An Assessment of CO2 Capture Technologies towards Global Carbon Net Neutrality
Abstract
1. Introduction
2. Research Methodology
2.1. Overall Strategy
2.2. Multi-Criteria Decision Method—The TOPSIS Technique
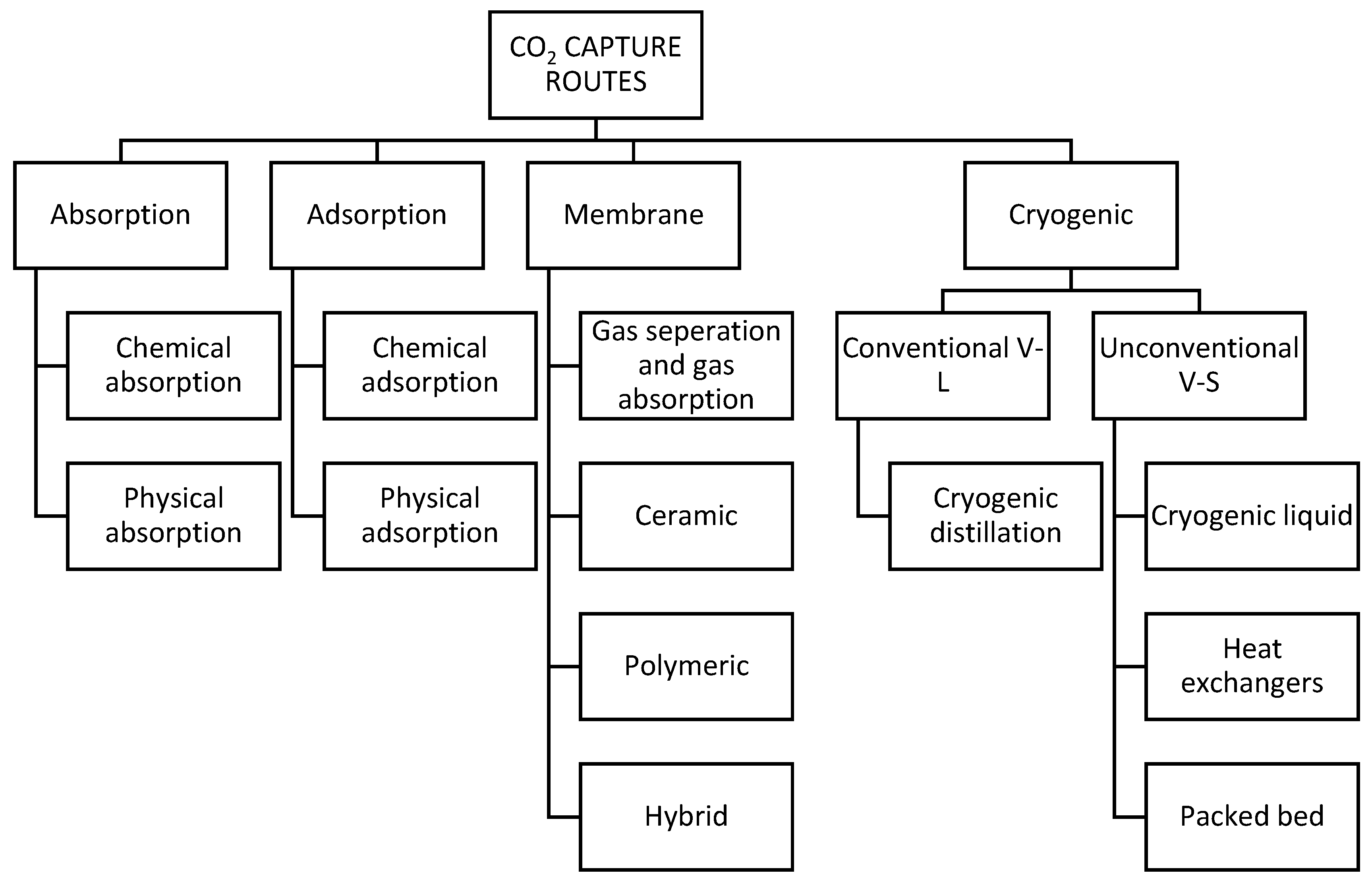
3. Results and Discussion
3.1. Absorption
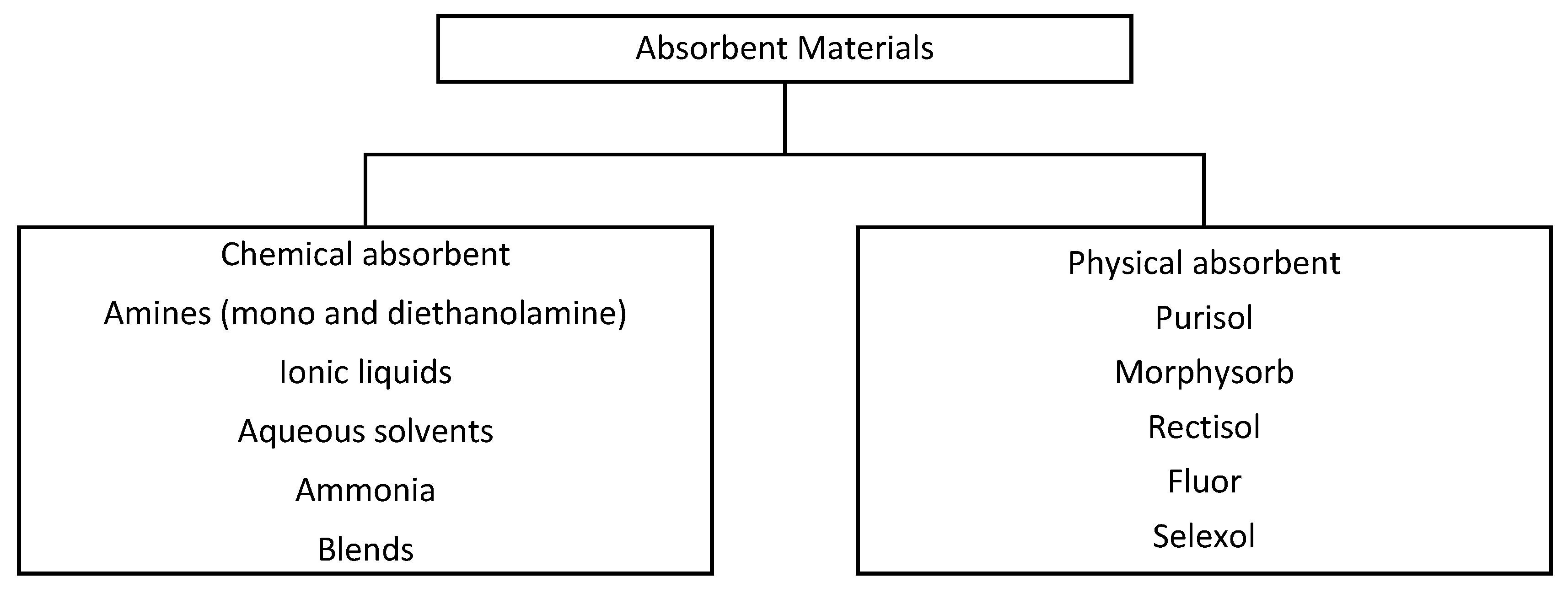
3.1.1. Chemical Absorption
3.1.2. Physical Absorption
3.2. Adsorption
3.2.1. Physical Adsorption
3.2.2. Chemical Adsorption
3.3. Membrane Separation
3.4. Cryogenic Carbon Capture
3.4.1. Conventional Cryogenic-Based Vapour–Liquid Separation
3.4.2. Unconventional Cryogenic-Based Vapour–Solid Separation
3.4.3. Heat Exchangers
3.4.4. Cryogenic Liquid
3.4.5. Packed Beds for Cryogenic Capture
3.5. Strengths, Weaknesses, Opportunities, and Threats (SWOT) Analyses
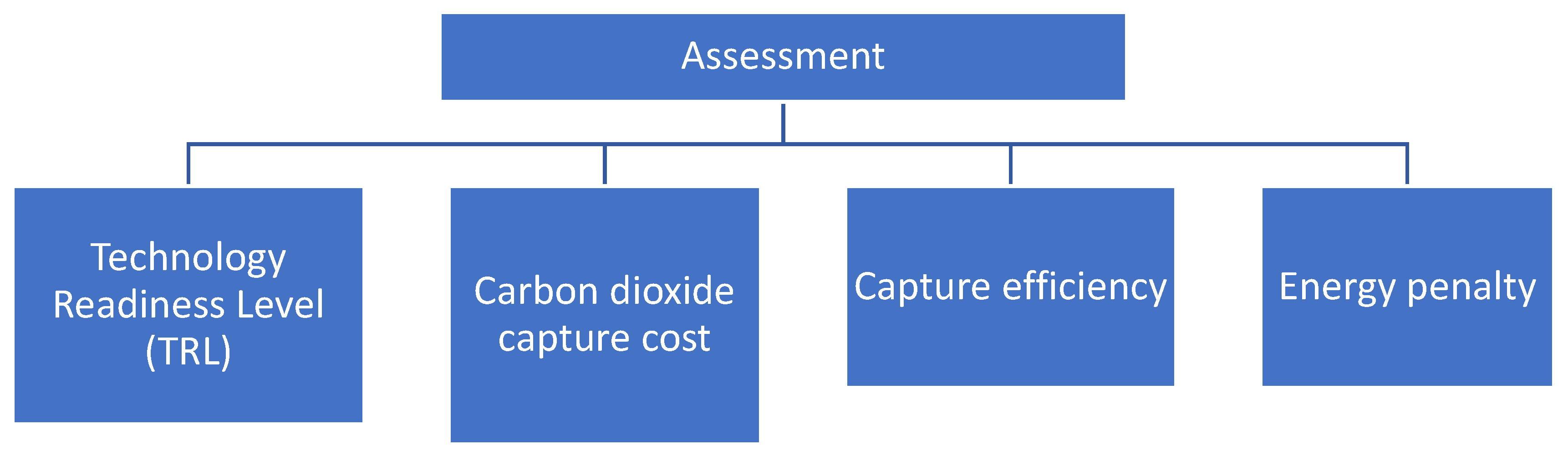
3.6. Assessment
3.6.1. Technology Readiness Level
3.6.2. Capture Efficiency
3.6.3. Capture Cost
3.6.4. Energy Penalty
3.7. Carbon Capture Forecast towards Net-Zero
3.8. Multicriteria Ranking of Carbon Capture Technologies (Using TOPSIS)
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A. Case 2: Developed Countries with Low Emissions
| Capture Route (Weightage) | TRL (0.2) | Capture Efficiency, % (0.5) | Energy Penalty, GJ/tCO2 (0.2) | Cost, USD/tCO2 (0.1) |
|---|---|---|---|---|
| Absorption | 4.1 | 77.5 | 5.75 | 70 |
| Adsorption | 3.6 | 87.5 | 5 | 100 |
| Membrane | 4 | 75 | 3.25 | 35 |
| Cryogenic | 3.7 | 99.99 | 3.8 | 92.5 |
| Capture Route | TRL | Capture Efficiency (%) | Energy Penalty (GJ/tCO2) | Cost (USD/tCO2) |
|---|---|---|---|---|
| Absorption | 0.531706 | 0.452872 | 0.6309 | 0.446 |
| Adsorption | 0.466864 | 0.511307 | 0.5486 | 0.637 |
| Membrane | 0.518737 | 0.438263 | 0.3566 | 0.223 |
| Cryogenic | 0.479832 | 0.584292 | 0.4169 | 0.589 |
| Capture Route | TRL | Capture Efficiency (%) | Energy Penalty (GJ/tCO2) | Cost (USD/tCO2) |
|---|---|---|---|---|
| Absorption | 0.106341 | 0.181149 | 0.1262 | 0.089 |
| Adsorption | 0.093373 | 0.204523 | 0.1097 | 0.127 |
| Membrane | 0.103747 | 0.175305 | 0.0713 | 0.045 |
| Cryogenic | 0.095966 | 0.233717 | 0.0834 | 0.118 |
| V+ | 0.106341 | 0.233717 | 0.0713 | 0.045 |
| V− | 0.093373 | 0.175305 | 0.1262 | 0.127 |
| Capture Route | Si+ | Si− | Pi | Rank |
|---|---|---|---|---|
| Absorption | 0.088 | 0.041 | 0.316 | 3 |
| Adsorption | 0.097 | 0.034 | 0.258 | 4 |
| Membrane | 0.058 | 0.1 | 0.631 | 1 |
| Cryogenic | 0.075 | 0.073 | 0.494 | 2 |
Appendix B. Case 3: Developing Countries with High Emissions
| Capture Route (Weightage) | TRL (0.2) | Capture Efficiency, % (0.5) | Energy Penalty, GJ/tCO2 (0.2) | Cost, USD/tCO2 (0.1) |
|---|---|---|---|---|
| Absorption | 4.1 | 77.5 | 5.75 | 70 |
