Validity and Reproducibility of Counter Electrodes for Linear Sweep Voltammetry Test in Microbial Electrolysis Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrode Fabrication
2.2. MEC Configuration and Operation
2.3. Electrochemical Test
2.4. Statistics
3. Results
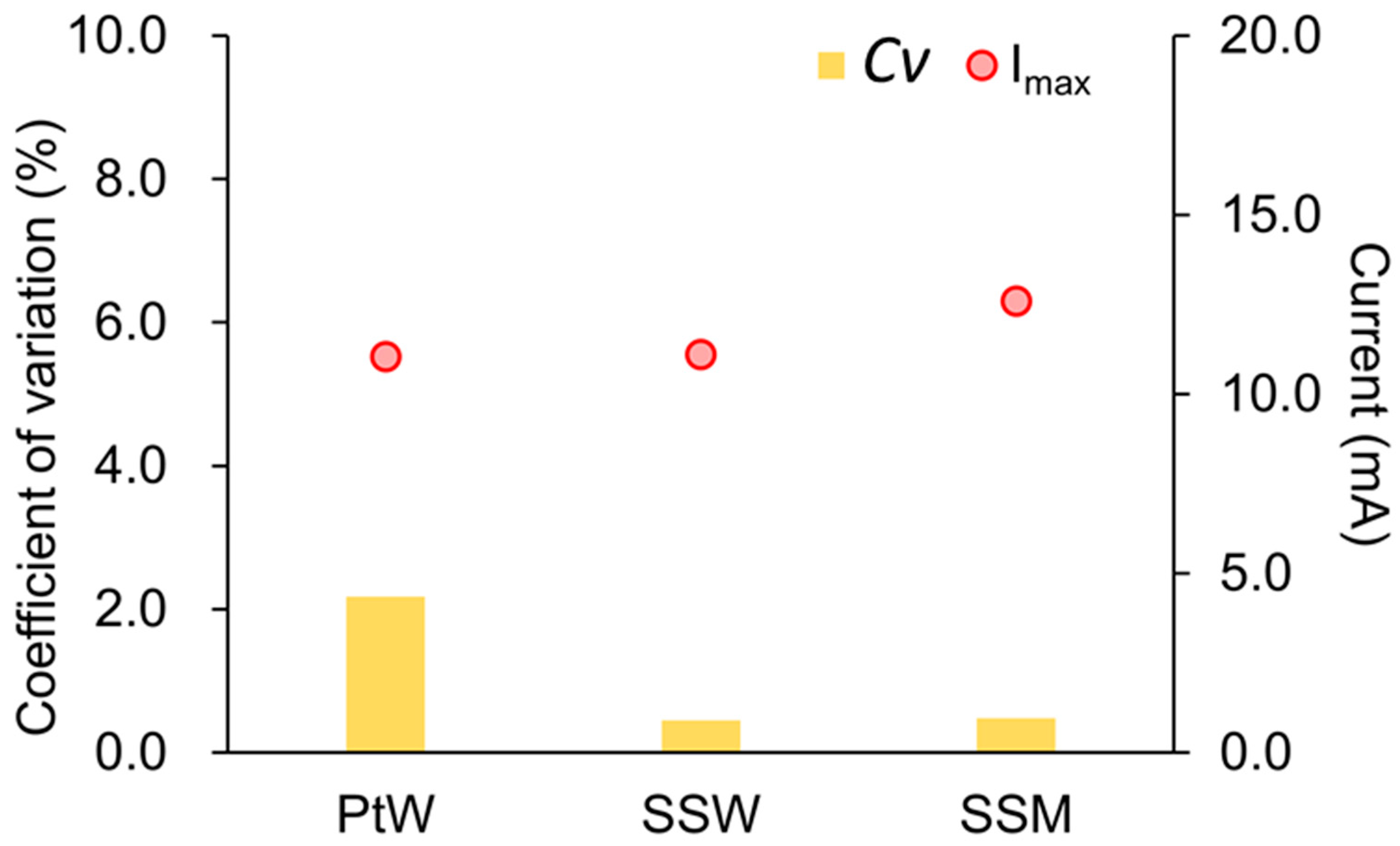
3.1. Anode Linear Sweep Voltammetry Test
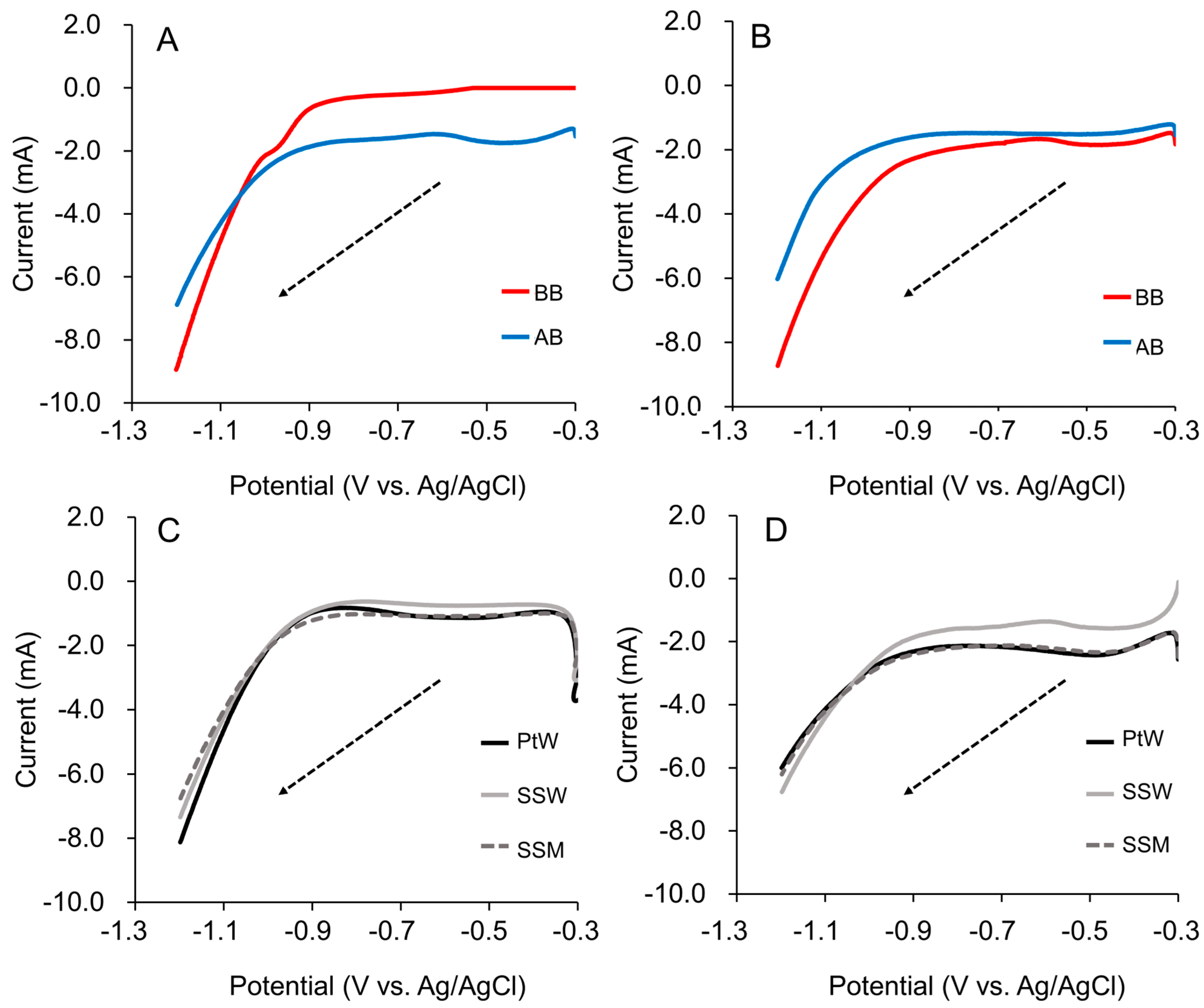
3.2. Stainless Steel Mesh (SSM) Cathode Linear Sweep Voltammetry Test
3.3. Ni-AC-SSM Cathode Linear Sweep Voltammetry Test
4. Discussion
4.1. Reproducibility of Anode Linear Sweep Voltammetry Test
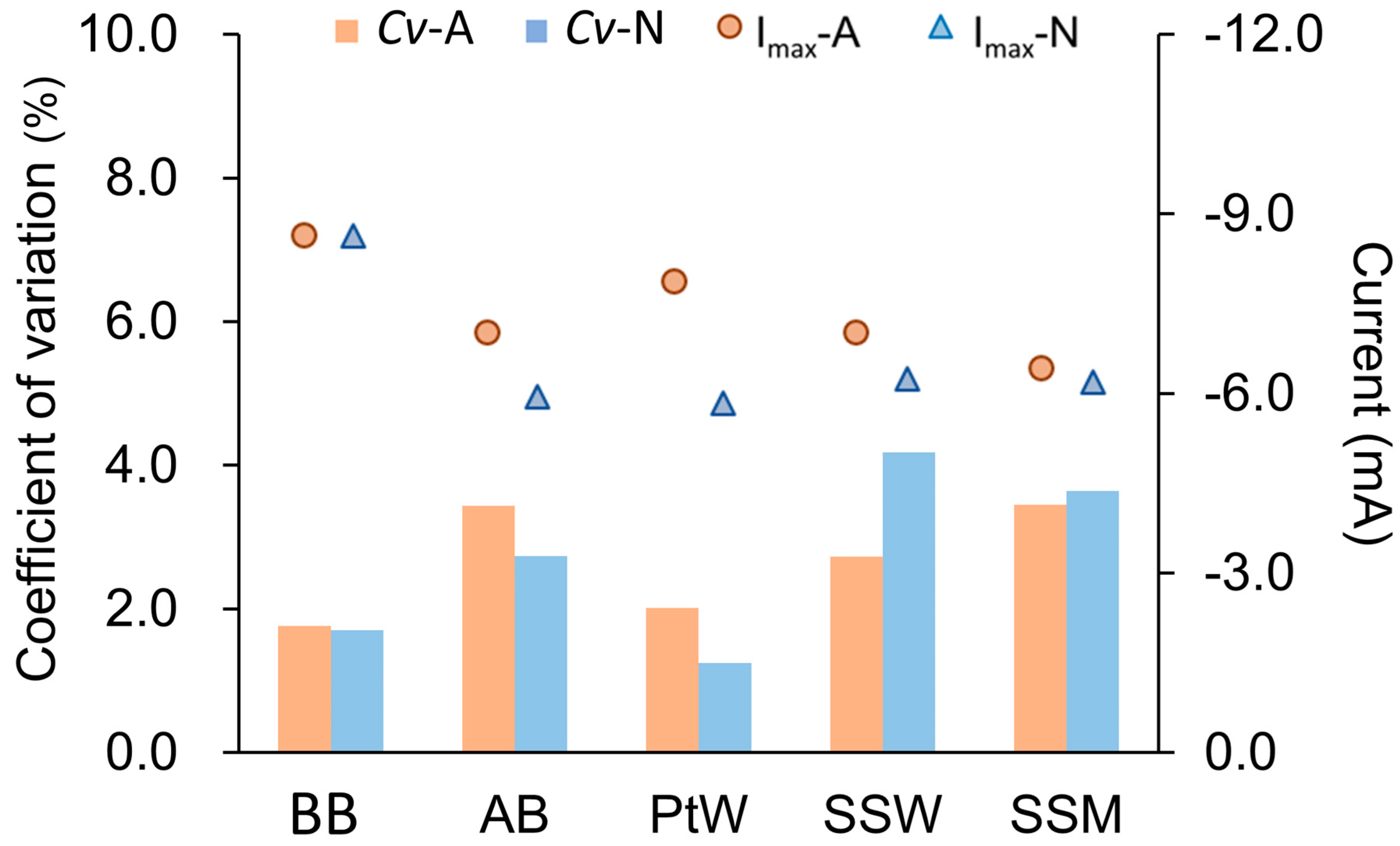
4.2. Reproducibility of Cathode Linear Sweep Voltammetry Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Duce, R.A.; LaRoche, J.; Altieri, K.; Arrigo, K.R.; Baker, A.R.; Capone, D.; Cornell, S.; Dentener, F.; Galloway, J.; Ganeshram, R.S. Impacts of atmospheric anthropogenic nitrogen on the open ocean. Science 2008, 320, 893–897. [Google Scholar] [CrossRef] [PubMed]
- Im, S.-W.; Lee, H.-J.; Chung, J.-W.; Ahn, Y.-T. The Effect of Electrode Spacing and Size on the Performance of Soil Microbial Fuel Cells (SMFC). J. Korean Soc. Environ. Eng. 2014, 36, 758–763. [Google Scholar] [CrossRef]
- Park, Y.; Yang, H.; Yu, J.; Lee, T. Effect of COD and HRT Changes in Submerged Microbial Fuel Cells on Nitrogen Removal at the Level of Domestic Wastewater. J. Korean Soc. Environ. Eng. 2018, 40, 314–319. [Google Scholar] [CrossRef]
- Park, Y.; Yu, J.; Widyaningsih, E.; Lee, T. Effect of Reactor Width and HRT on Simultaneous Removal of Organic Compounds and Nitrogen in Flat-Type Air-Cathode Microbial Fuel Cell. J. Korean Soc. Environ. Eng. 2019, 41, 69–75. [Google Scholar] [CrossRef]
- Shin, Y.; Lee, M.-E.; Park, C.-H.; Ahn, Y. Effect of Electrode Configuration on the Substrate Degradation in Microbial Fuel Cells. J. Korean Soc. Environ. Eng. 2017, 39, 489–493. [Google Scholar] [CrossRef]
- Logan, B.E.; Rabaey, K. Conversion of wastes into bioelectricity and chemicals by using microbial electrochemical technologies. Science 2012, 337, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Logan, B.E.; Call, D.; Cheng, S.; Hamelers, H.V.; Sleutels, T.H.; Jeremiasse, A.W.; Rozendal, R.A. Microbial electrolysis cells for high yield hydrogen gas production from organic matter. Environ. Sci. Technol. 2008, 42, 8630–8640. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ren, Z.J. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol. Adv. 2013, 31, 1796–1807. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.; Bakonyi, P.; Zhen, G.; Sivagurunathan, P.; Koók, L.; Kim, S.-H.; Tóth, G.; Nemestóthy, N.; Bélafi-Bakó, K. Microbial electrochemical systems for sustainable biohydrogen production: Surveying the experiences from a start-up viewpoint. Renew. Sustain. Energy Rev. 2017, 70, 589–597. [Google Scholar] [CrossRef]
- Liu, H.; Logan, B.E. Electricity generation using an air-cathode single chamber microbial fuel cell in the presence and absence of a proton exchange membrane. Environ. Sci. Technol. 2004, 38, 4040–4046. [Google Scholar] [CrossRef]
