Abstract
Sewage sludge (SS) holds promise for environmental, agricultural, and energy applications. However, its direct use is limited due to contaminant concerns. Pyrolysis can turn SS into beneficial products like bio-oil and biochar. This study explores biochar production from SS pyrolysis and its potential for pollutant adsorption. The effects of pyrolysis temperature (500, 650, 850 °C) and SS particle size (800–1000 µm, 400–800 µm, 100–400 µm, ≤100 µm) on biochar yield and adsorption capacity for methylene blue and mercury were investigated. Regardless of particle size and temperature, SS-derived biochar exhibited second-order adsorption kinetics. Biochar with a particle size of 100–400 µm displayed the highest potential for methylene blue adsorption. Subsequent alkali treatment (biochar:NaOH = 3:4) of these particles significantly increased specific surface area from 27.5 m2/g to 144.27 m2/g and further enhanced adsorption capacities for both methylene blue (from 9 mg/g to 35 mg/g) and mercury (from 17 mg/g to 36 mg/g). These findings suggest that SS-derived biochar, particularly the 100–400 µm fraction with alkali treatment, presents a promising cost-effective adsorbent for water treatment, aligning with circular economy principles.
1. Introduction
Wastewater treatment facilities constantly produce significant amounts of municipal sewage sludge (SS). Handling and disposing of wastewater sludge are challenging environmental issues, as sewage sludge contains bacteria, organic contaminants, and inorganic components in various oxides and salts [1]. The Environmental Protection Agency (US EPA) classifies SS as a contaminant and thus strongly regulates its disposal [2]. Currently, a large quantity of dewatered, unstable SS is landfilled in Kazakhstan. In 2020, approximately 362,000 m3 of wastewater were treated daily in the Almaty region of Kazakhstan, resulting in millions of tons of sewage sludge (SS) produced over the year [3]. Astana, the capital city of Kazakhstan, generated around 350 tons of SS with over 70% moisture content daily in 2023 [4]. Landfilling of SS ultimately allows hazardous substances to permeate the food chain and accumulate, posing a potential risk to human health [5]. Therefore, careful treatment and disposal of SS are imperative. While historically, SS had been used for soil remediation, recent rules, such as standard NY525-2021 [6], prohibit sludge usage as a natural organic fertilizer. Conversely, choosing thermochemical methods for the treatment of SS depends on the project specifics, production cost, and environmental assessments. These methods, such as gasification, pyrolysis, incineration, and hydrothermal carbonization, are simple to execute and can significantly reduce the amount of sewage sludge, mitigate harmful compounds, and eradicate pathogens [7,8]. Among these methods, the pyrolysis of SS has emerged as a proven technology that converts roughly 50% of the organic matter in sewage sludge into helpful bioenergy and yields a value-added stabilized pyrolytic residue known as biochar [8]. Biochar derived from SS holds promise as an adsorbent for the removal of pollutants such as methylene blue and heavy metals from wastewater [9,10].
Methylene Blue (MB) is a synthetic dye that is commonly found in the effluents of the dyeing industries [11]. It is primarily distinguished by its dark blue color, complex ring structure, and alkaline characteristics and is generally non-biodegradable. On the other hand, mercury is a highly persistent, volatile, and toxic metal and is also hazardous to human health and the health of the biome [12]. The primary sources of mercury released into the environment are wastewater treatment facilities of non-ferrous metal production and electronics manufacturing. Typical wastewater treatment techniques include coagulation [13], adsorption [14], membrane separation [15], and chemical degradation such as advanced oxidation processes [16]. Among them, adsorption is regarded as the preferable method for removing persistent chemicals owing to its exceptional efficacy, cost efficiency, wide availability of raw materials, and adaptability of adsorbent to modification [14]. In this context, biochar produced from pyrolysis is a promising material for adsorption. It can be used without any prior treatment [17] or can be further treated with acidic, alkaline, or metallic substances to improve biochar’s properties [18]. According to Fan et al. [19], sewage sludge–derived biochar could adsorb up to 210 mg/g of methylene blue. In contrast, Park et al. [20] recently observed 95.2 mg/g mercury adsorption in sulfur-modified SS-derived biochar.
The utilization of sewage sludge–derived biochar offers numerous advantages in adsorption technologies; however, it is essential to acknowledge that specific SS biochar variants may habor leachable heavy metals, posing potential environmental challenges [21,22]. Previous work has examined the leaching toxicity of heavy metals in SS-derived biochar with varying particle sizes of >0.830 mm, 0.180–0.830 mm, and <0.180 mm before fast pyrolysis [23]. The investigation indicated that Cu, Zn, and As in the biochar from the larger SS particles exhibited increased susceptibility to leaching into the environment [23]. Furthermore, Huang et al. [24] conducted a heterogeneous Fenton-like examination to analyze Cu, Fe, and Zn leachability from SS biochar at distinct pH levels. According to their findings, the leaching of metals decreased as the solution’s pH was increased from 3 to 7. Additionally, Tay et al. [25] demonstrated that sludge carbonized at 600 °C possessed an increasingly porous structure with a relatively high pore volume (0.504 mL/g) compared with those pyrolyzed at 800 °C and 1000 °C. These results suggest the usage of smaller-sized biochar particles and maintaining the operating conditions and a neutral pH for better adsorption. However, adsorption characteristics under these conditions remain obscured.
SS has a highly intricate composition, and when subjected to pyrolysis, it generates a substantial quantity of inorganic and organic materials [26]. Sludge biochar predominantly consists of elements like Silicon (Si), aluminum (Al), various forms of iron (Fe3O4, Fe2O3, FeO, and Fe3C), as well as organic components such as O-H, and C=O functional groups [26]. Metals including Fe2+, Zn2+, and Mn2+ in SS biochar act as active sites for adsorption [9,27]. However, high metal loading decreases the density of active sites on the material’s surface and disrupts the regular structures [27]. Furthermore, it has been discovered that oxygen-based functional groups increased with an increase in the pyrolysis temperature from 300 to 600 °C but decreased as the temperature further rose to 800 °C [28]. Additionally, SS biochar produced in nitrogen, argon, and ammonia environments has demonstrated a potent catalytic activity toward peroxymonosulfate, enabling the swift degradation of bisphenol A [29]. Moreover, biochar can be treated with acids, bases, or metals using modifying reagents, including sulfur-containing ligands [20], Fe salts [30], halides [31], KOH, NaOH, and ZnCl2, to enhance the SS biochar’s characteristics [32]. These modifications have significantly improved the adsorption capabilities of biochar.
However, despite these advances, less attention has been paid to the effect of sewage sludge particle sizes on biochar characteristics [33,34,35,36]. Most of the occasions, a broader range of the particle size of the SS has been used for pyrolysis [34,35]. To address this gap, we focus on the influence of particle sizes on SS biochar characteristics and adsorption properties, a factor that has yet been largely overlooked in previous studies. While studies have explored variables like pyrolysis temperature and chemical modifications, the relationship between particle size, metal content, and adsorption capacity remains unexplored. Our study fills this gap by (1) characterizing biochar from pyrolysis under varying SS particle sizes, (2) evaluating the effect of parameters including pyrolysis temperature and particle size on biochar yield, and (3) assessing the adsorption capacity of methylene blue and mercury onto SS biochar.