| Adsorption | 3.6 | 87.5 | 5 | 100 |
| Membrane | 4 | 75 | 3.25 | 35 |
| Cryogenic | 3.7 | 99.99 | 3.8 | 92.5 |
| Capture Route | TRL | Capture Efficiency (%) | Energy Penalty (GJ/tCO2) | Cost (USD/tCO2) |
|---|---|---|---|---|
| Absorption | 0.531706 | 0.452872 | 0.6309 | 0.446 |
| Adsorption | 0.466864 | 0.511307 | 0.5486 | 0.637 |
| Membrane | 0.518737 | 0.438263 | 0.3566 | 0.223 |
| Cryogenic | 0.479832 | 0.584292 | 0.4169 | 0.589 |
| Capture Route | TRL | Capture Efficiency (%) | Energy Penalty (GJ/tCO2) | Cost (USD/tCO2) |
|---|---|---|---|---|
| Absorption | 0.106341 | 0.181149 | 0.1262 | 0.089 |
| Adsorption | 0.093373 | 0.204523 | 0.1097 | 0.127 |
| Membrane | 0.103747 | 0.175305 | 0.0713 | 0.045 |
| Cryogenic | 0.095966 | 0.233717 | 0.0834 | 0.118 |
| V+ | 0.106341 | 0.233717 | 0.0713 | 0.045 |
| V− | 0.093373 | 0.175305 | 0.1262 | 0.127 |
| Capture Route | Si+ | Si− | Pi | Rank |
|---|---|---|---|---|
| Absorption | 0.088 | 0.041 | 0.316 | 3 |
| Adsorption | 0.097 | 0.034 | 0.258 | 4 |
| Membrane | 0.058 | 0.1 | 0.631 | 1 |
| Cryogenic | 0.075 | 0.073 | 0.494 | 2 |
Appendix C. Case 4: Developing Countries with Low Emissions
| Capture Route (Weightage) | TRL (0.2) | Capture Efficiency, % (0.5) | Energy Penalty, GJ/tCO2 (0.2) | Cost, USD/tCO2 (0.1) |
|---|---|---|---|---|
| Absorption | 4.1 | 77.5 | 5.75 | 70 |
| Adsorption | 3.6 | 87.5 | 5 | 100 |
| Membrane | 4 | 75 | 3.25 | 35 |
| Cryogenic | 3.7 | 99.99 | 3.8 | 92.5 |
| Capture Route | TRL | Capture Efficiency (%) | Energy Penalty (GJ/tCO2) | Cost (USD/tCO2) |
|---|---|---|---|---|
| Absorption | 0.531706 | 0.452872 | 0.6309 | 0.446 |
| Adsorption | 0.466864 | 0.511307 | 0.5486 | 0.637 |
| Membrane | 0.518737 | 0.438263 | 0.3566 | 0.223 |
| Cryogenic | 0.479832 | 0.584292 | 0.4169 | 0.589 |
| Capture Route | TRL | Capture Efficiency (%) | Energy Penalty (GJ/tCO2) | Cost (USD/tCO2) |
|---|---|---|---|---|
| Absorption | 0.106341 | 0.090574 | 0.1262 | 0.178 |
| Adsorption | 0.093373 | 0.102261 | 0.1097 | 0.255 |
| Membrane | 0.103747 | 0.087653 | 0.0713 | 0.089 |
| Cryogenic | 0.095966 | 0.116858 | 0.0834 | 0.236 |
| V+ | 0.106341 | 0.116858 | 0.0713 | 0.089 |
| V− | 0.093373 | 0.087653 | 0.1262 | 0.255 |
| Capture Route | Si+ | Si− | Pi | Rank |
|---|---|---|---|---|
| Absorption | 0.108 | 0.078 | 0.418 | 2 |
| Adsorption | 0.171 | 0.022 | 0.114 | 4 |
| Membrane | 0.029 | 0.175 | 0.856 | 1 |
| Cryogenic | 0.147 | 0.055 | 0.273 | 3 |
References
- Table 2-8 Inventory of U.S. Greenhouse Gas Emissions and Sinks, 1990 to 2020. United States Environmental Protection Agency. 2020. Available online: https://www.epa.gov/ghgemissions/inventory-us-greenhouse-gas-emissions-and-sinks-1990-2020 (accessed on 10 January 2023).
