Impact of Organic Load on Methane Yields and Kinetics during Anaerobic Digestion of Sugarcane Bagasse: Optimal Feed-to-Inoculum Ratio and Total Solids of Reactor Working Volume
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Biochemical Methane Potential Test
2.3. Kinetic Modelling
2.4. Theoretical Methane Yield and Experimental Biodegradability
2.5. Analytical Methods
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition of Substrate and Inoculum
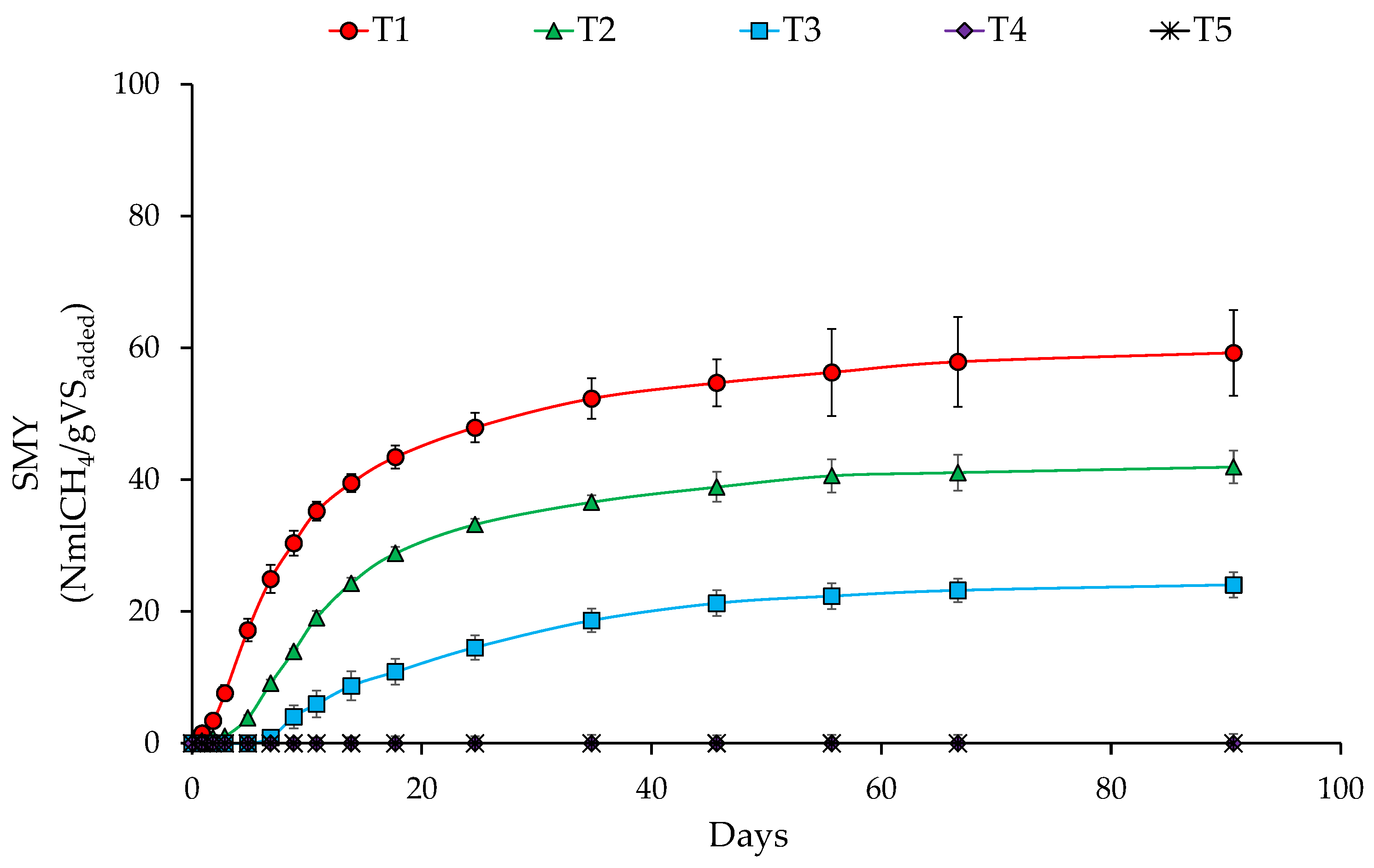
3.2. Effect of Organic Load on SMY of SB
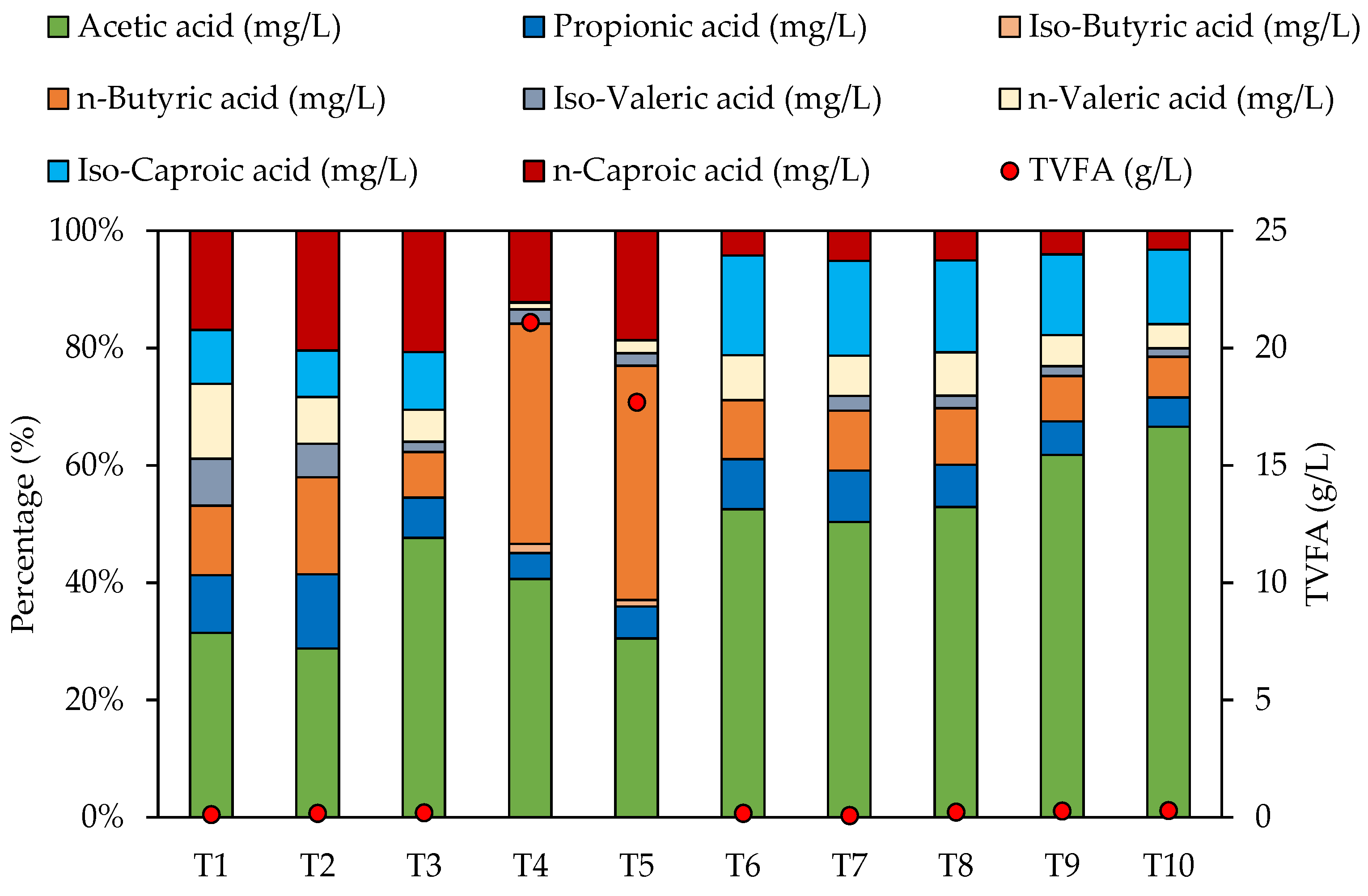
3.3. Effect of Organic Load on Chemical Composition of Digestates
3.4. Effect of Organic Load on Biodegradability Index and VS Removal
3.5. Effect of Organic Load on Methane Production Kinetics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pan, S.; Zabed, H.M.; Wei, Y.; Qi, X. Technoeconomic and environmental perspectives of biofuel production from sugarcane bagasse: Current status, challenges and future outlook. Ind. Crop. Prod. 2022, 188, 115684. [Google Scholar] [CrossRef]
- Toscano Miranda, N.; Lopes Motta, I.; Maciel Filho, R.; Wolf Maciel, M.R. Sugarcane bagasse pyrolysis: A review of operating conditions and products properties. Renew. Sustain. Energy Rev. 2021, 149, 111394. [Google Scholar] [CrossRef]
- Paulose, P.; Kaparaju, P. Anaerobic mono-digestion of sugarcane trash and bagasse with and without pretreatment. Ind. Crop. Prod. 2021, 170, 113712. [Google Scholar] [CrossRef]
- Gabov, K.; Hemming, J.; Fardim, P. Sugarcane bagasse valorization by fractionation using a water-based hydrotropic process. Ind. Crop. Prod. 2017, 108, 495–504. [Google Scholar] [CrossRef]
- Mukherjee, T.; Trably, E.; Kaparaju, P. Critical Assessment of Hydrogen and Methane Production from 1G and 2G Sugarcane Processing Wastes Using One-Stage and Two-Stage Anaerobic Digestion. Energies 2023, 16, 4919. [Google Scholar] [CrossRef]
- Sahu, O. Assessment of sugarcane industry: Suitability for production, consumption, and utilization. Ann. Agrar. Sci. 2018, 16, 389–395. [Google Scholar] [CrossRef]
- George, P.; Cabello Eras, J.; Sagastume, A.; Hens, L.; Vandecasteele, C. Residue from Sugarcane Juice Filtration (Filter Cake): Energy Use at the Sugar Factory. Waste Biomass Valorization 2010, 1, 407–413. [Google Scholar] [CrossRef]
- Prasara-A, J.; Gheewala, S.H.; Silalertruksa, T.; Pongpat, P.; Sawaengsak, W. Environmental and social life cycle assessment to enhance sustainability of sugarcane-based products in Thailand. Clean Technol. Environ. Policy 2019, 21, 1447–1458. [Google Scholar] [CrossRef]
- Cai, M.; Javed, J.; Wu, H.; Zhou, Y.; Liyang, H.; Yang, C.; Tsui, T.-H.; Song, B.; Zhang, Q. Valorizing waste activated sludge incineration ash to S-doped Fe2+@Zeolite 4A catalyst for the treatment of emerging contaminants exemplified by sulfamethoxazole. J. Environ. Manag. 2024, 369, 122382. [Google Scholar] [CrossRef]
- Arelli, V.; Mamindlapelli, N.K.; Juntupally, S.; Begum, S.; Anupoju, G.R. Solid-state anaerobic digestion of sugarcane bagasse at different solid concentrations: Impact of bio augmented cellulolytic bacteria on methane yield and insights on microbial diversity. Bioresour. Technol. 2021, 340, 125675. [Google Scholar] [CrossRef]
