Comprehensive Review of Biomass Pyrolysis: Conventional and Advanced Technologies, Reactor Designs, Product Compositions and Yields, and Techno-Economic Analysis
Abstract
1. Introduction
2. Conventional Pyrolysis
- (i)
- (ii)
- (iii)
- (iv)
2.1. Slow Pyrolysis
2.2. Intermediate Pyrolysis
2.3. Fast Pyrolysis
2.4. Flash Pyrolysis
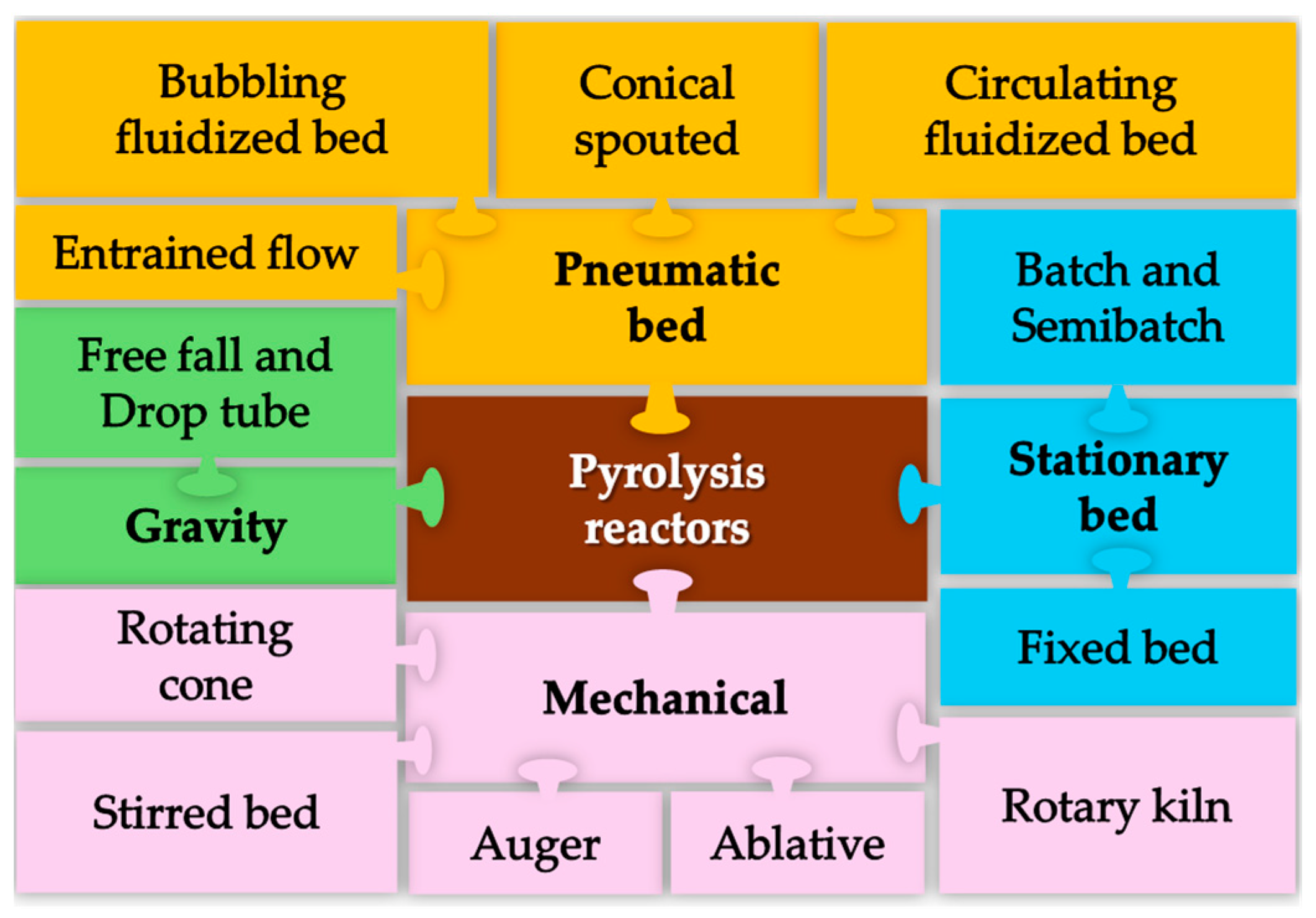
3. Reactors Type and Design
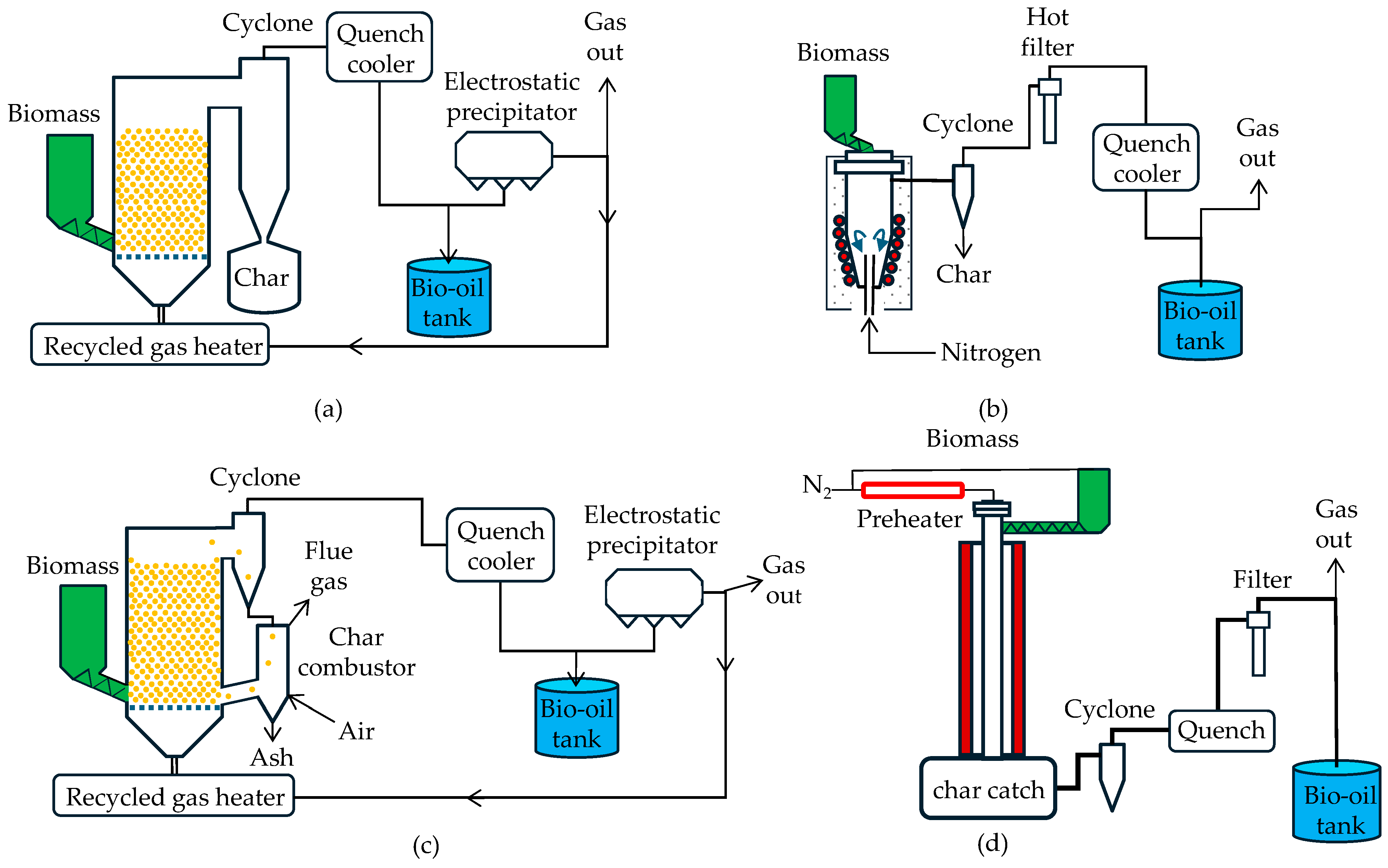
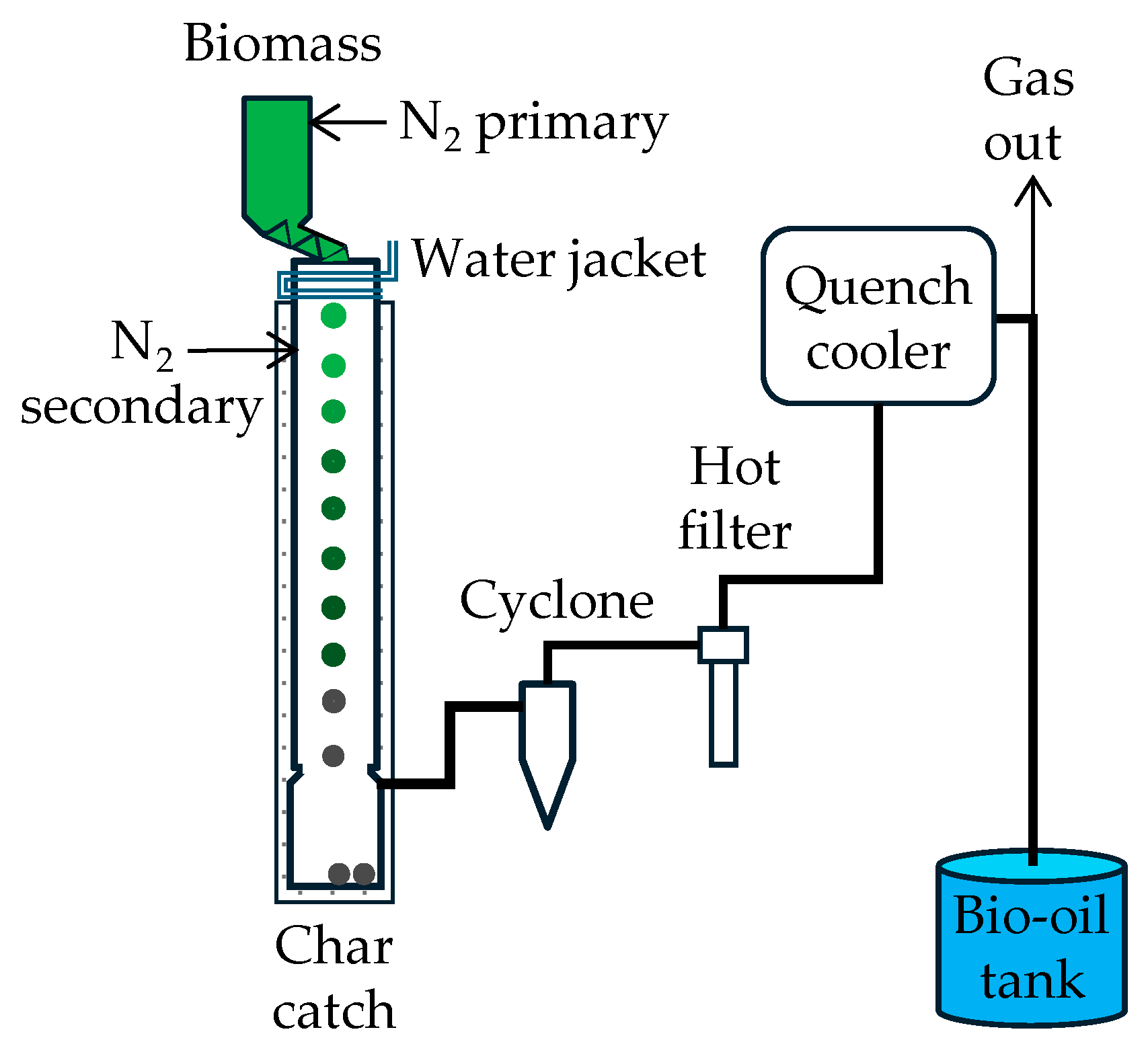
3.1. Pneumatic Bed Reactors
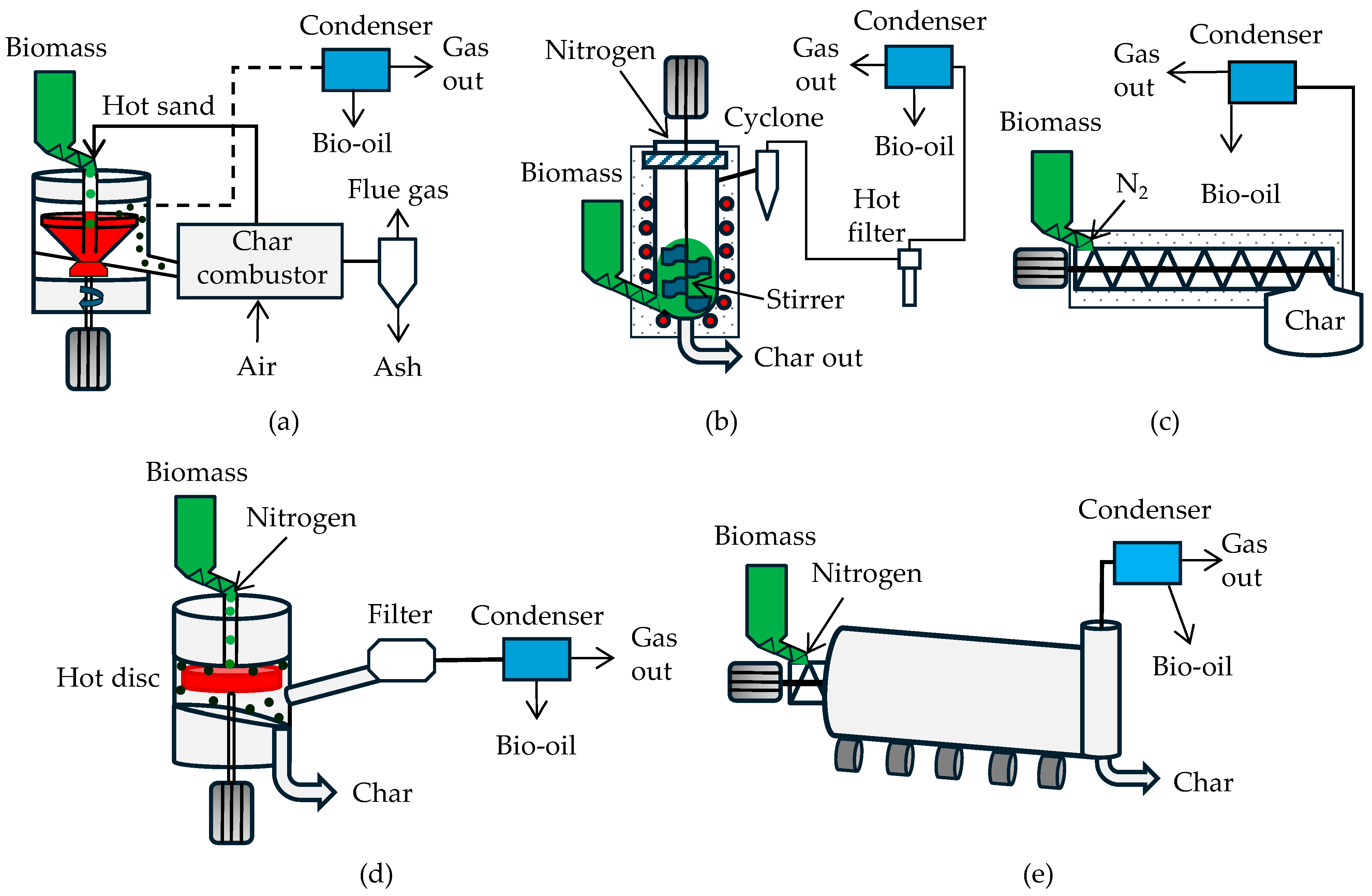
3.2. Gravity Reactors
3.3. Stationary Bed Reactors
3.4. Mechanical Reactors
4. Advanced Technologies
