Thermodynamic Feasibility Evaluation of Alkaline Thermal Treatment Process for Hydrogen Production and Carbon Capture from Biomass by Process Modeling
Abstract
1. Introduction
2. Modeling and Simulation
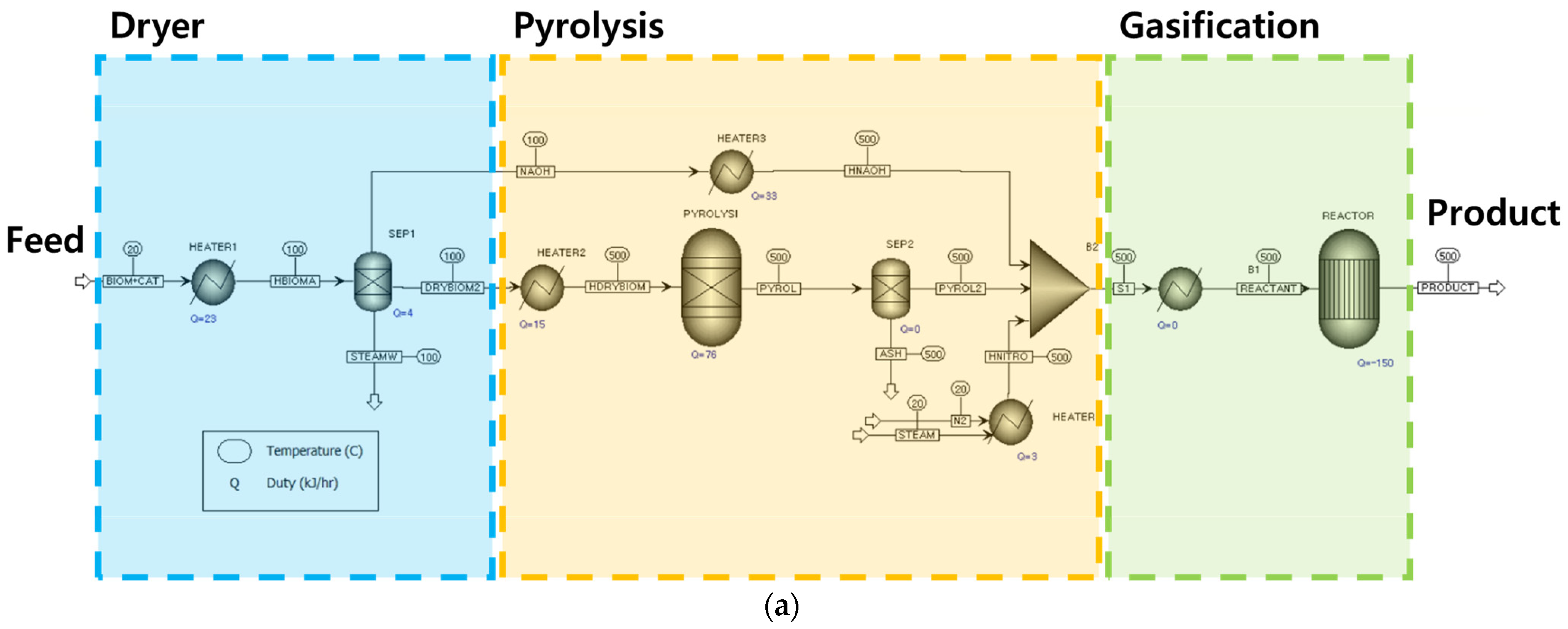
2.1. Hydrogen Production Process Modeling
2.1.1. Gasification Modeling Based on Thermodynamics
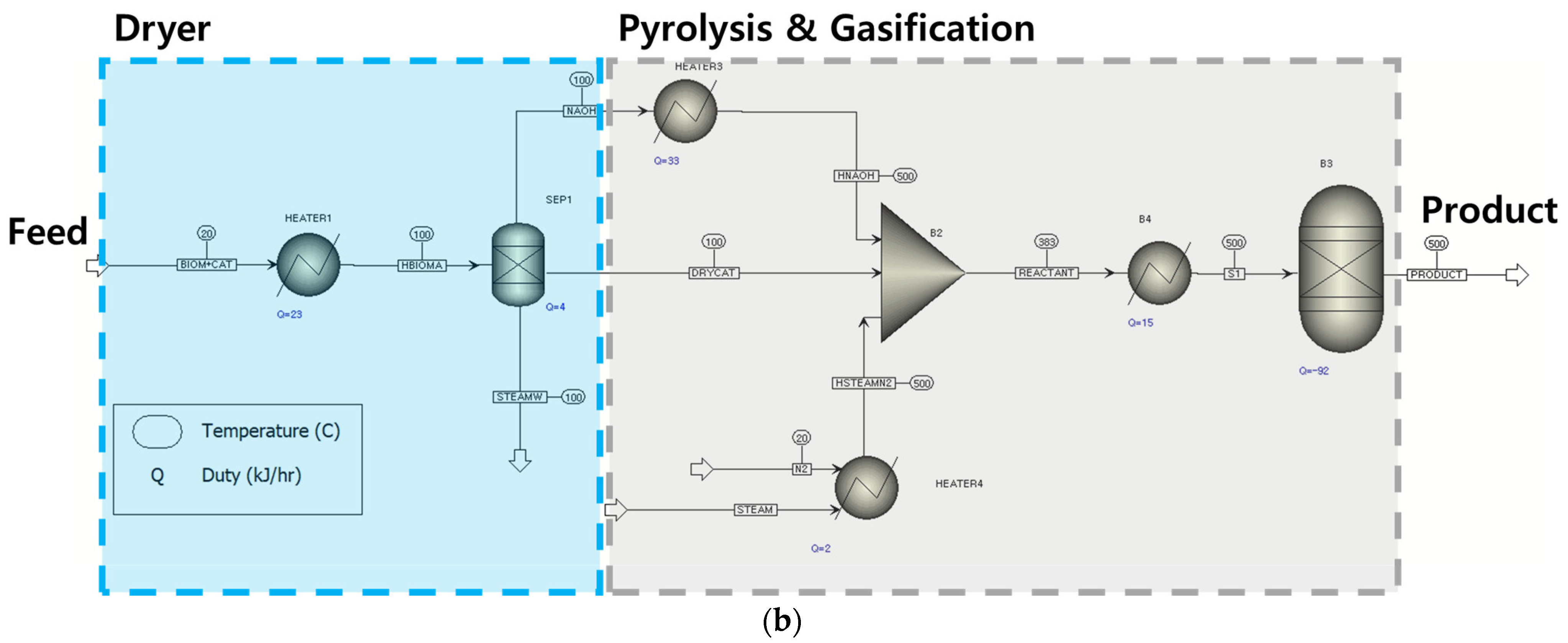
2.1.2. Gasification Modeling Based on Literature Data