2. Materials and Methods
2.1. Sample Preparation
Municipal SS samples were collected from Astana Su Arnasy, the wastewater treatment plant in Astana city. The SS samples were air-dried in a clean environment for one week, then pulverized, sieved, and sorted into four particle sizes characterized as S1 (800–1000 µm); S2 (400–800 µm); S3 (100–400 µm); S4 (d ≤ 100 µm). The sorted samples were then stored in air-tight glass tubes for further analysis. Before pyrolysis, the samples received additional drying treatment in a Naberthem furnace at 105 °C for 5 h [37].
2.2. Pyrolysis Reactor
The pyrolysis was conducted in a quartz horizontal tube reactor depicted in Figure 1. The details of the quartz tube reactor can be found elsewhere [38]. Slow pyrolysis, employing a heating rate of 5 °C/min was carried out in a horizontal fixed-bed reactor for each SS particle size (S1, S2, S3, and S4). A 20 g of each sample was weighed using the calibrated balance (Sartorius 3716 MP; Data Weighing Systems, Wood Dale, IL, USA), and the samples were placed in a quartz tube (length: 20 cm, diameter: 1.2 cm). The first tube with the sample was then inserted in the middle of a larger quartz tube (length: 100 cm, diameter: 5.4 cm) to minimize the contamination of the biochar by produced bio-oil. Pyrolysis was carried out at temperatures of 500 °C, 650 °C, and 800 °C, using nitrogen (purity of 99.5% and 1 MPa) introduced at a flowrate of 0.3 L/min to maintain a residence time of 20 min in a heated zone. The temperature of the rig was recorded using a thermocouple inserted into the inner quartz tube containing the sample. The biochar yield was calculated using Equation (1). The experiment procedure was repeated twice, and the average of the results was reported. The obtained biochar was also further sieved into the above-mentioned four sizes for further characterization and adsorption experiments.
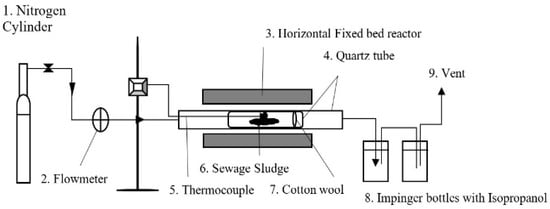
Figure 1.
Schematic diagram of a horizontal quartz tube reactor used for pyrolysis of SS.
2.3. Modification of Biochar
The samples of this work were prepared according to the procedure noted by [39]. The sludge-derived biochar obtained at 500 °C, 650 °C, and 800 °C was treated with NaOH at a mass ratio 3:4 (biochar: NaOH) to improve the surface area. In the process, the biochar-NaOH mixture was pyrolyzed again with a retention time of 20 min at the same temperatures as before, under 5 °C/min. After, the modified biochar was allowed to cool. The biochar was heated using a Fisher Scientific Isotemp top hot plate stirrer with 600 mL of deionized water at 120 °C for 1 h to remove the excess NaOH and reduce its alkalinity. After obtaining the solid material, the biochar was rinsed with deionized water until the pH of the resulting solution was neutral. The resulting solid material was dried for 18 h in a furnace at 100 °C and further sieved to maintain the particle size before using for the adsorption experiment.
2.4. Batch Adsorption Studies
Methylene Blue Adsorption: Batch adsorption experiments were carried out using 30 mg of SS biochar and 50 mL of methylene blue (MB) solution with a concentration from 10–50 mg/L concentration. The solution was agitated at 150 rpm in a dark environment. The sample was withdrawn every hour, filtered through a 0.22 µm membrane, and the absorbance of the filtered solution was measured using a 664 nm ultra-violet visible spectrophotometer. Before this, a calibration curve was prepared to determine methylene blue concentration accurately.
Mercury Adsorption: A 400 mg/L mercury [Hg (II)] working solution was prepared from Hg(NO3)2·H2O. The total mercury concentration in the biochar was determined according to ASTM method D6722-11 [40], using an analytical complex consisting of an RA-915M atomic absorption spectrometer and a PYRO 915+ pyrolytic (Lumex Ltd., St. Petersburg, Russia). In the process, 0.5 g of the biochar was added to 50 mL of mercury solutions in a polypropylene tube. The mixture was placed on a vibrating shaker (Heidolph, Schwabach, Germany) at a speed of 200 rpm. A 20 mL adsorbate solution was taken every 15 min, soaked onto carbon material, and inserted into the PYRO-915+ furnace to measure Hg (II) amount (Figure 2).
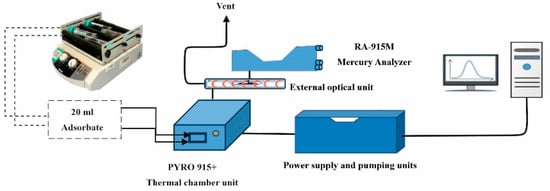
Figure 2.
Schematic diagram of mercury thermal decomposition experimental system.
All experiments were conducted at room temperature and at a pH of 6.5 because mercury is highly available for sorption in its ionic form as Hg2+ at a slightly acidic pH. The removal efficiency (R%) and adsorption capacity (Qe) were determined using Equations (2) and (3), respectively. The procedure was repeated twice with a maximum standard error of 0.1 mg/g.
where
: adsorption capacity as a function of time, mg/g
: initial MB concentration, mg/L
: final MB concentration, mg/L
volume of MB solution, L
mass of sewage sludge–derived char, g
The adsorption kinetics data were later fitted to the following four commonly used models to explore the adsorption mechanisms:
where
adsorption amount at a specific time, mg/g
equilibrium adsorption capacity, mg/g
pseudo-first-order adsorption rate constant,
pseudo-second-order adsorption rate constant, (g/(mg·))
intraparticle diffusion rate constant, (mg/g·
initial adsorption coefficient, (mg/g·
desorption coefficient, (g/mg)
contact time, (min)
constant related to the effect of the thickness of the boundary layer.
2.5. Material Characterization
The morphologies of SS, SS-derived biochar, and modified biochar were analyzed using SEM (JEOL JSM-IT200, LA, Akishima, Japan). FTIR (Bruker Alpha II, Billerica, MA, USA) was employed to examine surface functional groups of the samples with a spectral resolution of 5 cm−1 and a wave number range of 400–4000 cm−1. The biochar’s elemental composition was determined using inductively coupled plasma (ICP) analysis (iCAPTM RQ, Thermo Fisher Scientific, Waltham, MA, USA). The solutions for ICP were made using an ETHOS UP high-performance microwave digestion system (Milestone Srl, Sorisole, Italy), wherein 100 mg of sample was digested in a mixture of 3 mL of HCl, 1 mL of HNO3, 2 mL of HClO4, and 4 mL of HF at 200 °C for 1 h, using the ETHOS UP microwave at 1800 kW, US EPA, SW-846, Method 6010C. The solution was later neutralized with 10 mL of H3BO3 and was heated for 15 min at 160 °C. The resulting solution was further cooled, dissolved in 30 mL deionized water, filtered using a grade of 0.22 µm, and diluted in 10×. All the reagents used were commercial grade. Pore particle size properties were analyzed on N2 adsorption-desorption isotherms at 77 K (Micromeritics 3Flex high-performance adsorption analyzer, SciTest LLP, Almaty, Kazakhstan).