4.1. Co-Pyrolysis
4.2. Catalytic Pyrolysis
4.3. Microwave Pyrolysis
4.4. Hydrothermal Pyrolysis
4.5. Plasma Pyrolysis
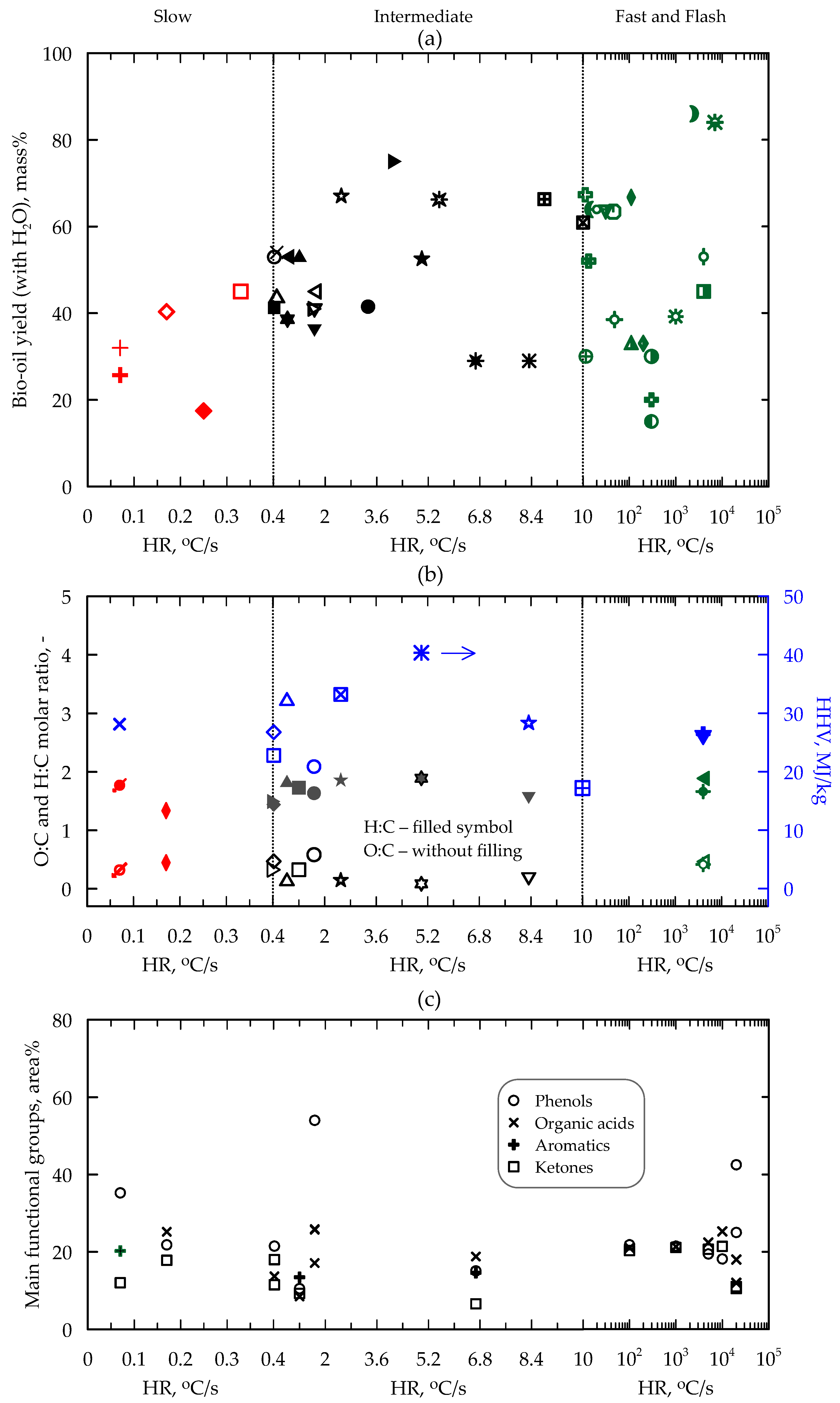
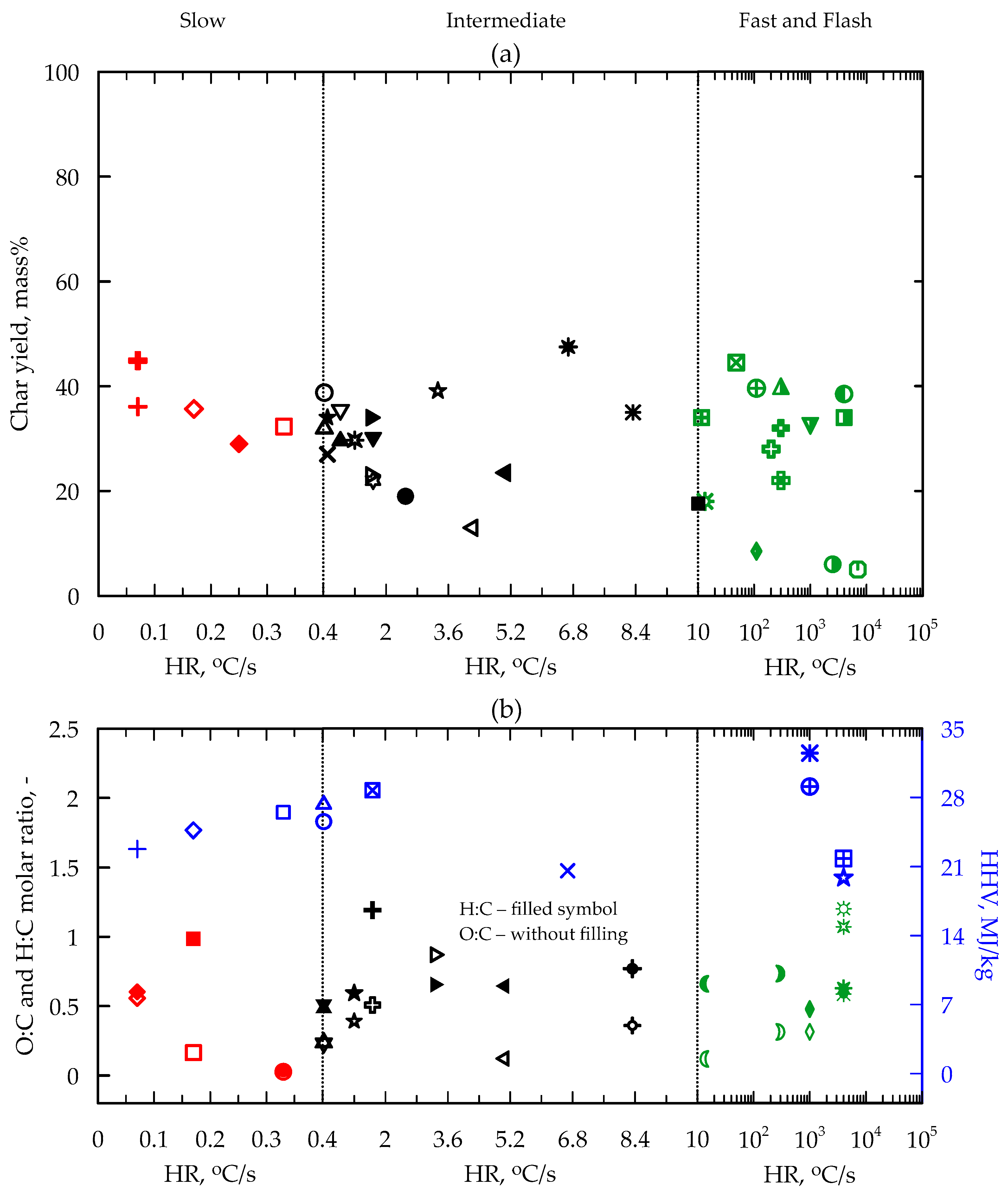
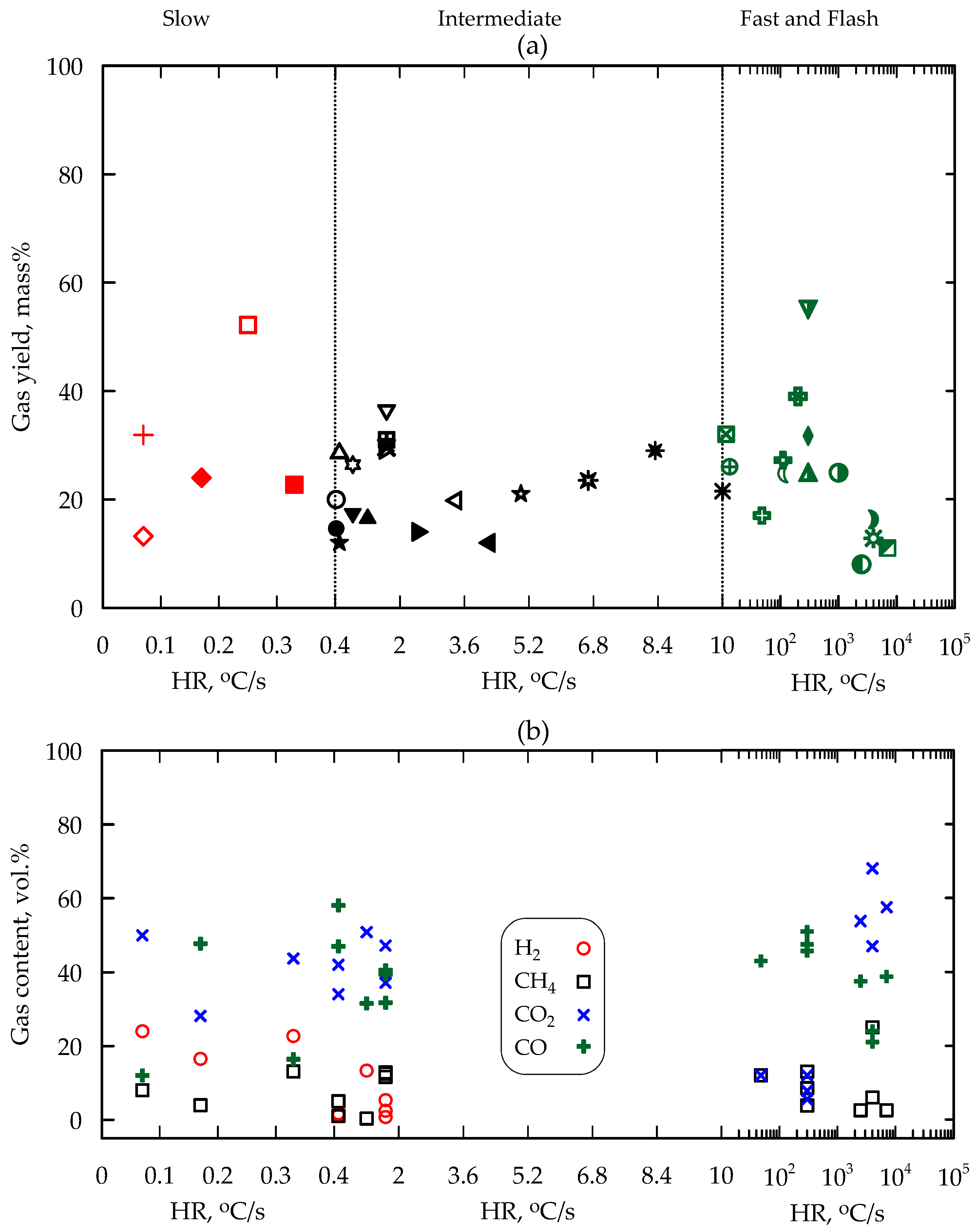
5. Yield and Composition of Products
5.1. Bio-Oil
5.2. Char
5.3. Non-Condensable Gas
6. Techno-Economic Analysis
7. Future Outlooks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- IEA. World Energy Outlook 2023. 2023. Available online: https://www.iea.org/news/the-energy-world-is-set-to-change-significantly-by-2030-based-on-today-s-policy-settings-alone (accessed on 10 June 2024).
