Gasification of Sewage Sludge—A Review
Abstract
1. Introduction
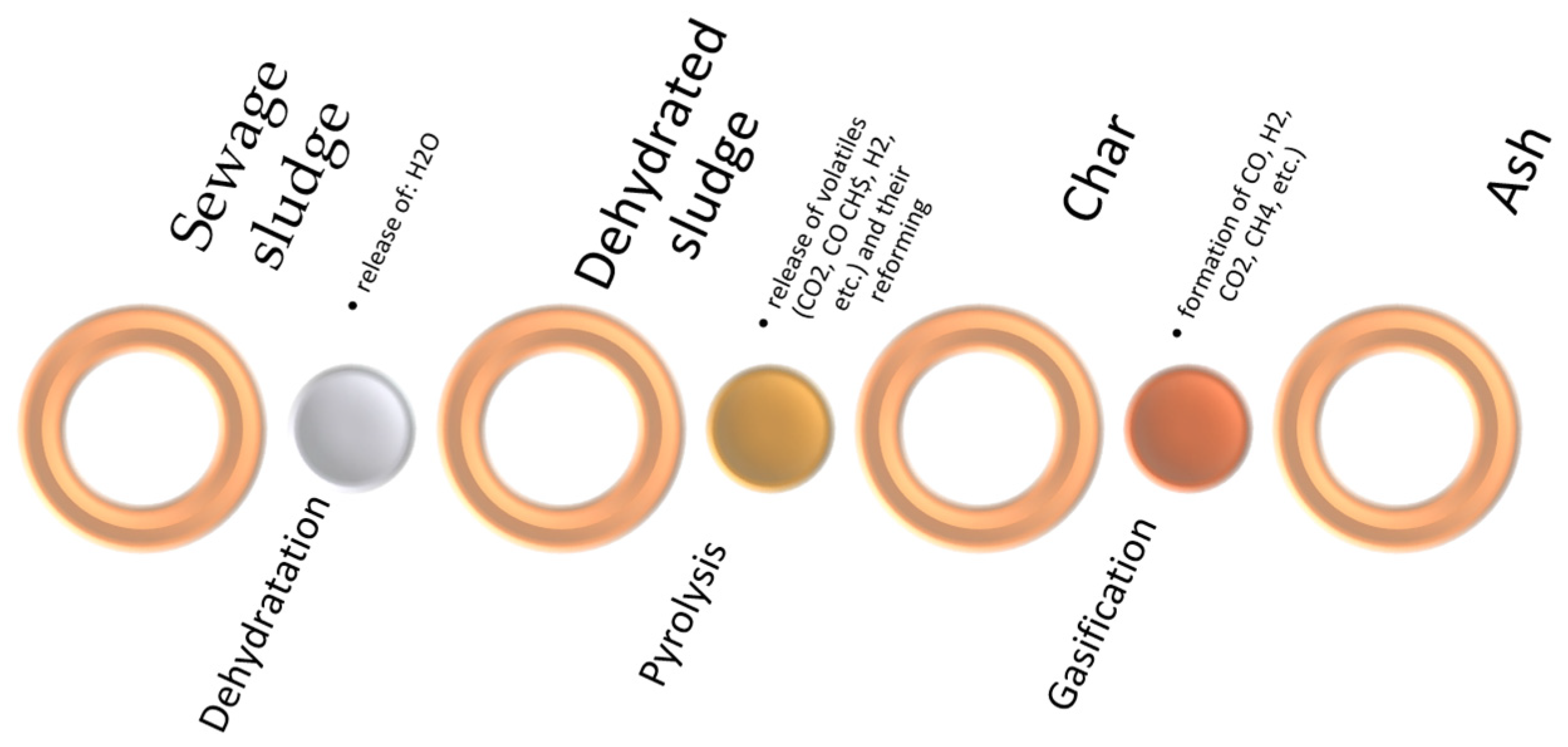
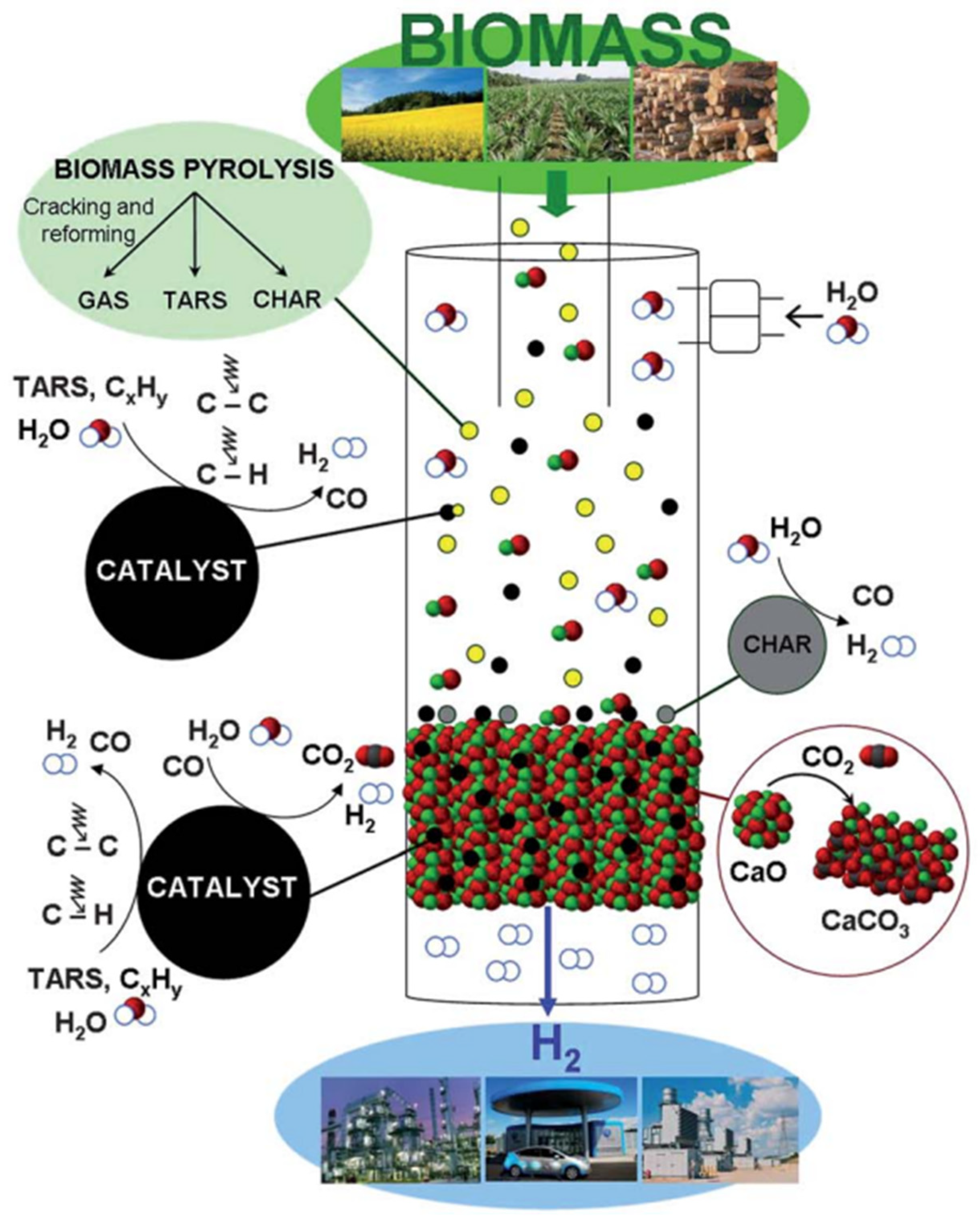
2. Gasification of Sewage Sludge
- (1)
- Dehydration—moisture evaporation occurring at 70–200 °C
- (2)
- Pyrolysis—thermal decomposition of sewage sludge occurring between 350–600 °C
- (3)
- Char gasification (800–1200 °C)—conversion of char into CO and H2 due to carbon-steam gasification reaction (C + H2O → CO + H2), Bouduard reaction (C + CO2 → 2 CO), hydrogasification (C + H2 → CH4), and partial oxidation (C + 0.5 O2 → CO). This stage is the slowest and limits the rate of the entire process.
