Abstract
Driven by environmental and economic considerations, this study explores the viability of utilizing coconut juice residues (CJRs), a byproduct from coconut milk production, as a carbon source for bacterial cellulose (BC) synthesis in the form of a versatile bio-membrane. This work investigates the use of optimization modeling as a tool to find the optimal conditions for BC cultivation in consideration of waste minimization and resource sustainability. Optimization efforts focused on three parameters, including pH (4–6), cultivation temperature (20–30 °C), and time (6–10 days) using Design Expert (DE) V.13. The maximum yield of 9.31% (g/g) was achieved when the cultivation took place at the optimal conditions (pH 6, 30 °C, and 8 days). This approach aligns with circular economy principles, contributing to sustainable resource management and environmental impact reduction. The experimental and predicted optimal conditions from DE V.13 were in good agreement, validating the study’s outcomes. The predictive model gave the correlations of the optimal conditions in response to the highest yield and maximum eco-efficiency. The use of prediction modeling resulted in a useful tool for forecasting and obtaining guidelines that can assist other researchers in calculating optimal conditions for a desired yield. Acetylation of the BC resulted in cellulose acetate (CA) membranes. The CA membrane exhibited the potential to separate CO2 from a CH4/CO2 mixed gas with a CO2 selectivity of 1.315 in a membrane separation. The promising gas separation results could be further explored to be utilized in biogas purification applications.
1. Introduction
As the world seeks innovative and sustainable solutions, the exploration of JCRs as a carbon source for circular economy membrane material synthesis represents a forward-thinking approach that aligns with the ethos of green technology, promoting resource efficiency, waste reduction, and the development of eco-friendly alternatives. In this study, we harness the byproduct generated during the production of coconut milk as a carbon source for the preparation of the bio-bacterial cellulose membrane. BC membranes can be enriched with bioactive compounds from sustainable sources. Their versatility and advantageous properties make BC membranes valuable for a wide range of applications, including medical treatments and environmental protection [1]. By converting this waste into a valuable and versatile product, we actively contribute to waste minimization efforts, aligning with circular economy principles and sustainable resource utilization. Our work underscores the potential for sustainable practices in repurposing waste streams and aligns with broader environmental goals by mitigating the environmental footprint associated with waste disposal.
Coconut milk production is an important industry in southern Thailand, where coconuts are one of the major agricultural products that are harvested in the area. Each year, more than 650 MTs of coconut were produced on approximately 900 k hectares of agricultural plantation area [2]. The process of making coconut milk begins with cleaning and storing coconut flesh in different temperature conditions: freezing at −16 °C (±2 °C) for 24 h, refrigeration at 6 °C (±2 °C) for 24 h, and storing at room temperature at 27 °C (±2 °C). The thawed coconut flesh is grated and then pressed using a hydraulic press to extract coconut milk. The extracted milk is filtered to remove impurities before testing for yield, water content, pH, and viscosity [3]. A study by Srivina et al. suggested that one coconut contains roughly 15% of coconut juice [4]. Recognizing the scale of the coconut milk production industry, a substantial amount of CJRs are being annually generated from the extraction process, which requires great attention for appropriate waste management.
Many agricultural wastes have been reported that can be potentially utilized as carbon sources in bioprocesses involving microorganisms such as Acetobacter xylinum (A. xylinum), which take part in breaking down the more complex lignocellulosic structure into bio-cellulose. Studies have explored a variety of carbon substrates, including CJRs, also known as nata de coco [5], rambutan juice [6], banana stems [7], sugarcane bagasse [8], and pineapple juice [9], for BC production with A. xylinum.
A. xylinum, now classified as Komagataeibacter xylinus, is a key bacterium utilized for bacterial cellulose (BC) production due to its efficient synthesis of BC with unique properties [10]. Studies have explored various substrates for BC production, such as sucrose, lactose, starch, coconut water, rice-soaking water, and their combinations, showcasing the versatility of A. xylinum in utilizing different carbon sources for BC synthesis [10,11]. Furthermore, genetic modifications of A. xylinum strains have been investigated to enhance BC properties, with mutated strains showing improvements in BC molecular weight, crystallinity, and thermal stability, leading to the production of BC composites with enhanced mechanical properties [12].
Cellulose synthesized from natural carbon sources occurs via multiple reactions, including enzymes, catalytic complexes, and regulatory proteins [13]. There are four essential enzymatic steps to produce BC from glucose using A. xylinum, as shown in Figure 1. As a precursor to BC synthesis, UDP-glucose production is a major component of the mechanistic pathways of BC production. After this procedure, individual glucose units are polymerized to produce β-1→4 glucan chains. Because UGPase is around 100 times more active in cellulose-producing bacteria than in non-cellulose-producing bacteria, it is believed to play an important role in the production of cellulose [14,15]. Individual chains are excreted through TCs and form ribbon-like structures composed of hundreds and thousands of individual chains. The bacterial cellulose fibrils are formed by the bundling of these ribbon-like structures [16,17]. However, BC may have distinct hereditary physical characteristics (such as molecular weight, crystallinity, and thermal stability) as a result of cultivating A. xylinum strains [18,19].
Various factors influence BC cultivation, including the bacterial strain used, nutrient medium composition, pH, temperature, cultivation time, and culture conditions. Studies have shown that optimizing these factors can significantly impact BC production. For instance, research has demonstrated that factors such as inoculum concentration and incubation time play a crucial role in determining the BC yield [20]. Furthermore, studies utilizing experimental designs such as Plackett-Burman and Box-Behnken have highlighted the importance of factors such as sucrose concentration and fermentation conditions in enhancing BC yield [21]. Moreover, the use of specific fermentation conditions, such as glucose and citric acid additives, along with optimal inoculation amounts, has been shown to maximize BC yield [22]. Among these parameters, pH is one of the important parameters in BC synthesis utilizing A. xylinum. Previous studies [6,7,23,24] discovered that in the rather acidic conditions of BC production, i.e., a pH range of 3–6, the growth of A. xylinum was improved. Furthermore, N. Pa’e et al. [25] discovered that the optimal pH for growing A. xylinum for BC manufacturing was 4 and 5 for static fermentation and rotary disk reactor (RDR), respectively. Adjusting the pH to 7 for BC incubation from natural carbon sources such as bagasse, dried jackfruit leaves, and palm oil bunches resulted in BC wet yields ranging from 63.8 to 81.71% using the phase inversion method [8,26]. As a result, this study focused on pH levels ranging from 4 to 6. The second affecting parameter examined in this study was cultivating temperature, which is critical for growing A. xylinum. Previous research revealed that a desirable optimum temperature ranged between 25 and 35 °C [6,8,24,27,28]. Thus, in this study, the affecting temperature ranges from 20 to 30 °C were investigated. Among all factors, cultivating time is one of the most essential influences on A. xylinum growth. The cultivating time in this study ranged from 6–10 days.
Numerous contemporary studies have delved into BC production, examining the utilization of natural carbon sources. Notably, a study conducted by [29] focused on optimizing bacterial cellulose production from pineapple waste using response surface methodology (RSM). Additionally, Cheng et al. [30] noted that the utilization of easily available raw materials such as sugarcane bagasse, bananas, and paddy straws can serve as an alternative source to produce BC. However, there remains a notable gap in the literature regarding BC production from CJRs, and limited information exists on the application of Design Expert (DE) V.13 for experimental design and optimization. Addressing this gap, this study aims to contribute insights gained from utilizing DE V.13, filling a void in research related to bacterial cellulose production. The fitting model obtained from DE V.13 provides a systematic and efficient approach to ensuring optimal conditions, scalability, and enhanced productivity in larger-scale BC manufacturing processes.
Current studies on the elimination of CO2 from biomass have concentrated on utilizing cellulose acetate CA membranes, incorporating different additives to enhance the effectiveness of the separation process. For instance, adding cellulose nanocrystals (CNCs) to CA membranes has been demonstrated to enhance both CO2 selectivity and permeability [31]. In addition to this, efforts have been made in research using biomass-based cellulose nanofibers, which revealed that the best separation performance is achieved when 0.5% succinate-functionalized cellulose nanofiber is utilized. In addition, UiO-66-NH2 and ZIF-8 nanoparticles doped in CA mixed matrix membranes (MMMs) show significant improvements in terms of selectivity and permeability for CO2 removal from biogas [32,33]. These advancements explain how these materials can be more effective in CO2 removal from biogas.