- IEA. CO2 Emissions in 2023. 2024. Available online: https://www.iea.org/reports/world-energy-outlook-2023 (accessed on 10 June 2024).
- Olatunji, K.O.; Ahmed, N.A.; Ogunkunle, O. Optimization of Biogas Yield from Lignocellulosic Materials with Different Pretreatment Methods: A Review. Biotechnol. Biofuels 2021, 14, 159. [Google Scholar] [CrossRef] [PubMed]
- Su, G.; Ong, H.C.; Gan, Y.Y.; Chen, W.H.; Chong, C.T.; Ok, Y.S. Co-Pyrolysis of Microalgae and Other Biomass Wastes for the Production of High-Quality Bio-Oil: Progress and Prospective. Bioresour. Technol. 2022, 344, 126096. [Google Scholar] [CrossRef]
- IEA Bioenergy Report 2022. How Bioenergy Contributes to a Sustainable Future. ISBN 979-12-80907-19-6. Available online: https://www.ieabioenergyreview.org/ (accessed on 10 June 2024).
- Jerzak, W.; Kuźnia, M. Examination of Inorganic Gaseous Species and Condensed Phases during Coconut Husk Combustion Based on Thermodynamic Equilibrium Predictions. Renew. Energy 2021, 167, 497–507. [Google Scholar] [CrossRef]
- Abioye, K.J.; Harun, N.Y.; Sufian, S.; Yusuf, M.; Jagaba, A.H.; Ekeoma, B.C.; Kamyab, H.; Sikiru, S.; Waqas, S.; Ibrahim, H. A Review of Biomass Ash Related Problems: Mechanism, Solution, and Outlook. J. Energy Inst. 2024, 112, 101490. [Google Scholar] [CrossRef]
- Variny, M.; Varga, A.; Rimár, M.; Janošovský, J.; Kizek, J.; Lukáč, L.; Jablonský, G.; Mierka, O. Advances in Biomass Co-Combustion with Fossil Fuels in the European Context: A Review. Processes 2021, 9, 100. [Google Scholar] [CrossRef]
- Nagarajan, J.; Prakash, L. Preparation and Characterization of Biomass Briquettes Using Sugarcane Bagasse, Corncob and Rice Husk. Mater. Today Proc. 2021, 47, 4194–4198. [Google Scholar] [CrossRef]
- Begum, Y.A.; Kumari, S.; Jain, S.K.; Garg, M.C. A Review on Waste Biomass-to-Energy: Integrated Thermochemical and Biochemical Conversion for Resource Recovery. Environ. Sci. Adv. 2024, in press. [CrossRef]
- Cheng, Y.-L.; Lee, C.-Y.; Huang, Y.-L.; Buckner, C.A.; Lafrenie, R.M.; Dénommée, J.A.; Caswell, J.M.; Want, D.A.; Gan, G.G.; Leong, Y.C.; et al. Recent Advances in Pyrolysis; Al-Haj Ibrahim, H., Ed.; IntechOpen: London, UK, 2020; ISBN 978-1-78984-942-4. [Google Scholar] [CrossRef]
- Wang, Y.; Li, B.; Gao, A.; Ding, K.; Xing, X.; Wei, J.; Huang, Y.; Chun-Ho Lam, J.; Subramanian, K.A.; Zhang, S. Volatile-Char Interactions during Biomass Pyrolysis: Effect of Biomass Acid-Washing Pretreatment. Fuel 2023, 340, 127496. [Google Scholar] [CrossRef]
- Pinheiro Pires, A.P.; Arauzo, J.; Fonts, I.; Domine, M.E.; Fernández Arroyo, A.; Garcia-Perez, M.E.; Montoya, J.; Chejne, F.; Pfromm, P.; Garcia-Perez, M. Challenges and Opportunities for Bio-Oil Refining: A Review. Energy Fuels 2019, 33, 4683–4720. [Google Scholar] [CrossRef]
- Gogoi, D.; Kumar, M.; Lakshmi, Y.G. A Comprehensive Review on “Pyrolysis” for Energy Recovery. Bioenergy Res. 2023, 16, 1417–1437. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Varsha Vuppaladadiyam, S.S.; Sikarwar, V.S.; Ahmad, E.; Pant, K.K.; Murugavelh, S.; Pandey, A.; Bhattacharya, S.; Sarmah, A.; Leu, S.Y. A Critical Review on Biomass Pyrolysis: Reaction Mechanisms, Process Modeling and Potential Challenges. J. Energy Inst. 2023, 108, 101236. [Google Scholar] [CrossRef]
- Matamba, T.; Tahmasebi, A.; Yu, J.; Keshavarz, A.; Abid, H.R.; Iglauer, S. A Review on Biomass as a Substitute Energy Source: Polygeneration Influence and Hydrogen Rich Gas Formation via Pyrolysis. J. Anal. Appl. Pyrolysis 2023, 175, 106221. [Google Scholar] [CrossRef]
- Bianasari, A.A.; Khaled, M.S.; Hoang, T.D.; Reza, M.S.; Bakar, M.S.A.; Azad, A.K. Influence of Combined Catalysts on the Catalytic Pyrolysis Process of Biomass: A Systematic Literature Review. Energy Convers. Manag. 2024, 309, 118437. [Google Scholar] [CrossRef]
- El Bari, H.; Fanezoune, C.K.; Dorneanu, B.; Arellano-Garcia, H.; Majozi, T.; Elhenawy, Y.; Bayssi, O.; Hirt, A.; Peixinho, J.; Dhahak, A.; et al. Catalytic Fast Pyrolysis of Lignocellulosic Biomass: Recent Advances and Comprehensive Overview. J. Anal. Appl. Pyrolysis 2024, 178, 106390. [Google Scholar] [CrossRef]
- Kumar Mishra, R.; Singh, B.; Acharya, B. A Comprehensive Review on Activated Carbon from Pyrolysis of Lignocellulosic Biomass: An Application for Energy and the Environment. Carbon Resour. Convers. 2024, 7, 100228. [Google Scholar] [CrossRef]
- Nadarajah, K.; Asharp, T.; Jeganathan, Y. Biochar from Waste Biomass, Its Fundamentals, Engineering Aspects, and Potential Applications: An Overview. Water Sci. Technol. 2024, 89, 1211–1239. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, K.; Tao, Y.; Yang, Q.; Xu, L.; Liu, C.; Ma, L.; Xiao, R. Biomass Directional Pyrolysis Based on Element Economy to Produce High-Quality Fuels, Chemicals, Carbon Materials—A Review. Biotechnol. Adv. 2023, 69, 108262. [Google Scholar] [CrossRef]
- Velvizhi, G.; Jacqueline, P.J.; Shetti, N.P.; Latha, K.; Mohanakrishna, G.; Aminabhavi, T.M. Emerging Trends and Advances in Valorization of Lignocellulosic Biomass to Biofuels. J. Environ. Manag. 2023, 345, 118527. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Vuppaladadiyam, S.S.V.; Awasthi, A.; Sahoo, A.; Rehman, S.; Pant, K.K.; Murugavelh, S.; Huang, Q.; Anthony, E.; Fennel, P.; et al. Biomass Pyrolysis: A Review on Recent Advancements and Green Hydrogen Production. Bioresour. Technol. 2022, 364, 128087. [Google Scholar] [CrossRef]
- Sharma, T.; Hakeem, I.G.; Gupta, A.B.; Joshi, J.; Shah, K.; Vuppaladadiyam, A.K.; Sharma, A. Parametric Influence of Process Conditions on Thermochemical Techniques for Biochar Production: A State-of-the-Art Review. J. Energy Inst. 2024, 113, 101559. [Google Scholar] [CrossRef]
- Russo, C.; Cerciello, F.; Senneca, O.; Apicella, B. Challenges and Progresses in the Chemical Investigation of High Molecular Weight Species in Condensed Pyrolysis Products of Coal and Biomass. J. Anal. Appl. Pyrolysis 2024, 177, 106280. [Google Scholar] [CrossRef]
- Raza, M.; Inayat, A.; Ahmed, A.; Jamil, F.; Ghenai, C.; Naqvi, S.R.; Shanableh, A.; Ayoub, M.; Waris, A.; Park, Y.K. Progress of the Pyrolyzer Reactors and Advanced Technologies for Biomass Pyrolysis Processing. Sustainability 2021, 13, 11061. [Google Scholar] [CrossRef]
- Roy, P.; Dias, G. Prospects for Pyrolysis Technologies in the Bioenergy Sector: A Review. Renew. Sustain. Energy Rev. 2017, 77, 59–69. [Google Scholar] [CrossRef]
- Slezak, R.; Unyay, H.; Szufa, S.; Ledakowicz, S. An Extensive Review and Comparison of Modern Biomass Reactors Torrefaction vs. Biomass Pyrolizers—Part 2. Energies 2023, 16, 2212. [Google Scholar] [CrossRef]
- Hasan, M.M.; Rasul, M.G.; Khan, M.M.K.; Ashwath, N.; Jahirul, M.I. Energy Recovery from Municipal Solid Waste Using Pyrolysis Technology: A Review on Current Status and Developments. Renew. Sustain. Energy Rev. 2021, 145, 111073. [Google Scholar] [CrossRef]
- Pahnila, M.; Koskela, A.; Sulasalmi, P.; Fabritius, T. A Review of Pyrolysis Technologies and the Effect of Process Parameters on Biocarbon Properties. Energies 2023, 16, 6936. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Iwuchukwu, F.U.; Eyankware, O.E.; Iwuozor, K.O.; Olotu, K.; Bright, O.C.; Igwegbe, C.A. Flash Pyrolysis of Biomass: A Review of Recent Advances. Clean Technol. Environ. Policy 2022, 24, 2349–2363. [Google Scholar] [CrossRef]
- Zha, Z.; Wu, K.; Ge, Z.; Ma, Y.; Zeng, M.; Wu, Y.; Tao, Y.; Zhang, H. Effect of Oxygen on Thermal Behaviors and Kinetic Characteristics of Biomass during Slow and Flash Pyrolysis Processes. Combust. Flame 2023, 247, 112481. [Google Scholar] [CrossRef]
- Treedet, W.; Suntivarakorn, R. Design and Operation of a Low Cost Bio-Oil Fast Pyrolysis from Sugarcane Bagasse on Circulating Fluidized Bed Reactor in a Pilot Plant. Fuel Process. Technol. 2018, 179, 17–31. [Google Scholar] [CrossRef]
- Landrat, M.; Abawalo, M.; Pikoń, K.; Fufa, P.A.; Seyid, S. Assessing the Potential of Teff Husk for Biochar Production through Slow Pyrolysis: Effect of Pyrolysis Temperature on Biochar Yield. Energies 2024, 17, 1988. [Google Scholar] [CrossRef]
- Nam, H.; Capareda, S.C.; Ashwath, N.; Kongkasawan, J. Experimental Investigation of Pyrolysis of Rice Straw Using Bench-Scale Auger, Batch and Fluidized Bed Reactors. Energy 2015, 93, 2384–2394. [Google Scholar] [CrossRef]
- Santos, J.L.; Centeno, M.A.; Odriozola, J.A. Biochar Production from Cellulose under Reductant Atmosphere: Influence of the Total Pyrolysis Time. RSC Adv. 2023, 13, 21071–21079. [Google Scholar] [CrossRef] [PubMed]
- Jalalabadi, T.; Glenn, M.; Tremain, P.; Moghtaderi, B.; Donne, S.; Allen, J. Modification of Biochar Formation during Slow Pyrolysis in the Presence of Alkali Metal Carbonate Additives. Energy Fuels 2019, 33, 11235–11245. [Google Scholar] [CrossRef]
- Jouiad, M.; Al-Nofeli, N.; Khalifa, N.; Benyettou, F.; Yousef, L.F. Characteristics of Slow Pyrolysis Biochars Produced from Rhodes Grass and Fronds of Edible Date Palm. J. Anal. Appl. Pyrolysis 2015, 111, 183–190. [Google Scholar] [CrossRef]
