Transforming Wastewater into Biofuel: Nutrient Removal and Biomass Generation with Chlorella vulgaris
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalga Strain
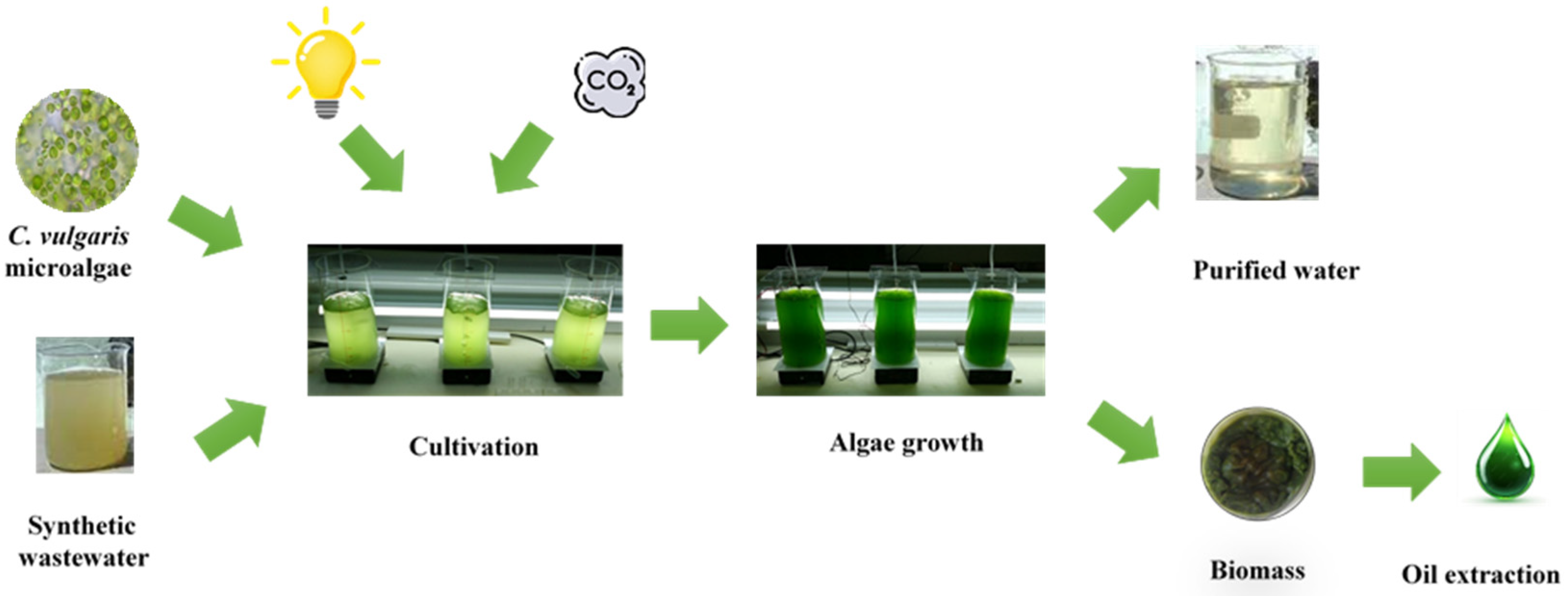
2.2. Experiment Conditions
2.3. Analytical Methods
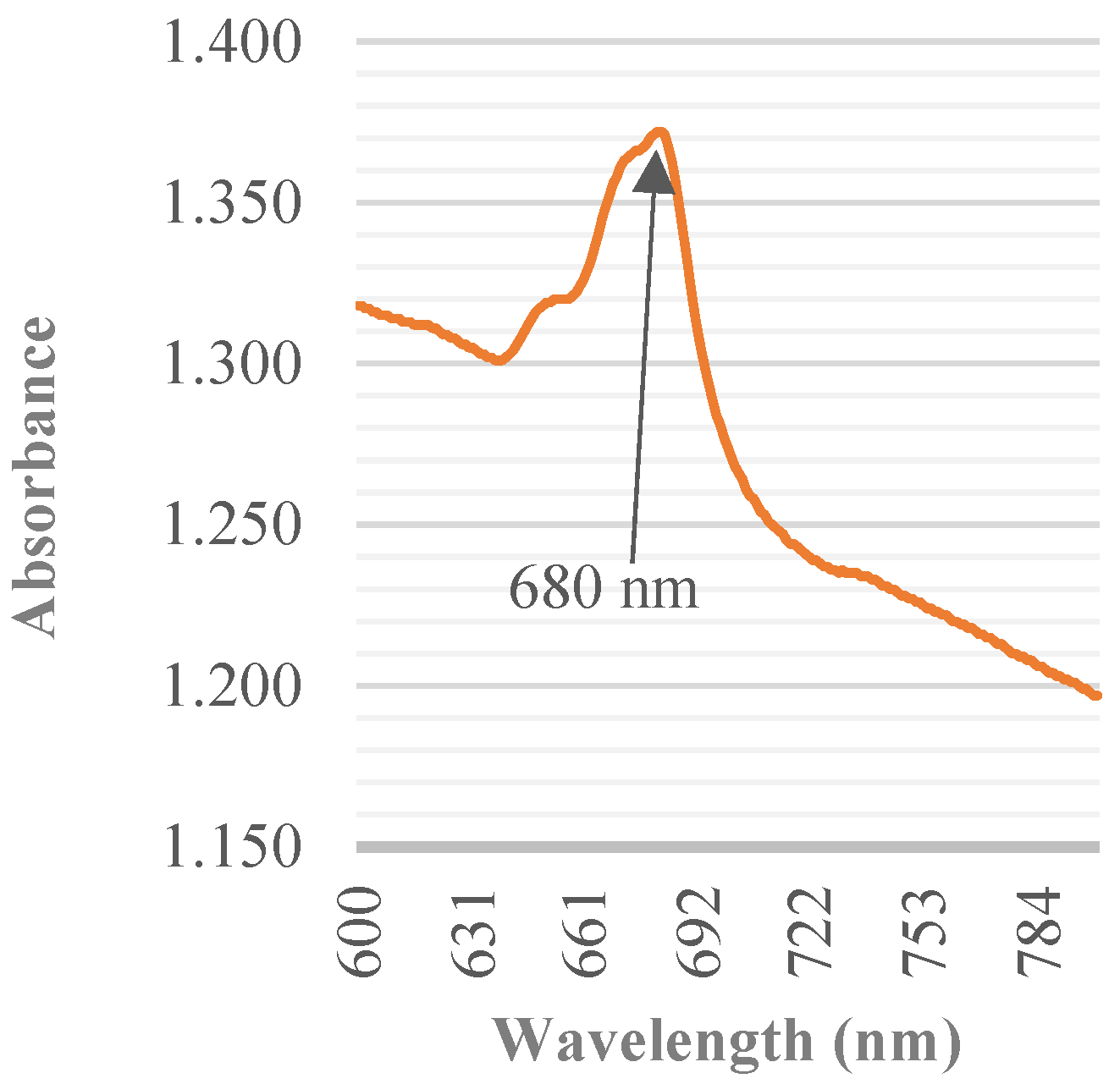
2.3.1. Biomass Determination
2.3.2. Physico-Chemical Water Characteristics
2.3.3. Lipid Extraction Method
2.3.4. Lipid Analysis
3. Results and Discussion
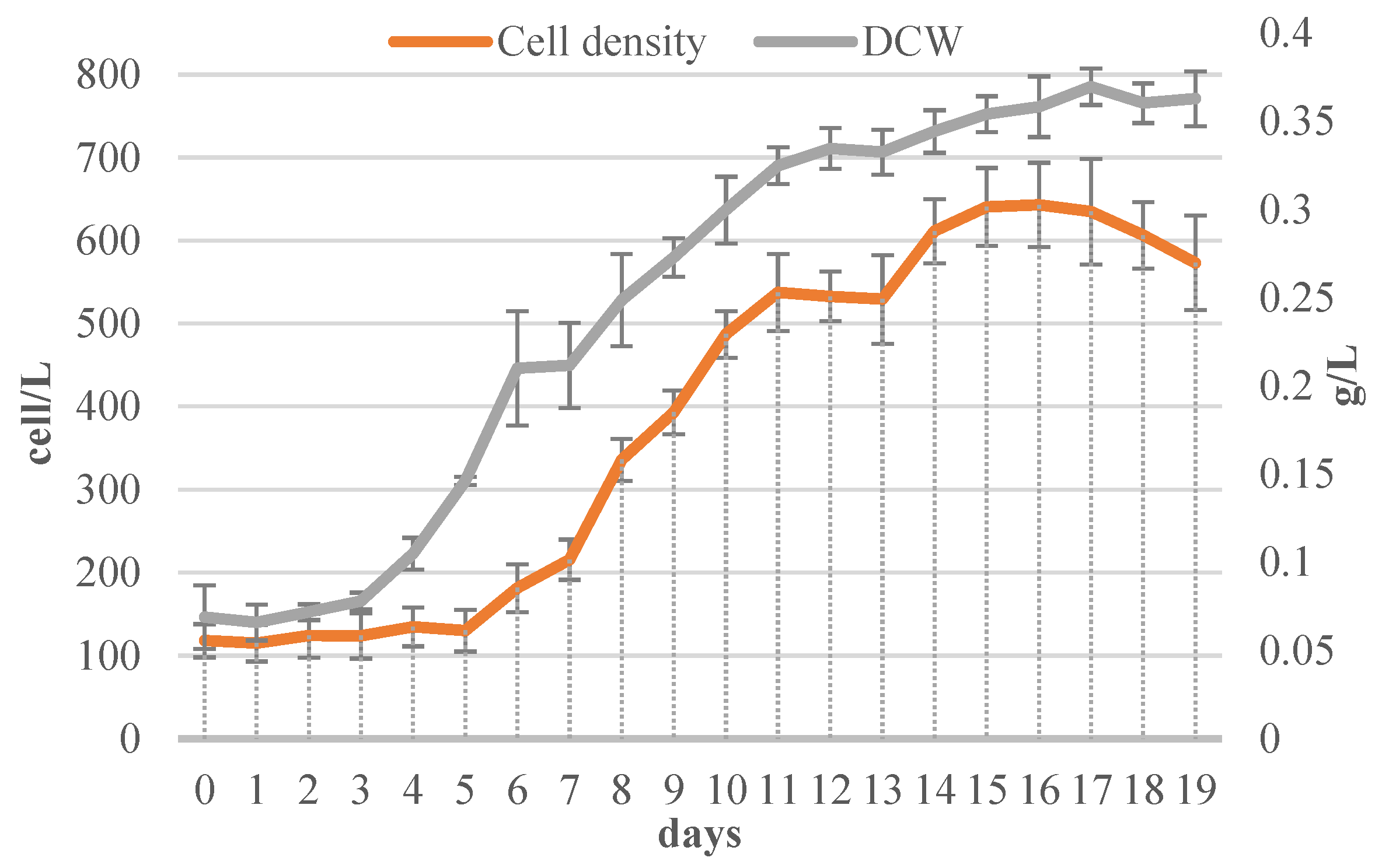
3.1. Evaluation of Algal Growth
3.2. Removal of Nutrients
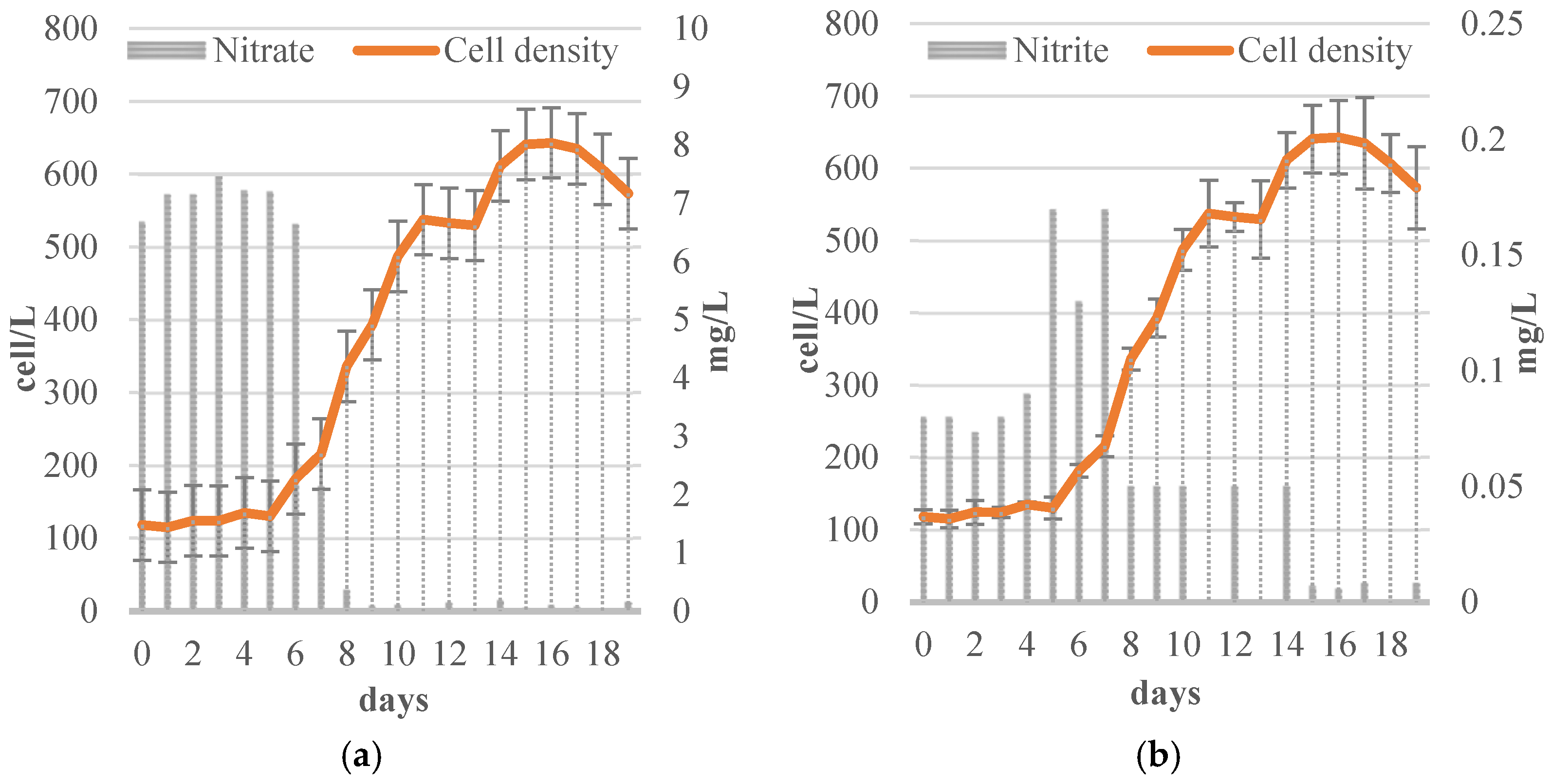
3.2.1. Nitrogen
3.2.2. Phosphate
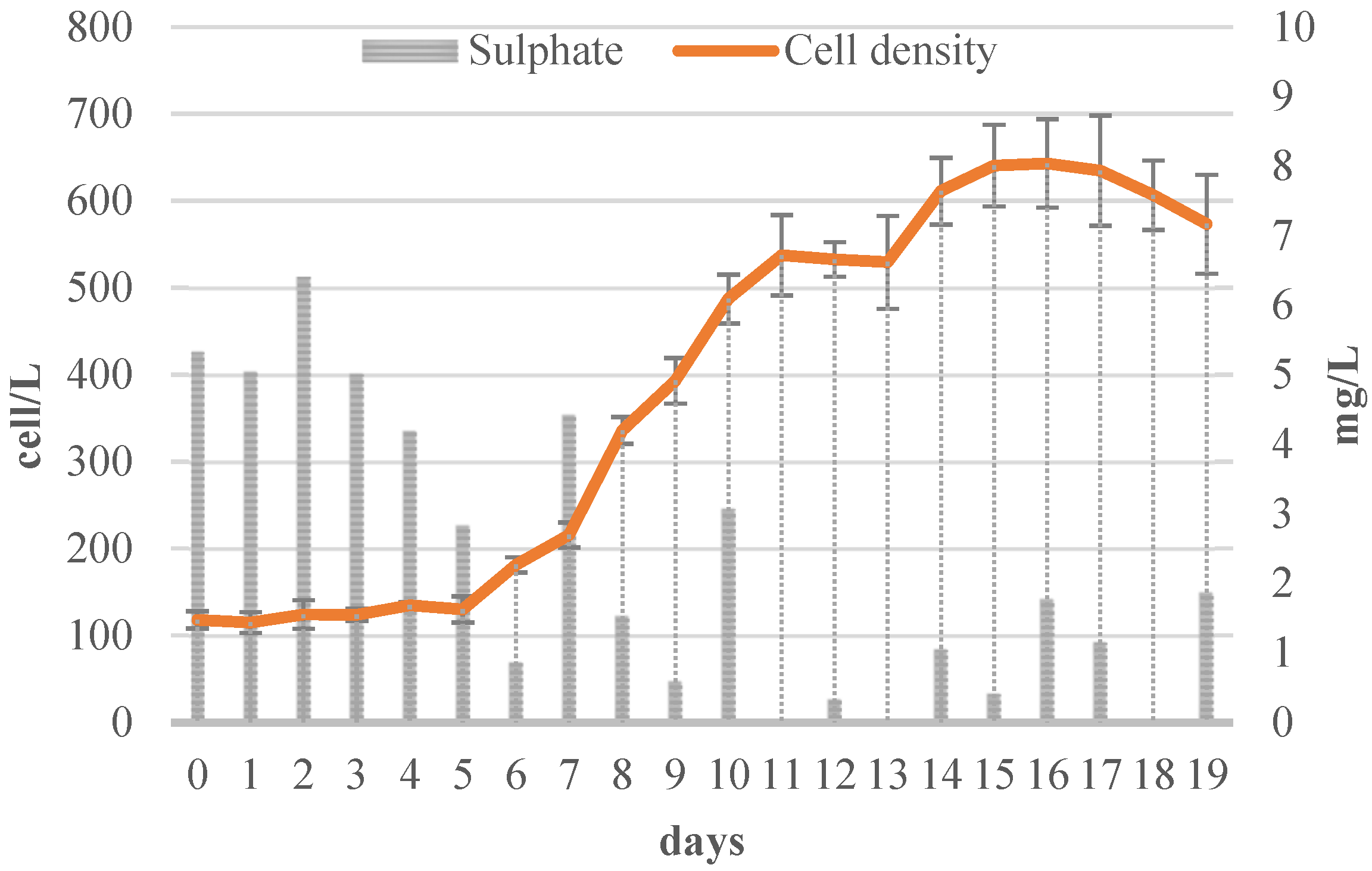
3.2.3. Sulfate
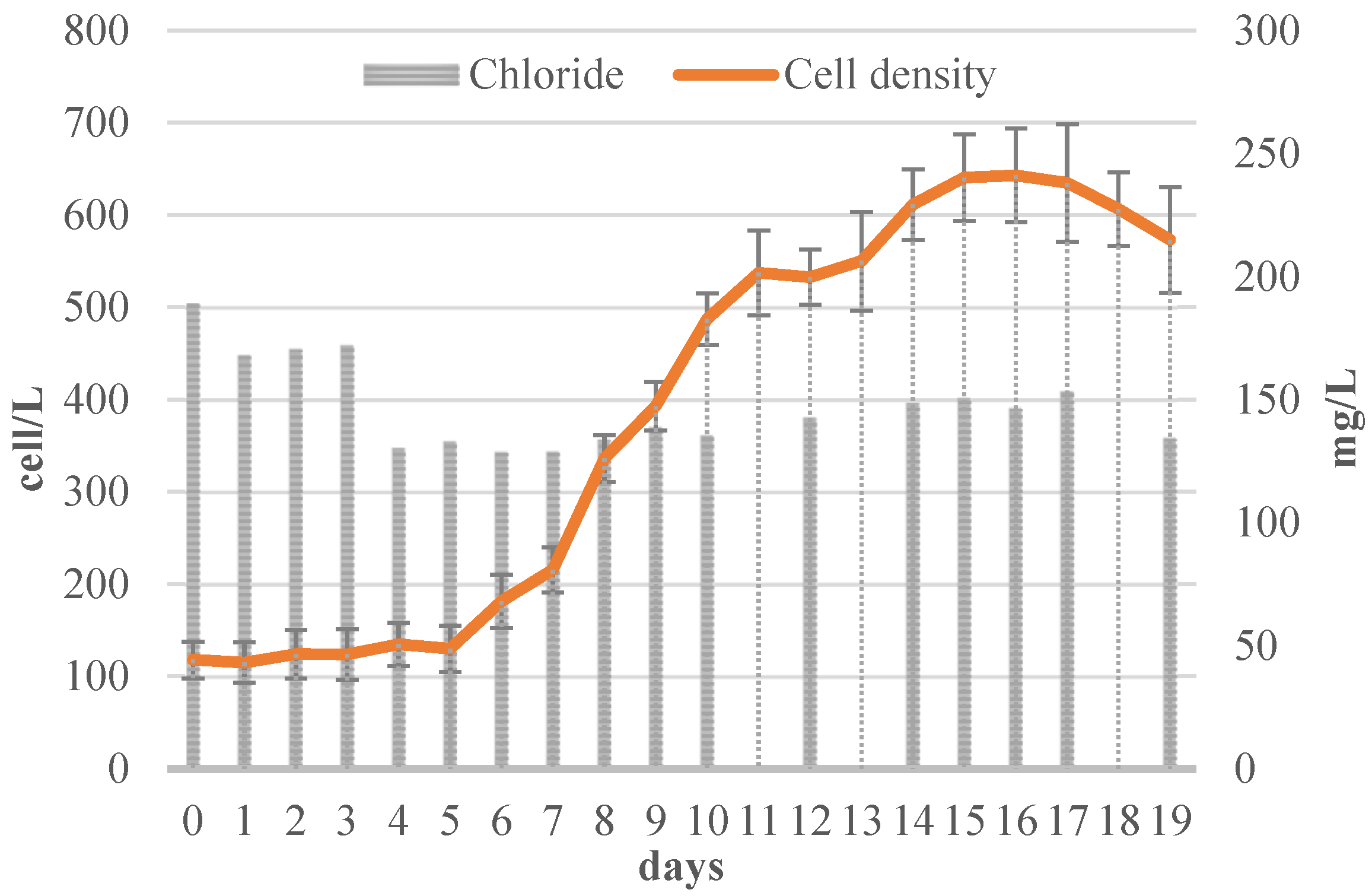
3.2.4. Chloride
3.2.5. Fluoride
3.2.6. COD
3.3. Lipid Extraction
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jayaseelan, M.; Usman, M.; Somanathan, A.; Palani, S.; Muniappan, G.; Jeyakumar, R.B. Microalgal Production of Biofuels Integrated with Wastewater Treatment. Sustainability 2021, 13, 8797. [Google Scholar] [CrossRef]
- Chew, K.W.; Yap, J.Y.; Show, P.L.; Suan, N.H.; Juan, J.C.; Ling, T.C.; Lee, D.-J.; Chang, J.-S. Microalgae biorefinery: High value products perspectives. Bioresour. Technol. 2017, 229, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Umamaheswari, J.; Shanthakumar, S. Efficacy of microalgae for industrial wastewater treatment: A review on operating conditions, treatment efficiency and biomass productivity. Rev. Environ. Sci. Bio/Technol. 2016, 15, 265–284. [Google Scholar] [CrossRef]