- Zhu, J.; Zheng, Y.; Xu, F.; Li, Y. Solid-state anaerobic co-digestion of hay and soybean processing waste for biogas production. Bioresour. Technol. 2014, 154, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Lehtomäki, A.; Huttunen, S.; Lehtinen, T.M.; Rintala, J.A. Anaerobic digestion of grass silage in batch leach bed processes for methane production. Bioresour. Technol. 2008, 99, 3267–3278. [Google Scholar] [CrossRef] [PubMed]
- Sawatdeenarunat, C.; Surendra, K.C.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic digestion of lignocellulosic biomass: Challenges and opportunities. Bioresour. Technol. 2015, 178, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.M.; Tyagi, V.K.; Ahmed, B.; Kazmi, A.; Ojha, C.S.P.; Singh, R. Critical insights into anaerobic co-digestion of wheat straw with food waste and cattle manure: Synergistic effects on biogas yield and kinetic modeling. Environ. Res. 2022, 212, 113382. [Google Scholar] [CrossRef]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Caramiello, C.; Lancellotti, I.; Righi, F.; Tatàno, F.; Taurino, R.; Barbieri, L. Anaerobic digestion of selected Italian agricultural and industrial residues (grape seeds and leather dust): Combined methane production and digestate characterization. Environ. Technol. 2013, 34, 1225–1237. [Google Scholar] [CrossRef]
- Sarode, S.D.; Kumar, D.; Mathias, D.; McNeill, D.; Kaparaju, P. Anaerobic Digestion of Spoiled Maize, Lucerne and Barley Silage Mixture with and without Cow Manure: Methane Yields and Kinetic Studies. Energies 2023, 16, 6179. [Google Scholar] [CrossRef]
- Agarwal, N.K.; Kumar, M.; Ghosh, P.; Kumar, S.S.; Singh, L.; Vijay, V.K.; Kumar, V. Anaerobic digestion of sugarcane bagasse for biogas production and digestate valorization. Chemosphere 2022, 295, 133893. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhao, J.; Xu, F.; Li, Y. Pretreatment of lignocellulosic biomass for enhanced biogas production. Prog. Energy Combust. Sci. 2014, 42, 35–53. [Google Scholar] [CrossRef]
- Vivekanand, V.; Olsen, E.F.; Eijsink, V.G.; Horn, S.J. Methane potential and enzymatic saccharification of steam-exploded bagasse. BioResources 2014, 9, 1311–1324. [Google Scholar] [CrossRef]
- Dastyar, W.; Azizi, S.M.M.; Dhadwal, M.; Dhar, B.R. High-solids anaerobic digestion of organic fraction of municipal solid waste: Effects of feedstock to inoculum ratio and percolate recirculation time. Bioresour. Technol. 2021, 337, 125335. [Google Scholar] [CrossRef] [PubMed]
- Carlos-Pinedo, S.; Wang, Z.; Eriksson, O. Methane yield from SS-AD: Experiences to learn by a full spectrum analysis at laboratory-, pilot-and full-scale. Biomass Bioenergy 2019, 127, 105270. [Google Scholar] [CrossRef]
- Abid, M.; Wu, J.; Seyedsalehi, M.; Hu, Y.-y.; Tian, G. Novel insights of impacts of solid content on high solid anaerobic digestion of cow manure: Kinetics and microbial community dynamics. Bioresour. Technol. 2021, 333, 125205. [Google Scholar] [CrossRef]