3. Results and Discussion
3.1. Effects of Pyrolysis Temperature on Biochar Yield with Different Particle Sizes
Figure 3 shows the biochar yield for the various sludge particle sizes at different temperatures. The standard deviations upon repetitions were less than ±0.5%. The biochar mass loss was observed between 55 and 65 wt.% for all particle sizes and varied non-monotonically with the size of the particles. Figure 3 depicts a noticeable trend of a decreased biochar yield in the S3 sample range (100–400 µm) at both 500 °C and 650 °C pyrolysis temperatures. This observation aligns with the findings by [41], who explored particle sizes categorized as (d < 0.25 mm, 0.25 mm < d < 0.83 mm, and d > 0.83 mm). Their study concluded that regardless of the heating rate employed, particularly at 5 °C/min, the most significant total mass loss occurred in particle size d < 0.25 mm at a temperature range of approximately 400–650 °C. This was attributed to the larger surface area of smaller particles facilitating faster and more complete heat transfer. However, exceptions were noted based on our studies for powder particles (S4: d ≤ 100 µm), which showed a marginal increase in biochar yield at 500 °C and 650 °C and a significant reduction of biochar yield at 800 °C. In addition, [42] also observed lower mass loss (%) at the pyrolysis active regime (205–383 °C) of municipal solid waste (MSDW) of particle size 26.5 µm and higher mass loss (%) in the pyrolysis passive regime (383–900 °C) of 26.5 μm. This phenomenon could be due to more interstices in larger particles of SS primarily allowing gas substances to escape without allowing them to participate in secondary decomposition reactions. Hence, the yield tends to stabilize at higher temperatures, such as 650 and 800 °C, because most easily volatile components have already been released, leading to a plateau in biochar yield. In comparison, smaller particles with fewer interstices tend to trap molecules within the sample structure and release them during temperature increases, allowing for secondary decomposition reactions [42]. The pyrolysis temperatures at 500 °C, 650 °C, and 800 °C were also found to change the particle sizes of the obtained biochar, with larger particles of sewage sludge experiencing a more significant size reduction. Hence, for any post-experiments on the adsorption studies, the biochar was further sieved and categorized according to the four sizes as used above.
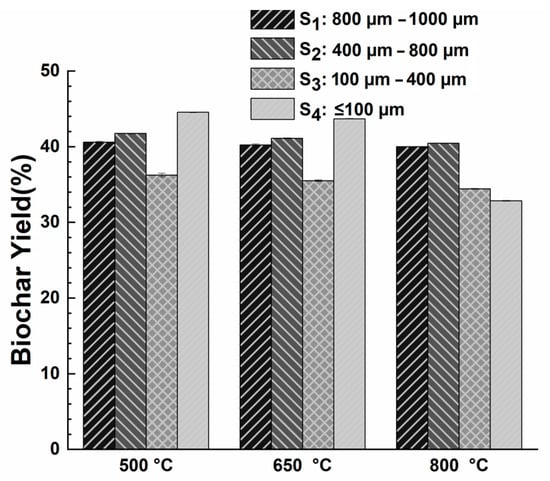
Figure 3.
Effect of different SS particle sizes on the biochar yield (%) at different pyrolysis temperatures. Standard deviations were all less than ±0.5%.
3.2. Characterization of Sludge-Derived Char
3.2.1. Heavy Metals Distribution in Different Particle Sizes of SS Biochar
As mentioned above, the presence of metals such as Fe2+, Zn2+, and Mn2+ in biochar creates active sites for adsorption. Despite the significance of heavy metal content in the biochar, some leachable toxic heavy metals in sewage sludge also raise concerns regarding potential environmental impacts [21,22]. Hence, it is essential to have a comprehensive composition of the major elements in the biochar. Figure 4 below shows the heavy metal content of Fe2+, Mn2+, and Zn2+ in the SS and the different-sized biochar obtained at different temperatures. These elements were mainly chosen because of their high metal content. Other key elements, including Ca, Mg, Na, K, Si, Ti, Pb, and Co, are presented in the (Supplementary Material Table S1). The results indicate that these heavy metals were low in the raw SS but approximately doubled in the obtained biochar because the heavy metals are enriched in solids with the decomposition of the organic matter. These findings were similar to earlier studies [23,43]. Jina et al. (2014) reported zinc concentration of 0.4–0.5 mg/g raw sewage sludge values, with its concentration doubling to 1–2 mg/g in the SS-derived biochar at 500 °C [23]. Lu et al. (2013) found increased metal concentrations between 300 and 500 °C with Mn and Fe concentrations of 0.25–0.47 mg/g and 14.25–23.2 mg/g of raw SS with 0.25–0.7 mg/g and 14.25–44.17 mg/g in the SS-derived biochar, respectively [43]. We do highlight that the metal composition will depend on the type of SS, which varies widely in its constituents. Interestingly, in our study, the Fe content ranged between 15 and 25 mg/g of biochar, whereas the Zn and Mn range was 0.6–1.8 mg/g and 0.3–0.8 mg/g, respectively, which was closer to the earlier reported values. Among different particle sizes, S3 retained the highest amount of heavy metals, followed by S1, S4, and S2, at pyrolysis temperatures of 500 °C and 650 °C. However, several exceptions existed at 800 °C, with S4 retaining more heavy metals. This is because as carbonization progresses, carbonaceous structures with high surface area and porous structures are formed in biochar, causing the heavy metal to be immobilized in their aromatic structures. However, at high temperatures such as 800 °C, metals like Fe, Zn, and Mn are also volatilized, as also highlighted in the literature for copper, cadmium, etc. [44]. Additionally, the content of other heavy toxic metals, including Co and Pb, was in the 0.01–0.10 mg/g range (Supplementary Material Table S1). Mercury was absent in the SS and its biochar. Considering the application of biochar to the soil directly, the concentrations of Fe, Zn, and Mn meet European Union Council Directive 86/278/EEC (The Sewage Sludge Directive); however, Zn (1.6 mg/g) exceeded the stricter Chinese GB4284–84 control standard [45].
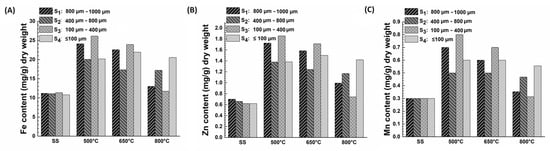
Figure 4.
Total amount of Fe (A), Zn (B), and Mn (C) in the different particle sizes of sewage sludge at 500, 650, and 800 °C.
3.2.2. Surface Morphology of the SS Biochar and NaOH-Modified Biochar
A crucial parameter of an adsorbent material is its surface morphology, which highlights its porous structures. Among all samples, the SEM analysis was performed only for the S3-sized fraction of the biochar for brevity, as they showed better adsorption characteristics. Figure 5A–C show the SEM images of biochar (S3; top), and Figure 5D–F shows an alkali-modified biochar (MS3; bottom) surface at 500, 650, and 800 °C. The pictures highlight that fractures on the surface increased with pyrolysis temperature from 500 to 650 °C and that the hole sizes and distribution increased. However, an increase in temperature to 800 °C led to a decrease in the number of surface holes, as seen in Figure 5C,F. After modification of sewage sludge–derived biochar by NaOH, rough granular structures were observed, as seen in the bottom panel of Figure 5. However, modified biochar at 800 °C also showed much less porous structures.
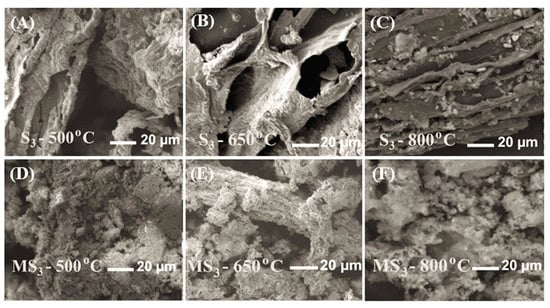
Figure 5.