2.2. Efficiency Calculation
2.3. Sensitivity Analysis
2.3.1. Gasification Modeling Based on Thermodynamics
2.3.2. Gasification Modeling Based on Literature Data
3. Results and Discussion
3.1. Process Modeling Based on Thermodynamic Equilibrium
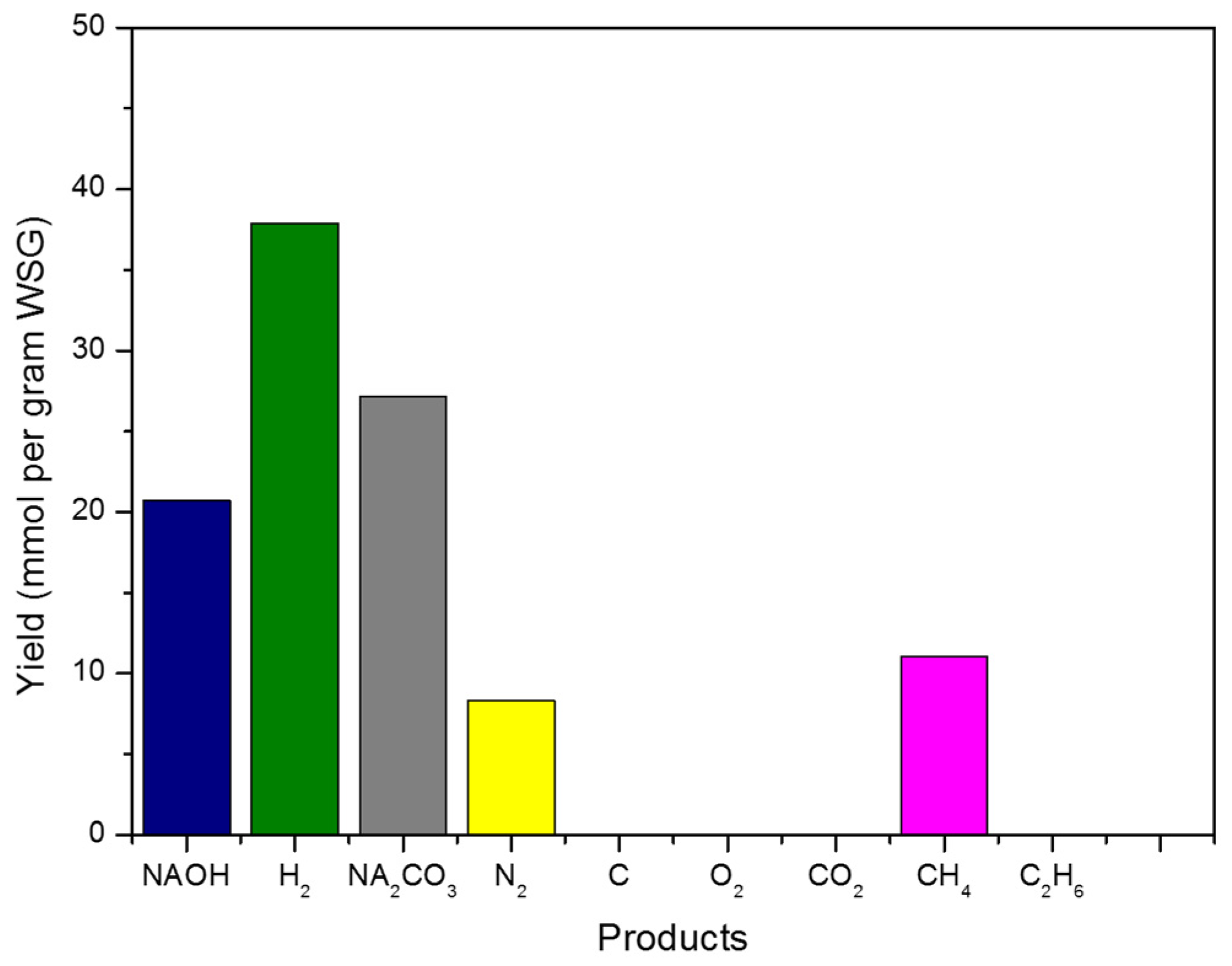
3.1.1. Results Based on Basic Conditions
3.1.2. Sensitivity Analysis for ATT Based on Thermodynamic Equilibrium
Effects of RGibbs Reactor Temperature