The outcomes of this research are poised to offer valuable contributions in the form of prediction modeling for BC production using CJRs, in which the BC production is illustrated in Figure 1. This includes establishing and optimizing the predictive models that elucidate the relationship between influential parameters for the maximum dry BC yield and eco-efficiency simultaneously. In this study, the key factors (pH, time, and temperature) that greatly affect the bioprocess of making BC membrane from JCRs by A. xylinum, were systematically investigated. The optimization models were proposed to predict the optimal conditions for BC production in which the maximum dry yield and eco-efficiency can be achieved. The bio-CA was then modified from obtained BC membranes by the acetylation and was utilized in the CO2 removal from the CO2/CH4 gas mixture in the membrane separation unit. By addressing these aspects, this study seeks to bridge a significant gap in the current literature, enhancing our understanding of the intricate dynamics involved in BC production, its economic implications, and its application in CO2 gas separation.
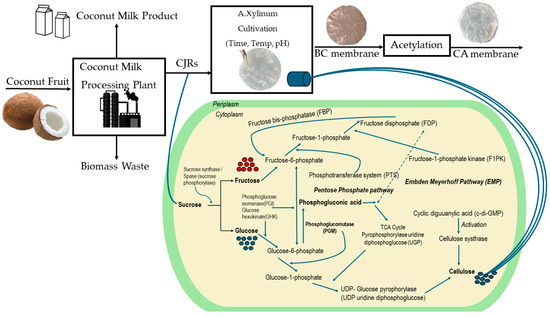
Figure 1.
Schematic of synthesis of BC and CA membranes from CJRs, the cultivation mechanism pathways by A. xylinum were adapted from Khami et al. [24].
2. Materials and Methods
2.1. Materials
A. xylinum from the Thailand Institute of Scientific and Technological Research (TISTR), Bangkok, Thailand, was prepared for use in the standard plate counts (SPC). CJRs were obtained from a coconut milk manufacturer. Ammonium sulfate [(NH4)2SO4] with a purity of more than 99.99% was purchased from QRec (Thailand). Ethanol (96%) was purchased from Vdells Siam (Bangkok, Thailand). Mitr Phol Sugar Corp., Ltd. was the supplier of sugar (Bangkok, Thailand). The aqueous solution of 5% acetic acid was obtained from Aorsorror Food Co., Ltd., located in Bangkok, Thailand.
2.2. Experimental Design
RSM is mainly used for the modeling and optimization of process parameters [34]. Apart from optimization, the software can also interpret the factors in the experiment. In the software, the operation is divided into three choices, which are screening, characterization, and optimization options [35]. Optimization options were used in this study to find the optimal conditions of each parameter that affects the BC yield and eco-efficiency simultaneously. BC cultivation from CJRs was conducted using the Box-Behnken Design, with 15 experimental runs. BBD was used in this study for several reasons, the primary one being its effective exploration. With a comparatively small number of experimental runs, optimal conditions were attained by applying a sequence of factorial and fractional factorial design points [36,37]. Additionally, BBD works well for fitting models that are second-order, or quadratic. Regarding their ability to approximate the curvature of the response surface, which enables a more precise understanding of how the factors affect the response, these models are probably suitable for RSM [38]. The studying conditions for all runs suggested by the software cover the varying pH (4 to 6), the cultivating temperature (20 to 35 °C), and the cultivation time (6 to 10 days). The yields were calculated by Equation (1). In the optimization, both BC dry yield and Eco-efficiency were maximized at the same time.
Yield % (g/g) = mass of BC/mass of coconut substrate × 100
2.3. A. xylinum Preparation
CJRs are preserved by filtering to remove any contaminants. Before use, the CJRs are placed in a sterilized container and kept in a refrigerator at 6 °C. CJRs and agar powder were combined to culture A. xylinum. The mixture contained 1000 mL of filtered CJRs, 28 g of nutrient agar, 100 g of sugar, 1 g of ammonium sulfate, 100 mL of 40% by volume ethanol, and 5 mL of acetic acid. A 1000-mL beaker was used to hold the mixture and swirled with a magnetic stirrer until it mixed. The liquid was then put into a 1000-mL Duran container. A pH of 6 and 12% brix was maintained for the cultivation. The mixture was then sterilized for 15 min at 121 °C in an autoclave [39]. After cooling to room temperature, the mixture was transferred to a vertical laminar airflow apparatus. To prevent cross-contamination during this operation, the machine was sanitized beforehand using a UV light that was switched on for 30 min before the mixture entered the machine. This method was used to check the colony-forming units (CFU) by using the pour plate technique, and the colonies were counted after 7 days. This is the starting point for further A. xylinum usage during this research.
2.4. BC and CA Membrane Preparation
The prepared coconut substrate was obtained from a mixture of 1000 mL of filtered CJRs, 50 g of sugar, 1 g of ammonium sulfate, 100 mL of 40% ethanol by volume, and 5 mL of acetic acid. This mixture was then divided into separate 20-mL sterilized Petri plates (8 cm in diameter), each containing 10 mL of A. xylinum obtained from the previous step. The Petri plates were then moved into an incubator to incubate at 20, 25, and 30 °C for 6, 8, and 10 days, respectively. Following cultivation, the BC pellicle on the surface of the substrate was collected and left to soak for a day in distilled water at room temperature. The BC was thereafter placed in an oven set at 60 °C for 24 h to eliminate any extra moisture that could have been present in the BC. After the BC had dried for 24 h, weight measurements were taken to calculate the yield. Temperature, cultivation time, and pH were all influencing variables in the production of BC. The following factors were taken into consideration when choosing the BC culture parameters for this study. The experiment’s results were used to compute the yields of BC. Following that, DE V.13 was used to identify the BC’s optimal conditions. Growing BC underwent semiacetylation to produce the bio-CA membrane. The extent of acetylation is determined by the accessibility of cellulose fibers and the sensitivity of individual cellulose crystallites [40]. After removing the BC pellicle from the substrate surface, it was left to soak for three days at room temperature in distilled water. By boiling the BC for three hours at 60 °C in 40 mL of 2% NaOH, bacterial cell debris was removed. Subsequently, 40 mL of 40% ethanol was added, and the BC was boiled for an additional three hours at the same temperature. The BC was then combined with 40 mL of acetic acid, 50 mL of toluene, and 0.2 mL of 60% perchloric acid after being dehydrated for three hours at 60 °C in an oven. To get rid of any remaining residues of chemicals and impurities, the BC was submerged in 40 milliliters of 75% ethanol for two hours. The BC was last thoroughly cleaned with distilled water and then dried in a hot air oven (Memmert GmbH + Co. KG, Bangkok, Thailand) that was set to 60 °C for eight hours [24].
2.5. Membrane Characterizations
2.5.1. Fourier Transform Infrared (FTIR) Spectra Analysis
The functional groups of the BC membrane’s chemical structure were examined with an attenuated total multiple reflection (ATR) Fourier Transform IR (FTIR) spectrometer (model Tensor 27; Bruker, Germany). The ATR-FTIR technique was employed for the analysis. The wavelength range for the spectrum used in the investigation was 400–4000 cm−1.
2.5.2. Morphological Properties
The microstructure of the CJR BC membrane was investigated using a scanning electron microscope (SEM, Model Merlin Compact, Carl Zeiss Co., Ltd., Bangkok, Thailand). The BC membrane was first dried in a hot air oven (model FD115; Franz Binder GmbH & Co., Bangkok, Thailand) at 60 °C for 24 h. After drying, it was cooled to room temperature in a desiccator (model RT-48C; Eureka Design Co., Ltd., Pathum Thani, Thailand). The dried BC membrane was then placed on carbon stubs, followed by gold sputtering. The samples were analyzed with a Jeol Jx A-840 SEM (MA, USA). SEM images were further analyzed using Microsun 2000/s image analysis software to obtain detailed information about the nanostructure of the BC membrane. The image analysis was performed at a magnification of 10,000× and an accelerating voltage of 5 kV [41].