Scanning electron microscope of sludge-derived biochar (S3 ; A–C) and modified biochar (MS3 ; D–F) at 500 °C, 650 °C, and 800 °C.
3.2.3. Specific Surface Area of the SS-Derived Biochar
The pore structure of the particle size S3 of sludge-derived biochar and modified biochar (MS3) at different temperatures were analyzed (Table 1).

Table 1.
Specific surface area and pore characteristics for S3 particle size biochar and modified biochar (MS3) at different temperatures.
Table 1 reveals that biochar MS3-650 demonstrated the highest specific surface area at 144.27 m2/g with a total pore volume of 0.25 cm3/g. The results also clearly suggest alkali activation to be an effective method for increasing the specific surface area of the base material [32]. For instance, even for biochar produced at 800 °C, which has a low BET area of 2.72 m2/g, the alkali activation increased the BET area to 27.78 m2/g. According to [46], pyrolysis temperature is crucial in determining biochar’s structural characteristics, particularly its carbon content. Their study found that the optimal temperature range for shaping sludge biochar is between 600 °C and 800 °C. Below 600 °C, incomplete carbonization of the sludge may occur, while temperatures exceeding 800 °C can lead to carbon contraction, impeding pore development by distorting the carbon structure and reducing pore volume. Zhang et al., 2019 suggested that biochar pores collapse beyond a temperature of 700 °C, reducing the specific area [47]. Similarly, the study by Liu et al. examined the surface area of the pharmaceutical sewage sludge [48]. Their results also confirmed that biochar produced at 600 °C exhibited the highest specific surface area within the temperature range of 400–800 °C.
3.2.4. FTIR Spectrum of Sewage Sludge, Sewage Sludge–Derived Biochar, and Modified Biochar
The structural characterization of SS, SS-derived biochar (S3-500 °C, S3-650 °C, S3-800 °C), and modified biochar (MS3-500 °C, MS3-650 °C, MS3-800 °C) was conducted using Fourier Transform Infrared (FTIR), as illustrated in Figure 6. The analysis evaluated the effect of pyrolysis temperature and alkali activation temperature on the composition of functional groups within the materials. The identified functional groups in the sewage sludge includes O-H (3277 cm−1), methyl (-CH2; 2917 cm−1) and aliphatic group (C-H; 2849 cm−1) [49], C=C group (1633 cm−1) [50], N-O group (1539 cm−1 and 1575 cm−1) [51], O-H plane bending in the carboxyl group (-COOH) (1466/1418 cm−1) [52], -Si-O-Si- bonds (419 cm−1/464 cm−1) [53] and Si-O group (1024 cm−1) [54], and (-CHO) carbonyl group and C-N-C bend amines [52]. The analysis revealed notable transformations in functional groups at different temperatures. For instance, at 500 °C, a prominent peak at 3345 cm−1 was observed and diminished progressively at 650 °C and 800 °C, suggesting the decomposition of large hydroxyl groups during pyrolysis. Furthermore, the disappearance of aliphatic bands in the sewage sludge–derived biochar at 500 °C, 650 °C, and 800 °C indicated the decomposition of organic fatty hydrocarbons into gases such as methane and carbon dioxide. The study also highlighted the changes in the O-H plane bending in the carboxyl group, with a decrease at 500 °C (1417 cm−1) and 650 °C (1394 cm−1) and complete absence at 800 °C. These peak bands were found in modified biochar’s MS3-500 °C, MS3-650 °C, and MS3-800 °C at 1411 cm−1, 1410 cm-1, and 1430 cm−1 respectively. The Si-O group in the SS decreased when the temperature increased from 500 °C (1004 cm−1) to 650 °C (981 cm−1) and disappeared at 800 °C. These peak bands were later found in modified biochar MS3-500 °C, MS3-650 °C, and MS3-800 °C at 989 cm−1, 958 cm−1, 945 cm−1, respectively. The peak bands from 871 cm−1 to 877 cm−1 were assigned to the heteroatoms of alkyl halides found in all biochar except S3-800 °C and MS3-650 °C. However, the appearance of peak bands 778 cm−1 and 776 cm−1 in the biochar produced at 500 °C (S3-500 °C) and 650 °C (S3-650 °C) were assigned to the (-CHO) carbonyl group [52] and was absent in modified biochar’s. Furthermore, the peak bands at 564 cm−1, 568 cm−1, and 564 cm−1 were found in modified biochar and absent in the sewage sludge–derived biochar at 500 °C, 650 °C, and 800 °C. These peak bands were assigned to the C-N-C bend amines [52]. According to [53], the peak band from 448 cm−1 to 419 cm−1, found in all biochar samples, could be attributed to -Si-O-Si-, which may act as active sites for adsorption through n-π interaction.
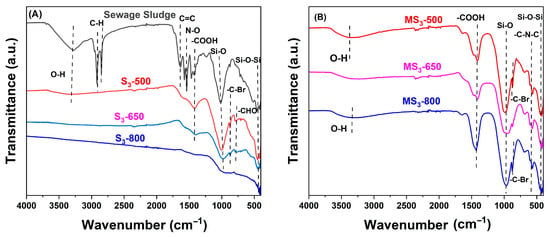
Figure 6.
Structural characterization of sewage sludge, (A) sludge-derived biochar (S3), and (B) modified sludge–derived biochar (MS3) for S3-sized particles at 500, 650, and 800 °C.
3.3. Adsorption of Methylene Blue (MB) and Mercury (Hg2+)
3.3.1. Influence of Biochar Particle Size on MB Adsorption
The MB absorption was first performed on various particle sizes (S1, S2, S3, and S4) to investigate the adsorption mechanism at low concentrations of the contaminants. As shown in Figure 7A, the experiment employed biochar samples without pretreatment to assess the inherent behavior of each size fraction produced at a pyrolysis temperature of 500 °C. The results revealed an exciting interplay between biochar particle size and MB adsorption from a solution of 10 mg/L of MB. The adsorption kinetics was followed for 600 min, wherein all the four sizes of particles reached equilibrium capacity, characterized by a constant MB concentration of 10 mg/L. Intuitively, smaller particle sizes should have had the highest adsorption capacity due to the larger surface area. However, our results revealed that the biochar particle of size S3, (100–400 μm) demonstrated the highest adsorption capacity, reaching 14.6 mg/g. In contrast, the smaller-sized biochar particle S4 (<100 μm) and larger-sized particles S1 (800–1000 μm) and S2 (400–800 μm) had a low adsorption capacity of 11 mg/g, 5.5 mg/g, and 3.5 mg/g, respectively. The excellent performance of the particle size of sludge-derived biochar (S3) can be attributed to more active sites, as observed from the ICP analysis. The standard deviation was within ±0.2%.
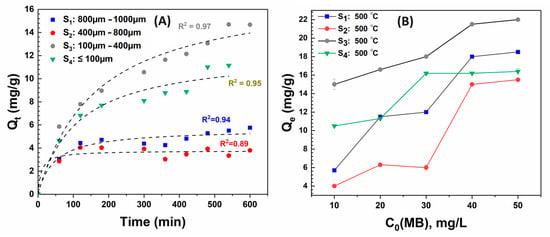
Figure 7.
Adsorption of MB over different-sized SS-derived biochar particles produced at 500 °C, pH: 6.5, 30 mg dose. (A) Adsorption kinetics of 10 mg/L of MB along with fit using Pseudo-second-order kinetics model. (B) Effect of initial MB concentration from 10 to 50 mg/L over equilibrium adsorption capacity.