- Sharma, A.; Johnson, B. The Ten Point Plan for a Green Industrial Revolution; Department for Energy Security and Net Zero; Prime Minister’s Office, 10 Downing Street; Department for Business, Energy & Industrial Strategy; HM Government, 2020; pp. 1–38.
- Delbeke, J.; Runge-Metzger, A.; Slingenberg, Y.; Werksman, J. The Paris Agreement; United Nations: New York, NY, USA, 2019; pp. 24–45. [Google Scholar]
- Madejski, P.; Chmiel, K.; Subramanian, N.; Kus, T. Methods and Techniques for CO2 Capture: Review of Potential. Energies 2022, 15, 887. [Google Scholar] [CrossRef]
- International Energy Agency. Achieving Net Zero Electricity Sectors in G7 Members; Organisation for Economic Co-Operation and Development (OECD): Paris, France, 2021. [Google Scholar]
- Sreedhar, I.; Nahar, T.; Venugopal, A.; Srinivas, B. Carbon Capture by Absorption—Path Covered and Ahead. Renew. Sustain. Energy Rev. 2017, 76, 1080–1107. [Google Scholar] [CrossRef]
- Chakma, A. Formulated Solvents: New Opportunities for Energy Efficient Separation of Acid Gases. Energy Sources 1999, 21, 51–62. [Google Scholar] [CrossRef]
- Aroonwilas, A.; Tontiwachwuthikul, P. High-Efficiency Structured Packing for CO2 Separation Using 2-Amino-2-Methyl-1-Propanol (AMP). Sep. Purif. Technol. 1997, 12, 67–79. [Google Scholar] [CrossRef]
- Vega, F.; Cano, M.; Camino, S.; Fernández, L.M.G.; Portillo, E.; Navarrete, B. Solvents for Carbon Dioxide Capture. In Carbon Dioxide Chemistry, Capture and Oil Recovery; IntechOpen: London, UK, 2018; pp. 142–163. [Google Scholar] [CrossRef]
- Sifat, N.S.; Haseli, Y. A Critical Review of CO2 Capture Technologies and Prospects for Clean Power Generation. Energies 2019, 12, 4143. [Google Scholar] [CrossRef]
- Yu, C.-H.; Huang, C.-H.; Tan, C.-S. A Review of CO2 Capture by Absorption and Adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Theo, W.L.; Lim, J.S.; Hashim, H.; Mustaffa, A.A.; Ho, W.S. Review of Pre-Combustion Capture and Ionic Liquid in Carbon Capture and Storage. Appl. Energy 2016, 183, 1633–1663. [Google Scholar] [CrossRef]
- Jansen, D.; Gazzani, M.; Manzolini, G.; van Dijk, E.; Carbo, M. Pre-Combustion CO2 Capture. Int. J. Greenh. Gas Control 2015, 40, 167–187. [Google Scholar] [CrossRef]
- Sanni, S.E.; Agboola, O.; Fagbiele, O.; Yusuf, E.O.; Emetere, M.E. Optimization of Natural Gas Treatment for the Removal of CO2 and H2S in a Novel Alkaline-DEA Hybrid Scrubber. Egypt. J. Pet. 2020, 29, 83–94. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, S.J.; Lee, J.W.; Chun, S.N.; Bin Lee, J. The Quantitative Evaluation of Two-Stage Pre-Combustion CO2 Capture Processes Using the Physical Solvents with Various Design Parameters. Energy 2015, 81, 47–55. [Google Scholar] [CrossRef]
- Chen, W.-H.; Chen, S.-M.; Hung, C.-I. Carbon Dioxide Capture by Single Droplet Using Selexol, Rectisol and Water as Absorbents: A Theoretical Approach. Appl. Energy 2013, 111, 731–741. [Google Scholar] [CrossRef]
- Ferris, L.E.; Naylor, C.D. Physician Remuneration in Industry-Sponsored Clinical Trials: The Case for Standardized Clinical Trial Budgets. Can. Med. Assoc. J. 2004, 171, 883–886, Erratum in Can. Med. Assoc. J. 2004, 171, 1327. [Google Scholar] [CrossRef]
- Chao, C.; Deng, Y.; Dewil, R.; Baeyens, J.; Fan, X. Post-Combustion Carbon Capture. Renew. Sustain. Energy Rev. 2021, 138, 110490. [Google Scholar] [CrossRef]
- Modak, A.; Jana, S. Advancement in Porous Adsorbents for Post-Combustion CO2 Capture. Microporous Mesoporous Mater. 2019, 276, 107–132. [Google Scholar] [CrossRef]
- Hu, X.; Liu, L.; Luo, X.; Xiao, G.; Shiko, E.; Zhang, R.; Fan, X.; Zhou, Y.; Liu, Y.; Zeng, Z.; et al. A Review of N-Functionalized Solid Adsorbents for Post-Combustion CO2 Capture. Appl. Energy 2020, 260, 114244. [Google Scholar] [CrossRef]
- Wang, S.; Yan, S.; Ma, X.; Gong, J. Recent Advances in Capture of Carbon Dioxide Using Alkali-Metal-Based Oxides. Energy Environ. Sci. 2011, 4, 3805–3819. [Google Scholar] [CrossRef]
- International Energy Agency. Energy Technology Perspectives 2020—Special Report on Carbon Capture Utilisation and Storage; Organisation for Economic Co-Operation and Development (OECD): Paris, France, 2020. [Google Scholar]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.-C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Sayari, A.; Belmabkhout, Y.; Serna-Guerrero, R. Flue Gas Treatment via CO2 Adsorption. Chem. Eng. J. 2011, 171, 760–774. [Google Scholar] [CrossRef]
- Plaza, M.G.; García, S.; Rubiera, F.; Pis, J.J.; Pevida, C. Post-Combustion CO2 Capture with a Commercial Activated Carbon: Comparison of Different Regeneration Strategies. Chem. Eng. J. 2010, 163, 41–47. [Google Scholar] [CrossRef]
- Thiruvenkatachari, R.; Su, S.; An, H.; Yu, X.X. Post Combustion CO2 Capture by Carbon Fibre Monolithic Adsorbents. Prog. Energy Combust. Sci. 2009, 35, 438–455. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, L.; Otto, A.; Robinius, M.; Stolten, D. A Review of Post-combustion CO2 Capture Technologies from Coal-fired Power Plants. Energy Procedia 2017, 114, 650–665. [Google Scholar] [CrossRef]
- Kárászová, M.; Zach, B.; Petrusová, Z.; Červenka, V.; Bobák, M.; Šyc, M.; Izák, P. Post-Combustion Carbon Capture by Membrane Separation, Review. Sep. Purif. Technol. 2020, 238, 116448. [Google Scholar] [CrossRef]
- Brunetti, A.; Scura, F.; Barbieri, G.; Drioli, E. Membrane Technologies for CO2 Separation. J. Membr. Sci. 2010, 359, 115–125. [Google Scholar] [CrossRef]
- Mondal, M.K.; Balsora, H.K.; Varshney, P. Progress and Trends in CO2 Capture/Separation Technologies: A Review. Energy 2012, 46, 431–441. [Google Scholar] [CrossRef]
- Holmes, I.A.S.; Ryan, J.M. Cryogenic Distillative Separation of Acid Gases from Methane. US Patent US4318723A, 14 November 1979. [Google Scholar]
