Unveiling the Potential of Cryogenic Post-Combustion Carbon Capture: From Fundamentals to Innovative Processes
Abstract
1. Introduction
- Improvement of fossil fuel-based energy efficiency;
- Enhancement of nuclear and renewable energy, as well as increasing use of biofuel-based energy;
- Development of environmental engineering works, such as afforestation and reforestation.
- CCUS can be easily integrated into existing energy and utility systems without invasive or complex retrofits;
- CCUS can be implemented for the production of low-carbon hydrogen (blue hydrogen): currently, around 76% of hydrogen (corresponding to 75 Mt y−1) is produced worldwide from natural gas, generating overall CO2 emissions exceeding 800 Mt y−1 [8]; green hydrogen produced by electrolysis is still expensive having a cost of USD 2.3–6.9 tH2−1 versus USD 1.4–2.4 tH2−1 from steam methane reforming (SMR) coupled with CCS [8,9];
- The technology features extremely high selectivity and thus minimizes the competitive capture of other components in the flue gases;
- The separation process is driven by temperature differences and does not rely on solvents, adsorbents or membranes;
- The CO2 product is generally extracted in the liquid phase so as to avoid the downstream compression work;
- Scalability potential and a wide range of applications have been demonstrated. In addition, CCC can be integrated into existing industrial processes with minimal retrofit requirements;