Further, the adsorption process was examined by applying pseudo-first-order kinetics, pseudo-second-order kinetics, Webber–Morris, and Elovich Models. The supplementary material delineates the fit results (Supplementary Figure S1 and Table S2). Among the Pseudo kinetic models, the second order provides a better fit with the highest averaged R2 values and least averaged error of less than 11%, predicting that chemisorption is the rate-limiting process [55]. Figure 7A also shows a pseudo-second-order model and the corresponding R2 (Table S2). Since the pseudo-second-order model explained the surface adsorption, intra-particle diffusion, and exterior liquid film diffusion processes [56], this model gave a more thorough and accurate portrayal of the MB adsorption kinetics of the sludge-derived biochar. According to the Webber–Morris model [55], intraparticle diffusion strongly controls adsorption if the adsorption capacity, qt, is linearly proportional with the square root of time and passes through the origin. However, the results herein (Supplementary Figure S1) showed that the Webber–Morris fit for all SS particle sizes did not pass through the origin, although it had good R2 values between 0.83 and 0.98 (except for S2). The intraparticle diffusion (Kd) rate of the sludge-derived biochar produced at 500 °C for sized fraction S3: 100–400 µm had a higher Kd value of 0.57 compared to S1: 800–1000 µm (0.2), S2: 400–800 µm (0.1), and S4 (0.44) suggesting a greater mass transfer resistance to intraparticle diffusion and potentially longer times to reach equilibrium. The lower constant value (C) 0.79 of the sewage sludge biochar (S3: 100–400 µm) suggests a thicker boundary layer. Furthermore, the higher correlation value of 0.99 of the particle size fraction S3: 100–400 µm compared to S1: 800–1000 µm (0.83), S2: 400–800 µm (0.5), and S4 (0.95) indicates that the model provided an adequate description of the experimental data. Despite the complexities of the pore structures of particle size fraction (S3: 100–400 µm), the model could still capture the underlying adsorption kinetics reasonably well. Hence, intra-particle diffusion processes, along with liquid film diffusion, are crucial factors, as noted earlier in the literature [19]. Furthermore, the Elovich plot’s (Supplementary Figure S1) strong fit to the kinetic data and the high correlation coefficient values obtained suggest that MB adsorption on biochar is not a straightforward first or second-order reaction but a complex process [57]. In addition, the model fit parameters (Supplementary Table S2) “α and β” reveal comparable initial adsorption and desorption rates of 0.17 and 0.22 for S3, indicating better adsorption characteristics. Furthermore, the concentration of MB was varied from 10 mg/L to 50 mg/L. Figure 7B reveals that the equilibrium capacity of S3 increased from 14.6 to 22 mg/g with increasing the dye concentration from 10 to 50 mg/L and seemingly saturating at above 40 mg/L of MB. Likewise, the adsorption capacity increased for other particle sizes, with a maximum change occurring for size S2 (6 to 14 mg/g) when increasing MB concentration from 30 to 40 mg/L. This phenomenon indicates that the active sites were being progressively occupied to near saturation.
3.3.2. Effect of Adsorbent Dosages
The effect of adsorbent (S3: 100–400 µm) dosage was studied using 10 mg/L of methylene blue and varied adsorbent masses of 0.01 g, 0.03 g, 0.05 g, and 0.1 g. The equilibrium adsorption capacity of methylene blue onto the sewage sludge–derived biochar increased with increasing adsorbent mass from 0.2 g/L to 0.6 g/L (Figure 8); however, it decreased in equilibrium adsorption capacity with a continual mass dose from 0.6 g/L to 2 g/L and approximately 95% removal efficiency. Hence, the results suggest that 0. 6 g/L is an optimal adsorbent mass required to effectively remove 10 mg/L of methylene blue from aqueous water.
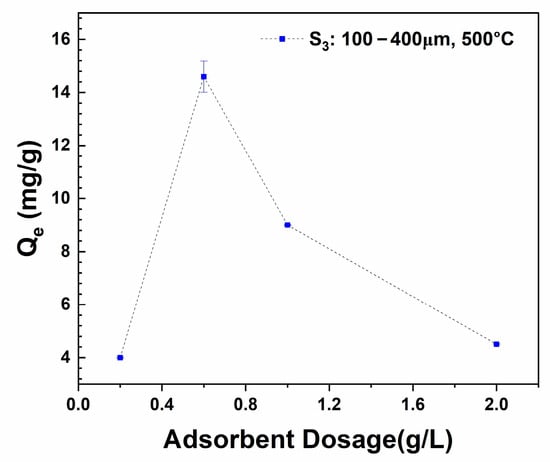
Figure 8.
Equilibrium adsorption capacity with adsorbent dose of S3: 100–400 µm in 10 mg/L of MB, pH: 6.5.
3.3.3. Influence of Pyrolysis Temperature and Alkali Activation on Adsorption
The study also investigated the impact of pyrolysis temperatures (500, 650, and 800 °C) and alkali activation on particle size fraction (S3: 100–400 µm) adsorption for individually removing 400 mg/L of methylene blue and mercury from an aqueous solution with an adsorbent mass of 500 mg. The sampling interval was 15 min at an agitation speed of 200 rev/min, following a consistent experimental procedure for methylene blue and mercury removal studies. Figure 9 shows the adsorption capacity of methylene blue (Figure 9A) and mercury (Figure 9B) with time. The modified biochar MS3-650 °C exhibited the highest adsorption capacity for methylene blue at 35 mg/g and mercury at 36 mg/g, potentially attributed to its elevated surface area of 144.27 m2/g (Table 1). Despite modified biochar MS3-800 °C possessing a surface area of 27.78 m2/g, in comparison to sewage sludge biochar S3-500 °C of 26.38 m2/g, the adsorption capacity of methylene blue onto S3-500 °C (15 mg/g) exceeded that of MS3-800 °C (10 mg/g). This disparity was associated with larger pore diameter of 5.81 nm of S3-500 °C as compared to 1.90 nm of MS3-800 °C, indicating that the total pore diameter of the biochar influenced the quantity of methylene blue molecules to be adsorbed. Further, the removal of the O-H group from the surface of the biochar at temperatures of 650 °C and 800 °C led to a decrease in methylene blue and mercury adsorption capacity of S3-650 °C and S3-800 °C, consistent with the findings of [54]. From Figure 9B, the modified biochars (MS3-500 °C, MS3-650 °C, MS3-800 °C) showed superior mercury adsorption capacity than sewage sludge–derived biochars (S3-500 °C, S3-650 °C, S3-800 °C), with surface area being the predominant factor.
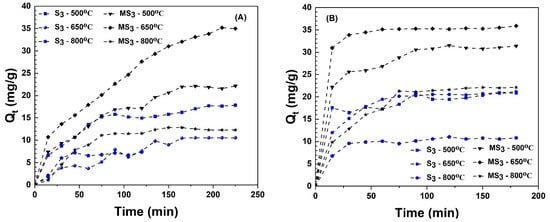
Figure 9.
Adsorption capacity of methylene blue (A) and mercury (B) with time (Co (MB): 400 mg/L of MB, pH: 6.5, 500 mg mass dose).