- Ferreira, S.D.; Junges, J.; Bassanesi, G.R.; Lazzarotto, I.P.; Osório, E.; Godinho, M. Investigation of the Structure of the Biochar Obtained by Slow Pyrolysis of Elephant Grass during Its Steam Gasification. Chem. Eng. Technol. 2019, 42, 2546–2555. [Google Scholar] [CrossRef]
- Trada, A.; Chaudhary, A.; Patel, D.; Upadhyay, D.S. An Alternative Fuel Production from Sawdust through Batch-Type Pyrolysis Reactor: Fuel Properties and Thermodynamic Analysis. Process Saf. Environ. Prot. 2022, 167, 332–342. [Google Scholar] [CrossRef]
- Suresh Babu, K.K.B.; Nataraj, M.; Tayappa, M.; Vyas, Y.; Mishra, R.K.; Acharya, B. Production of Biochar from Waste Biomass Using Slow Pyrolysis: Studies of the Effect of Pyrolysis Temperature and Holding Time on Biochar Yield and Properties. Mater. Sci. Energy Technol. 2024, 7, 318–334. [Google Scholar] [CrossRef]
- Safavi, A.; Richter, C.; Unnthorsson, R. Mathematical Modeling and Experiments on Pyrolysis of Walnut Shells Using a Fixed-Bed Reactor. ChemEngineering 2022, 6, 93. [Google Scholar] [CrossRef]
- Caballero, B.M.; López-Urionabarrenechea, A.; Pérez, B.; Solar, J.; Acha, E.; de Marco, I. Potentiality of “Orujillo” (Olive Oil Solid Waste) to Produce Hydrogen by Means of Pyrolysis. Int. J. Hydrogen Energy 2020, 45, 20549–20557. [Google Scholar] [CrossRef]
- Ochieng, R.; Cerón, A.L.; Konist, A.; Sarker, S. Experimental and Modeling Studies of Intermediate Pyrolysis of Wood in a Laboratory-Scale Continuous Feed Retort Reactor. Bioresour. Technol. Rep. 2023, 24, 101650. [Google Scholar] [CrossRef]
- Ahmed, A.; Abu Bakar, M.S.; Azad, A.K.; Sukri, R.S.; Phusunti, N. Intermediate Pyrolysis of Acacia Cincinnata and Acacia Holosericea Species for Bio-Oil and Biochar Production. Energy Convers. Manag. 2018, 176, 393–408. [Google Scholar] [CrossRef]
- Safdari, M.S.; Amini, E.; Weise, D.R.; Fletcher, T.H. Heating Rate and Temperature Effects on Pyrolysis Products from Live Wildland Fuels. Fuel 2019, 242, 295–304. [Google Scholar] [CrossRef]
- Desideri, U.; Lazaroiu, G.; Stroe, C. Conventional Pyrolysis of Spruce Wood and Hazelnut Shell Delivering Oily Products. J. Sustain. Energy 2011, 2, 55–58. [Google Scholar]
- Ibrahim, M.D.; Abakr, Y.A.; Gan, S.; Lee, L.Y.; Thangalazhy-Gopakumar, S. Intermediate Pyrolysis of Bambara Groundnut Shell (BGS) in Various Inert Gases (N2, CO2, and N2/CO2). Energies 2022, 15, 8421. [Google Scholar] [CrossRef]
- Tabal, A.; Belyazid, O.; Dahman, H.; Berrich, E.; Jeguirim, M.; El Achaby, M.; El Harfia, K.; Aboulkas, A. Intermediate Pyrolysis of Ficus Nitida Wood in a Fixed-Bed Reactor: Effect of Pyrolysis Parameters on Bio-Oil and Bio-Char Yields and Properties. C. R. Chim. 2023, 26, 7–23. [Google Scholar] [CrossRef]
- Debdoubi, A.; El Amarti, A.; Colacio, E.; Blesa, M.J.; Hajjaj, L.H. The Effect of Heating Rate on Yields and Compositions of Oil Products from Esparto Pyrolysis. Int. J. Energy Res. 2006, 30, 1243–1250. [Google Scholar] [CrossRef]
- Jerzak, W.; Gao, N.; Kalemba-Rec, I.; Magdziarz, A. Catalytic Intermediate Pyrolysis of Post-Extraction Rapeseed Meal by Reusing ZSM-5 and Zeolite Y Catalysts. Catal. Today 2022, 404, 63–77. [Google Scholar] [CrossRef]
- Funke, A.; Tomasi Morgano, M.; Dahmen, N.; Leibold, H. Experimental Comparison of Two Bench Scale Units for Fast and Intermediate Pyrolysis. J. Anal. Appl. Pyrolysis 2017, 124, 504–514. [Google Scholar] [CrossRef]
- Torri, I.D.V.; Paasikallio, V.; Faccini, C.S.; Huff, R.; Caramão, E.B.; Sacon, V.; Oasmaa, A.; Zini, C.A. Bio-Oil Production of Softwood and Hardwood Forest Industry Residues through Fast and Intermediate Pyrolysis and Its Chromatographic Characterization. Bioresour. Technol. 2016, 200, 680–690. [Google Scholar] [CrossRef] [PubMed]
- Onay, O. Influence of Pyrolysis Temperature and Heating Rate on the Production of Bio-Oil and Char from Safflower Seed by Pyrolysis, Using a Well-Swept Fixed-Bed Reactor. Fuel Process. Technol. 2007, 88, 523–531. [Google Scholar] [CrossRef]
- Morgano, M.T.; Bergfeldt, B.; Leibold, H.; Richter, F.; Stapf, D. Intermediate Pyrolysis of Agricultural Waste: A Decentral Approach towards Circular Economy. Chem. Eng. Trans. 2018, 65, 649–654. [Google Scholar] [CrossRef]
- Hornung, U.; Schneider, D.; Hornung, A.; Tumiatti, V.; Seifert, H. Sequential Pyrolysis and Catalytic Low Temperature Reforming of Wheat Straw. J. Anal. Appl. Pyrolysis 2009, 85, 145–150. [Google Scholar] [CrossRef]
- Boscagli, C.; Tomasi Morgano, M.; Raffelt, K.; Leibold, H.; Grunwaldt, J.D. Influence of Feedstock, Catalyst, Pyrolysis and Hydrotreatment Temperature on the Composition of Upgraded Oils from Intermediate Pyrolysis. Biomass Bioenergy 2018, 116, 236–248. [Google Scholar] [CrossRef]
- Klinger, J.L.; Westover, T.L.; Emerson, R.M.; Williams, C.L.; Hernandez, S.; Monson, G.D.; Ryan, J.C. Effect of Biomass Type, Heating Rate, and Sample Size on Microwave-Enhanced Fast Pyrolysis Product Yields and Qualities. Appl. Energy 2018, 228, 535–545. [Google Scholar] [CrossRef]
- Uzun, B.B.; Apaydin-Varol, E.; Ateş, F.; Özbay, N.; Pütün, A.E. Synthetic Fuel Production from Tea Waste: Characterisation of Bio-Oil and Bio-Char. Fuel 2010, 89, 176–184. [Google Scholar] [CrossRef]
- Demirbas, A. Determination of Calorific Values of Bio-Chars and Pyro-Oils from Pyrolysis of Beech Trunkbarks. J. Anal. Appl. Pyrolysis 2004, 72, 215–219. [Google Scholar] [CrossRef]
- Tsai, W.T.; Lee, M.K.; Chang, Y.M. Fast Pyrolysis of Rice Husk: Product Yields and Compositions. Bioresour. Technol. 2007, 98, 22–28. [Google Scholar] [CrossRef]
- Rueangsan, K.; Heman, A.; Kraisoda, P.; Tasarod, H.; Duanguppama, K.; Trisupakitti, S.; Morris, J. Bio-Oil Production via Fast Pyrolysis of Cassava Residues Combined with Ethanol and Volcanic Rock in a Free-Fall Reactor. Cogent Eng. 2023, 10, 2156054. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Ioannidou, O.; Skoulou, V. Rapeseed Residues Utilization for Energy and 2nd Generation Biofuels. Fuel 2008, 87, 1492–1502. [Google Scholar] [CrossRef]
- Zinchik, S.; Klinger, J.L.; Westover, T.L.; Donepudi, Y.; Hernandez, S.; Naber, J.D.; Bar-Ziv, E. Evaluation of Fast Pyrolysis Feedstock Conversion with a Mixing Paddle Reactor. Fuel Process. Technol. 2018, 171, 124–132. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Damartzis, T. Modelling the Intra-Particle Transport Phenomena and Chemical Reactions of Olive Kernel Fast Pyrolysis. J. Anal. Appl. Pyrolysis 2007, 80, 187–194. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Ioannidou, O.; Antonakou, E.; Lappas, A. Experimental Study of Pyrolysis for Potential Energy, Hydrogen and Carbon Material Production from Lignocellulosic Biomass. Int. J. Hydrogen Energy 2008, 33, 2433–2444. [Google Scholar] [CrossRef]
- Morgan, T.J.; Turn, S.Q.; Sun, N.; George, A. Fast Pyrolysis of Tropical Biomass Species and Influence of Water Pretreatment on Product Distributions. PLoS ONE 2016, 11, e0151368. [Google Scholar] [CrossRef] [PubMed]
- Sadakata, M.; Takahashi, K.; Saito, M.; Sakai, T. Production of Fuel Gas and Char from Wood, Lignin and Holocellulose by Carbonization. Fuel 1987, 66, 1667–1671. [Google Scholar] [CrossRef]
- Li, B.; Song, M.; Xie, X.; Wei, J.; Xu, D.; Ding, K.; Huang, Y.; Zhang, S.; Hu, X.; Zhang, S.; et al. Oxidative Fast Pyrolysis of Biomass in a Quartz Tube Fluidized Bed Reactor: Effect of Oxygen Equivalence Ratio. Energy 2023, 270, 126987. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Jensen, P.A.; Jensen, A.D.; Steibel, M.; Spliethoff, H.; Glarborg, P. Influence of Fast Pyrolysis Conditions on Yield and Structural Transformation of Biomass Chars. Fuel Process. Technol. 2015, 140, 205–214. [Google Scholar] [CrossRef]
- Wang, B.; Xu, F.; Zong, P.; Zhang, J.; Tian, Y.; Qiao, Y. Effects of Heating Rate on Fast Pyrolysis Behavior and Product Distribution of Jerusalem Artichoke Stalk by Using TG-FTIR and Py-GC/MS. Renew. Energy 2019, 132, 486–496. [Google Scholar] [CrossRef]
- Urban, B.; Shirazi, Y.; Maddi, B.; Viamajala, S.; Varanasi, S. Flash Pyrolysis of Oleaginous Biomass in a Fluidized-Bed Reactor. Energy Fuels 2017, 31, 8326–8334. [Google Scholar] [CrossRef]
- Hoekstra, E.; Van Swaaij, W.P.M.; Kersten, S.R.A.; Hogendoorn, K.J.A. Fast Pyrolysis in a Novel Wire-Mesh Reactor: Decomposition of Pine Wood and Model Compounds. Chem. Eng. J. 2012, 187, 172–184. [Google Scholar] [CrossRef]
- Boateng, A.A.; Mullen, C.A.; Goldberg, N.; Hicks, K.B.; Jung, H.J.G.; Lamb, J.F.S. Production of Bio-Oil from Alfalfa Stems by Fluidized-Bed Fast Pyrolysis. Ind. Eng. Chem. Res. 2008, 47, 4115–4122. [Google Scholar] [CrossRef]
- Marathe, P.S.; Westerhof, R.J.M.; Kersten, S.R.A. Effect of Pressure and Hot Vapor Residence Time on the Fast Pyrolysis of Biomass: Experiments and Modeling. Energy Fuels 2020, 34, 1773–1780. [Google Scholar] [CrossRef]