- Jang, J.K.; Kim, K.M.; Byun, S.; Ryou, Y.S.; Chang, I.S.; Kang, Y.K.; Kim, Y.H. Current generation from microbial fuel cell using stainless steel wire as anode electrode. J. Korean Soc. Environ. Eng. 2014, 36, 753–757. [Google Scholar] [CrossRef]
- Shin, W.; Park, J.; Lee, B.; Kim, Y.; Jun, H. Evaluation of Biogas Production Rate by using Various Electrodes Materials in a Combined Anaerobic Digester and Microbial Electrochemical Technology (MET). J. Korean Soc. Environ. Eng. 2017, 39, 82–88. [Google Scholar] [CrossRef]
- Kim, T.; Kang, S.; Chang, I.S.; Kim, H.W.; Sung, J.H.; Paek, Y.; Kim, Y.H.; Jang, J.K. Prevention of Power Overshoot and Reduction of Cathodic Overpotential by Increasing Cathode Flow Rate in Microbial Fuel Cells used Stainless Steel Scrubber Electrode. J. Korean Soc. Environ. Eng. 2017, 39, 591–598. [Google Scholar] [CrossRef]
- Yoon, H.-S.; Song, Y.-C.; Choi, T.-S. Improvement of anodic performance by using CTP binder containg nickel. J. Korean Soc. Environ. Eng. 2015, 37, 499–504. [Google Scholar] [CrossRef]
- Hassan, M.; Zhu, G.; Lu, Y.-Z.; AL-Falahi, A.H.; Lu, Y.; Huang, S.; Wan, Z. Removal of antibiotics from wastewater and its problematic effects on microbial communities by bioelectrochemical Technology: Current knowledge and future perspectives. Environ. Eng. Res. 2020, 26, 190405. [Google Scholar] [CrossRef]
- Savla, N.; Pandit, S.; Khanna, N.; Mathuriya, A.S.; Jung, S.P. Microbially Powered Electrochemical Systems Coupled with Membrane-based Technology for Sustainable Desalination and Efficient Wastewater Treatment. J. Korean Soc. Environ. Eng. 2020, 42, 360–380. [Google Scholar] [CrossRef]
- Guo, S.; Asset, T.; Atanassov, P. Catalytic hybrid electrocatalytic/biocatalytic cascades for carbon dioxide reduction and valorization. ACS Catal. 2021, 11, 5172–5188. [Google Scholar] [CrossRef]
- Li, H.; Opgenorth, P.H.; Wernick, D.G.; Rogers, S.; Wu, T.-Y.; Higashide, W.; Malati, P.; Huo, Y.-X.; Cho, K.M.; Liao, J.C. Integrated electromicrobial conversion of CO2 to higher alcohols. Science 2012, 335, 1596. [Google Scholar] [CrossRef] [PubMed]
- Quraishi, M.; Wani, K.; Pandit, S.; Gupta, P.K.; Rai, A.K.; Lahiri, D.; Jadhav, D.A.; Ray, R.R.; Jung, S.P.; Thakur, V.K. Valorisation of CO2 into value-added products via microbial electrosynthesis (MES) and electro-fermentation technology. Fermentation 2021, 7, 291. [Google Scholar] [CrossRef]
- Eaktasang, N.; Kang, C.S.; Ryu, S.J.; Suma, Y.; Kim, H.S. Enhanced current production by electroactive biofilm of sulfate-reducing bacteria in the microbial fuel cell. Environ. Eng. Res. 2013, 18, 277–281. [Google Scholar] [CrossRef]
- Haque, N.; Cho, D.; Kwon, S. Performances of metallic (sole, composite) and non-metallic anodes to harness power in sediment microbial fuel cells. Environ. Eng. Res. 2014, 19, 363–367. [Google Scholar] [CrossRef]
- Kamel, M.S.; Abd-Alla, M.H.; Abdul-Raouf, U.M.; Kamel, M.S.; Abd-Alla, M.H.; Abdul-Raouf, U.M. Characterization of anodic biofilm bacterial communities and performance evaluation of a mediator-free microbial fuel cell. Environ. Eng. Res. 2019, 25, 862–870. [Google Scholar] [CrossRef]
