application on crop productivity, soil carbon sequestration, and global warming potential controlled by biochar C: N ratio and soil pH: A global meta-analysis. Soil Tillage Res. 2021, 213, 105125. [Google Scholar] [CrossRef]
- Ding, Y.; Liu, Y.; Liu, S.; Li, Z.; Tan, X.; Huang, X.; Zeng, G.; Zhou, L.; Zheng, B. Biochar to improve soil fertility: A review. Agron. Sustain. Dev. 2016, 36, 36. [Google Scholar] [CrossRef]
- Shinogi, Y.; Kanri, Y. Pyrolysis of plant, animal and human waste: Physical and chemical characterization of the pyrolytic products. Bioresour. Technol. 2003, 90, 241–247. [Google Scholar] [CrossRef]
- Zhao, S.X.; Na, T.; Wang, X.D. Effect of temperature on the structural and physicochemical properties of biochar with apple tree branches as feedstock material. Energies 2017, 10, 1293. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokołowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Usevičiūtė, L.; Baltrėnaitė-Gedienė, E. Dependence of pyrolysis temperature and lignocellulosic physical-chemical properties of biochar on its wettability. Biomass Conv. Bioref. 2021, 11, 2775–2793. [Google Scholar] [CrossRef]
- Rasa, K.; Heikkinen, J.; Hannula, M.; Arstila, K.; Kulju, S.; Hyvaluoma, J. How and why does willow biochar increase a clay soil water tention capacity? Biomass Bioenergy 2018, 119, 346–353. [Google Scholar] [CrossRef]
- Das, O.; Sarmah, A.K. The love-hate relationship of pyrolysis biochar and water: A perspective. Sci. Total Environ. 2015, 512, 682–685. [Google Scholar] [CrossRef]

| Species | Yield Percentages of PCMs | ||||
|---|---|---|---|---|---|
| 300 °C | 350 °C | 400 °C | 450 °C | 500 °C | |
| D. panamensis | 44.48 | 33.80 | 31.42 | 28.47 | 28.18 |
| G. arborea | 56.63 | 41.63 | 37.18 | 32.97 | 30.46 |
| H. alchorneoides | 50.21 | 48.11 | 35.64 | 32.57 | 33.69 |
| T. grandis | 46.74 | 51.00 | 36.56 | 33.78 | 32.47 |
| Parameter | Species Effect | Site Effect | Interaction |
|---|---|---|---|
| Density | 443.02 ** | 444.80 ** | 16.02 ** |
| Moisture content | 3.06 * | 1821.77 ** | 28.83 ** |
| Compression strength | 98.51 ** | 1438.63 ** | 93.68 ** |
| GCV | 5.26 ** | 113.24 ** | 1.81 ** |
| Volatile matter (%) | 130.20 ** | 776.24 * | 4.91 ** |
| Ash (%) | 78.35 ** | 18.56 ** | 8.25 ** |
| C (%) | 16.13 ** | 493.50 ** | 3.16 ** |
| H (%) | 5.02 ** | 399.83 ** | 3.32 ** |
| O (%) | 33.21 ** | 459.26 ** | 3.34 ** |
| N (%) | 14.59 ** | 35.28 ** | 9.30 ** |
| C/N ratio | 16.65 ** | 30.04 ** | 10.19 ** |
| C/H ratio | 2.51 NS | 239.14 ** | 3.72 ** |
| O/C molar | 20.36 ** | 982.02 ** | 2.49 ** |
| H/C molar | 5.07 ** | 1273.33 ** | 3.39 ** |
| Conductivity (µS/cm) | 104.83 ** | 3.07 * | 4.69 ** |
| pH | 133.34 ** | 104.37 ** | 14.42 ** |
| Initial contact angle | 1.11 NS | 1.60 NS | 5.58 ** |
| Wetting rate | 5.55 ** | 7.88 ** | 4.22 ** |
| Species | Variable | Pyrolysis Temperature (°C) | |||||
|---|---|---|---|---|---|---|---|
| Parent Wood | 300 | 350 | 400 | 450 | 500 | ||