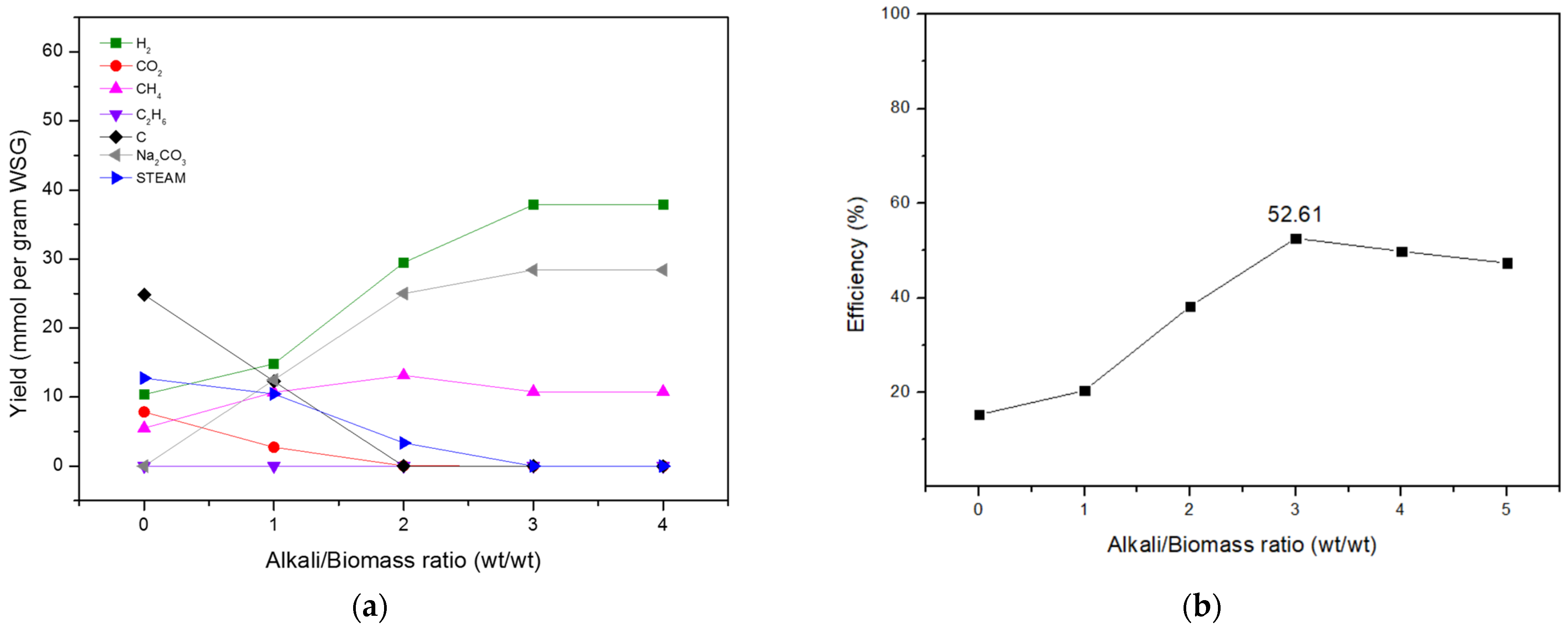
Effects of Alkali/Biomass Mass Ratio
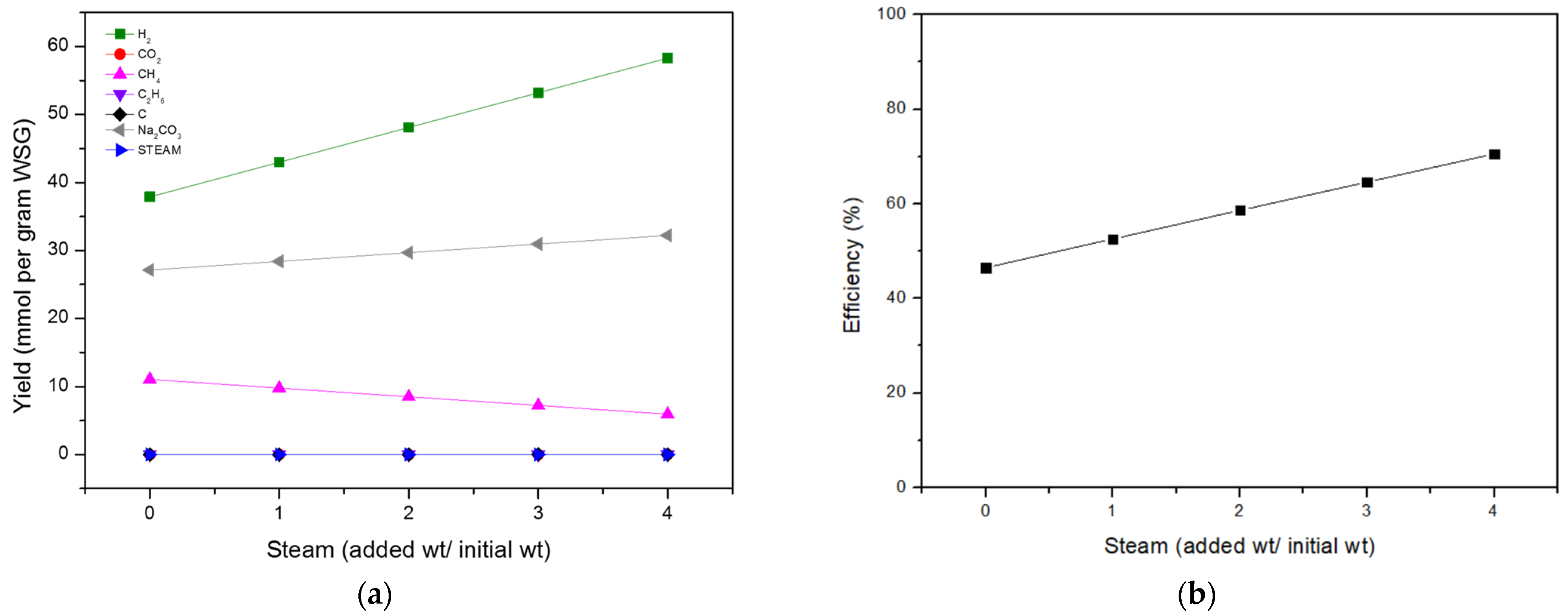
Effects of Steam Flow Rate
3.2. Process Modeling Based on Experimental Data
3.3. Comparisons of Gasification Results: Stoichiometric Equation, Thermodynamic Equilibrium, and Literature Data
3.3.1. Efficiency Calculation of a Stoichiometric Model
3.3.2. Efficiency Calculation of a Thermodynamic-based Model
3.3.3. Efficiency Calculation of the Experiment-based Model
3.4. Implications and Limitations
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Masson-Delmotte, V.; Zhai, P.; Pörtner, H.; Roberts, D.; Skea, J.; Shukla, P.; Pirani, A.; Moufouma-Okia, W.; Péan, C.; Pidcock, R. Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty. Intergovernmental Panel on Climate Change (IPCC). 2018. Available online: https://www.ipcc.ch/sr15/ (accessed on 1 October 2023).