- Kim, I.-S.; Chae, K.-J.; Choi, M.-J.; Verstraete, W. Microbial fuel cells: Recent advances, bacterial communities and application beyond electricity generation. Environ. Eng. Res. 2008, 13, 51–65. [Google Scholar] [CrossRef]
- Lee, K.-Y.; Choi, I.-K.; Lim, K.-H.; Lee, K.-Y.; Choi, I.-K.; Lim, K.-H. Nitrogen removal and electrochemical characteristics depending on separators of two-chamber microbial fuel cells. Environ. Eng. Res. 2018, 24, 443–448. [Google Scholar] [CrossRef]
- Nam, J.-Y.; Kim, H.-W.; Lim, K.-H.; Shin, H.-S. Electricity generation from MFCs using differently grown anode-attached bacteria. Environ. Eng. Res. 2010, 15, 71–78. [Google Scholar] [CrossRef]
- Nam, J.-Y.; Moon, C.; Jeong, E.; Lee, W.-T.; Shin, H.-S.; Kim, H.-W.; Nam, J.-Y.; Moon, C.; Jeong, E.; Lee, W.-T. Optimal metal dose of alternative cathode catalyst considering organic substances in single chamber microbial fuel cells. Environ. Eng. Res. 2013, 18, 145–150. [Google Scholar] [CrossRef]
- Nam, T.; Son, S.; Kim, E.; Tran, H.V.H.; Koo, B.; Chai, H.; Kim, J.; Pandit, S.; Gurung, A.; Oh, S.-E. Improved structures of stainless steel current collector increase power generation of microbial fuel cells by decreasing cathodic charge transfer impedance. Environ. Eng. Res. 2018, 23, 383–389. [Google Scholar] [CrossRef]
- Wang, Z.; Lim, B. Electric power generation from sediment microbial fuel cells with graphite rod array anode. Environ. Eng. Res. 2020, 25, 238–242. [Google Scholar] [CrossRef]
- Wang, Z.J.; Lim, B.S.; Wang, Z.J.; Lim, B.S. Electric power generation from treatment of food waste leachate using microbial fuel cell. Environ. Eng. Res. 2016, 22, 157–161. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Wu, H.-J.; Fu, H.-Y.; Dai, Z.; Wang, Z.-J. Burial depth of anode affected the bacterial community structure of sediment microbial fuel cells. Environ. Eng. Res. 2019, 25, 870–876. [Google Scholar] [CrossRef]
- Reinikovaite, V.; Zukauskas, S.; Zalneravicius, R.; Ratautaite, V.; Ramanavicius, S.; Bucinskas, V.; Vilkiene, M.; Ramanavicius, A.; Samukaite-Bubniene, U. Assessment of Rhizobium anhuiense bacteria as a potential biocatalyst for microbial biofuel cell design. Biosensors 2022, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Call, D.; Logan, B.E. Hydrogen production in a single chamber microbial electrolysis cell lacking a membrane. Environ. Sci. Technol. 2008, 42, 3401–3406. [Google Scholar] [CrossRef] [PubMed]
- Son, S.; Koo, B.; Chai, H.; Tran, H.V.H.; Pandit, S.; Jung, S.P. Comparison of hydrogen production and system performance in a microbial electrolysis cell containing cathodes made of non-platinum catalysts and binders. J. Water Process Eng. 2021, 40, 101844. [Google Scholar] [CrossRef]
- Koo, B.; Jung, S.P. Recent Trends of Oxygen Reduction Catalysts in Microbial Fuel Cells: A Review. J. Korean Soc. Environ. Eng. 2019, 41, 657–675. [Google Scholar] [CrossRef]
- Koo, B.; Jung, S.P. Improvement of air cathode performance in microbial fuel cells by using catalysts made by binding metal-organic framework and activated carbon through ultrasonication and solution precipitation. Chem. Eng. J. 2021, 424, 130388. [Google Scholar] [CrossRef]