2.5.3. Membrane Composition Analysis
The compositions of BC and CA membranes were analyzed through the lignocellulose analysis. The analysis focuses on determining the content of key components cellulose, lignin, and hemicellulose that collectively form the complex structure of membrane materials. In this study, 80 mL of acetone was placed in a soxlet extractor and heated to 90 °C for an hour. The acetone was then allowed to pass through the extractor for 30 min at room temperature. To analyze the hemicellulose, 15 cm3 test tubes were filled with NaOH and heated to 80 °C for 210 min. Then the BC residue was filtered through Whatman No. 1 filter papers and was dried in an oven set at 105 °C for three hours. To calculate the amount of lignin, 0.2 g of BC and 3 mL of H2SO4 were prepared in a 500 mL flask and heated to 30 °C for one hour. Following this, the mixture was autoclaved for 15 min at 121 °C after 56 cm3 of DI water was added. The resulting solution was filtered using Whatman papers and dried at 80 °C for 3 h. Equations (2)–(4) were utilized to calculate the composition of BC and CA membranes [42,43].
Cellulose Content:
Cellulose (%) = NDF (%) − ADF (%)
Hemicellulose Content:
Hemicellulose (%) = NDF (%) − ADF (%)
Lignin Content:
where NDF is the weight of neutral detergent fiber, and ADF is the weight of acid detergent fiber.
Lignin (%) = NDF (%) − Cellulose (%) − Hemicellulose (%)
2.6. Eco-Efficiency
Eco-efficiency is a comprehensive matrix that considers resource utilization, economic development, and environmental implications when evaluating the performance of product, process, and service (PPS) systems [44]. It is a useful tool for encouraging sustainable growth since it conforms with the eco-efficiency assessment of product systems required by the ISO14045 standard for environmental management [45]. Eco-efficiency is defined as the value of a product or service in relation to its environmental impact, encompassing factors such as energy, material, and water consumption, as well as greenhouse gas and CO2 emissions. This technique provides for an in-depth investigation that considers both economic and environmental elements. The conceptual equation for calculating eco-efficiency is represented as Equation (5) [46].
Eco-efficiency = Value of the product, process, or service (PPS)
/Environmental impact of the PPS
/Environmental impact of the PPS
The coupling of sustainability and eco-efficiency concepts in this study necessitated a change to the eco-efficiency Equation (6). The BC yield was employed to represent the PPS value in this context. The fixed operational expenses were determined using the cost of BC synthesis used in the experiment. The total operational cost of the bio-CA membrane, including chemical and electricity expenditures, was calculated using the equation below.
where Ed is the yield of BC and Ec is the cost of CA membrane production, including chemical uses and operating costs.
Eco-efficiency = Ed/Ec
2.7. CO2 Separation by CA Membrane
Several studies have shown that the CA membrane can remove the CO2 from gas mixtures. A membrane separation unit was designed to examine the CO2 separation performance of the bio-CA membrane, as shown in Figure 2. This unit, constructed from aluminum, is comprised of five layers. The feed inlet and the retentate outlet were both attached to one end of the device. Between the feed and permeate chambers was a porous stainless-steel disc on which the CA membranes were placed. On the permeate chamber side, the permeate outlet was connected to the endplate. Each compartment’s rubber O-rings were used to stop gas leakage. The gas mixture was controlled by a regulator at the top of the membrane separation unit, as shown in Figure 2. With the use of a rotameter, the gas flow rate was monitored and regulated. The gas mixture of 40/60% CO2/CH4 was used to examine the CO2 selectivity of the BC and CA membranes in the separation unit. The separation conditions were set at 0.3 MPa of gas mixture pressure, a temperature of 30 °C, and CA membrane thicknesses of 0.04 ± 0.005 and 0.05 ± 0.005 mm. Using the Agilent GC model 7890B gas chromatography technique, the gas compositions of the permeate and retentate streams were examined.
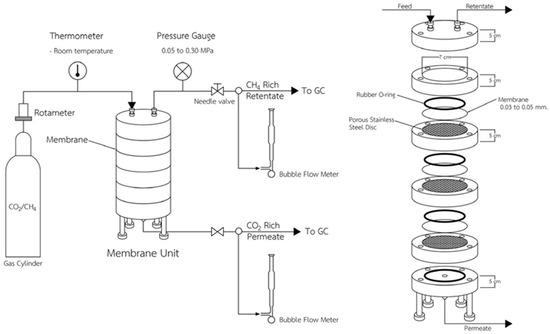
Figure 2.
Membrane separation unit.
The separation performance of the CO2 separation from the binary mixed gas was analyzed in terms of the permeability of the CH4 and CO2 components and the CO2 selectivity. For membrane separation of a mixed gas system, the permeability of each component can be calculated by Equation (7) [47].
where Pi is the permeability of component i, Q is the feed flowrate, Xperm,i is the mole fraction of component i in the permeate stream, Xfeed,i is the mole fraction of component i in the feed stream, l is the membrane thickness, and A is the membrane area.
Pi = Q × Xperm,i × l/(A(PfeedXfeed,i − PpermXperm,i))
The separation capability in terms of the selectivity (Sa/b) is obtained from Equation (8):
where Pa is the permeability of component a, and Pb is the permeability of component b.
Sa/b = Pa/Pb
3. Results
3.1. Membrane Preparation
In the early stage of the cultivation, a wet gel-like thin membrane of BC was formed.
The membrane became denser and thicker as the cultivation process proceeded until the cultivating medium was depleted. Figure 3a–c depicts the wet and dry BC and dry CA membranes, respectively. The BC yield and Eco-efficiency result (dry basis) for all 15 experiments designed by DE V.13 are summarized in Table 1. The amount of BC that was produced, in terms of dry yields, depended largely on the set-forth cultivating conditions with various pH, time, and temperature. All three parameters play important roles in the cultivation process that affected the resulting yields to different degrees of extent. From the data, the maximum dry yield of 9.31% (g/g) was obtained from the experiment when the cultivation process took place at 30 °C for 8 days with a controlled pH value of 6. The results conclude that the CJRs could be potentially used as a carbon source for BC production. The yield of BC production from CJR in this study was converted to g/l by using a coconut juice density of 1020 g/L. The yield obtained from CJRs (94.96 g/L) proves to be significantly higher (approximately 10 folds) when compared to other carbon sources, as shown in Table 2. According to a previous study [48], optimized substrate conditions have identified coconut juice as a more suitable substrate for BC production compared to pineapple juice. A. xylinum exhibits high BC formation rates and efficiently converts glucose to biomass and BC when using a coconut juice substrate. To determine the optimal conditions that correspond to the maximum yields, correlations between the yields and the affecting factors must have been established. The statistical analysis by experimental design allows for the determination of the optimal condition in any given condition.
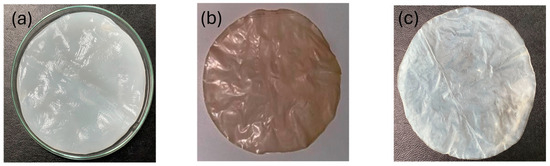
Figure 3.
(a) wet BC; (b) dry BC; (c) dry CA.

Table 1.
Experimental and predicted BC yields eco-efficiency.

Table 2.
Comparison of BC synthesis prepared from various natural carbon sources.
3.2. Membrane Characteristics
3.2.1. FTIR Analysis
FTIR analysis was used to verify the existence of the achieved BC and CA membranes. Figure 4 shows the FTIR spectrum of the membranes. The results revealed that both BC and CA membranes show similar distinct spectrum peaks of the cellulose backbones, including a sharp peak at 2925 cm−1 (C-H stretching), 1654 cm−1 (C=O stretching in the cellulose structure), 1314 cm−1 (C-O-C asymmetric stretching), and 1161 cm−1 (C-O-C asymmetric stretching). However, the peak at around 1210–1150 cm−1 was observed in the CA sample. These peaks are associated with C-C-O stretches in saturated esters such as those in cellulose acetate. The FTIR results show a good correlation with other studies on bacterial cellulose and cellulose acetate materials, undertaken by [52,53,54,55].