- CCC is characterized by low water consumption and offers a large heat integration potential. This, in turn, minimizes the disposal of waste and enhances safety and environmental aspects.
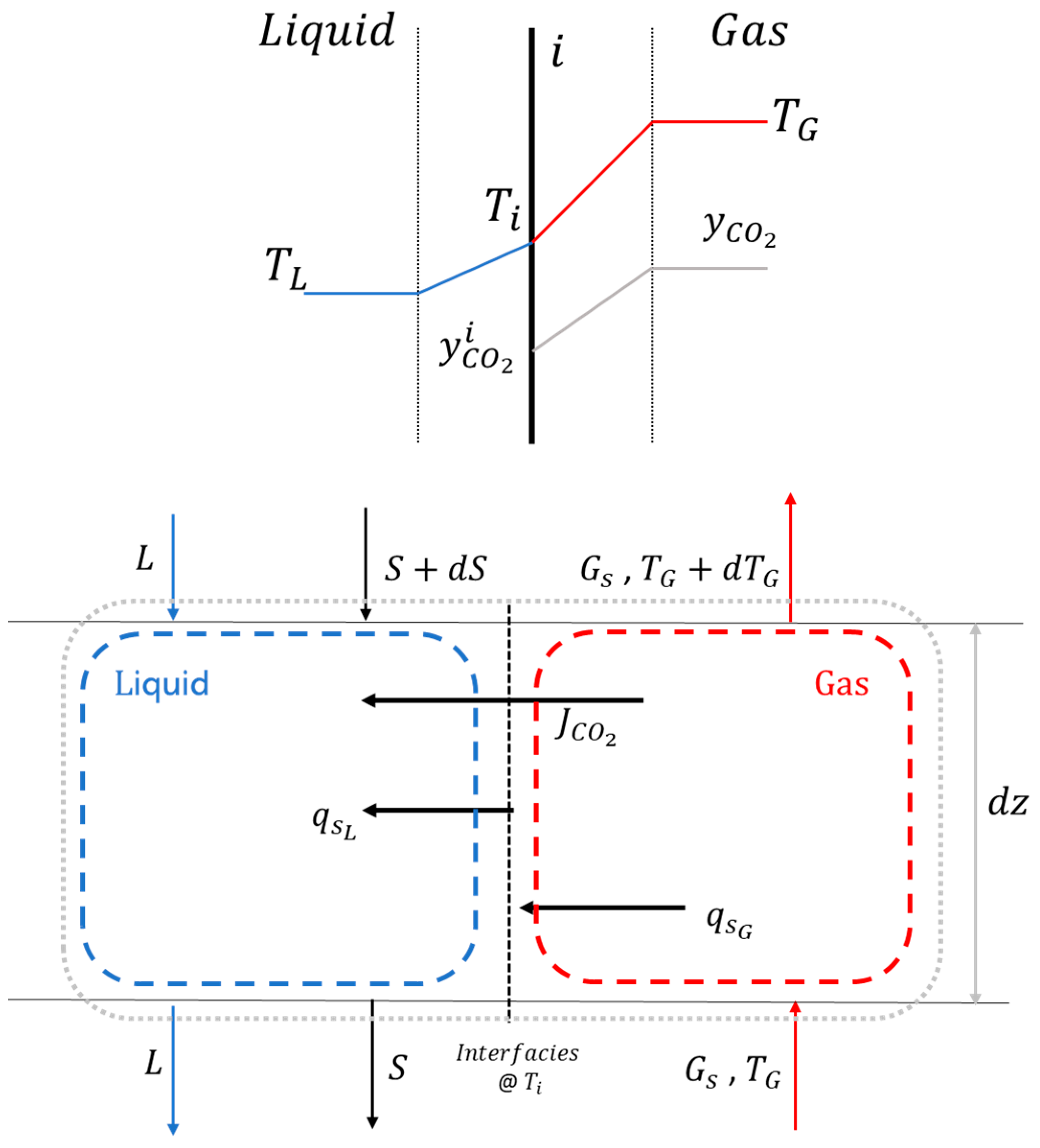
2. Fundamentals of Cryogenic Desublimation
3. Processes Based on Cryogenic Desublimation
3.1. Dynamic Packed Bed
3.2. External Cooling Loop
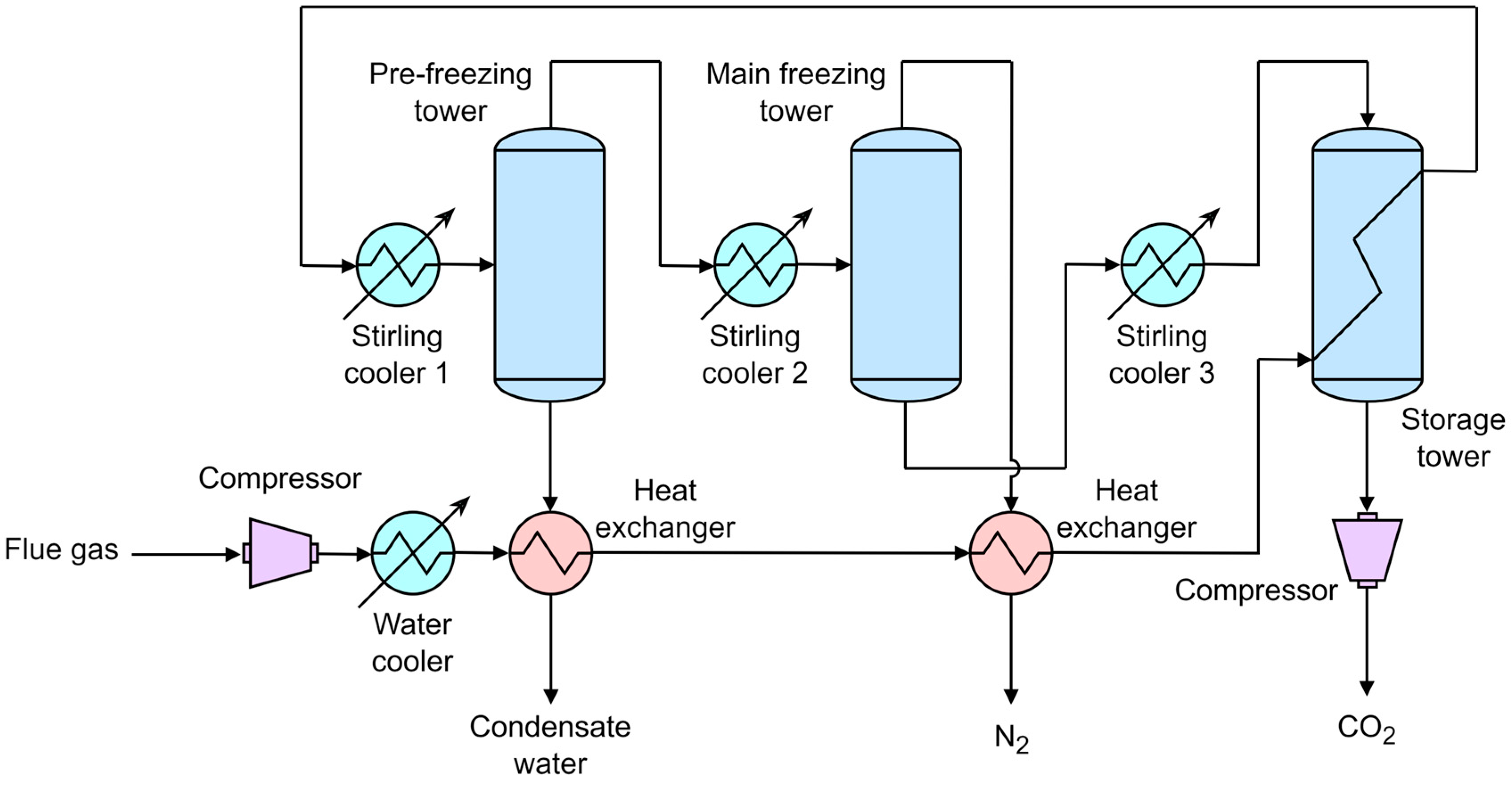
3.3. Stirling Cooler
3.4. Antisublimation (AnSU)
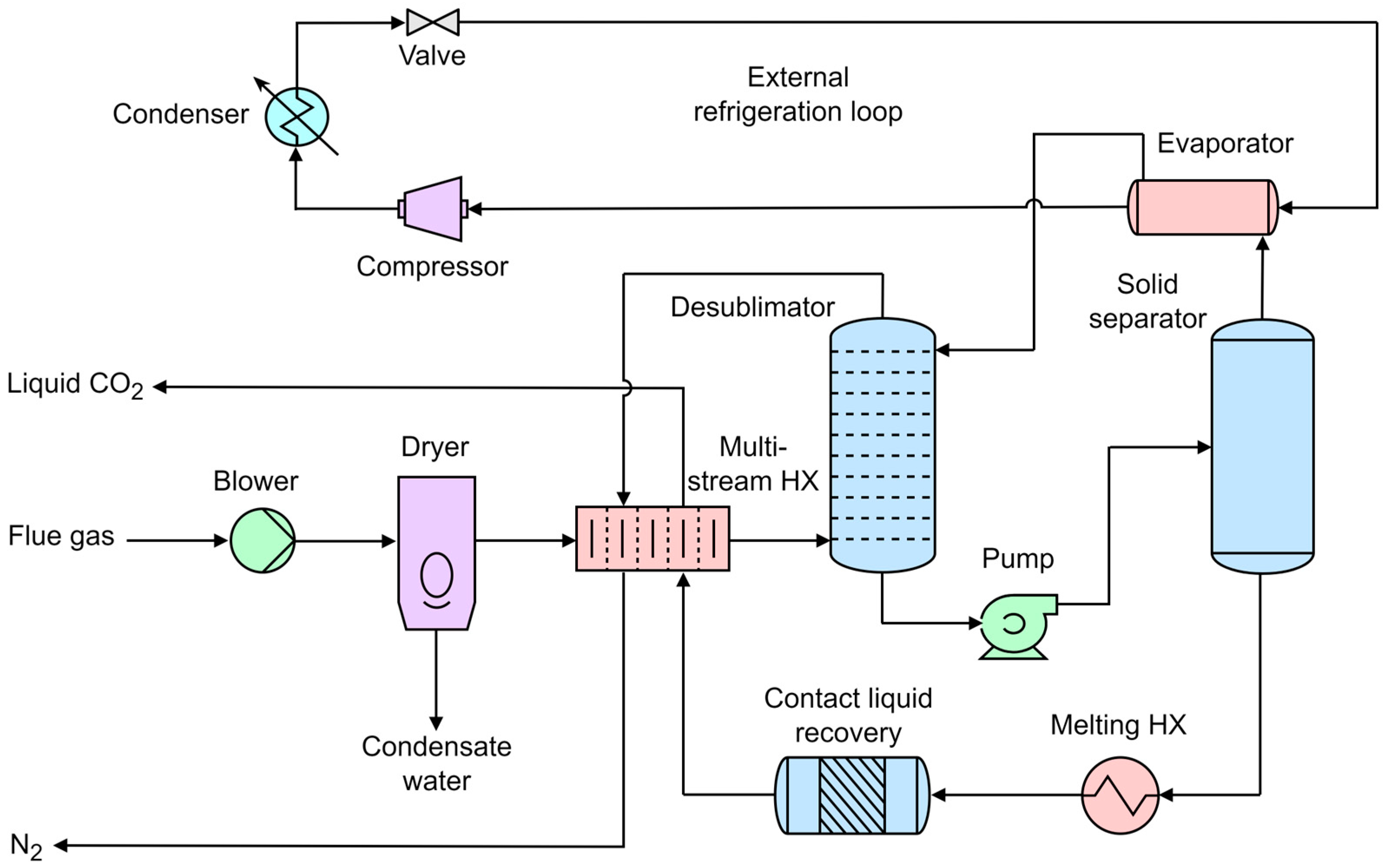
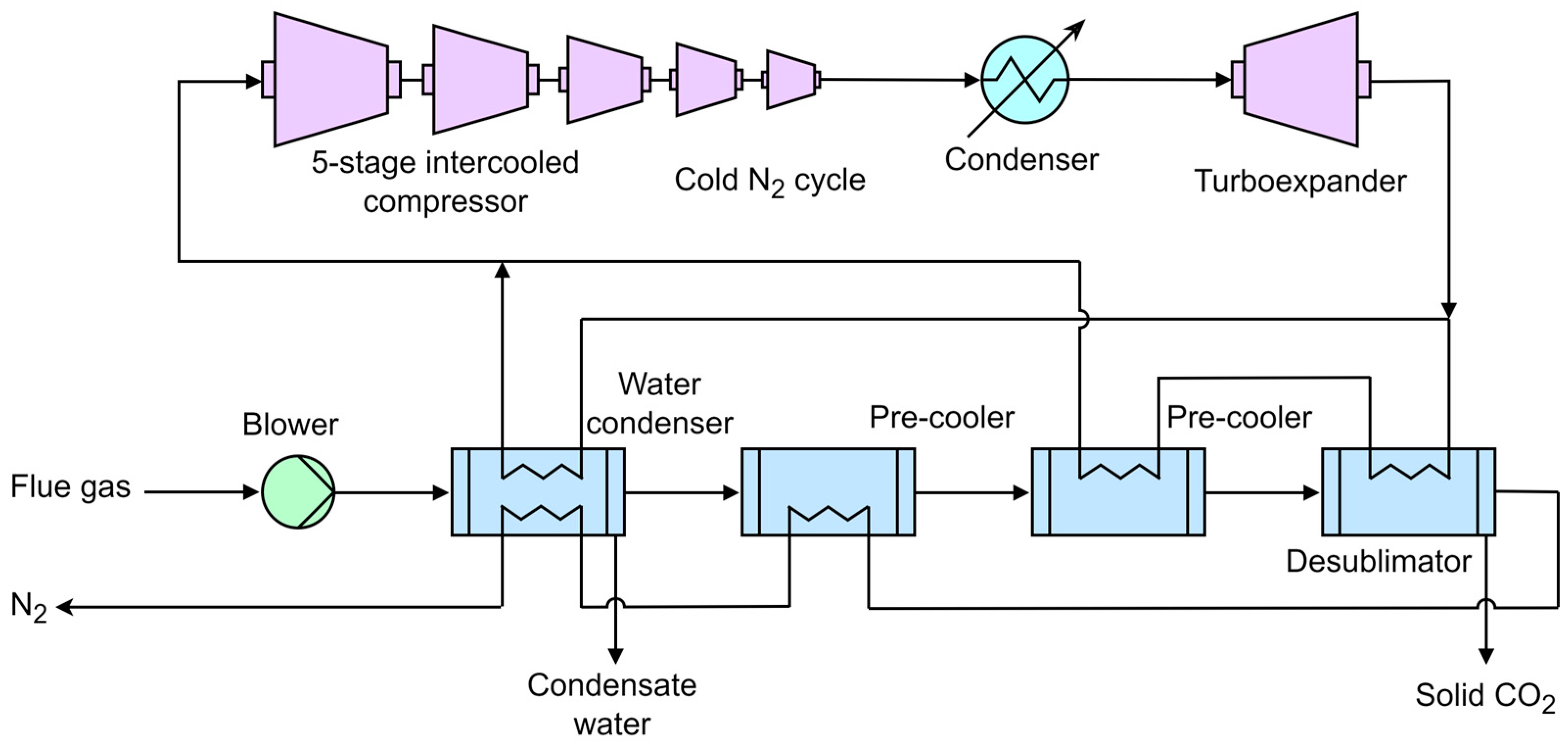
3.5. Novel Low-Cost CO2 Capture Technology (NLCCT)
3.6. Comparison of Cryogenic Desublimation Processes
4. Conclusions and Technological Challenges
- Reduced water (and steam) consumption
- Possibility of coupling with large-scale energy storage (with benefit for grid stabilization)
- Solutions as bolt-on retrofit technology or as an integrated system.
- Possibility of ancillary pollutants’ separation (such as SOx, NOx, and Hg): in this way, a green-field, fully integrated plant can redirect the capital, operating cost, and footprint resources currently dedicated to pollutant control and redirect these toward the carbon capture system.
- Material Challenges and Extreme Temperatures: From the point of view of durability, cryogenic temperatures can cause materials to become brittle and prone to failure, necessitating the use of specialized materials. Repeated cooling and heating cycles can lead to material fatigue and failure over time. The austenitic stainless steels such as 304 and 316 (an alloy with at least 9% nickel) retain their engineering properties at cryogenic temperatures and are commonly used for transport and storage of cryogenic liquids. Aluminum and aluminum alloys can be suitable for extreme cold scenarios, too, since they exhibit very good stability against temperature variations, and some of them even show an increase in ultimate tensile strength and fracture toughness. On the other hand, the insulation system is fundamental for cryogenic applications to avoid energy losses and to prevent accidents and/or external water vapor desublimation. The insulation types and materials are chosen based on their thermal performance and mechanical properties. The insulation systems can be made of foam and fibrous, multilayers, and vacuum jacketed systems. Materials implemented for this purpose are fiberglass, mineral wool, aerogel composite, and powder and granular. Perlite and Hollow glass or ceramic microspheres are often implemented to fill spaces between vacuum walls in insulating cryogenic vessels. For example, the equipment of the cited pilot plants was often installed in a cold box filled with perlite or glass powder insulation and vacuum-jacketed tubing was realized for some of the key fluid transfer lines; at small scales, the main equipment is placed inside blankets of gray fiberglass insulation.