2.1. Sewage Sludge as Feedstock for Gasification
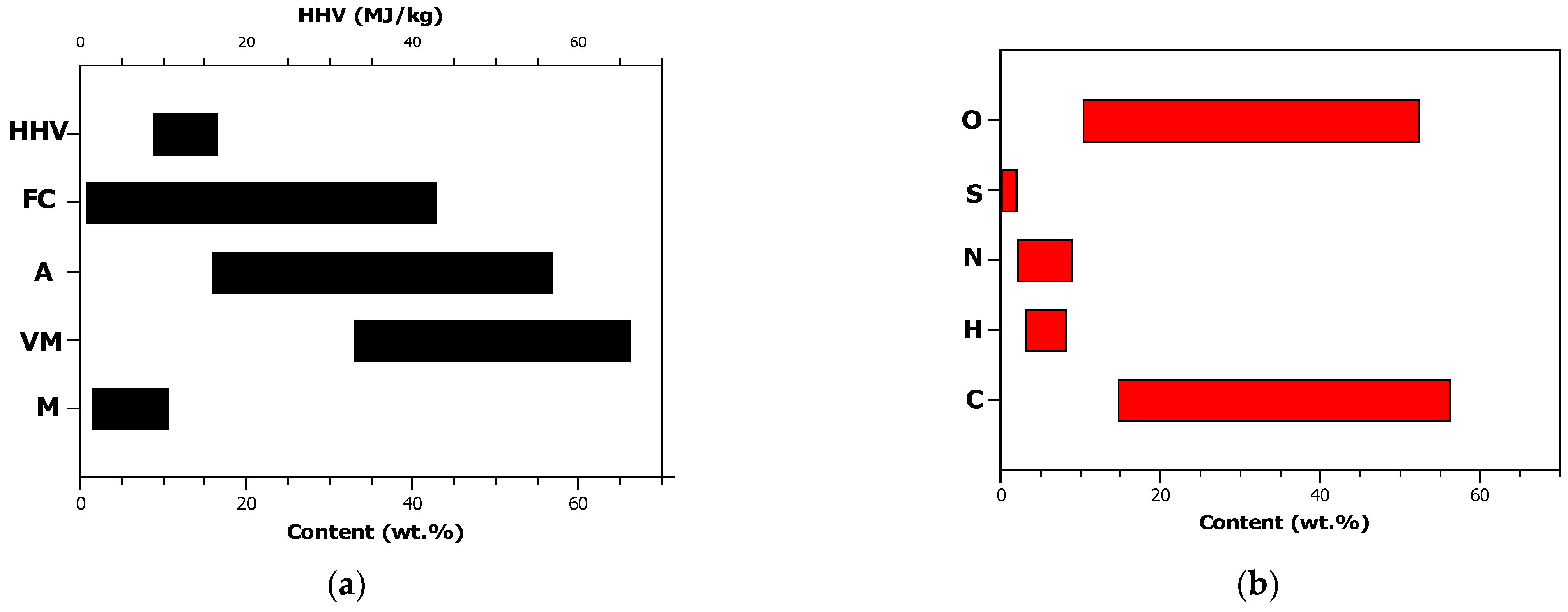
2.1.1. Proximate and Ultimate Composition of Sewage Sludge
2.1.2. Composition of the Inorganic Substance of Sewage Sludge
2.1.3. Morphology and Chemical Structure of Sewage Sludge
2.1.4. Comparison of Sewage Sludge with Its Chars
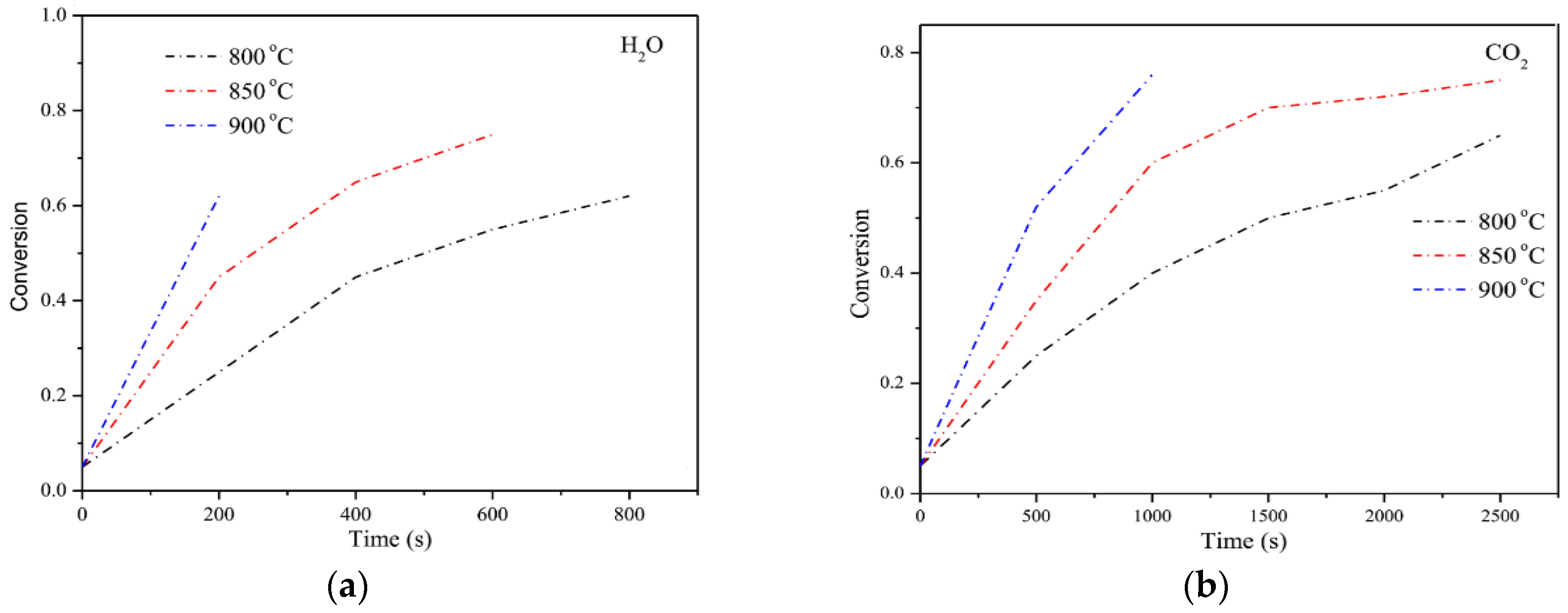
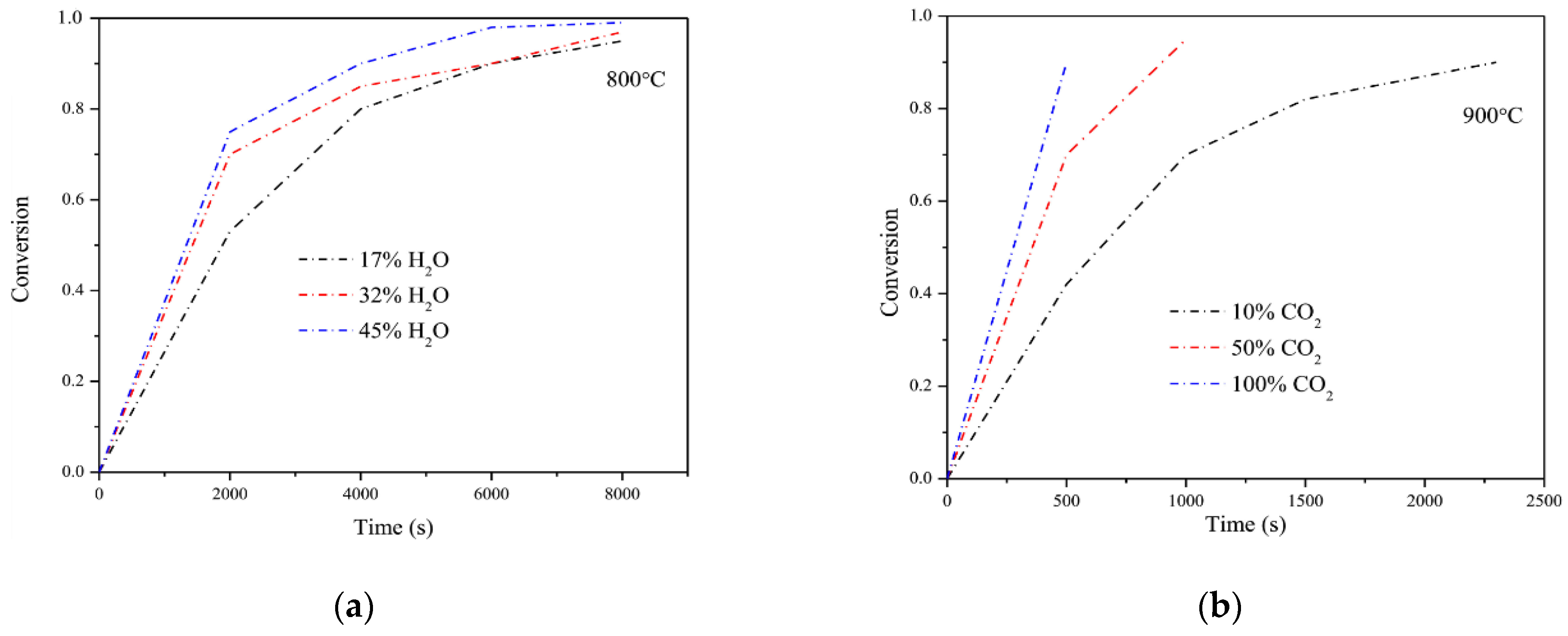
2.2. Kinetics of Sewage Sludge Gasification
2.3. Effect of Gasification Conditions on the Quality and Quantity of the Resulting Gas
2.3.1. Effect of Sewage Sludge Properties
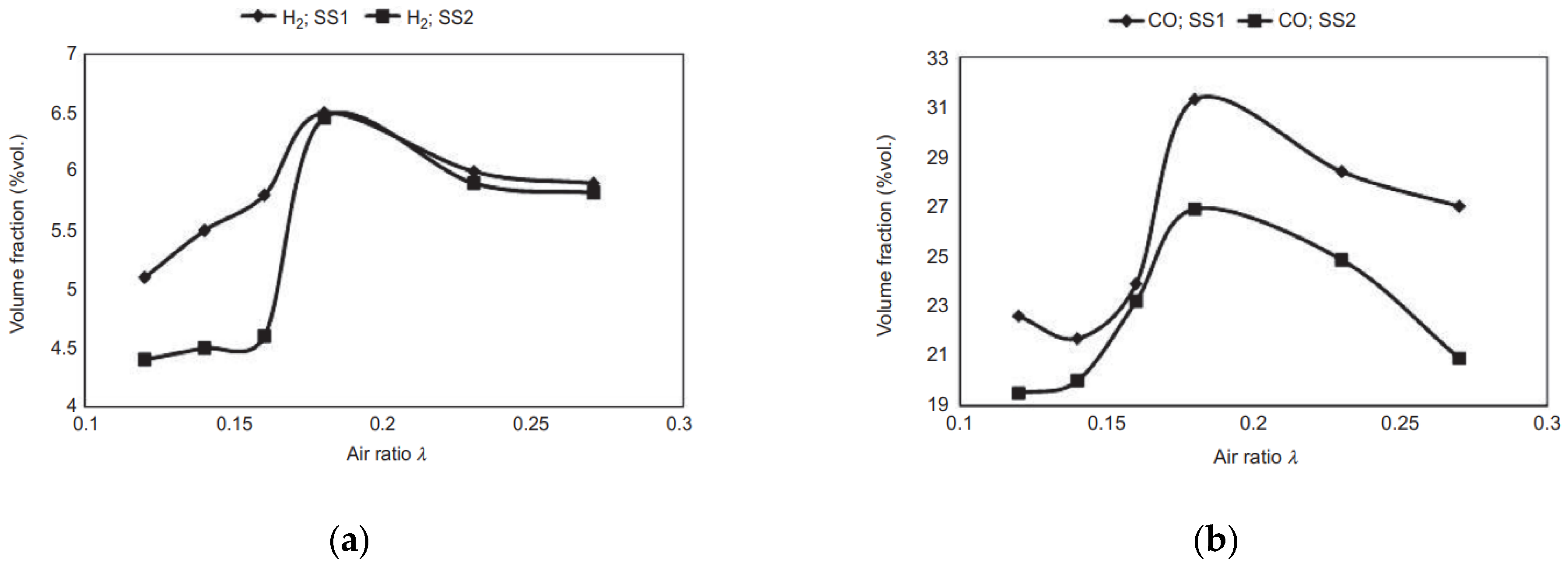
2.3.2. Effect of Operating Conditions
- Temperature
- Type and amount of gasifying agent
- Equivalence ratio (actual air to sewage sludge mass ratio)
- Presence of catalyst
2.4. Co-Gasification of Sewage Sludge
2.5. Supercritical and Plasma Gasification of Sewage Sludge
2.6. Management of Sewage Sludge By-Products
- ∘
- N-aromatics (benzo-nitrile, methyl-pyridine, quinoline, phenyl-pyridine, pyridine) state approx. 50%
- ∘
- PAH (naphthalene, methyl naphthalene, biphenylene, biphenyl, phenanthrene, fluorine) state nearly 36%
- ∘
- Light aromatics state about 6.5%
- ∘
- S-compounds (2-benzothiophene, propane nitrile) state approx. 4.9%
- ∘
- O-aromatics (phenol, benzofuran) state about 2.6%.
2.7. Environmental Impact of Sewage Sludge Gasification
2.8. Future Work
3. Conclusions
Funding
Conflicts of Interest
References
- Mishra, A.; Siddiqi, H.; Meikap, B.C. Elucidating sustainable waste management approaches along with waste-to-energy pathways: A critical review. Energy Waste 2022, 28, 83–96. [Google Scholar]
- Perazzini, H.; Freire, F.B.; Freire, J.T. Thermal Treatment of Solid Wastes Using Drying Technologies: A Review. Dry. Technol. 2016, 34, 39–52. [Google Scholar] [CrossRef]
- Żogała, A. Technological and environmental problems connected with thermal conversion of sewage sludge. Inżynieria Ekol. 2016, 46, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Delibacak, S.; Voronina, L.; Morachevskaya, E.; Ongun, A.R. Use of sewage sludge in agricultural soils: Useful or harmful. Eurasian J. Soil Sci. 2020, 9, 126–139. [Google Scholar] [CrossRef]
- Zuo, W.; Gu, C.; Zhang, W.; Xu, K.; Wang, Y.; Bai, Y.; Shan, Y.; Dai, Q. Sewage sludge amendment improved soil properties and sweet sorghum yield and quality in a newly reclaimed mudflat land. Sci. Total Environ. 2019, 654, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Fijalkowski, K.; Rorat, A.; Grobelak, A.; Kacprzak, M.J. The presence of contaminations in sewage sludge—The current situation. J. Environ. Manag. 2017, 203, 1126–1136. [Google Scholar] [CrossRef]
- Zhang, Q.; Hu, J.; Lee, D.-J.; Chang, Y.; Lee, Y.-J. Sludge treatment: Current research trends. Bioresour. Technol. 2017, 243, 1159–1172. [Google Scholar] [CrossRef]
- World Population Prospects. 2024. Available online: https://population.un.org/wpp/Download/Standard/MostUsed/ (accessed on 25 August 2024).