- Jung, S.P.; Son, S.; Koo, B. Reproducible polarization test methods and fair evaluation of polarization data by using interconversion factors in a single chamber cubic microbial fuel cell with a brush anode. J. Clean. Prod. 2023, 390, 136157. [Google Scholar] [CrossRef]
- Lu, L.; Hou, D.; Fang, Y.; Huang, Y.; Ren, Z.J. Nickel based catalysts for highly efficient H2 evolution from wastewater in microbial electrolysis cells. Electrochim. Acta 2016, 206, 381–387. [Google Scholar] [CrossRef]
- Kim, K.-Y.; Logan, B.E. Nickel powder blended activated carbon cathodes for hydrogen production in microbial electrolysis cells. Int. J. Hydrogen Energy 2019, 44, 13169–13174. [Google Scholar] [CrossRef]
- Santoro, C.; Serov, A.; Gokhale, R.; Rojas-Carbonell, S.; Stariha, L.; Gordon, J.; Artyushkova, K.; Atanassov, P. A family of Fe-NC oxygen reduction electrocatalysts for microbial fuel cell (MFC) application: Relationships between surface chemistry and performances. Appl. Catal. B Environ. 2017, 205, 24–33. [Google Scholar] [CrossRef]
- Kang, H.; Jeong, J.; Gupta, P.L.; Jung, S.P. Effects of brush-anode configurations on performance and electrochemistry of microbial fuel cells. Int. J. Hydrogen Energy 2017, 42, 27693–27700. [Google Scholar] [CrossRef]
- Nam, T.; Son, S.; Koo, B.; Tran, H.V.H.; Kim, J.R.; Choi, Y.; Jung, S.P. Comparative evaluation of performance and electrochemistry of microbial fuel cells with different anode structures and materials. Int. J. Hydrogen Energy 2017, 42, 27677–27684. [Google Scholar] [CrossRef]
- Jung, S.P.; Kim, E.; Koo, B. Effects of wire-type and mesh-type anode current collectors on performance and electrochemistry of microbial fuel cells. Chemosphere 2018, 209, 542–550. [Google Scholar] [CrossRef] [PubMed]
- Koo, B.; Lee, S.-M.; Oh, S.-E.; Kim, E.J.; Hwang, Y.; Seo, D.; Kim, J.Y.; Kahng, Y.H.; Lee, Y.W.; Chung, S.-Y. Addition of reduced graphene oxide to an activated-carbon cathode increases electrical power generation of a microbial fuel cell by enhancing cathodic performance. Electrochim Acta 2019, 297, 613–622. [Google Scholar] [CrossRef]
- Jung, S.; Mench, M.M.; Regan, J.M. Impedance characteristics and polarization behavior of a microbial fuel cell in response to short-term changes in medium pH. Environ. Sci. Technol. 2011, 45, 9069–9074. [Google Scholar] [PubMed]
- Kenjo, T.; Yamakoshi, Y.; Wada, K. An Estimation of the Electrode-Electrolyte Contact Area by Linear Sweep Voltammetry in Pt/ZrO2 Oxygen Electrodes. J. Electrochem. Soc. 1993, 140, 2151. [Google Scholar] [CrossRef]
- Zago, M.; Baricci, A.; Bisello, A.; Jahnke, T.; Yu, H.; Maric, R.; Zelenay, P.; Casalegno, A. Experimental analysis of recoverable performance loss induced by platinum oxide formation at the polymer electrolyte membrane fuel cell cathode. J. Power Sources 2020, 455, 227990. [Google Scholar]
- Gao, S.; Wang, B.; Liu, Z. Enhanced hydrogen production of PbTe-PbS/TNAs electrodes modified with ordered mesoporous carbon. J. Colloid. Interface Sci. 2017, 504, 652–659. [Google Scholar] [PubMed]