- Gonçalves, A.L.; Pires, J.C.M.; Simões, M. A review on the use of microalgal consortia for wastewater treatment. Algal Res. 2017, 24, 403–415. [Google Scholar] [CrossRef]
- Salbitani, G.; Carfagna, S. Ammonium Utilization in Microalgae: A Sustainable Method for Wastewater Treatment. Sustainability 2021, 13, 956. [Google Scholar] [CrossRef]
- Gouveia, L.; Graça, S.; Sousa, C.; Ambrosano, L.; Ribeiro, B.; Botrel, E.P.; Castro Neto, P.; Ferreira, A.F.; Silva, C.M. Microalgae biomass production using wastewater: Treatment and costs: Scale-up considerations. Algal Res. 2016, 16, 167–176. [Google Scholar] [CrossRef]
- Zoppi, G.; Pipitone, G.; Pirone, R.; Bensaid, S. Aqueous phase reforming process for the valorization of wastewater streams: Application to different industrial scenarios. Catal. Today 2022, 387, 224–236. [Google Scholar] [CrossRef]
- Fahmideh, L.; Khodadadi, E.; Khodadadi, E. A review of applications of biotechnology in the environment. Int. J. Farming 2014, 12, 1319–1325. [Google Scholar]
- Alazaiza, M.; He, S.; Su, D.; Abu, S.S.; Yi, P.; Bashir, M. Sewage Water Treatment Using Chlorella vulgaris Microalgae for Simultaneous Nutrient Separation and Biomass Production. Separations 2023, 10, 229. [Google Scholar] [CrossRef]
- Marjakangas, J.M.; Chen, C.-Y.; Lakaniemi, A.-M.; Puhakka, J.A.; Whang, L.-M.; Chang, J.-S. Simultaneous nutrient removal and lipid production with Chlorella vulgaris on sterilized and non-sterilized anaerobically pretreated piggery wastewater. Biochem. Eng. J. 2015, 103, 177–184. [Google Scholar] [CrossRef]