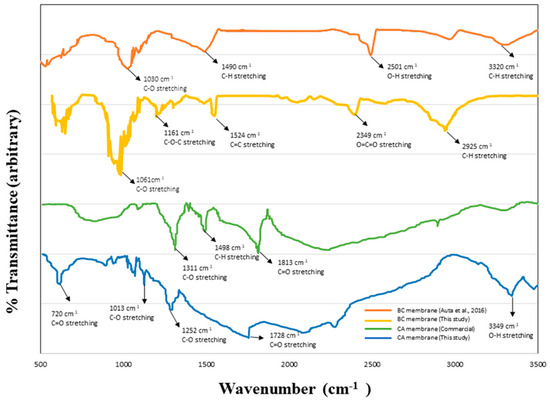
Figure 4.
FTIR analysis of the BC and CA membrane [52].
3.2.2. Morphology and Structural Analysis
The BC membranes were fabricated using the established cultivation process as previously described. The morphological analysis of BC was conducted using Scanning Electron Microscopy (SEM), encompassing an assessment of fibril density, size, and arrangement. These aspects are known to be influenced by factors such as medium composition, viscosity, and the activity of the BC-generating bacteria, as discussed in the works of [56]. Following freeze-drying, two distinct BC samples were subjected to SEM examination. The SEM micrographs of the highest and lowest yield BC membranes, illustrating their normal and cross-sectional surfaces, respectively, are presented in Figure 5 and Figure 6. Figure 5 illustrates the membrane with the highest potential yield obtained through the employed method. In general, the membrane exhibits a multilayer-nanofibrillar structure. It is composed of tightly packed nanofibrils arranged in a 3D network in which the cellulose fibers are randomly intertwined to form overlapping woven patterns, which were similar to [57] that BC obtained from different media all had uniform three-dimensional networks of cellulose nanofibers and microfibers, as depicted in Figure 5a. The cross-sectional view reveals a multi-stack-like structure comprising multiple thin layers of BC fiber sheets arranged in superimposition, with porosity introduced by the interlayer gaps, as shown in Figure 5b (10 k× magnification) and Figure 5c (30 k× magnification). In contrast, Figure 6 displays the SEM micrograph of the membrane with the lowest yield, exhibiting a less dense arrangement of fibers compared to Figure 5. Figure 5b (the highest yield) shows that the cellulose fibers are denser with less void compared to Figure 6b (the lowest BC yield). This might result from incompletely developed BC due to the shorter cultivation period. The cross-sectional profile also shows evident gaps between the layers of BC fiber sheets, indicative of reduced compactness compared to the higher-yield counterpart. From the results, the degree of porosity of the membrane could be manipulated by adjusting the appropriate cultivating time. The unique interconnected woven nano-fibrillar structure gives good mechanical strength and duration to the biodegradable BC membranes. In addition, the porous structure would allow excellent absorption and gas permeability. Proper modifications to the based BC membranes could be further tailored for such particular membrane separation applications.
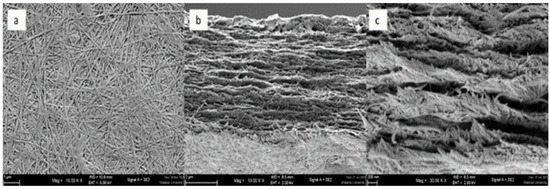
Figure 5.
SEM results of the BC membrane sample with the highest yield of (a) surface, (b) cross-section at 10 k× magnification, (c) cross-section at 30 k× magnification.
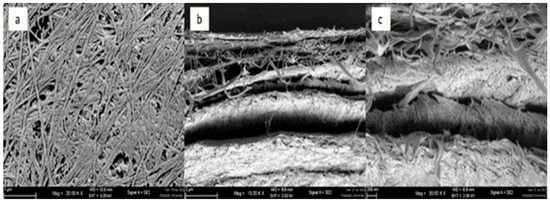
Figure 6.
SEM results of the BC membrane sample with the lowest yield of (a) surface, (b) cross-section at 10 k× magnification, (c) cross-section at 30 k× magnification
3.2.3. Lignocellulose Compositions
The compositions of the BC and CA membranes with the comparison with those of previous studies are summarized in Table 3. The BC sample with the highest yield demonstrated a cellulose content of 46.20%, along with hemicellulose at 22.13% and lignin at 31.67%. Conversely, the sample with the lowest yield gave the cellulose, hemicellulose, and lignin content of 25.54%, 44.29%, and 30.17%, respectively. For CA membrane samples, cellulose, hemicellulose, and lignin contents ranged from 37.45–48.08%, 28.59–29.22%, and 23.33–33.33%, respectively. The compositions of BC and CA membranes in this study are comparable with those from other studies, as illustrated in Table 3. From the results, cellulose was considered to be the main component, typically constituting 25–46% of the dry weight. The cellulose constituent is typically arranged in a highly crystalline and interconnected network, contributing to the membrane’s strength and rigidity. The hemicellulose consisted of 22–44% of the dry weight. This polysaccharide component is less crystalline than cellulose and consists of various sugar monomers (e.g., xylose, mannose, galactose) with a branched structure, which can enhance the water-holding capacity and flexibility of the membranes. The phenolic lignin made up the rest of the composition of the membranes. Since the coconut juice residues were used as the carbon source, the lignin content could have stemmed from the bioprocess of suspended lignocellulose biomass presented in the cultivating media. At the longer cultivation time employed for BC synthesis, a higher yield could be achieved at which the hemicellulose constituent was shifted towards the increase in the cellulose structure. It is important to note that the specific lignocellulosic composition of the BC membranes can be tailored to some extent by optimizing the fermentation process and carbon source selection. These varying constituent ratios underscore the intricate nature of BC synthesis, prompting further investigation into the underlying mechanisms at play. Understanding the composition and its relation to desired properties is crucial for developing BC membranes with targeted applications in various fields such as biofiltration, wound healing, and composite materials. Acetylation of lignocellulose involves the replacement of hydroxyl (OH) groups in cellulose with acetyl (CH₃CO) groups. In the context of cellulose acetate (CA) membranes, this acetylation process subtly modifies the lignocellulose composition, potentially affecting the constituents that originally contained hydroxyl groups.

Table 3.
BC and CA membrane composition.
3.3. Statistical Analysis for BC Yield and Eco-Efficiency
Model summary statistics of dry yields and eco-efficiency in Table 4 show the coefficient of determination (R2) of the proposed model is greater than 0.9. By focusing on the model maximizing the adjusted R2 and the predicted R2, it was shown that, as suggested, 2FI can be used to predict the dry BC yield. The quadratic model of BC production was also suggested by earlier research [62] for the affecting factors of fructose concentration, corn steep liquor (CSL) concentration, dissolved oxygen, and agar content.

Table 4.
Model summary statistics of dry yields.
The ANOVA was used to examine the model’s significance, and the results are shown in Table 5. The significance of the model for the dry BC yield is also presented in Table 5. The model’s F-value of 18.09 indicates its significance. The likelihood of an F-value of this substantial occurring by chance is only 0.66%. Model terms with p-values less than 0.0500 are deemed statistically significant. In this situation, B, C, and BC are important model terms. The lack of fit F-value of 0.17 indicates that the lack of fit is not substantial in comparison to the pure error. A large lack of fit F-value could arise due to noise 85.35 percent of the time. A non-significant lack of fit is a positive factor.

Table 5.
Analysis of variance (ANOVA) for the response surface model of dry BC yield.
Figure 7 shows the normal percent probability and externally studentized residuals for dry BC yields. The linear model demonstrates a significant correlation between input and output variables. The time of fermentation plays a crucial role in the production of bacterial cellulose from coconut water, with different studies suggesting different optimal fermentation times [63]. The temperature plays a significant role in BC production as well [20]. It has been found that A. xylinum culture needs warm and static conditions for growth [64]. Cultivation time and temperature are key factors that were identified when using DE V.13. On the other hand, compared to the other factors, pH in the range of 4–6 was not as significant for the cultivation of BC. Nevertheless, others reported that decreasing the pH level would reduce BC production; decreasing the pH level lower than 4 will have a significant effect on BC production [43,65].