- Almeida, M.C.P.D.S.; Silva, J.E.D.; Batista, W.G.D.S.; Alves, J.L.F.; Melo, D.M.D.A.; Pimenta, A.S.; Braga, R.M. Valorization of Wood Residues from Vegetation Suppression during Wind Energy Plant Implementation and Its Potential for Renewable Phenolic Compounds through Flash Pyrolysis: A Case Study in Northeast Brazil’s Semi-Arid Region. Forests 2024, 15, 621. [Google Scholar] [CrossRef]
- Maduskar, S.; Facas, G.G.; Papageorgiou, C.; Williams, C.L.; Dauenhauer, P.J. Five Rules for Measuring Biomass Pyrolysis Rates: Pulse-Heated Analysis of Solid Reaction Kinetics of Lignocellulosic Biomass. ACS Sustain. Chem. Eng. 2018, 6, 1387–1399. [Google Scholar] [CrossRef]
- Shuangning, X.; Zhihe, L.; Baoming, L.; Weiming, Y.; Xueyuan, B. Devolatilization Characteristics of Biomass at Flash Heating Rate. Fuel 2006, 85, 664–670. [Google Scholar] [CrossRef]
- Greenhalf, C.E.; Nowakowski, D.J.; Harms, A.B.; Titiloye, J.O.; Bridgwater, A.V. A Comparative Study of Straw, Perennial Grasses and Hardwoods in Terms of Fast Pyrolysis Products. Fuel 2013, 108, 216–230. [Google Scholar] [CrossRef]
- Kaur, R.; Kumar, A.; Biswas, B.; Krishna, B.B.; Bhaskar, T. Investigations into Pyrolytic Behaviour of Spent Citronella Waste: Slow and Flash Pyrolysis Study. Bioresour. Technol. 2022, 366, 128202. [Google Scholar] [CrossRef]
- Bridgwater, T. Challenges and Opportunities in Fast Pyrolysis of Biomass: Part II. Johns. Matthey Technol. Rev. 2018, 62, 150–160. [Google Scholar] [CrossRef]
- Okolie, J.A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Chemistry and Specialty Industrial Applications of Lignocellulosic Biomass. Waste Biomass Valorization 2021, 12, 2145–2169. [Google Scholar] [CrossRef]
- Mohan, D.; Pittman, C.U.; Steele, P.H. Pyrolysis of Wood /Biomass for Bio-Oil. Energy Fuels 2006, 20, 848–889. [Google Scholar] [CrossRef]
- Qureshi, K.M.; Kay Lup, A.N.; Khan, S.; Abnisa, F.; Wan Daud, W.M.A. A Technical Review on Semi-Continuous and Continuous Pyrolysis Process of Biomass to Bio-Oil. J. Anal. Appl. Pyrolysis 2018, 131, 52–75. [Google Scholar] [CrossRef]
- Carrascosa, M.S. Reactor Design and Characterization of Biochar; University of Agder: Kristiansand, Norway, 2016. [Google Scholar]
- Lewandowski, W.M.; Januszewicz, K.; Kosakowski, W. Efficiency and Proportions of Waste Tyre Pyrolysis Products Depending on the Reactor Type—A Review. J. Anal. Appl. Pyrolysis 2019, 140, 25–53. [Google Scholar] [CrossRef]
- Lopez, G.; Alvarez, J.; Amutio, M.; Hooshdaran, B.; Cortazar, M.; Haghshenasfard, M.; Hosseini, S.H.; Olazar, M. Kinetic Modeling and Experimental Validation of Biomass Fast Pyrolysis in a Conical Spouted Bed Reactor. Chem. Eng. J. 2019, 373, 677–686. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, J.K.; Oh, C.H.; Park, J.W.; Kwon, E.E. Production of Bio-Oil from Fast Pyrolysis of Biomass Using a Pilot-Scale Circulating Fluidized Bed Reactor and Its Characterization. J. Environ. Manag. 2019, 234, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Tahmasebi, A.; Maliutina, K.; Matamba, T.; Kim, J.H.; Jeon, C.H.; Yu, J. Pressurized Entrained-Flow Pyrolysis of Lignite for Enhanced Production of Hydrogen-Rich Gas and Chemical Raw Materials. J. Anal. Appl. Pyrolysis 2020, 145, 104741. [Google Scholar] [CrossRef]
- Clissold, J.; Jalalifar, S.; Salehi, F.; Abbassi, R.; Ghodrat, M. Fluidisation Characteristics and Inter-Phase Heat Transfer on Product Yields in Bubbling Fluidised Bed Reactor. Fuel 2020, 273, 117791. [Google Scholar] [CrossRef]
- Meier, D.; Faix, O. State of the Art of Applied Fast Pyrolysis of Lignocellulosic Materials—A Review. Bioresour. Technol. 1999, 68, 71–77. [Google Scholar] [CrossRef]
- Amutio, M.; Lopez, G.; Artetxe, M.; Elordi, G.; Olazar, M.; Bilbao, J. Influence of Temperature on Biomass Pyrolysis in a Conical Spouted Bed Reactor. Resour. Conserv. Recycl. 2012, 59, 23–31. [Google Scholar] [CrossRef]
- Amutio, M.; Lopez, G.; Alvarez, J.; Moreira, R.; Duarte, G.; Nunes, J.; Olazar, M.; Bilbao, J. Flash Pyrolysis of Forestry Residues from the Portuguese Central Inland Region within the Framework of the BioREFINA-Ter Project. Bioresour. Technol. 2013, 129, 512–518. [Google Scholar] [CrossRef]
- Fernandez-Akarregi, A.R.; Makibar, J.; Lopez, G.; Amutio, M.; Olazar, M. Design and Operation of a Conical Spouted Bed Reactor Pilot Plant (25 Kg/h) for Biomass Fast Pyrolysis. Fuel Process. Technol. 2013, 112, 48–56. [Google Scholar] [CrossRef]
- Peterson, B.; Engtrakul, C.; Evans, T.J.; Iisa, K.; Watson, M.J.; Jarvis, M.W.; Robichaud, D.J.; Mukarakate, C.; Nimlos, M.R. Optimization of Biomass Pyrolysis Vapor Upgrading Using a Laminar Entrained-Flow Reactor System. Energy Fuels 2020, 34, 6030–6040. [Google Scholar] [CrossRef]
- Tchapda, A.H.; Pisupati, S.V. Characterization of an Entrained Flow Reactor for Pyrolysis of Coal and Biomass at Higher Temperatures. Fuel 2015, 156, 254–266. [Google Scholar] [CrossRef]
- Newalkar, G.; Iisa, K.; Damico, A.D.; Sievers, C.; Agrawal, P. Effect of Temperature, Pressure, and Residence Time on Pyrolysis of Pine in an Entrained Flow Reactor. Energy Fuels 2014, 28, 5144–5157. [Google Scholar] [CrossRef]
- Li, S.; Xu, S.; Liu, S.; Yang, C.; Lu, Q. Fast Pyrolysis of Biomass in Free-Fall Reactor for Hydrogen-Rich Gas. Fuel Process. Technol. 2004, 85, 1201–1211. [Google Scholar] [CrossRef]
- Bieniek, A.; Sieradzka, M.; Jerzak, W.; Magdziarz, A. Fast Pyrolysis of Agricultural Biomass in Drop Tube Reactor for Bio-Oil Production: Numerical Calculations. J. Anal. Appl. Pyrolysis 2023, 176, 106241. [Google Scholar] [CrossRef]
- Pattiya, A.; Sukkasi, S.; Goodwin, V. Fast Pyrolysis of Sugarcane and Cassava Residues in a Free-Fall Reactor. Energy 2012, 44, 1067–1077. [Google Scholar] [CrossRef]
- Magalhães, D.; Akgül, A.; Kazanç, F.; Costa, M. Interactions during CO2 Co-Gasification of Biomass and Coal Chars Obtained from Fast Pyrolysis in a Drop Tube Furnace. Energy Fuels 2021, 35, 7065–7076. [Google Scholar] [CrossRef]
- Ellens, C.J.; Brown, R.C. Optimization of a Free-Fall Reactor for the Production of Fast Pyrolysis Bio-Oil. Bioresour. Technol. 2012, 103, 374–380. [Google Scholar] [CrossRef]
- Jerzak, W.; Wądrzyk, M.; Sieradzka, M.; Magdziarz, A. Valorisation of Tyre Waste from a Vulcanisation Plant by Catalytic Pyrolysis—Experimental Investigations Using Pyrolysis–Gas Chromatography–Mass Spectrometry and Drop-Tube–Fixed-Bed Reactor. Energy Convers. Manag. 2024, 313, 118642. [Google Scholar] [CrossRef]
- Salehi, E.; Abedi, J.; Harding, T. Bio-Oil from Sawdust: Pyrolysis of Sawdust in a Fixed-Bed System. Energy Fuels 2009, 23, 3767–3772. [Google Scholar] [CrossRef]
- Kwapinski, W.; Byrne, C.M.P.; Kryachko, E.; Wolfram, P.; Adley, C.; Leahy, J.J.; Novotny, E.H.; Hayes, M.H.B. Biochar from Biomass and Waste. Waste Biomass Valorization 2010, 1, 177–189. [Google Scholar] [CrossRef]
- Varma, A.K.; Mondal, P. Pyrolysis of Sugarcane Bagasse in Semi Batch Reactor: Effects of Process Parameters on Product Yields and Characterization of Products. Ind. Crops Prod. 2017, 95, 704–717. [Google Scholar] [CrossRef]
- Ateş, F.; Miskolczi, N.; Borsodi, N. Comparision of Real Waste (MSW and MPW) Pyrolysis in Batch Reactor over Different Catalysts. Part I: Product Yields, Gas and Pyrolysis Oil Properties. Bioresour. Technol. 2013, 133, 443–454. [Google Scholar] [CrossRef]
- Milhé, M.; Van De Steene, L.; Haube, M.; Commandré, J.M.; Fassinou, W.F.; Flamant, G. Autothermal and Allothermal Pyrolysis in a Continuous Fixed Bed Reactor. J. Anal. Appl. Pyrolysis 2013, 103, 102–111. [Google Scholar] [CrossRef]
- Efika, C.E.; Onwudili, J.A.; Williams, P.T. Influence of Heating Rates on the Products of High-Temperature Pyrolysis of Waste Wood Pellets and Biomass Model Compounds. Waste Manag. 2018, 76, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Mlonka-Mędrala, A.; Evangelopoulos, P.; Sieradzka, M.; Zajemska, M.; Magdziarz, A. Pyrolysis of Agricultural Waste Biomass towards Production of Gas Fuel and High-Quality Char: Experimental and Numerical Investigations. Fuel 2021, 296, 120611. [Google Scholar] [CrossRef]
- Guo, X.; Yang, H.; Wenga, T.; Zhang, R.; Liu, B.; Chen, G.; Hou, L. Catalytic Fast Pyrolysis of Arundo Donax in a Two-Stage Fixed Bed Reactor over Metal-Modified HZSM-5 Catalysts. Biomass Bioenergy 2022, 156, 106316. [Google Scholar] [CrossRef]
- Wang, F.; Gao, N.; Magdziarz, A.; Quan, C. Co-Pyrolysis of Biomass and Waste Tires under High-Pressure Two-Stage Fixed Bed Reactor. Bioresour. Technol. 2022, 344, 126306. [Google Scholar] [CrossRef]
- Wagenaar, B.M.; Prins, W.; van Swaaij, W.P.M. Pyrolysis of Biomass in the Rotating Cone Reactor: Modelling and Experimental Justification. Chem. Eng. Sci. 1994, 49, 5109–5126. [Google Scholar] [CrossRef]
- BTG Bioliquids. Available online: https://www.btg-bioliquids.com/plant/empyro-hengelo/ (accessed on 10 June 2024).