- Technological Readiness and Cost: Assessing the economic viability of cryogenic carbon capture and identifying strategies to reduce costs are critical knowledge gaps. Research should focus on the development of cost-effective materials and innovative engineering solutions at cryogenic conditions to enhance the overall economic feasibility. This should include the insulation materials and ancillary equipment. Among the process equipment characterizing cryogenic carbon capture, there are some critical apparatuses such as solid–liquid separation (e.g., screw press and centrifugal separators), dehydrator systems for water vapor removal, cryogenic distillation to reduce impurities, and losses in closed cycle (direct-contact) heat transfer configurations, as well as slurry pumps, packed beds, bubble columns and turboexpanders. Continued innovation is needed to overcome current technological barriers, but this requires sustained investment and research. Many cryogenic carbon capture technologies are still in the developmental or pilot stages, with limited real-world deployment; transitioning from laboratory or pilot-scale systems to full-scale industrial applications presents significant engineering challenges. For example, the processes of start-up and shut-down, as well as the process control (maintaining precise control over temperature and pressure within the cryogenic system), are technically demanding. Moreover, handling cryogenic fluids poses safety risks, including frostbite, asphyxiation, and equipment failure. The research group that first made these steps forward is that of Baxter et al. [30,40,64,65,66,67], which recently published industrial data for a medium-sized pilot plant (around a few tons of CO2 per day). They also produced a techno-economic analysis to compare the economic performances against the baseline for fossil energy plants. The estimated cost of CO2 avoidance is below USD 40 per ton, and LCOE is around USD 9 per kWh. Recently, similar values (even lower) were disclosed by De et al. [80] for their system. Their results overcame the ones reported by the group of Tuinier at al. [58], who compared the economics of their innovative cryogenic packed bed to the performances of absorption and membrane technology.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Nomenclature
| Acronyms | ||
| AnSU | Antisublimation | |
| BECCS | Bioenergy with carbon capture and storage | |
| CCC | Cryogenic carbon capture | |
| CCS | Carbon capture and storage | |
| CCU | Carbon capture and utilization | |
| CCUS | Carbon capture, utilization and storage | |
| COP | Coefficient of performance | |
| DAC | Direct air capture | |
| EoS | Equation of state | |
| NLCCT | Novel low-cost CO2 capture technology | |
| PSA | Pressure swing adsorption | |
| RIC | Refrigeration integrated cascade | |
| SMR | Steam methane reforming | |
| SVE | Solid-vapor equilibrium | |
| SLVE | Solid-liquid-vapor equilibrium | |
| TRL | Technology readiness level | |
| TSA | Temperature swing adsorption | |
| Symbols | Unit | Description |
| Pa m6 mol−2 | Energy parameter in cubic EoS | |
| m3 mol−1 | Covolume in cubic EoS | |
| W m−2 K−1 | Heat transfer coefficient of the gas phase | |
| W m−2 K−1 | Heat transfer coefficient of the liquid phase | |
| kg m−2 s−1 | Mass transfer coefficient of the gas phase | |
| mol | Moles of CO2 in the feed | |
| mol | Moles of CO2 in the product 1 | |
| mol | Moles of CO2 in the product 2 | |
| mol | Moles of inert in the feed | |
| mol | Moles of inert in the product 1 | |
| mol | Moles of inert in the product 2 | |
| Pa | Total pressure | |
| Pa | Sublimation vapor pressure of CO2 | |
| Pa | Initial pressure of compression | |
| Pa | Final pressure of compression | |
| W | Thermal duty of condensation | |
| kJ kg−1 | Specific thermal duty of separation | |
| W m−2 | Sensible heat flux of the gas phase | |
| W m−2 | Latent heat flux of the gas phase | |
| W m−2 | Sensible heat flux of the liquid phase | |
| J mol−1 K−1 | Ideal gas constant | |
| m2 | Interface surface | |
| K | Temperature | |
| K | Ambient temperature | |
| K | Temperature of liquefaction | |
| K | Temperature of the gas phase | |
| K | Temperature of the interphase | |
| K | Temperature of the liquid phase | |
| K | Temperature of the hot source used for separation | |
| m3 mol−1 | Molar volume in cubic EoS | |
| m3 mol−1 | Molar volume of solid CO2 | |
| kJ kgCO2−1 | Minimum work rate of compression | |
| kJ kgCO2−1 | Actual work of compression | |
| kJ kgCO2−1 | Minimum work of liquefaction | |
| kJ kgCO2−1 | Actual work of liquefaction | |
| kJ kgCO2−1 | Minimum work of separation | |
| kJ kgCO2−1 | Actual work of separation | |
| – | Mole fraction of CO2 in the vapor phase | |
| – | Mole fraction of CO2 in the interface | |
| – | Mole fraction of CO2 in the feed | |
| – | Mole fraction of CO2 in the product 1 | |
| – | Mole fraction of CO2 in the product 2 | |
| – | Mole fraction of inert in the feed | |
| – | Mole fraction of inert in the product 1 | |
| – | Mole fraction of inert in the product 2 | |
| m | Axial dimension | |
| Greek letters | ||
| – | II law efficiency | |
| J kg−1 | Heat of liquefaction of CO2 | |
| – | Fugacity coefficient of CO2 at the saturated solid pressure | |
| – | Fugacity coefficient of CO2 in the vapor phase | |
References
- IPCC. Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V.P., Zhai, A., Pirani, S.L., Connors, C., Péan, S., Berger, N., Caud, Y., Chen, L., Goldfarb, M.I., Gomis, M., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; pp. 3–32. [Google Scholar]
- Gabrielli, P.; Gazzani, M.; Mazzotti, M. The role of carbon capture and utilization, carbon capture and storage, and biomass to enable a net-zero-CO2 emissions chemical industry. Ind. Eng. Chem. Res. 2020, 59, 7033–7045. [Google Scholar] [CrossRef]
- Keyßer, L.T.; Lenzen, M. 1.5 °C degrowth scenarios suggest the need for new mitigation pathways. Nat. Commun. 2021, 12, 2676. [Google Scholar] [CrossRef] [PubMed]
- Bednar, J.; Obersteiner, M.; Baklanov, A.; Thomson, M.; Wagner, F.; Geden, O.; Allen, M.; Hall, J.W.A. Operationalizing the net-negative carbon economy. Nature 2021, 596, 377–383. [Google Scholar] [CrossRef]
- IEA. Energy Technology Perspectives, 2020. IEA, Paris. Available online: https://www.iea.org/reports/energy-technology-perspectives-2020 (accessed on 1 May 2024).