- Song, C.; Liu, Q.; Deng, S.; Li, H.; Kitamura, Y. Cryogenic-Based CO2 Capture Technologies: State-of-the-Art Developments and Current Challenges. Renew. Sustain. Energy Rev. 2019, 101, 265–278. [Google Scholar] [CrossRef]
- Font-Palma, C.; Cann, D.; Udemu, C. Review of Cryogenic Carbon Capture Innovations and Their Potential Applications. C 2021, 7, 58. [Google Scholar] [CrossRef]
- Jensen, M.J.; Russell, C.S.; Bergeson, D.; Hoeger, C.D.; Frankman, D.J.; Bence, C.S.; Baxter, L.L. Prediction and Validation of External Cooling Loop Cryogenic Carbon Capture (CCC-ECL) for Full-Scale Coal-Fired Power Plant Retrofit. Int. J. Greenh. Gas Control 2015, 42, 200–212. [Google Scholar] [CrossRef]
- Tuinier, M.J.; Annaland, M.v.S.; Kuipers, J.A.M. A Novel Process for Cryogenic CO2 Capture Using Dynamically Operated Packed Beds—An Experimental and Numerical Study. Int. J. Greenh. Gas Control 2011, 5, 694–701. [Google Scholar] [CrossRef]
- Willson, P.; Lychnos, G.; Clements, A.; Michailos, S.; Font-Palma, C.; Diego, M.E.; Pourkashanian, M.; Howe, J. Evaluation of the Performance and Economic Viability of a Novel Low Temperature Carbon Capture Process. Int. J. Greenh. Gas Control 2019, 86, 1–9. [Google Scholar] [CrossRef]
- Bui, M.; Adjiman, C.S.; Bardow, A.; Anthony, E.J.; Boston, A.; Brown, S.; Fennell, P.S.; Fuss, S.; Galindo, A.; Hackett, L.A.; et al. Carbon capture and storage (CCS): The way forward. Energy Environ. Sci. 2018, 11, 1062–1176. [Google Scholar] [CrossRef]
- Barzagli, F.; Lai, S.; Mani, F. Novel Non-Aqueous Amine Solvents for Reversible CO2 Capture. Energy Procedia 2014, 63, 1795–1804. [Google Scholar] [CrossRef]
- Mahi, M.-R.; Mokbel, I.; Negadi, L.; Dergal, F.; Jose, J. Experimental Solubility of Carbon Dioxide in Monoethanolamine, or Diethanolamine or N-methyldiethanolamine (30 wt%) Dissolved in Deep Eutectic Solvent (Choline Chloride and Ethylene Glycol Solution). J. Mol. Liq. 2019, 289, 111062. [Google Scholar] [CrossRef]
- Resnik, K.P.; Yeh, J.T.; Pennline, H.W. Aqua Ammonia Process for Simultaneous Removal of CO2, SO2 and NOx. Int. J. Environ. Technol. Manag. 2004, 4, 89. [Google Scholar] [CrossRef]
- Haszeldine, R.S. Carbon Capture and Storage: How Green Can Black Be? Science 2009, 325, 1647–1652. [Google Scholar] [CrossRef]
- Vega, F.; Baena-Moreno, F.M.; Fernández, L.M.G.; Portillo, E.; Navarrete, B.; Zhang, Z. Current Status of CO2 Chemical Absorption Research Applied to CCS: Towards Full Deployment at Industrial Scale. Appl. Energy 2020, 260, 114313. [Google Scholar] [CrossRef]
- Merkel, T.C.; Lin, H.; Wei, X.; Baker, R. Power Plant Post-Combustion Carbon Dioxide Capture: An Opportunity for Membranes. J. Membr. Sci. 2010, 359, 126–139. [Google Scholar] [CrossRef]
- Lin, H.; He, Z.; Sun, Z.; Kniep, J.; Ng, A.; Baker, R.W.; Merkel, T.C. CO2-Selective Membranes for Hydrogen Production and CO2 Capture—Part II: Techno-Economic Analysis. J. Membr. Sci. 2015, 493, 794–806. [Google Scholar] [CrossRef]
- Tuinier, M.J.; Hamers, H.P.; Annaland, M.v.S. Techno-Economic Evaluation of Cryogenic CO2 Capture—A Comparison with Absorption and Membrane Technology. Int. J. Greenh. Gas Control 2011, 5, 1559–1565. [Google Scholar] [CrossRef]
- Grande, C.A.; Blom, R.; Andreassen, K.A.; Stensrød, R.E. Experimental Results of Pressure Swing Adsorption (PSA) for Pre-Combustion CO2 Capture with Metal Organic Frameworks. Energy Procedia 2017, 114, 2265–2270. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S. An Overview of Activated Carbons Utilization for the Post-Combustion Carbon Dioxide Capture. J. CO2 Util. 2016, 13, 1–16. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, J.; Zhong, Z.; Borgna, A. CO2 Capture by Solid Adsorbents and Their Applications: Current Status and New Trends. Energy Environ. Sci. 2011, 4, 42–55. [Google Scholar] [CrossRef]
- Millward, A.R.; Yaghi, O.M. Metal−Organic Frameworks with Exceptionally High Capacity for Storage of Carbon Dioxide at Room Temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef]
- Kuppler, R.J.; Timmons, D.J.; Fang, Q.-R.; Li, J.-R.; Makal, T.A.; Young, M.D.; Yuan, D.; Zhao, D.; Zhuang, W.; Zhou, H.-C. Potential Applications of Metal-Organic Frameworks. Coord. Chem. Rev. 2009, 253, 3042–3066. [Google Scholar] [CrossRef]
- Pires, J.C.M.; Martins, F.G.; Alvim-Ferraz, M.C.M.; Simões, M. Recent Developments on Carbon Capture and Storage: An Overview. Chem. Eng. Res. Des. 2011, 89, 1446–1460. [Google Scholar] [CrossRef]
- Freeman, B.C.; Bhown, A.S. Assessment of the Technology Readiness of Post-Combustion CO2 Capture Technologies. Energy Procedia 2011, 4, 1791–1796. [Google Scholar] [CrossRef]
- Global CCS Institute. CO2 Capture Technologies—PostCombustion Capture (PCC). 2012, pp. 1–16. Available online: https://www.globalccsinstitute.com/resources/publications-reports-research/co2-capture-technologies-post-combustion-capture-pcc/ (accessed on 10 January 2023).
- Bhown, A.S.; Freeman, B.C. Analysis and Status of Post-Combustion Carbon Dioxide Capture Technologies. Environ. Sci. Technol. 2011, 45, 8624–8632. [Google Scholar] [CrossRef] [PubMed]
- NETL Petra Nova. W.A. Parish Post-Combustion CO2 Capture and Sequestration Demonstration Project; Petra Nova LLC: Thompsons, TX, USA, 2020; p. 12. [Google Scholar]
- Que, Z.; Jian, C.; Shujuan, W. Carbon Dioxide Capture from Coal-Fired Power Plants in China. Summ. Eport NZEC Work Package 2009, 3, 2010-10. [Google Scholar]
- Rochelle, G.T.; Goff, G.S.; Cullinane, T. Method for Recovery of CO2 from Gas Streams. US Patent US7056482B2, 12 June 2003. Available online: https://patents.google.com/patent/US7056482B2/en (accessed on 10 January 2023).