- Ji, M.-K.; Yun, H.-S.; Park, Y.-T.; Kabra, A.N.; Oh, I.-H.; Choi, J. Mixotrophic cultivation of a microalga Scenedesmus obliquus in municipal wastewater supplemented with food wastewater and flue gas CO2 for biomass production. J. Environ. Manag. 2015, 159, 115–120. [Google Scholar] [CrossRef]
- Liu, X.; Ying, K.; Chen, G.; Zhou, C.; Zhang, W.; Zhang, X.; Cai, Z.; Holmes, T.; Tao, Y. Growth of Chlorella vulgaris and nutrient removal in the wastewater in response to intermittent carbon dioxide. Chemosphere 2017, 186, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.K.; Yusoff, M.I.; Uemura, Y.; Lim, J.W.; Khoo, C.G.; Lee, K.T.; Ong, H.C. Cultivation of Chlorella vulgaris using nutrients source from domestic wastewater for biodiesel production: Growth condition and kinetic studies. Renew. Energy 2017, 103, 197–207. [Google Scholar] [CrossRef]
- Torres, E.; Bertoldo, L.; Bender, C.; Medianeira, T.; Cassia de Souza, R. Removal of organic contaminants in water bodies or wastewater by microalgae of the genus Chlorella: A review. Case Stud. Chem. Environ. Eng. 2023, 8, 100476. [Google Scholar] [CrossRef]
- Znad, H.; Al Ketife, A.M.D.; Judd, S.; AlMomani, F.; Vuthaluru, H.B. Bioremediation and nutrient removal from wastewater by Chlorella vulgaris. Ecol. Eng. 2018, 110, 1–7. [Google Scholar] [CrossRef]