- Schmitt, T.; Homsy, S.; Mantripragada, H.; Woods, M.; Hoffman, H.; Shultz, T.; Fout, T.; Hackett, G. Cost and Performance of Retrofitting NGCC Units for Carbon Capture; U.S. Department of Energy Office of Scientific and Technical Information: Pittsburgh, PA, USA, 2023.
- Capocelli, M.; Luberti, M.; Inno, S.; D’Antonio, F.; Di Natale, F.; Lancia, A. Post-combustion CO2 capture by RVPSA in a large-scale steam reforming plant. J. CO2 Util. 2019, 32, 53–65. [Google Scholar] [CrossRef]
- Luberti, M.; Brown, A.; Balsamo, M.; Capocelli, M. Numerical analysis of VPSA technology retrofitted to steam reforming hydrogen plants to capture CO2 and produce blue H2. Energies 2022, 15, 1091. [Google Scholar] [CrossRef]
- Luberti, M.; Ahn, H. Review of Polybed pressure swing adsorption for hydrogen purification. Int. J. Hydrogen Energy 2022, 47, 10911–10933. [Google Scholar] [CrossRef]
- Barba, D.; Brandani, F.; Capocelli, M.; Luberti, M.; Zizza, A. Process analysis of an industrial waste-to-energy plant: Theory and experiments. Process Saf. Environ. Prot. 2015, 96, 61–73. [Google Scholar] [CrossRef]
- Butnar, I.; Cronin, J.; Pye, S. Review of Carbon Capture Utilisation and Carbon Capture and Storage in Future EU Decarbonisation Scenarios; Final Report Prepared for The Carbon Capture and Storage Association; UCL Energy Institute: London, UK, 2020. [Google Scholar]
- Koelbl, B.S.; van den Broek, M.; Faaij, A.P.C.; van Vuuren, D.P. Uncertainty in Carbon Capture and Storage (CCS) deployment projections: A cross-model comparison exercise. Clim. Chang. 2014, 123, 461–476. [Google Scholar] [CrossRef]
- Capocelli, M.; De Falco, M. Generalized penalties and standard efficiencies of carbon capture and storage processes. Int. J. Energy Res. 2022, 46, 4808–4824. [Google Scholar] [CrossRef]
- Sifat, N.S.; Yousef, H. A critical review of CO2 capture technologies and prospects for clean power generation. Energies 2019, 12, 4143. [Google Scholar] [CrossRef]
- Kearns, D.; Liu, H.; Consoli, C. Technology Readiness and Costs of CCS. Global CCS Institute, 2021; Available online: https://www.globalccsinstitute.com/wp-content/uploads/2021/03/Technology-Readiness-and-Costs-for-CCS-2021-1.pdf (accessed on 27 May 2024).
- Ahn, H.; Luberti, M.; Liu, Z.; Brandani, S. Process simulation of aqueous MEA plants for post-combustion capture from coal-fired power plants. Energy Procedia 2013, 37, 1523–1531. [Google Scholar] [CrossRef]
- Erto, A.; Silvestre-Albero, A.; Silvestre-Albero, J.; Rodríguez-Reinoso, F.; Balsamo, M.; Lancia, A.; Montagnaro, F. Carbon-supported ionic liquids as innovative adsorbents for CO₂ separation from synthetic flue-gas. J. Colloid. Interface Sci. 2015, 15, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Luberti, M.; Oreggioni, G.D.; Ahn, H. Design of a rapid vacuum pressure swing adsorption (RVPSA) process for post-combustion CO2 capture from a biomass-fuelled CHP plant. J. Environ. Chem. Eng. 2017, 5, 3973–3982. [Google Scholar] [CrossRef]
- Luberti, M.; Ahn, H. Design of an industrial multi-bed (V)PSA unit for argon concentration. Sep. Purif. Technol. 2021, 261, 118254. [Google Scholar] [CrossRef]
- Brunetti, A.; Scura, F.; Barbieri, G.; Drioli, E. Membrane technologies for CO2 separation. J. Membr. Sci. 2010, 359, 115–125. [Google Scholar] [CrossRef]
- Font-Palma, C.; Cann, D.; Udemu, C. Review of cryogenic carbon capture innovations and their potential applications. J. Carbon Res. 2021, 7, 58. [Google Scholar] [CrossRef]
- Swanson, C.E.; Elzey, J.W.; Hershberger, R.E.; Donnelly, R.J.; Pfotenhauer, J. Thermodynamic analysis of low-temperature carbon dioxide and sulfur dioxide capture from coal-burning power plants. Phys. Rev. E 2012, 86, 016103. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.; Ryan, J.M. Cryogenic Distillative Separation of Acid Gases from Methane. US Patent 4,318,723, 9 March 1982. [Google Scholar]
- Hart, A.; Gnanendran, N. Cryogenic CO2 capture in natural gas. Energy Procedia 2009, 1, 697–706. [Google Scholar] [CrossRef]
- Yousef, A.M.; El-Maghlany, W.M.; Eldrainy, Y.A.; Attia, A. New approach for biogas purification using cryogenic separation and distillation process for CO2 capture. Energy 2018, 156, 328–351. [Google Scholar] [CrossRef]
- Shen, M.; Tong, L.; Yin, S.; Liu, C.; Wang, L.; Feng, W.; Ding, Y. Cryogenic technology progress for CO2 capture under carbon neutrality goals: A review. Sep. Purif. Technol. 2022, 299, 121734. [Google Scholar] [CrossRef]