3.3.4. Comparison with Other Adsorbents
This study involved a comparative analysis of methylene blue (MB) and mercury equilibrium adsorption capacity from an aqueous solution using various established adsorbents reported in the literature and the included novel alkali activation biochar of MS3-650 °C. Table 2 shows the equilibrium adsorption capacity results of mixed municipal discarded materials (MMDM) derived char [58], chitosan nanocomposite [59], kaolin [60], soybean stalk [61], coconut activated carbon [62], corn-straw biochar [63], bagasse/hickory chips (HCB) [64], sugarcane bagasse [65], and activated carbon [66]. Remarkably, pyrolysis of municipal sewage sludge waste at 650 °C and NaOH activation at the same temperature emerged as a comparable standout candidate as it offers a cost-effective material effective for water treatments. This biochar exhibits auspicious potential for future environmental endeavors. MS3-650 °C utilization not only tackles pollution issues but also adheres to the principles of a circular economy. By repurposing waste materials, MS3-650 °C demonstrates sustainability and resource optimization, leading to a more robust and ecologically responsible future.

Table 2.
Comparison of adsorption capacity of MS3 650 °C with other known adsorbents with their characteristics and properties. * Current work.
4. Conclusions
The study emphasizes the importance of preparation conditions (particle size and pyrolysis temperature) in improving the heavy metal content and adsorption capacity of biochar derived from sewage sludge. Slow pyrolysis of smaller particle sizes (100–400 µm and ≤100 µm) showed better Fe2+, Mn2+, Zn2+, and Ti2+ retention, making them effective adsorbents for treating methylene blue and mercury from water. Pyrolysis at 800 °C resulted in a higher percent mass loss of sewage sludge–derived biochar. However, pyrolysis at 650 °C with NaOH activation optimized methylene blue and mercury adsorption. Remarkably, particle sizes 100–400 µm exhibited the best adsorption performance, with significant enhancements through surface modification. These findings highlight the potential of optimizing sewage sludge biochar for efficient pollutant removal and evaluating the performance of this biochar in different persistent pollutants to promote its engineering applications in wastewater treatment. Future research is needed to explore the adsorption mechanism of modified biochar (MS3-650 °C) toward methylene blue and mercury adsorption. Additionally, evaluating its long-term stability and effectiveness in real-world scenarios involving low concentrations of pollutants will provide valuable insights for practical applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/en17184554/s1, Figure S1: Adsorption of 10 mg/L of MB over different sized SS biochar particles produced at 500 oC, pH 6.5, 10 mg mass dose, fitted to A) Pseudo first-order kinetics model, B) Pseudo second-order kinetics model, C) Webber-Morris Model and d) Elovich Model, along with the corresponding R2. Figure S2: Physical nature of particle sizes produced at pyrolysis temperature of 500 °C during MB adsorption (A: top) and towards MB equilibrium adsorption stage (B: bottom) in 10 mg/L of MB (pH 6.5, 10 mg mass dose). Table S1: Concentration of the other elements in raw SS and SS-derived biochar at different temperatures and different sized-fraction S1 (800–1000 µm); S2 (400–800 µm); S3 (100–400 µm); S4 (d ≤ 100 µm). Table S2: The fit parameters for adsorption of 10 mg/L of MB over different particles sized SS biochar for Pseudo first-order kinetics model, Pseudo second-order kinetics model, Webber-Morris Model and Elovich Model, along with the corresponding R2 (pH 6.5, 10 mg mass dose).
Author Contributions
A.K.A.: conceptualization, investigation, methodology, visualization, writing–original draft preparation, review and editing. S.G.P.: conceptualization, validation, methodology, writing–reviewing and editing. Y.S.: conceptualization, validation, writing—reviewing and editing. D.S.: conceptualization, validation, writing—reviewing and editing, funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Science and Higher Education of the Republic of Kazakhstan, Project number IRN: AP14872091 (Project name: Comparative assessment of municipal sewage sludge pyrolysis, gasification and incineration in the pilot-scale fluidized bed rig) and a Nazarbayev University research grant: 11022021FD2905 (Project name: Efficient thermal valorization of municipal sewage sludge in fluidized bed systems: Advanced experiments with process modeling).
Data Availability Statement
The data supporting this study’s findings are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Du, Z.L.; Hu, A.B.; Wang, Q.D.; Ai, J.; Zhang, W.J.; Liang, Y.; Cao, M.X.; Wu, H.J.; Wang, D.S. Molecular composition and biotoxicity effects of dissolved organic matters in sludge- based carbon: Effects of pyrolysis temperature. J. Hazard. Mater. 2022, 424, 12734. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Zhang, W.; Liao, G.; Chen, F.; Wang, D. A novel waste activated sludge multistage utilization strategy for preparing carbon-based Fenton-like catalyst: Catalytic performance assessment and micro-interfacial mechanisms. Water Res. 2019, 150, 473–487. [Google Scholar] [CrossRef] [PubMed]
- Ospanov, K.; Kuldeyev, E.; Kenzhaliyev, B.; Korotunov, A. Wastewater Treatment Methods and Sewage Treatment Facilities in Almaty, Kazakhstan. J. Ecol. Eng. 2022, 23, 240–251. [Google Scholar] [CrossRef]
- Temireyeva, A.; Zhunussova, K.; Aidabulov, M.; Venetis, C.; Sarbassov, Y.; Shah, D. Greenhouse Gas Emissions-Based Development and Characterization of Optimal Scenarios for Municipal Solid and Sewage Sludge Waste Management in Astana City. Sustainability 2023, 14, 15850. [Google Scholar] [CrossRef]
- Yuan, S.J.; Dai, X.H. Sewage sludge-based functional nanomaterials: Development and applications. Environ. Sci. Nano 2017, 4, 17–26. [Google Scholar] [CrossRef]
- NY525-2021; Water Soluble Fertilizers–Determination of Water Insoluble Matter Content and pH. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2021.