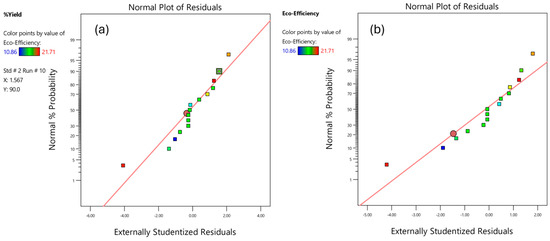
Figure 7.
Normal percent probability of residuals versus externally studentized residuals of (a) dry BC yields and (b) eco-efficiency.
A three-dimensional surface diagram is a graphical representation of the regression equation that can be used to optimize the parameters and examine the interactions between them [66]. The results of the interaction effect between the three independent parameters and the dependent parameter are shown in Figure 8. Figure 8a shows the interaction effect of cultivation time and pH on the dry yield, which depicts a 2FI response surface graph. The effect of cultivation time and pH on the yield was significant for the value of the yield in the range. The interaction effect of temperature and pH on the yield depicted a 2FI curve as shown in Figure 8b. The effect of temperature and cultivation time on the dry BC yield is shown in Figure 8c. Since there is no significant variation when utilizing pH 4–6 for the same length of time and temperature, the pH impact has no significant influence on the dry BC yield. Both trials validated the projected outcomes, which revealed the same general patterns.
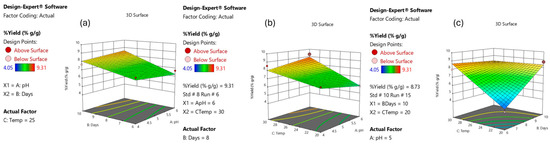
Figure 8.
Response surface plot demonstrates the interaction effect of (a) pH and days; (b) pH and temperature; (c) days and temperature for dry yield BC.
The considerable decline in BC production could be attributed to a decrease in pH culture as a result of the synthesis of gluconic acid as a byproduct during the static fermentation process [67]. Model fitting with DE V.13 was utilized to generate the best-fitted model to maximize the optimal conditions of dry BC yields. The 2FI equation was derived from the fitting experimental data and stated in terms of actual elements as follows in Equation (9):
where A is the pH, B is the cultivation time (days), and C is the temperature (°C).
Dry BC yield = −6.79533 − 3.3575A + 2.5525B + 0.6355C + 0.1325AB + 0.092AC − 0.1105BC
3.4. Eco-Efficiency Analysis
Comparing the costs of carbon sources to synthesize one BC membrane sheet (50 cm2) reveals that utilizing CJRs is more cost-effective than using sugar. The cost of producing BC membranes using sugar amounts to 2.336 THB/sheet for dry BC. In contrast, the cost of using CJRs is approximately in the range of 0.37 to 0.50 THB/sheet for dry CA. The cost of BC and CA production using CJRs and carbon sources is composed of fixed and operating costs. The fixed cost covers chemicals and materials used in the synthesis. While operating cost includes the utility of the synthetic process. Table 1 displays the costs and eco-efficiency of each set of experiments. From Table 1, the cost for conditions with longer incubating periods is higher. However, the eco-efficiency analysis results reveal a positive correlation wherein an increase in yield corresponds to an elevated level of eco-efficiency, which confirms the optimal conditions for BC formulation suggested by the DE V.13 optimization. The model F-value of 12.66 implies the model is significant. There is only a 0.11% chance that an F-value this large could occur due to noise. p-values (0.0011) that are less than 0.0500 indicate model terms are significant. In this case, C, and BC are significant model terms for eco-efficiency.
Figure 9a shows the interaction effect of pH and cultivation time on the eco-efficiency, which depicts a 2FI response surface graph. The interaction effect of pH temperature on the eco-efficiency depicted a 2FI curve, as shown in Figure 9b. The effect of temperature and cultivation time on the eco-efficiency is shown in Figure 9c. Since there is no significant variation when utilizing pH 4–6 for the same length of time and temperature, the pH impact has no significant influence on the eco-efficiency. The 2FI equation was derived from the fitting experimental data and stated in terms of actual elements as follows in Equation (10):
where A is the pH, B is the cultivation time (days), and C is the temperature (°C).
Eco-efficiency = −12.8464 − 7.76375A + 5.46313B + 1.757C + 0.295AB + 0.2145AC − 0.28725BC

Figure 9.
Response surface plot demonstrates the interaction effect of (a) pH and days; (b) pH and temperature; (c) days and temperature for eco-efficiency.
3.5. Optimal Condition and Validation of Bio-BC Membrane
The optimal conditions for the synthesis of BC were investigated and accomplished using the response optimizer function in a statistical package. The optimal conditions were chosen with the highest value of the composite desirability (D) function, which has a value between 0 and 1. If the D value is equal to 1, then the result is completely satisfied [68]. 80 conditions were extracted from the model based on the dry BC yield response optimizer results. As demonstrated in Figure 10 with the dry BC yield of 8.92% and the D value of 1.000, the optimal conditions for BC yield suggested by the model are 30 °C, pH 6, and a cultivation time of 7.35 days as exponential growth is observed at 48 h, indicating a longer cultivation period is needed for higher yield [48], and a temperature of 30 °C [63].
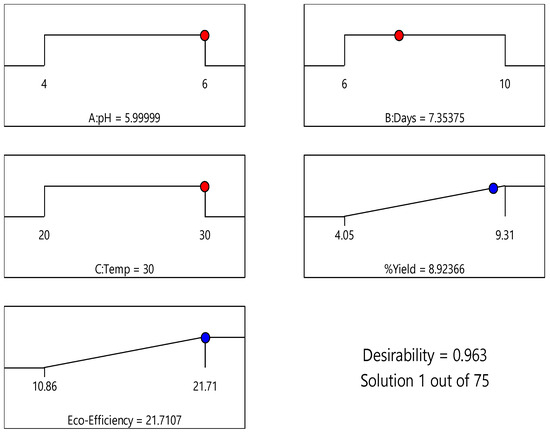
Figure 10.
Desirability ramps of dry BC yield and eco-efficiency.
A set of experiments was conducted to validate the model received from the DE V.13 optimization to predict the dry BC yields. As seen from Table 6, the average value of dry BC yields obtained from the experiment was 9.03%, respectively, which is in good agreement with that from the model (9.36%). The results validate the model’s excellent prediction for dry BC yield optimization.

Table 6.
Confirmation of conditional optimizations.
3.6. CO2 Removal from CH4/CO2 Gas Mixture
As previously discussed, modification of the BC membrane by acetylation was performed to graft the acetyl functional group onto the cellulose main chains to some degree of extent. The synthetic biogas containing binary CH4/CO2 (60/40% v) mixture was fed into the membrane unit. The GC results of the permeate were used in calculations to investigate the potential of using the achieved cellulose acetate (CA) membrane for CO2 removal from the biogas. Table 7 shows the CO2/CH4 separation capability of the BC and bio-CA membranes in terms of CH4 permeability, CO2 permeability, and CO2 selectivity (SCO2). Under a feed pressure of 0.3 Mpa, the SCO2 values for the CA membranes having the thickness of 0.04 mm and 0.05 mm were 1.116 and 1.315, respectively. While the base BC membrane exhibited an insignificant shift in the gas composition since the SCO2 was roughly about 1. The SCO2 greater than 1 indicates that the CO2 is more selective to the CA membrane than the CH4 when transported through the membrane, resulting in a more CO2-rich composition on the permeate side. The transport of CO2 and CH4 through the dense multi-layer fibrous CA membranes could be explained by the sorption-desorption mechanism of the diffusing pair components, which was induced by the concentration differences between the feed and the permeate side [69]. Compared to the BC structure, the presence of carbonyl in addition to the hydroxyl groups in the CA backbones allowed stronger interactions towards the polar CO2 than the non-polar CH4 molecules. The CO2 solubility within the CA matrix was increased as a result; therefore, more of the CO2 could selectively diffuse through the CA membrane when compared to the non-polar CH4 components [70,71]. The observed CO2 selectivity (1.116–1.315) of the CA membranes was comparable and in good agreement with the separation results reported in a study by [72], at which the SCO2 of 1.2 was achieved in a CO2/CH4 separation by the unmodified cellulose triacetate hollow membrane. Cellulose acetate with a higher degree of acetylation would demonstrate improved CO2 interaction with the matrix membrane [73]. In other words, the affinity of the membrane towards CO2 is higher compared to CH4, which could result in better gas separation capabilities [74]. Modifications of the pristine cellulose acetate membrane to improve its CO2 interactions have led to significantly improved CO2/CH4 separation performance with high CO2 selectivity and permeability [75].