- Berstad, D.; Skaugen, G.; Roussanaly, S.; Anantharaman, R.; Nekså, P.; Jordal, K.; Trædal, S.; Gundersen, T. CO2 capture from IGCC by low-temperature synthesis gas separation. Energies 2022, 15, 515. [Google Scholar] [CrossRef]
- Eide, L.I.; Anheden, M.; Lyngfelt, A.; Abanades, C.; Younes, M.; Clodic, D.; Bill, A.A.; Feron, P.H.M.; Rojey, A.; Giroudiere, F. Novel capture processes. Oil Gas Sci. Technol. 2005, 60, 497–508. [Google Scholar] [CrossRef]
- Tuinier, M.J.; van Sint Annaland, M.; Kramer, G.J.; Kuipers, J.A.M. Cryogenic CO2 capture using dynamically operated packed beds. Chem. Eng. Sci. 2010, 65, 114–119. [Google Scholar] [CrossRef]
- Jensen, M.J.; Russell, C.S.; Bergeson, D.; Hoeger, C.D.; Frankman, D.J.; Bence, C.S.; Baxter, L.L. Prediction and validation of external cooling loop cryogenic carbon capture (CCC-ECL) for full-scale coal-fired power plant retrofit. Int. J. Greenh. Gas Control 2015, 45, 200–212. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Deng, S.; Li, H.; Kitamura, Y. Cryogenic-based CO2 capture technologies: State-of-the-art developments and current challenges. Renew. Sustain. Energy Rev. 2019, 101, 265–278. [Google Scholar] [CrossRef]
- Asgharian, H.; Iov, F.; Araya, S.S.; Pedersen, T.H.; Nielsen, M.P.; Baniasadi, E.; Liso, V. A Review on process modeling and simulation of cryogenic carbon capture for post-combustion treatment. Energies 2023, 16, 1855. [Google Scholar] [CrossRef]
- Aneesh, A.M.; Sam, A.A. A mini-review on cryogenic carbon capture technology by desublimation: Theoretical and modeling aspects. Front. Energy Res. 2023, 11, 1167099. [Google Scholar] [CrossRef]
- Yang, W.; Li, S.; Li, X.; Liang, Y.; Zhang, X. Analysis of a new liquefaction combined with desublimation system for CO2 separation based on N2/CO2 phase equilibrium. Energies 2015, 8, 9495–9508. [Google Scholar] [CrossRef]
- De Guido, G.; Langè, S.; Moioli, S.; Pellegrini, L.A. Thermodynamic method for the prediction of solid CO2 formation from multicomponent mixtures. Process Saf. Environ. Prot. 2014, 92, 70–79. [Google Scholar] [CrossRef]
- De Guido, G.; Pellegrini, L.A. Phase equilibria analysis in the presence of solid carbon dioxide. Chem. Eng. Trans. 2021, 86, 1261–1266. [Google Scholar]
- Wang, J.; Wang, Z.; Sun, B. Improved equation of CO2 Joule–Thomson coefficient. J. CO2 Util. 2017, 19, 296–307. [Google Scholar] [CrossRef]
- Pellegrini, L.; De Guido, G.; Ingrosso, S. Thermodynamic Framework for Cryogenic Carbon Capture. In Proceedings of the 30th European Symposium on Computer Aided Process Engineering 2020 (ESCAPE30), Milano, Italy, 24–27 May 2020. [Google Scholar]
- Jensen, M. Energy Process Enabled by Cryogenic Carbon Capture. Ph.D. Thesis, BYU Theses and Dissertations, Brigham Young University, Provo, UT, USA, 2015. [Google Scholar]
- Baxter, L.; Baxter, A.; Burt, S. Cryogenic CO2 capture as a cost-effective CO2 capture process. In Proceedings of the International Pittsburgh Coal Conference, Pittsburgh, PA, USA, 20–23 September 2009. [Google Scholar]
- Yurata, T.; Lei, H.; Tang, L.; Lu, M.; Patel, J.; Lim, S.; Piumsomboon, P.; Chalermsinsuwan, B.; Li, C. Feasibility and sustainability analyses of carbon dioxide—Hydrogen separation via de-sublimation process in comparison with other processes. Int. J. Hydrogen Energy 2019, 44, 23120–23134. [Google Scholar] [CrossRef]
- Seader, J.D.; Henley, E. Separation Process Principles; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2006. [Google Scholar]
- Zonfrilli, M.; Facchino, M.; Serinelli, R.; Chesti, M.; De Falco, M.; Capocelli, M. Thermodynamic analysis of cold energy recovery from LNG regasification. J. Clean. Prod. 2023, 420, 138443. [Google Scholar] [CrossRef]
- House, K.Z.; Harvey, C.F.; Aziz, M.J.; Schrag, D.P. The Energy Penalty of Post-Combustion CO2 Capture & Storage and Its Implications for Retrofitting the U.S. Installed Base. Energy Environ. Sci. 2009, 2, 193. [Google Scholar]
- Bejan, A. Advanced Engineering Thermodynamics, 4th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016. [Google Scholar]
- Yu, Z.; Miller, F.; Pfotenhauer, J.M. Numerical modeling and analytical modeling of cryogenic carbon capture in a de-sublimating heat exchanger. IOP Conf. Ser. Mater. Sci. Eng. 2017, 278, 012032. [Google Scholar] [CrossRef]