- Freeman, S.A.; Dugas, R.; Van Wagener, D.H.; Nguyen, T.; Rochelle, G.T. Carbon Dioxide Capture with Concentrated, Aqueous Piperazine. Int. J. Greenh. Gas Control 2010, 4, 119–124. [Google Scholar] [CrossRef]
- Perry, R.J.; Grocela-Rocha, T.A.; O’Brien, M.J.; Genovese, S.; Wood, B.R.; Lewis, L.N.; Lam, H.; Soloveichik, G.; Rubinsztajn, M.; Kniajanski, S.; et al. Aminosilicone Solvents for CO2 Capture. ChemSusChem 2010, 3, 919–930. [Google Scholar] [CrossRef]
- Bara, J.E.; Carlisle, T.K.; Gabriel, C.J.; Camper, D.; Finotello, A.; Gin, D.L.; Noble, R.D. Guide to CO2 Separations in Imidazolium-Based Room-Temperature Ionic Liquids. Ind. Eng. Chem. Res. 2009, 48, 2739–2751. [Google Scholar] [CrossRef]
- Sjostrom, S.; Krutka, H. Evaluation of Solid Sorbents as a Retrofit Technology for CO2 Capture. Fuel 2010, 89, 1298–1306. [Google Scholar] [CrossRef]
- Radosz, M.; Hu, X.; Krutkramelis, K.; Shen, Y. Flue-Gas Carbon Capture on Carbonaceous Sorbents: Toward a Low-Cost Multifunctional Carbon Filter for “Green” Energy Producers. Ind. Eng. Chem. Res. 2008, 47, 3783–3794. [Google Scholar] [CrossRef]
- Demessence, A.; D’alessandro, D.M.; Foo, M.L.; Long, J.R. Strong CO2 Binding in a Water-Stable, Triazolate-Bridged Metal−Organic Framework Functionalized with Ethylenediamine. J. Am. Chem. Soc. 2009, 131, 8784–8786. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Doonan, C.J.; Furukawa, H.; Ferreira, R.B.; Towne, J.; Knobler, C.B.; Wang, B.; Yaghi, O.M. Multiple Functional Groups of Varying Ratios in Metal-Organic Frameworks. Science 2010, 327, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Gupta, H.; Fan, L.-S. Carbonation−Calcination Cycle Using High Reactivity Calcium Oxide for Carbon Dioxide Separation from Flue Gas. Ind. Eng. Chem. Res. 2002, 41, 4035–4042. [Google Scholar] [CrossRef]
- Baker, R.W.; Lokhandwala, K. Natural Gas Processing with Membranes: An Overview. Ind. Eng. Chem. Res. 2008, 47, 2109–2121. [Google Scholar] [CrossRef]
- Pakala, N.R.; Bhown, A.S. Effect of Particulate Matter on Mass Transfer Through Microporous Hollow Fiber Membranes. J. Membr. Sci. 1996, 111, 71–79. [Google Scholar] [CrossRef]
- Lively, R.P.; Koros, W.J.; Johnson, J. Enhanced Cryogenic CO2 Capture Using Dynamically Operated Low-Cost Fiber Beds. Chem. Eng. Sci. 2012, 71, 97–103. [Google Scholar] [CrossRef]
- Clodic, D.; Hitti, R.E.; Paris, M.D.; Bill, A.; Environment, A.P. CO2 Capture by Anti-Sublimation Thermo-Economic Process Evaluation Center for Energy and Processes. In Proceedings of the 4th Annual Conference on Carbon Capture & Sequestration, Alexandria, VA, USA, 2–5 May 2005. [Google Scholar]
- Hart, A.; Gnanendran, N. Cryogenic CO2 Capture in Natural Gas. Energy Procedia 2009, 1, 697–706. [Google Scholar] [CrossRef]
- Baxter, L.; Baxter, A.; Burt, S. Cryogenic CO2 Capture as a Cost-Effective CO2 Capture Process. In Proceedings of the International Pittsburgh Coal Conference, Pittsburgh, PA, USA, 20–23 September 2009; Volume 1, pp. 762–775. [Google Scholar]
- Song, C.-F.; Kitamura, Y.; Li, S.-H.; Ogasawara, K. Design of a Cryogenic CO2 Capture System Based on Stirling Coolers. Int. J. Greenh. Gas Control 2012, 7, 107–114. [Google Scholar] [CrossRef]
- Song, C.F.; Kitamura, Y.; Li, S.H. Evaluation of Stirling Cooler System for Cryogenic CO2 Capture. Appl. Energy 2012, 98, 491–501. [Google Scholar] [CrossRef]
- Arstad, B.; Blom, R.; Swang, O. CO2 Absorption in Aqueous Solutions of Alkanolamines: Mechanistic Insight from Quantum Chemical Calculations. J. Phys. Chem. A 2007, 111, 1222–1228. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.N.; Jensen, J.N.; Vilhelmsen, P.-J.; Biede, O. Experience with CO2 Capture from Coal Flue Gas in Pilot-Scale: Testing of Different Amine Solvents. Energy Procedia 2009, 1, 783–790. [Google Scholar] [CrossRef]
- Gurkan, B.E.; de la Fuente, J.C.; Mindrup, E.M.; Ficke, L.E.; Goodrich, B.F.; Price, E.A.; Schneider, W.F.; Brennecke, J.F. Equimolar CO2 Absorption by Anion-Functionalized Ionic Liquids. J. Am. Chem. Soc. 2010, 132, 2116–2117. [Google Scholar] [CrossRef] [PubMed]
- Yancheshmeh, M.S.; Radfarnia, H.R.; Iliuta, M.C. High Temperature CO2 Sorbents and Their Application for Hydrogen Production by Sorption Enhanced Steam Reforming Process. Chem. Eng. J. 2016, 283, 420–444. [Google Scholar] [CrossRef]
- Ebner, A.D.; Gray, M.L.; Chisholm, N.G.; Black, Q.T.; Mumford, D.D.; Nicholson, M.A.; Ritter, J.A. Suitability of a Solid Amine Sorbent for CO2 Capture by Pressure Swing Adsorption. Ind. Eng. Chem. Res. 2011, 50, 5634–5641. [Google Scholar] [CrossRef]
- Bamdad, H.; Hawboldt, K.; MacQuarrie, S. A Review on Common Adsorbents for Acid Gases Removal: Focus on Biochar. Renew. Sustain. Energy Rev. 2018, 81, 1705–1720. [Google Scholar] [CrossRef]
- Takamura, Y.; Aoki, J.; Uchida, S.; Narita, S. Application of High-Pressure Swing Adsorption Process for Improvement of CO2 Recovery System from Flue Gas. Can. J. Chem. Eng. 2001, 79, 812–816. [Google Scholar] [CrossRef]
- Clausse, M.; Merel, J.; Meunier, F. Numerical Parametric Study on CO2 Capture by Indirect Thermal Swing Adsorption. Int. J. Greenh. Gas Control 2011, 5, 1206–1213. [Google Scholar] [CrossRef]
- Rodrigues, G.; Raventos, M.; Dubettier, R.; Ruban, S. Adsorption Assisted Cryogenic Carbon Capture: An Alternate Path to Steam Driven Technologies to Decrease Cost and Carbon Footprint (March 18, 2021). International Energy Agency Greenhouse Gas R&D Programme (IEAGHG), 15th Greenhouse Gas Control Technologies Conference 2020 (GHGT-15). Available online: https://ssrn.com/abstract=3820744 (accessed on 10 January 2023).