- Mujtaba, G.; Lee, K. Treatment of real wastewater using co-culture of immobilized Chlorella vulgaris and suspended activated sludge. Water Res. 2017, 12, 174–184. [Google Scholar] [CrossRef]
- Zaini, N.; Kasmuri, N. The growth and development of Chlorella vulgaris in the batch culture system. World Sustain. Constr. Conf. Series. IOP Conf. Ser. Earth Environ. Sci. 2023, 1140, 012009. [Google Scholar] [CrossRef]
- Santos, K.; Mariano, A. Determination of optimal algae concentration for continuous growth of Scenedesmus sp. in chu and modified chu media. Therm. Eng. 2014, 13, 52–58. [Google Scholar] [CrossRef]
- Santos-Ballardo, D.U.; Rossi, S.; Hernández, V.; Vázquez Gómez, R.; Rendón-Unceta, M.C.; Caro-Corrales, J.; Valdez-Ortiz, A. A simple spectrophotometric method for biomass measurement of important microalgae species in aquaculture. Aquaculture 2015, 448, 87–92. [Google Scholar] [CrossRef]
- Loyola-Vargas, V.M.; Vazquez-Flota, F.A. Growth measurements: Estimation of cell division and cell expansion. Methods Mol. Biol. 2006, 318, 51–58. [Google Scholar] [CrossRef]
- UNE-EN ISO 12966-2:2017; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. European Committee for Standardization: Brussels, Belgium, 2017.
- Sacristán de Alva, M.; Luna-Pabello, V.M.; Cadena, E.; Ortíz, E. Green microalga Scenedesmus acutus grown on municipal wastewater to couple nutrient removal with lipid accumulation for biodiesel production. Bioresour. Technol. 