- Liu, Z.; Cao, X.; Wang, B.; Xia, M.; Lin, S.; Guo, Z.; Zhang, X.; Gao, S. Coupling thermoelectricity and electrocatalysis for hydrogen production via PbTePbS/TiO2 heterojunction. J. Power Sources 2017, 342, 452–459. [Google Scholar] [CrossRef]
- Skoog, D.A.; Holler, F.J.; Crouch, S.R. Principles of Instrumental Analysis; Cengage Learning: Boston, MA, USA, 2017. [Google Scholar]
- Jafary, T.; Daud, W.R.W.; Ghasemi, M.; Bakar, M.H.A.; Sedighi, M.; Kim, B.H.; Carmona-Martínez, A.A.; Jahim, J.M.; Ismail, M. Clean hydrogen production in a full biological microbial electrolysis cell. Int. J. Hydrogen Energy 2019, 44, 30524–30531. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Liu, X.-W.; Sun, X.-F.; Sheng, G.-P.; Zhang, Y.-Y.; Yan, G.-M.; Wang, S.-G.; Xu, A.-W.; Yu, H.-Q. A new cathodic electrode deposit with palladium nanoparticles for cost-effective hydrogen production in a microbial electrolysis cell. Int. J. Hydrogen Energy 2011, 36, 2773–2776. [Google Scholar] [CrossRef]
- Li, F.; Liu, W.; Sun, Y.; Ding, W.; Cheng, S. Enhancing hydrogen production with Ni–P coated nickel foam as cathode catalyst in single chamber microbial electrolysis cells. Int. J. Hydrogen Energy 2017, 42, 3641–3646. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Huang, Q.; Feng, Y.; Zhu, S.; Shen, S. Hydrogen production with carbon nanotubes based cathode catalysts in microbial electrolysis cells. J. Chem. Technol. Biotechnol. 2012, 87, 1150–1156. [Google Scholar] [CrossRef]
- Cheng, S.; Wu, J. Air-cathode preparation with activated carbon as catalyst, PTFE as binder and nickel foam as current collector for microbial fuel cells. Bioelectrochemistry 2013, 92, 22–26. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.-A.; Wang, B.-S.; Wang, Y.-H. Increasing efficiencies of microbial fuel cells for collaborative treatment of copper and organic wastewater by designing reactor and selecting operating parameters. Bioresour. Technol. 2013, 147, 332–337. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, J.; Li, D.; Wang, H.; Qu, Y.; Wang, X.; Feng, Y. The electrochemical behavior of three air cathodes for microbial electrochemical system (MES) under meter scale water pressure. J. Power Sources 2014, 267, 219–226. [Google Scholar] [CrossRef]
- Wu, D.; Huang, L.; Quan, X.; Puma, G.L. Electricity generation and bivalent copper reduction as a function of operation time and cathode electrode material in microbial fuel cells. J. Power Sources 2016, 307, 705–714. [Google Scholar] [CrossRef]
- Pasupuleti, S.B.; Srikanth, S.; Mohan, S.V.; Pant, D. Development of exoelectrogenic bioanode and study on feasibility of hydrogen production using abiotic VITO-CoRE™ and VITO-CASE™ electrodes in a single chamber microbial electrolysis cell (MEC) at low current densities. Bioresour. Technol. 2015, 195, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Roubaud, E.; Lacroix, R.; Da Silva, S.; Bergel, A.; Basséguy, R.; Erable, B. Catalysis of the hydrogen evolution reaction by hydrogen carbonate to decrease the voltage of microbial electrolysis cell fed with domestic wastewater. Electrochim Acta 2018, 275, 32–39. [Google Scholar] [CrossRef]