| D. panamensis | GCV (MJ/kg) | 19.1 C | 29.1 B | 29.1 B | 30.1 A | 30.9 A | 29.9 A |
| Volatile matter (%) | 87.9 D | 32.2 A | 31.8 A | 28.0 B | 24.4 C | 28.5 B | |
| Ash (%) | 1.5 B | 1.3 B | 1.5 B | 2.2 A | 2.0 A | 2.0 A | |
| C (%) | 47.3 C | 76.1 B | 77.6 B | 80.2 A | 82.0 A | 81.98 A | |
| H (%) | 6.6 C | 4.1 A | 4.1 A | 3.7 B | 3.5 B | 3.7 B | |
| O (%) | 44.5 C | 17.9 A | 16.4 A | 13.6 B | 12.2 B | 12.03 B | |
| N (%) | 0.07 C | 0.6 A | 0.33 B | 0.28 B | 0.30 B | 0.28 B | |
| C/N ratio | 708.0 C | 142.7 A | 236.9 A | 291.8 A | 279.7 A | 297.2 A | |
| C/H ratio | 7.2 D | 18.4 C | 19.1 C | 21.8 B | 23.8 A | 22.32 B | |
| O/C molar | 0.73 F | 0.19 D | 0.17 C | 0.15 B | 0.13 A | 0.13 A | |
| H/C molar | 1.67 D | 0.65 C | 0.63 C | 0.55 A | 0.50 B | 0.54 A | |
| G. arborea | GCV (MJ/kg) | 18.4 C | 28.1 B | 27.5 B | 28.6 A | 29.4 A | 29.8 A |
| Volatile matter (%) | 93.7 D | 51.5 A | 44.7 B | 43.7 B | 36.6 C | 35.6 C | |
| Ash (%) | 1.2 C | 2.8 B | 3.6 A | 3.8 A | 4.0 A | 2.7 B | |
| C (%) | 47.6 E | 72.2 D | 77.0 C | 79.4 BC | 81.5 AB | 84.8 A | |
| H (%) | 6.9 D | 4.7 A | 4.2 A | 4.0 B | 3.8 B | 3.27 C | |
| O (%) | 45.5 F | 22.8 A | 18.5 B | 16.4 C | 14.4 D | 11.6 E | |
| N (%) | 0.12 B | 0.23 A | 0.23 A | 0.23 A | 0.26 A | 0.25 A | |
| C/N ratio | 412.8 B | 330.3 A | 350.1 A | 359.6 A | 324.3 A | 346.3 A | |
| C/H ratio | 6.9 E | 15.3 D | 18.4 C | 20.3 B | 21.6 B | 26.2 A | |
| O/C molar | 0.72 F | 0.24 E | 0.18 D | 0.16 C | 0.13 B | 0.10 A | |
| H/C molar | 1.73 F | 0.78 E | 0.65 D | 0.60 C | 0.56 B | 0.46 A | |
| H. alchorneoides | GCV (MJ/kg) | 18.6 C | 28.0 A | 28.1 A | 29.6 A | 29.4 A | 29.4 A |
| Volatile matter (%) | 83.4 D | 28.5 A | 29.4 A | 25.2 B | 29.6 A | 17.9 C | |
| Ash (%) | 1.6 B | 1.3 B | 1.4 B | 1.5 B | 2.0 A | 1.6 B | |
| C (%) | 46.9 C | 73.2 B | 73.0 B | 77.4 A | 75.4 A | 78.8 A | |
| H (%) | 6.5 D | 4.4 A | 4.1 A | 3.8 B | 3.5 C | 3.5 C | |
| O (%) | 46.4 E | 22.2 A | 22.6 A | 18.5 D | 20.8 B | 17.4 D | |
| N (%) | 0.13 E | 0.24 D | 0.27 C | 0.31 A | 0.29 B | 0.31 A | |
| C/N ratio | 371.7 C | 306.6 A | 270.5 B | 254.6 B | 266.4 B | 261.5 B | |
| C/H ratio | 7.2 D | 16.9 C | 17.8 C | 20.5 B | 21.6 A | 22.8 A | |
| O/C molar | 0.74 D | 0.23 C | 0.23 C | 0.21 B | 0.18 A | 0.17 A | |
| H/C molar | 1.67 F | 0.72 E | 0.67 D | 0.59 C | 0.56 B | 0.53 A | |
| T. grandis | GCV (MJ/kg) | 19.6 C | 27.6 B | 27.4 B | 28.6 A | 28.2 A | 30.4 A |
| Volatile matter (%) | 84.0 E | 29.3 B | 34.8 A | 26.7 C | 23.8 D | 22.1 D | |
| Ash (%) | 1.2 B | 2.6 A | 3.1 A | 2.4 A | 2.2 A | 2.6 A | |
| C (%) | 47.0 D | 74.7 C | 72.9 C | 76.6 B | 80.2 A | 82.1 A | |
| H (%) | 6.7 C | 4.2 A | 4.4 A | 3.8 B | 3.6 B | 3.5 B | |
| O (%) | 46.2 F | 20.6 A | 22.4 A | 19.2 B | 15.8 C | 14.0 D | |
| N (%) | 0.14 D | 0.41 A | 0.24 C | 0.31 B | 0.31 B | 0.30 B | |
| C/N ratio | 346.0 D | 188.9 C | 310.1 B | 248.7 A | 263.2 A | 274.8 A | |
| C/H ratio | 7.0 E | 18.4 CD | 16.5 D | 20.2 B | 22.3 AB | 23.3 A | |
| O/C molar | 0.74 E | 0.21 CD | 0.23 D | 0.19 C | 0.15 B | 0.13 A | |