- Yave, W.; Car, A.; Funari, S.S.; Nunes, S.P.; Peinemann, K.-V. CO2-Philic Polymer Membrane with Extremely High Separation Performance. Macromolecules 2010, 43, 326–333. [Google Scholar] [CrossRef]
- Aaron, D.; Tsouris, C. Separation of CO2 from Flue Gas: A Review. Sep. Sci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Caramanna, G.; Maroto-Valer, M.M. An Overview of Current Status of Carbon Dioxide Capture and Storage Technologies. Renew. Sustain. Energy Rev. 2014, 39, 426–443. [Google Scholar] [CrossRef]
- Khalilpour, R.; Mumford, K.; Zhai, H.; Abbas, A.; Stevens, G.; Rubin, E.S. Membrane-Based Carbon Capture from Flue Gas: A Review. J. Clean. Prod. 2015, 103, 286–300. [Google Scholar] [CrossRef]
- Hussain, A.; Hägg, M.-B. A Feasibility Study of CO2 Capture from Flue Gas by a Facilitated Transport Membrane. J. Membr. Sci. 2010, 359, 140–148. [Google Scholar] [CrossRef]
- Zhai, H.; Rubin, E.S. Techno-Economic Assessment of Polymer Membrane Systems for Postcombustion Carbon Capture at Coal-Fired Power Plants. Environ. Sci. Technol. 2013, 47, 3006–3014. [Google Scholar] [CrossRef] [PubMed]
- Kelley, B.T.; Valencia, J.A.; Northrop, P.S.; Mart, C.J. Controlled Freeze Zone™ for Developing Sour Gas Reserves. Energy Procedia 2011, 4, 824–829. [Google Scholar] [CrossRef]
- Ebrahimzadeh, E.; Matagi, J.; Fazlollahi, F.; Baxter, L.L. Alternative Extractive Distillation System for CO2–Ethane Azeotrope Separation in Enhanced Oil Recovery Processes. Appl. Therm. Eng. 2016, 96, 39–47. [Google Scholar] [CrossRef]
- Song, C.F.; Kitamura, Y.; Li, S.H.; Jiang, W.Z. Parametric Analysis of a Novel Cryogenic CO2 Capture System Based on Stirling Coolers. Environ. Sci. Technol. 2012, 46, 12735–12741. [Google Scholar] [CrossRef]
- Roussanaly, S.; Berghout, N.; Fout, T.; Garcia, M.; Gardarsdottir, S.; Nazir, S.M.; Ramirez, A.; Rubin, E.S. Towards improved cost evaluation of Carbon Capture and Storage from industry. Int. J. Greenh. Gas Control 2021, 106, 103263. [Google Scholar] [CrossRef]
- Rubin, E.; Booras, G.; Davison, J.; Ekstrom, C.; Matuszewski, M.; McCoy, S.; Short, C. Toward a Common Method of Cost Estimation for CO2 Capture and Storage at Fossil Fuel Power Plants; Global CCS Institute: Canberra, Australia, 2013; pp. 1–36. Available online: https://www.globalccsinstitute.com/resources/publications-reports-research/toward-a-common-method-of-cost-estimation-for-co2-capture-and-storage-at-fossil-fuel-power-plants/ (accessed on 12 December 2022).
- Hetti, R.K.; Karunathilake, H.; Hewage, K.; Sadiq, R. A Comparison of Carbon Capturing Technologies for Community Energy Systems: A Techno-Economic Assessment. In Proceedings of the 2nd International Conference on New Horizons in Green Civil Engineering (NHICE-02), Victoria, BC, Canada, 24–26 August 2020; pp. 1–5. [Google Scholar]
- Gielen, D. CO2 Removal in the Iron and Steel Industry. Energy Convers. Manag. 2003, 44, 1027–1037. [Google Scholar] [CrossRef]
- Davis, J.; Rochelle, G. Thermal Degradation of Monoethanolamine at Stripper Conditions. Energy Procedia 2009, 1, 327–333. [Google Scholar] [CrossRef]
- Ferrara, G.; Lanzini, A.; Leone, P.; Ho, M.T.; Wiley, D.E. Exergetic and Exergoeconomic Analysis of Post-Combustion CO2 Capture Using MEA-Solvent Chemical Absorption. Energy 2017, 130, 113–128. [Google Scholar] [CrossRef]
- Abu Zahra, M.; Niederer, J.P.; Feron, P.H.; Versteeg, G.F. CO2 Capture from Power Plants: Part II. A Parametric Study of the Economical Performance Based on Mono-Ethanolamine. Int. J. Greenh. Gas Control 2007, 1, 135–142. [Google Scholar] [CrossRef]
- Huang, C.-H.; Chang, K.-P.; Yu, C.-T.; Chiang, P.-C.; Wang, C.-F. Development of High-Temperature CO2 Sorbents Made of CaO-Based Mesoporous Silica. Chem. Eng. J. 2010, 161, 129–135. [Google Scholar] [CrossRef]
- Kulkarni, A.R.; Sholl, D.S. Analysis of Equilibrium-Based TSA Processes for Direct Capture of CO2 from Air. Ind. Eng. Chem. Res. 2012, 51, 8631–8645. [Google Scholar] [CrossRef]
- James, R.; Zoelle, A.; Keairns, D.; Turner, M.; Woods, M.; Kuehn, N. Cost and Performance Baseline for Fossil Energy Plants Volume 1: Bituminous Coal and Natural Gas to Electricity; NETL Rep. Pub-22638; National Energy Technology Laboratory (NETL): Pittsburgh, PA, USA, 2019; Volume 1, p. 598. [Google Scholar]
- Li, G.; Bai, P. New Operation Strategy for Separation of Ethanol–Water by Extractive Distillation. Ind. Eng. Chem. Res. 2012, 51, 2723–2729. [Google Scholar] [CrossRef]
- International Energy Agency. Improvements in Power Generation with Post-Combustion Capture of CO2; IEA Greenhouse Gas R&D Programme: Cheltenham, UK, 2004; pp. 1–153. [Google Scholar]
- Li, J.; You, C.; Chen, L.; Ye, Y.; Qi, Z.; Sundmacher, K. Dynamics of CO2 Absorption and Desorption Processes in Alkanolamine with Cosolvent Polyethylene Glycol. Ind. Eng. Chem. Res. 2012, 51, 12081–12088. [Google Scholar] [CrossRef]
- Lombardo, G.; Agarwal, R.; Askander, J. Chilled Ammonia Process at Technology Center Mongstad—First Results. Energy Procedia 2014, 51, 31–39. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Chowdhury, S.; Balasubramanian, R. Biomass Derived Low-Cost Microporous Adsorbents for Efficient CO2 Capture. Fuel 2015, 148, 246–254. [Google Scholar] [CrossRef]
- Luberti, M.; Oreggioni, G.D.; Ahn, H. Design of a Rapid Vacuum Pressure Swing Adsorption (RVPSA) Process for Post-Combustion CO2 Capture from a Biomass-Fuelled CHP Plant. J. Environ. Chem. Eng. 2017, 5, 3973–3982. [Google Scholar] [CrossRef]
- Mendes, P.A.; Ribeiro, A.M.; Gleichmann, K.; Ferreira, A.F.; Rodrigues, A.E. Separation of CO2/N2 on Binderless 5A Zeolite. J. CO2 Util. 2017, 20, 224–233. [Google Scholar] [CrossRef]