2013, 146, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.A.; Curtis, B.S.; Curtis, W.R. Improving accuracy of cell and chromophore concentration measurements using optical density. BMC Biophys. 2013, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Mujtaba, G.; Rizwan, M.; Lee, K. Removal of nutrients and COD from wastewater using symbiotic co-culture of bacterium Pseudomonas putida and immobilized microalga Chlorella vulgaris. J. Ind. Eng. Chem. 2017, 49, 145–151. [Google Scholar] [CrossRef]
- Das, C.; Naseera, K.; Ram, A.; Meena, R.M.; Ramaiah, N. Bioremediation of tannery wastewater by a salt-tolerant strain of Chlorella vulgaris. J. Appl. Phycol. 2017, 29, 235–243. [Google Scholar] [CrossRef]
- Delgadillo-Mirquez, L.; Lopes, F.; Taidi, B.; Pareau, D. Nitrogen and phosphate removal from wastewater with a mixed microalgae and bacteria culture. Biotechnol. Rep. 2016, 11, 18–26. [Google Scholar] [CrossRef]
- Taziki, M.; Ahmadzadeh, H.; Murry, M.A.; Lyon, S.R. Nitrate and Nitrite Removal from Wastewater using Algae. Curr. Biotechnol. 2016, 4, 426–440. [Google Scholar] [CrossRef]
- Su, Y.; Mennerich, A.; Urban, B. Municipal wastewater treatment and biomass accumulation with a wastewater-born and settleable algal-bacterial culture. Water Res. 2011, 45, 3351–3358. [Google Scholar] [CrossRef]
- Wang, L.; Min, M.; Li, Y.; Chen, P.; Chen, Y.; Liu, Y.; Wang, Y.; Ruan, R. Cultivation of Green Algae Chlorella sp. in Different Wastewaters from Municipal Wastewater Treatment Plant. Appl. Biochem. Biotechnol. 2010, 162, 1174–1186. [Google Scholar] [CrossRef]