- Jang, Y.-H.; Lee, S.; Shin, H.Y.; Bae, J. Development and evaluation of a 3-cell stack of metal-based solid oxide fuel cells fabricated via a sinter-joining method for auxiliary power unit applications. Int. J. Hydrogen Energy 2018, 43, 16215–16229. [Google Scholar] [CrossRef]
- Lee, H.; Lee, S. Economic Analysis on Hydrogen Pipeline Infrastructure Establishment Scenarios: Case Study of South Korea. Energies 2022, 15, 6824. [Google Scholar] [CrossRef]
- Lee, S.; Lee, K.; Lee, J.; Lee, J.; Kim, T.; Bae, J. Evaluation of Electrolyte Materials of Gd-and Ce-Doped Scandia-Stabilized Zirconia and Yb-and Bi-Doped Gadolinium-Doped Ceria for Highly Durable Solid Oxide Fuel Cells. Int. J. Precis. Eng. Manuf.-Green Technol. 2023, 1–12. [Google Scholar] [CrossRef]
- Park, H.; Kim, K.; Yu, M.; Yun, Z.; Lee, S. Economic analysis of the circular economy based on waste plastic pyrolysis oil: A case of the university campus. Environ. Dev. Sustain. 2023, 26, 6293–6313. [Google Scholar] [CrossRef]
- Ramachandran, R.; Menon, R.K. An overview of industrial uses of hydrogen. Int. J. Hydrog. Energy 1998, 23, 593–598. [Google Scholar] [CrossRef]
- Furusawa, Y.; Taguchi, H.; Ismail, S.N.; Thangavel, S.; Matsuoka, K.; Fushimi, C. Estimation of cold gas efficiency and reactor size of low-temperature gasifier for advanced-integrated coal gasification combined cycle systems. Fuel Process Technol. 2019, 193, 304–316. [Google Scholar] [CrossRef]
- Ultanir, M. Hidrojenin yakıt olarak kullanımı ve özellikleri. In Proceedings of the Çevre-Enerji Kongresi, TMMOB Makine Mühendisleri Odası, Ankara, Turkey, 5–7 June 1997; pp. 195–315. [Google Scholar]
- Oh, G.; Jang, J.Y.; Ra, H.W.; Seo, M.W.; Mun, T.Y.; Lee, J.-G.; Yoon, S.J. Gasification of Coal and Torrefied Biomass Mixture. Trans. Korean Hydrog. New Energy Soc. 2017, 28, 190–199. [Google Scholar] [CrossRef]
- Felseghi, R.-A.; Carcadea, E.; Raboaca, M.S.; Trufin, C.N.; Filote, C. Hydrogen fuel cell technology for the sustainable future of stationary applications. Energies 2019, 12, 4593. [Google Scholar] [CrossRef]
- Ishaq, H.; Dincer, I.; Crawford, C. A review on hydrogen production and utilization: Challenges and opportunities. Int. J. Hydrog. Energy 2022, 47, 26238–26264. [Google Scholar] [CrossRef]
- Cook, B. Introduction to fuel cells and hydrogen technology. Eng. Sci. Educ. J. 2002, 11, 205–216. [Google Scholar] [CrossRef]
- Roh, G.; Kim, H.; Jeon, H.; Yoon, K. Fuel consumption and CO2 emission reductions of ships powered by a fuel-cell-based hybrid power source. J. Mar. Sci. Eng. 2019, 7, 230. [Google Scholar] [CrossRef]
- Dutta, S. A review on production, storage of hydrogen and its utilization as an energy resource. J. Ind. Eng. Chem. 2014, 20, 1148–1156. [Google Scholar] [CrossRef]
- IEA. Global Hydrogen Review 2023; IEA: Paris, France, 2023; Available online: https://www.iea.org/reports/global-hydrogen-review-2023 (accessed on 1 October 2023).