Table 7.
Membrane separation results.
4. Conclusions
CJRs have been shown as a potential carbon source for BC production using A. xylinum in the cultivation process. Both experimental and optimizing results suggested that the most crucial parameter among the three factors in this study is the cultivation period. The longer period in cultivation time is reflected in the increasing BC yields and eco-efficiency. The statistical modeling of yield predictions was successfully achieved from DE V.13. The optimal conditions for BC synthesis should occur at a pH of 6 for 10 days at 30 °C for dry BC. These conditions produced the best yield of dry BC at 9.31% g/g or 94.96 g/L. The synthesis of BC membrane from CJRs was cost-effective. The cost for the fabrication using CJRs as the raw material was found to be 3 to 10 times less than when compared to the process using sucrose. In addition, the obtained BC membrane, which possesses a morphology with multiple layers of entangled cellulose fibers becomes attractive as the base membrane to be utilized in many promising separation processes. Modification of the obtained BC by acetylation reaction resulted in the formation of a selective cellulose acetate (CA) membrane that exhibited the capability to remove CO2 from a binary gas mixture consisting of CH4 and CO2 in membrane separation. The separation capability in terms of the selectivity of the CO2 component (SCO2) of the CA membrane was significantly improved (SCO2 = 1.315) compared to that of the unmodified BC membrane (SCO2 = 0.997). The preparation of bio-CA membranes from the proposed high eco-efficiency route could become attractive to be utilized in biogas purification applications. However, further study on how to improve the separation efficiency of these bio-CA membranes would be required to cope with limitations. The implications of this work are promising and could serve as the foundation for creating an economically attractive and environmentally friendly bio-based membrane from bio-waste. Such a membrane would be useful for biogas upgrading and purification, opening wider opportunities for affordable renewable biogas and other related applications.
Author Contributions
W.D. and A.K.: conceptualized and supervised the study, designed and validated the experiments, analyzed and interpreted the results, wrote and edited the manuscript; K.W.: performed the experiments, analyzed and visualized the experimental results, prepared the original draft, wrote and edited the manuscript; J.H.L.: curated and assisted on language editing, prepared the original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This work has been supported by The Ph.D. Excellence Scholarship, Walailak University, Contract No. 02/2020.
Data Availability Statement
The data and code supporting the results presented in this paper, as well as additional findings from this study, are accessible from the corresponding author upon reasonable request.
Acknowledgments
The authors would like to thank the Biomass and Oil Palm Center of Excellence (BoP-CoE), Walailak University, for facilitating laboratory support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wis, A.A.; Kodal, M.; Ozturk, S.; Ozkoc, G. Overmolded polylactide/jute-mat eco-composites: A new method to enhance the properties of natural fiber biodegradable composites. J. Appl. Polym. Sci. 2020, 137, 48692. [Google Scholar] [CrossRef]
- Office of Agricultural Economics, Coconut Production. S.E.O., Percentage and Monthly Output Volume. Including Countries, Regions and Provinces in 2022. Available online: https://www.oae.go.th/assets/portals/1/fileups/prcaidata/files/Coconut%20percent%2065.pdf (accessed on 13 June 2024).
- Alfian, R.N.; Choirunnisa, N.I.; Susilo, B.; Sandra. Effect of storage temperatures treatment of coconut flesh (Cocos nucifera L.) based on the quality of coconut milk. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2023. [Google Scholar] [CrossRef]
- Srivastava, Y.; Semwal, A.D.; Sharma, G.K. Virgin Coconut Oil as Functional Oil. In Therapeutic, Probiotic, and Unconventional Foods; Elsevier: Amsterdam, The Netherlands, 2018; pp. 291–301. [Google Scholar] [CrossRef]
- Mandey, L.; Kandou, J.E.A.; Tarore, D. Nata De Coco Quality Development Model from Different Inoculum Sources. Int. J. Life Sci. Agric. Res. 2024, 3, 259–266. [Google Scholar] [CrossRef]
- Yardrung, S.; Waritchon, N.; Rungtiwa, S.; Komsan, M. Production of Bacterial Cellulose from Acetobacter xylinum by using Rambutan Juice as a Carbon Source. Int. J. Agric. Technol. 2017, 13, 1361–1369. [Google Scholar]
- Sulastri, A.; Rahmidar, L. Fabrication of Biomembrane from Banana Stem for Lead Removal Fabrication of Biomembrane from Banana Stem for Lead Removal. Indones. J. Sci. Technol. 2016, 1, 115–131. [Google Scholar] [CrossRef]
- Candido, R.G.; Godoy, G.G.; Gonçalves, A. Characterization and application of cellulose acetate synthesized from sugarcane bagasse. Carbohydr. Polym. 2017, 167, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Kongruang, S. Bacterial Cellulose Production by Acetobacter xylinum Strains from Agricultural Waste Products. Appl. Biochem. Biotechnol. 2008, 148, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Przygrodzka, K.; Charęza, M.; Banaszek, A.; Zielińska, B.; Ekiert, E.; Drozd, R. Bacterial Cellulose Production by Komagateibacter xylinus with the Use of Enzyme-Degraded Oligo- and Polysaccharides as the Substrates. Appl. Sci. 2022, 12, 12673. [Google Scholar] [CrossRef]
- Liany, S.A.; Syafira, W.; Putri, A.; Khasanah, A.U. Effect of Bacterial Cellulose (BC) Formation on Various Substrate Variations and Combinations. Berk. Ilm. Biol. 2022, 13, 13–20. [Google Scholar] [CrossRef]
- Liang, X.; Hu, W.; Zhong, J.-J. Use of bacterial cellulose (BC) from a mutated strain for BC-starch composite film preparation. Process Biochem. 2023, 131, 1–12. [Google Scholar] [CrossRef]
- Huang, H.Y.; Cheng, Y.S. Heterologous overexpression, purification and functional analysis of plant cellulose synthase from green bamboo. Plant Methods 2019, 15, 80. [Google Scholar] [CrossRef]
- Aloni, Y.; Delmer, D.P.; Benzimant, M. Achievement of high rates of in vitro synthesis of 1,4-13-D-glucan: Activation by cooperative interaction of the Acetobacter xylinum enzyme system with GTP, polyethylene glycol, and a protein factor. Proc. Natl. Acad. Sci. USA 1982, 79, 6448–6452. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Zheng, X. The crystal structure of human UDP-glucose pyrophosphorylase reveals a latch effect that influences en-zymatic activity. Biochem. J. 2012, 442, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Penttilä, P.A.; Imai, T.; Capron, M.; Mizuno, M.; Amano, Y.; Schweins, R.; Sugiyama, J. Multimethod approach to understand the assembly of cellulose fibrils in the biosynthesis of bacterial cellulose. Cellulose 2018, 25, 2771–2783. [Google Scholar] [CrossRef]
- Andrade, F.K.; Morais, J.P.S.; Muniz, C.R.; Nascimento, J.H.O.; Vieira, R.S.; Gama, F.M.P.; Rosa, M.F. Stable microfluidized bacterial cellulose suspension. Cellulose 2019, 26, 5851–5864. [Google Scholar] [CrossRef]
- Zahan, K.A.; Anuar, A.H.I.; Ring, L.C.; Yenn, T.W.; Mustapha, M. Characterization of bacterial cellulose produced via fermentation of Acetobacter xylinum 0416. Int. J. Adv. Appl. Sci. 2017, 4, 19–24. [Google Scholar] [CrossRef][Green Version]
- Pham, T.T.; Tran, T.T.A. Evaluation of the crystallinity of bacterial cellulose produced from pineapple waste solution by using acetobacter xylinum. ASEAN Eng. J. 2023, 13, 81–91. [Google Scholar] [CrossRef]
- Uğurel, C.; Öğüt, H. Optimization of Bacterial Cellulose Production by Komagataeibacter rhaeticus K23. Fibers 2024, 12, 29. [Google Scholar] [CrossRef]
- Lima, N.F.; Maciel, G.M.; de Andrade Arruda Fernandes, I.; Haminiuk, C.W.I. Optimising the Production Process of Bacterial Nanocellulose: Impact on Growth and Bioactive Compounds. Food Technol. Biotechnol. 2023, 61, 494–504. [Google Scholar] [CrossRef]
- Zhu, B.; Zhang, Z.; Wang, H.; Ma, X. Isolation and culture conditions optimization of a new bacterial cellulose producing strain komagataeibacter intermedius 6-5. In IOP Conference Series: Earth and Environmental Science; IOP Publishing Ltd.: Bristol, UK, 2021. [Google Scholar] [CrossRef]
- Khami, S.; Khamwichit, W.; Suwannahong, K.; Sanongraj, W. Characteristics of bacterial cellulose production from agricultural wastes. Adv. Mater. Res. 2014, 931, 693–697. [Google Scholar] [CrossRef]
- Khami, S.; Khamwichit, W.; Suwannahong, K. Synthesis of cellulose acetate nanofiber (CANF) from bacterial cellulose (BC) incubated from cannery seafood wastewater (CSW) using acetobacter xylinum. ARPN J. Eng. Appl. Sci. 2019, 14, 3038–3045. Available online: https://www.arpnjournals.com (accessed on 15 September 2024).