| H/C molar | 1.72 F | 0.67 E | 0.73 D | 0.59 C | 0.54 B | 0.52 A | |
| Species | Variable | Pyrolysis Temperature (°C) | ||||
|---|---|---|---|---|---|---|
| 300 | 350 | 400 | 450 | 500 | ||
| D. panamensis | Conductivity (µS/cm) | 237.3 A | 240.3 A | 337.3 A | 319.3 A | 297.3 A |
| pH | 6.5 B | 6.8 B | 7.7 A | 7.8 A | 7.7 A | |
| G. arborea | Conductivity (µS/cm) | 286.3 A | 308.3 A | 299.0 A | 163.5 B | 291.0 A |
| pH | 6.2 B | 9.53 A | 9.42 A | 8.69 A | 9.03 A | |
| H. alchorneoides | Conductivity (µS/cm) | 106.8 A | 104.0 A | 85.0 B | 51.0 C | 61.0 C |
| pH | 5.7 CD | 5.2 D | 6.6 BC | 7.6 AB | 7.6 A | |
| T. grandis | Conductivity (µS/cm) | 107.0 A | 91.3 B | 71.3 D | 73.3 D | 71.3 D |
| pH | 5.3 D | 5.7 D | 6.4 BC | 7.0 AB | 7.6 A | |
| Parameters | Variable | CP-1 | CP-2 |
|---|---|---|---|
| Statistical parameters of principal components | Eigenvalue | 6.06 | 4.47 |
| % Total of variance | 36.0 | 26.0 | |
| Total cumulative | 36.0 | 62.0 | |
| Correlations of characteristics measured and principal components | |||
| Variables | Parameters | CP1 | CP2 |
| Physical and mechanical properties | Density | −0.01 | −0.77 * |
| Moisture content | 0.37 | 0.01 | |
| Compression strength | −0.27 | −0.65 | |
| Charcoal characteristics | GCV | 0.80 ** | −0.38 |
| Volatility | −0.31 | 0.77 * | |
| Ash (%) | 0.18 | 0.86 ** | |
| Chemical characteristics | N | −0.15 | −0.68 |
| C | 0.96 ** | 0.02 | |
| H | 0.89 ** | 0.27 | |
| S | −0.09 | −0.47 | |
| O (%) | 0.95 ** | −0.03 | |
| C/N ratio | 0.26 | 0.83 ** | |
| C/H ratio | 0.95 ** | −0.15 | |
| Biochar characteristics | pH | 0.76 * | 0.50 |
| Conductivity | 0.31 | 0.37 | |
| Initial contact angle | 0.47 | −0.12 | |
| Wetting rate | 0.77 * | −0.34 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moya, R.; Tenorio, C.; Quesada-Kimzey, J.; Másis-Meléndez, F. Pyrogenic Carbonaceous Materials Production of Four Tropical Wood Produced by Slow Pyrolysis at Different Temperatures: Charcoal and Biochar Properties. Energies 2024, 17, 1953. https://doi.org/10.3390/en17081953
Moya R, Tenorio C, Quesada-Kimzey J, Másis-Meléndez F. Pyrogenic Carbonaceous Materials Production of Four Tropical Wood Produced by Slow Pyrolysis at Different Temperatures: Charcoal and Biochar Properties. Energies. 2024; 17(8):1953. https://doi.org/10.3390/en17081953
Chicago/Turabian StyleMoya, Róger, Carolina Tenorio, Jaime Quesada-Kimzey, and Federico Másis-Meléndez. 2024. "Pyrogenic Carbonaceous Materials Production of Four Tropical Wood Produced by Slow Pyrolysis at Different Temperatures: Charcoal and Biochar Properties" Energies 17, no. 8: 1953. https://doi.org/10.3390/en17081953
APA StyleMoya, R., Tenorio, C., Quesada-Kimzey, J., & Másis-Meléndez, F. (2024). Pyrogenic Carbonaceous Materials Production of Four Tropical Wood Produced by Slow Pyrolysis at Different Temperatures: Charcoal and Biochar Properties. Energies, 17(8), 1953. https://doi.org/10.3390/en17081953