- Soltani, S.M.; Lahiri, A.; Bahzad, H.; Clough, P.; Gorbounov, M.; Yan, Y. Sorption-enhanced steam methane reforming for combined CO2 capture and hydrogen production: A state-of-the-art review. Carbon Capture Sci. Technol. 2021, 1, 100003. [Google Scholar] [CrossRef]
- Sobolyeva, T. On the Microstructure of PEM Fuel Cell Catalyst Layers. Ph.D. Thesis, Simon Fraser University, Burnaby, BC, Canada, 2010. [Google Scholar]
- Younas, M.; Shafique, S.; Hafeez, A.; Javed, F.; Rehman, F. An overview of hydrogen production: Current status, potential, and challenges. Fuel 2022, 316, 123317. [Google Scholar] [CrossRef]
- Kalinci, Y.; Hepbasli, A.; Dincer, I. Biomass-based hydrogen production: A review and analysis. Int. J. Hydrog. Energy 2009, 34, 8799–8817. [Google Scholar] [CrossRef]
- Franchi, G.; Capocelli, M.; De Falco, M.; Piemonte, V.; Barba, D. Hydrogen production via steam reforming: A critical analysis of MR and RMM technologies. Membranes 2020, 10, 10. [Google Scholar] [CrossRef]
- Bocci, E.; Di Carlo, A.; Vecchione, L.; Villarini, M.; De Falco, M.; Dell’Era, A. Technical-economic analysis of an innovative cogenerative small scale biomass gasification power plant. In Proceedings of the Computational Science and Its Applications–ICCSA 2013: 13th International Conference, Ho Chi Minh City, Vietnam, 24–27 June 2013; pp. 256–270. [Google Scholar]
- Rosa, L.; Mazzotti, M. Potential for hydrogen production from sustainable biomass with carbon capture and storage. Renew Sustain. Energy Rev. 2022, 157, 112123. [Google Scholar] [CrossRef]
- Chandra, R.; Takeuchi, H.; Hasegawa, T.; Kumar, R. Improving biodegradability and biogas production of wheat straw substrates using sodium hydroxide and hydrothermal pretreatments. Energy 2012, 43, 273–282. [Google Scholar] [CrossRef]
- Mani, T.; Murugan, P.; Abedi, J.; Mahinpey, N. Pyrolysis of wheat straw in a thermogravimetric analyzer: Effect of particle size and heating rate on devolatilization and estimation of global kinetics. Chem. Eng. Res. Des. 2010, 88, 952–958. [Google Scholar] [CrossRef]
- Babatabar, M.A.; Saidi, M. Hydrogen production via integrated configuration of steam gasification process of biomass and water-gas shift reaction: Process simulation and optimization. Int. J. Energy Res. 2021, 45, 19378–19394. [Google Scholar] [CrossRef]
- Doranehgard, M.H.; Samadyar, H.; Mesbah, M.; Haratipour, P.; Samiezade, S. High-purity hydrogen production with in situ CO2 capture based on biomass gasification. Fuel 2017, 202, 29–35. [Google Scholar] [CrossRef]