- Pa’e, N.; Zahan, K.A.; Muhamad, I.I. Production of Biopolymer from Acetobacter xylinum Using Different Fermentation Methods. Int. J. Eng. Technol. 2011, 15, 90–98. [Google Scholar]
- Tristantini, D.; Sandra, C. Synthesis of cellulose acetate from palm oil bunches and dried jackfruit leaves. In E3S Web of Conferences; EDP Sciences: Les Ulis, France, 2018. [Google Scholar] [CrossRef]
- Gillis, M.; Kersters, K.; Hoste, B.; Janssens, D.; Kroppenstedt, R.M.; Stephan, M.P.; Teixeira, K.R.S.; Dobereiner, J.; De Ley, J. Acetobactev diazotrophicus sp. nov., a Nitrogen-Fixing Acetic Acid Bacterium Associated with Sugarcane. Int. J. Syst. Evol. Microbiol. 1989, 39, 361–364. [Google Scholar]
- da Silva Meireles, C.; Filho, G.R.; Ferreira, M.F.; Cerqueira, D.A.; Assunção, R.M.N.; Ribeiro, E.A.M.; Poletto, P.; Zeni, M. Characterization of asymmetric membranes of cellulose acetate from biomass: Newspaper and mango seed. Carbohydr. Polym. 2010, 80, 954–961. [Google Scholar] [CrossRef]
- Zakaria, J. Optimization of Bacterial Cellulose Production from Pineapple Waste: Effect of Temperature, pH and Concentration. 2012. Available online: https://www.researchgate.net/publication/326550365 (accessed on 15 September 2024).
- Cheng, K.C.; Catchmark, J.M.; Demirci, A. Enhanced production of bacterial cellulose by using a biofilm reactor and its material property analysis. J. Biol. Eng. 2009, 3, 12. [Google Scholar] [CrossRef] [PubMed]
- Mithra, S.N.; Ahankari, S.S. Biogas purification by CO2 removal using facilitated transport mechanism of cellulose nanocrystals/polyvinyl alcohol layered hollow fiber membranes. J. Appl. Polym. Sci. 2024, 141, e55536. [Google Scholar] [CrossRef]
- Tanvidkar, P.; Jonnalagedda, A.; Kuncharam, B.V.R. Investigation of cellulose acetate and ZIF-8 mixed matrix membrane for CO2 separation from model biogas. Environ. Technol. 2024, 45, 2867–2878. [Google Scholar] [CrossRef]
- Tanvidkar, P.; Jonnalagedda, A.; Kuncharam, B.V.R. Fabrication and testing of mixed matrix membranes of UiO-66-NH2 in cellulose acetate for CO2 separation from model biogas. J. Appl. Polym. Sci. 2023, 140, e53264. [Google Scholar] [CrossRef]
- Kayarogannam, P. Introductory Chapter: Response Surface Methodology, n.d. Available online: https://www.intechopen.com (accessed on 15 September 2024).
- Sopyan, I.; Gozali, D.; Sriwidodo; Guntina, R.K. Design-expert software (DOE): an application tool for optimization in pharmaceutical preparations formulation. Int. J. Appl. Pharm. 2022, 14, 55–63. [Google Scholar] [CrossRef]
- Aliemeke, B.N.G.; Okwudibe, H.A.; Omoakhalen, A.I.; Major, P. Box-behnken optimization of pin-on-disc wear test process parameters. Niger. J. Technol. 2023, 42, 464–471. [Google Scholar] [CrossRef]
- Kiwu, L.C.; Kiwu-Lawrence, F.C.; Boniface, D.C.O.I.; Onyemachi, E. Appropriate Number of Center Points for Response Surface Exploration Using Small Box Behnken Design. Arch. Curr. Res. Int. 2022, 22, 1–6. [Google Scholar] [CrossRef]
- Mwangi, W.P.; Anapapa, A.; Otieno, A. Selection of Second Order Models’ Design Using D-, A-, E-, T-Optimality Criteria. Asian J. Probab. Stat. 2019, 5, 1–15. [Google Scholar] [CrossRef]
- Khamwichit, A.; Wattanasit, S.; Dechapanya, W. Synthesis of Bio-Cellulose Acetate Membrane From Coconut Juice Residues for Carbon Dioxide Removal from Biogas in Membrane Unit. Front. Energy Res. 2021, 9, 670904. [Google Scholar] [CrossRef]
- Barud, H.S.; de Araújo Júnior, A.M.; Santos, D.B.; de Assunção, R.M.N.; Meireles, C.S.; Cerqueira, D.A.; Filho, G.R.; Ribeiro, C.A.; Messaddeq, Y.; Ribeiro, S.J.L. Thermal behavior of cellulose acetate produced from homogeneous acetylation of bacterial cellulose. Thermochim. Acta 2008, 471, 61–69. [Google Scholar] [CrossRef]
- Dechapanya, W.; Khamwichit, A. Biosorption of aqueous Pb(II) by H3PO4-activated biochar prepared from palm kernel shells (PKS). Heliyon 2023, 9, e17250. [Google Scholar] [CrossRef] [PubMed]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber. Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists; Latimer, G.W.; Horwitz, W. Official Methods of Analysis of AOAC International; AOAC International: Rockville, MD, USA, 2006. [Google Scholar]
- Liu, K.; Catchmark, J.M. Bacterial cellulose/hyaluronic acid nanocomposites production through co-culturing Gluconacetobacter hansenii and Lactococcus lactis under different initial pH values of fermentation media. Cellulose 2020, 27, 2529–2540. [Google Scholar] [CrossRef]
- Changwichan, K.; Silalertruksa, T.; Gheewala, S.H. Eco-efficiency assessment of bioplastics production systems and end-of-life options. Sustainability 2018, 10, 952. [Google Scholar] [CrossRef]
- Heilala, J.; Ruusu, R.; Montonen, J.; Vatanen, S.; Kavka, C.; Asnicar, F.; Scholze, S.; Armiojo, A.; Insunza, M. Eco-Process Engineering System for Collaborative Product Process System Optimisation; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Basu, S.; Khan, A.L.; Cano-Odena, A.; Liu, C.; Vankelecom, I.F.J. Membrane-based technologies for biogas separations. Chem. Soc. Rev. 2010, 39, 750–768. [Google Scholar] [CrossRef]
- Lestari, P.; Elfrida, N.; Suryani, A.; Suryadi, Y. Study on the Production of Bacterial Cellulose from Acetobacter xylinum using Agro-Waste. Jordan J. Biol. Sci. 2014, 7, 75–85. [Google Scholar] [CrossRef]
- Hungund, B.; Prabhu, S.; Shetty, C.; Acharya, S.; Prabhu, V.; Gupta, S.G. Production of bacterial cellulose from gluconacetobacter persimmonis GH-2 using dual and cheaper carbon sources. J. Microb. Biochem. Technol. 2013, 5, 31–33. [Google Scholar] [CrossRef]
- Tabaii, M.J.; Emtiazi, G. Comparison of Bacterial Cellulose Production among Different Strains and Fermented Media. Appl. Food Biotechnol. 2016, 3, 35–41. Available online: www.journals.sbmu.ac.ir/afb (accessed on 15 September 2024).