- El Ouadrhiri, F.; Elyemni, M.; Lahkimi, A.; Lhassani, A.; Chaouch Mehdi Taleb, M. Mesoporous Carbon from Optimized Date Stone Hydrochar by Catalytic Hydrothermal Carbonization Using Response Surface Methodology: Application to Dyes Adsorption. Int. J. Chem. Eng. 2021, 5555406. [Google Scholar] [CrossRef]
- Hu, M.; Ye, Z.; Zhang, H.; Chen, B.; Pan, Z.; Wang, J. Thermochemical conversion of sewage sludge for energy and resource recovery: Technical challenges and prospects. Environ. Pollut. Bioavailab. 2021, 33, 145–163. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Shao, Y.; Guo, H.; Liu, Z.; Hu, G.; Xiang, H.; Hu, J. Removal of elemental mercury using magnetic Fe-containing carbon prepared from sludge flocculated with ferrous sulfate by Zinc chloride activation. J. Energy Inst. 2021, 98, 98–106. [Google Scholar] [CrossRef]
- Zaini, M.A.A.; Zakaria, M.; Mohd-Setapar, S.H.; Che-Yunus, M.A. Sludge-adsorbents from palm oil mill effluent for methylene blue removal. J. Environ. Chem. Eng. 2013, 1, 1091–1098. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Najeeb, J.; Hussain, Z. Fundamentals and photocatalysis of methylene blue dye using various nanocatalytic assemblies- a critical review. J. Clean Prod. 2021, 298, 126567. [Google Scholar] [CrossRef]
- Ali, J.; Wang, H.; Ifthikar, J.; Khan, A.; Wang, T.; Zhan, K. Efficient, stable, and selective adsorption of heavy metals by the-functionalized layered double hydroxide in diverse types of water. Chem. Eng. J. 2018, 332, 387–397. [Google Scholar] [CrossRef]
- Ye, G.; Zhou, J.; Huang, R.; Ke, Y.; Peng, Y.; Zhou, Y. Magnetic sludge-based biochar derived from Fenton sludge as an efficient heterogeneous Fenton catalyst for degrading Methylene blue. J. Environ. Chem. Eng. 2022, 10, 107242. [Google Scholar] [CrossRef]
- Chen, W.; Liu, Z.; Tang, Q.; Du, B.; Huang, X.; Mo, Y.; Fan, L.; Luo, H.; Chen, F. Assessment of a novel aminated magnetic adsorbent with excellent adsorption capacity for dyes and drugs. J. Environ. Manag. 2021, 293, 112809. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Chowdhary, P.; Bharagava, R.N. Conventional methods for the removal of industrial pollutants, their merits and demerits. In Emerging and Eco-Friendly Approaches for Waste Management; Springer: Singapore, 2018; pp. 1–31. [Google Scholar] [CrossRef]
- El Ouadrhiri, F.; Althomali, R.H.; Adachi, A.; Saleh, E.A.M.; Husain, K.; Lhassani, A.; Hassan, I.; Moharam, M.M.; Kassem, A.F.; Chaouch, M.; et al. Nitrogen and phosphorus co-doped carbocatalyst for efficient organic pollutant removal through persulfate- based advanced oxidation processes. J. Saudi Chem. Soc. 2023, 27, 101648. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, S.; Chen, D.; Dai, G.; Wei, D.; Shu, Y. Activation of persulfate with char for degradation of bisphenol A in soil. J. Chem. Eng. 2020, 381, 122637. [Google Scholar] [CrossRef]
- Lei, Y.; Guo, X.; Jiang, M.; Sun, W.; He, H.; Chen, Y.; Thummavichai, K.; Ola, O.; Zhu, Y.; Wang, N. Co-ZIF reinforced cow manure biochar (CMB) as an effective peroxymonosulfate activator for degradation of carbamazepine. Appl. Catal. B Environ. 2022, 319, 121932. [Google Scholar] [CrossRef]
- Fana, S.; Wanga, Y.; Wanga, Z.; Tanga, J.; Tanga, J.; Lia, X. Removal of methylene blue from aqueous solution by sewage sludge-derived biochar: Adsorption kinetics, equilibrium, thermodynamics, and mechanism. J. Environ. Chem. Eng. 2017, 5, 601–611. [Google Scholar] [CrossRef]
- Park, J.H.; Wang, J.J.; Zhou, B.; Mikhael, J.E.R.; DeLaune, R.D. Removing mercury from aqueous solution using sulfurized biochar and associated mechanisms. Environ. Pollut. 2019, 244, 627–635. [Google Scholar] [CrossRef]
- Ren, J.; Cao, J.; Zhao, X.; Liu, Y. Fundamentals and applications of char in biomass tar reforming. Fuel Process Technol. 2021, 216, 106782. [Google Scholar] [CrossRef]
- Wang, X.; Li, C.; Li, Z.; Yu, G.; Wang, Y. Effect of pyrolysis temperature on characteristics, chemical speciation, and risk evaluation of heavy metals in biochar derived from textile dyeing sludge. Ecotox. Environ. Safe 2019, 168, 45–52. [Google Scholar] [CrossRef]
- Jina, H.; Arazo, R.O.; Gaoe, J.; Capareda, S.; Changa, Z. Leaching of heavy metals from fast pyrolysis residues produced from different particle sizes of sewage sludge. J. Anal. Appl. Pyrolysis 2014, 109, 168–175. [Google Scholar] [CrossRef]
- Huang, Y.F.; Huang Chiueh, P.T.; Lo, S.L. Heterogeneous Fenton oxidation of trichloroethylene catalyzed by sewage sludge biochar: Experimental study and life cycle assessment. Chemosphere 2020, 249, 126139. [Google Scholar] [CrossRef] [PubMed]
- Tay, J.H.; Chen, X.G.; Jeyaseelan, S.; Graham, N. Optimizing the preparation of activated carbon from digested sewage sludge and coconut husk. Chemosphere 2001, 44, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Quan, C.; Liu, B.; Li, Z.; Wu, C.; Li, A. Continuous Pyrolysis of sewage sludge in a screw-feeding reactor: Products characterization and ecological risk assessment of heavy metals. Energy Fuels 2017, 31, 5063–5072. [Google Scholar] [CrossRef]
- Huang, Y.X.; Sun, Y.R.; Xu, Z.H.; Luo, M.Y.; Zhu, C.L.; Li, L. Removal of aqueous oxalic acid by heterogeneous catalytic ozonation with MnOx/sewage sludge-derived activated carbon as catalysts. Sci. Total Environ. 2017, 575, 50–57. [Google Scholar] [CrossRef]
- Wang, X.P.; Gu, L.; Zhou, P.; Zhu, N.W.; Li, C.X.; Tao, H.; Wen, H.F.; Zhang, D.F. Pyrolytic temperature-dependent conversion of sewage sludge to carbon catalyst and their Performance in persulfate degradation of 2-Naphthol. Chem. Eng. J. 2017, 324, 203–215. [Google Scholar] [CrossRef]
- Huang, B.C.; Jiang, J.; Huang, G.X.; Yu, H.Q. Sludge biochar-based catalysts for improved pollutant degradation by activating peroxymonosulfate. J. Mater. Chem. 2018, 6, 8978–8985. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Ifthikar, J.; Shi, L.; Khan, A.; Chen, Z.; Chen, Z. Towards a better understanding on mercury adsorption by magnetic bio-adsorbents with gamma-Fe2O3 from pinewood sawdust derived hydrochar: Influence of atmosphere in heat treatment. Bioresour. Technol. 2018, 256, 269–276. [Google Scholar] [CrossRef]
- Wang, T.; Liu, J.; Zhang, Y.; Zhang, H.; Chen, W.; Norris, P.; Pan, W. Use of a non-thermal plasma technique to increase the number of chlorine active sites on biochar for improved mercury removal. J. Chem. Eng. 2018, 331, 536–544. [Google Scholar] [CrossRef]
- Mian, M.M.; Liu, G.; Fu, B. Conversion of sewage sludge into environmental catalyst and microbial fuel cell electrode material: A review. Sci. Total Environ. 2019, 666, 525–539. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, N.; Giri, B.S.; Chowdhary, P.; Chaturvedi, P. Removal of methylene blue dye using rice husk, cow dung, and sludge biochar: Characterization, application, and kinetic studies. Bioresour. Technol. 2020, 306, 12320. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Deng, W.; Hu, M.; Chen, G.; Zhou, P.; Zhou, Y.; Su, Y. Preparation of binder-less activated char briquettes from pyrolysis of sewage sludge for liquid-phase adsorption of methylene blue. J. Environ. Manag. 2021, 299, 113601. [Google Scholar] [CrossRef] [PubMed]
- Lia, Y.H.; Chang, F.M.; Huang, B.; Song, Y.P.; Zhao, H.Y.; Wang, K.J. Activated carbon preparation from pyrolysis char of sewage sludge and its adsorption performance for organic compounds in sewage. Fuel 2020, 266, 117053. [Google Scholar] [CrossRef]
- Zeng, H.; Qi, W.; Zhai, L.; Wang, F.; Zhang, J.; Li, D. Preparation and Characterization of Sludge-Based Magnetic Biochar by Pyrolysis for Methylene Blue Removal. Nanomaterials 2021, 11, 2473. [Google Scholar] [CrossRef]
- Agrafioti, E.; Bouras, G.; Kalderis, D.; Diamadopoulos, E. Biochar production by sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 72–78. [Google Scholar] [CrossRef]
- Tursunov, O.; Suleimenova, B.; Kuspangaliyeva, B.; Inglezakis, V.J.; Anthony, E.J.; Sarbassov, Y. Characterization of tar generated from the mixture of municipal solid waste and coal pyrolysis at 800 °C. Energy Rep. 2020, 6, 147–152. [Google Scholar] [CrossRef]
- Kalampaliki, D.; Jayasinghe, G.D.T.M.; Avramiotis, E.; Manariotis, I.D.; Venier, D.; Poulopoulos, S.G.; Szpunar, J.; Vakros, J.; Mantzavinos, D. Application of KOH-activated biochar for the activation of persulfate and the degradation of sulfamethoxazole. J. Chem. Eng. Res. Des. 2023, 194, 306–317. [Google Scholar] [CrossRef]
- ASTM method D6722-11; Standard Test Method for Total Mercury in Coal and Coal Combustion Residues by Direct Combustion Analysis. ASTM: West Conshohocken, PA, USA, 2011.