- Qasem, N.A.; Ben-Mansour, R. Energy and Productivity Efficient Vacuum Pressure Swing Adsorption Process to Separate CO2 from CO2/N2 Mixture Using Mg-MOF-74: A CFD Simulation. Appl. Energy 2018, 209, 190–202. [Google Scholar] [CrossRef]
- Matsumiya, N.; Teramoto, M.; Kitada, S.; Matsuyama, H. Evaluation of Energy Consumption for Separation of CO2 in Flue Gas by Hollow Fiber Facilitated Transport Membrane Module with Permeation of Amine Solution. Sep. Purif. Technol. 2005, 46, 26–32. [Google Scholar] [CrossRef]
- Bounaceur, R.; Lape, N.; Roizard, D.; Vallieres, C.; Favre, E. Membrane Processes for Post-Combustion Carbon Dioxide Capture: A Parametric Study. Energy 2006, 31, 2556–2570. [Google Scholar] [CrossRef]
- Jensen, M. Energy Process Enabled by Cryogenic Carbon Capture; Brigham Young University: Provo, UT, USA, 2015. [Google Scholar]
- Safdarnejad, S.M.; Hedengren, J.D.; Baxter, L.L. Plant-Level Dynamic Optimization of Cryogenic Carbon Capture with Conventional and Renewable Power Sources. Appl. Energy 2015, 149, 354–366. [Google Scholar] [CrossRef]
- Maqsood, K.; Ali, A.; Shariff, A.B.; Ganguly, S. Process Intensification Using Mixed Sequential and Integrated Hybrid Cryogenic Distillation Network for Purification of High CO2 Natural Gas. Chem. Eng. Res. Des. 2017, 117, 414–438. [Google Scholar] [CrossRef]
- Maqsood, K.; Pal, J.; Turunawarasu, D.; Pal, A.J.; Ganguly, S. Performance Enhancement and Energy Reduction Using Hybrid Cryogenic Distillation Networks for Purification of Natural Gas with High CO2 Content. Korean J. Chem. Eng. 2014, 31, 1120–1135. [Google Scholar] [CrossRef]
- Song, C.; Kitamura, Y.; Li, S. Energy Analysis of the Cryogenic CO2 Capture Process Based on Stirling Coolers. Energy 2014, 65, 580–589. [Google Scholar] [CrossRef]
- Turan, G.; Zapantis, A.; Kearns, D.; Tamme, E.; Staib, C.; Zhang, T.; Burrows, J.; Gillespie, A.; Havercroft, I.; Rassool, D.; et al. Global Status of CCS 2021; Global CCS Institute: Melbourne, Australia, 2021; pp. 1–43. [Google Scholar]
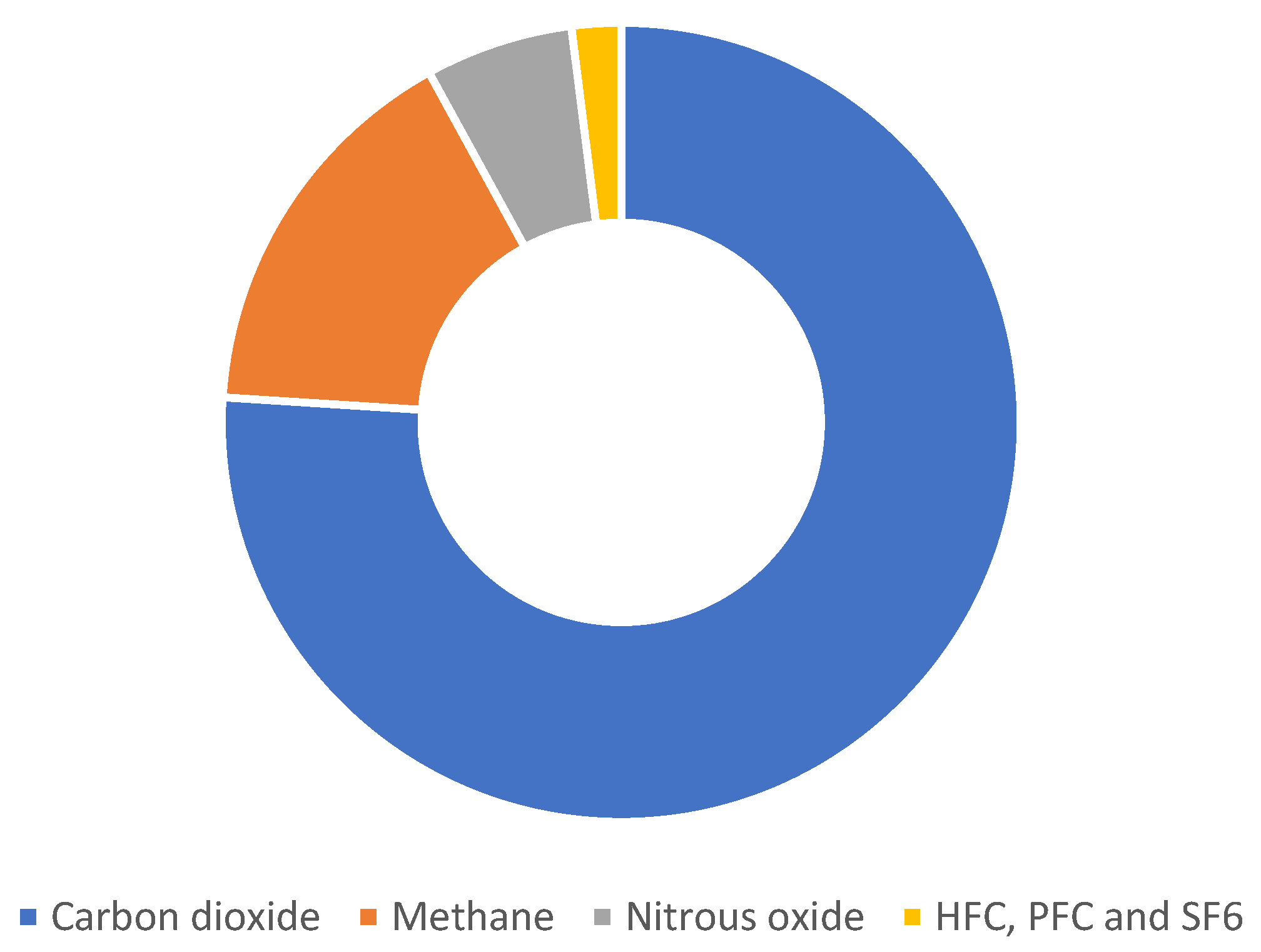



| Process | Advantages | Disadvantages |
|---|---|---|
| Selexol | Hydrogen sulphide selectivity is high. No washing is required to recover solvent. High stability. Pollution level in exhaust will be low [13] Capable of moisture removal. Minimal capital and operational expenditures [14]. | Reduced absorption rate at low temperature [15]. CO2 concentration should be high for efficient operation [14]. |
| Rectisol | Efficiency is high. Enable simultaneous capture of H2S and CO2. No solvent wastage [13]. Reduced energy penalty. Stable and resistant to corrosion [14]. | Expensive due to complex process [14,16]. |
| Purisol | Can be used for both H2S and CO2 removal [13]. | Low efficiency and stability for solvent [13]. |
| Morphysorb process | High absorption and corrosion-resistant solvent. Reduced Capex and Opex. Energy efficient. | Technology readiness level is low. |
| Fluor process | High absorption and resistance to corrosion [14]. | Expensive. Contactor technology is not mature [14]. |
| Type of Adsorbents | Merits | Demerits |
|---|---|---|
| Zeolite | Highly porous. Stability with high capture rate. | Susceptible to corrosion. |
| Activated carbon [19] | Compact and economical. High stability and corrosion resistance | Efficient only at high carbon dioxide concentration. Prior water separation is required. |
| Amine-functionalised adsorbents [20] | Works at low flue gas pressure. High capture capacity. | Not stable at high operating temperatures. |
| Metal–Organic Framework [19] | High capture capacity and efficiency. | Costly and susceptible to corrosion. |
| Alkali-metal-based oxides [21] | Cheap and available. Works on a wide temperature range. | Low stability and capture capacity. |
| Absorption | Adsorption | Membranes | Cryogenic | |
|---|---|---|---|---|
| Strengths | High carbon capture efficiency. Could be used to filter out other pollutants as well. Easily adaptable at power stations and other point sources. Availability of cheap solvents. Suitable for high-temperature applications | Sorbents could be regenerated and reused. Low cost of sorbents. Mature technology. | Energy-efficient and eco-friendly. High purity and recovery of CO2. Short startup time and low energy requirement. Low-cost separation of CO2. | High carbon recovery rate. Could be used to filter out other pollutants as well. Easily adaptable at power stations and other point sources. Energy required for compression can be saved. |
| Weaknesses | Solvent degradation. Increased energy consumption for regeneration. Amines are prone to corrosion. Solvent regeneration efficiency. Huge plant equipment size. Large capital cost for high-performance sorbent. | Frequent regeneration would lead to quick replacement. Less carbon capture capacity. Huge energy consumption for regeneration. | Membranes have strict temperature requirements. Susceptible to corrosion. Low selectivity for CO2 capture. High capital expense. High carbon concentration is required in flue gas. | Moisture removal from flue gas is required. Substantial energy requirements for cryogenic process. High installation cost. Not economical when carbon dioxide concentration is low |