- Mera, R.; Torres, E.; Abalde, J. Sulphate, more than a nutrient, protects the microalga Chlamydomonas moewusii from cadmium toxicity. Aquat. Toxicol. 2014, 148, 92–103. [Google Scholar] [CrossRef]
- Mera, R.; Torres, E.; Abalde, J. Effects of sodium sulfate on the freshwater microalga Chlamydomonas moewusi: Implications for the optimization of algal culture media. J. Phycol. 2016, 52, 75–88. [Google Scholar] [CrossRef]
- Van Den Brand, T.P.H.; Roest, K.; Chen, G.-H.; Brdjanovic, D.; van Loosdrecht, M.C.M. Effects of Chemical Oxygen Demand, Nutrients and Salinity on Sulfate—Reducing Bacteria. Environ. Eng. Sci. 2014, 32, 858–864. [Google Scholar] [CrossRef]
- Li, Q.; Wu, Y.Y.; Wu, Y.D. Effects of fluoride and chloride on the growth of Chlorella pyrenoidosa. Water Sci. Technol. 2013, 68, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Kothari, R.; Prasad, R.; Kumar, V.; Singh, D.P. Production of biodiesel from microalgae Chlamydomonas polypyrenoideum grown on dairy industry wastewater. Bioresour. Technol. 2013, 144, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Chae, Y.; Kim, D.; An, Y.-J. Effect of fluoride on the cell viability, cell organelle potential, and photosynthetic capacity of freshwater and soil algae. Environ. Pollut. 2016, 219, 359–367. [Google Scholar] [CrossRef]
- Ali, G. Fluoride and aluminium tolerance in planktonic microalgae. Fluoride 2004, 37, 88–95. [Google Scholar]