- Zhai, Y.; Peng, W.; Zeng, G.; Fu, Z.; Lan, Y.; Chen, H.; Wang, C.; Fan, X. Pyrolysis characteristics and kinetics of sewage sludge for different sizes and heating rates. J. Therm. Anal. Calorim. 2012, 107, 1015–1022. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Vinu, R. Effects of Biomass Particle Size on Slow Pyrolysis Kinetics and Fast Pyrolysis Product Distribution. Waste Biomass Valorization 2018, 9, 465–477. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, W.; Wang, S.; Zhuang, L.; Yang, Y.; Qiu, R. Characterization of sewage sludge-derived biochars from different feedstocks and pyrolysis temperatures. J. Anal. Appl. Pyrolysis 2013, 102, 137–143. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, Y.; Guo, Z.; Xiao, X.; Peng, C.; Zeng, P. Optimizing pyrolysis temperature of contaminated rice straw biochar: Heavy metal(loid) deportment, properties evolution, and Pb adsorption/immobilization. J. Saudi Chem. Soc. 2022, 26, 101439. [Google Scholar] [CrossRef]
- GB4284-84; Control Standards for Pollutants in Sludges from Agricultural Use. Ministry of Ecology and Environment of The People’s Republic of China: Beijing, China, 1984.
- Yuan, Y.; Yuan, T.; Wang, D.; Tang, J.; Zhou, S. Sewage sludge biochar as an efficient catalyst for oxygen reduction in a microbial fuel cell. Bioresour. Technol. 2013, 144, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Li, Y.; Cao, Y.; Han, L. Characteristics of tetracycline adsorption by cow manure biochar prepared at different pyrolysis temperatures. Bioresour. Technol. 2019, 285, 121348. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, G.; Li, G. The characteristics of pharmaceutical sludge-derived biochar and its application for the adsorption of tetracycline. Sci. Total Environ. 2020, 747, 141492. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Oleszczuk, P. The conversion of sewage sludge into biochar reduces polycyclic aromatic hydrocarbon content and ecotoxicity but increases trace metal content. Biomass Bioenergy 2015, 75, 235–244. [Google Scholar] [CrossRef]
- Peng, H.; Gao, P.; Chu, G.; Pan, B.; Peng, J.; Xing, B. Enhanced adsorption of Cu (II) and Cd (II) by phosphoric acid-modified biochars. Environ. Pollut. 2017, 229, 846–853. [Google Scholar] [CrossRef]
- Liu, X.Q.; Ding, H.S.; Wang, Y.Y.; Liu, W.J.; Jiang, H. Pyrolytic temperature dependent and ash catalyzed formation of sludge char with ultra-high adsorption to 1-naphthol. Environ. Sci. Technol. 2016, 50, 2602–2609. [Google Scholar] [CrossRef]
- Raj, A.; Yadav, A.; Arya, S.; Sirohi, R.; Kumar, S.; Rawat, A.P.; Thakur, R.S.; Patel, D.K.; Bahadur, L.; Pandey, A. Preparation, characterization and agri applications of biochar produced by pyrolysis of sewage sludge at different temperatures. Sci. Total Environ. 2021, 795, 148722. [Google Scholar] [CrossRef]
- Nematollahzadeh, A.; Seraj, S.; Mirzayi, B. Catecholamine-coated maghemite nanoparticles for the environmental remediation: Hexavalent chromium ions removal. Chem. Eng. J. 2015, 277, 21–29. [Google Scholar] [CrossRef]
- Khraisheh, M.; Al-Ghouti, M.; Allen, S.; Ahmad, M. Effect of O.H. and silanol groups in removing dyes from aqueous solution using diatomite. Water Res. 2005, 39, 922–932. [Google Scholar] [CrossRef]
- Sun, Y.; Ding, C.; Cheng, W.; Wang, X. Simultaneous adsorption and reduction of U (VI) on reduced graphene oxide-supported nanoscale zerovalent iron. J. Hazard. Mater. 2014, 280, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.C.; Tseng, R.-L.; Juang, R.-S. Characteristics of Elovich equation used to analyze adsorption kinetics in dye-chitosan systems. Chem. Eng. J. 2009, 150, 366–373. [Google Scholar] [CrossRef]
- Hoslett, J.; Ghazzal, H.; Mohamad, N.; Johura, H. Removal of methylene blue from aqueous solutions by biochar prepared from the pyrolysis of mixed municipal discarded material. Sci. Total Environ. 2020, 714, 136832. [Google Scholar] [CrossRef] [PubMed]
- Ismaturrahmi, R.; Mustafa, I. Methylene blue removal from water using H2SO4 crosslinked magnetic chitosan nanocomposite beads. Microchem. J. 2019, 144, 397–402. [Google Scholar] [CrossRef]
- Mouni, L.; Belkhiri, L.; Bollinger, J.C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Ma dani, K.; Remini, H. Removal of methylene blue from aqueous solutions by adsorption on kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Kong, H.; He, J.; Gao, Y. Cosorption of phenanthrene and mercury (II) from aqueous solution by soybean stalk-based biochar. J. Agric. Food Chem. 2011, 59, 12116–12123. [Google Scholar] [CrossRef]
- Lu, X.; Jiang, J.; Sun, K. Influence of the pore structure and surface chemical properties of activated carbon on the adsorption of mercury from aqueous solutions. Mar. Pollut. Bull. 2014, 78, 69–76. [Google Scholar] [CrossRef]
- Tan, G.; Sun, W.; Xu, Y. Bioresource technology sorption of mercury (II) and atrazine by biochar, modified biochars and biochar based activated carbon in aqueous solution. Bioresour. Technol. 2016, 211, 727–735. [Google Scholar] [CrossRef]
- Xu, X.; Schierz, A.; Xu, N.; Cao, X. Comparison of the characteristics and mechanisms of Hg (II) sorption by biochars and activated carbon. J. Colloid Interface Sci. 2016, 463, 55–60. [Google Scholar] [CrossRef]
- Khoramzadeh, E.; Nasernejad, B.; Halladj, R. Mercury biosorption from aqueous solutions by sugarcane bagasse. J. Taiwan Inst. Chem. Eng. 2013, 44, 266–269. [Google Scholar] [CrossRef]
- Rao, M.M.; Reddy, D.H.K.K.; Venkateswarlu, P.; Seshaiah, K. Removal of mercury from aqueous solutions using activated carbon prepared from agricultural by-product/waste. J. Environ. Manag. 2009, 90, 634–643. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).