- Arelli, V.; Mamindlapelli, N.K.; Begum, S.; Juntupally, S.; Anupoju, G.R. Solid state anaerobic digestion of food waste and sewage sludge: Impact of mixing ratios and temperature on microbial diversity, reactor stability and methane yield. Sci. Total Environ. 2021, 793, 148586. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Zhang, D.; Li, G.; Lu, J.; Li, S. Solid state anaerobic co-digestion of tomato residues with dairy manure and corn stover for biogas production. Bioresour. Technol. 2016, 217, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Tang, F.; Lin, Y.; Xu, Z.; Xie, Z.; Tian, J. Solid-state anaerobic digestion of rice straw pretreated with swine manure digested effluent. J. Clean. Prod. 2022, 348, 131252. [Google Scholar] [CrossRef]
- Xu, F.; Li, Y. Solid-state co-digestion of expired dog food and corn stover for methane production. Bioresour. Technol. 2012, 118, 219–226. [Google Scholar] [CrossRef]
- Paritosh, K.; Yadav, M.; Kesharwani, N.; Pareek, N.; Karthyikeyan, O.P.; Balan, V.; Vivekanand, V. Strategies to improve solid state anaerobic bioconversion of lignocellulosic biomass: An overview. Bioresour. Technol. 2021, 331, 125036. [Google Scholar] [CrossRef]
- Ghimire, A.; Trably, E.; Frunzo, L.; Pirozzi, F.; Lens, P.N.; Esposito, G.; Cazier, E.A.; Escudié, R. Effect of total solids content on biohydrogen production and lactic acid accumulation during dark fermentation of organic waste biomass. Bioresour. Technol. 2018, 248, 180–186. [Google Scholar] [CrossRef]
- Paul, S.; Dutta, A. Challenges and opportunities of lignocellulosic biomass for anaerobic digestion. Resour. Conserv. Recycl. 2018, 130, 164–174. [Google Scholar] [CrossRef]
- Yan, J.; Zhao, Y.; He, H.; Cai, Y.; Zhao, Y.; Wang, H.; Zhu, W.; Yuan, X.; Cui, Z. Anaerobic co-digestion of dairy manure and maize stover with different total solids content: From the characteristics of digestion to economic evaluation. J. Environ. Chem. Eng. 2022, 10, 107602. [Google Scholar] [CrossRef]
- Gandhi, B.P.; Otite, S.V.; Fofie, E.A.; Lag-Brotons, A.J.; Ezemonye, L.I.; Semple, K.T.; Martin, A.D. Kinetic investigations into the effect of inoculum to substrate ratio on batch anaerobic digestion of simulated food waste. Renew. Energy 2022, 195, 311–321. [Google Scholar] [CrossRef]
- Wang, Z.; Jiang, Y.; Wang, S.; Zhang, Y.; Hu, Y.; Hu, Z.-h.; Wu, G.; Zhan, X. Impact of total solids content on anaerobic co-digestion of pig manure and food waste: Insights into shifting of the methanogenic pathway. Waste Manag. 2020, 114, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Motte, J.C.; Escudié, R.; Bernet, N.; Delgenes, J.P.; Steyer, J.P.; Dumas, C. Dynamic effect of total solid content, low substrate/inoculum ratio and particle size on solid-state anaerobic digestion. Bioresour. Technol. 2013, 144, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Jia, Z.; Wang, P.; Yang, X.; Lin, P.; Ren, L.; Farghali, M. Restoration of acidified dry anaerobic digestion of food waste: Bioaugmentation of butyric acid-resistant microbes. J. Environ. Chem. Eng. 2022, 10, 106935. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, Y.; Wang, S.; Wu, G.; Zhan, X. A critical review on dry anaerobic digestion of organic waste: Characteristics, operational conditions, and improvement strategies. Renew. Sustain. Energy Rev. 2023, 176, 113208. [Google Scholar] [CrossRef]