- Venkata Mohan, S.; Ramanaiah, S.V.; Rajkumar, B.; Sarma, P.N. Biosorption of fluoride from aqueous phase onto algal Spirogyra IO1 and evaluation of adsorption kinetics. Bioresour. Technol. 2007, 98, 1006–1011. [Google Scholar] [CrossRef]
- Chen, J.; Liu, S.; Qi, X.; Yan, S.; Guo, Q. Study and Design on Chemical Oxygen Demand Measurement Based on Ultraviolet Absorption. Sens. Actuators B Chem. 2017, 254, 778–784. [Google Scholar] [CrossRef]
- Ren, H.-Y.; Liu, B.-F.; Kong, F.; Zhao, L.; Ren, N. Hydrogen and lipid production from starch wastewater by co-culture of anaerobic sludge and oleaginous microalgae with simultaneous COD, nitrogen and phosphorus removal. Water Res. 2015, 85, 404–412. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, W.; Yen, H.-W.; Ho, S.-H.; Lo, Y.-C.; Cheng, C.-L.; Ren, N.; Chamg, J.-S. Cultivation of Chlorella vulgaris JSC-6 with swine wastewater for simultaneous nutrient/COD removal and carbohydrate production. Bioresour. Technol. 2015, 198, 619–625. [Google Scholar] [CrossRef]
- Rezende dos Santos, R.; Mendonça, D.; Norie, C.; Gomes, D.A.; Lapa, C.M.L. Comparison between several methods of total lipid extraction from Chlorella vulgaris biomass. Ultrason. Sonochem. 2015, 22, 95–99. [Google Scholar] [CrossRef]
- Yeong, T.; Mee, C.; Ling, W.; Subramaniam, G. A review on extraction of lipid from microalgae using microwave-assisted ex-traction. Malays. J. Anal. Sci. 2023, 27, 292–303. [Google Scholar]