- Yu, Q.; Xiong, W.; Huang, D.; Luo, C.; Yang, Q.; Guo, T.; Wei, Q.; Yu, Q.; Xiong, W.; Huang, D. Cathodic reduction characteristics of 2-chloro-4-nitrophenol in microbial electrolysis cell. Environ. Eng. Res. 2019, 25, 854–861. [Google Scholar] [CrossRef]
- Wu, D.; Pan, Y.; Huang, L.; Zhou, P.; Quan, X.; Chen, H. Complete separation of Cu (II), Co (II) and Li (I) using self-driven MFCs–MECs with stainless steel mesh cathodes under continuous flow conditions. Sep. Purif. Technol. 2015, 147, 114–124. [Google Scholar] [CrossRef]
- Lamp, J.L.; Guest, J.S.; Naha, S.; Radavich, K.A.; Love, N.G.; Ellis, M.W.; Puri, I.K. Flame synthesis of carbon nanostructures on stainless steel anodes for use in microbial fuel cells. J. Power Sources 2011, 196, 5829–5834. [Google Scholar] [CrossRef]
- Selembo, P.A.; Merrill, M.D.; Logan, B.E. Hydrogen production with nickel powder cathode catalysts in microbial electrolysis cells. Int. J. Hydrogen Energy 2010, 35, 428–437. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Q.; Wang, X.; Logan, B.E. Treatment of carbon fiber brush anodes for improving power generation in air–cathode microbial fuel cells. J. Power Sources 2010, 195, 1841–1844. [Google Scholar] [CrossRef]
- Tran, H.V.; Kim, E.; Jung, S.P. Anode biofilm maturation time, stable cell performance time, and time-course electrochemistry in a single-chamber microbial fuel cell with a brush-anode. J. Ind. Eng. Chem. 2022, 106, 269–278. [Google Scholar] [CrossRef]
- Zhang, Y.; Merrill, M.D.; Logan, B.E. The use and optimization of stainless steel mesh cathodes in microbial electrolysis cells. Int. J. Hydrogen Energy 2010, 35, 12020–12028. [Google Scholar] [CrossRef]
- Cheng, S.; Logan, B.E. Evaluation of catalysts and membranes for high yield biohydrogen production via electrohydrogenesis in microbial electrolysis cells (MECs). Water Sci. Technol. 2008, 58, 853–857. [Google Scholar] [CrossRef]
- Chorbadzhiyska, E.; Bardarov, I.; Hubenova, Y.; Mitov, M. Modified graphite electrodes as potential cathodic electrocatalysts for microbial electrolysis cells. Bulg. Chem. Commun. 2019, 51, 282–286. [Google Scholar] [CrossRef]
- Nishanthi, R.; Malathi, S.; Palani, P. Green synthesis and characterization of bioinspired silver, gold and platinum nanoparticles and evaluation of their synergistic antibacterial activity after combining with different classes of antibiotics. Mater. Sci. Eng. C 2019, 96, 693–707. [Google Scholar]
- Holt-Hindle, P.; Yi, Q.; Wu, G.; Koczkur, K.; Chen, A. Electrocatalytic activity of nanoporous Pt–Ir materials toward methanol oxidation and oxygen reduction. J. Electrochem. Soc. 2007, 155, K5. [Google Scholar] [CrossRef]
- Call, D.F.; Merrill, M.D.; Logan, B.E. High surface area stainless steel brushes as cathodes in microbial electrolysis cells. Environ. Sci. Technol. 2009, 43, 2179–2183. [Google Scholar] [CrossRef]
- Yang, J.; Cheng, S.; Li, C.; Sun, Y.; Huang, H. Shear stress affects biofilm structure and consequently current generation of bioanode in microbial electrochemical systems (MESS). Front. Microbiol. 2019, 10, 398. [Google Scholar] [CrossRef] [PubMed]