- Berger, A.H.; Hoeger, C.; Baxter, L.; Bhown, A.S. Evaluation of cryogenic systems for post combustion CO2 capture. In Proceedings of the 14th International Conference on Greenhouse Gas Control Technologies, GHGT-14, Melbourne, Australia, 21–25 October 2018. [Google Scholar]
- Cann, D.G. Experimental Exploration of Cryogenic CO2 Capture Utilising a Moving Bed. Ph.D. Thesis, The University of Chester, Chester, UK, 2021. [Google Scholar]
- James, D.W. Failing Drop CO2 Deposition (Desublimation) Heat Exchanger for the Cryogenic Carbon Capture Process. Ph.D. Thesis, Brigham Young University, Provo, UT, USA, 2011. [Google Scholar]
- Sun, B.; Ghatage, S.; Evans, G.M.; Bhatelia, T.; Utikar, R.P.; Pareek, V.K. Dynamic study of frost formation on cryogenic surface. Int. J. Heat Mass Transf. 2020, 150, 119372. [Google Scholar] [CrossRef]
- Wu, X.; Ma, Q.; Chu, F.; Hu, S. Phase change mass transfer model for frost growth and densification. Int. J. Heat Mass Transf. 2016, 96, 11–19. [Google Scholar] [CrossRef]
- Wu, X.M.; Webb, R.L. Investigation of the possibility of frost release from a cold surface. Exp. Therm. Fluid Sci. 2001, 24, 151–156. [Google Scholar] [CrossRef]
- Lei, T.; Luo, K.H.; Hernández Pérez, F.E.; Wang, G.; Wang, Z.; Cano, J.R.; Im, H.G. Study of CO2 desublimation during cryogenic carbon capture using the lattice Boltzmann method. J. Fluid Mech. 2023, 964, A1. [Google Scholar] [CrossRef]
- Pan, X.; Clodic, D.; Toubassy, J. CO2 capture by antisublimation process and its technical economic analysis. Greenh. Gas Sci. Technol. 2013, 3, 8–20. [Google Scholar] [CrossRef]
- Rezaei, S.; Liu, A.; Hovington, P. Emerging technologies in post-combustion carbon dioxide capture & removal. Catal. Today 2023, 423, 114286. [Google Scholar]
- Tuinier, M.J.; van Sint Annaland, M.; Kuipers, J.A.M. A novel process for cryogenic CO2 capture using dynamically operated packed beds—An experimental and numerical study. Int. J. Greenh. Gas Control 2011, 5, 694–701. [Google Scholar] [CrossRef]
- Luberti, M. Oxygen recovery from ozone generators by adsorption processes. Adsorption 2023, 29, 73–86. [Google Scholar] [CrossRef]
- Tuinier, M.J.; Hamers, H.P.; van Sint Annaland, M. Techno-economic evaluation of cryogenic CO2 capture—A comparison with absorption and membrane technology. Int. J. Greenh. Gas Control 2011, 5, 1559–1565. [Google Scholar] [CrossRef]
- Lively, R.P.; Koros, W.J.; Johnson, J.R. Enhanced cryogenic CO2 capture using dynamically operated low-cost fiber beds. Chem. Eng. Sci. 2012, 71, 97–103. [Google Scholar] [CrossRef]
- Willson, P.; Lychnos, G.; Clements, A.; Michailos, S.; Font-Palma, C.; Diego, M.E.; Pourkashanian, M.; Howe, J. Evaluation of the performance and economic viability of a novel low temperature carbon capture process. Int. J. Greenh. Gas Control 2019, 86, 1–9. [Google Scholar] [CrossRef]
- Cann, D.; Font-Palma, C.; Willson, P. Experimental analysis of CO2 frost front behaviour in moving packed beds for cryogenic CO2 capture. Int. J. Greenh. Gas Control 2021, 107, 103291. [Google Scholar] [CrossRef]
- Cann, D.; Font-Palma, C.; Willson, P. Moving packed beds for cryogenic CO2 capture: Analysis of packing material and bed precooling. Carbon Capture Sci. Technol. 2021, 1, 100017. [Google Scholar] [CrossRef]
- Cann, D.; Font-Palma, C. Evaluation of mathematical models for CO2 frost formation in a cryogenic moving bed. Energies 2023, 16, 2314. [Google Scholar] [CrossRef]
- Sayre, A.; Frankman, D.; Baxter, A.; Stitt, K. Field testing of cryogenic carbon capture. In Proceedings of the Carbon Management Technology Conference, Houston, TX, USA, 17–20 July 2017. [Google Scholar]
- Baxter, L. Systems and Methods for Separating Condensable Vapors from Light Gases or Liquids by Recuperative Cryogenic Process. US Patent No. US 10,724,793 B2, 28 July 2020. [Google Scholar]
- Safdarnejad, S.M.; Hedengren, J.D.; Baxter, L.L. Plant-level dynamic optimization of Cryogenic Carbon Capture with conventional and renewable power sources. Appl. Energy 2015, 149, 354–366. [Google Scholar] [CrossRef]
- Baxter, L.L.; Baxter, A.; Bever, E.; Burt, S.; Chamberlain, S.; Frankman, D.; Hoeger, C.; Mansfield, E.; Parkinson, D.; Sayre, A.; et al. Cryogenic Carbon Capture Development; Final Technical Report Submitted to US DoE NETL; National Energy Technology Laboratory: Pittsburgh, PA, USA, 2019.