- Ajayi-Banji, A.; Rahman, S. A review of process parameters influence in solid-state anaerobic digestion: Focus on performance stability thresholds. Renew. Sustain. Energy Rev. 2022, 167, 112756. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, C.; Nges, I.A.; Liu, J. The effects of pre-aeration and inoculation on solid-state anaerobic digestion of rice straw. Bioresour. Technol. 2017, 224, 78–86. [Google Scholar] [CrossRef]
- Li, Y.; Park, S.Y.; Zhu, J. Solid-state anaerobic digestion for methane production from organic waste. Renew. Sustain. Energy Rev. 2011, 15, 821–826. [Google Scholar] [CrossRef]
- Brown, D.; Li, Y. Solid state anaerobic co-digestion of yard waste and food waste for biogas production. Bioresour. Technol. 2013, 127, 275–280. [Google Scholar] [CrossRef]
- Rocamora, I.; Wagland, S.T.; Villa, R.; Simpson, E.W.; Fernández, O.; Bajón-Fernández, Y. Dry anaerobic digestion of organic waste: A review of operational parameters and their impact on process performance. Bioresour. Technol. 2020, 299, 122681. [Google Scholar] [CrossRef] [PubMed]
- Rouches, E.; Escudié, R.; Latrille, E.; Carrère, H. Solid-state anaerobic digestion of wheat straw: Impact of S/I ratio and pilot-scale fungal pretreatment. Waste Manag. 2019, 85, 464–476. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Dennehy, C.; Lawlor, P.G.; Hu, Z.; McCabe, M.; Cormican, P.; Zhan, X.; Gardiner, G.E. Inhibition of volatile fatty acids on methane production kinetics during dry co-digestion of food waste and pig manure. Waste Manag. 2018, 79, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Basinas, P.; Rusín, J.; Chamrádová, K. Assessment of high-solid mesophilic and thermophilic anaerobic digestion of mechanically-separated municipal solid waste. Environ. Res. 2021, 192, 110202. [Google Scholar] [CrossRef] [PubMed]
- Achinas, S.; Li, Y.; Achinas, V.; Euverink, G.J.W. Biogas potential from the anaerobic digestion of potato peels: Process performance and kinetics evaluation. Energies 2019, 12, 2311. [Google Scholar] [CrossRef]
- Nielfa, A.; Cano, R.; Fdz-Polanco, M. Theoretical methane production generated by the co-digestion of organic fraction municipal solid waste and biological sludge. Biotechnol. Rep. 2015, 5, 14–21. [Google Scholar] [CrossRef]
- Yasim, N.S.E.M.; Buyong, F. Comparative of experimental and theoretical biochemical methane potential generated by municipal solid waste. Environ. Adv. 2023, 11, 100345. [Google Scholar] [CrossRef]
- Conde, E.; Kaparaju, P. Effect of Temporal Variation in Chemical Composition on Methane Yields of Rendering Plant Wastewater. Energies 2022, 15, 7252. [Google Scholar] [CrossRef]
- Association, A.P.H.; Association, A.P.H. Standard methods for the Examination ofWater andWastewater, APHA. In American Water Works Association and Water Environment Federation, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Kruk, D.; Elektorowicz, M.; Oleszkiewicz, J. Struvite Precipitation and Phosphorus Removal Using Magnesium Sacrificial Anode. Chemosphere 2013, 101, 28–33. [Google Scholar] [CrossRef]