- Sewage Sludge Production and Disposal from Urban Wastewater. Available online: https://ec.europa.eu/eurostat/databrowser/view/ten00030/default/table?lang=en (accessed on 25 August 2024).
- Feng, J.; Burke, I.T.; Chen, X.; Stewart, D.I. Assessing metal contamination and speciation in sewage sludge: Implications for soil application and environmental risk. Rev. Environ. Sci. Bio/Technol. 2023, 22, 1037–1058. [Google Scholar] [CrossRef]
- Peccia, J.; Westerhoff, P. We Should Expect More out of Our Sewage Sludge. Environ. Sci. Technol. 2015, 49, 8271–8276. [Google Scholar] [CrossRef]
- Kołodziej, B.; Antonkiewicz, J.; Bielińska, E.J.; Witkowicz, R.; Dubis, B. Recovery of microelements from municipal sewage sludge by reed canary grass and giant miscanthus. Int. J. Phytoremediation 2023, 25, 441–454. [Google Scholar] [CrossRef]
- Zoghlami, R.I.; Hamdi, H.; Mokni-Tlili, S.; Hechmi, S.; Khelil, M.N.; Ben Aissa, N.; Moussa, M.; Bousnina, H.; Benzarti, S.; Jedidi, N. Monitoring the variation of soil quality with sewage sludge application rates in absence of rhizosphere effect. Int. Soil Water Conserv. Res. 2020, 8, 245–252. [Google Scholar] [CrossRef]
- Hechmi, S.; Zoghlami, R.I.; Khelil, M.N.; Mokni-Tlili, S.; Kallel, A.; Trabelsi, I.; Jedidi, N. Cumulative effect of sewage sludge application on soil adsorption complex and nutrient balance: A field study in semi-arid region (oued Souhil, Tunisia). Arab. J. Geosci. 2022, 15, 54. [Google Scholar] [CrossRef]
- Saxlund. Sludge Cake Storage, Reclaiming and Conveying: Piece of Cake. 2022. Available online: https://www.saxlundgroup.com/en/industries/waste-water/ (accessed on 23 October 2022).
- Barraoui, D.; Blais, J.F.; Labrecque, M. Cleanup of sewage sludge spiked with Cd, Cu, and Zn: Sludge quality and distribution of metals in the “soil-plant-water” system. Chemosphere 2021, 267, 129223. [Google Scholar] [CrossRef]
- Nahar, N.; Hossen, S. Influence of sewage sludge application on soil properties, carrot growth and heavy metal uptake. Commun. Soil Sci. Plant Anal. 2021, 52, 1–10. [Google Scholar] [CrossRef]
- Charlton, A.; Sakrabani, R.; Tyrrel, S.; Casado, M.R.; McGrath, S.; Crooks, B.; Cooper, P.; Campbell, C.D. Long-term impact of sewage sludge application on soil microbial biomass: An evaluation using meta-analysis. Environ. Pollut. 2016, 219, 1021–1035. [Google Scholar] [CrossRef] [PubMed]
- Hasnine, M.T.; Huda, M.E.; Khatun, R.; Saadat, A.H.M.; Ahasan, M.; Akter, S.; Uddin, M.F.; Monika, A.N.; Rahman, M.A.; Ohiduzzaman, M. Heavy Metal Contamination in Agricultural Soil at DEPZA, Bangladesh. Environ. Ecol. Res. 2017, 5, 510–516. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, C.; Champagne, P.; Mabee, W. Overview of current biological and thermo-chemical treatment technologies for sustainable sludge management. Waste Manag. Res. J. Sustain. Circ. Econ. 2014, 32, 586–600. [Google Scholar] [CrossRef]
- Aracil, C.; Haro, P.; Fuentes-Cano, D.; Gómez-Barea, A. Implementation of waste-to-energy options in landfill-dominated countries: Economic evaluation and GHG impact. Waste Manag. 2018, 76, 443–456. [Google Scholar] [CrossRef]
- Roy, M.M.; Dutta, A.; Corscadden, K.; Havard, P.; Dickie, L. Review of biosolids management options and co-incineration of a biosolid-derived fuel. Waste Manag. 2011, 31, 2228–2235. [Google Scholar] [CrossRef]
- Fonts, I.; Gea, G.; Azuara, M.; Ábrego, J.; Arauzo, J. Sewage sludge pyrolysis for liquid production: A review. Renew. Sustain. Energy Rev. 2012, 16, 2781–2805. [Google Scholar] [CrossRef]
- Fytili, D.; Zabaniotou, A. Utilization of sewage sludge in EU application of old and new methods—A review. Renew. Sustain. Energy Rev. 2008, 12, 116–140. [Google Scholar] [CrossRef]
- Rulkens, W. Sewage Sludge as a Biomass Resource for the Production of Energy: Overview and Assessment of the Various Options. Energy Fuels 2008, 22, 9–15. [Google Scholar] [CrossRef]
- Adar, E.; Karatop, B.; İnce, M.; Bilgili, M.S. Comparison of methods for sustainable energy management with sewage sludge in Turkey based on SWOT-FAHP analysis. Renew. Sustain. Energy Rev. 2016, 62, 429–440. [Google Scholar] [CrossRef]
- Syed-Hassan, S.S.A.; Wang, Y.; Hu, S.; Su, S.; Xiang, J. Thermochemical processing of sewage sludge to energy and fuel: Fundamentals, challenges and considerations. Renew. Sustain. Energy Rev. 2017, 80, 888–913. [Google Scholar] [CrossRef]
- Bora, R.R.; Richardson, R.E.; You, F. Resource recovery and waste-to-energy from wastewater sludge via thermochemical conversion technologies in support of circular economy: A comprehensive review. BMC Chem. Eng. 2020, 2, 8. [Google Scholar] [CrossRef]
- Wądrzyk, M.; Janus, R.; Jakóbiec, J. Upłynnianie odpadowej materii organicznej do biooleju w wodzie w warunkach podkrytycznych. Przemysł Chem. 2017, 1, 107–112. [Google Scholar] [CrossRef]
- Środa, K.; Kijo-Kleczkowska, A.; Otwinowski, H. Termiczne unieszkodliwianie osadów ściekowych. Inżynieria Ekol. 2012, 28, 67–81. [Google Scholar]
- Quan, L.M.; Kamyab, H.; Yuzir, A.; Ashokkumar, V.; Hosseini, S.E.; Balasubramanian, B.; Kirpichnikova, I. Review of the application of gasification and combustion technology and waste-to-energy technologies in sewage sludge treatment. Fuel 2022, 316, 123199. [Google Scholar] [CrossRef]
- Mohamed, B.A.; Li, L.Y. Biofuel production by co-pyrolysis of sewage sludge and other materials: A review. Environ. Chem. Lett. 2023, 21, 153–182. [Google Scholar] [CrossRef]
- Racek, J.; Sevcik, J.; Chorazy, T.; Kucerik, J.; Hlavinek, P. Biochar—Recovery Material from Pyrolysis of Sewage Sludge: A Review. Waste Biomass-Valorization 2020, 11, 3677–3709. [Google Scholar] [CrossRef]
- Ghodke, P.K.; Sharma, A.K.; Pandey, J.; Chen, W.-H.; Patel, A.; Ashokkumar, V. Pyrolysis of sewage sludge for sustainable biofuels and value-added biochar production. J. Environ. Manag. 2021, 298, 113450. [Google Scholar] [CrossRef] [PubMed]
- Muzyka, R.; Chrubasik, M.; Stelmach, S.; Sajdak, M. Preliminary studies on the treatment of wastewater from biomass gasification. Waste Manag. 2015, 44, 135–146. [Google Scholar] [CrossRef]
- Yaman, S. Pyrolysis of biomass to produce fuels and chemical feedstocks. Energy Convers. Manag. 2004, 45, 651–671. [Google Scholar] [CrossRef]
- Werle, S.; Dudziak, M. Gasification of sewage sludge. In Industrial and Municipal Sludge; Butterworth-Heinemann: Oxford, UK, 2019; pp. 575–593. [Google Scholar]
- Śpiewak, K.; Czerski, G.; Bijak, K. The Effect of Temperature-Pressure Conditions on the RDF Gasification in the Atmosphere of Steam and Carbon Dioxide. Energies 2021, 14, 7502. [Google Scholar] [CrossRef]
- Rauch, R.; Hrbek, J.; Hofbauer, H. Biomass gasification for synthesis gas production and applications of the syngas. In Advances in Bioenergy: The Sustainability Challenge; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2016; pp. 73–91. [Google Scholar]
- You, S.; Ok, Y.S.; Tsang, D.C.W.; Kwon, E.E.; Wang, C.-H. Towards practical application of gasification: A critical review from syngas and biochar perspectives. Crit. Rev. Environ. Sci. Technol. 2018, 48, 1165–1213. [Google Scholar] [CrossRef]
- Reddy, S.N.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Supercritical water gasification of biomass for hydrogen production. Int. J. Hydrogen Energy 2014, 39, 6912–6926. [Google Scholar] [CrossRef]
- Werle, S. Gasification of a dried sewage sludge in a laboratory scale fixed bed reactor. Energies 2015, 8, 8562–8572. [Google Scholar] [CrossRef]
- Thomsen, T.P.; Sárossy, Z.; Gøbel, B.; Stoholm, P.; Ahrenfeldt, J.; Frandsen, F.J.; Henriksen, U.B. Low temperature circulating fluidized bed gasification and co-gasification of municipal sewage sludge. Part 1: Process performance and gas product characterization. Waste Manag. 2017, 66, 123–133. [Google Scholar] [CrossRef]
- Freda, C.; Cornacchia, G.; Romanelli, A.; Valerio, V.; Grieco, M. Sewage sludge gasification in a bench scale rotary kiln. Fuel 2018, 212, 88–94. [Google Scholar] [CrossRef]
- Striūgas, N.; Valinčius, V.; Pedišius, N.; Poškas, R.; Zakarauskas, K. Investigation of sewage sludge treatment using air plasma assisted gasification. Waste Manag. 2017, 64, 149–160. [Google Scholar] [CrossRef]
- Gao, N.; Kamran, K.; Quan, C.; Williams, P.T. Thermochemical conversion of sewage sludge: A critical review. Prog. Energy Combust. Sci. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Vishwajeet; Pawlak-Kruczek, H.; Baranowski, M.; Czerep, M.; Chorążyczewski, A.; Krochmalny, K.; Ostrycharczyk, M.; Ziółkowski, P.; Madejski, P.; Mączka, T.; et al. Entrained Flow Plasma Gasification of Sewage Sludge–Proof-of-Concept and Fate of Inorganics. Energies 2022, 15, 1948. [Google Scholar] [CrossRef]
- Nikolaisen, L.; Jensen, P.D.; Bech, K.S.; Dahl, J.; Busk, J.; Brødsgaard, T.; Rasmussen, M.B.; Bruhn, A.; Bjerre, A.B.; Nielsen, H.B.; et al. Energy Production from Marine Biomass (Ulva Lactuca); Danish Technological Institute: Taastrup, Danmark, 2011. [Google Scholar]
- Chiang, K.-Y.; Lu, C.-H.; Liao, C.-K.; Ger, R.H.-R. Characteristics of hydrogen energy yield by co-gasified of sewage sludge and paper-mill sludge in a commercial scale plant. Int. J. Hydrogen Energy 2016, 41, 21641–21648. [Google Scholar] [CrossRef]
- Winchell, L.J.; Ross, J.J.; Brose, D.A.; Pluth, T.B.; Fonoll, X.; Norton Jr, J.W.; Bell, K.Y. Pyrolysis and gasification at water resource recovery facilities: Status of the industry. Water Environ. Res. 2022, 94, e10701. [Google Scholar] [CrossRef]
- Application of Aries Process Technology to the Problem of PFAS Contamination. Available online: https://ariescleantech.com/wp-content/uploads/2020/11/Addressing-PFAS-with-Aries-Process-Technology-rev.-2-2020.pdf (accessed on 25 August 2024).