- Qi, P.; Su, Y.; Zhang, S.; Jiang, M.; Sun, X.; Shi, L.; Xiong, Y. An innovative strategy on co-production of porous carbon and high purity hydrogen by alkaline thermal treatment of rice husk. Int. J. Hydrog. Energy 2022, 47, 23151–23164. [Google Scholar] [CrossRef]
- Stonor, M.R.; Ouassil, N.; Chen, J.G.; Park, A.-H.A. Investigation of the role of Ca(OH)2 in the catalytic Alkaline Thermal Treatment of cellulose to produce H2 with integrated carbon capture. J. Energy Chem. 2017, 26, 984–1000. [Google Scholar] [CrossRef]
- Zhao, M.; Cui, X.; Ji, G.; Zhou, H.; Vuppaladadiyam, A.K.; Zhao, X. Alkaline thermal treatment of cellulosic biomass for H2 production using Ca-based bifunctional materials. ACS Sustain. Chem. Eng. 2018, 7, 1202–1209. [Google Scholar] [CrossRef]
- Channiwala, S.; Parikh, P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Zhou, H.; Park, A.-H.A. Bio-energy with carbon capture and storage via alkaline thermal Treatment: Production of high purity H2 from wet wheat straw grass with CO2 capture. Appl. Energy 2020, 264, 114675. [Google Scholar] [CrossRef]
- Kirsanovs, V.; Blumberga, D.; Veidenbergs, I.; Rochas, C.; Vigants, E.; Vigants, G. Experimental investigation of downdraft gasifier at various conditions. Energy Procedia 2017, 128, 332–338. [Google Scholar] [CrossRef]
- Waheed, Q.M.; Williams, P.T. Hydrogen production from high temperature pyrolysis/steam reforming of waste biomass: Rice husk, sugar cane bagasse, and wheat straw. Energy Fuels 2013, 27, 6695–6704. [Google Scholar] [CrossRef]
- Kayfeci, M.; Keçebaş, A.; Bayat, M. Hydrogen production. In Solar Hydrogen Production; Elsevier: Amsterdam, The Netherlands, 2019; pp. 45–83. [Google Scholar]
- Pan, S.-Y.; Chang, E.; Chiang, P.-C. CO2 capture by accelerated carbonation of alkaline wastes: A review on its principles and applications. Aerosol Air Qual. Res. 2012, 12, 770–791. [Google Scholar] [CrossRef]
- Li, J.; Cheng, W. Comparative life cycle energy consumption, carbon emissions and economic costs of hydrogen production from coke oven gas and coal gasification. Int. J. Hydrog. Energy 2020, 45, 27979–27993. [Google Scholar] [CrossRef]

| Element | Ultimate Analysis wt% (Dry Ash-Free Basis) | |
|---|---|---|
| C | 49.0 | [31] |
| H | 6.8 | |
| O | 44.2 | |
| N | 0.0 |
| Type | Proximate Analysis wt% | |
|---|---|---|
| Moisture (wet basis) | 2.3 | [30,31] |
| Ash (dry basis) | 4.2 | |
| FC (dry basis) | 10.98 | |
| VM (dry basis) | 82.12 |
| Variables | Basic Process Conditions (WSG Supply 250 mg/min) | |
|---|---|---|
| Reactor temperature | 500 °C | [31] |
| Alkali/Biomass mass ratio | 3/1 | |
| Steam flow rates (mg/min) | 5.75 |