- Bressy, F.C.; Brito, G.B.; Barbosa, I.S.; Teixeira, L.S.G.; Korn, M.G.A. Determination of trace element concentrations in tomato samples at different stages of maturation by ICP OES and ICP-MS following microwave-assisted digestion. Microchem. J. 2013, 109, 145–149. [Google Scholar] [CrossRef]
- Trzcinski, A.P.; Stuckey, D.C. Treatment of municipal solid waste leachate using a submerged anaerobic membrane bioreactor at mesophilic and psychrophilic temperatures: Analysis of recalcitrants in the permeate using GC-MS. Water Res. 2010, 44, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, R.; Liu, X.; Chen, C.; Xiao, X.; Feng, L.; He, Y.; Liu, G. Evaluating Methane Production from Anaerobic Mono- and Co-digestion of Kitchen Waste, Corn Stover, and Chicken Manure. Energy Fuels 2013, 27, 2085–2091. [Google Scholar] [CrossRef]
- Anderson, R.; Conway, H.; Pfeifer, V.; Griffin, L.E.J. Roll and extrusion-cooking of grain sorghum grits. Cereal Sci. Today 1969, 14, 372–375. [Google Scholar]
- Flores-Maltos, D.A.; Mussatto, S.I.; Contreras Esquivel, J.; Buenrostro, J.J.; Rodríguez, R.; Teixeira, J.A.; Aguilar, C.N. Typical Mexican agroindustrial residues as supports for solid-state fermentation. Am. J. Agric. Biol. Sci. 2014, 9, 289–293. [Google Scholar] [CrossRef][Green Version]
- Orzua, M.C.; Mussatto, S.I.; Contreras-Esquivel, J.C.; Rodriguez, R.; de la Garza, H.; Teixeira, J.A.; Aguilar, C.N. Exploitation of agro industrial wastes as immobilization carrier for solid-state fermentation. Ind. Crop. Prod. 2009, 30, 24–27. [Google Scholar] [CrossRef]
- Mathias, D.J.; Edwiges, T.; Ketsub, N.; Singh, R.; Kaparaju, P. Sweet Sorghum as a Potential Fallow Crop in Sugarcane Farming for Biomethane Production in Queensland, Australia. Energies 2023, 16, 6497. [Google Scholar] [CrossRef]
- Benbelkacem, H.; Bollon, J.; Bayard, R.; Escudié, R.; Buffière, P. Towards optimization of the total solid content in high-solid (dry) municipal solid waste digestion. Chem. Eng. J. 2015, 273, 261–267. [Google Scholar] [CrossRef]
- Bollon, J.; Benbelkacem, H.; Gourdon, R.; Buffière, P. Measurement of diffusion coefficients in dry anaerobic digestion media. Chem. Eng. Sci. 2013, 89, 115–119. [Google Scholar] [CrossRef]
- Khadka, A.; Parajuli, A.; Dangol, S.; Thapa, B.; Sapkota, L.; Carmona-Martínez, A.A.; Ghimire, A. Effect of the substrate to inoculum ratios on the kinetics of biogas production during the mesophilic anaerobic digestion of food waste. Energies 2022, 15, 834. [Google Scholar] [CrossRef]
- Capson-Tojo, G.; Trably, E.; Rouez, M.; Crest, M.; Steyer, J.-P.; Delgenès, J.-P.; Escudié, R. Dry anaerobic digestion of food waste and cardboard at different substrate loads, solid contents and co-digestion proportions. Bioresour. Technol. 2017, 233, 166–175. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Poulsen, T.G.; Xia, Y.; Sheng, K. Combinations of fungal and milling pretreatments for enhancing rice straw biogas production during solid-state anaerobic digestion. Bioresour. Technol. 2017, 224, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Neves, L.; Oliveira, R.; Alves, M. Influence of inoculum activity on the bio-methanization of a kitchen waste under different waste/inoculum ratios. Process Biochem. 2004, 39, 2019–2024. [Google Scholar] [CrossRef]