- Oladejo, J.; Shi, K.; Luo, X.; Yang, G.; Wu, T. A Review of Sludge-to-Energy Recovery Methods. Energies 2018, 12, 60. [Google Scholar] [CrossRef]
- Sikarwar, V.S.; Zhao, M.; Fennell, P.S.; Shah, N.; Anthony, E.J. Progress in biofuel production from gasification. Prog. Energy Combust. Sci. 2017, 61, 189–248. [Google Scholar] [CrossRef]
- Haghighat, M.; Majidian, N.; Hallajisani, A.; Samipourgiri, M. Production of bio-oil from sewage sludge: A review on the thermal and catalytic conversion by pyrolysis. Sustain. Energy Technol. Assess. 2020, 42, 100870. [Google Scholar] [CrossRef]
- Castello, D.; Haider, M.S.; Rosendahl, L.A. Catalytic upgrading of hydrothermal liquefaction biocrudes: Different challenges for different feedstocks. Renew. Energy 2019, 141, 420–430. [Google Scholar] [CrossRef]
- Djandja, O.S.; Wang, Z.-C.; Wang, F.; Xu, Y.-P.; Duan, P.-G. Pyrolysis of Municipal Sewage Sludge for Biofuel Production: A Review. Ind. Eng. Chem. Res. 2020, 59, 16939–16956. [Google Scholar] [CrossRef]
- Xu, Q.; Tang, S.; Wang, J.; Ko, J.H. Pyrolysis kinetics of sewage sludge and its biochar characteristics. Process. Saf. Environ. Prot. 2018, 115, 49–56. [Google Scholar] [CrossRef]
- Alvarez, J.; Amutio, M.; Lopez, G.; Bilbao, J.; Olazar, M. Fast co-pyrolysis of sewage sludge and lignocellulosic biomass in a conical spouted bed reactor. Fuel 2015, 159, 810–818. [Google Scholar] [CrossRef]
- Wang, Z.; Shu, X.; Zhu, H.; Xie, L.; Cheng, S.; Zhang, Y. Characteristics of biochars prepared by co-pyrolysis of sewage sludge and cotton stalk intended for use as soil amendments. Environ. Technol. 2020, 41, 1347–1357. [Google Scholar] [CrossRef]
- Zaker, A.; Chen, Z.; Zaheer-Uddin, M.; Guo, J. Co-pyrolysis of sewage sludge and low-density polyethylene—A thermogravimetric study of thermo-kinetics and thermodynamic parameters. J. Environ. Chem. Eng. 2021, 9, 104554. [Google Scholar] [CrossRef]
- Soria-Verdugo, A.; Goos, E.; Morato-Godino, A.; García-Hernando, N.; Riedel, U. Pyrolysis of biofuels of the future: Sewage sludge and microalgae—Thermogravimetric analysis and modelling of the pyrolysis under different temperature conditions. Energy Convers. Manag. 2017, 138, 261–272. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Q.; Hu, H.; Liu, P.; Hu, X.; Li, A.; Yao, H. Catalytic role of conditioner CaO in nitrogen transformation during sewage sludge pyrolysis. Proc. Combust. Inst. 2015, 35, 2759–2766. [Google Scholar] [CrossRef]
- Wang, K.; Zheng, Y.; Zhu, X.; Brewer, C.E.; Brown, R.C. Ex-situ catalytic pyrolysis of wastewater sewage sludge—A micro-pyrolysis study. Bioresour. Technol. 2017, 232, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Luo, G.-Q.; Hu, H.-Y.; Zhang, Q.; Yang, J.-K.; Yao, H. Emission characteristics of nitrogen- and sulfur-containing odorous compounds during different sewage sludge chemical conditioning processes. J. Hazard. Mater. 2012, 235–236, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Karouki, E.; Sfakiotakis, S. Gasification of waste biomass chars by carbon dioxide via thermogravimetry. Part I: Effect of mineral matter. Fuel 2011, 90, 1120–1127. [Google Scholar]
- Lahijani, P.; Zainal, Z.A.; Mohamed, A.R.; Mohammadi, M. CO2 gasification reactivity of biomass char: Catalytic influence of alkali, alkaline earth and transition metal salts. Bioresour. Technol. 2013, 144, 288–295. [Google Scholar] [CrossRef]
- Śpiewak, K.; Czerski, G.; Soprych, P. Steam gasification of tire char supported by catalysts based on biomass ashes. Energy 2023, 285, 129378. [Google Scholar] [CrossRef]
- Bouraoui, Z.; Dupont, C.; Jeguirim, M.; Limousy, L.; Gadiou, R. CO2 gasification of woody biomass chars: The influence of K and Si on char reactivity. Comptes Rendus Chim. 2016, 19, 457–465. [Google Scholar] [CrossRef]
- Payá, J.; Monzó, J.; Borrachero, M.V.; Soriano, L. Sewage sludge ash. In New Trends in Eco-Efficient and Recycled Concrete; Woodhead Publishing: Sawston, UK, 2019; pp. 121–152. [Google Scholar]
- Donatello, S.; Freeman-Pask, A.; Tyrer, M.; Cheeseman, C. Effect of milling and acid washing on the pozzolanic activity of incinerator sewage sludge ash. Cem. Concr. Compos. 2010, 32, 54–61. [Google Scholar] [CrossRef]
- Donatello, S.; Tyrer, M.; Cheeseman, C. Comparison of test methods to assess pozzolanic activity. Cem. Concr. Compos. 2010, 32, 121–127. [Google Scholar] [CrossRef]
- Dyer, T.D.; Halliday, J.E.; Dhir, R.K. Hydration Chemistry of Sewage Sludge Ash Used as a Cement Component. J. Mater. Civ. Eng. 2011, 23, 648–655. [Google Scholar] [CrossRef]
- Lin, K.L.; Chang, W.C.; Lin, D.F.; Luo, H.L.; Tsai, M.C. Effects of nano-SiO2 and different ash particle sizes on sludge ash–cement mortar. J. Environ. Manag. 2008, 88, 708–714. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Carrión, M.; Baeza-Brotons, F.; Payá, J.; Saval, J.M.; Zornoza, E.; Borrachero, M.V.; Garcés, P. Potential use of sewage sludge ash (SSA) as a cement replacement in precast concrete blocks. Mater. De Construccion 2014, 64, e002. [Google Scholar] [CrossRef]
- Baeza, F.; Payá, J.; Galao, O.; Saval, J.; Garcés, P. Blending of industrial waste from different sources as partial substitution of Portland cement in pastes and mortars. Constr. Build. Mater. 2014, 66, 645–653. [Google Scholar] [CrossRef]
- Czerski, G.; Śpiewak, K.; Makowska, D.; Grycova, B. Study on Steam Co-Gasification of Waste Tire Char and Sewage Sludge. Energies 2023, 16, 2156. [Google Scholar] [CrossRef]
- Śpiewak, K.; Czerski, G.; Porada, S. Effect of K, Na and Ca-based catalysts on the steam gasification reactions of coal. Part I: Type and amount of one-component catalysts. Chem. Eng. Sci. 2021, 229, 116024. [Google Scholar] [CrossRef]
- Śpiewak, K.; Czerski, G.; Porada, S. Effect of K, Na and Ca-based catalysts on the steam gasification reactions of coal. Part II: Composition and amount of multi-component catalysts. Chem. Eng. Sci. 2021, 229, 116023. [Google Scholar] [CrossRef]
- Martins, M.N.; de Souza, V.V.; da Silva Souza, T. Genotoxic and mutagenic effects of sewage sludge on higher plants. Ecotoxicol. Environ. Saf. 2016, 124, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Kończak, M.; Oleszczuk, P. Application of biochar to sewage sludge reduces toxicity and improve organisms growth in sewage sludge-amended soil in long term field experiment. Sci. Total Environ. 2018, 625, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Mulchandani, A.; Westerhoff, P. Recovery opportunities for metals and energy from sewage sludges. Bioresour. Technol. 2016, 215, 215–226. [Google Scholar] [CrossRef]
- Li, L.; Xu, Z.; Zhang, C.; Bao, J.; Dai, X. Quantitative evaluation of heavy metals in solid residues from sub- and super-critical water gasification of sewage sludge. Bioresour. Technol. 2012, 121, 169–175. [Google Scholar] [CrossRef]
- Shi, W.; Liu, C.; Ding, D.; Lei, Z.; Yang, Y.; Feng, C.; Zhang, Z. Immobilization of heavy metals in sewage sludge by using subcritical water technology. Bioresour. Technol. 2013, 137, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Oleszczuk, P.; Charmas, B.; Skubiszewska-Zięba, J.; Pasieczna-Patkowska, S. Effect of sewage sludge properties on the biochar characteristic. J. Anal. Appl. Pyrolysis 2015, 112, 201–213. [Google Scholar] [CrossRef]
- Yuan, H.; Lu, T.; Huang, H.; Zhao, D.; Kobayashi, N.; Chen, Y. Influence of pyrolysis temperature on physical and chemical properties of biochar made from sewage sludge. J. Anal. Appl. Pyrolysis 2015, 112, 284–289. [Google Scholar] [CrossRef]
- Figueiredo, C.; Lopes, H.; Coser, T.; Vale, A.; Busato, J.; Aguiar, N.; Novotny, E.; Canellas, L. Influence of pyrolysis temperature on chemical and physical properties of biochar from sewage sludge. Arch. Agron. Soil Sci. 2018, 64, 881–889. [Google Scholar] [CrossRef]
- Antunes, E.; Schumann, J.; Brodie, G.; Jacob, M.V.; Schneider, P.A. Biochar produced from biosolids using a single-mode microwave: Characterisation and its potential for phosphorus removal. J. Environ. Manag. 2017, 196, 119–126. [Google Scholar] [CrossRef]
- Moulijn, J.A.; Kapteijn, F. Towards a unified theory of reactions of carbon with oxygen-containing molecules. Carbon 1995, 33, 1155–1165. [Google Scholar] [CrossRef]
- Kowalski, M.; Kowalska, K.; Wiszniowski, J.; Turek-Szytow, J. Qualitative analysis of activated sludge using FT-IR technique. Chem. Pap. 2018, 72, 2699–2706. [Google Scholar] [CrossRef] [PubMed]
- Grube, M.; Lin, J.; Lee, P.; Kokorevicha, S. Evaluation of sewage sludge-based compost by FT-IR spectroscopy. Geoderma 2006, 130, 324–333. [Google Scholar] [CrossRef]