- Su, S.-G.; Cheng, H.-Y.; Zhu, T.-T.; Wang, H.-C.; Wang, A.-J. Kinetic competition between microbial anode respiration and nitrate respiration in a bioelectrochemical system. Bioelectrochemistry 2018, 123, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Rozenfeld, S.; Ouaknin Hirsch, L.; Gandu, B.; Farber, R.; Schechter, A.; Cahan, R. Improvement of microbial electrolysis cell activity by using anode based on combined plasma-pretreated carbon cloth and stainless steel. Energies 2019, 12, 1968. [Google Scholar] [CrossRef]
- Cai, W.; Liu, W.; Han, J.; Wang, A. Enhanced hydrogen production in microbial electrolysis cell with 3D self-assembly nickel foam-graphene cathode. Biosens. Bioelectron. 2016, 80, 118–122. [Google Scholar] [CrossRef]
- Kim, B.H.; Lim, S.S.; Daud, W.R.W.; Gadd, G.M.; Chang, I.S. The biocathode of microbial electrochemical systems and microbially-influenced corrosion. Bioresour. Technol. 2015, 190, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Rozendal, R.A.; Jeremiasse, A.W.; Hamelers, H.V.; Buisman, C.J. Hydrogen production with a microbial biocathode. Environ. Sci. Technol. 2008, 42, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.S.; Kim, B.H.; Li, D.; Feng, Y.; Daud, W.R.W.; Scott, K.; Yu, E.H. Effects of applied potential and reactants to hydrogen-producing biocathode in a microbial electrolysis cell. Front. Chem. 2018, 6, 318. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Jia, J.; Lu, J.; Yang, L.; Hou, D.; Li, G.; Chen, S. Recent developments of carbon-based electrocatalysts for hydrogen evolution reaction. Nano Energy 2016, 28, 29–43. [Google Scholar] [CrossRef]
- Mitov, M.; Chorbadzhiyska, E.; Rashkov, R.; Hubenova, Y. Novel nanostructured electrocatalysts for hydrogen evolution reaction in neutral and weak acidic solutions. Int. J. Hydrogen Energy 2012, 37, 16522–16526. [Google Scholar] [CrossRef]
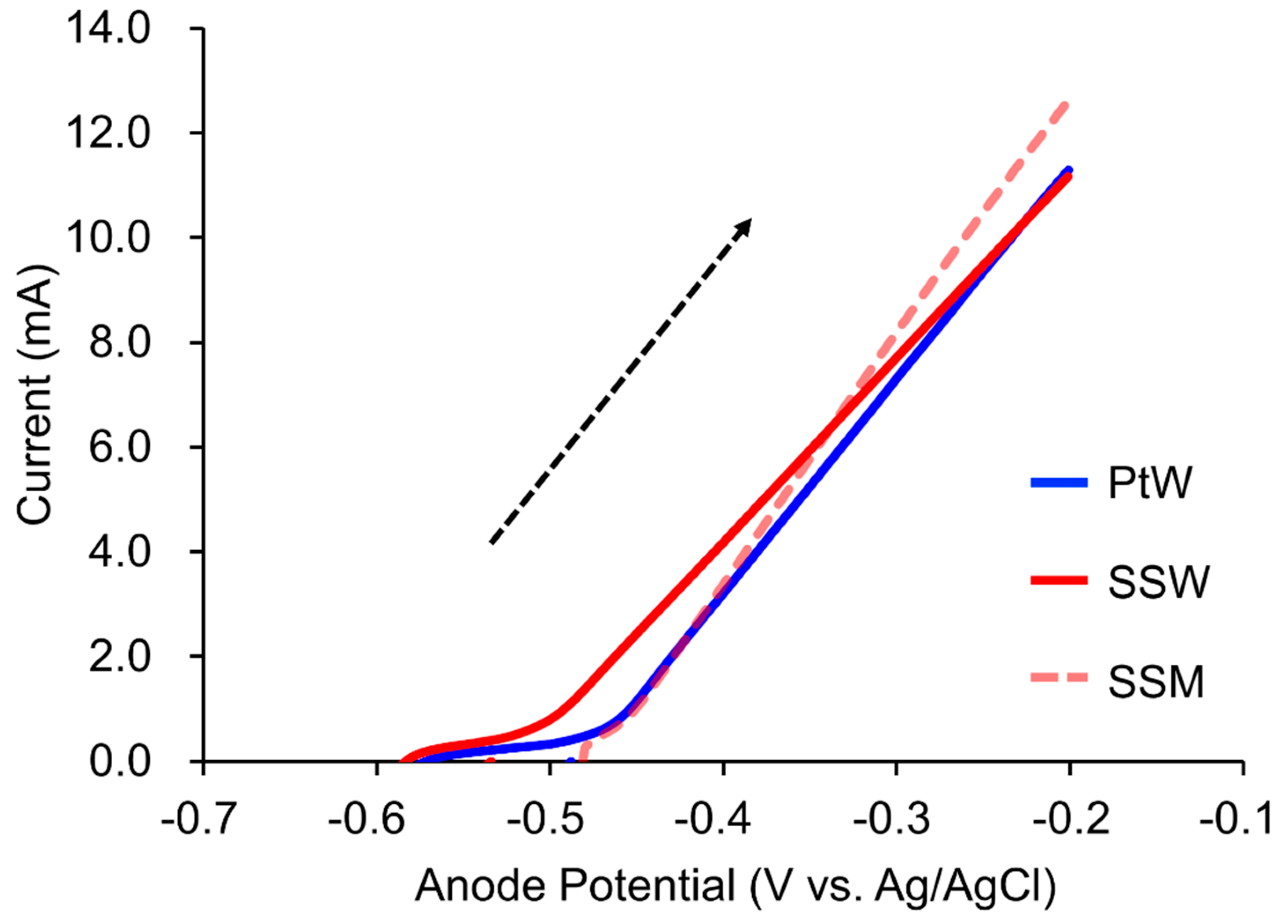


| IB Anode LSV Test (20 mM Acetate) | ||||||
|---|---|---|---|---|---|---|
| Counter Electrode | Imax (mA) | Cv (%) | Ef (V) | Cv (%) | m (mA/V) | Cv (%) |
| PtW | 11.05 ± 0.24 | 2.17 | −0.45 ± 0.00 | 0.03 | 39.86 ± 0.99 | 2.49 |
| SSW | 11.11 ± 0.05 | 0.45 | −0.49 ± 0.00 | 0.42 | 35.27 ± 0.12 | 0.34 |
| SSM | 12.59 ± 0.06 | 0.48 | −0.45 ± 0.00 | 0.47 | 47.09 ± 0.05 | 0.09 |
| SSM cathode LSV test (20 mM acetate) | ||||||
| Counter electrode | Imax (mA) | Cv (%) | Ef (V) | Cv (%) | m (mA/V) | Cv (%) |
| IB | −8.79 ± 0.15 | 1.76 | −0.87 ± 0.10 | 1.24 | 25.10 ± 0.90 | 3.58 |
| AB | −7.12 ± 0.25 | 3.43 | −0.93 ± 0.01 | 1.02 | 22.82 ± 1.18 | 5.18 |
| PtW | −7.96 ± 0.16 | 2.01 | −0.88 ± 0.01 | 1.13 | 22.35 ± 1.15 | 5.14 |
| SSW | −7.15 ± 0.19 | 2.72 | −0.87 ± 0.01 | 0.81 | 19.50 ± 0.30 | 1.54 |
| SSM | −6.53 ± 0.23 | 3.44 | −0.89 ± 0.00 | 0.19 | 18.70 ± 0.20 | 1.07 |
| SSM cathode LSV test (non-acetate) | ||||||
| Counter electrode | Imax (mA) | Cv (%) | Ef (V) | Cv (%) | m (mA/V) | Cv (%) |
| IB | −8.87 ± 0.15 | 1.71 | −0.91 ± 0.00 | 0.38 | 16.85 ± 0.45 | 2.67 |
| AB | −6.19 ± 0.17 | 2.73 | −0.99 ± 0.04 | 1.02 | 20.94 ± 1.31 | 6.25 |
| PtW | −6.07 ± 0.07 | 1.24 | −0.94 ± 0.01 | 0.56 | 16.00 ± 0.80 | 5.00 |