- Yildiz, G.; Ronsse, F.; Venderbosch, R.; van Duren, R.; Kersten, S.R.A.; Prins, W. Effect of Biomass Ash in Catalytic Fast Pyrolysis of Pine Wood. Appl. Catal. B Environ. 2015, 168–169, 203–211. [Google Scholar] [CrossRef]
- Xi, Y.; Chen, Q.; You, C. Flow Characteristics of Biomass Particles in a Horizontal Stirred Bed Reactor: Part II. Modeling Studies on Particle Residence Time Distribution and Axial Mixing. Powder Technol. 2015, 269, 585–595. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Raghavan, V. Pyrolysis in Auger Reactors for Biochar and Bio-Oil Production: A Review. Biosyst. Eng. 2017, 161, 80–92. [Google Scholar] [CrossRef]
- Gupta, M.; Nguyen, L.; Bronson, B.; Jeaidi, J.; Funke, A.; Thorson, M. Country Reports 2023 Direct Thermochemical Liquefaction of Biomass in Canada, Germany and the United States. IEA Bioenergy. 2024. Available online: https://www.ieabioenergy.com/blog/publications/overview-of-thermochemical-liquefaction-activities-in-canada-germany-and-the-united-states/ (accessed on 10 June 2024).
- Campuzano, F.; Brown, R.C.; Martínez, J.D. Auger Reactors for Pyrolysis of Biomass and Wastes. Renew. Sustain. Energy Rev. 2019, 102, 372–409. [Google Scholar] [CrossRef]
- Solar, J.; Caballero, B.M.; López-Urionabarrenechea, A.; Acha, E.; Arias, P.L. Pyrolysis of Forestry Waste in a Screw Reactor with Four Sequential Heating Zones: Influence of Isothermal and Nonisothermal Profiles. Ind. Eng. Chem. Res. 2021, 60, 18627–18639. [Google Scholar] [CrossRef]
- Brown, J.N.; Brown, R.C. Process Optimization of an Auger Pyrolyzer with Heat Carrier Using Response Surface Methodology. Bioresour. Technol. 2012, 103, 405–414. [Google Scholar] [CrossRef]
- Papari, S.; Hawboldt, K. Development and Validation of a Process Model to Describe Pyrolysis of Forestry Residues in an Auger Reactor. Energy Fuels 2017, 31, 10833–10841. [Google Scholar] [CrossRef]
- Kapoor, L.; Bose, D.; Mekala, A. Biomass Pyrolysis in a Twin-Screw Reactor to Produce Green Fuels. Biofuels 2020, 11, 101–107. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification, Pyrolysis and Torrefaction: Practical Design and Theory; Elsevier Inc.: Amsterdam, The Netherlands, 2013; ISBN 9780123964885. [Google Scholar]
- Luo, G.; Chandler, D.S.; Anjos, L.C.A.; Eng, R.J.; Jia, P.; Resende, F.L.P. Pyrolysis of Whole Wood Chips and Rods in a Novel Ablative Reactor. Fuel 2017, 194, 229–238. [Google Scholar] [CrossRef]
- Peacocke, G.V.C.; Bridgwater, A.V. Ablative Plate Pyrolysis of Biomass for Liquids. Biomass Bioenergy 1994, 7, 147–154. [Google Scholar] [CrossRef]
- Mankeed, P.; Khuenkaeo, N.; Malik, F.R.; Tippayawong, N. Temperature Evolution and Heating Rates of Biomass Undergoing Ablative Pyrolysis. Eng. Technol. Appl. Sci. Res. 2023, 13, 10301–10305. [Google Scholar] [CrossRef]
- Khuenkaeo, N.; Tippayawong, N. Production and Characterization of Bio-Oil and Biochar from Ablative Pyrolysis of Lignocellulosic Biomass Residues. Chem. Eng. Commun. 2020, 207, 153–160. [Google Scholar] [CrossRef]
- Bojanovský, J.; Máša, V.; Hudák, I.; Skryja, P.; Hopjan, J. Rotary Kiln, a Unit on the Border of the Process and Energy Industry—Current State and Perspectives. Sustainability 2022, 14, 13903. [Google Scholar] [CrossRef]
- Fantozzi, F.; Colantoni, S.; Bartocci, P.; Desideri, U. Rotary Kiln Slow Pyrolysis for Syngas and Char Production from Biomass and Waste—Part I: Working Envelope of the Reactor. J. Eng. Gas Turbines Power 2007, 129, 901–907. [Google Scholar] [CrossRef]
- Hornung, A. (Ed.) Transformation of Biomass Theory to Practice, 4. Pyrolysis; Wiley: Hoboken, NJ, USA, 2014; ISBN 9781466594579. [Google Scholar]
- Tanoh, T.S.; Ait Oumeziane, A.; Lemonon, J.; Escudero Sanz, F.J.; Salvador, S. Green Waste/Wood Pellet Pyrolysis in a Pilot-Scale Rotary Kiln: Effect of Temperature on Product Distribution and Characteristics. Energy Fuels 2020, 34, 3336–3345. [Google Scholar] [CrossRef]
- Sanginés, P.; Domínguez, M.P.; Sánchez, F.; San Miguel, G. Slow Pyrolysis of Olive Stones in a Rotary Kiln: Chemical and Energy Characterization of Solid, Gas, and Condensable Products. J. Renew. Sustain. Energy 2015, 7, 043103. [Google Scholar] [CrossRef]
- Mei, Y.; Liu, R.; Yang, Q.; Yang, H.; Shao, J.; Draper, C.; Zhang, S.; Chen, H. Torrefaction of Cedarwood in a Pilot Scale Rotary Kiln and the Influence of Industrial Flue Gas. Bioresour. Technol. 2015, 177, 355–360. [Google Scholar] [CrossRef]
- Hassan, H.; Lim, J.K.; Hameed, B.H. Recent Progress on Biomass Co-Pyrolysis Conversion into High-Quality Bio-Oil. Bioresour. Technol. 2016, 221, 645–655. [Google Scholar] [CrossRef]
- Abnisa, F.; Wan Daud, W.M.A. A Review on Co-Pyrolysis of Biomass: An Optional Technique to Obtain a High-Grade Pyrolysis Oil. Energy Convers. Manag. 2014, 87, 71–85. [Google Scholar] [CrossRef]
- Sharma, A.; Pareek, V.; Zhang, D. Biomass Pyrolysis—A Review of Modelling, Process Parameters and Catalytic Studies. Renew. Sustain. Energy Rev. 2015, 50, 1081–1096. [Google Scholar] [CrossRef]
- Adrados, A.; Lopez-Urionabarrenechea, A.; Acha, E.; Solar, J.; Caballero, B.M.; de Marco, I. Hydrogen Rich Reducing Gases Generation in the Production of Charcoal from Woody Biomass Carbonization. Energy Convers. Manag. 2017, 148, 352–359. [Google Scholar] [CrossRef]
- Pang, S. Advances in Thermochemical Conversion of Woody Biomass to Energy, Fuels and Chemicals. Biotechnol. Adv. 2019, 37, 589–597. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Z.; Sun, J.; Guo, W.; Wang, Q. Effects of Phosphorus-Modified HZSM-5 on Distribution of Hydrocarbon Compounds from Wood-Plastic Composite Pyrolysis Using Py-GC/MS. J. Anal. Appl. Pyrolysis 2015, 116, 223–230. [Google Scholar] [CrossRef]
- Dorado, C.; Mullen, C.A.; Boateng, A.A. H-ZSM5 Catalyzed Co-Pyrolysis of Biomass and Plastics. ACS Sustain. Chem. Eng. 2014, 2, 301–311. [Google Scholar] [CrossRef]
- Cai, W.; Luo, Z.; Zhou, J.; Wang, Q. A Review on the Selection of Raw Materials and Reactors for Biomass Fast Pyrolysis in China. Fuel Process. Technol. 2021, 221, 106919. [Google Scholar] [CrossRef]
- Asomaning, J.; Haupt, S.; Chae, M.; Bressler, D.C. Recent Developments in Microwave-Assisted Thermal Conversion of Biomass for Fuels and Chemicals. Renew. Sustain. Energy Rev. 2018, 92, 642–657. [Google Scholar] [CrossRef]
- Kappe, C.O. Unraveling the Mysteries of Microwave Chemistry Using Silicon Carbide Reactor Technology. Acc. Chem. Res. 2012, 46, 1579–1587. [Google Scholar] [CrossRef] [PubMed]
- Perreux, L.; Loupy, A.; Petit, A. Nonthermal Effects of Microwaves in Organic Synthesis. In Microwaves in Organic Synthesis, 2nd ed.; Antonio de la Hoz, A.L., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinhei, Germany, 2012; Volume 1, pp. 127–207. ISBN 3527314520. [Google Scholar]
- Kremsner, J.M.; Kappe, C.O. Silicon Carbide Passive Heating Elements in Microwave-Assisted Organic Synthesis. J. Org. Chem. 2006, 71, 4651–4658. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Liu, S.; Peng, P.; Min, M.; Cheng, Y.; Anderson, E.; Zhou, N.; Fan, L.; Liu, C.; et al. Effects of Feedstock Characteristics on Microwave-Assisted Pyrolysis—A Review. Bioresour. Technol. 2017, 230, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Sekar, M.; Mathimani, T.; Alagumalai, A.; Chi, N.T.L.; Duc, P.A.; Bhatia, S.K.; Brindhadevi, K.; Pugazhendhi, A. A Review on the Pyrolysis of Algal Biomass for Biochar and Bio-Oil—Bottlenecks and Scope. Fuel 2021, 283, 119190. [Google Scholar] [CrossRef]
- Mohd Mokhta, Z.; Ong, M.Y.; Salman, B.; Nomanbhay, S.; Salleh, S.F.; Chew, K.W.; Show, P.L.; Chen, W.H. Simulation Studies on Microwave-Assisted Pyrolysis of Biomass for Bioenergy Production with Special Attention on Waveguide Number and Location. Energy 2020, 190, 116474. [Google Scholar] [CrossRef]
- Ellison, C.R.; Hoff, R.; Mărculescu, C.; Boldor, D. Investigation of Microwave-Assisted Pyrolysis of Biomass with Char in a Rectangular Waveguide Applicator with Built-in Phase-Shifting. Appl. Energy 2020, 259, 114217. [Google Scholar] [CrossRef]
- Alper, K.; Tekin, K.; Karagöz, S.; Ragauskas, A.J. Sustainable Energy and Fuels from Biomass: A Review Focusing on Hydrothermal Biomass Processing. Sustain. Energy Fuels 2020, 4, 4390–4414. [Google Scholar] [CrossRef]
- Brindhadevi, K.; Anto, S.; Rene, E.R.; Sekar, M.; Mathimani, T.; Thuy Lan Chi, N.; Pugazhendhi, A. Effect of Reaction Temperature on the Conversion of Algal Biomass to Bio-Oil and Biochar through Pyrolysis and Hydrothermal Liquefaction. Fuel 2021, 285, 119106. [Google Scholar] [CrossRef]
- Huang, X.; Cheng, D.G.; Chen, F.; Zhan, X. Reaction Pathways of Hemicellulose and Mechanism of Biomass Pyrolysis in Hydrogen Plasma: A Density Functional Theory Study. Renew. Energy 2016, 96, 490–497. [Google Scholar] [CrossRef]
- Lin, K.C.; Lin, Y.C.; Hsiao, Y.H. Microwave Plasma Studies of Spirulina Algae Pyrolysis with Relevance to Hydrogen Production. Energy 2014, 64, 567–574. [Google Scholar] [CrossRef]