- Wiercik, P.; Kuśnierz, M.; Kabsch-Korbutowicz, M.; Plucińska, A.; Chrobot, P. Evaluation of changes in activated sludge and sewage sludge quality by FTIR analysis and laser diffraction. Desalination Water Treat. 2022, 273, 114–125. [Google Scholar] [CrossRef]
- Callegari, A.; Capodaglio, A.G. Properties and Beneficial Uses of (Bio)Chars, with Special Attention to Products from Sewage Sludge Pyrolysis. Resources 2018, 7, 20. [Google Scholar] [CrossRef]
- Gai, C.; Chen, M.; Liu, T.; Peng, N.; Liu, Z. Gasification characteristics of hydrochar and pyrochar derived from sewage sludge. Energy 2016, 113, 957–965. [Google Scholar] [CrossRef]
- Cao, X.; Harris, W. Properties of dairy-manure-derived biochar pertinent to its potential use in remediation. Bioresour. Technol. 2010, 101, 5222–5228. [Google Scholar] [CrossRef]
- Yuan, J.-H.; Xu, R.-K.; Zhang, H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011, 102, 3488–3497. [Google Scholar] [CrossRef]
- Fuertes, A.B.; Arbestain, M.C.; Sevilla, M.; Maciá-Agulló, J.A.; Fiol, S.; López, R.; Smernik, R.J.; Aitkenhead, W.P.; Arce, F.; Macías, F. Chemical and structural properties of carbonaceous products obtained by pyrolysis and hydrothermal carbonisation of corn stover. Soil Res. 2010, 48, 618–626. [Google Scholar] [CrossRef]
- Yoshida, T.; Antal, M.J., Jr. Sewage sludge carbonization for terra preta applications. Energy Fuels 2009, 23, 5454–5459. [Google Scholar] [CrossRef]
- Saadatkhah, N.; Garcia, A.C.; Ackermann, S.; Leclerc, P.; Latifi, M.; Samih, S.; Patience, G.S.; Chaouki, J. Experimental methods in chemical engineering: Thermogravimetric analysis—TGA. Can. J. Chem. Eng. 2020, 98, 34–43. [Google Scholar] [CrossRef]
- Nowicki, L.; Markowski, M. Gasification of pyrolysis chars from sewage sludge. Fuel 2015, 143, 476–483. [Google Scholar] [CrossRef]
- Hernández, A.B.; Okonta, F.; Freeman, N. Thermal decomposition of sewage sludge under N2, CO2 and air: Gas characterization and kinetic analysis. J. Environ. Manag. 2017, 196, 560–568. [Google Scholar] [CrossRef]
- Stolarek, P.; Ledakowicz, S. Thermal processing of sewage sludge by drying, pyrolysis, gasification and combustion. Water Sci. Technol. 2001, 44, 333–339. [Google Scholar] [CrossRef]
- Jayaraman, K.; Gökalp, I. Pyrolysis, combustion and gasification characteristics of miscanthus and sewage sludge. Energy Convers. Manag. 2015, 89, 83–91. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, J.; Ying, Z.; Ji, S.; Feng, Y.; Wang, B.; Dou, B. Thermochemical conversion of sewage sludge-derived hydrochars: Volatiles release and char gasification kinetics. J. Anal. Appl. Pyrolysis 2021, 156, 105138. [Google Scholar] [CrossRef]
- Urych, B.; Smoliński, A. Kinetics of Sewage Sludge Pyrolysis and Air Gasification of Its Chars. Energy Fuels 2016, 30, 4869–4878. [Google Scholar] [CrossRef]
- Nowicki, L.; Antecka, A.; Bedyk, T.; Stolarek, P.; Ledakowicz, S. The kinetics of gasification of char derived from sewage sludge. J. Therm. Anal. Calorim. 2011, 104, 693–700. [Google Scholar] [CrossRef]
- Vamvuka, D.; Karouki, E.; Sfakiotakis, S.; Salatino, P. Gasification of Waste Biomass Chars by Carbon Dioxide via Thermogravimetry—Effect of Catalysts. Combust. Sci. Technol. 2012, 184, 64–77. [Google Scholar] [CrossRef]
- Śpiewak, K.; Czerski, G.; Grzywacz, P.; Makowska, D. Assessment of sewage sludge as a component for the tire char co-gasification process. Energy 2024, 308, 132892. [Google Scholar] [CrossRef]
- Lundberg, L.; Tchoffor, P.A.; Pallarès, D.; Johansson, R.; Thunman, H.; Davidsson, K. Influence of surrounding conditions and fuel size on the gasification rate of biomass char in a fluidized bed. Fuel Process. Technol. 2016, 144, 323–333. [Google Scholar] [CrossRef]
- Nilsson, S.; Gómez-Barea, A.; Cano, D.F. Gasification reactivity of char from dried sewage sludge in a fluidized bed. Fuel 2012, 92, 346–353. [Google Scholar] [CrossRef]
- Zhong, M.; Gao, S.; Zhou, Q.; Yue, J.; Ma, F.; Xu, G. Characterization of char from high temperature fluidized bed coal pyrolysis in complex atmospheres. Particuology 2016, 25, 59–67. [Google Scholar] [CrossRef]
- Sattar, A.; Leeke, G.A.; Hornung, A.; Wood, J. Steam gasification of rapeseed, wood, sewage sludge and miscanthus biochars for the production of a hydrogen-rich syngas. Biomass-Bioenergy 2014, 69, 276–286. [Google Scholar] [CrossRef]
- Gil-Lalaguna, N.; Sánchez, J.; Murillo, M.; Ruiz, V.; Gea, G. Air-steam gasification of char derived from sewage sludge pyrolysis. Comparison with the gasification of sewage sludge. Fuel 2014, 129, 147–155. [Google Scholar] [CrossRef]
- Schwitalla, D.; Reinmöller, M.; Forman, C.; Wolfersdorf, C.; Gootz, M.; Bai, J.; Guhl, S.; Neuroth, M.; Meyer, B. Ash and slag properties for co-gasification of sewage sludge and coal: An experimentally validated modeling approach. Fuel Process. Technol. 2018, 175, 1–9. [Google Scholar] [CrossRef]
- González, J.; Román, S.; Bragado, D.; Calderón, M. Investigation on the reactions influencing biomass air and air/steam gasification for hydrogen production. Fuel Process. Technol. 2008, 89, 764–772. [Google Scholar] [CrossRef]
- Orío, A.; Corella, J.; Narváez, I. Performance of Different Dolomites on Hot Raw Gas Cleaning from Biomass Gasification with Air. Ind. Eng. Chem. Res. 1997, 36, 3800–3808. [Google Scholar] [CrossRef]
- Kwon, E.E.; Yi, H.; Kwon, H.-H. Thermo-chemical process with sewage sludge by using CO2. J. Environ. Manag. 2013, 128, 435–440. [Google Scholar] [CrossRef]
- Lee, U.; Dong, J.; Chung, J. Experimental investigation of sewage sludge solid waste conversion to syngas using high temperature steam gasification. Energy Convers. Manag. 2018, 158, 430–436. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Ko, J.-H.; Kim, J.-S. A new type three-stage gasification of dried sewage sludge: Effects of equivalence ratio, weight ratio of activated carbon to feed, and feed rate on gas composition and tar, NH 3, and H 2 S removal and results of approximately 5 h gasification. Energy 2017, 118, 139–146. [Google Scholar] [CrossRef]
- Arjharn, W.; Hinsui, T.; Liplap, P.; Raghavan, G.S.V. Evaluation of an Energy Production System from Sewage Sludge Using a Pilot-Scale Downdraft Gasifier. Energy Fuels 2013, 27, 229–236. [Google Scholar] [CrossRef]
- Werle, S. Sewage sludge gasification process for clean and sustainable environment. Renew. Energy Environ. Sustain. 2016, 1, 35. [Google Scholar] [CrossRef]
- Gai, C.; Guo, Y.; Liu, T.; Peng, N.; Liu, Z. Hydrogen-rich gas production by steam gasification of hydrochar derived from sewage sludge. Int. J. Hydrogen Energy 2016, 41, 3363–3372. [Google Scholar] [CrossRef]
- Xu, Z.R.; Zhu, W.; Gong, M.; Zhang, H.W. Direct gasification of dewatered sewage sludge in supercritical water. Part 1: Effects of alkali salts. Int. J. Hydrogen Energy 2013, 38, 3963–3972. [Google Scholar] [CrossRef]
- Farooq, A.; Ko, C.H.; Park, Y.-K. Sewage sludge steam gasification over bimetallic mesoporous Al-MCM48 catalysts for efficient hydrogen generation. Environ. Res. 2023, 224, 115553. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.C. Catalytic hydrothermal gasification of biomass. Biofuels Bioprod. Biorefining 2008, 2, 254–265. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.; Wang, L.; Xu, D. Catalytic hydrogen production from municipal sludge in supercritical water with partial oxidation. In Challenges of Power Engineering and Environment; Proceedings of the International Conference on Power Engineering 2007; Springer: Berlin/Heidelberg, Germany, 2007; pp. 1252–1255. [Google Scholar]
- Ratnasari, D.K.; Bijl, A.; Yang, W.; Jönsson, P.G. Effect of H-ZSM-5 and Al-MCM-41 Proportions in Catalyst Mixtures on the Composition of Bio-Oil in Ex-Situ Catalytic Pyrolysis of Lignocellulose Biomass. Catalysts 2020, 10, 868. [Google Scholar] [CrossRef]
- Wang, L.; Li, D.; Koike, M.; Watanabe, H.; Xu, Y.; Nakagawa, Y.; Tomishige, K. Catalytic performance and characterization of Ni–Co catalysts for the steam reforming of biomass tar to synthesis gas. Fuel 2013, 112, 654–661. [Google Scholar] [CrossRef]
- Wang, L.; Li, D.; Koike, M.; Koso, S.; Nakagawa, Y.; Xu, Y.; Tomishige, K. Catalytic performance and characterization of Ni-Fe catalysts for the steam reforming of tar from biomass pyrolysis to synthesis gas. Appl. Catal. A Gen. 2011, 392, 248–255. [Google Scholar] [CrossRef]
- Hong, S.-P.; Dong, J.-I.; Yeo, S.-K.; Park, I.-H.; Chung, M.-S.; Kim, D.-I.; Park, Y.-K. Reduction of tar using cheap catalysts during sewage sludge gasification. J. Mater. Cycles Waste Manag. 2011, 13, 186–189. [Google Scholar] [CrossRef]
- De Andres, J.M.; Narros, A.; Rodríguez, M.E. Behaviour of dolomite, olivine and alumina as primary catalysts in air–steam gasification of sewage sludge. Fuel 2011, 90, 521–527. [Google Scholar] [CrossRef]