| Opportunities | Cheap solvent availability with high capture capacity. Amine absorption is the most widely used technology in carbon capture. Recent progress in technology readiness levels. Advanced amines and Ionic liquids. | Development in composite adsorbents. Low-cost reusable sorbents make this technology promising. Noncorrosive. Gas products are dry. | High commercial availability and separation efficiency. Development in composite hollow-fibre membranes, mixed matrix membranes, and hybrid membrane–cryogenic processes. Low cost of separation and energy requirements could drive this technology in the future. Low footprint, good for offshore use. | Long track record for industrial CO2 recovery. Captured carbon dioxide could be used for industrial purposes. High purity of product increases the economic value. |
| Threats | Large capital costs and high energy penalties would be challenging. Potential corrosion issues. Amine degradation. Environmental impact | Achieving optimum operating temperature. Low technology readiness level. Increased energy penalty along with periodic sorbent regeneration. | Energy intensive. Low selectivity and temperature sensitivity. Requires multiple stages of removal and compression. Operational issues like low fluxes and fouling. | High energy consumption and installation costs could serve as a deterrent for this technology. Only viable for high carbon dioxide concentration. Subzero temperature requirement. |
| References | [11,12,42,43] | [11,12,30] | [29,43,44] | [32,35,45] |
| Technology | Absorption | Membrane | Adsorption | Cryogenic |
|---|---|---|---|---|
| Technology Readiness Level | 4.1 | 4 | 3.6 | 3.7 |
| Capture Cost/tCO2 | USD 40–100 | USD 15–55 | USD 50–150 | USD 55–130 |
| CO2 recovery | 60–95% | 60–90% | 80–95% | 99.99% |
| Energy consumption (GJ/tCO2) | 2.3–9.2 GJ/tCO2 | 0.5–6 GJ/tCO2 | 4–6 GJ/tCO2 | 2.4–5.2 GJ/tCO2 |
| References | [11,12,42,43] | [29,43,44] | [11,12,30] | [32,35,45] |
| Demonstration | 9 | Normal commercial service |
| 8 | Commercial demonstration, full-scale deployment in final form | |
| 7 | Sub-scale demonstration, fully functional prototype | |
| Development | 6 | Fully integrated pilot tested in a relevant environment |
| 5 | Sub-system validation in a relevant environment | |
| 4 | System validation in a laboratory environment | |
| Research | 3 | Proof-of-concept tests, component level |
| 2 | Formulation of the design | |
| 1 | Basic principles observed, initial concept |
| Type of Capture Technology | Overall TRL | References |
|---|---|---|
| Absorption | 4.1 | [52,54] |
| Adsorption | 3.6 | [52,54] |
| Membrane | 4 | [52,54] |
| Cryogenic | 3.7 | [52,54] |
| Type of Cryogenic | Recovery Rate | Reference |
|---|---|---|
| Packed bed | 99% | [35] |
| Anti-sublimation | 90% | [69] |
| Controlled freeze zone | 98–99% | [89] |
| External cooling loop | 95.6% | [90] |
| Stirling cooler | 85% | [91] |
| Type of Capture Technology | Overall Capture Rate | References |
|---|---|---|
| Absorption | 60–95% | [11,12,42,43] |
| Adsorption | 80–95% | [11,12,30,82] |
| Membrane | 60–90% | [29,43,44] |
| Cryogenic | 99.99% | [32,35,45] |
| Type of Capture Technology | Overall Capture Cost | References |
|---|---|---|
| Absorption | USD 40–100 | [11,12,42,43] |
| Adsorption | USD 50–150 | [11,12,30,82] |
| Membrane | USD 15–55 | [29,43,44] |
| Cryogenic | USD 55–130 | [32,35,45] |
| Type of Capture Technology | Overall Energy Consumption | References |
|---|---|---|
| Absorption | 2.3–9.2 GJ/tCO2 | [11,12,42,43] |
| Adsorption | 4–6 GJ/tCO2 | [11,12,30,82] |
| Membrane | 0.5–6 GJ/tCO2 | [29,43,44] |
| Cryogenic | 2.4–5.2 GJ/tCO2 | [32,35,45] |
| Type of Cryogenic | Energy Requirement | Reference |
|---|---|---|
| Packed bed | 1.8 GJ/tCO2 | [35] |
| Anti-sublimation | 1.18 GJ/tCO2 | [69] |
| Controlled freeze zone | 5.2 GJ/tCO2 | [89] |
| External cooling loop | 1.48 GJ/tCO2 | [90] |
| Stirling cooler | 3.4 GJ/tCO2 | [91] |
| Capture Route (Weightage) | TRL (0.2) | Capture Efficiency, % (0.5) | Energy Penalty, GJ/tCO2 (0.2) | Cost, USD/tCO2 (0.1) |
|---|---|---|---|---|
| Absorption | 4.1 | 77.5 | 5.75 | 70 |
| Adsorption | 3.6 | 87.5 | 5 | 100 |
| Membrane | 4 | 75 | 3.25 | 35 |
| Cryogenic | 3.7 | 99.99 | 3.8 | 92.5 |
| Capture Route | TRL | Capture Efficiency (%) | Energy Penalty (GJ/tCO2) | Cost (USD/tCO2) |
|---|---|---|---|---|
| To Absorption | 0.531706 | 0.452872 | 0.6309 | 0.446 |
| Adsorption | 0.466864 | 0.511307 | 0.5486 | 0.637 |
| Membrane | 0.518737 | 0.438263 | 0.3566 | 0.223 |
| Cryogenic | 0.479832 | 0.584292 | 0.4169 | 0.589 |
| Capture Route | TRL | Capture Efficiency (%) | Energy Penalty (GJ/tCO2) | Cost (USD/tCO2) |
|---|---|---|---|---|
| Absorption | 0.106341 | 0.226436 | 0.1262 | 0.045 |
| Adsorption | 0.093373 | 0.255653 | 0.1097 | 0.064 |
| Membrane | 0.103747 | 0.219132 | 0.0713 | 0.022 |
| Cryogenic | 0.095966 | 0.292146 | 0.0834 | 0.059 |
| V+ | 0.106341 | 0.292146 | 0.0713 | 0.022 |
| V− | 0.093373 | 0.219132 | 0.1262 | 0.064 |
| Capture Route | Rank | |||
|---|---|---|---|---|
| Absorption | 0.088 | 0.024 | 0.215 | 4 |
| Adsorption | 0.068 | 0.04 | 0.369 | 3 |
| Membrane | 0.073 | 0.069 | 0.487 | 2 |
| Cryogenic | 0.04 | 0.085 | 0.68 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karayil, A.; Elseragy, A.; Aliyu, A.M. An Assessment of CO2 Capture Technologies towards Global Carbon Net Neutrality. Energies 2024, 17, 1460. https://doi.org/10.3390/en17061460
Karayil A, Elseragy A, Aliyu AM. An Assessment of CO2 Capture Technologies towards Global Carbon Net Neutrality. Energies. 2024; 17(6):1460. https://doi.org/10.3390/en17061460
Chicago/Turabian StyleKarayil, Amith, Ahmed Elseragy, and Aliyu M. Aliyu. 2024. "An Assessment of CO2 Capture Technologies towards Global Carbon Net Neutrality" Energies 17, no. 6: 1460. https://doi.org/10.3390/en17061460
APA StyleKarayil, A., Elseragy, A., & Aliyu, A. M. (2024). An Assessment of CO2 Capture Technologies towards Global Carbon Net Neutrality. Energies, 17(6), 1460. https://doi.org/10.3390/en17061460