- Muvhiiwa, R.F.; Sempuga, B.; Hildebrandt, D.; Van Der Walt, J. Study of the Effects of Temperature on Syngas Composition from Pyrolysis of Wood Pellets Using a Nitrogen Plasma Torch Reactor. J. Anal. Appl. Pyrolysis 2018, 130, 249–255. [Google Scholar] [CrossRef]
- Zhang, H.; Xiao, R.; Wang, D.; He, G.; Shao, S.; Zhang, J.; Zhong, Z. Biomass Fast Pyrolysis in a Fluidized Bed Reactor under N2, CO2, CO, CH4 and H2 Atmospheres. Bioresour. Technol. 2011, 102, 4258–4264. [Google Scholar] [CrossRef]
- Cortazar, M.; Lopez, G.; Alvarez, J.; Amutio, M.; Bilbao, J.; Olazar, M. Advantages of Confining the Fountain in a Conical Spouted Bed Reactor for Biomass Steam Gasification. Energy 2018, 153, 455–463. [Google Scholar] [CrossRef]
- Bridgwater, A. Thermal Biomass Conversion and Utilization—Biomass Information System; European Communities: Luxembourg, 1996; ISBN 92-827-7207-1. [Google Scholar]
- Bridgwater, A.V. Review of Fast Pyrolysis of Biomass and Product Upgrading. Biomass Bioenergy 2012, 38, 68–94. [Google Scholar] [CrossRef]
- Wang, Z.; Burra, K.G.; Lei, T.; Gupta, A.K. Co-Pyrolysis of Waste Plastic and Solid Biomass for Synergistic Production of Biofuels and Chemicals-A Review. Prog. Energy Combust. Sci. 2021, 84, 100899. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Zhang, H.; Chu, C.; Zheng, K.; Ju, M.; Liu, L. Liquefaction of Biomass and Upgrading of Bio-Oil: A Review. Molecules 2019, 24, 2250. [Google Scholar] [CrossRef]
- Annamalai, K.; Thanapal, S.S.; Ranjan, D. Ranking Renewable and Fossil Fuels on Global Warming Potential Using Respiratory Quotient Concept. J. Combust. 2018, 2018, 1270708. [Google Scholar] [CrossRef]
- Stelmach, S.; Ignasiak, K.; Czardybon, A.; Bigda, J. Evaluation of Bio-Oils in Terms of Fuel Properties. Processes 2023, 11, 3317. [Google Scholar] [CrossRef]
- Roy, R.; Rahman, M.S.; Amit, T.A.; Jadhav, B. Recent Advances in Lignin Depolymerization Techniques: A Comparative Overview of Traditional and Greener Approaches. Biomass 2022, 2, 130–154. [Google Scholar] [CrossRef]
- Barbosa, A.S.; Siqueira, L.A.M.; Medeiros, R.L.B.A.; Melo, D.M.A.; Melo, M.A.F.; Freitas, J.C.O.; Braga, R.M. Renewable Aromatics through Catalytic Flash Pyrolysis of Pineapple Crown Leaves Using HZSM-5 Synthesized with RHA and Diatomite. Waste Manag. 2019, 88, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Lachos-Perez, D.; Martins-Vieira, J.C.; Missau, J.; Anshu, K.; Siakpebru, O.K.; Thengane, S.K.; Morais, A.R.C.; Tanabe, E.H.; Bertuol, D.A. Review on Biomass Pyrolysis with a Focus on Bio-Oil Upgrading Techniques. Analytica 2023, 4, 182–205. [Google Scholar] [CrossRef]
- Grams, J. Surface Analysis of Solid Products of Thermal Treatment of Lignocellulosic Biomass. J. Anal. Appl. Pyrolysis 2022, 161, 105429. [Google Scholar] [CrossRef]
- Standardized Product Definition and Product Testing Guidelines for Biochar That Is Used in Soil. Product Definition and Specification Standards. IBI Biochar Standards Version 2.1. 2015. Available online: http://www.biochar-international.org (accessed on 10 June 2024).
- Klasson, K.T. Biochar Characterization and a Method for Estimating Biochar Quality from Proximate Analysis Results. Biomass Bioenergy 2017, 96, 50–58. [Google Scholar] [CrossRef]
- European Biochar Foundation (EBC). 2012–2023 “European Biochar Certificate—Guidelines for a Sustainable Production of Biochar”. Carbon Standards International (CSI), Version 10.3 from 5 April 2022. Available online: http://european-biochar.org (accessed on 10 June 2024).
- Azzi, E.S.; Li, H.; Cederlund, H.; Karltun, E.; Sundberg, C. Modelling Biochar Long-Term Carbon Storage in Soil with Harmonized Analysis of Decomposition Data. Geoderma 2024, 441, 116761. [Google Scholar] [CrossRef]
- Chen, D.; Li, Y.; Cen, K.; Luo, M.; Li, H.; Lu, B. Pyrolysis Polygeneration of Poplar Wood: Effect of Heating Rate and Pyrolysis Temperature. Bioresour. Technol. 2016, 218, 780–788. [Google Scholar] [CrossRef]
- Cheng, J.; Hu, S.C.; Sun, G.T.; Geng, Z.C.; Zhu, M.Q. The Effect of Pyrolysis Temperature on the Characteristics of Biochar, Pyroligneous Acids, and Gas Prepared from Cotton Stalk through a Polygeneration Process. Ind. Crops Prod. 2021, 170, 113690. [Google Scholar] [CrossRef]
- Zhao, C.; Jiang, E.; Chen, A. Volatile Production from Pyrolysis of Cellulose, Hemicellulose and Lignin. J. Energy Inst. 2017, 90, 902–913. [Google Scholar] [CrossRef]
- Yu, Y.; Li, X.; Shao, S.; Zhang, P.; Jiang, J. Hydrogen Production via Biomass Fast Pyrolysis and In-Line Steam Reforming Using Carbon Reduced Cathode Material of Spent LiCoO2 Batteries as Catalyst. Fuel 2024, 357, 129659. [Google Scholar] [CrossRef]
- Alonso-Gómez, L.A.; Celis-Carmona, D.D.; Rodríguez-Sánchez, Y.F.; Castro-Ladino, J.R.; Solarte-Toro, J.C. Biochar Production from Cassava Waste Biomass: A Techno-Economic Development Approach in the Colombian Context. Bioresour. Technol. Rep. 2024, 26, 101872. [Google Scholar] [CrossRef]
- Setiawan, A.; Faisal, F.; Anshar, K.; Hasibuan, R.; Riskina, S. Alchalil Techno-Economic Assessment of Densified Arabica Coffee Pulp Pyrolysis in a Pilot-Scale Reactor. Biomass Convers. Biorefinery 2024, in press. [Google Scholar] [CrossRef]
- Shahbaz, M.; AlNouss, A.; Parthasarathy, P.; Abdelaal, A.H.; Mackey, H.; McKay, G.; Al-Ansari, T. Investigation of Biomass Components on the Slow Pyrolysis Products Yield Using Aspen Plus for Techno-Economic Analysis. Biomass Convers. Biorefinery 2022, 12, 669–681. [Google Scholar] [CrossRef]
- Rahul, S.; Dhanuprabha, D.; Prabakaran, S.; Arumugam, A. An Integrated Biorefinery of Madhuca Indica for Co-Production of Biodiesel, Bio-Oil, and Biochar: Towards a Sustainable Circular Bioeconomy. Ind. Crops Prod. 2024, 221, 119409. [Google Scholar] [CrossRef]
- Hu, M.; Guo, K.; Zhou, H.; Zhu, W.; Deng, L.; Dai, L. Techno-Economic Assessment of Swine Manure Biochar Production in Large-Scale Piggeries in China. Energy 2024, 308, 133037. [Google Scholar] [CrossRef]
- van Schalkwyk, D.L.; Mandegari, M.; Farzad, S.; Görgens, J.F. Techno-Economic and Environmental Analysis of Bio-Oil Production from Forest Residues via Non-Catalytic and Catalytic Pyrolysis Processes. Energy Convers. Manag. 2020, 213, 112815. [Google Scholar] [CrossRef]
- Al Yahya, S.; Iqbal, T.; Omar, M.M.; Ahmad, M. Techno-Economic Analysis of Fast Pyrolysis of Date Palm Waste for Adoption in Saudi Arabia. Energies 2021, 14, 6048. [Google Scholar] [CrossRef]
- Ma, Y.; Tian, H.; Cheng, H.; Xuan, Y.; Shang, L.; Yang, Y. The Economic and Environmental Sustainability of Converting Miscanthus to Hydrocarbon Biofuel by Pyrolysis and Catalytic Hydrotreatment. Biomass Bioenergy 2024, 181, 107041. [Google Scholar] [CrossRef]
- Brigagão, G.V.; de Queiroz Fernandes Araújo, O.; de Medeiros, J.L.; Mikulcic, H.; Duic, N. A Techno-Economic Analysis of Thermochemical Pathways for Corncob-to-Energy: Fast Pyrolysis to Bio-Oil, Gasification to Methanol and Combustion to Electricity. Fuel Process. Technol. 2019, 193, 102–113. [Google Scholar] [CrossRef]
- Ramirez, J.A.; Rainey, T.J. Comparative Techno-Economic Analysis of Biofuel Production through Gasification, Thermal Liquefaction and Pyrolysis of Sugarcane Bagasse. J. Clean. Prod. 2019, 229, 513–527. [Google Scholar] [CrossRef]
- Pighinelli, A.L.M.T.; Schaffer, M.A.; Boateng, A.A. Utilization of Eucalyptus for Electricity Production in Brazil via Fast Pyrolysis: A Techno-Economic Analysis. Renew. Energy 2018, 119, 590–597. [Google Scholar] [CrossRef]
- Wang, W.C.; Jan, J.J. From Laboratory to Pilot: Design Concept and Techno-Economic Analyses of the Fluidized Bed Fast Pyrolysis of Biomass. Energy 2018, 155, 139–151. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, H.; Xiao, R. Mobile Autothermal Pyrolysis System for Local Biomass Conversion: Process Simulation and Techno-Economic Analysis. Energy Fuels 2018, 32, 4178–4188. [Google Scholar] [CrossRef]
- Khan, S.R.; Ciolkosz, D.; Vasco-Correa, J.; Zeeshan, M. A Techno-Economic Study to Evaluate the Impacts of Feedstock Ratio on Commercial Scale Co-Pyrolysis Plants of Biomass and Waste Tire. J. Anal. Appl. Pyrolysis 2022, 167, 105699. [Google Scholar] [CrossRef]
- O’Boyle, M.; Mohamed, B.A.; Li, L.Y. Co-Pyrolysis of Sewage Sludge and Biomass Waste into Biofuels and Biochar: A Comprehensive Feasibility Study Using a Circular Economy Approach. Chemosphere 2024, 350, 141074. [Google Scholar] [CrossRef]



| Main Research Topics | References |
|---|---|
| Biomass pyrolysis for energy recovery, recent advances, feedstock compositions, techno-economic analyses, and future research paths. | [14] |
| Pyrolysis reaction mechanisms, process modelling, and challenges (e.g., aerosol and tar formation), enhancing process control and performance. | [15] |
| Hydrogen production via pyrolysis, polygeneration systems, impact of reaction conditions on hydrogen and chemical formation, and two-stage pyrolysis. | [16] |