- de Andrés, J.M.; Narros, A.; Rodríguez, M.E. Air-steam gasification of sewage sludge in a bubbling bed reactor: Effect of alumina as a primary catalyst. Fuel Process. Technol. 2011, 92, 433–440. [Google Scholar] [CrossRef]
- Gil, J.; Caballero, M.A.; Martín, J.A.; Aznar, M.-P.; Corella, J. Biomass Gasification with Air in a Fluidized Bed: Effect of the In-Bed Use of Dolomite under Different Operation Conditions. Ind. Eng. Chem. Res. 1999, 38, 4226–4235. [Google Scholar] [CrossRef]
- Rapagnà, S.; Jand, N.; Kiennemann, A.; Foscolo, P. Steam-gasification of biomass in a fluidised-bed of olivine particles. Biomass-Bioenergy 2000, 19, 187–197. [Google Scholar] [CrossRef]
- Fermoso, J.; Rubiera, F.; Chen, D. Sorption enhanced catalytic steam gasification process: A direct route from lignocellulosic biomass to high purity hydrogen. Energy Environ. Sci. 2012, 5, 6358–6367. [Google Scholar] [CrossRef]
- Migliaccio, R.; Brachi, P.; Montagnaro, F.; Papa, S.; Tavano, A.; Montesarchio, P.; Ruoppolo, G.; Urciuolo, M. Sewage Sludge Gasification in a Fluidized Bed: Experimental Investigation and Modeling. Ind. Eng. Chem. Res. 2021, 60, 5034–5047. [Google Scholar] [CrossRef]
- Khan, M.A.; Naqvi, S.R.; Taqvi, S.A.; Shahbaz, M.; Ali, I.; Mehran, M.T.; Khoja, A.H.; Juchelková, D. Air gasification of high-ash sewage sludge for hydrogen production: Experimental, sensitivity and predictive analysis. Int. J. Hydrogen Energy 2022, 47, 37374–37384. [Google Scholar] [CrossRef]
- Chen, G.-B.; Wu, F.-H.; Lin, S.-P.; Hsu, Y.-T.; Lin, T.-H. A study of sewage sludge Co-gasification with waste shiitake substrate. Energy 2022, 259, 124991. [Google Scholar] [CrossRef]
- Jeong, Y.-S.; Mun, T.-Y.; Kim, J.-S. Two-stage gasification of dried sewage sludge: Effects of gasifying agent, bed material, gas cleaning system, and Ni-coated distributor on product gas quality. Renew. Energy 2022, 185, 208–216. [Google Scholar] [CrossRef]
- Tezer, Ö.; Karabağ, N.; Öngen, A.; Ayol, A. Syngas production from municipal sewage sludge by gasification Process: Effects of fixed bed reactor types and gasification agents on syngas quality. Sustain. Energy Technol. Assess. 2023, 56, 103042. [Google Scholar] [CrossRef]
- Roche, E.; de Andrés, J.M.; Narros, A.; Rodríguez, M.E. Air and air-steam gasification of sewage sludge. The influence of dolomite and throughput in tar production and composition. Fuel 2014, 115, 54–61. [Google Scholar] [CrossRef]
- de Andrés, J.M.; Roche, E.; Narros, A.; Rodríguez, M.E. Characterisation of tar from sewage sludge gasification. Influence of gasifying conditions: Temperature, throughput, steam and use of primary catalysts. Fuel 2016, 180, 116–126. [Google Scholar] [CrossRef]
- Gil-Lalaguna, N.; Sánchez, J.L.; Murillo, M.B.; Rodríguez, E.; Gea, G. Air–steam gasification of sewage sludge in a fluidized bed. Influence of some operating conditions. Chem. Eng. J. 2014, 248, 373–382. [Google Scholar] [CrossRef]
- Nipattummakul, N.; Ahmed, I.; Kerdsuwan, S.; Gupta, A.K. High temperature steam gasification of wastewater sludge. Appl. Energy 2010, 87, 3729–3734. [Google Scholar] [CrossRef]
- Gong, M.; Zhu, W.; Zhang, H.; Ma, Q.; Su, Y.; Fan, Y. Influence of NaOH and Ni catalysts on hydrogen production from the supercritical water gasification of dewatered sewage sludge. Int. J. Hydrogen Energy 2014, 39, 19947–19954. [Google Scholar] [CrossRef]
- Havilah, P.R.; Sharma, A.K.; Govindasamy, G.; Matsakas, L.; Patel, A. Biomass Gasification in Downdraft Gasifiers: A Technical Review on Production, Up-Gradation and Application of Synthesis Gas. Energies 2022, 15, 3938. [Google Scholar] [CrossRef]
- Speight, J.G. Handbook of Gasification Technology: Science, Processes, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Chen, G.-B.; Wu, F.-H.; Fang, T.-L.; Lin, H.-T.; Chao, Y.-C. A study of Co-gasification of sewage sludge and palm kernel shells. Energy 2021, 218, 119532. [Google Scholar] [CrossRef]
- Szwaja, S.; Poskart, A.; Zajemska, M.; Szwaja, M. Theoretical and Experimental Analysis on Co-Gasification of Sewage Sludge with Energetic Crops. Energies 2019, 12, 1750. [Google Scholar] [CrossRef]
- Peng, L.; Wang, Y.; Lei, Z.; Cheng, G. Co-gasification of wet sewage sludge and forestry waste in situ steam agent. Bioresour. Technol. 2012, 114, 698–702. [Google Scholar] [CrossRef]
- Ong, Z.; Cheng, Y.; Maneerung, T.; Yao, Z.; Tong, Y.W.; Wang, C.H.; Dai, Y. Co-gasification of woody biomass and sewage sludge in a fixed-bed downdraft gasifier. AIChE J. 2015, 61, 2508–2521. [Google Scholar] [CrossRef]
- Ramachandran, S.; Yao, Z.; You, S.; Massier, T.; Stimming, U.; Wang, C.-H. Life cycle assessment of a sewage sludge and woody biomass co-gasification system. Energy 2017, 137, 369–376. [Google Scholar] [CrossRef]
- Hu, Q.; Dai, Y.; Wang, C.-H. Steam co-gasification of horticultural waste and sewage sludge: Product distribution, synergistic analysis and optimization. Bioresour. Technol. 2020, 301, 122780. [Google Scholar] [CrossRef] [PubMed]
- García, G.; Arauzo, J.; Gonzalo, A.; Sánchez, J.; Ábrego, J. Influence of feedstock composition in fluidised bed co-gasification of mixtures of lignite, bituminous coal and sewage sludge. Chem. Eng. J. 2013, 222, 345–352. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Liu, Y.; Lv, M.; You, P.; Wang, X.; Zhou, H.; Wang, J. Co-Gasification Synergistic Characteristics of Sewage Sludge and High-Sodium Coal. ACS Omega 2023, 8, 6571–6583. [Google Scholar] [CrossRef] [PubMed]
- Smoliński, A.; Howaniec, N. Co-gasification of coal/sewage sludge blends to hydrogen-rich gas with the application of simulated high temperature reactor excess heat. Int. J. Hydrogen Energy 2016, 41, 8154–8158. [Google Scholar] [CrossRef]
- Li, L.; Li, X.; Cao, W. Reaction pathway and kinetics study on supercritical water gasification of oily sludge. J. Anal. Appl. Pyrolysis 2023, 170, 105920. [Google Scholar] [CrossRef]
- Chen, Y.; Guo, L.; Cao, W.; Jin, H.; Guo, S.; Zhang, X. Hydrogen production by sewage sludge gasification in supercritical water with a fluidized bed reactor. Int. J. Hydrogen Energy 2013, 38, 12991–12999. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, W.; Gong, M.; Su, Y.; Fan, Y. Influence of H2O2 and Ni catalysts on hydrogen production and PAHs inhibition from the supercritical water gasification of dewatered sewage sludge. J. Supercrit. Fluids 2017, 130, 183–188. [Google Scholar] [CrossRef]
- Li, Z.; Gong, M.; Wang, M.; Feng, A.; Wang, L.; Ma, P.; Yuan, S. Influence of AlCl3 and oxidant catalysts on hydrogen production from the supercritical water gasification of dewatered sewage sludge and model compounds. Int. J. Hydrogen Energy 2021, 46, 31262–31274. [Google Scholar] [CrossRef]
- Peterson, A.A.; Vogel, F.; Lachance, R.P.; Fröling, M.; Antal, M.J., Jr.; Tester, J.W. Thermochemical biofuel production in hydrothermal media: A review of sub-and supercritical water technologies. Energy Environ. Sci. 2008, 1, 32–65. [Google Scholar] [CrossRef]
- Yang, C.; Wang, S.; Li, Y.; Zhang, Y.; Cui, C. Thermodynamic analysis of hydrogen production via supercritical water gasification of coal, sewage sludge, microalga, and sawdust. Int. J. Hydrogen Energy 2021, 46, 18042–18050. [Google Scholar] [CrossRef]
- Adar, E.; Ince, M.; Bilgili, M.S. Supercritical water gasification of sewage sludge by continuous flow tubular reactor: A pilot scale study. Chem. Eng. J. 2020, 391, 123499. [Google Scholar] [CrossRef]
- Chen, Y.; Yi, L.; Li, S.; Yin, J.; Jin, H. Catalytic gasification of sewage sludge in near and supercritical water with different catalysts. Chem. Eng. J. 2020, 388, 124292. [Google Scholar] [CrossRef]
- Yan, M.; Hantoko, D.; Kanchanatip, E.; Zheng, R.; Zhong, Y.; Mubeen, I. Valorization of sewage sludge through catalytic sub- and supercritical water gasification. J. Energy Inst. 2020, 93, 1419–1427. [Google Scholar] [CrossRef]
- Cai, X.; Wei, X.; Du, C. Thermal Plasma Treatment and Co-processing of Sludge for Utilization of Energy and Material. Energy Fuels 2020, 34, 7775–7805. [Google Scholar] [CrossRef]
- Cvetinović, D.; Erić, A.; Mladenović, M.; Buha-Marković, J.; Janković, B. Thermal plasma gasification of sewage sludge: Optimisation of operating parameters and economic evaluation. Energy Convers. Manag. 2024, 313, 118639. [Google Scholar] [CrossRef]
- Evangelisti, S.; Tagliaferri, C.; Clift, R.; Lettieri, P.; Taylor, R.; Chapman, C. Life cycle assessment of conventional and two-stage advanced energy-from-waste technologies for municipal solid waste treatment. J. Clean. Prod. 2015, 100, 212–223. [Google Scholar] [CrossRef]