- Song, C.; Lu, J.; Kitamura, Y. Study on the COP of free piston Stirling cooler (FPSC) in the anti-sublimation CO2 capture process. Renew. Energy 2015, 74, 948–954. [Google Scholar] [CrossRef]
- Song, C.; Kitamura, Y.; Li, S.; Ogasawara, K. Design of a cryogenic CO2 capture system based on Stirling coolers. Int. J. Greenh. Gas Control 2012, 7, 107–114. [Google Scholar] [CrossRef]
- Song, C.; Kitamura, Y.; Li, S.; Jiang, W. Parametric analysis of a novel cryogenic CO2 capture system based on Stirling coolers. Environ. Sci. Technol. 2012, 46, 12735−12741. [Google Scholar] [CrossRef] [PubMed]
- Song, C.; Kitamura, Y.; Li, S. Optimization of a novel cryogenic CO2 capture process by response surface methodology (RSM). J. Taiwan Inst. Chem. Eng. 2014, 45, 1666–1676. [Google Scholar] [CrossRef]
- Song, C.; Kitamura, Y.; Li, S.; Lu, J. Deposition CO2 capture process using a free piston Stirling cooler. Ind. Eng. Chem. Res. 2013, 52, 14936−14943. [Google Scholar] [CrossRef]
- Song, C.; Liu, Q.; Ji, N.; Deng, S.; Zhao, J.; Kitamura, Y. Advanced cryogenic CO2 capture process based on Stirling coolers by heat integration. Appl. Therm. Eng. 2017, 114, 887–895. [Google Scholar] [CrossRef]
- Clodic, D.; Younes, M. A new method for CO2 capture: Frosting CO2 at atmospheric pressure. Greenh. Gas Control Technol. 2003, I, 155–160. [Google Scholar]
- Clodic, D.; El Hitti, R.; Younes, M.; Bill, A.; Casier, F. CO2 capture by anti-sublimation: Thermo-economic process evaluation. In Proceedings of the 4th Annual Conference on Carbon Capture & Sequestration, Alexandria, VA, USA, 2–5 May 2005. [Google Scholar]
- Clodic, D.; Younes, M. Method and System for Extracting carbon Dioxide by Anti-Sublimation for Storage Thereof. US Patent No. 7,073,348 B2, 11 July 2006. [Google Scholar]
- Clodic, D.; Younes, M.; Bill, A. Test results of CO2 capture by anti-sublimation: Capture efficiency and energy consumption for boiler plants. Greenh. Gas Control Technol. 2005, II, 1775–1780. [Google Scholar]
- Hees, W.G.; Monroe, C.M. Method and System for Extracting Carbon Dioxide by Anti-Sublimation at RAISED Pressure. US Patent No. 8,163,070 B2, 24 April 2012. [Google Scholar]
- De, D.K.; Oduniyi, I.A. Highly Cost Effective Technology for Capture of Industrial Emissions without Reagent for Clean Energy and Clean Environment Applications. US Patent No. 10,670,334 B2, 2 June 2020. [Google Scholar]
- De, D.K.; Oduniyi, I.A.; Sam, A.A. A novel cryogenic technology for low-cost carbon capture from NGCC power plants for climate change mitigation. Therm. Sci. Eng. Prog. 2022, 36, 101495. [Google Scholar] [CrossRef]






| Reference | Main Content and Findings |
|---|---|
| Asgarian et al. [32] | They reviewed thermodynamic framework, general models, and process simulator works for calculating equipment for cryogenic process and apparatus. They also included heat exchanger modeling, particle velocity model for heat transfer from or to droplets, plug flow reactor modeling, and Aspen Plus simulations for modeling the LNG storage. |
| De Guido et al. [35], Pellegrini et al. [38] | They implemented SRK and PR EoS to calculate solid CO2 solubilities and proposed a mathematical algorithm developed for the calculation of Solid Vapor Equilibrium stages and compared their results to the Aspen RGibbs reactor. |
| Yu et al. [46] | Numerical analysis of a 1-dimensional desublimating heat exchanger. They calculated the rate of desublimation and thickness of solid formation on the walls as a function of time and location. Both the inert gas (in the gaseous mixture with CO2) and the cryogenic liquid are nitrogen. They analyzed the effect of fluid mass flowrates and temperatures; they neglected the thermal resistance of the solid CO2 layer and pressure drops. |
| Berger et al. [47] | They proposed a conceptual framework to address the work of separation through cryogenic desublimation; they defined the methodologies for energy balances and energy penalty calculation and proposed the comparison to the minimum work of separation. |
| Cann [48] | The frost front velocity experiments in a fixed packed bed allowed the design of a moving packed bed (setting the bed flowrate to match the frost front velocity) to prevent the excessive accumulation of CO2 frost. He reported heat transfer coefficients, pressure drops, and other fundamental correlations to describe experimental data on desublimation in a moving bed. |
| James [49] | He proposed a model that predicted the behavior of a falling sphere heat exchanger in a desublimating column for CO2 capture. Desublimation and condensation of molecules in flue gas streams occurring in a countercurrent falling sphere heat exchanger have been modeled and partially validated with experiments. The model is interesting for spray chambers but is limited by the fact that the majority of the properties used to calculate heat transfer are at film temperature. |
| Sun et al. [50], Wu et al. [51], Wu and Webb [52] | Frost formation and frost release on surfaces, frost growth and densification, and frost release: detailed modeling of experimental results. |
| Lei et al. [53] | CO2 desublimation on a cooled cylinder surface by means of lattice Boltzmann model with 2D simulations, various behaviors in response to different operation conditions. |
| Cryogenic Process | Advantages | Challenges |
|---|---|---|
| Dynamic packed bed |
|
|
| External cooling loop |
|
|
| Stirling cooler |
|
|
| AnSU |
|
|
| NLCCT |
|
|
| Cryogenic Process | Feed CO2 Concentration (mol%) | Cold Energy Source | CO2 Recovery (%) | Minimum Specific Energy Consumption (MJe kgCO2−1) | Type of Study | Reference |
|---|---|---|---|---|---|---|
| Dynamic packed bed | 10 | LNG | 99 | 3.60 | Experimental and Modeling | Tuinier et al. [56] |
| External cooling loop | 16 | Multiple refrigerants | 90 | 0.74 | Experimental and Modeling | Jensen et al. [30] |
| Stirling cooler | 13 | Stirling cooler | 95 | 0.55 | Experimental and Modeling | Song et al. [71] |
| AnSU | 12 | LNG | 90 | 1.18 | Experimental | Pan et al. [54] |
| NLCCT | 6.7 | Cold N2 gas | 99 | 0.63 | Modeling | De et al. [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luberti, M.; Ballini, E.; Capocelli, M. Unveiling the Potential of Cryogenic Post-Combustion Carbon Capture: From Fundamentals to Innovative Processes. Energies 2024, 17, 2673. https://doi.org/10.3390/en17112673
Luberti M, Ballini E, Capocelli M. Unveiling the Potential of Cryogenic Post-Combustion Carbon Capture: From Fundamentals to Innovative Processes. Energies. 2024; 17(11):2673. https://doi.org/10.3390/en17112673
Chicago/Turabian StyleLuberti, Mauro, Erika Ballini, and Mauro Capocelli. 2024. "Unveiling the Potential of Cryogenic Post-Combustion Carbon Capture: From Fundamentals to Innovative Processes" Energies 17, no. 11: 2673. https://doi.org/10.3390/en17112673
APA StyleLuberti, M., Ballini, E., & Capocelli, M. (2024). Unveiling the Potential of Cryogenic Post-Combustion Carbon Capture: From Fundamentals to Innovative Processes. Energies, 17(11), 2673. https://doi.org/10.3390/en17112673