| Role of catalysts in pyrolysis and benefits and drawbacks of catalytic pyrolysis by combining different catalysts for enhanced performance. | [17] |
| Catalytic fast pyrolysis (CFP), optimization of bio-oil production, challenges in CFP, advanced upgrading methods, and applications of CFP products. | [18] |
| Production and properties of activated carbon (AC) from biomass pyrolysis, and applications of AC in the adsorption of pollutants and gases. | [19] |
| Production and applications of char from waste biomass, environmental remediation, engineering aspects, and water treatment. | [20] |
| Directional pyrolysis based on the element economy (carbon, hydrogen, oxygen, and nitrogen), the production of high-quality fuels, chemicals, carbon materials, and the enviro-economic assessment. | [21] |
| Advanced technologies for biomass conversion to biofuels, thermochemical and biochemical methods, efficiency improvements, and environmental impact. | [22] |
| Research Topic | [14] | [15] | [16] | [17] | [18] | [19] | [20] | [21] | [22] | This Review |
|---|---|---|---|---|---|---|---|---|---|---|
| Conventional Pyrolysis | ✓ | ✓ | ✗ | ✓ | ✓ | ✗ | ✓ | ✗ | ✓ | ✓ |
| Reactors Design | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✗ | ✓ |
| Advanced Technologies | ✓ | ✓ | ✓ | ✓ | ✓ | ✗ | ✗ | ✓ | ✓ | ✓ |
| Product Compositions and Yields | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Techno-economic Analysis | ✓ | ✓ | ✓ | ✗ | ✗ | ✗ | ✗ | ✓ | ✗ | ✓ |
| Future Outlooks | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ |
| Pyrolysis Type | HR, °C/s | T, °C | HVRT, s | SRT, min | PS, mm | CG, - | References |
|---|---|---|---|---|---|---|---|
| Slow | 0.07 | 400, 500 | 7200 a | 120 | <0.5 | N2 ᵇ | [34] |
| 0.07 | 500 | 1800 a | 30 | 2 | N2 ᵇ | [35] | |
| 0.08–0.33 | 700 | — | 71–310 | 0.075–0.25 | N2:H2 (1:1) | [36] | |
| 0.083 | 600 | — | 120 | 0.075–0.15 | N2 | [37] | |
| 0.083 | 400 | — | 740 | ~2 | N2 | [38] | |
| 0.083 | 900 | — | 180 | 19 | N2 | [39] | |
| 0.17 | 300–500 | 3600 a | 60 | <2 | N2ᵇ | [40] | |
| 0.17 | 400, 600, 800 | — | 30–60 | 0.7–0.8 | N2 | [41] | |
| 0.25 | 300–600 | — | 1.7 | 1–2 | Ar | [42] | |
| 0.33 | 500, 700 | — | 55–65 | — | N2 | [43] | |
| – | 450–650 | 1–60 | 1–20 | 0.25–1.4 | N2 | [44] | |
| Intermediate | 0.42 | 500 | 17 c | — | 0.5–1 | N2 | [45] |
| 0.5 | 500 | — | — | — | N2 | [46] | |
| 0.5, 1, 5 | 300–550 | — | — | 0.18–0.25 | N2 | [47] | |
| 0.55 | 600 | — | 78 | 1.18 | N2, CO2, and N2:CO2 (3:1) | [48] | |
| 0.83, 1.33, 1.67 | 350–600 | — | 10 | 0.1–0.2 | N2 | [49] | |
| 0.83, 2.5, 4.17 | 400–700 | — | — | <1.0 | Ar | [50] | |
| 1.2 | 500 | 20 | 7 | 0.3–0.75 | N2 | [51] | |
| 1.67 | 500 | <20 | 1.2 | 0.5–1.0 | N2 | [52] | |
| 1.67 | 400–700 | — | 9–12 | 0.4–0.92 | N2 | [53] | |
| 1.67, 5 | 400–700 | — | 10 | 0.85–1.25 | N2 | [54] | |
| 3.33 | 350–500 | — | 2.5–40 | — | flue gas | [55] | |
| — | 450 | 2 | 10 | — | N2 | [56] | |
| — | 350–500 | 20–40 | 10 | 2–4.5 | N2 | [57] | |
| Intermediate and Fast | 1.5–48 | 500 | 0.5–0.8 | — | 2–3 | N2 | [58] |
| 5, 8.33, 11.6 | 400–700 | — | 10 | ~0.85 | N2 | [59] | |
| 5–100 | 550–800 | — | — | 0.25–0.43 | N2 | [60] | |
| 6.67 | 500 | — | 1 | <0.5 | N2 | [61] | |
| 10 | 450–550 | 3.6 | 0.2–0.5 | N2 | [62] | ||
| Fast | 48 | 480–790 | — | — | <1 | He | [63] |
| 110 | 500 | — | 0.072 | <2 | N2 | [64] | |
| 166.7 | 500 | <1 | — | 0.5–1 | N2 | [52] | |
| 180 | 765 | 0.1 | — | — | flue gas | [46] | |
| 200 | 300–600 | 0.5–1 | 0.025 | 0.175 | He | [65] | |
| 300 | 300–750 | 0.5–1 | — | <1 | N2 | [66] | |
| 400 | 400–600 | 1.2–12 | — | 0.2 | N2 | [67] | |
| 1000 | 400–900 | — | — | 5 | N2 | [68] | |
| — | 500 | 3.3 | 10 | 0.09–0.180 | N2:air (2:3) | [69] | |
| Fast and Flash | 10–3000 | 350–1400 | — | 0.017–0.067 | 0.05–2 | N2 | [70] |
| 100, 1000, 5000, 10,000 | 600 | — | 0.17 | 0.4 | He | [71] | |
| 600–2200 | 250–610 | 0.2–0.32 | — | 0.84–1 | N2 | [72] | |
| Flash | 2500, 7000 | 500 | 0.015–0.025 | 0.016 | 0.074 | N2 | [73] |
| 3826–4578 | 439–521 | — | — | <2 | N2 | [74] | |
| 5000 | 485, 515 | 0.02 | 0.083 | <0.15 | N2 | [75] | |
| 5000 | 485 | 1 | — | 1–2 | N2 | [75] | |
| 10,000 | 500 | — | 0.34 | 0.15 | N2 | [76] | |
| 11,875 | 400–500 | 0.05–2 | — | <0.07 | N2 | [77] | |
| 13,000–21,000 | 477–627 | 0.115–0.240 | — | 0.05–0.07 | Ar | [78] | |
| 20,000 | 520 | — | 0.25 | 0.15–0.25 | He | [79] | |
| 20,000 | 300–500 | — | — | 0.5–1.5 | He | [80] |
| Pyrolysis Type | Biomass or Raw Material | Feed Rate | Reactor Type | Payback Period in Years | Economic Analysis Findings | Cost of Bio-Char/Bio-Oil | Refs. |
|---|---|---|---|---|---|---|---|
| Slow | Cassava branches (CB) and peels (CP) | 2.9 t/h (CB) 0.2 t/h (CP) | Muffle | 5 (CB) 8 (CP) | Economic feasibility is achieved with processing scales above 3.4 t/h. Competitive selling price if feedstock cost reduced >40%. | Biochar: USD 1.6/kg (target USD 1.25/kg for CB) | [176] |
| Slow | Coffee pulp | 30 L, 100 L and 200 L batch | BR | 2 | Highest NPV with 200 L batch at USD 9781. Profit in 10 years: USD 15,399 | Biochar: USD 0.7/kg Bio-oil: USD 1.03/kg | [177] |
| Slow | Cellulose, hemicellulose, lignin | 100 t/h | CSTR | — | The revenues from char production show a positive net profit for all pyrolysis cases, with profits exceeding USD 90/t for lignin, cellulose, and hemicellulose. | Biochar: USD 110/t (lignin), USD 285/t (cellulose), USD 296/t (hemicellulose) | [178] |
| Slow | Madhuca indica tree | — | CSTR | 3.14 | At the targeted interest rate of 20%, the total capital cost was found to be USD 10,753,500 per year. | Bio-oil: USD 1.11/kg | [179] |
| Slow | Swine manure | 1 t/h | — | 4.6 | If the selling price of biochar decreases by 20%, the investment payback period extends beyond 8 years. | Biochar: USD 116/t Bio-oil: USD 154/t | [180] |
| Intermediate | Forest residues | 338–2549 t/day | RK | — | The MSP (10% IRR) of upgraded bio-oil was more than double that of crude bio-oil. Economy-of-scale benefits are evident. | Bio-oil: USD 0.71/L (crude), USD 1.25/L (upgraded) | [181] |
| Fast | Date palm waste | 10 t/day | BFB | 2.57 | Net savings: USD 556.8/t of waste. Potential earnings of USD 44.8 million annually with 50% waste processed | — | [182] |
| Fast | Miscanthus | 2000 t/day | BFB | 7.6–8.9 | The best economic performance with IRR: 11.3%, ROI: 13.1%. | Bio-oil: CNY 6.89/L | [183] |
| Fast | Corncob | 96.8 t/h | CFB | 13 | Pyrolysis shows a positive NPV, provided the biomass cost is below USD 75.5/t. | Bio-oil: USD 1.47/gasoline gallon equivalent | [184] |
| Fast | Sugarcane bagasse | 10 t/h | SBR | — | The cost to build the pyrolysis plant was USD 52 million. The NPV was negative with USD 65.7 million. | Bio-oil: USD 1.19/L | [185] |
| Fast | Eucalyptus | 2000 t/day | BFB | 10 | MSPs for bio-oil: USD 1.04/L (single facility), USD 0.58/L (distributed). Single facility more economically favorable. | Bio-oil: USD 1.04/L (single), USD 0.58/L (distributed) | [186] |
| Fast | Rice husk | 1000 t/day | BFB | — | Double the profit results in 57% higher of the selling price. 75% of operating cost is on utilities. | Bio-oil: USD 0.55/L | [187] |
| Fast | Forestry and agricultural residues | 100 kg/h (mobile) 4000 kg/h (fixed) | BFB | 6 years (mobile) | Total capital investment: CNY 0.86 million (mobile). | Biochar: CNY 1.2/kg, Bio-oil: CNY 1.25 /kg. | [188] |
| Fast | Rice straw (RS), waste tire (WT) | 20 t/h | BFB | 6.23 | Plant (20% RS, 80% WT) most economical: NPV, USD 5.63 million. | Biochar (20% RS, 80% WT): USD 0.07/kg, Bio-oil: USD 0.36/kg. | [189] |
| Fast | Sewage sludge (SS), wheat straw (WS), sawdust (SD) | 1.2–4.0 t/campaign | — | — | Net present worth (40% SD, 60% SS): CAD 8.71 million. Single pyrolysis of SS not profitable. | SS Biochar: (CAD 1.33/kg) and from WS (CAD 4.99/kg). | [190] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerzak, W.; Acha, E.; Li, B. Comprehensive Review of Biomass Pyrolysis: Conventional and Advanced Technologies, Reactor Designs, Product Compositions and Yields, and Techno-Economic Analysis. Energies 2024, 17, 5082. https://doi.org/10.3390/en17205082
Jerzak W, Acha E, Li B. Comprehensive Review of Biomass Pyrolysis: Conventional and Advanced Technologies, Reactor Designs, Product Compositions and Yields, and Techno-Economic Analysis. Energies. 2024; 17(20):5082. https://doi.org/10.3390/en17205082
Chicago/Turabian StyleJerzak, Wojciech, Esther Acha, and Bin Li. 2024. "Comprehensive Review of Biomass Pyrolysis: Conventional and Advanced Technologies, Reactor Designs, Product Compositions and Yields, and Techno-Economic Analysis" Energies 17, no. 20: 5082. https://doi.org/10.3390/en17205082
APA StyleJerzak, W., Acha, E., & Li, B. (2024). Comprehensive Review of Biomass Pyrolysis: Conventional and Advanced Technologies, Reactor Designs, Product Compositions and Yields, and Techno-Economic Analysis. Energies, 17(20), 5082. https://doi.org/10.3390/en17205082