- Balgaranova, J. Plasma chemical gasification of sewage sludge. Waste Manag. Res. J. Sustain. Circ. Econ. 2003, 21, 38–41. [Google Scholar] [CrossRef]
- Ruya, P.M.; Purwadi, R.; Lim, S.S. Supercritical water gasification of sewage sludge for power generation– thermodynamic study on auto-thermal operation using Aspen Plus. Energy Convers. Manag. 2020, 206, 112458. [Google Scholar] [CrossRef]
- Qian, L.; Wang, S.; Wang, S.; Zhao, S.; Zhang, B. Supercritical water gasification and partial oxidation of municipal sewage sludge: An experimental and thermodynamic study. Int. J. Hydrogen Energy 2021, 46, 89–99. [Google Scholar] [CrossRef]
- Jeništa, J.; Hirka, I.; Živný, O. Modelling of gasification of organic waste in thermal-plasma chemical reactors. Plasma Phys. Technol. 2024, 11, 17–27. [Google Scholar] [CrossRef]
- Han, H.; Li, A.; Zhu, M.; Hu, S.; Xu, J.; Xiong, Z.; Ren, Q.; Wang, Y.; Jiang, L.; Su, S.; et al. Heavy tar evolution characteristics during advanced sludge pyrolysis and biomass gasification integrated process. Sci. Total Environ. 2022, 853, 158107. [Google Scholar] [CrossRef]
- Werle, S.; Sobek, S. Gasification of sewage sludge within a circular economy perspective: A Polish case study. Environ. Sci. Pollut. Res. 2019, 26, 35422–35432. [Google Scholar] [CrossRef] [PubMed]
- Werle, S.; Dudziak, M. The assessment of sewage sludge gasification by-products toxicity by ecotoxicologial test. Waste Manag. Res. J. A Sustain. Circ. Econ. 2015, 33, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Krzywicka, A.; Kwarciak-Kozłowska, A. Advanced oxidation processes with coke plant wastewater treatment. Water Sci. Technol. 2014, 69, 1875–1878. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.-S.; Choi, Y.-K.; Park, K.-B.; Kim, J.-S. Air co-gasification of coal and dried sewage sludge in a two-stage gasifier: Effect of blending ratio on the producer gas composition and tar removal. Energy 2019, 185, 708–716. [Google Scholar] [CrossRef]
- Viader, R.P.; Jensen, P.E.; Ottosen, L.M.; Ahrenfeldt, J.; Hauggaard-Nielsen, H. Sequential electrodialytic recovery of phosphorus from low-temperature gasification ashes of chemically precipitated sewage sludge. Waste Manag. 2017, 60, 211–218. [Google Scholar] [CrossRef]
- Thomsen, T.P.; Hauggaard-Nielsen, H.; Gøbel, B.; Stoholm, P.; Ahrenfeldt, J.; Henriksen, U.B.; Müller-Stöver, D.S. Low temperature circulating fluidized bed gasification and co-gasification of municipal sewage sludge. Part 2: Evaluation of ash materials as phosphorus fertilizer. Waste Manag. 2017, 66, 145–154. [Google Scholar] [CrossRef]
- Hannl, T.K.; Häggström, G.; Hedayati, A.; Skoglund, N.; Kuba, M.; Öhman, M. Ash transformation during single-pellet gasification of sewage sludge and mixtures with agricultural residues with a focus on phosphorus. Fuel Process. Technol. 2022, 227, 107102. [Google Scholar] [CrossRef]
- Hannl, T.K.; Sefidari, H.; Kuba, M.; Skoglund, N.; Öhman, M. Thermochemical equilibrium study of ash transformation during combustion and gasification of sewage sludge mixtures with agricultural residues with focus on the phosphorus speciation. Biomass-Convers. Biorefinery 2021, 11, 57–68. [Google Scholar] [CrossRef]
- Hartmann, T.E.; Möller, K.; Meyer, C.; Müller, T. Partial replacement of rock phosphate by sewage sludge ash for the production of superphosphate fertilizers. J. Plant Nutr. Soil Sci. 2020, 183, 233–237. [Google Scholar] [CrossRef]
- Gil-Lalaguna, N.; Sánchez, J.; Murillo, M.; Gea, G. Use of sewage sludge combustion ash and gasification ash for high-temperature desulphurization of different gas streams. Fuel 2015, 141, 99–108. [Google Scholar] [CrossRef]
- Cao, Y.; Pawłowski, A. Life cycle assessment of two emerging sewage sludge-to-energy systems: Evaluating energy and greenhouse gas emissions implications. Bioresour. Technol. 2013, 127, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Fericelli, P.D. Comparison of sludge treatment by gasification vs. incineration. In Proceedings of the Ninth LACCEI Latin American and Caribbean Conference (LACCEI’2011), Engineering for a Smart Planet, Innovation, Information Technology and Computational Tools for Sustainable Development, Medellín, Colombia, 3–5 August 2011. [Google Scholar]
- Buonocore, E.; Mellino, S.; De Angelis, G.; Liu, G.; Ulgiati, S. Life cycle assessment indicators of urban wastewater and sewage sludge treatment. Ecol. Indic. 2018, 94, 13–23. [Google Scholar] [CrossRef]
- Teoh, S.K.; Li, L.Y. Feasibility of alternative sewage sludge treatment methods from a lifecycle assessment (LCA) perspective. J. Clean. Prod. 2020, 247, 119495. [Google Scholar] [CrossRef]
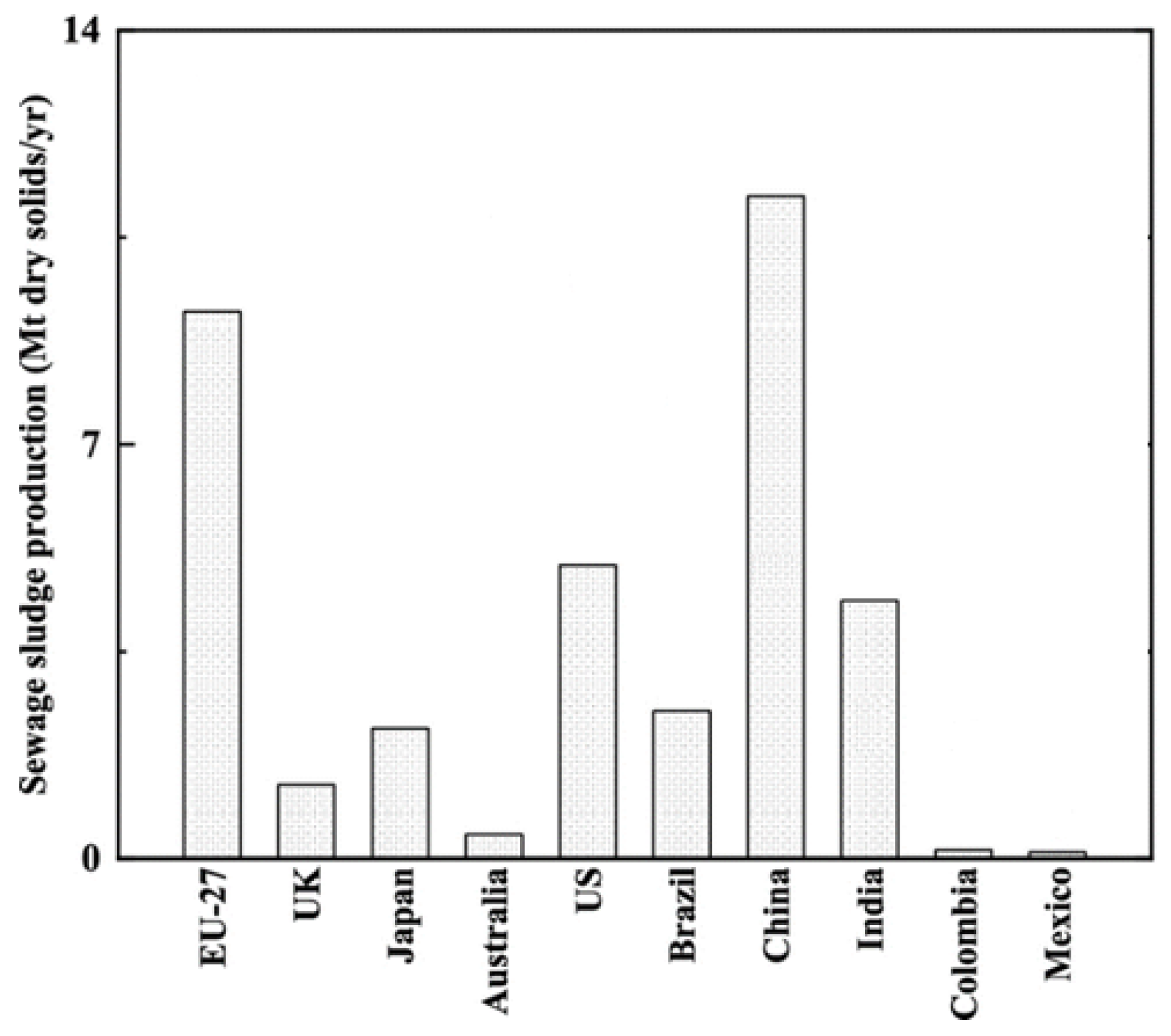



| Location | Reactor | Fuel | Other Information | Ref. |
|---|---|---|---|---|
| Denmark, Kalundborg | LT-CFBR | Blended sewage sludge and local residues | Temperature in reactor: 730 °C; Gas temperature: ~650 °C; Capacity: 6 MWth; Ash is used for fertilizer field tests. | [43,48] |
| Taiwan, Tao-Yuan | BFBR | Blended sewage sludge, paper-mill sludge | Temperature in reactor: 900 °C; Gas temperature: 816 and 858 °C; Capacity: 3 MWth. | [49] |
| USA, City of Lebanon | Downdraft-bed reactor | Blended sewage sludge and local waste (waste wood, scrap tires) | Temperature in reactor: 1250 °F; Gas combusted in thermal oxidizer; Production of 420 kW of electricity. | [50,51] |
| Ash Composition (wt.%) | Heavy Metals Content (mg/kg) | ||
|---|---|---|---|
| SiO2 | 14.4–36.19 | As | 5.6–56.1 |
| Al2O3 | 4.02–14.9 | Ba | 41.5–1300 |
| Fe2O3 | 5.43–15.6 | Cd | 0.83 ± 0.06 |
| TiO2 | 0.58–1.07 | Cr | 18.6 ± 2.2 |
| CaO | 4.73–49.94 | Pb | 4.0–429.8 |
| MgO | 1.4–6.33 | Hg | 0.1–1.1 |
| K2O | 0.42–2.84 | Mo | 1.7–75 |
| Na2O | 0.2–1.99 | Ni | 8.6–420 |
| MnO | 0.003–0.39 | Se | 2 |
| P2O5 | 0.11–26.8 | Zn | 0.0–7500 |
| SO3 | 1.3–13.1 | Cu | 75.8 ± 7.0 |
| Specific surface area | |||
| SBET (m2/g) | ~1–18.2 | ||
| Surface functional groups | |||
| Wavelenght (cm−1) | Band assigned | Wavelength (cm−1) | Band assigned |
| 630–726 | H2O rocking, C-H bending of alcohol, hydrocarbons, aromatic groups | 1450 | aliphatic C–H deformation; C–H bond vibration in saccharides, N–H group in amides, and C–H in alkenes |
| 974–1028 | C-N stretching of amine | 1535 | stretching vibration of C–N; deformation vibration N–H of the peptidic bond of proteins; cell wall of G+ and G– bacteria |
| 1030–1080 | C–O stretching of polysaccharides or polysaccharide substances | 1540 | asymmetric stretching of C=O in carboxylic groups; N–H bending in amide or amino group |
| 1030 | Si–O stretching in the mineral phase of the sludge (silicate impurities/clay minerals) | 1540–1520 | NH2 deformation of amide |
| 1060 | C–O bond stretching vibration in glycerol | 1575 | COO– in carboxylic functional groups |
| 1160 | C–O–C stretching at the glycosidic linkages | 1634 | C=O groups of carboxylic acids; C=C in alkenes; O–H group (adsorbed water) |
| 1230 | C–N stretching of amide II | 1650–1590 | N–H bend in amide (I) |
| 1234 | cell wall of G+ and G– bacteria | 1690–1630 | C=O stretch in amide |
| 1235, 1230 | vibration of C=O in fats and carboxylic acids | 1730, 1720 | C=O in carboxylic acids and ketonic carbonyls |
| 1245 | deforming vibration of NH+ in peptides and proteins | 2008–2258 | C–O stretching |
| 1338–1397 | C-H bending in aromatic and aliphatic hydrocarbons | 2265–2413 | C=O asymmetric stretching |
| 1384, 1380 | N=O in nitrates | 2641–2770 | C-H stretching |
| 1410 | CH2 in polyalcohol | 2925, 2855 | aliphatic methylene groups in fats and lipids |
| 1419 | vibration of C=O group of carboxylates and carboxylic acids; cell wall of G+ and G– bacteria | 3000–2800 | aliphatic C–H stretching in saccharides, polyalcohols fats and lipids |
| 1445–1380 | deforming skeletal vibration of C–H in saccharides | 3300–2800 | N–H in amines, proteins, peptides |
| 1440 | CH2 deformation in fats | 3600–3200 | O–H group in polymeric compounds (polysaccharides, phenols, etc.) and water |
| Sewage Sludge | Chars | |||||
|---|---|---|---|---|---|---|
| Content of: | 300 °C | 400 °C | 500 °C | 600 °C | 700 °C | |
| A (wt.%) | 55.7 | 65.8 | 75.5 | 80.6 | 83.8 | 86.8 |
| VM (wt.%) | 39.7 | 27.4 | 16.0 | 10.2 | 8.6 | 5.5 |
| FC (wt.%) | 7.7 | 4.6 | 6.8 | 8.5 | 9.2 | 7.6 |
| SBET (m2/g) | 11.85 | 14.37 | 22.68 | 24.53 | 26.66 | 26.70 |
| K (g/kg) | 7.47 | 7.47 | 8.99 | 10.1 | 13.3 | 16.6 |
| Mg (g/kg) | 5.62 | 6.19 | 6.96 | 7.47 | 7.86 | 8.06 |
| Ca (g/kg) | 17.4 | 20.6 | 22.7 | 23.9 | 24.0 | 25.8 |
| Fe (g/kg) | 30 | 34.5 | 38.4 | 40.8 | 41.7 | 43.1 |
| Pb (mg/kg) | 3740 | 4410 | 4900 | 5120 | 5250 | 5200 |