| SSW | −6.49 ± 0.27 | 4.17 | −0.95 ± 0.00 | 0.15 | 16.75 ± 0.25 | 1.49 |
| SSM | −6.44 ± 0.23 | 3.64 | −0.93 ± 0.00 | 0.04 | 15.95 ± 0.05 | 0.31 |
| Ni-AC-SSM cathode LSV test (20 mM acetate) | ||||||
| Counter electrode | Imax (mA) | Cv (%) | Ef (V) | Cv (%) | m (mA/V) | Cv (%) |
| IB | −16.01 ± 1.41 | 8.81 | N/D | N/D | 11.17 ± 2.09 | 18.67 |
| AB | −16.99 ± 3.73 | 21.95 | N/D | N/D | 22.35 ± 8.37 | 37.43 |
| PtW | −13.26 ± 0.47 | 3.54 | N/D | N/D | 14.72 ± 1.02 | 6.94 |
| SSW | −13.96 ± 1.27 | 9.13 | N/D | N/D | 15.62 ± 0.48 | 3.04 |
| SSM | −13.59 ± 2.02 | 14.82 | N/D | N/D | 15.50 ± 0.29 | 1.89 |
| Ni-AC-SSM cathode LSV test (non-acetate) | ||||||
| Counter electrode | Imax (mA) | Cv (%) | Ef (V) | Cv (%) | m (mA/V) | Cv (%) |
| IB | −15.73 ± 1.15 | 7.31 | N/D | N/D | 14.03 ± 2.11 | 15.09 |
| AB | −12.44 ± 0.30 | 2.43 | N/D | N/D | 21.18 ± 7.57 | 35.75 |
| PtW | −13.11 ± 0.08 | 0.61 | N/D | N/D | 14.04 ± 0.44 | 3.16 |
| SSW | −11.95 ± 0.15 | 1.21 | N/D | N/D | 15.77 ± 0.16 | 1.04 |
| SSM | −13.57 ± 0.70 | 5.15 | N/D | N/D | 13.16 ± 1.33 | 10.12 |
| Anode LSV Tests | |||||||
|---|---|---|---|---|---|---|---|
| Counter Electrode | Imax (mA) | Cv (%) | Ef (V) | Cv (%) | m (mA/V) | Cv (%) | Reference |
| SSW | 11.11 ± 0.05 | 0.45 | −0.49 ± 0.00 | 0.42 | 35.27 ± 0.12 | 0.34 | This study |
| Pt/C a | 16.36 ± 0.32 | 1.93 | N/D | N/D | N/D | N/D | [74] |
| Ni foam b | 2.80 ± 0.18 | 6.25 | −0.32 | N/D | N/D | N/D | [72] |
| AB c | N/D | N/D | −0.41 ± 0.01 | 2.44 | N/D | N/D | [73] |
| Cathode LSV tests (non-acetate) | |||||||
| Counter electrode | Imax (mA) | Cv (%) | Ef (V) | Cv (%) | m (mA/V) | Cv (%) | Reference |
| PtW | −13.11 ± 0.08 | 0.61 | N/D | N/D | 14.04 ± 0.44 | 3.16 | This study |
| PtW d | −2.17 ± 0.01 | 0.28 | 0.02 ± 0.00 | 9.52 | N/D | N/D | [55] |
| PtW e | N/D | N/D | −0.40 | N/D | 5.44 | N/D | [68] |
| Pt plate f | −8.90 ± 0.50 | 5.61 | N/D | N/D | N/D | N/D | [54] |
| Pt plate g | −2.26 ± 0.09 | 4.16 | N/D | N/D | N/D | N/D | [56] |
| Pt-Ti mesh h | −74.25 ± 1.35 | 1.82 | −0.69 ± 0.01 | 1.16 | 43.8 ± 1.7 | 3.88 | [80] |
| Pt foil i | −5.95 | N/D | 0.08 | N/D | N/D | N/D | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, H.; Koo, B.; Son, S.; Jung, S.P. Validity and Reproducibility of Counter Electrodes for Linear Sweep Voltammetry Test in Microbial Electrolysis Cells. Energies 2024, 17, 2674. https://doi.org/10.3390/en17112674
Chai H, Koo B, Son S, Jung SP. Validity and Reproducibility of Counter Electrodes for Linear Sweep Voltammetry Test in Microbial Electrolysis Cells. Energies. 2024; 17(11):2674. https://doi.org/10.3390/en17112674
Chicago/Turabian StyleChai, Hyungwon, Bonyoung Koo, Sunghoon Son, and Sokhee Philemon Jung. 2024. "Validity and Reproducibility of Counter Electrodes for Linear Sweep Voltammetry Test in Microbial Electrolysis Cells" Energies 17, no. 11: 2674. https://doi.org/10.3390/en17112674
APA StyleChai, H., Koo, B., Son, S., & Jung, S. P. (2024). Validity and Reproducibility of Counter Electrodes for Linear Sweep Voltammetry Test in Microbial Electrolysis Cells. Energies, 17(11), 2674. https://doi.org/10.3390/en17112674