- Zhao, H.; Xia, J.; Wang, J.; Yan, X.; Wang, C.; Lei, T.; Xian, M.; Zhang, H. Production of bacterial cellulose using polysaccharide fermentation wastewater as inexpensive nutrient sources. Biotechnol. Biotechnol. Equip. 2018, 32, 350–356. [Google Scholar] [CrossRef]
- Auta, R.; Adamus, G.; Kwiecien, M.; Radecka, I.; Hooley, P. Production and characterization of bacterial cellulose before and after enzymatic hydrolysis. Afr. J. Biotechnol. 2017, 16, 470–482. [Google Scholar] [CrossRef]
- Akintunde, M.O.; Adebayo-Tayo, B.C.; Ishola, M.M.; Zamani, A.; Horváth, I.S. Bacterial Cellulose Production from agricultural Residues by two Komagataeibacter sp. Strains. Bioeng. 2022, 13, 10010–10025. [Google Scholar] [CrossRef] [PubMed]
- Kuzminova, A.; Dmitrenko, M.; Dubovenko, R.; Puzikova, M.; Mikulan, A.; Korovina, A.; Koroleva, A.; Selyutin, A.; Semenov, K.; Su, R.; et al. Development and Study of Novel Ultrafiltration Membranes Based on Cellulose Acetate. Polymers 2024, 16, 1236. [Google Scholar] [CrossRef]
- Afzal, A.; Liaqat, W.; Ahsan, F. Synthesis and anti-microbial investigations of CZ6 composite reinforced CA mixed matrix membranes. Phys. B Condens. Matter 2023, 652, 414642. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Khan, T.; Park, J.K. Water holding and release properties of bacterial cellulose obtained by in situ and ex situ modification. Carbohydr. Polym. 2012, 88, 596–603. [Google Scholar] [CrossRef]
- Feng, X.; Ge, Z.; Wang, Y.; Xia, X.; Zhao, B.; Dong, M. Production and characterization of bacterial cellulose from kombucha-fermented soy whey. Food Production. Process. Nutr. 2024, 6, 20. [Google Scholar] [CrossRef]
- Kuhad, R.C.; Singh, A.; Eriksson, K.E. Microorganisms and Enzymes Involved in the Degradation of Plant Fiber Cell Walls. Biotechnol. Pulp Pap. Ind. 2006, 57, 45–125. [Google Scholar]
- Bi, S.; Peng, L.; Chen, K.; Zhu, Z. Enhanced enzymatic saccharification of sugarcane bagasse pretreated by combining O2 and NaOH. Bioresour. Technol. 2016, 214, 692–699. [Google Scholar] [CrossRef]
- Laluce, C.; Roldan, I.U.; Pecoraro, E.; Igbojionu, L.I.; Ribeiro, C.A. Effects of pretreatment applied to sugarcane bagasse on composition and morphology of cellulosic fractions. Biomass Bioenergy 2019, 126, 231–238. [Google Scholar] [CrossRef]
- Nu, D.T.T.; Hung, N.P.; Van Hoang, C.; Van der Bruggen, B. Preparation of an asymmetric membrane from sugarcane bagasse using DMSO as green solvent. Appl. Sci. 2019, 9, 3347. [Google Scholar] [CrossRef]
- Bae, S.; Shoda, M. Statistical optimization of culture conditions for bacterial cellulose production using Box-Behnken design. Biotechnol. Bioeng. 2005, 90, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Indrianingsih, A.W.; Rosyida, V.T.; Jatmiko, T.H.; Prasetyo, D.J.; Poeloengasih, C.D.; Apriyana, W.; Nisa, K.; Nurhayati, S.; Darsih, C.; Pratiwi, D.; et al. Preliminary study on biosynthesis and characterization of bacteria cellulose films from coconut water. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Bristol, UK, 2017. [Google Scholar] [CrossRef]
- Aswini, K.; Gopal, N.O.; Uthandi, S. Optimized culture conditions for bacterial cellulose production by Acetobacter senegalensis MA1. BMC Biotechnol. 2020, 20, 46. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Chung, J.; Jiang, Q.; Sun, R.; Zhang, J.; Zhong, Y.; Ren, N. Characteristics of rumen microorganisms involved in anaerobic degradation of cellulose at various pH values. RSC Adv. 2017, 7, 40303–40310. [Google Scholar] [CrossRef]
- Lye, L.M.; Popescu, R. Application of response surface methodology in numerical geotechnical analysis. In Proceedings of the 55th Canadian Society for Geotechnical Conference, Hamilton, Ontario, 2 October 2002. [Google Scholar] [CrossRef]
- Hishammuddin, N.; Radzun, K.A.; Syafiq, M.H.; Rahman, S.A.; Bahari, S.A.; Osman, S.; Abu, F.; Zakaria, M.N. Green environment: Effects of acetate buffer on cellulose production by Acetobacter xylinum 0416 in fermented static cultivation. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2022; Volume 951, p. 012025. [Google Scholar] [CrossRef]
- Suwannahong, K.; Wongcharee, S.; Kreanuarte, J.; Kreetachat, T. Pre-treatment of acetic acid from food processing wastewater using response surface methodology via fenton oxidation process for sustainable water reuse. J. Sustain. Dev. Energy. Water Environ. Syst. 2021, 9, 1080363. [Google Scholar] [CrossRef]
- Ismail, A.F.; Lorna, W. Penetrant-induced plasticization phenomenon in glassy polymers for gas separation membrane. Sep. Purif. Technol. 2002, 27, 173–194. [Google Scholar] [CrossRef]
- Selvaraj, G.; Wilfred, C.D. CO2/CH4 Separation in Amino Acid Ionic Liquids, Polymerized Ionic Liquids, and Mixed Matrix Membranes. Molecules 2024, 29, 1357. [Google Scholar] [CrossRef]
- Li, C.; Ding, Y.; Xu, W.; Li, M.; Li, W. Cellulose acetate mixed-matrix membranes doped with high CO2 affinity zeolitic tetrazolate-imidazolate framework additives. React. Funct. Polym. 2023, 182, 105463. [Google Scholar] [CrossRef]
- Sunder, N.; Fong, Y.-Y.; Bustam, M.A.; Lau, W.-J. Study on the Performance of Cellulose Triacetate Hollow Fiber Mixed Matrix Membrane Incorporated with Amine-Functionalized NH2-MIL-125(Ti) for CO2 and CH4 Separation. Separations 2023, 10, 41. [Google Scholar] [CrossRef]
- Puleo, A.C.; Paul, D.R.; Kelley, S.S. The effect of degree of acetylation on gas sorption and transport behavior in cellulose acetate. J. Memb. Sci. 1989, 47, 301–332. [Google Scholar] [CrossRef]
- Chen, G.-J.; Lee, D.-J. Synthesis of asymmetrical cellulose acetate/cellulose triacetate forward osmosis membrane: Optimization. J. Taiwan Inst. Chem. Eng. 2019, 96, 299–304. [Google Scholar] [CrossRef]
- Zhang, Y.; Sunarso, J.; Liu, S.; Wang, R. Current status and development of membranes for CO2/CH4 separation: A review. Int. J. Greenh. Gas. Control 2013, 12, 84–107. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).