| Zn (mg/kg) | 735 | 875 | 986 | 1040 | 1090 | 1090 |
| Ni (mg/kg) | 72.4 | 86.3 | 95.4 | 97.7 | 101 | 103 |
| Cd (mg/kg) | 169 | 197 | 225 | 235 | 229 | 123 |
| As (mg/kg) | 26 | 27 | 31 | 32 | 35 | 37 |
| Cu (mg/kg) | 172 | 195 | 213 | 215 | 209 | 227 |
| Cr (mg/kg) | 100 | 105 | 118 | 116 | 106 | 103 |
| Atmosphere of Pyrolysis */Gasification | Heating Rate K/min | Approach | Temperature Range (°C) | Ea (Model Used) (kJ/mol) | Ref. |
|---|---|---|---|---|---|
| Ar/CO2 | 2, 5, 10, 15, 20 | In-situ | 600–1000 | 285.45 (VRM) | [101] |
| Steam | 40 | In-situ | 800–940 | 59.39 (FM) 45.7 (CRM) | [102] |
| Steam + air + O2 | 40 | In-situ | 800–940 | 51.7 (FM) 46.5 (CRM) | [102] |
| He/CO2 | 10 | In-situ | 800–1100 | 467.37 (VRM) | [103] |
| CO2 + N2 | 20 | In-situ | 430–570 | 267.3 (VRM) | [100] |
| N2/Air | ** | In-situ | 700 800 900 | 17 (A-EM) 15 (A-EM) 12 (A-EM) | [104] |
| Ar/O2 | 10 | Ex-situ | 400–600 | 114 (VRM) | [105] |
| Ar/CO2 | 10 | Ex-situ | 800–1000 | 227 (VRM) | [105] |
| Ar/steam | 10 | Ex-situ | 750–950 | 193 (VRM) | [105] |
| Ar/steam | 10 | Ex-situ | 620–950 | 180 (VRM) 177 (VRM) | [99] |
| Ar/CO2 | 10 | Ex-situ | 700–1000 | 211 (SCM) 234 (SCM) | [99] |
| He/CO2 | 10 | Ex-situ | 517–914 | 180 (ICM) | [65,106] |
| He/CO2 | 10 | Ex-situ | 827–972 | 168 (ICM) | [65] |
| Reactor | Temperature °C | Other Parameters | Observed Effect | Ref. |
|---|---|---|---|---|
| Laboratory fixed-bed reactor | 700 | Gasifying agent: atmospheric air and O2-enriched air; ER: 0.12, 0.14, 0.16, 0.18, 0.23, 0.27; SS1 properties: VM: 44.2%, A: 49%, C: 27.72%; SS2 properties: VM: 36.5%, A: 51.5%, C: 31.79% | Optimal ER value: 0.18, resulting in the highest CO content (31.3% and 26.9% for two analyzed sewage sludge) and the highest LHV (~5 MJ/m3) | [37] |
| Bench-scale fluidized-bed reactor | 850 | Gasifying agent: N2/air; ER: 0.1–0.2; SS properties: VM: 54.3%, A: 30.6%, C: 49.16% | An increase in ER reduces tar content in gas (from 37.5 g/Nm3 at 0.1 EA to 29.4 g/Nm3 at 0.2 ER) but decreases its lower heating value (from 12.1 to 5.8 MJ/Nm3) | [135] |
| Lab-scaled bub-bling fluidized-bed gasifier | 700–850 | Gasifying agent: air; ER: 0.2–0.35; SS properties: VM: 44.6%, A: 44.6%, C: 40.4% | Optimal temperature: 850 °C (the highest cold gas efficiency was achieved—24%); Optimal ER: 0.35 (the highest LHV of gas was obtained, equal to 3.3 MJ/Nm3) | [136] |
| Lab-scaled bubbling fluidized-bed gasifier | 750–900 | Gasifying agent: air; ER: 0.1–0.4; SS properties: VM: 50.1%, A: 40.4%, C: 19.85% | The higher temperature increased the gas yield. Maximum combustible gas yield (H2, CO, and CH4) was obtained at ER = 0.25 and 900 °C. | [137] |
| Fluidized bed and fixed bed | 650, 810, 830 | Gasifying agent: air; ER: 0.22–0.5; SS properties: VM: 50.3%, A: 34.7%, C: 29.88% | The highest H2 content in produced gas (29 vol.%) was obtained at the equivalence ratio of 0.35. The highest ER (0.5) strongly decreased impurity contents (tar, NH3, and H2S). | [118] |
| Fluidized-bed gasifier | 800 | Gasifying agent: air, steam/O2; SS properties: VM: 48.74%, A: 39.9%, C: 30.64% | Steam gasification resulted in the following:
| [138] |
| Two fixed-bed gasifiers (downdraft and updraft) | 700, 800, 900 | Gasifying agent: air, pure O2; SS properties: VM: 47.53%, A: 39.63%, C: 32.22% | An increase in temperature increased the volumetric percentage of the H2, whereas the effect of the gasifying agent was almost insignificant. The highest H2 was obtained at 900 °C, and it was 42 and 40% for air and O2, respectively (updraft gasifier), and 46% and 45% for air and O2, respectively (downdraft gasifier). | [139] |
| Fluidized-bed gasifier | 800 | Gasifying agent: air, air + steam; SS properties: VM: 58.3%, A: 41.7%, C: 29.5% | Steam addition increased the H2 and CO2 content and decreased the content of CO and tar (due to the steam reforming reactions). | [140] |
| Fluidized-bed gasifier | 750, 850 | Gasifying agent: air, air + steam; SS properties: VM: 58.3%, A: 41.7%, C: 29.5% | Temperature increase up to 850 °C:
| [141] |
| Quartz tubular reactor | 650–850 | Gasifying agent: steam (various flow rates); SS properties: C: 30% | An increase in temperature and steam flow significantly raises the gas yield and carbon conversion, but too high of a temperature reduces H2 content (the highest H2 content was obtained at 750 °C (~57%)). | [111] |
| Tubular reactor | 770–850 | Gasifying agent: air, steam + air; SS properties: VM: 50.09%, A: 39.04%, C: 29.5% | Higher temperatures reduce tar content and improve the gas yield (including H2 and CO yields). In turn, the addition and increase of steam in the gasifying agent favor the formation of gas with better heating value and H2/CO molar ratio. | [142] |
| Laboratory-scale semi-batch scale experimental facility | 900 | Gasifying agent: steam (various amounts represented by various steam/carbon ratios): 3.05, 5.62, and 7.38; SS properties: VM: 44.3%, A: 33.91%, C: 45.79% | Optimum S/C ratio: 5.62 (the highest syngas and H2 yields). Further increase in S/C decreases the H2 content since an increase in the steam flow rate has a twofold competing effect—(1) the tendency to accelerate steam-reforming reactions and (2) the tendency to decrease the reactants’ residence time. Thus, the time for the reaction between steam and condensable hydrocarbons is decreased. | [143] |
| Laboratory-scale quartz tubular reactor | 700–1000 | Gasifying agent: steam; Catalysts: KOH, K2CO3, NaOH, and Na2CO3; SS properties: VM: 48.51%, A: 43.11%, C: 27.69% | The greatest improvement in H2 content at low temperatures was obtained by using NaOH and Na2CO3 catalysts. In turn, K2CO3 enhanced in the greatest extent the total gas yield, whereas Na2CO3 was the most effective for improving energy density for sewage sludge. | [121] |
| Quartz reactor | 700, 750, 800 | Gasifying agent: steam Catalysts: Ni-Fe and Ni-Co/Al-MCM48; SS properties: VM: 57.74%, A: 29.58%, C: 27.19% | The temperature of 800 °C resulted in the highest gas yield as well as H2 and CO yields (35.3% and 11.7%). The presence of the catalyst resulted in the enhancement of this effect, especially Ni-Co (the highest H2 content ∼52 vol%) due to the improved Ni dispersion and synergy between catalyst components. | [123] |
| Laboratory-scale fixed-bed reactor | 600–800 | Gasifying agent: air, ER: 0.2, Catalysts: dolomite, steel slag, and calcium oxide; SS properties: VM: 66.53%, A: 27.33%, C: 39.98% | The temperature increase results in higher gas yield and lower tar content. Catalysts used additionally reduce tar contents (due to the racking of the hydrocarbon structure), especially dolomite. In turn, calcium oxide results in the highest H2 and CH4 content in the resulting gas. | [129] |
| Fluidized-bed reactor | 750–850 | Gasifying agent: air + steam Catalysts: dolomite, olivine, alumina; SS properties: VM: 56.0%, A: 44.0%, C: 27.3% | Dolomite was characterized by the greatest activity in tar destruction, followed by alumina and olivine. The presence of steam and the catalysts increased the H2 content in the gases by nearly 60%. | [130] |
| Fluidized-bed reactor | 750–850 | Gasifying agent: steam, ER: 0.2–0.4, Catalyst: alumina; SS properties: VM: 53.3%, A: 46.7%, C: 25.9% | The addition of 10 wt.% of alumina significantly reduces tar production (improvement up to 42%) and increases the carbon conversion and LHV of the gas. | [131] |
| Batch reactor | 450 | Gasifying agent: steam (supercritical gasification), catalysts: NaOH, KOH, K2CO3, Na2CO3, and Ca(OH)2; SS properties: VM: 57.4%, A: 42.6% | Most catalysts increased H2 content in the gas (especially K2CO3). The exception was Ca(OH)2 (no catalytic effect on H2 yields) but it affected the CO2 yield. | [122] |
| Batch reactor | 400 | Gasifying agent: steam (supercritical gasification), catalysts: NaOH, NaOH + Ni; SS properties: VM: 59.52%, A: 41.19%; C: 25.05 | Both catalysts increase the yield of H2 in the resulting gas, whereas the effect of NaOH + Ni was greater (the H2 yield was almost five times as much as without catalyst). | [144] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Śpiewak, K. Gasification of Sewage Sludge—A Review. Energies 2024, 17, 4476. https://doi.org/10.3390/en17174476
Śpiewak K. Gasification of Sewage Sludge—A Review. Energies. 2024; 17(17):4476. https://doi.org/10.3390/en17174476
Chicago/Turabian StyleŚpiewak, Katarzyna. 2024. "Gasification of Sewage Sludge—A Review" Energies 17, no. 17: 4476. https://doi.org/10.3390/en17174476
APA StyleŚpiewak, K. (2024). Gasification of Sewage Sludge—A Review. Energies, 17(17), 4476. https://doi.org/10.3390/en17174476