| 300 °C | 400 °C | 500 °C | 600 °C | |
|---|---|---|---|---|
| H2 (mmol/g WSG) | 24.174 | 29.652 | 37.262 | 37.913 |
| CH4 (mmol/g WSG) | 0.083 | 5.222 | 5.253 | 7.250 |
| C2H6 (mmol/g WSG) | 0.002 | 0.001 | 0.001 | 0.140 |
| CO2 (mmol/g WSG) | 0.079 | 0.103 | 0.252 | 0.448 |
| NaOH (mmol/g WSG) | Unknown | Unknown | 58.303 | Unknown |
| Na2CO3 (mmol/g WSG) | Unknown | Unknown | 8.570 | Unknown |
| 0:1 | 1:1 | 2:1 | 3:1 | 5:1 | |
|---|---|---|---|---|---|
| H2 (mmol/g WSG) | 3.191 | 15.837 | 29.416 | 37.262 | 36.342 |
| CH4 (mmol/g WSG) | 0 | 0 | 1.477 | 5.253 | 5.036 |
| C2H6 (mmol/g WSG) | 0.002 | 0.253 | 0.001 | 0.001 | 0 |
| CO2 (mmol/g WSG) | 3.310 | 2.046 | 0.948 | 0.252 | 0.107 |
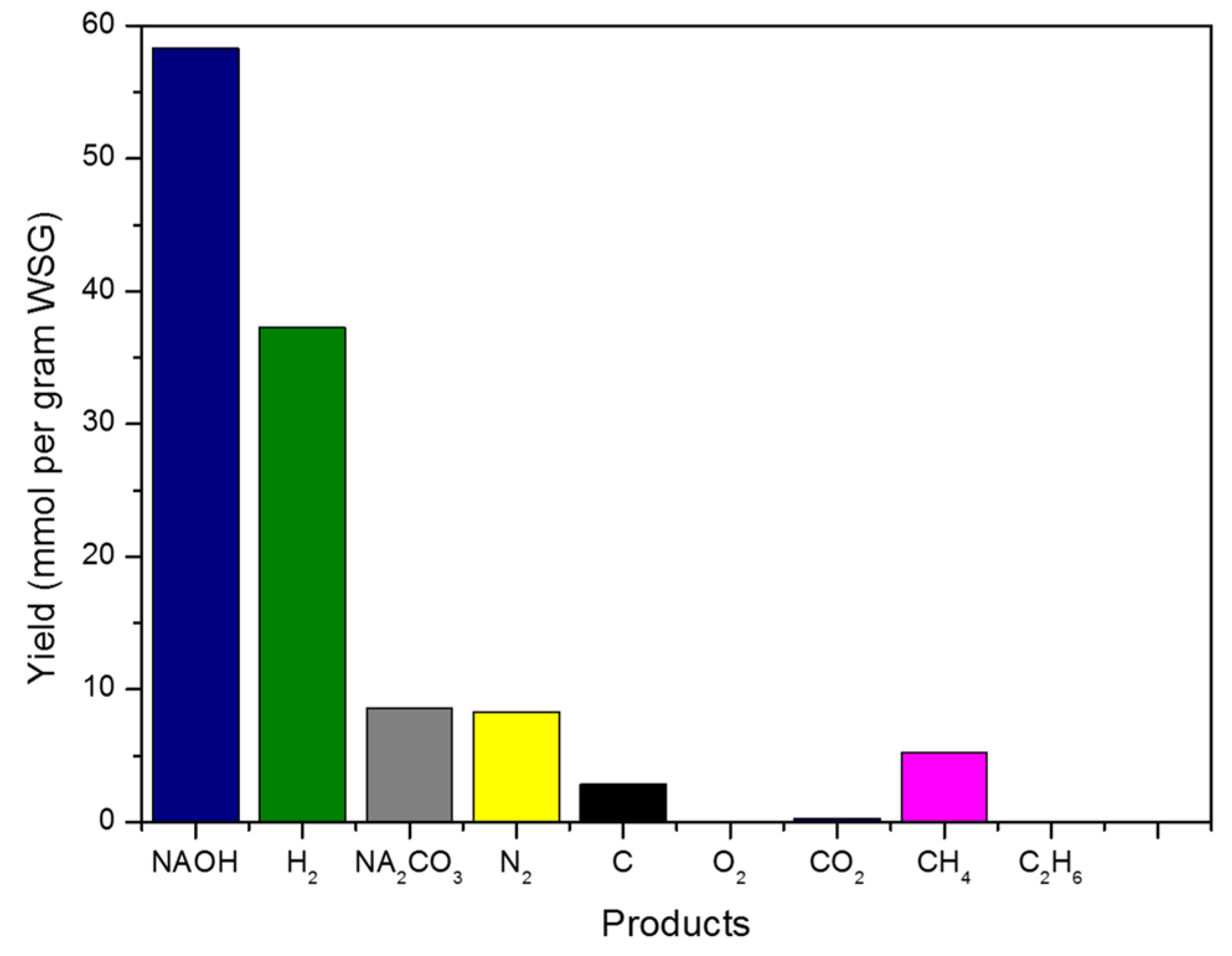
| Products | Yields (mmol/g WSG) |
|---|---|
| H2 | 37.88 |
| CH4 | 11.05 |
| C2H6 | 4.53 × 10−5 |
| CO2 | 0 |
| NaOH | 18.18 |
| Na2CO3 | 28.41 |
| Products | Yields (mmol/g WSG) |
|---|---|
| H2 | 37.262 |
| CH4 | 5.253 |
| C2H6 | 0.001 |
| CO2 | 0.252 |
| NaOH | 58.303 |
| Na2CO3 | 8.570 |
| Products | Yields (at 500 °C) |
|---|---|
| H2 (mmol/g WSG) | 42.90 |
| Na2CO3 (mmol/g WSG) | 26.37 |
| CH4 (mmol/g WSG) | 7.364 |
| C (mmol/g WSG) | 6.323 |
| CO2 (mmol/g WSG) | 0.1895 |
| C2H6 (mmol/g WSG) | 0.1380 |
| Heat Required (kJ/h) | H2 Production (mmol/g WSG) | System Efficiency (%) | CGE (%) | GY (g/1 g WSG) | |
|---|---|---|---|---|---|
| Stoichiometry | 77 | 42.90 | 52.40 | 67.73 | 0.2171 |
| Thermodynamic | 143 | 37.88 | 39.08 | 59.80 | 0.2537 |
| Experiment | 77 | 37.26 | 45.76 | 58.82 | 0.1705 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, Y.; Lee, S. Thermodynamic Feasibility Evaluation of Alkaline Thermal Treatment Process for Hydrogen Production and Carbon Capture from Biomass by Process Modeling. Energies 2024, 17, 1661. https://doi.org/10.3390/en17071661
Jung Y, Lee S. Thermodynamic Feasibility Evaluation of Alkaline Thermal Treatment Process for Hydrogen Production and Carbon Capture from Biomass by Process Modeling. Energies. 2024; 17(7):1661. https://doi.org/10.3390/en17071661
Chicago/Turabian StyleJung, Yujung, and Sanghun Lee. 2024. "Thermodynamic Feasibility Evaluation of Alkaline Thermal Treatment Process for Hydrogen Production and Carbon Capture from Biomass by Process Modeling" Energies 17, no. 7: 1661. https://doi.org/10.3390/en17071661
APA StyleJung, Y., & Lee, S. (2024). Thermodynamic Feasibility Evaluation of Alkaline Thermal Treatment Process for Hydrogen Production and Carbon Capture from Biomass by Process Modeling. Energies, 17(7), 1661. https://doi.org/10.3390/en17071661