- Mitra, S.; Kaparaju, P. Feasibility of Food Organics and Garden Organics as a Promising Source of Biomethane: A Review on Process Optimisation and Impact of Nanomaterials. Energies 2024, 17, 4198. [Google Scholar] [CrossRef]
- Abbassi-Guendouz, A.; Brockmann, D.; Trably, E.; Dumas, C.; Delgenès, J.-P.; Steyer, J.-P.; Escudié, R. Total solids content drives high solid anaerobic digestion via mass transfer limitation. Bioresour. Technol. 2012, 111, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Pastor-Poquet, V.; Papirio, S.; Steyer, J.-P.; Trably, E.; Escudié, R.; Esposito, G. High-solids anaerobic digestion model for homogenized reactors. Water Res. 2018, 142, 501–511. [Google Scholar] [CrossRef]
- Motte, J.-C.; Trably, E.; Escudié, R.; Hamelin, J.; Steyer, J.-P.; Bernet, N.; Delgenes, J.-P.; Dumas, C. Total solids content: A key parameter of metabolic pathways in dry anaerobic digestion. Biotechnol. Biofuels 2013, 6, 164. [Google Scholar] [CrossRef]
- Kaparaju, P.; Rintala, J. Anaerobic co-digestion of potato tuber and its industrial by-products with pig manure. Resour. Conserv. Recycl. 2005, 43, 175–188. [Google Scholar] [CrossRef]
- Li, D.; Liu, S.; Mi, L.; Li, Z.; Yuan, Y.; Yan, Z.; Liu, X. Effects of feedstock ratio and organic loading rate on the anaerobic mesophilic co-digestion of rice straw and cow manure. Bioresour. Technol. 2015, 189, 319–326. [Google Scholar] [CrossRef]
- Benabdallah El Hadj, T.; Astals, S.; Galí, A.; Mace, S.; Mata-Álvarez, J. Ammonia influence in anaerobic digestion of OFMSW. Water Sci. Technol. 2009, 59, 1153–1158. [Google Scholar] [CrossRef]
- Liew, L.N.; Shi, J.; Li, Y. Methane production from solid-state anaerobic digestion of lignocellulosic biomass. Biomass Bioenergy 2012, 46, 125–132. [Google Scholar] [CrossRef]
- de Albuquerque, F.P.; Dastyar, W.; Azizi, S.M.M.; Zakaria, B.S.; Kumar, A.; Dhar, B.R. Carbon cloth amendment for boosting high-solids anaerobic digestion with percolate recirculation: Spatial patterns of microbial communities. Chemosphere 2022, 307, 135606. [Google Scholar] [CrossRef] [PubMed]
- Dastyar, W.; Mirsoleimani Azizi, S.M.; Meshref, M.N.A.; Dhar, B.R. Powdered activated carbon amendment in percolate tank enhances high-solids anaerobic digestion of organic fraction of municipal solid waste. Process Saf. Environ. Prot. 2021, 151, 63–70. [Google Scholar] [CrossRef]
- Li, Y.; Jin, Y.; Borrion, A.; Li, J. Influence of feed/inoculum ratios and waste cooking oil content on the mesophilic anaerobic digestion of food waste. Waste Manag. 2018, 73, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Dong, B.; Jin, J.W.; Dai, X.H. Effect of Increasing Total Solids Contents on Anaerobic Digestion of Food Waste under Mesophilic Conditions: Performance and Microbial Characteristics Analysis. PLoS ONE 2014, 9, e0102548. [Google Scholar] [CrossRef]



| Treatments | F/I Ratio | TS (%) | Organic Load (gVS/L) |
|---|---|---|---|
| T1 | 0.3 | 10 | 19.9 |
| T2 | 0.5 | 10 | 28.5 |
| T3 | 1.0 | 10 | 50.0 |
| T4 | 2.0 | 10 | 80.3 |
| T5 | 3.0 | 10 | 106.1 |
| T6 | 1.0 | 3 | 13.6 |
| T7 | 1.0 | 8 | 40.3 |
| T8 | 1.0 | 12 | 65.2 |
| T9 | 1.0 | 14 | 88.4 |
| T10 | 1.0 | 16 | 110.0 |
| Parameter | SB | LI | SI |
|---|---|---|---|