- Vijay, R.; Butler, T.; Pandhal, J.; Vaidyanathan, S. Microwave-Assisted Extraction for Microalgae: From Biofuels to Biorefinery. Biology 2018, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.A.; Cheng, Y.M.; Huang, J.W.; Jen, J.F.; Huang, Y.S.; Yu, C.C. Effects of ultrasonic and microwave pretreatments on lipid extraction of micro-algae. Bioprocess Biosyst. Eng. 2014, 37, 1543–1549. [Google Scholar] [CrossRef] [PubMed]
- 91/271/EEC; Council Directive 91/271/EEC of 21 May 1991 Concerning Urban Waste-Water Treatment. European Commission: Brussels, Belgium, 1991.




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salgueiro, J.L.; Perez-Rial, L.; Maceiras, R.; Sanchez, A.; Cancela, A. Transforming Wastewater into Biofuel: Nutrient Removal and Biomass Generation with Chlorella vulgaris. Energies 2024, 17, 4911. https://doi.org/10.3390/en17194911
Salgueiro JL, Perez-Rial L, Maceiras R, Sanchez A, Cancela A. Transforming Wastewater into Biofuel: Nutrient Removal and Biomass Generation with Chlorella vulgaris. Energies. 2024; 17(19):4911. https://doi.org/10.3390/en17194911
Chicago/Turabian StyleSalgueiro, Jose Luis, Leticia Perez-Rial, Rocio Maceiras, Angel Sanchez, and Angeles Cancela. 2024. "Transforming Wastewater into Biofuel: Nutrient Removal and Biomass Generation with Chlorella vulgaris" Energies 17, no. 19: 4911. https://doi.org/10.3390/en17194911
APA StyleSalgueiro, J. L., Perez-Rial, L., Maceiras, R., Sanchez, A., & Cancela, A. (2024). Transforming Wastewater into Biofuel: Nutrient Removal and Biomass Generation with Chlorella vulgaris. Energies, 17(19), 4911. https://doi.org/10.3390/en17194911