| TS (%ww) | 80.9 | 2.8 | 20.4 |
| VS (%ww) | 72.3 | 2.0 | 14.6 |
| VS/TS | 0.9 | 0.7 | 0.7 |
| Density (g/mL) | 0.2 | 1.0 | 1.0 |
| TVFA (g/L) | NA | 0.2 | 0.6 |
| WAI (g/gTS) | 10.5 | NA | NA |
| TKP (gP/kgTS) | 0.2 | 27.7 | 24.7 |
| TKN (gN/kgTS) | 2.1 | 60.4 | 53.5 |
| Parameter | SB |
|---|---|
| Carbon (%TS) | 44.5 |
| Hydrogen (%TS) | 5.7 |
| Nitrogen (%TS) | 0.5 |
| Oxygen (%TS) | 44.4 |
| Sulphur (%TS) | 0.1 |
| Parameter | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 |
|---|---|---|---|---|---|---|---|---|---|---|
| TS (%w/w) | 8.93 | 9.18 | 8.13 | 10.06 | 10.49 | 3.02 | 8.83 | 16.45 | 21.69 | 26.97 |
| VS (%w/w) | 6.02 | 6.31 | 5.74 | 8.22 | 8.97 | 2.30 | 6.57 | 12.53 | 17.13 | 21.36 |
| VS/TS | 0.67 | 0.69 | 0.71 | 0.82 | 0.85 | 0.76 | 0.74 | 0.76 | 0.79 | 0.79 |
| pH | 7.95 | 7.92 | 7.81 | 5.19 | 5.25 | 7.66 | 7.79 | 8.22 | 8.40 | 8.47 |
| NO2-N (mg/L) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 10.10 | 5.06 | 11.80 | 9.94 | 5.03 |
| NO3-N (mg/L) | 253.05 | 249.58 | 340.47 | 327.39 | 287.91 | 372.12 | 166.14 | 440.43 | 299.78 | 430.74 |
| NH4-N (mg/L) | 2464.08 | 2387.20 | 2231.95 | 1882.52 | 1372.48 | 1009.74 | 1961.03 | 3201.83 | 3578.28 | 4071.59 |
| PO4-P (mg/L) | 158.16 | 172.25 | 127.68 | 854.86 | 705.96 | 148.32 | 128.92 | 105.77 | 120.27 | 114.47 |
| TKP (gP/kgTS) | 34.00 | 31.95 | 27.75 | 18.80 | 14.05 | 17.42 | 15.33 | 14.61 | 13.73 | 12.37 |
| TKN (gN/kgTS) | 55.00 | 51.00 | 46.75 | 32.10 | 23.40 | 28.83 | 26.56 | 24.67 | 23.58 | 21.85 |
| Total VFA (g/L) | 0.12 | 0.17 | 0.20 | 21.10 | 17.69 | 0.17 | 0.08 | 0.22 | 0.27 | 0.30 |
| Acetic acid (mg/L) | 36.89 | 49.07 | 93.17 | 8571.13 | 5396.51 | 91.09 | 40.22 | 118.29 | 165.12 | 196.64 |
| Propionic acid (mg/L) | 11.48 | 21.58 | 13.41 | 931.04 | 956.30 | 14.77 | 6.99 | 16.06 | 15.23 | 14.73 |
| Iso-Butyric acid (mg/L) | 0.00 | 0.00 | 0.00 | 334.96 | 202.66 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| n-Butyric acid (mg/L) | 13.92 | 28.19 | 15.19 | 7914.72 | 7067.40 | 17.48 | 8.17 | 21.62 | 20.69 | 20.49 |
| Iso-Valeric acid (mg/L) | 9.40 | 9.75 | 3.43 | 512.34 | 375.65 | 0.00 | 2.01 | 4.79 | 4.41 | 4.24 |
| n-Valeric acid (mg/L) | 14.96 | 13.57 | 10.73 | 234.56 | 395.56 | 13.27 | 5.45 | 16.60 | 14.20 | 12.21 |
| Iso-Caproic acid (mg/L) | 10.79 | 13.57 | 19.20 | 23.41 | 0.00 | 29.46 | 12.91 | 35.01 | 36.73 | 37.39 |
| n-Caproic acid (mg/L) | 19.83 | 34.80 | 40.49 | 2573.41 | 3300.39 | 7.34 | 4.14 | 11.33 | 10.87 | 9.60 |
| Total Alcohol (mg/L) | 40.71 | 46.64 | 69.76 | 58.98 | 374.09 | 0.00 | 11.62 | 13.65 | 2.73 | 0.00 |
| Ethanol (mg/L) | 40.71 | 44.90 | 69.76 | 58.98 | 297.55 | 0.00 | 11.62 | 13.65 | 2.73 | 0.00 |
| Propanol (mg/L) | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Butanol (mg/L) | 0.00 | 0.00 | 0.00 | 0.00 | 58.18 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 1-Hexanol (mg/L) | 0.00 | 1.74 | 0.00 | 0.00 | 18.35 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Treatments | BMPth | SMY | BDI | VSi | VSf | VSR % |
|---|---|---|---|---|---|---|
| (NmLCH4/gVSadded) | (NmLCH4/gVSadded) | (%) | (gVS) | (gVS) | (%) | |
| T1 | 437.64 | 59.27 | 13.54 | 8.81 | 6.67 | 24.32 |
| T2 | 437.64 | 41.95 | 9.59 | 9.92 | 7.33 | 26.12 |
| T3 | 437.64 | 24.05 | 5.49 | 13.22 | 7.59 | 42.57 |
| T4 | 437.64 | 0.00 | 0.00 | 9.92 | 6.76 | 31.82 |
| T5 | 437.64 | 0.00 | 0.00 | 14.33 | 9.31 | 35.03 |
| T6 | 437.64 | 57.47 | 13.13 | 2.77 | 2.34 | 15.31 |
| T7 | 437.64 | 27.87 | 6.37 | 8.54 | 6.96 | 18.55 |
| T8 | 437.64 | 15.09 | 3.45 | 14.34 | 13.77 | 3.98 |
| T9 | 437.64 | 8.73 | 1.99 | 20.14 | 19.52 | 3.11 |
| T10 | 437.64 | 7.06 | 1.61 | 25.94 | 25.19 | 2.89 |
| First-Order Kinetic Model | |||||||||
| SMY | Bo | Diff % | khyd | Tdelay | T90 | Tef | rRMSE | R2 | |
| (NmLCH4/gVSadded) | (NmLCH4/gVSadded) | (%) | (d−1) | (d) | (d) | (d) | (%) | ||
| T1 | 59.27 | 56.69 | −4.37 | 0.09 | 1.02 | 39.44 | 38.41 | 5.23 | 0.9958 |
| T2 | 41.95 | 40.94 | −2.48 | 0.08 | 1.67 | 40.24 | 38.57 | 2.67 | 0.9981 |
| T3 | 24.05 | 24.36 | −0.12 | 0.05 | 5.55 | 49.16 | 43.61 | 1.00 | 0.9992 |
| T4 | 0.00 | NA | |||||||
| T5 | 0.00 | ||||||||
| T6 | 57.47 | 57.82 | −0.75 | 0.05 | 2.13 | 51.35 | 49.22 | 3.63 | 0.9980 |
| T7 | 27.87 | 27.09 | −4.48 | 0.05 | 3.77 | 55.69 | 51.92 | 1.36 | 0.9959 |
| T8 | 15.09 | 14.71 | −3.60 | 0.05 | 2.37 | 60.13 | 57.76 | 3.26 | 0.9926 |
| T9 | 8.73 | 8.55 | −3.53 | 0.05 | 3.24 | 60.22 | 56.78 | 0.61 | 0.9975 |
| T10 | 7.06 | 7.17 | −3.31 | 0.03 | 1.97 | 63.37 | 61.40 | 0.90 | 0.9907 |
| Modified Gompertz Model | |||||||||
| SMY | Bo | Diff % | Rmax | λ | rRMSE | R2 | |||
| (NmLCH4/gVSadded) | (NmLCH4/gVSadded) | (%) | (NmLCH4/gVSadded.day) | (d) | (%) | ||||
| T1 | 59.27 | 54.98 | 7.22 | 3.41 | 0.51 | 10.51 | 0.9829 | ||
| T2 | 41.95 | 39.73 | 5.31 | 2.20 | 2.91 | 5.26 | 0.9928 | ||
| T3 | 24.05 | 22.99 | 4.47 | 0.82 | 4.95 | 3.43 | 0.9908 | ||
| T4 | 0.00 | NA | |||||||
| T5 | 0.00 | ||||||||
| T6 | 57.47 | 54.08 | 5.93 | 1.97 | 1.69 | 8.96 | 0.9875 | ||
| T7 | 27.87 | 24.92 | 10.61 | 0.90 | 2.06 | 6.10 | 0.9739 | ||
| T8 | 15.09 | 13.77 | −3.60 | 0.54 | 3.26 | 2.64 | 0.9846 | ||
| T9 | 8.73 | 7.95 | 8.93 | 0.29 | 2.82 | 0.61 | 0.9838 | ||
| T10 | 7.06 | 6.43 | 9.34 | 0.17 | 1.34 | 1.69 | 0.9669 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puthumana, A.B.; Kaparaju, P. Impact of Organic Load on Methane Yields and Kinetics during Anaerobic Digestion of Sugarcane Bagasse: Optimal Feed-to-Inoculum Ratio and Total Solids of Reactor Working Volume. Energies 2024, 17, 5083. https://doi.org/10.3390/en17205083
Puthumana AB, Kaparaju P. Impact of Organic Load on Methane Yields and Kinetics during Anaerobic Digestion of Sugarcane Bagasse: Optimal Feed-to-Inoculum Ratio and Total Solids of Reactor Working Volume. Energies. 2024; 17(20):5083. https://doi.org/10.3390/en17205083
Chicago/Turabian StylePuthumana, Amal Babu, and Prasad Kaparaju. 2024. "Impact of Organic Load on Methane Yields and Kinetics during Anaerobic Digestion of Sugarcane Bagasse: Optimal Feed-to-Inoculum Ratio and Total Solids of Reactor Working Volume" Energies 17, no. 20: 5083. https://doi.org/10.3390/en17205083
APA StylePuthumana, A. B., & Kaparaju, P. (2024). Impact of Organic Load on Methane Yields and Kinetics during Anaerobic Digestion of Sugarcane Bagasse: Optimal Feed-to-Inoculum Ratio and Total Solids of Reactor Working Volume. Energies, 17(20), 5083. https://doi.org/10.3390/en17205083







